Abstract
In a high‐risk patient with subacute heparin‐induced thrombocytopenia (HIT) type A (platelet count recovery following acute HIT but with persisting platelet‐activating antibodies), in whom urgent cardiac surgery was required, a key clinical question arose: could intraoperative heparin be given safely with “platelet anesthesia” provided with high‐dose intravenous immunoglobulin (IVIG) plus cangrelor (ultra‐short‐acting antiplatelet agent)? This approach proved successful, without unexpected postoperative thrombocytopenia or thromboembolism. In vitro studies confirmed that both IVIG and cangrelor contributed to perioperative inhibition of HIT antibody‐induced platelet activation. Interestingly, despite the patient testing strongly positive in 4 HIT immunoassays (latex immunoturbidimetric assay and 3 enzyme‐immunoassays), the serotonin‐release assay (SRA) was consistently negative. Nevertheless, platelet‐activating HIT antibodies were detectable using modified (platelet factor 4–enhanced) SRA. Our protocol of heparin rechallenge following IVIG/cangrelor provides both intraoperative and early postoperative inhibition of HIT antibody‐induced platelet activation and is applicable to patients with circulating functional HIT antibodies requiring urgent heart surgery, including those with “SRA‐negative HIT.”
Keywords: antibodies, cardiopulmonary bypass, heparin, intravenous immunoglobulin, thrombocytopenia, thrombosis
Essentials.
Strategies are required for safe heparin use for cardiac surgery in subacute heparin‐induced thrombocytopenia (HIT) type A.
High‐dose intravenous immunoglobulinand cangrelor platelet “anesthesia” permitted successful surgery with heparin.
In vitro studies showed that each treatment contributed to successful platelet inhibition.
This case indicates that serotonin‐release assay–negative HIT can trigger severe thrombosis
1. INTRODUCTION
Subacute heparin‐induced thrombocytopenia (HIT) type A indicates platelet count recovery following acute HIT but where heparin‐dependent platelet‐activating antibodies remain detectable by platelet activation assay. 1 Reexposure to unfractionated heparin (UFH) in this setting risks triggering rapid‐onset HIT. For such a patient who requires urgent cardiac surgery, nonheparin anticoagulation for cardiopulmonary bypass (CPB) can be performed with bivalirudin, a short‐acting direct thrombin inhibitor; however, this strategy is primarily used for lower‐risk patients, 2 as its use in higher‐risk patients carries risk of severe bleeding. 3 Other options include administering heparin after reducing HIT antibody titers using therapeutic plasma exchange, 4 or with inhibition of HIT‐associated platelet activation with concomitant intraoperative administration of a short‐acting antiplatelet agent (“platelet anesthesia”). 1 , 5 However, rebound HIT‐associated hypercoagulability could occur once the intraoperative anticoagulant and antiplatelet effects wear off.
High‐dose intravenous immunglobulin (IVIG) inhibits HIT antibody‐induced platelet activation in a predictable, dose‐dependent fashion, usually for several days. 6 , 7 , 8 We report using the combined strategy of high‐dose IVIG plus cangrelor (ultra‐short‐acting antiplatelet agent) for a patient with subacute HIT type A who required urgent cardiac reoperation for HIT‐associated intracardiac thrombosis. Despite such severe HIT‐associated complications, our patient had serotonin‐release assay (SRA)‐negative HIT. 9 , 10 , 11 We therefore used a modified SRA to demonstrate inhibition of platelet‐activating HIT antibodies in this patient.
2. CASE REPORT
A 64‐year‐old male with severely impaired cardiac and renal function underwent urgent coronary artery bypass grafting and mitral valve replacement (mechanical prosthesis), with postoperative anticoagulation with UFH. On postoperative day (POD) 8, the platelet count fell below 100 × 109 L−1; HIT was suspected (high‐probability 4Ts score), and the diagnosis was confirmed with a strong‐positive IgG‐specific chemiluminescence immunoassay 12 (CLIA; HemosIL AcuStar, Werfen) result of 17.6 U mL−1 (Figure 1). Anticoagulation was changed to argatroban. On POD 10, routine echocardiography revealed a large, shell‐like thrombosis involving the wall and roof of the left atrium requiring urgent surgical thrombectomy. Repeat CLIA (POD 15) was 10.8 U mL−1, indicating likely persistence of heparin‐dependent platelet‐activating antibodies. As bivalirudin risk seemed too great, the strategy chosen was to await platelet count recovery (ie, allowing the patient to transition from acute to subacute HIT) and perform intraoperative anticoagulation with UFH and a new strategy combining IVIG and cangrelor for perioperative inhibition of HIT. We administered IVIG (Privigen, CSL Behring) 1 g kg−1 (given postanesthesia induction) at the time of redo surgery on POD 16. Ten minutes before systemic heparinization for CPB (400 IU kg−1), a bolus of 30 µg kg−1 of the P2Y12 (ADP) receptor antagonist, cangrelor, was administered, with a constant cangrelor infusion of 4 µg kg−1min−1. Thrombectomy of the large thromboses from the left atrial wall was performed. After 95 minutes of CPB, protamine was given and cangrelor infusion stopped. During the intraoperative period red cell concentrates (6 units) and 30 IU kg‐1 of prothrombin complex concentrate (not containing heparin [Cofact 500, Sanquin]) were transfused. No platelet transfusions were given. Argatroban infusion was restarted in the intensive care unit 6 hours after surgery. The 12‐hour postoperative drainage was 1100 mL. The preoperative platelet count was 375 × 109 L−1; the immediate postoperative platelet count was 206 × 109 L−1, with a further decline to 129 over the next 4 days (expected period of postoperative thrombocytopenia). No thromboembolic complications were observed, and the patient was discharged to home 3 weeks after redo surgery. Histopathology of the atrial thrombus revealed granulocyte‐rich and fibrin‐rich hemorrhagic material.
Figure 1.
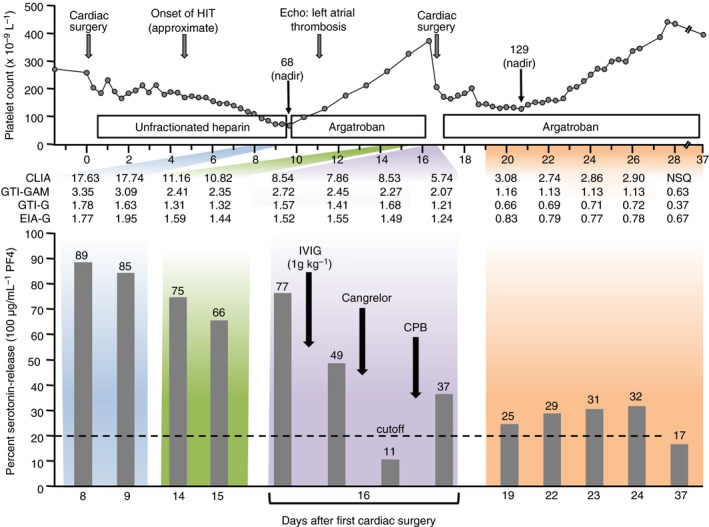
Clinical and laboratory data in patient with post–cardiac surgery SRA‐negative HIT who required urgent repeat cardiac surgery. The top panel shows the serial platelet counts, anticoagulant administration, and other key events. The middle section shows serial CLIA, GTI‐GAM (commercial polyspecific anti‐PF4/polyvinyl sulfonate EIA [LIFECODES PF4 Enhanced, Immucor GTI Diagnostics,Inc]), GTI‐G (commercial IgG specific anti‐PF4/polyvinyl sulfonate EIA [LIFECODES PF4 IgG,Immucor GTI Diagnostics,Inc]), and EIA‐G (in‐house IgG‐specific EIA of the McMaster Platelet Immunology Laboratory). The lower panel shows the percent serotonin‐release (100 ug mL‐1 PF4). CLIA, chemiluminenscence immunoassay; CPB, cardiopulmonary bypass; Echo, echocardiogram; EIA‐G, IgG‐specific enzyme‐immunoassay; GTI‐G, IgG‐specific enzyme‐immunoassay from GTI Diagnostics; GTI‐GAM, polyspecific enzyme‐immunoassay from GTI Diagnostics; HIT, heparin‐induced thrombocytopenia; IVIG, intravenous immunoglobulin; NSQ, not sufficient quantity; PF4, platelet factor 4
The patient’s serum and plasma tested consistently negative in the SRA. However, patient serum (POD 8 sample) induced strong serotonin‐release (~88%) in the platelet factor 4 (PF4)‐SRA 10 as well as in the PF4/heparin‐SRA (data not shown). 11 Figure 1 (lower panel) shows the results of the PF4‐SRA at multiple time points. Immediately prior to repeat CPB with UFH, patient serum caused 77% serotonin‐release by PF4‐SRA; this was reduced to 49% release after IVIG, and further reduced to 11% after starting cangrelor. Following CPB and stopping cangrelor, there was rebound in serotonin release to 37%, with low levels of serum‐induced serotonin release (ranging from 25% to 32%) observed in the subsequent days. Serial enzyme immunoassay (EIA) reactivity did not appreciably change on POD 16 despite the abrupt decrease in platelet activation associated with IVIG and cangrelor administration.
3. DISCUSSION
In patients with acute or recent (subacute) HIT, where platelet‐activating HIT antibodies continue to circulate, the concept of anticoagulation for CPB with UFH and concomitant platelet anesthesia is an established strategy. 1 , 6 The ultra‐short‐acting platelet P2Y12 (ADP) receptor inhibitor cangrelor appears ideal for this indication. This agent has no adverse hemodynamic effects, potent inhibition of platelet aggregation is immediately achieved, and elimination is independent of renal/liver function. After infusion termination, platelet function recovers within 40‐60 minutes. Moreover, ADP is known to potentiate HIT antibody‐induced platelet activation. 13 Thus, combined treatment with IVIG provides dual inhibition of HIT antibody‐induced platelet activation. Additionally, cangrelor could attenuate platelet activation caused by CPB itself. 14
However, cangrelor monotherapy has potential limitations in the setting of heparin reexposure for patients with subacute HIT type A. First, rapid loss of antiplatelet activity of cangrelor could lead to rebound in HIT antibody‐induced platelet activation, given that PF4/heparin immune complexes formed during intraoperative heparin exposure—or that could form postoperatively due to heparin “rebound” phenomenon 15 —could activate platelets and coagulation prior to achieving postoperative anticoagulation with an agent such as argatroban. Second, HIT pathogenesis reflects more than FcγIIa receptor–mediated platelet activation: Recent concepts include Fcγ receptor–mediated activation of monocytes and neutrophils, including formation of neutrophil extracellular traps (NETosis). 16 , 17 Unlike the platelet tropic effects of cangrelor, high‐dose IVIG likely attenuates HIT antibody‐induced pancellular activation (platelets, monocytes, neutrophils). 7 , 8 Thus, combined IVIG/cangrelor is an ideal combination for enhancing platelet anesthesia (through dual inhibition of platelet activation) including beyond the transient period of intraoperative cangrelor administration.
Three observations warrant further comment. First, despite reexposure to UFH for redo cardiac surgery, there was no “boosting” of HIT antibody levels over the ensuing days/weeks, as reactivity in all immunoassays (CLIA and 3 EIAs) continued to decrease following the redo surgery (Figure 1). (Unlike platelet activation assays, immunoassay results are not affected by administration of high‐dose IVIG. 7 , 8 ) This observation is in line with a previous report describing brief UFH reexposure for CPB in patients with subacute HIT type B (ie, where platelet‐activating HIT antibodies are no longer detectable, although immunoassay reactivity remains positive). 18 However, these previously reported patients underwent heart transplantation and therefore received immunosuppressive and immunomodulating therapies. Our observations indicate that HIT antibody restimulation does not necessarily occur even in the absence of immunosuppressive therapy.
Second, despite our patient’s high‐probability clinical picture of HIT (4Ts, 8 points), 19 and strong‐positive CLIA and EIA results, our patient’s blood unexpectedly yielded a negative SRA. We therefore investigated the patient for heparin‐dependent platelet‐activating antibodies using 2 other platelet activation tests, the PF4‐SRA 10 and the PF4/H‐SRA. 11 These modified SRAs both yielded strong‐positive results, indicating a diagnosis of SRA‐negative HIT. A recent study 10 from the McMaster Platelet Immunology Laboratory found that <5% of HIT patients test negative in the conventional SRA but give strong results in a modified SRA, either the PF4‐SRA (performed with high concentrations of PF4, rather than heparin), or in the PF4/H‐SRA (performed with optimal concentrations of PF4 and heparin). Nevertheless, by performing the PF4‐SRA and the PF4/H‐SRA using serial blood samples obtained from the patient, we were able to demonstrate that high‐dose IVIG was able to inhibit the patient’s HIT antibody‐induced platelet‐activating properties, as shown using the conventional SRA in previous patients treated with high‐dose IVIG. 7 , 8 Our report thus shows for the first time efficacy of high‐dose IVIG in a patient with SRA‐negative HIT.
Third, the important role of neutrophils and NETosis in selected fields of arterial and venous thrombosis is increasingly appreciated. 20 The thrombotic material of our patient was not a typical platelet‐rich “white clot,” but predominantly consisted of granulocytes and fibrin. This finding provides clinical evidence that neutrophils may play an important role in HIT‐associated thrombosis. This observation may support contemporary models of “dynamic intercellular HIT.” 16 , 17
Fourth, the optimal dose for IVIG in the setting of acute/subacute type A thrombotic HIT remains uncertain. 6 , 7 , 8 In our patient, inhibition of the PF4‐SRA after 1g kg−1 IVIG bolus was incomplete, with further inhibition provided by short‐term cangrelor. We also observed some rebound of patient serum‐induced platelet activation after surgery (Figure 1). Although the clinical course during and following the second surgery was favorable, without evidence of HIT‐associated complications, it is plausible that a higher dose of IVIG (either an initial dose of 2g kg−1 or a second 1g kg−1 bolus given after surgery) may have provided a greater degree of inhibition of platelet activation (through effects on ex vivo data as presented in Figure 1 with presumably parallel in vivo effects), given the dose‐dependent action of IVIG upon platelet activation. 6 , 7 , 8
Our patient’s successful clinical outcome suggests that dual platelet inhibition with high‐dose IVIG and cangrelor is a reasonable treatment approach for patients with subacute HIT type A. The distinct platelet‐inhibitory mechanisms of cangrelor and IVIG, their differing time periods of action (minutes and days, respectively), and potential for pancellular inhibition by IVIG, are compelling concepts. Our experience also shows how the effects of these platelet‐inhibiting therapies can be evaluated in patients such as ours with the recently reported entity of SRA‐negative HIT.
RELATIONSHIP DISCLOSURE
TEW has received lecture honoraria from Instrumentation Laboratory and royalties from Informa (Taylor & Francis); has provided consulting services to Aspen Global, Bayer, CSL Behring, Ergomed, and Octapharma; has received research funding from Instrumentation Laboratory; and has provided expert witness testimony relating to HIT and non‐HIT thrombocytopenic and coagulopathic disorders. AK, IN, IEB, JWS, and JIS declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
AK and IB delivered patient care. AK developed the clinical protocol, abstracted the clinical and laboratory data, and arranged for collection of patient serum and plasma. JIS performed the immunoassays and prepared the figure. JWS performed the SRA, PF4‐SRA, and PF4/H‐SRA. IN and TEW supervised the laboratory studies. AK and TEW jointly prepared the initial draft of the manuscript. All authors reviewed and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank Tobias Flieder for taking care of the samples and providing some of the clinical data.
Koster A, Nazy I, Birschmann IE, Smith JW, Sheppard J-AI, Warkentin TE. High-dose IVIG plus cangrelor platelet “anesthesia” during urgent heparin-CPB in a patient with recent SRA-negative HIT-thrombosis with persisting platelet-activating antibodies. Res Pract Thromb Haemost. 2020;4:1060–1064. 10.1002/rth2.12348
Handling Editor: Dr Neil Zakai.
REFERENCES
- 1. Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin‐induced thrombocytopenia. Blood Adv. 2018;2:3360–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koster A, Dyke CM, Aldea G, Smedira NG, McCarthy HL II, Aronson S, et al. Bivalirudin during cardiopulmonary bypass inpatients with previous or acute heparin‐induced thrombocytopenia and heparin antibodies: Results of the CHOOSE‐ON trial. Ann Thorac Surg. 2007;83:572–7. [DOI] [PubMed] [Google Scholar]
- 3. Nhieu S, Nguyen L, Pretorius V, Ovando J, Moore D, Banks D, et al. CASE 1–2015: left ventricular assist device insertion in a patient with heparin‐induced thrombocytopenia and renal failure. J Cardiothorac Vasc Anesth. 2015;29:210–20. [DOI] [PubMed] [Google Scholar]
- 4. Warkentin TE, Sheppard JI, Chu FV, Kapoor A, Crowther MA, Gangji A. Plasma exchange to remove HIT antibodies: dissociation between enzyme‐immunoassay and platelet activation test reactivities. Blood. 2015;125:195–8. [DOI] [PubMed] [Google Scholar]
- 5. Gernhofer YK, Banks DA, Golts E. Pretorius V. Novel use of cangrelor with heparin during cardiopulmonary bypass in patients with heparin‐induced thrombocytopenia who require cardiovascular surgery: A case series. Semin Thorac Cardiovasc Surg. 2019; 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6. Padmanabahn A, Jones CG, Pechauer SM, Curtis BR, Bougie DW, Irani MS, et al. IVIg for treatment of severe refractory heparin‐induced thrombocytopenia. Chest. 2017;152:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Warkentin TE, Climans TH, Morin PA. Intravenous immune globulin to prevent heparin‐induced thrombocytopenia. N Engl J Med. 2018;378:1845–8. [DOI] [PubMed] [Google Scholar]
- 8. Warkentin TE. High‐dose intravenous immunoglobulin for the treatment and prevention of heparin‐induced thrombocytopenia: a review. Exp Rev Hematol. 2019;12:685–98. [DOI] [PubMed] [Google Scholar]
- 9. Pandya KA, Johnson EG, Davis GA, Padmanabhan A. Serotonin release assay (SRA)‐negative HIT, a newly recognized entity: implications for diagnosis and management. Thromb Res. 2018;172:169–71. [DOI] [PubMed] [Google Scholar]
- 10. Warkentin TE, Nazy I, Sheppard JI, Smith JW, Kelton JG, Arnold DM. Serotonin‐release assay‐negative heparin‐induced thrombocytopenia. Am J Hematol. 2020;95:38–47. [DOI] [PubMed] [Google Scholar]
- 11. Vayne C, Guery EA, Kizlik‐Masson C, Rollin J, Bauters A, Gruel Y, et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin‐induced thrombocytopenia antibodies. Br J Haematol. 2017;179:811–9. [DOI] [PubMed] [Google Scholar]
- 12. Warkentin TE, Sheppard JI, Linkins LA, Arnold DM. Nazy I. High sensitivity and specificity of an automated IgG‐specific chemiluminescence immunoassay for diagnosis of HIT. Blood. 2018;132:1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polgár J, Eichler P, Greinacher A, Clemetson KJ. Adenosine diphosphate (ADP) and ADP receptor play a major role in platelet activation/aggregation induced by sera from heparin‐induced thrombocytopenia patients. Blood. 1998;91:549–545. [PubMed] [Google Scholar]
- 14. Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–33. [DOI] [PubMed] [Google Scholar]
- 15. Jia Z, Tian G, Ren Y, Sun Z, Lu W, Hou X. Pharmacokinetic model of unfractionated heparin during and after cardiopulmonary bypass in cardiac surgery. J Transl Med. 2015;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tutwiler V, Madeeva D, Ahn HS, Andrianova I, Hayes V, Zheng XL, et al. Platelet transactivation by monocytes promotes thrombosis in heparin‐induced thrombocytopenia. Blood. 2016;127:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perdomo J, Leung HHL, Ahmadi Z, Yan F, Chong JJH, Passam FH, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin‐induced thrombocytopenia. Nat Commun. 2019;10(1):1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selleng S, Haneya A, Hirt S, Selleng K, Schmid C, Greinacher A. Management of anticoagulation in patients with subacute heparin‐induced thrombocytopenia scheduled for heart transplantation. Blood. 2008;112:4024–7. [DOI] [PubMed] [Google Scholar]
- 19. Warkentin TE, Linkins LA. Non‐necrotizing heparin‐induced skin lesions and the 4T’s score. J Thromb Haemost. 2010;8:1483–5. [DOI] [PubMed] [Google Scholar]
- 20. Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. 2019;45:86–93. [DOI] [PubMed] [Google Scholar]


