Abstract
Objective
Intracranial pineoblastomas are rare neoplasms with poor prognosis. The aim of this study was to describe the independent prognostic factors and treatment strategies for overall survival in pediatric and adult patients.
Methods
Sixty-four patients were surgically treated between January 2012 and December 2018.
Results
The series included 37 (57.8%) males and 27 (42.2%) females. Gross total resection was achieved in 41 (64.1%) cases, and the 1-, 3-, and 5-year rates of overall survival were 86.3, 52.3, and 36.6%, respectively. In the pediatric group (n=42), 28 patients (66.7%) were male, with the median, and the mean age was 4 and 6.2±4.7 years, respectively. After a median follow-up of 25.0 months, twenty-six patients (61.9%) died, and the 1-, 3-, and 5-year rates of overall survival were 84.9, 46.4, and 26.7%, respectively. Postoperative radiotherapy (p=0.058) and postoperative chemotherapy (p=0.183) had a positive influence on the increased overall survival. Meanwhile, postoperative radiotherapy combined with chemotherapy following surgery had a positive impact on overall survival (p=0.174, Log rank). In the adult group, the mean overall survival was 67.3±9.3 months (range, 0.8–95.3 months), and the 1-, 3-, and 5-year rates of overall survival were 89.5, 64.4, and 64.4%, respectively. In this group, no statistical association was observed between clinical factors and outcomes. However, patients who received postoperative radiotherapy (60.7 vs 57.6 month, mean survival; p=0.510, Log rank) or chemotherapy (63.0 vs 59.9 month, mean survival; p=0.404, Log rank) had better survival rates compared with those who declined.
Conclusion
In the pediatric group, surgery with postoperative radiotherapy and chemotherapy was a favorable factor for overall survival. In the adult group, a positive trend in overall survival was found when patients received radiation and/or chemotherapy following surgery.
Keywords: pediatric patients, adult patients, treatment strategy, overall survival
Introduction
Four grades of tumors of the pineal region, including pineocytoma, pineal parenchymal tumor of intermediate differentiation, papillary pineal tumor and pineoblastoma (PB), were classified by the World Health Organization (WHO) classification of tumors affecting the central nervous system (CNS).1 PB, recognized as WHO grade IV, is inclined to aggressive behavior with distant metastasis and constitutes 40% of parenchymal pineal cancers, and this tumor occurs more frequently in pediatric patients than in adult patients.2 To date, only a limited number of literature reports of PB in adults or pediatric patients have been reported, the majority of which were case studies.3–5 As a result of such an exceedingly low incidence of PBs in the general population, considerable dispute over factors affecting the prognosis and treatment strategies was present.6,7 In addition, the relationship of clinical behaviors between age-related subgroups was not identified. Therefore, this study aimed to explore adverse factors of overall survival (OS), multimodal treatment outcomes, and different clinical distributions between pediatric and adult patients based on our experience with surgical treatment in an institution.
Materials and Methods
Sixty-four consecutive patients surgically treated with PBs were pathologically diagnosed between January 2012 and December 2018. Three patients were excluded from these patients due to loss to follow-up; thus, 61 patients were included in the further analysis. Approval for this study was obtained from the Beijing Tiantan Hospital. All follow-up patients provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Data Collection
Clinical and radiologic data were obtained retrospectively from electronic medical records. As with all studies, there were some weaknesses and limitations to this retrospective study. The following information was recorded: sex, age at diagnosis, preoperative clinical presentation, pre- and postoperative Karnofsky Performance Scale (KPS), follow-up KPS, duration of symptoms, tumor traits (size, volume and MRI feature), treatment strategies (extent of resection and use of radiation and/or chemotherapy) and follow-up status.
We calculated both preoperative and postoperative magnetic resonance imagings (MRIs) using the cubature formula= (a×b×c/2). Tumor size was evaluated as the largest tumor diameter. Postoperative MRIs were reviewed to assess whether gross total resection (GTR) or subtotal resection (STR) was achieved. GTR was defined as complete removal of a tumor (without residue), and STR was set as any residual tumor revealed in the postoperative MRIs (>90% of excision of the lesion). Progression-free survival (PFS) was calculated from surgery date to the time of recurrence on follow-up MRI. OS was calculated from the date of surgery to the date of the death or most recent follow-up. Patient outcome and follow-up status were estimated through telephone interviews with outpatients.
Statistics
Survival analysis was performed using the Cox model. Kaplan–Meier curves were used to depict survival curves, and Log rank tests were performed to compare different survival functions according to clinical, radiological, and therapeutic factors. To assess the distinct clinical behavior distributions based on the age, the patients were divided into subgroups: the pediatric group (age≤18 years) and adult group (age>18 years). The correlation between these two groups was analysed by the Chi-square test for categorical variables and independent -sample t-test for continuous variables. Data were analysed using SPSS version 22.0 (IBM, Armonk, New York, USA), and a p value<0.05 was considered statistically significant.
Results
The clinical characteristics of 64 patients, including pediatric and adult patients, are summarized and shown in Table 1. This study included 42 (65.6%) pediatric patients and 22 (34.4%) adult patients, ranging in age from 11 months to 67 years with a mean age of 17.8 years. The most commonly presenting signs and symptoms were headache (n=47, 73.4%), vomiting (n=32, 50%), and weakness, as well as unsteady walking (n=20, 31.3%); less commonly presenting symptoms included dizziness (n=6, 9.4%) and double vision (n=5, 7.8%). Only 1 (1.6%) patient was discovered by physical examination. Fifty-seven (89.1%) patients experienced obstructive hydrocephalus, and 39 (60.9%) patients received an emergency operation, endoscopic, and/or ventriculostomy to achieve the mitigation.
Table 1.
Clinical Characteristics in Pediatric and Adult Patients
| Clinical Characteristics Overall n (%) | All 64 | Pediatric 42 (65.6) | Adult 22 (34.4) | P |
|---|---|---|---|---|
| Male | 37 (57.8) | 28 (66.7) | 9 (40.9) | 0.048†* |
| Duration of symptoms, mos | 0.004‡* | |||
| Range | 0.1–24.0 | 0.1–24.0 | 0.1–24.0 | |
| Mean | 3.9±5.6 | 2.3±4.0 | 7.1±6.7 | |
| Median | 2.0 | 1.0 | 6.0 | |
| KPS, mean | ||||
| Preoperative operation | 70.5±13.6 | 67.4±13.6 | 78.6±10.8 | 0.001‡* |
| At discharge | 79.0±16.1 | 78.3±14.1 | 83.1±19.9 | 0.263‡ |
| Diameter, cm | 0.086‡ | |||
| Range | 1.7–9.0 | 1.9–6.5 | 1.7–9.0 | |
| Mean | 3.7±1.2 | 3.8±1.0 | 3.3±1.5 | |
| Median | 3.5 | 4.7 | 3.2 | |
| GTR | 41 (64.1) | 26 (61.9) | 15 (68.2) | 0.619† |
| Radiotherapy§ | 32 (52.5) | 18 (42.9) | 14 (73.7) | 0.026†* |
| Chemotherapy§ | 25 (41.0) | 14 (30.0) | 11 (57.9) | 0.071† |
| Death§ | 32 (50.0) | 26 (61.9) | 6 (31.6) | 0.028†* |
Notes: (%) is the percentage of the proportion in this group. *P<0.05. †Chi-square test. ‡Independent sample t-test. §Three patients were lost to follow-up.
Abbreviations: GTR, gross total resection; KPS, Karnofsky Performance Scale.
Compared with the adult group, the pediatric group revealed a more obvious male predominance (p=0.048). The duration of symptoms with the median time of 1 month was significantly shorter in the pediatric group than with a median time of 6 months in the adult group (p=0.004). The adult group had a higher preoperative KPS than the pediatric group (78.6±10.8 vs 67.4±13.6 mean score; p=0.001). Larger tumor size, with a mean of 3.8±1.0 cm, was found in the pediatric group than in the adult group, with a mean of 3.3±1.5 cm (p=0.086). Compared with pediatric patients, who received radiotherapy (18/42, 42.9%) or chemotherapy (14/42, 30.0%) (radiotherapy, p=0.026; chemotherapy, p=0.071), more adult patients (14/19, 73.7%) received radiotherapy or chemotherapy (11/19, 57.9%). In this series, patients who survived usually had a better follow-up KPS score with a median of 90 (range, from 60 to 100). The most common postoperative chronic symptoms were headaches (n=2, 6.9%), unsteadiness%), and weak walk (n=3, 10.3%).
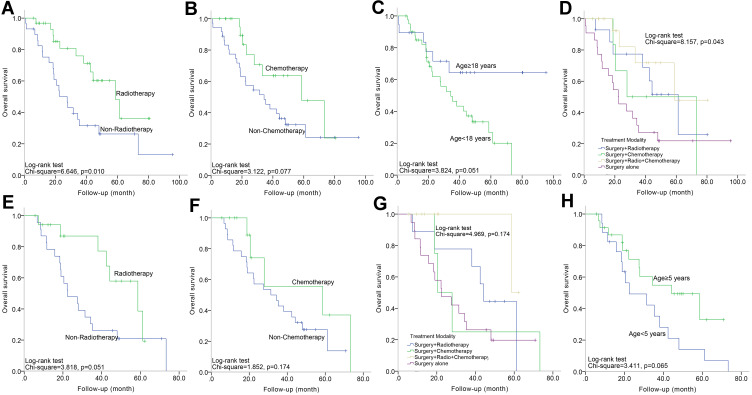
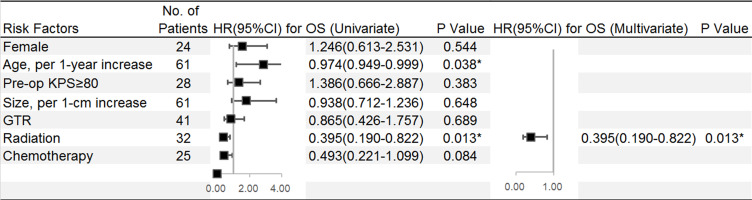
In this overall cohort (n=61), the rates of OS at 1, 3, and 5 years were 86.3, 52.3 and 36.6%, respectively, with a median time of 42.4 months, and these values were significantly related to age (per 1-year increase) (95% CI=0.949–0.999, HR=0.974; p=0.038). There was a significant difference in OS between patients who received adjuvant radiation and their counterparts (95% CI=0.190–0.822, HR=0.395; p=0.013) (Figure 1A) after the univariate analysis. Patients who received chemotherapy (58.7 vs 34.3 months, median survival; p=0.077, Log rank) (Figure 1B) or those younger than 18 years (67.3 vs 39.1 months, mean survival; p=0.051, Log rank) (Figure 1C) had a better OS. Multivariate analysis revealed that radiation remained a significantly independent risk factor (95% CI=0.190–0.822, HR=0.395; p=0.013). Patients were further divided into four groups based on the treatment strategy: surgery alone, surgery with radiation, surgery with chemotherapy, surgery with radiation, and chemotherapy. The survival curves showed that there were significant differences among patients stratified by treatment protocols (Figure 1D). Those who received surgery with radiation and chemotherapy had the most prolonged OS, whereas those who underwent surgery alone had the worst survival (58.7 vs 22.2 months, median survival; p=0.043) (Figure 2).
Figure 1.
(A–D) Kaplan–Meier curve analysis (Log rank test) displaying the different OS rates of all patients (n=61) between radiotherapy and non-radiotherapy (A); between chemotherapy and non-chemotherapy (B); between age<18 years and age≥18 years (C); among different four treatment protocols (D). E-H Kaplan–Meier curve analysis (Log rank test) displaying the different OS rates of pediatric patients (n=42) between radiotherapy and non-radiotherapy (E); between chemotherapy and non-chemotherapy (F); among different four treatment protocols (G); between age<18 years and age≥18 years (H).
Figure 2.
Univariate and multivariate Cox regression analyses were used to estimate the adverse factors for OS of patients. Black squares indicate the hazard ratio (HR). Error bars represent the 95% confidence intervals (CIs) and *Indicates p< 0.05.
Both pre- and postoperative MRIs were collected. The signal intensities of the tumor on T1-weighted and T2-weighted MRIs were classified as hypo-, iso-, hyper-, or mix-intense. The most common presentation on T1-weighted images was hypo-intense (n=36, 56.3%), and the most common presentation on T2-weighted images was hyper-intense (n=32, 50.0%); heterogeneous enhancement on contrast images was seen in 54 (84.4%) cases. (Table 2)
Table 2.
Tumor Characteristics
| Characteristics | Number n (%) |
|---|---|
| Overall | 64 |
| Lesion Volume in cm | |
| Range | 1.7–9.0 |
| Mean ± SD | 3.7±1.2 |
| Median | 3.5 |
| Lesion Volume in cm3 | |
| Range | 1.0–216.0 |
| Mean ± SD | 22.0±15.8 |
| Median | 13.5 |
| MRI Feature | |
| Hypo T1 and Hypo T2 | 2 (3.1) |
| Hypo T1 and Iso T2 | 2 (3.1) |
| Hypo T1 and Hyper T2 | 28 (43.8) |
| Hypo T1 and Mixed T2 | 4 (6.3) |
| Iso T1 and Iso T2 | 9 (14.1) |
| Iso T1 and Hyper T2 | 2 (3.1) |
| Iso T1 and Mixed T2 | 1 (1.6) |
| Mixed T1 and Hyper T2 | 2 (3.1) |
| Mixed T1 and Mixed T2 | 14 (21.9) |
| Enhancement | |
| Heterogeneous | 50 (78.1) |
| Homogeneous | 10 (15.6) |
| Circular | 4 (6.3) |
Note: (%) is the percentage of the proportion in this group.
Abbreviations: Hyper, hyperintensity; Hypo, hypointensity; Iso, isointensity.
Individual Data for the Pediatric Group
Twenty-eight patients (66.7%) were male with the median and the mean age was 4 and 6.2±4.7 years, respectively, ranging from 11 months to 17 years. The most common preoperative symptoms were vomiting (n=32, 76.2%) and headache (n=29, 69.0%), and the median period of symptoms was 1.0 month (Range, 3 days to 24 months). Twenty-six (61.9%) patients had GTR, and 16 (38.1%) patients had STR. Of 23 patients (54.8%) receiving adjuvant therapy, 18 patients (42.9%) received postoperative radiation, 14 patients (30.0%) received postoperative chemotherapy, and 9 (21.4%) patients received postoperative radiotherapy combined with chemotherapy (Table 1).
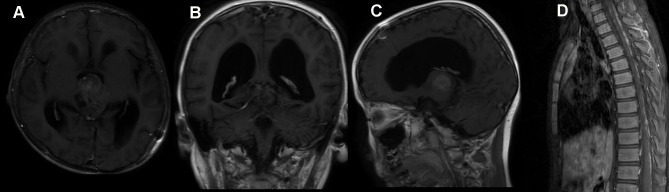
In this group, the median clinical follow-up time was 22.3 months (range, 5.6–73.3 months). Two patients developed an extra-cranial metastasis (Figure 3). Twenty-six (61.9%) died at a median of 25.0 months after surgery, and the OS rates at 1, 3 and 5 years were 84.9, 46.4, and 26.7%, respectively.
Figure 3.
A 5-year-old male, with two weeks of headache and vomiting, received surgery. (A–C) were preoperative MR images. After STR, he declined any adjuvant treatment. The MR image (D) at 1.5 months after surgery showed spinal metastasis.
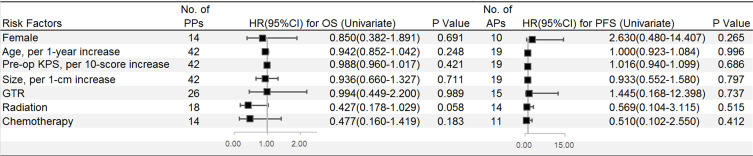
On the univariate analysis, the use of postoperative radiotherapy (58.7 vs 22.5 months, median survival; p=0.058) (Figure 1E) and postoperative chemotherapy (58.7 vs.31.4 months, median survival; p=0.183) (Figure 1F) were favorable factors. Survival curves did not show any significant difference among treatment strategies (Table 3), but postoperative radiotherapy combined with chemotherapy following surgery influenced OS (p=0.174, Log rank) (Figure 1G) (Figure 4).
Table 3.
Overall Survival of Different Treatment Protocols
| Treatment | No. of Pts | No. of Deaths | 5-Year OS | PPts | APts | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPts | APts | PPts | APts | PPts | APts | p value | HR (95% CI)* | p value | HR (95% CI)* | |
| Overall | 42 | 19 | 26 (61.9%) | 6 (31.6%) | 26.7% | 64.4% | 0.091 | 0.751 (0.539–1.047) | 0.065 | 0.610 (0.268–1.388) |
| Surgery alone | 19 | 3 | 15 (78.9%) | 2 (66.7%) | 19.7% | 33.3% | Reference† | Reference† | ||
| Surgery+ RT | 9 | 5 | 6 (66.7%) | 1 (20.0%) | 44.4% | 75.0% | 0.208 | 0.542 (0.208–1.408) | 0.185 | 0.196 (0.018–2186) |
| Surgery+ CT | 5 | 2 | 4 (80.0%) | 0 | 25.0% | 100 | 0.628 | 0.773 (0.208–2.581) | 0.989 | NA |
| Surgery+ RCT | 9 | 9 | 1 (11.1%) | 3 (33.3%) | 50.0% | 62.5% | 0.077 | 0.159 (0.021–1.217) | 0.150 | 0.264 (0.043–1.616) |
Notes: The overall cohort was divided into 4 groups, surgery alone, surgery+ radiotherapy, surgery+ chemotherapy and surgery+ radiochemotherapy. *Cox regression method. †We selected the subgroup of GTR alone as the reference (dummy variable).
Abbreviations: APts, adult patients; CT, chemotherapy; PPts, pediatric patients; Pts, patients; RT, radiotherapy; RCT, radiochemotherapy.
Figure 4.
Univariate Cox regression analyses were used to estimate the adverse factors for the OS of pediatric patients and adult patients separately. Black squares indicate the hazard ratio (HR), error bars represent the 95% confidence intervals (CIs).
Interestingly, neither tumor size (per 1-cm increase) nor the age at diagnosis (per 1-year increase) influenced OS. To further examine this phenomenon, tumor size was divided into two groups (<3.5 cm vs ≥3.5 cm) and age was divided into two groups (<5 years vs ≥5 years). However, difference trending significance was only observed in the age-related subgroup by Kaplan–Meier analysis (p=0.065, Log rank) (Figure 1H).
Individual Data for the Adult Group
This group included 9 (40.9%) males and 13 (59.1%) females, ranging in age from 23 to 67 years, with a mean and median age of 39.7±10.8 and 40.5 years. The most commonly preoperative presentation was headache (n=18, 81.8%), and the duration of symptoms ranged from 3 days to 24 months, with a median time of 6.0 months. GTR and STR were achieved in 15 (68.2%) and 7 (31.8%) patients, respectively. Radiation was administered to 14 (73.7%) patients, chemotherapy was administered to 11 (57.9%) patients, and radiation therapy combined with chemotherapy was administered to 3 (15.8%) patients. The remaining patients (n=5, 26.3%) declined any adjuvant treatment (Table 1).
The mean follow-up duration was 33.2 months (range 0.8–95.3 months). No patients had distant metastasis, and six patients (31.6%) had died by the follow-up. The median KPS at follow-up was 90 (Range 60–100). The mean OS was 67.3±9.3 months (range, 0.8–95.3 months) with 1-, 3- and 5-year OS rates of 89.5, 64.4, and 64.4%, respectively. Although patients who received postoperative radiotherapy (60.7 vs 57.6 months, mean survival; p=0.510, Log rank) or chemotherapy (63.0 vs 59.9 months, mean survival; p=0.404, Log rank) had better survival compared with those who declined, there was no significant difference among these factors in survival (Table 3) (Figure 4).
Discussion
The purpose of this study was to identify risk factors of OS and to describe outcomes following treatment modalities for patients with PBs in one of the most extensive series. In addition, this current study differed from many previous studies in the aspect of age (pediatric group vs adult group), which was assessed separately and compared.
This study indicated that PBs in pediatric patients might be gender-related, as the incidence in males tended to be much higher than that in females; the study also revealed that the gender ratio of PBs in pediatric patients was different from that of adult patients, which was nearly equal by gender. This finding in our younger group, a male predominance, was inconsistent with previous reports.6,8
Previous studies showed that heterogeneous enhancement is common in PBs.4,9 Our study also confirmed this observation and found that hypo-intense on T1 weighted images and hyper-intense on T2 weighted images occurred mainly for the radiologic features.
Tumor size played a paramount role in determining surgical treatment strategies for PBs. The more massive tumors commonly had a very large mass effect and involved more brain tissues, making it difficult for initial surgical resection. In that case, primary emergency measures were usually taken to mitigate the obstructive hydrocephalus caused by the effect through endoscopic third ventriculostomy and/or ventricular peritoneal shunt. Not surprisingly, larger tumor size in pediatric and adult patients in our series did not reach statistical significance for poor OS.
Surgery
Despite the conservative surgical strategy in the pineal region reported in early literature providing that at a high risk of mortality.10,11 There was no perioperative mortality or severe complication in our large cohort, even if GTR achieved 41 (64.1%) patients. With the popularization of micro-neurosurgery and a better understanding of local microanatomy, this cohort was more likely to achieve GTR. Because of this, patients usually had a better postoperative status as assessed by the KPS score. The surgical approach was one of the critical surgical techniques: the route closest to the tumor could expose the tumor and its surrounding structure via the natural fissure to protect the pontic vein and brain tissue.
However, unfortunately, in this series of pediatric patients, there was no significant association between the extent of resection and OS. Similarly, for pediatric patients, in a study by Parikh et al,6 the GTR did not significantly improve OS in patients with PBs; Cuccia et al8 failed to identify the outcome of the extent of resection. In contrast, in a study of pediatric patients GTR conferred an improvement in OS.13
In the analysis of adult patients, the significant impact of the extent of resection on OS in adult patients was notably described by Lee,12 simultaneously with an emphasis that no patients died of GTR. However, the significant role of GTR described in some studies remains unclear, including in our research.12,13
It was quite challenging to prove the significant association between GTR and outcome in either adult or pediatric patients; however, a higher OS by GTR was achieved than OS by STR reached in our study, consistent with other reports.13,14 However, Mynarek et al did not find that the extent of resection was a risk factor for PFS and OS.16 Additionally, along with the development of surgical skills contributing to better postoperative status, aggressive surgery was considered. Moreover, maximal surgery in time, if feasible, could alleviate the obstructive hydrocephalus and obtain more pathological specimens to allow an accurate diagnosis.
Adjuvant Therapy
Pediatric Group
The use of postoperative radiation has been related to higher OS.15–18 Our data indicated a better impact of radiation on favorable prognosis, which did not reach statistical significance, but the tendency that patients with adjuvant radiotherapy had a better survival was notable. Meanwhile, in agreement with previous studies, our cohort demonstrated that adjuvant chemotherapy was a favorable factor for survival.19,20 However, Jakacki et al thought that chemotherapy without radiotherapy was an ineffective therapy for young children.21 In consistent with our study, combined radiation and chemotherapy following surgery was better for survival.16
Considering the long-term side effects of radiotherapy in children, the decision as to whether to receive radiation mainly depended on patients’ age, especially in those younger than 3 years.22 As a result of insufficient adjuvant therapy, in line with previous reports, our study demonstrated that pediatric patients who were at age<5 years had worse survival.6,23,24
Adult Group
While the administration of adjuvant radiotherapy for PBs in adults has been identified to be useful to increase OS, a statistically significant effect on OS was not demonstrated owing to limited case reports.6,9,12 One case report advocated adjuvant chemotherapy for adult patients.15 In a previous large series of 34 adult cases, the contribution of chemotherapy to a favorable OS remained unclear.12 Unfortunately, our study had the same difficulties in solving the problem of improved survival after radiation/chemotherapy in adults with PBs. Indeed, a limited series assessed the association between postoperative radiotherapy/chemotherapy and survival, even though it did not provide the estimated OS rates of patients with adjuvant therapies.12,25 Our study failed to reach statistical significance mainly due to the small sample size. Nevertheless, we observed a trend towards improvement of OS when radiation or radiation was combined with chemotherapy.
Inconsistent with other studies,18 the adjuvant strategies for PBs may be helpful. Unfortunately, our study did not address the problem of the significant association between adjuvant therapy and outcome. However, we revealed that adjuvant radiotherapy and chemotherapy following surgery led to a better result in pediatric patients. Meanwhile, a positive trend in OS was found when patients received radiation and/or chemotherapy following surgery in the adult group. However, adjuvant therapy was not advocated when the patients could not tolerate the side effects of adjuvant therapy. In addition, the standard of chemotherapy regimens was questionable. Mynarek et al insisted that the response to chemotherapy seemed to be improved after more dose-intense chemotherapy.16 The chemotherapy regimens that Ghim et al gave were etoposide 100 mg/m2 days 1 to 3, cisplatin 100 mg/m2 day 1, and vincristine 1.5 mg/m2 day 1, repeated every 4 weeks; this chemotherapy regimen had a positive impact on survival.20 In another study, the protocols of chemotherapy drugs were different; combined chemotherapy and radiotherapy were feasible and effective in the older age group while younger children had a poorer response to neoadjuvant postoperative chemotherapy.15 In addition, the optimal radiation dose remained under dispute. Lee et al reported that patients who received a radiation dose of ≥40 Gy had a significantly better survival rate than those who received a lower radiation dose (29.8 vs 4.1 months, median survival).12 In a study by Mynarek et al, most patients received a radiation dose of 35 Gy, but they did not find an association between radiation dose and survival.16 Complete radiation data and the standard of chemotherapy were difficult to obtain in this retrospective study, which remains to be solved in the future.
We usually performed aggressive surgery to alleviate intracranial hypertension caused by tumor and hydrocephalus. When tumors compressed vital brain tissue, nerve, and vessel structures, we had to make a conservative choice. Patients were always recommended adjuvant therapy after surgery. Small cases hindered us from acquiring a statistical result for survival rates comparing patients undergoing GTR without adjuvant therapy vs those suffering STR with adjuvant therapy or those suffering GTR/STR with radiation and chemotherapy vs those suffering GTR/STR and radiation or chemotherapy alone. We need to perform a prospective subgroup analysis to determine which strategy offered a survival advantage.
Study Limitations
There are some weaknesses and limitations to the current study. First, it is a retrospective review of case reports; thus, the rate of missing detailed data for analysis is relatively high. Second, given the rarity of intracranial PBs, there was a lack of randomized data, and potential bias may exist in the statistical analysis data. Third, the subjective consistency measurement reflected a single-institute experience and was a limitation of the study; therefore, we defined it as a semi-quantitative consistency measurement according to intraoperative findings and the hardest part of the lesion. Given the intralesional heterogeneous consistency and the retrospective nature of the study, an objective quantitative consistency measurement was unavailable. Therefore, a prospective multicentre study with a large series of intracranial PBs is recommended.
Conclusion
Outcome benefit, especially for pediatric patients, was derived from adjuvant radiotherapy and chemotherapy following surgery. Small series with adult cases, to some degree, impeded any conclusion regarding the relationship between prognostic factors and outcome. However, a positive effect of multi-model treatment on the OS of adult patients was observed. Nevertheless, appropriate treatment modalities remain to be further investigated in both pediatric patients and adult patients.
Abbreviations
CI, confidence interval; GTR, gross total resection; HR, hazard ratio; KPS, Karnofsky performance scale; MRI, magnetic resonance imaging; OS, overall survival; PB, Pineoblastoma; PFS, progression-free survival; STR, subtotal resection.
Author contributions
Conception and experimental design: all authors. Acquisition of data and execution: Gui-Jun Zhang, Xu-Lei Huo, Bo Wang. Analysis and interpretation of data: all authors. Statistical analysis: Gui-Jun Zhang, Xu-Lei Huo, Xiao-Ying Xu, Xiao-Jie Li. Drafting the article: Gui-Jun Zhang, Xu-Lei Huo, Bo Wang. Critically revising the article: all authors. Agreed on the journal to which the article will be submitted: all authors. Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage: all authors. Agreed to take responsibility and be accountable for the contents of the article: all authors. Administrative/technical/material support: Jun-Ting Zhang, Zhen Wu, Liang Wang. Study supervision: all authors.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2.Tamrazi B, Nelson M, Blüml S. Pineal region masses in pediatric patients. Neuroimaging Clin N Am. 2017;27(1):85–97. doi: 10.1016/j.nic.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 3.Cuccia F, Mortellaro G, Cespuglio D, et al. A case report of adult pineoblastoma occurring in a pregnant woman. Anticancer Res. 2019;39(5):2627–2631. doi: 10.21873/anticanres.13386 [DOI] [PubMed] [Google Scholar]
- 4.Fujita A, Asada M, Saitoh M, et al. Pineoblastoma showing unusual ventricular extension in a young adult–case report. Neurol Med Chir (Tokyo). 1999;39(8):612–616. doi: 10.2176/nmc.39.612 [DOI] [PubMed] [Google Scholar]
- 5.Lesnick JE, Chayt KJ, Bruce DA, et al. Familial pineoblastoma. Report of two cases. J Neurosurg. 1985;62(6):930–932. doi: 10.3171/jns.1985.62.6.0930 [DOI] [PubMed] [Google Scholar]
- 6.Parikh KA, Venable GT, Orr BA, et al. Pineoblastoma-the experience at St. Jude Children’s Research Hospital. Neurosurgery. 2017;81(1):120–128. doi: 10.1093/neuros/nyx005 [DOI] [PubMed] [Google Scholar]
- 7.Biswas A, Mallick S, Purkait S, et al. Treatment outcome and patterns of failure in patients of pinealoblastoma: review of literature and clinical experience from a regional cancer centre in north India. Childs Nerv Syst. 2015;31(8):1291–1304. doi: 10.1007/s00381-015-2751-1 [DOI] [PubMed] [Google Scholar]
- 8.Cuccia V, Rodríguez F, Palma F, Zuccaro G. Pinealoblastomas in children. Childs Nerv Syst. 2006;22(6):577–585. doi: 10.1007/s00381-006-0095-6 [DOI] [PubMed] [Google Scholar]
- 9.Ai P, Peng X, Jiang Y, et al. Complete regression of adult pineoblastoma following radiotherapy: a case report and review of the literature. Oncol Lett. 2015;10(4):2329–2332. doi: 10.3892/ol.2015.3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jooma R, Kendall BE. Diagnosis and management of pineal tumors. J Neurosurg. 1983;58(5):654–665. doi: 10.3171/jns.1983.58.5.0654 [DOI] [PubMed] [Google Scholar]
- 11.Kunicki A. Operative experiences in 8 cases of pineal tumor. J Neurosurg. 1960;17:815–823. doi: 10.3171/jns.1960.17.5.0815 [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Wakabayashi T, Yoshida J. Management and survival of pineoblastoma: an analysis of 34 adults from the brain tumor registry of Japan. Neurol Med Chir (Tokyo). 2005;45(3):132–141, 141–142. doi: 10.2176/nmc.45.132 [DOI] [PubMed] [Google Scholar]
- 13.Tate M, Sughrue ME, Rutkowski MJ, et al. The long-term postsurgical prognosis of patients with pineoblastoma. Cancer. 2012;118(1):173–179. doi: 10.1002/cncr.26300 [DOI] [PubMed] [Google Scholar]
- 14.Jakacki RI, Burger PC, Kocak M, et al. Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(5):776–783. doi: 10.1002/pbc.25405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinkes BG, von Hoff K, Deinlein F, et al. Childhood pineoblastoma: experiences from the prospective multicenter trials HIT-SKK87, HIT-SKK92 and HIT91. J Neurooncol. 2007;81(2):217–223. doi: 10.1007/s11060-006-9221-2 [DOI] [PubMed] [Google Scholar]
- 16.Mynarek M, Pizer B, Dufour C, et al. Evaluation of age-dependent treatment strategies for children and young adults with pineoblastoma: analysis of pooled European Society for Paediatric Oncology (SIOP-E) and US Head Start data. Neurol Oncol. 2017;4(19):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Rojas T, Bautista F, Flores M, et al. Management and outcome of children and adolescents with non-medulloblastoma CNS embryonal tumors in Spain: room for improvement in standards of care. J Neurooncol. 2018;137(1):205–213. doi: 10.1007/s11060-017-2713-4 [DOI] [PubMed] [Google Scholar]
- 18.Selvanathan SK, Hammouche S, Smethurst W, Salminen HJ, Jenkinson MD. Outcome and prognostic features in adult pineoblastomas: analysis of cases from the SEER database. Acta Neurochir (Wien). 2012;154(5):863–869. doi: 10.1007/s00701-012-1330-4 [DOI] [PubMed] [Google Scholar]
- 19.Farnia B, Allen PK, Brown PD, et al. Clinical outcomes and patterns of failure in pineoblastoma: a 30-year, single-institution retrospective review. World Neurosurg. 2014;82(6):1232–1241. doi: 10.1016/j.wneu.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Ghim TT, Davis P, Seo JJ, et al. Response to neoadjuvant chemotherapy in children with pineoblastoma. Cancer. 1993;72(5):1795–1800. doi: [DOI] [PubMed] [Google Scholar]
- 21.Jakacki RI, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: a report of the Childrens Cancer Group. J Clin Oncol. 1995;13(6):1377–1383. doi: 10.1200/JCO.1995.13.6.1377 [DOI] [PubMed] [Google Scholar]
- 22.Fontana EJ, Garvin J, Feldstein N, Anderson RC. Pediatric considerations for pineal tumor management. Neurosurg Clin N Am. 2011;22(3):395–402. doi: 10.1016/j.nec.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 23.Tate MC, Rutkowski MJ, Parsa AT. Contemporary management of pineoblastoma. Neurosurg Clin N Am. 2011;22(3):409–412. doi: 10.1016/j.nec.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 24.Deng X, Yang Z, Zhang X, et al. Prognosis of pediatric patients with pineoblastoma: a SEER analysis 1990–2013. World Neurosurg. 2018;118:e871–e879. doi: 10.1016/j.wneu.2018.07.079 [DOI] [PubMed] [Google Scholar]
- 25.Chang SM, Lillis-Hearne PK, Larson DA, et al. Pineoblastoma in adults. Neurosurgery. 1995;3(3). [DOI] [PubMed] [Google Scholar]