Abstract
Introduction
Cancer-associated fibroblasts (CAFs) promote tumor progression; thus, drugs that can modify CAFs need to be identified.
Methods
To test the effect of cinnamaldehyde on prostate CAFs, the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2H-tetrazolium bromide assay was used to determine their survival. When spleen cells were treated with CAF supernatant, the proliferation of T cells was inhibited as determined by flow cytometry. After cinnamaldehyde treatment, this immunosuppressive function of CAFs was partially reversed. To explore the molecular mechanism, Western blotting and the quantitative real-time polymerase chain reaction were applied, and TLR4-dependent signaling pathway-related protein and mRNA levels were quantified.
Results
Cinnamaldehyde acted on the TLR4-dependent signaling pathway, altering the function of CAFs such that its supernatant no longer inhibited the proliferation of T cells.
Conclusion
These data indicate that cinnamaldehyde can modify the functions of CAFs, which may be helpful for treating tumors. Cinnamaldehyde can suppress CAF T-cell inhibition.
Keywords: cinnamaldehyde, cancer-associated fibroblasts, prostate cancer, TLR4
Introduction
Cancer-associated fibroblasts (CAFs) are a dominant component in the tumor stroma and function as architects of cancer pathogenesis.1,2 CAFs not only promote tumor progression via communication with cancer cells, but also affect cell motility by secreting growth factors.3 For example, the secretion of transforming growth factor (TGF-β) can reduce the activation, functions, and survival of T cells.4 In addition, our previous study indicated that fibroblasts in the tumor stroma promote growth in different tumor types.5 Together, these studies uncovered crucial functions of CAFs in the tumor stroma.
Prostate cancer is a severe disease that impacts many people. In the USA, among men under the age of 55 years, the risk of developing prostate cancer increased in the previous two decades.6 In 2018, nearly 1.3 million new prostate cancer cases were diagnosed. For men, it is estimated that prostate cancer is the second most commonly diagnosed cancer, just lower than lung cancer, and causes 350,000 deaths annually worldwide.7
Clinical studies indicate the primary mechanism of castration-resistant prostate cancer is sustained androgen receptor signaling.8 In addition, there are an increasing number of reports that reveal an association between the tumor microenvironment and castration-resistant prostate cancer.9,10 Some studies demonstrated that heterogeneity in the expression of androgen receptors in CAFs among prostate carcinomas may be relevant in tumorigenesis and castration resistance, such as high expression of the interleukin-17 receptor during tumor development, and highly expressed matrix metalloproteinase-11 in castration resistance.11 These findings inspired us to study cells in the tumor microenvironment.
Cinnamaldehyde is a natural compound that was first isolated in the 1800s. Cinnamaldehyde is a widely known flavor in food. Importantly, its biological effects were discovered in recent decades.12–14 Cinnamon is prescribed for the treatment of diseases with symptoms that are similar to tumors in Ge Hong’s A Handbook of Prescriptions for Emergencies. This may indicate the beneficial effects of cinnamaldehyde against cancer or tumors. In addition, increasing evidence of the effects of cinnamaldehyde against tumors and fibrosis have been unraveled in recent decades,15,16 suggesting that cinnamaldehyde might affect CAFs in the tumor microenvironment.
In this study, an in vitro prostate cancer model was used to investigate the effect of cinnamaldehyde on the immunosuppressive function of prostate CAFs and the potential molecular mechanism.
Materials and Methods
Prostate CAFs
Prostate CAFs (PF179T-CAF, isolated from a prostatectomy specimen marginal to prostate cancer by Prof. V Rotter; hTERT immortalized; designated PF179; 179 was the patient number) were purchased from the American Type Culture Collection (Manassas, VA, USA). These CAFs have a higher proliferation ability than normal-associated fibroblasts. In addition, expression of some proteins, such as interleukin 17, is upregulated. Unlike the general notion that high levels of α-smooth muscle actin serve as a marker for CAFs, these prostate CAFs express it at a lower level compared with prostate normal-associated fibroblasts.17 Furthermore, CAFs exhibit genomic stability.2
Cell Culture Medium
CAFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (HyClone, Logan, UT, USA) with 10% fetal bovine serum (PAN Biotech, Aidenbach, Germany) and 100 U/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). Spleen cells were cultured in RPMI-1640 (HyClone) with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin. All cells were incubated in a 37°C/5% CO2 incubator with a humidified atmosphere.
Cinnamaldehyde
Cinnamaldehyde (3-phenylacrylaldehyde, 98%) was purchased from Maclin (Shanghai, China). Cinnamaldehyde was diluted in 75% ethanol to a concentration of 10 mg/mL as a stock solution. Then, it was diluted in DMEM to a suitable concentration for treating the cells.
Cell Viability Assay
To test the viability of CAF and spleen cells after exposure to cinnamaldehyde, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2H-tetrazolium bromide, Amresco, Cleveland, OH, USA) was used. CAFs were plated onto 96-well plates at 5 × 103 cells per well. After exposure to cinnamaldehyde for 24 h, 10 μL MTT (diluted in phosphate-buffered saline [PBS], 5 mg/mL) were added to each well. The cells were incubated for 4 h, then 100 μL of a formazan dissolving solution (10% sodium dodecyl sulfate, 5% isobutanol, 0.012 mol/L hydrochloric acid, in double distilled water) were added to fully dissolve the formazan crystals. The absorbance at 570 nm was detected by a microplate reader (Bio-Rad, Hercules, CA, USA). For spleen cells, the protocol was similar except the plated cell density was 3 × 105 per well.
Western Blot Analyses
CAFs were exposed to 2.4 μM cinnamaldehyde for 0, 0.5, 1, 1.5, and 2 h. Then, the CAFs were lysed with radioimmunoprecipitation assay buffer (Beyotime, Haimen, China; 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate) supplemented with 50 nM NaF, 1 mM sodium orthovanadate, 25 μg/mL aprotinin, and 100 mM phenylmethylsulfonyl fluoride to isolate total protein. Protein samples (30 μg) were separated by electrophoresis on a 10% sodium dodecyl sulfate polyacrylamide gel, then transferred to a nitrocellulose membrane (GE Healthcare, Milwaukee, WI, USA). The membrane was blocked with 3% bovine serum albumin diluted with PBS-T (PBS with 0.1% Tween-20). Next, the membrane was incubated with the following primary antibodies: phospho-c-Jun N-terminal kinase (p-JNK) (Thr183/Tyr185), JNK, p-TAK1 (Ser412), TAK1, and p-c-Jun (Ser73) (1:1000, all from Cell Signaling Technology, Danvers, MA, USA) for 1 h. After washing with PBS-T three times, the membrane was incubated with the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG, 1:3000, Tianjin Sungene Biotech, Tianjin, China) for 1 h. Finally, the membrane was washed with PBS-T, incubated with the chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA), and the protein detected with a luminescence imaging system (Clinnx Science Instruments, Shanghai, China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Tianjin Sungene Biotech) was used as an internal standard. The toll-like receptor 4 (TLR4) signal was inhibited with1 μg/mL CLI-095 (Invitrogen).
Collection of the CAF Supernatant
CAFs were treated for 24 h with 0, 0.8, 1.6, and 2.4 μM cinnamaldehyde in 48-well plates at a density of 2.5 × 104 cells/well. The medium then was replaced with DMEM without cinnamaldehyde and cultured for 24 h. Finally, the supernatant was passed through a 0.22 μm filter and stored at −80 °C. For the supernatant used in the TLR4 inhibition assay, CAFs were treated with 1 μg/mL CIL-095 (InvivoGen, California, USA) for 6 h. Cinnamaldehyde was then added to the system.
C57 Mice
C57 mice were purchased from Weitonglihua (Beijing, China) and maintained in a pathogen-free environment at the Institute of Biophysics, Chinese Academy of Sciences. All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA) and were approved by the Biological Research Ethics Committee, Institute of Biophysics, Chinese Academy of Sciences.
Collection of Spleen Cells
Spleen of C57 mouse was sterilized in medical alcohol and PBS, then grinded in the cell culture medium. Next, blood cells were lysed with red blood cell lysate buffer. Finally the rest of the cells were collected and served as spleen cells.
Spleen Cell Proliferation Assay
Cells were acquired from C57 mouse spleens and labeled with carboxyfluorescein succinimidyl ester. The labeled cells were stimulated with 0.6 μg/mL concanavalin A. In addition, different types of CAF supernatant, which was treated by different concentrations of cinnamaldehyde or treated by CLI-095, were added into the system. In brief, the system contained carboxyfluorescein-labeled spleen cells, 0.6 μg/mL concanavalin A, 30% CAF supernatant, and RPMI-1640 medium. The cells were incubated for 3 days.
Flow Cytometry
A FACS Calibur was used to evaluate T cell proliferation. Phycoerythrin-CD4 and allophycocyanin-CD8 (diluted, 1:200 in PBS) were used to label the cells (BD Biosciences, Franklin Lakes, NJ, USA).
Quantitative Real-Time Polymerase Chain Reaction
In control and cinnamaldehyde group, CAFs were treated for 24 h with 0, 2.4 μM cinnamaldehyde in 6-well plates at a density of 3.0 × 105 cells/well. For the TLR4 inhibition group, CAFs were treated with 1 μg/mL CIL-095 for 6 h in advance, then discarded the medium and treated with 2.4 μM cinnamaldehyde for 24 h as well. Total RNA was extracted from CAFs by TRIzol reagent and quantified on a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). PrimeScript RT Master Mix (abm, Jiangsu, China) was used to reverse transcribe total RNA to cDNA. The amounts of GAPDH and α-smooth muscle actin mRNA were determined using the SYBR Green II Mix (Genstar, Beijing, China) on a Corbett 6200. All reactions were carried out for 40 cycles in a total volume of 10 μL (3 μL H2O, 5 μL SYBR Green II Mix, 1 μL of each primer, 1 μL cDNA). GAPDH was used as the internal control. Primer sequences were: GAPDH (forward: 5′-GTTGCCATCAATGACCCCTT-3′; reverse: 5′-CTCCACGACGTACTCAGCG-3′); Jun (forward: 5′- TCCAAGTGCCGAAAAAGGAAG-3′; reverse: 5′- CGAGTTCTGAGCTTTCAAGGT-3′).
Statistical Analysis
Data are presented as means ± standard deviation and were evaluated by a one-way analysis of variance. And Student’s t test was used to check the differences between two groups. P < 0.05 was considered statistically significant. All experiments were repeated independently at least three times.
Results
Cinnamaldehyde Did Not Influence the Viability of CAF and Spleen Cells in vitro
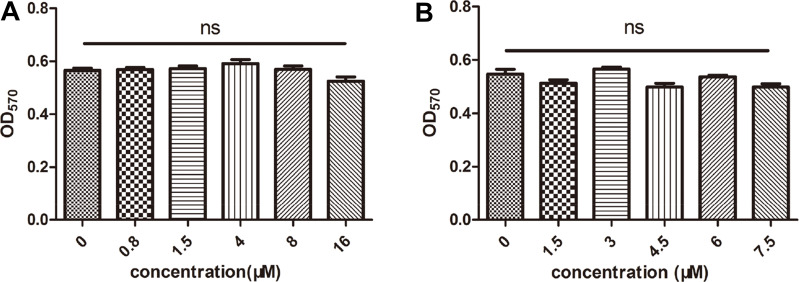
After exposure to 0, 0.8, 1.5, 4, 8, or 16 μM cinnamaldehyde for 24 h, the viability of CAFs was evaluated. Figure 1A shows that cinnamaldehyde did not inhibit CAF growth at any of the tested concentrations. This result indicated that CAFs could survive in an environment with cinnamaldehyde.
Figure 1.
Effects of cinnamaldehyde on cancer-associated fibroblast (CAF) and spleen cell growth in vitro. (A) CAFs were treated with different concentrations of cinnamaldehyde, and cell viability was tested after 24 h. (B) Spleen cells were treated with different concentrations of cinnamaldehyde, and cell viability was tested after 72 h.
To determine whether longer incubations with cinnamaldehyde affected the proliferation of spleen cells, CAFs were treated with 0, 1.5, 3, 4.5, 6, or 7.5 μM for 72 h. There were no statistically significant differences in spleen cell proliferation at any of these concentrations (Figure 1B).
Cinnamaldehyde Reversed CAF‐mediated T Cell Inhibition
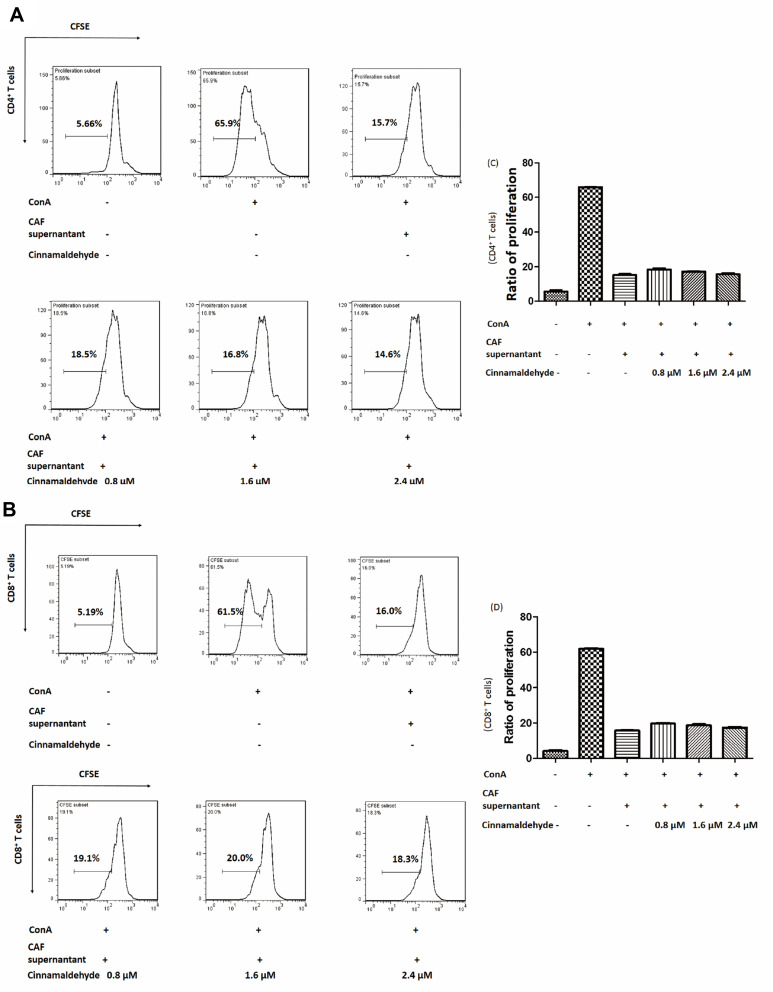
Although cinnamaldehyde did not kill spleen cells at a low concentration (≤ 2.4 μM), to preclude any direct effects on T cells, the culture containing cinnamaldehyde was discarded and replaced with fresh culture medium. Then, CAFs were cultured for an additional 24 h and the supernatant was used to treat spleen cells. When spleen cells were treated with CAF supernatant, the proliferation of T cells was inhibited (Figure 2A). CD4+ T cell proliferation recovered, to some degree, compared to cells treated with the supernatant collected from CAFs without cinnamaldehyde treatment, and this effect was concentration-dependent (Figure 2B and D). Analogously, there was a similar effect on T cells CD8+ T cell (Figure 2C and E). These results indicated that cinnamaldehyde could relieve CAF‐mediated T cell inhibition through some factors other than cinnamaldehyde in the supernatant.
Figure 2.
Effect of cinnamaldehyde on the immunosuppressive function of cancer-associated fibroblasts (CAFs). (A) CAFs were treated with 0, 0.8, 1.6, or 2.4 μM cinnamaldehyde for 24 h. The supernatant containing cinnamaldehyde was discarded and replaced with fresh medium. After culturing for an additional 24 h, the supernatant was used immediately or stored at −80°C. (B and C) Carboxyfluorescein succinimidyl ester (CFSE)-labeled spleen cells were stimulated with or without concanavalin A (ConA) and CAF supernatant. The ratio of T cell proliferation was analyzed by flow cytometry. Cinnamaldehyde suppressed CAF T-cell inhibition both in CD4+ T cells (B) and CD8+ T cells (C). (D and E) Quantitative results. Cinnamaldehyde inhibits the immunosuppressive function of CAFs in a concentration-dependent manner. **p < 0.01. ***p < 0.001.
Cinnamaldehyde Activated the TLR4-Dependent Pathway of CAFs
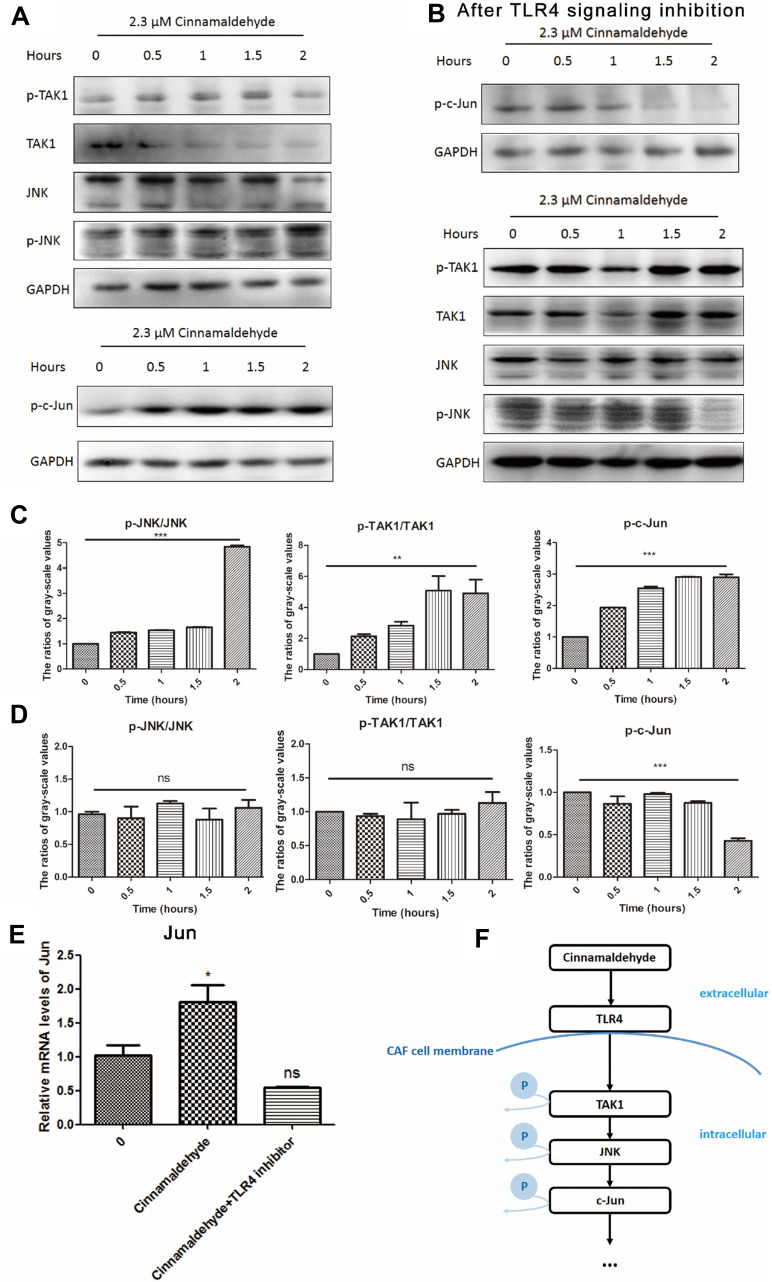
To determine how cinnamaldehyde reversed the immune-suppressive functions of CAFs, we examined the expression levels of TLR4-dependent pathway proteins (p-JNK, JNK, p-TAK1, TAK1, and p-c-Jun) using Western blotting. After exposure to cinnamaldehyde, changes in these proteins indicated that the TLR4-dependent pathway was stimulated in a time-dependent manner (Figure 3A and C). When TLR4 signaling inhibitor was applied to CAF, TLR4-dependent pathway could not be activated by cinnamaldehyde any more. (Figure 3B and D). In addition, qPCR was applied to test the changes in the mRNA level of Jun which also indicated that TLR4-dependent pathway was involved (Figure 3E). Therefore, a TLR4-dependent pathway was assumed in this process (Figure 3F).
Figure 3.
Cinnamaldehyde activates the toll-like receptor 4 (TLR4)-dependent pathway of cancer-associated fibroblasts (CAFs). (A) Protein levels of phospho-c-Jun N-terminal kinase (p-JNK), JNK, p-TAK1, TAK1, and p-c-Jun in whole cell lysates were assessed by Western blotting. The expression of GAPDH served as the control. After stimulation with cinnamaldehyde, time-dependent increases in the expression of p-c-Jun, p-JNK, and p-TAK1, and time-dependent decreases in the expression of JNK and TAK1 are evident. This indicates the TLR4-dependent pathway of CAFs is activated. (B) After pre-treatment with 1 µg/mL of a TLR4 signaling inhibitor (CLI-095), a slight time-dependent decrease in the expression of p-c-Jun is evident. Time-dependent changes in the levels of p-TAK1, p-JNK, JNK and TAK1 are no longer present. This indicates the TLR4-dependent pathway of signaling inhibitor-treated CAFs is not activated. (C–E) The Western blotting and quantitative real-time polymerase chain reaction results were analyzed quantitatively. (C) Factors downstream of TLR4, such as p-JNK, p-TAK1 and p-c-Jun, are significantly activated after treatment with cinnamaldehyde. (D) After pre-treatment with the TLR4 signaling inhibitor, the TLR4-dependent pathway of CAFs is no longer activated, even following treated with cinnamaldehyde. (E) The mRNA level of Jun is increased after stimulation with cinnamaldehyde. However, it is unchanged after pre-treatment with the TLR4 signaling inhibitor after cinnamaldehyde treatment. *p< 0.05. **p < 0.01. ***p < 0.001. ns represents a nonsignificant difference (p > 0.05). (F) The possible signaling pathway of the effect of cinnamaldehyde on CAFs.
TLR4-Signalling Was Necessary for the Effect of Cinnamaldehyde on CAFs
To evaluate the function of the TLR4-dependent pathway, we added 1 μg/mL CLI-095, a TLR4 signaling inhibitor, to the CAF culture and incubated the cells for 6 h at 37°C. Then, we added cinnamaldehyde and performed the spleen cell proliferation assay under the same conditions as the experiment described in section 3.2. After treatment with the TLR4 signaling inhibitor, CAFs retained their immunosuppressive function towards T cells. Even if CAFs were treated with a concentration gradient of cinnamaldehyde, the inhibitory effect of CAF on T cells cannot be attenuated since the TLR4 receptor of CAF is blocked (Figure 4). Both CD4+ T cells (Figure 4A and C) and CD8+ T cells (Figure 4B and D) were inhibited by CAF. In other words, cinnamaldehyde changed the properties of CAFs through a TLR4-dependent pathway.
Figure 4.
The effect of cinnamaldehyde is inhibited after treatment of cancer-associated fibroblasts (CAFs) with a toll-like receptor 4 (TLR4) signaling inhibitor. (A and B) The only aspect that differentiates this Figure from Figures 2A and 3A is the use of CAFs pretreated with 1 µg/mL of a TLR4 signaling inhibitor (CLI-095). Carboxyfluorescein succinimidyl ester (CFSE)-labeled spleen cells were stimulated with or without concanavalin A (ConA) and CAF supernatant. The ratio of T cell proliferation was analyzed by flow cytometry. (C and D) Quantitative results. ns represents a non-significant difference (p > 0.05).
Discussion
Cinnamon is an extremely common seasoning and is used as a traditional Chinese medicine. It is mentioned repeatedly in A Handbook of Prescriptions for Emergencies that cinnamon can be used to treat tumors. Cinnamaldehyde, the main active component of cinnamon oil, has many biological effects that are being gradually proven in different fields. For example, cinnamaldehyde can inhibit the progression of human oral squamous cell carcinoma by inducing apoptosis of cancer cells.18 In addition, cinnamaldehyde can reduce the progression of melanoma by decreasing the levels of vascular endothelial growth factor and hypoxia inducible factor-α in cancerous tissues, thereby inhibiting the formation of new blood vessels.19 In addition, cinnamaldehyde is a broad inhibitor of periostin expression and can be used to treat systemic fibrotic diseases.20 Because cinnamaldehyde has effects on both cancer and fibrosis, it is possible that it can affect CAFs in the tumor microenvironment and suppress cancer.
In this study, we found that CAFs were tolerant to cinnamaldehyde within a certain concentration range, and the immunosuppressive effect of CAFs was reversed after treatment with this compound. CAFs can shape the tumor microenvironment by secreting chemokines, cytokines, or other factors, and CAFs are considered an important source of TGF-β in the tumor microenvironment. Thus, CAFs have an immunosuppressive function in the tumor microenvironment.4
After cinnamaldehyde treatment, the inhibitory effect of the CAF supernatant on T cells was attenuated in a concentration-dependent manner (Figure 2B). This result suggested that cinnamaldehyde might reshape CAFs, inhibiting their function in the tumor microenvironment.
The TLR family is associated with the activation of several of transcription factors, such as members of the nuclear factor-kappa B and interferon families.21 In liver injury, hepatic stellate cells, the major precursor of liver myofibroblasts, regulate the secretion of TGF-β through a TLR4-related pathway, which is associated with the development of fibrosis.22 In addition, cinnamaldehyde can bind to TLR4 by the oxygen atom of the aldehyde group forming a hydrogen bond with the side chain of Gln589 in TLR4.23 Furthermore, some research has shown that cinnamaldehyde can target the oligomerization process of TLR4 and inhibit lipopolysaccharide-induced TLR4 activation.24 This also suggests that TLR4 might be the receptor that cinnamaldehyde acts upon in CAFs. However, that study suggested inhibition of TLR4 by cinnamaldehyde, while our results indicate activation. The difference may be due to the cell type and cinnamaldehyde concentration used in the two experiments, which was 50 μM on macrophages in the prior work,23 and 2.3 μM on CAFs in the current study.
TLR4 is a common receptor that is inextricably linked to inflammatory processes and tumors. TLR4 can regulate downstream proteins such as JNK, TAK1, and c-Jun, which have significant impacts on tumor development.25 Many studies have implicated a positive role of JNK in cancer as it can induce cancer cell apoptosis and inhibit tumor progression.26,27 In our study, the Western blotting results showed that levels of the TLR4 pathway-associated proteins were changed after cinnamaldehyde treatment (Figure 3A); specifically, the TLR4 pathway was activated. In addition, after blocking the pathway with a TLR4 signaling inhibitor, even with cinnamaldehyde treatment, CAFs showed an inhibitory effect on T cells (Figure 4). The TLR4 signaling inhibitor selectively suppresses TLR4-signaling by blocking the intracellular domain of TLR4. It potently suppresses both ligand-dependent and -independent signaling of TLR4.28 Both of these results indicated that TLR4 might be a receptor for cinnamaldehyde to act on CAFs. Importantly, these results revealed a portion of the mechanism by which cinnamaldehyde regulated CAFs and suggested that activation of the TLR4 pathway in CAFs might inhibit their immunosuppressive function. Besides, CIL-095 was quite TLR4-specific and showed little effect on other TLRs, TLR1/2, TLR2/6, TLR3, TLR5, TLR7 and TLR9.28 So we inferred that cinnamaldehyde might have little impact on other receptors.
The CAF signaling pathway appears to be activated for only 2 h (Figure 3A), and only when treated with cinnamaldehyde for a sufficient period of time (24 h) was the immunosuppressive effect of CAFs on T cells reduced (Figure 2). This suggests that the signal path was upstream, controlling the downstream effects on cells. Overall, cinnamaldehyde has potential effects on the transformation of CAFs. In future studies, the molecular mechanism by which cinnamaldehyde modifies the function of CAFs should be explored.
Conclusion
The results of the current study demonstrate that cinnamaldehyde relieves CAF‐mediated T cell inhibition in a TLR4-dependent manner. This suggests that cinnamaldehyde can modify the functions of CAFs which is potentially helpful for the treatment for tumors.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31370910).
Data Sharing Statement
The data is available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA) and were approved by the Biological Research Ethics Committee, Institute of Biophysics, Chinese Academy of Sciences.
Disclosure
Yuanyuan Wang, Minghua Hu, Fangli Ma are employees of Infinitus Chinese Herbal Immunity Research Center, Infinitus China Company Ltd. The authors report no other possible conflicts of interest in this work.
References
- 1.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta. 2013;1832(7):1070–1078. doi: 10.1016/j.bbadis.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 4.Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018;9:414. doi: 10.3389/fimmu.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Yang W, Qin C, et al. Responsiveness of stromal fibroblasts to IFN-gamma blocks tumor growth via angiostasis. J Immunol. 2009;183(10):6413–6421. doi: 10.4049/jimmunol.0901073 [DOI] [PubMed] [Google Scholar]
- 6.Salinas CA, Tsodikov A, Ishak-Howard M, Cooney KA. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11(6):317–323. doi: 10.1038/nrurol.2014.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 8.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–711. doi: 10.1038/nrc4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calcinotto A, Spataro C, Zagato E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–369. doi: 10.1038/s41586-018-0266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305. doi: 10.1038/nature08782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eiro N, Fernandez-Gomez J, Sacristan R, et al. Stromal factors involved in human prostate cancer development, progression and castration resistance. J Cancer Res Clin Oncol. 2017;143(2):351–359. doi: 10.1007/s00432-016-2284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George RC, Lew J, Graves DJ. Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2013;36(1):21–40. doi: 10.3233/JAD-122113 [DOI] [PubMed] [Google Scholar]
- 13.Shreaz S, Wani WA, Behbehani JM, et al. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia. 2016;112:116–131. doi: 10.1016/j.fitote.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Emont MP, Jun H, et al. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism. 2017;77:58–64. doi: 10.1016/j.metabol.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagenlocher Y, Satzinger S, Civelek M, et al. Cinnamon reduces inflammatory response in intestinal fibroblasts in vitro and in colitis in vivo leading to decreased fibrosis. Mol Nutr Food Res. 2017;61(9):1601085. doi: 10.1002/mnfr.201601085 [DOI] [PubMed] [Google Scholar]
- 16.Yu C, Liu SL, Qi MH, Zou X. Cinnamaldehyde/chemotherapeutic agents interaction and drug-metabolizing genes in colorectal cancer. Mol Med Rep. 2014;9(2):669–676. doi: 10.3892/mmr.2013.1830 [DOI] [PubMed] [Google Scholar]
- 17.Madar S, Brosh R, Buganim Y, et al. Modulated expression of WFDC1 during carcinogenesis and cellular senescence. Carcinogenesis. 2009;30(1):20–27. doi: 10.1093/carcin/bgn232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang WL, Cheng FC, Wang SP, Chou ST, Shih Y. Cinnamomum cassia essential oil and its major constituent cinnamaldehyde induced cell cycle arrest and apoptosis in human oral squamous cell carcinoma HSC-3 cells. Environ Toxicol. 2017;32(2):456–468. doi: 10.1002/tox.22250 [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Lu Y, Yang G, Wu J. Research on tumorigenicity of cinnamaldehyde in melanoma cell lines and its mechanism. Tumor Biol. 2014;35(6):5717–5722. doi: 10.1007/s13277-014-1757-8 [DOI] [PubMed] [Google Scholar]
- 20.Mitamura Y, Murai M, Mitoma C, Furue M. NRF2 activation inhibits both TGF-beta1- and IL-13-mediated periostin expression in fibroblasts: benefit of cinnamaldehyde for antifibrotic treatment. Oxid Med Cell Longev. 2018;2018:2475047. doi: 10.1155/2018/2475047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. doi: 10.1038/nri2079 [DOI] [PubMed] [Google Scholar]
- 22.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–1332. doi: 10.1038/nm1663 [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Zhang M, Zhou F, et al. Cinnamaldehyde ameliorates LPS-induced cardiac dysfunction via TLR4-NOX4 pathway: the regulation of autophagy and ROS production. J Mol Cell Cardiol. 2016;101:11–24. doi: 10.1016/j.yjmcc.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Youn HS, Lee JK, Choi YJ, et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75(2):494–502. doi: 10.1016/j.bcp.2007.08.033 [DOI] [PubMed] [Google Scholar]
- 25.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209 [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R. N-(4-hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006;25(19):2785–2794. doi: 10.1038/sj.onc.1209303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh MJ, Chien SY, Lin JT, Yang SF, Chen MK. Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomedicine. 2016;23(13):1545–1554. doi: 10.1016/j.phymed.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584(1):40–48. doi: 10.1016/j.ejphar.2008.01.026 [DOI] [PubMed] [Google Scholar]