Abstract
Background
Western blotting is used to measure protein expression in cells and tissues. Appropriate interpretation of resulting data is contingent upon antibody validation.
Objectives
We assessed several commercial anti‐human and anti‐mouse tissue factor (TF) antibodies for their ability to detect TF by western blotting.
Material and Methods
We used human pancreatic cancer cell lines expressing different levels of TF and a mouse pancreatic cancer cell line expressing TF with a matched knockout derivative.
Results
Human and mouse TF protein detected by western blotting correlated with levels of TF mRNA in these cell lines. The apparent molecular weight of TF is increased by N‐linked glycosylation and, as expected, deglycosylation decreased the size of TF based on western blotting. We found that four commercial anti‐human TF antibodies detected TF in a TF‐positive cell line HPAF‐II whereas no signal was observed in a TF‐negative cell line MIA PaCa‐2. More variability was observed in detecting mouse TF. Two anti‐mouse TF antibodies detected mouse TF in a TF‐positive cell line and no signal was observed in a TF knockout cell line. However, a third anti‐mouse TF antibody detected a nonspecific protein in both the mouse TF‐positive and TF‐negative cell lines. Two anti‐human TF antibodies that are claimed to cross react with mouse TF either recognized a nonspecific band or did not detect mouse TF.
Discussion
Our results indicate that there is a range in quality of commercial anti‐TF antibodies.
Conclusion
We recommend that all commercial antibodies should be validated to ensure that they detect TF.
Keywords: antibody, glycosylation, tissue factor, validation, western blotting
Essentials.
Western blotting is commonly used to detect tissue factor (TF).
We assessed commercial anti‐TF antibodies for their ability to detect either human or mouse TF.
Four commercial anti‐human TF antibodies successfully detected TF in cell extracts from cell lines.
Detection of mouse TF was more variable with some antibodies failing to recognize mouse TF.
1. INTRODUCTION
Concerns have been raised about the reproducibility of preclinical research. 1 , 2 This led the National Institutes of Health to describe the principles and guidelines for reporting preclinical research (https://www.nih.gov/research‐training/rigor‐reproducibility/principles‐guidelines‐reporting‐preclinical‐research). The section entitled “Consider Establishing Best Practice Guidelines” suggests that authors provide a description of biological material “with enough information to uniquely identify the reagent.” For antibodies, the source, dilution, and how they were validated should be included. These guidelines have been endorsed by many journals, including Arteriosclerosis, Thrombosis and Vascular Biology. 3
Scientific studies use western blotting to measure levels of a given protein in cells and tissues. However, this technique requires use of validated polyclonal or monoclonal antibodies, which can be obtained from investigators or companies. Polyclonal antibodies generally give a stronger signal in western blotting than monoclonal antibodies because they bind to multiple epitopes on the protein, whereas monoclonal antibodies have a higher specificity. In 2016, the International Working Group for Antibody Validation was convened to highlight the need for validating antibodies in common research applications. 4 It provided guidelines aimed to ensure antibody targeting and data reproducibility. For western blotting, several recommendations were made. Among them are (i) eliminating or reducing protein expression through genome editing or RNA interference to alter the levels of the target protein, (ii) performing comparative studies with antibodies that target the same protein at nonoverlapping epitopes, (iii) using an antibody‐independent method to analyze the protein, such as mass spectrometry, and (iv) labeling the target protein with an affinity tag to employ an independent method of detection.
Tissue factor (TF) is a transmembrane protein that functions as a high‐affinity receptor against factor VII (FVII) and FVIIa. 5 The TF‐FVIIa complex is the initiator of the coagulation protease cascade. The mature human TF is composed of 263 amino acids, and the predicted molecular weight without glycosylation is ~36 kDa. However, human TF has 3 N‐linked glycosylation sites (Asn 11, Asn 125, and Asn 137) in the extracellular domain that increase the apparent molecular weight to ~50 kDa. 6 , 7 , 8 , 9 , 10 Similarly, mature mouse TF is composed of 266 amino acids and has 4 N‐linked glycosylation sites (Asn 9, Asn 29, Asn 141, and Asn 172) that increase the apparent molecular weight from ~36 kDa to ~50 kDa. 11 , 12 Deglycosylation of human TF reduces its molecular weight to ~36 kDa. 13
An array of monoclonal antibodies have been generated against human TF. 14 , 15 , 16 , 17 , 18 In addition, numerous companies sell anti‐human TF monoclonal and polyclonal antibodies, including Novus Biologicals, Cell Signaling Technologies, Bio‐Rad Laboratories, Abcam, and BD Biosciences. There are far less well‐characterized antibodies against mouse TF. Three rat anti‐mouse TF monoclonal antibodies have been reported, including 2 that inhibit mouse TF (1H1 and 21E10). 19 , 20 Many companies sell anti‐mouse TF monoclonal and polyclonal antibodies, including R&D Systems, Abcam, and BioMedica Diagnostics.
In this study, we evaluated several commercial anti‐human and anti‐mouse TF antibodies for their ability to detect human and mouse TF by western blotting.
2. METHODS
2.1. Antibodies
Table 1 shows the in‐house and commercial anti‐human and mouse TF antibodies that were used in this study. The anti‐human TF mouse monoclonal antibody HTF1 has been described previously. 15 These antibodies were purchased from different companies or provided by colleagues (Dr C. Wu, University of Kentucky [Abcam ab189483] and Dr L. Sommerville, Duke University [BioMedica Diagnostics 4515]). A mouse anti‐human glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) polyclonal antibody (Santa Cruz Biotechnology, Cat #sc‐47724) and a goat anti‐mouse GAPDH polyclonal antibody (Santa Cruz Biotechnology, Cat #sc‐48167) were used as loading controls.
TABLE 1.
Anti‐human and anti‐mouse tissue factor antibodies that were assessed in the present study
| Target species | Host species | Ab type | Description | Company | Cat # | Lot # | Conc. | Application | WB validation |
|---|---|---|---|---|---|---|---|---|---|
| Human | Mouse | Monoclonal | TF9‐10H10 | In‐house | ‐ | ‐ | 1.0 mg/mL | IHC, WB | Y‐Human |
| Human | Mouse | Monoclonal | Mouse anti Human CD142 (TF9‐10H10) | Bio‐Rad | 9010‐5059 | 170131 | 1.0 mg/mL | FCM, IF, IHC, WB | Y‐Human |
| Human | Mouse | Monoclonal | Purified Mouse Anti‐Human CD142 (HTF‐1) | BD Biosciences | 550252 | 6357515 | 0.5 mg/mL | FCM, Inh | Y‐Human |
| Human | Rabbit | Polyclonal | Coagulation Factor III/Tissue Factor Antibody | Novus Biologicals | NBP2‐15139 | 42928 | 0.64 mg/mL | IHC, WB | Y‐Human |
| Human | Rabbit | Polyclonal | Tissue Factor/CD142 Antibody | Cell Signaling | 47769 | 1 | 0.055 mg/mL | WB | Y‐Human |
| Mouse | Goat | Polyclonal | Mouse Coagulation Factor III/Tissue Factor Antibody | R&D Systems | AF3178 | WQY0214032 | 0.2 mg/mL | FCM, IP, WB | Y‐Mouse |
| Mouse | Rabbit | Monoclonal | Recombinant Anti‐Tissue Factor antibody (ERP18160‐175) | Abcam | ab189483 | GR3196777‐2 | 0.576 mg/mL | IP, WB | Y‐Mouse |
| Mouse | Rabbit | Polyclonal | Rabbit anti‐mouse Tissue Factor IgG | Biomedica | 4515 | 150408 | 1.0 mg/mL | FCM, IHC, Inh, WB | N‐Mouse |
| Human a | Rabbit | Polyclonal | Anti‐Tissue Factor antibody | Abcam | ab104513 | GR3224342‐1 | 0.5 mg/mL | IHC, WB | N‐Mouse |
| Human b | Rabbit | Monoclonal | Anti‐Tissue Factor antibody (EPR8986) | Abcam | ab151748 | GR150188‐8 | 0.21 mg/mL | IHC, ICC, IF, WB | N‐Mouse |
FCM, flow cytometry; ICC, immunocytochemistry; IF, immunofluorescence; IHC, immunohistochemistry; Inh, inhibitory; IP, immunoprecipitation; N, no; WB, western blotting; Y, yes.
Predicted to cross react with mouse/rat.
Cross reacts with mouse/rat.
2.2. Cell lines
The human pancreatic cancer cell lines BxPC‐3, HPAF‐II, PANC‐1, and MIA PaCa‐2 were obtained from American Type Culture Collection (Manassas, VA, USA). AsPC‐1 was obtained from Dr Bruce A. Sullenger (Duke University). BxPC‐3 and AsPC‐1 cells were cultured in RPMI1640 medium (Thermo Fisher Scientific, Waltham, MA, USA; Cat #11875‐093). HPAF‐II cells were cultured in MEM (Thermo Fisher Scientific, Cat #11095‐080). PANC‐1 and MIA PaCa‐2 cells were cultured in Dulbecco’s Modified Eagle Medium (Thermo Fisher Scientific, Cat #11965‐092). All cells were grown in a 5% CO2 incubator. We established a mouse pancreatic cancer cell line called KPC2 from KPC mice (Kras G12D/+, p53 R172H/+, ElasC reER/+). 21 KPC2 cells were cultured in RPMI1640 medium (Thermo Fisher Scientific). All media contained 10% fetal bovine serum (Omega Scientific, Tarzana, CA, USA; Cat #FB‐02) and 1% antibiotics‐antimycotic (Thermo Fisher Scientific, Cat #15240‐062).
2.3. Generation of a mouse TF knockout cell line
The dimeric clustered regularly interspaced short palindromic repeats (CRISPR) RNA‐guided Fokl nuclease strategy was applied to knock out the mouse TF gene in KPC2 cells as described. 22 In brief, CRISPR plasmid pSQT1313 (dual guide RNA [gRNA] plasmid) and pSQT1601 (CRISPR‐associated protein 9 [Cas9] plasmid) were obtained from Addgene (Watertown, MA, USA; Cat #53370 and 53369). gRNA oligonucleotides were designed and generated to target the ATG start codon of the TF (F3) gene (TF gRNA plasmid). For transfection, two 6‐cm dishes of KPC2 cells with ~70% confluency were prepared the day before transfection. Two micrograms of Cas9 plasmid, 250 ng of linear puromycin plasmid (Clontech, Mountain View, CA, USA; Cat #631626) and 0.5 μg of TF gRNA plasmid were premixed in serum‐free RPMI1640 medium and cotransfected into KPC2 cells using Xtreme Gene 9 (Roche, Basel, Switzerland; Cat #XTG9‐RO) to generate TF knockout (KO) cells. Cas9 control cells were generated by only cotransfecting with the Cas9 plasmid and the linear puromycin plasmid. Transfected cells were subsequently cultured in medium containing 2 μg/mL of puromycin for selection. On day 10, 25 emerged cell colonies were selected using standard cloning cylinders (Fisher Scientific, Waltham, MA, USA; Cat #0790710). Standard PCR and cloning techniques were applied for screening positive colonies. Deletion of the TF gene was confirmed by sequencing each of the alleles.
2.4. Assessment of TF mRNA
We used the Genevestigator gene expression database (https://genevestigator.com/gv/) for assessment of TF mRNA levels in the human pancreatic cell lines. TF mRNA levels in the KPC2 Cas9 and KPC2 TF KO were measured using RT‐PCR and SYBR Green with primers that span the deletion site: forward 5′‐cttggacatggcgatcct‐3′ and reverse 5′‐gttgccactccaaattgtct‐3′.
2.5. Preparation of cell lysates and SDS‐PAGE
Cells were washed once with phosphate‐buffered saline (PBS), scraped, lysed in RIPA lysis buffer (MilliporeSigma, Billerica, MA, USA; Cat #20‐188) containing a protease inhibitor cocktail (Roche, Cat #11697498001) and lysates incubated on ice for 30 minutes. The protein concentration of samples was determined using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Cat #23227). Cell lysates were prepared for electrophoresis by addition of SDS sample buffer (Invitrogen, Carlsbad, CA, USA; Cat #NP0007) containing 2.5% β‐mercaptoethanol and heated to 95°C for 10 minutes. Forty micrograms of protein were loaded onto 4%‐20% gradient Mini‐PROTEAN TGX gels (BioRad Laboratories, Hercules, CA, USA; Cat #456‐1093). A protein marker (Gel Company, San Francisco, CA, USA; Cat #FPL‐008) was used as a standard. Proteins were separated by electrophoresis using Tris/Glycine/SDS buffer (BioRad Laboratories, Cat #161‐0732).
2.6. Western blotting
Proteins were transferred to polyvinylidene difluoride membranes (MilliporeSigma, Cat #IPFL00010) and blocked using protein‐free blocking buffer (Thermo Fisher Scientific, Cat #23227). Membranes were probed with various anti‐TF antibodies shown in Table 1. All primary antibodies were used at a 1:1000 dilution in blocking buffer. After washing with Tris‐buffered saline containing 0.1% Tween 20 (TBS‐T), membranes were incubated with either Alexa Fluor 647 chicken anti‐mouse IgG (Invitrogen, Cat #A21463), Alexa Fluor 680 rabbit anti‐goat IgG (Invitrogen, Cat #A21088), or IR Dye 680 RP goat anti‐rabbit IgG (LI‐COR Biosciences, Lincoln, NE, USA; Cat #926‐68071). All secondary antibodies were used at a 1:10 000 dilution in blocking buffer. Specific antigen‐antibody complexes were visualized using an Odyssey imaging system (LI‐COR Biosciences). Antibodies were removed with stripping buffer (Thermo Fisher Scientific, Cat #21059), and membranes were reprobed with either anti‐human GAPDH or anti‐mouse GAPDH antibodies. Both antibodies were diluted 1:1000 in blocking buffer. After washing with TBS‐T, membranes were incubated with either IR Dye 800 CW goat anti‐mouse IgG (LI‐COR Biosciences, Cat #926‐33210) or IR Dye 800 CW donkey anti‐goat IgG (LI‐COR Biosciences, Cat #926‐32214). An Odyssey imaging system was used to detect antibody‐protein complexes.
2.7. Deglycosylation
Deglycosylation of human and mouse TF was performed using a commercial enzymatic deglycosylation kit that contains incubation mix, denaturation solution, and detergent solution (Agilent Technologies, Santa Clara, CA, USA; Cat #GK80115). Cell lysates (100 μg) were dissolved in 30 μL of deionized water. Next, 10 μL of 5× incubation mix and 2.5 μL denaturation solution were added. The solution was mixed gently and incubated for 5 minutes at 100˚C. After cooling to room temperature, 2.5 μL of detergent solution was added and mixed. N‐Glycanase (2 μL), Sialidase A (3 μL), and O‐Glycanase (2 μL) were added to the solution, and samples were incubated at 37˚C for 3 hours.
2.8. Cellular TF activity assay
Cells were washed with PBS without calcium and magnesium (Thermo Fisher Scientific, Cat #14190‐250), reconstituted with Hank’s balanced salt solution without calcium and magnesium (Thermo Fisher Scientific, Cat #14170‐112), and disrupted by sonication (total of ~24 000 J) using a Q700 sonicator (Qsonica, Newtown, CT, USA). The clotting time was measured using a STart4 coagulation analyzer (Diagnostica Stago, Parsippany, NJ, USA). Briefly, 50 μL of sonicated cellular suspension were added to a mixture of 50 μL of 25 mmol/L CaCl2 solution and 50 μL of human (Pacific Hemostasis Coagulation Control Normal, Level 1, Thermo Fisher Scientific) or mouse plasma. Mouse plasma was prepared from whole blood of C57BL/6J mice by centrifugation at 4500 g for 15 minutes. TF activity was calculated using a standard curve generated by Innovin (Siemens, Munich, Germany). TF activity was normalized by protein concentration determined using a BCA assay.
3. RESULTS
3.1. Levels of TF mRNA, protein, and activity in various human and mouse pancreatic cancer cell lines
We selected 5 human pancreatic cancer cell lines that express high (BxPC‐3, HPAF‐II, AsPC‐1), very low (PANC‐1), and no (MIA PaCa‐2) TF. We used an anti‐human TF polyclonal antibody from Cell Signaling Technologies (Danvers, MA, USA) for western blotting. In general, levels of TF mRNA, protein and activity correlated with each other in the different lines (Figure 1). The only discrepancy was the relative levels of TF protein in the TF expressing lines vs their corresponding TF activity, which indicates a different TF protein:TF activity ratio in these lines. BxPC‐3, HPAF‐II, and AsPC‐1 expressed a ~50 kDa band on western blotting, which is consistent with the expected size of glycosylated TF (Figure 1B). 14 Several weaker nonspecific, higher‐molecular‐weight bands were observed in all the cell lines (Figure 1B).
FIGURE 1.
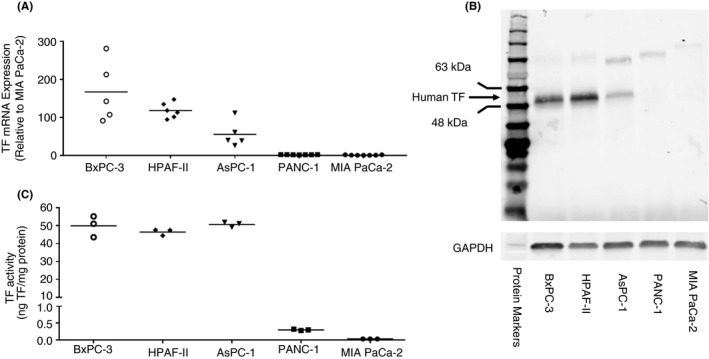
Tissue factor expression in human pancreatic cancer cell lines. We used five human pancreatic cancer cell lines that express different levels of tissue factor (TF). A, The level of TF mRNA in the different cell lines was from the Genevestigator gene expression database. B, The level of TF protein in the different cell lines was determined by western blotting using an anti‐human TF polyclonal antibody from Cell Signaling Technologies 47769 (1:1000 dilution, final concentration 0.055 μg/mL). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was detected using an anti‐human GAPDH antibody (1:1000 dilution, final concentration 0.2 μg/mL). Secondary antibodies were used at a 1:10 000 dilution in blocking buffer. A protein marker is shown on the left of the panel. C, The level of TF activity in the different cell lines was determined using a one stage clotting assay. Mean levels of TF mRNA and activity are shown
The mouse pancreatic cancer cell line KPC2 expresses a high level of TF. 21 For this study, 2 new derivative lines were generated for analysis. These include a KPC2 Cas9 control line and a KPC2 TF KO line. We used an anti‐mouse TF monoclonal antibody from Abcam (Cambridge, MA, USA; ab189483) for western blotting. Similar to the parental line KPC2 (not shown), KPC2 Cas9 cells expressed a high level of TF mRNA, protein, and activity, whereas no TF expression was detected in the KPC2 TF KO line (Figure 2). KPC2 Cas9 cells expressed an ~50‐kDa band on western blotting, which is consistent with the expected size of glycosylated mouse TF (Figure 2B). Two weaker and lower‐molecular‐weight bands (~35 and 30 kDa) were also observed in KPC2 Cas9 cell lysate. These may represent nonglycosylated forms of mouse TF. These data indicate that these human and mouse pancreatic cancer cell lines can be used to assess the ability of different commercial anti‐TF antibodies to detect TF by western blotting.
FIGURE 2.
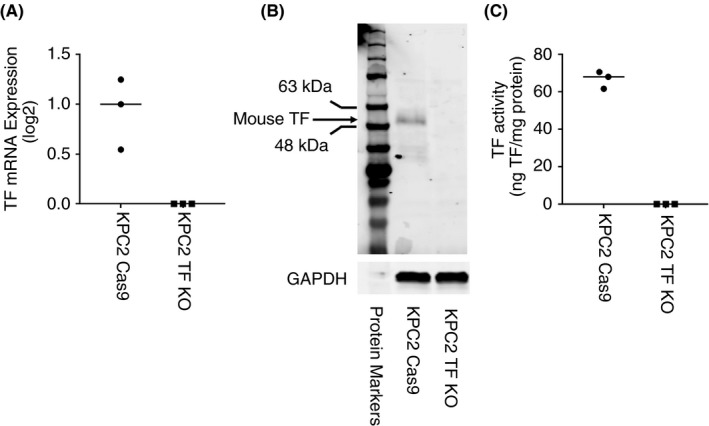
Tissue factor expression in mouse pancreatic cancer cell lines. We used a mouse pancreatic cancer cell line that expresses tissue factor (TF) (KPC2 Cas9) and a TF knockout line (KPC2 TF KO). A, The level of TF mRNA in the 2 cell lines was determined by RT‐PCR. B, The level of TF protein in the 2 cell lines was determined by western blotting using an anti‐mouse TF monoclonal antibody from Abcam ab189483 (1:1000 dilution, final concentration 0.576 μg/mL). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was detected using an anti‐mouse GAPDH antibody (1:1000 dilution, final concentration 0.2 μg/mL). Secondary antibodies were used at a 1:10 000 dilution in blocking buffer. A protein marker is shown on the left of the panel. C, The level of TF activity in the 2 cell lines was determined using a 1‐stage clotting assay. Mean levels of TF mRNA and activity are shown
3.2. Deglycosylation of human and mouse TF
Both human and mouse TF are glycosylated. Therefore, one would expect that the size of both proteins would be decreased by deglycosylation. We used an anti‐human TF polyclonal antibody from Cell Signaling Technologies and an anti‐mouse TF polyclonal antibody from Abcam (ab189483) for western blotting. Deglycosylation of human TF expressed by BxPC‐3 and HPAF‐II reduced the molecular weight from ~50 kDa to ~40 kDa (Figure 3A). An additional band was observed in the deglycosylated HPAF‐II sample that likely represents partially deglycosylated protein. Deglycosylation of mouse TF expressed by KPC2 Cas9 cells decreased the size to ~35 kDa (Figure 3B). A higher‐molecular‐weight band (~38 kDa) may be a partially deglycosylated product. In addition, 2 lower‐molecular‐weight bands were observed that might represent degradation products (Figure 3B). These results indicate that the ~50‐kDa band observed in TF expressing human and mouse pancreatic cancer cell lines is a glycosylated form of TF because its molecular weight is decreased by deglycosylation.
FIGURE 3.
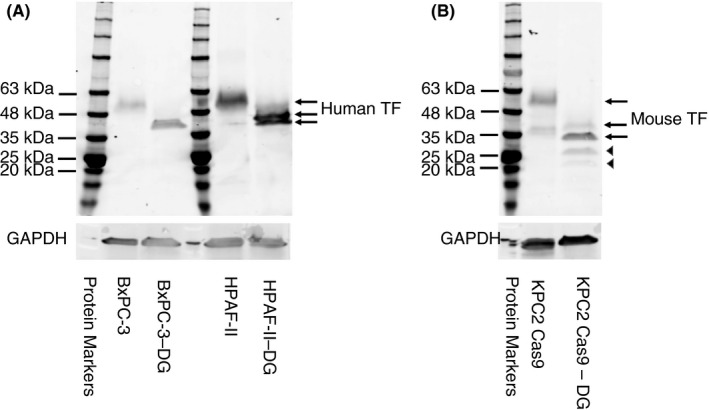
Deglycosylation of human and mouse tissue factor. We enzymatically deglycosylated cell lysates from human and mouse pancreatic cancer cell lines. We then determined the molecular weight of nondeglycosylated and deglycosylated human and mouse tissue factor (TF). The various forms of human and mouse TF are indicated by the arrows. Arrowheads indicate possible degradation products. Human and mouse TF were detected using Cell Signaling Technologies 49969 antibody (1:1000 dilution, final concentration 0.055 μg/mL) and Abcam ab189483 (1:1000 dilution, final concentration 0.576 μg/mL), respectively. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was detected using an anti‐human or anti‐mouse GAPDH antibody (1:1000 dilution, final concentration 0.2 μg/mL). Secondary antibodies were used at a 1:10 000 dilution in blocking buffer. A protein marker is shown on the left of each panel. DG, deglycosylation
3.3. Detection of human TF by western blotting using commercial anti‐human TF antibodies
Four commercially available anti‐human TF antibodies (2 monoclonal and 2 polyclonal antibodies) that are claimed to detect human TF by western blotting were randomly selected for validation. We used a TF‐positive cell line (HPAF‐II) and a TF‐negative cell line were used (MIA PaCa‐2). An “in‐house” anti‐human TF monoclonal antibody TF9‐10H10 served as a positive control. 14 , 15 All the commercial anti‐human TF antibodies detected a single band at ~50 kDa, which is the expected size for glycosylated TF (Figure 4).
FIGURE 4.
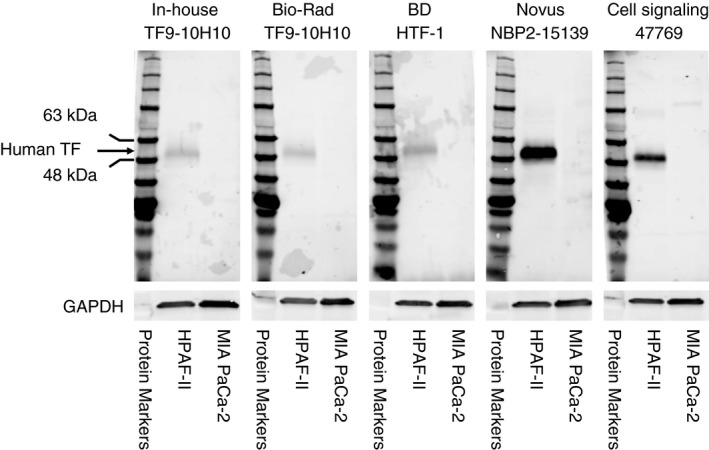
Detection of human tissue factor by western blotting using commercial antibodies. We used a tissue factor (TF)‐positive cell line (HPAF‐II) and a TF‐negative cell line (MIA PaCa‐2). Each primary antibody was diluted 1:1000. The final concentration of each antibody was as follows: Novus Biologicals (final concentration 0.64 μg/mL); Cell Signaling Technologies (final concentration 0.055 μg/mL), HTF‐1 (final concentration 0.55 μg/mL), in‐house TF9‐10H10 (final concentration 1.0 μg/mL), Bio‐Rad Laboratories TF9‐10H10 (final concentration 0.055 μg/mL). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was detected using an anti‐human GAPDH antibody (1:1000 dilution, final concentration 0.2 μg/mL). Secondary antibodies were used at a 1:10 000 dilution in blocking buffer. A protein marker is shown on the left of each panel
3.4. Detection of mouse TF by western blotting using commercial anti‐mouse TF and anti‐human TF antibodies
We used 3 commercial anti‐mouse TF antibodies and 2 anti‐human TF antibodies to detected mouse TF by western blotting. The mouse pancreatic cancer cell lines KPC2 Cas9 and KPC2 TF KO were used as positive and negative controls, respectively. The R&D Systems goat polyclonal antibody AF3178 and the Abcam ab189483 monoclonal antibody detected at band at ~50 kDa in KPC2 Cas9 cells, which is the expected size of glycosylated TF, but nothing was detected with KPC2 TF KO (Figure 5). The anti‐mouse TF polyclonal antibody from BioMedica Diagnostics detected a nonspecific band at ~48 kDa that was present in both the KPC2 Cas9 and KPC2 TF KO lines (Figure 5). Similarly, the anti‐human TF polyclonal antibody Abcam ab151748 detected a nonspecific band at ~50 kDa that was present in both the KPC2 Cas9 and KPC2 TF KO lines (Figure 5). Finally, the anti‐human TF polyclonal antibody ab104513 from Abcam failed to detect mouse TF (Figure 5). These data indicate that in our limited survey, 2 of 3 commercial anti‐mouse antibodies detected mouse TF by western blotting whereas the third anti‐mouse TF antibody recognized a nonspecific protein. Both anti‐human TF antibodies failed to detect mouse TF.
FIGURE 5.
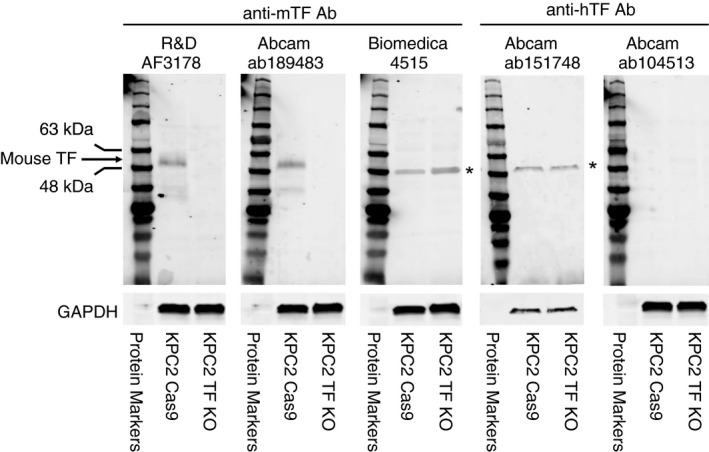
Detection of mouse tissue factor by western blotting using commercial antibodies. We used a tissue factor (TF)‐positive cell line (KPC2 Cas9) and a TF‐negative cell line (KPC2 TF KO). Each primary antibody was diluted 1:1000. The final concentration of each antibody was as follows: R&D Systems (final concentration 0.2 μg/mL), Abcam ab189483 (final concentration 0.576 μg/mL), BioMedica Diagnostics (final concentration 1.0 μg/mL), Abcam ab151748 (final concentration 0.212 μg/mL), and Abcam ab104513 (final concentration 0.55 μg/mL). Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was detected using an anti‐mouse GAPDH antibody (1:1000 dilution, final concentration 0.2 μg/mL). Secondary antibodies were used at a 1:10 000 dilution in blocking buffer. A protein marker is shown on the left of each panel. The asterisk marks a nonspecific band. All data was generated with blots run in parallel and probed in parallel except the data for ab151748 was obtained on a different day using different samples
4. DISCUSSION
In this study, we evaluated different commercial anti‐human TF and anti‐mouse TF antibodies for their ability to detect human and mouse TF by western blotting. In our limited survey, we found that all the commercial anti‐human polyclonal and monoclonal antibodies examined detected TF in human pancreatic cancer cell lines by western blotting. In contrast, only 2 of 3 commercial anti‐mouse TF antibodies detected mouse TF, and the other recognized a nonspecific band. Two anti‐human TF polyclonal antibodies that claimed to either cross react or predicted to cross react with mouse TF did not detect mouse TF by western blotting.
The cost of antibodies can be substantial with the price of TF antibodies ranging from approximately $130 to $450. Thus, investigators rely on company technical support services or other online resources when selecting an antibody. However, the quality of technical support provided by different companies can vary widely. A “best‐case scenario” was experienced by our research team with the technical service department of Cell Signaling Technologies. This group readily provided western blot data for their antibody using several cell lines, including BxPC‐3 and HPAF‐II. In addition, Cell Signaling Technologies has detailed information on their website ( https://www.cellsignal.com/products/primary‐antibodies/tissuefactor‐cd142‐antibody/47769 ). In contrast, the technical services department for a separate company told us that “we couldn’t get a QC [quality control] image from the antibody originator for you,” and we were not provided information about the antibody originator.
Another source available for selecting an antibody is the Human Protein Atlas (HPA; https://www.proteinatlas.org), which, among its various purposes, is designed to serve as a free, independent source for antibody validation. However, in our experience, this source must be reviewed with caution for information on anti‐TF antibodies. Data on the HPA website indicates that the TF antibody HPA049292 from MilliporeSigma recognized an ~50‐kDa band in a confirmed TF‐positive cell line U‐251 MG, and no signal was observed with known TF‐negative samples. This antibody should have received a “supported” score. Surprisingly, it was given an “uncertain” grade in the HPA. In contrast, a second antibody HPA069132 from MilliporeSigma that recognized a nonspecific protein at ~30 kDa in TF‐negative samples was given a “supported” grade. We expressed our concerns to the HPA organization and were told by a representative that “the initial scoring of western blots is based on the theoretical molecular weight of the unmodified proteins related to each gene,” which in the case of TF would be ~36 kDa. It is clear that HPA’s scoring criteria for anti‐TF antibodies does not account for posttranslational modifications, such as glycosylation, and does not consider nonspecific binding of antibodies to TF‐negative cell lines and samples. Therefore, such sources of antibody validation should be carefully evaluated prior to use.
We found a high degree of variability in the detection of mouse TF by western blotting using commercial antibodies. The R&D Systems goat anti‐mouse TF polyclonal antibody AF3178 and the Abcam ab189483 rabbit anti‐mouse monoclonal antibody detected TF by western blotting. Indeed, the R&D Systems antibody has been used by our laboratory and others and has the advantage of being antigen affinity purified to increase its specificity. 23 , 24 , 25 , 26 , 27 Human and mouse TF are highly homologous. 28 However, this does not mean that polyclonal antibodies raised against one species will cross react with TF from another species. For example, the anti‐human TF polyclonal antibody ab104513 from Abcam is marketed as predicted to cross react with mouse TF. We contacted Abcam and were told by the technical service department that this claim was based on a paper that used the antibody to detect mouse TF by immunohistochemistry. 29 The Abcam website (https://www.abcam.com/tissue‐factor‐antibody‐ab104513‐references.html) lists 13 publications that used this antibody to detect TF from a variety of species, including human, rat, mouse, and pig. Importantly, 2 papers used the antibody to measure levels of mouse TF protein by western blotting. 30 , 31 We found that this antibody did not recognize mouse TF by western blotting. Similarly, in 2014, Dr Moshe Shashar reported that ab104513 does not recognize mouse TF. 32 This example highlights the need for individual investigators and laboratories to perform in‐house validation of antibodies prior to publishing findings.
A major complicating factor in the use of antibodies for western blotting protocols to detect TF is the fact that some anti‐mouse TF polyclonal antibodies do not recognize mouse TF but rather bind to a nonspecific protein in western blots. In our study, we found that a rabbit anti‐mouse polyclonal antibody from BioMedica Diagnostics 4515 recognized a nonspecific protein in both TF‐positive and TF‐negative cell lines by western blotting. We could not find any papers that used the BioMedica Diagnostics antibody. Another antibody of concern is Abcam ab151748. This rabbit anti‐human monoclonal antibody was raised against a peptide from human TF. Abcam claimed that this antibody detects human, rat, and mouse TF by western blotting. However, the datasheet gave a predicted molecular weight of TF as 33 kDa rather than ~50 kDa for the glycosylated form. We found 29 papers that used this antibody to measure human, mouse, rabbit and canine TF by a variety of methods, including 15 that used the antibody for western blotting (Table S1). 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 Importantly, Abcam evaluated the ability of the antibody to detect human TF in wild‐type and KO cell lines by western blotting. 62 Based on the data they obtained, they recently withdrew ab151748 and stated that “after evaluation, using a HAP1 knockout cell line, ab151748 was not shown to react specifically with the target protein and has therefore been discontinued.” We confirmed that this antibody does not detect mouse TF by western blotting but rather a nonspecific band. This highlights the need for appropriate quality control to fully characterize antibodies. An important downstream implication of this revelation is that published western blot data obtained using ab151748 should be reevaluated.
A final consideration is that even for a given antibody, there may be variation based on the commercial provider. It is interesting to note that at least 15 companies sell TF9‐10H10, including Creative Biolabs, MilliporeSigma, Thermo Fisher Scientific, and Novus Biologicals. It would be good to know if these antibodies are all indeed equivalent. Sources of potential variation between vendors include different purification techniques, storage conditions, or buffers and carrier proteins. MilliporeSigma technical services reported that they obtain the antibody from Calbiochem, whereas Bio‐Rad, Novus Biologicals, and Thermo Fisher Scientific technical services told us that the source was proprietary.
The study has some limitations. We used human and mouse pancreatic cell lines as positive and negative controls for human and mouse TF expression. We would expect similar results using other cell lines, activated monocytes and TF‐expressing tissues but this was not tested. Our study focused on the ability of commercial antibodies to detect human and mouse TF by western and did not determine the ability of these antibodies to detect TF by other methods, such as immunohistochemistry. Interestingly, the rat anti‐mouse TF monoclonal antibody 1H1 detects mouse TF by immunohistochemistry but failed to detect mouse TF by western blotting (data not shown). 19 Therefore, our western blot data cannot be extrapolated to our techniques or vice versa.
The lessons from these examples indicate that full information should be available for commercial antibodies and that it is important to know TF is a glycosylated protein that is differentially expressed by various cell lines and tissues. 12 , 63 , 64 In the era of rigor and reproducibility, companies must do a better job validating their antibodies using a variety of methods. Investigators should provide the antibody source, including lot number, and independently validate commercial antibodies as recommended by the National Institutes of Health.
RELATIONSHIP DISCLOSURE
The authors declare nothing to report.
AUTHOR CONTRIBUTIONS
NM designed the study. AR, BM, YH, RC, GL, and YY performed the experiments. All authors analyzed data and wrote and approved the manuscript.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank Drs. Congqing Wu (University of Kentucky) and Laura J. Sommerville (Duke University) for providing antibodies.
Rosell A, Moser B, Hisada Y, et al. Evaluation of different commercial antibodies for their ability to detect human and mouse tissue factor by western blotting. Res Pract Thromb Haemost. 2020;4:1013–1023. 10.1002/rth2.12363
Axel Rosell and Bernhard Moser contributed equally to this work.
Funding information
This study was funded by the Japanese Society on Thrombosis and Hemostasis (YH), the John C. Parker Professorship (NM), the Swedish Society on Thrombosis and Haemostasis (AR), and the Regional ALF agreement on medical training and clinical research between the Stockholm County Council and Karolinska Institutet (AR).
Handling Editor: Yotis Senis
Contributor Information
Matthew J. Flick, @fib390_396A.
Nigel Mackman, Email: nmackman@med.unc.edu.
REFERENCES
- 1. Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature. 2012;483:531–3. [DOI] [PubMed] [Google Scholar]
- 2. Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712. [DOI] [PubMed] [Google Scholar]
- 3. Daugherty A, Hegele RA, Mackman N, Rader DJ, Schmidt AM, Weber C. Complying with the National Institutes of Health guidelines and principles for rigor and reproducibility: refutations. Arterioscler Thromb Vasc Biol. 2016;36:1303–4. [DOI] [PubMed] [Google Scholar]
- 4. Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, et al. A proposal for validation of antibodies. Nat Methods. 2016;13:823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–25. [DOI] [PubMed] [Google Scholar]
- 6. Paborsky LR, Harris RJ. Post‐translational modifications of recombinant human tissue factor. Thromb Res. 1990;60:367–76. [DOI] [PubMed] [Google Scholar]
- 7. Egorina EM, Sovershaev MA, Osterud B. Regulation of tissue factor procoagulant activity by post‐translational modifications. Thromb Res. 2008;122:831–7. [DOI] [PubMed] [Google Scholar]
- 8. Butenas S. Tissue factor structure and function. Scientifica (Cairo). 2012;2012:964862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spicer EK, Horton R, Bloem L, Bach R, Williams KR, Guha A, et al. Isolation of cDNA clones coding for human tissue factor: primary structure of the protein and cDNA. Proc Natl Acad Sci U S A. 1987;84:5148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrissey JH, Fakhrai H, Edgington TS. Molecular cloning of the cDNA for tissue factor, the cellular receptor for the initiation of the coagulation protease cascade. Cell. 1987;50:129–35. [DOI] [PubMed] [Google Scholar]
- 11. Ranganathan G, Blatti SP, Subramaniam M, Fass DN, Maihle NJ, Getz MJ. Cloning of murine tissue factor and regulation of gene expression by transforming growth factor type beta 1. J Biol Chem. 1991;266:496–501. [PubMed] [Google Scholar]
- 12. Hartzell S, Ryder K, Lanahan A, Lau LF, Nathan D. A growth factor‐responsive gene of murine BALB/c 3T3 cells encodes a protein homologous to human tissue factor. Mol Cell Biol. 1989;9:2567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kothari H, Rao LV, Pendurthi UR. Glycosylation of tissue factor is not essential for its transport or functions. J Thromb Haemost. 2011;9:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morrissey JH, Fair DS, Edgington TS. Monoclonal antibody analysis of purified and cell‐associated tissue factor. Thromb Res. 1988;52:247–61. [DOI] [PubMed] [Google Scholar]
- 15. Carson SD, Ross SE, Bach R, Guha A. An inhibitory monoclonal antibody against human tissue factor. Blood. 1987;70:490–3. [PubMed] [Google Scholar]
- 16. Flossel C, Luther T, Muller M, Albrecht S, Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry. 1994;101:449–53. [DOI] [PubMed] [Google Scholar]
- 17. Vallier L, Bouriche T, Bonifay A, Judicone C, Bez J, Franco C, et al. Increasing the sensitivity of the human microvesicle tissue factor activity assay. Thromb Res. 2019;182:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrissey JH, Agis H, Albrecht S, Dignat‐george F, Edgington TS, Luther T. CD142 (tissue factor) workshop panel report In: Kishimoto et al., editors Leukocyte Typing VI: White Cell Differentiation Antigens. New York, NY: Garland Publishing; 1997:742–6. [Google Scholar]
- 19. Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005;3:1098–9. [DOI] [PubMed] [Google Scholar]
- 20. Furlan‐Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor‐dependent thrombosis in mice. J Clin Invest. 2011;121:2932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Y, Stang A, Schweickert PG, Lanman NA, Paul EN, Monia BP, et al. Thrombin signaling promotes pancreatic adenocarcinoma through PAR‐1‐dependent immune evasion. Cancer Res. 2019;79:3417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, et al. Dimeric CRISPR RNA‐guided FokI nucleases for highly specific genome editing. Nat Biotechnol. 2014;32:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sebag SC, Bastarache JA, Ware LB. Mechanical stretch inhibits lipopolysaccharide‐induced keratinocyte‐derived chemokine and tissue factor expression while increasing procoagulant activity in murine lung epithelial cells. J Biol Chem. 2013;288:7875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaver CM, Grove BS, Putz ND, Clune JK, Lawson WE, Carnahan RH, et al. Regulation of alveolar procoagulant activity and permeability in direct acute lung injury by lung epithelial tissue factor. Am J Respir Cell Mol Biol. 2015;53:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rautou PE, Tatsumi K, Antoniak S, Owens AP 3rd, Sparkenbaugh E, Holle LA, et al. Hepatocyte tissue factor contributes to the hypercoagulable state in a mouse model of chronic liver injury. J Hepatol. 2016;64:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang S, Reeves B, Sparkenbaugh EM, Russell J, Soltys Z, Zhang H, et al. Protective and detrimental effects of neuroectodermal cell–derived tissue factor in mouse models of stroke. JCI Insight. 2016;1:e86663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, Soloviev DA, et al. Leukocyte integrin Mac‐1 regulates thrombosis via interaction with platelet GPIbalpha. Nat Commun. 2017;8:15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andrews BS, Rehemtulla A, Fowler BJ, Edgington TS, Mackman N. Conservation of tissue factor primary sequence among three mammalian species. Gene. 1991;98:265–9. [DOI] [PubMed] [Google Scholar]
- 29. Mizugishi K, Inoue T, Hatayama H, Bielawski J, Pierce JS, Sato Y, et al. Sphingolipid pathway regulates innate immune responses at the fetomaternal interface during pregnancy. J Biol Chem. 2015;290:2053–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Y, Zhang X, Yang W, Li C, Chu Y, Jiang H, et al. Mechanism of the protective effects of the combined treatment with rhynchophylla total alkaloids and sinapine thiocyanate against a prothrombotic state caused by vascular endothelial cell inflammatory damage. Exp Ther Med. 2017;13:3081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kessinger CW, Kim JW, Henke PK, Thompson B, McCarthy JR, Hara T, et al. Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS ONE. 2015;10:e0116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.[Accessed 2020 May 15] Available from https://www.abcam.com/tissue‐factor‐antibody‐ab104513.html?productWallTab=ShowAll
- 33. de la Fuente C, Pumarola M, Blasco E, Fernández F, Viu J, Añor S. Immunohistochemical evaluation of tissue factor, fibrin/fibrinogen and D‐dimers in canine gliomas. Vet J. 2014;200:387–92. [DOI] [PubMed] [Google Scholar]
- 34. Gong X, Duan R, Ao JE, Ai Q, Ge P, Lin L, et al. Metformin suppresses intrahepatic coagulation activation in mice with lipopolysaccharide/d‐galactosamine induced fulminant hepatitis. Mol Med Rep. 2015;12:6384–90. [DOI] [PubMed] [Google Scholar]
- 35. Font C, de la Fuente C, Pumarola M, Blasco E, Fernandez F, Viu J, et al. Canine intracranial meningiomas: immunohistochemical evaluation of tissue factor, fibrin/fibrinogen and D‐dimers. Vet J. 2015;206:426–8. [DOI] [PubMed] [Google Scholar]
- 36. Chang CY, Chen JY, Chen SH, Cheng TJ, Lin MT, Hu ML. Therapeutic treatment with ascorbate rescues mice from heat stroke‐induced death by attenuating systemic inflammatory response and hypothalamic neuronal damage. Free Radic Biol Med. 2016;93:84–93. [DOI] [PubMed] [Google Scholar]
- 37. Yasui H, Donahue DL, Walsh M, Castellino FJ, Ploplis VA. Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoesel B, Mussbacher M, Dikorman B, Salzmann M, Assinger A, Hell L, et al. Androgen receptor dampens tissue factor expression via nuclear factor‐kappaB and early growth response protein 1. J Thromb Haemost. 2018;16:749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi G, Zhao JW, Sun XX, Ma JF, Wang P, He FC, et al. TIPE2 is negatively correlated with tissue factor and thrombospondin‐1 expression in patients with bronchial asthma. Exp Ther Med. 2018;15:3449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu S, Zhou Z, Li H, Liu Z, Pan X, Wang F, et al. BMSCs ameliorate septic coagulopathy by suppressing inflammation in cecal ligation and puncture‐induced sepsis. J Cell Sci. 2018;131:jcs211151. [DOI] [PubMed] [Google Scholar]
- 41. Hennink I, van Leeuwen MW, Penning LC, Piek CJ. Increased number of tissue factor protein expressing thrombocytes in canine idiopathic immune mediated hemolytic anemia. Vet Immunol Immunopathol. 2018;196:22–9. [DOI] [PubMed] [Google Scholar]
- 42. Jing Y, Hu Y, Li H, Wang J, Si X, Zheng H, et al. Assessment of thrombotic risk in atrial fibrillation with ultrasound molecular imaging of P‐selectin. Thromb Haemost. 2018;118:388–400. [DOI] [PubMed] [Google Scholar]
- 43. Morrow JJ, Bayles I, Funnell APW, Miller TE, Saiakhova A, Lizardo MM, et al. Positively selected enhancer elements endow osteosarcoma cells with metastatic competence. Nat Med. 2018;24:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Honda K, Matoba T, Antoku Y, Koga JI, Ichi I, Nakano K, et al. Lipid‐lowering therapy with ezetimibe decreases spontaneous atherothrombotic occlusions in a rabbit model of plaque erosion: a role of serum oxysterols. Arterioscler Thromb Vasc Biol. 2018;38:757–71. [DOI] [PubMed] [Google Scholar]
- 45. Garrett N, Pombo J, Umpierrez M, Clark JE, Simmons M, Girardi G. Pravastatin therapy during preeclampsia prevents long‐term adverse health effects in mice. JCI Insight. 2018;3:e120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bondu V, Bitting C, Poland VL, Hanson JA, Harkins MS, Lathrop S, et al. Upregulation of P2Y2R, active uPA, and PAI‐1 are essential components of hantavirus cardiopulmonary syndrome. Front Cell Infect Microbiol. 2018;8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kroesen VM, Rodriguez‐Martinez P, Garcia E, Rosales Y, Diaz J, Martin‐Cespedes M, et al. A Beneficial effect of low‐dose aspirin in a murine model of active tuberculosis. Front Immunol. 2018;9:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rancourt RC, Rioux JS, Veress LA, Garlick RB, Croutch CR, Peters E, et al. Methyl isocyanate inhalation induces tissue factor‐dependent activation of coagulation in rats. Drug Chem Toxicol. 2019;42:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sachetto ATA, Rosa JG, Santoro ML. Rutin (quercetin‐3‐rutinoside) modulates the hemostatic disturbances and redox imbalance induced by Bothrops jararaca snake venom in mice. PLoS Negl Trop Dis. 2018;12:e0006774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Li H, Dong J, Ma L, Liao A, Rong Z, et al. mTOR and ERK regulate VKORC1 expression in both hepatoma cells and hepatocytes which influence blood coagulation. Clin Exp Med. 2019;19:121–32. [DOI] [PubMed] [Google Scholar]
- 51. Matsubara Y, Matsumoto T, Yoshiya K, Yoshida A, Ikeda S, Furuyama T, et al. Budding uninhibited by benzimidazole‐1 insufficiency prevents acute renal failure in severe sepsis by maintaining anticoagulant functions of vascular endothelial cells. Shock. 2019;51:364–71. [DOI] [PubMed] [Google Scholar]
- 52. Miranda S, Billoir P, Damian L, Thiebaut PA, Schapman D, Le Besnerais M, et al. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: role of reduced inflammation and endothelial dysfunction. PLoS ONE. 2019;14:e0212614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burzynski LC, Humphry M, Pyrillou K, Wiggins KA, Chan JNE, Figg N, et al. The coagulation and immune systems are directly linked through the activation of interleukin‐1alpha by thrombin. Immunity. 2019;50:1033–42.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50:1401–11.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei Q, Wang J, Shi W, Zhang B, Jiang H, Du M, et al. Improved in vivo detection of atherosclerotic plaques with a tissue factor‐targeting magnetic nanoprobe. Acta Biomater. 2019;90:324–36. [DOI] [PubMed] [Google Scholar]
- 56. Lou J, Hu Y, Wu MD, Che LQ, Wu YF, Zhao Y, et al. Endothelial cell‐specific anticoagulation reduces inflammation in a mouse model of acute lung injury. Acta Pharmacol Sin. 2019;40:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu S, Zhang Y, Zhao X, Wang J, Di C, Zhao Y, et al. Tumor‐specific silencing of tissue factor suppresses metastasis and prevents cancer‐associated hypercoagulability. Nano Lett. 2019;19:4721–30. [DOI] [PubMed] [Google Scholar]
- 58. Liu B, Wang Y, Wu Y, Cheng Y, Qian H, Yang H, et al. IKKbeta regulates the expression of coagulation and fibrinolysis factors through the NF‐kappaB canonical pathway in LPS‐stimulated alveolar epithelial cells type II. Exp Ther Med. 2019;18:2859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin C, Yuan H, Wang W, Zhu Z, Lu Y, Wang J, et al. Importance of PNO1 for growth and survival of urinary bladder carcinoma: role in core‐regulatory circuitry. J Cell Mol Med. 2020;24:1504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D‐dependent phosphatidylserine exposure. Immunity. 2019;51:983–96.e6. [DOI] [PubMed] [Google Scholar]
- 61. D'Onofrio N, Sardu C, Paolisso P, Minicucci F, Gragnano F, Ferraraccio F, et al. MicroRNA‐33 and SIRT1 influence the coronary thrombus burden in hyperglycemic STEMI patients. J Cell Physiol. 2020;235:1438–52. [DOI] [PubMed] [Google Scholar]
- 62.[Accessed 2020 May 15] Available from https://www.abcam.com/tissue‐factor‐antibody‐epr8986‐ab151748.html
- 63. Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, et al. Tumor‐derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ. Murine tissue factor gene expression in vivo. tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol. 1993;143:76–84. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


