Abstract
Initially recognised as an important factor for bone health, vitamin D is now known to have a range of effects on the immune system. Vitamin D deficiency is associated with an increased risk of multiple sclerosis (MS), a chronic immune‐mediated demyelinating disease of the CNS. In this review, we explore the links between vitamin D deficiency, MS risk, and disease activity. We also discuss the known immune effects of vitamin D supplementation and the relevance of these observations to the immunopathology of MS. Finally, we review the existing evidence for vitamin D supplementation as an MS therapy, highlighting several recent clinical studies and trials.
Keywords: vitamin D, cholecalciferol, multiple sclerosis, immune, transcriptome, genetic, supplementation, treatment, therapeutic
Abbreviations
- CDMS
clinically definite MS
- CIS
clinically isolated syndrome
- DC
dendritic cell
- FDE
first demyelinating event
- MS
multiple sclerosis
1. INTRODUCTION
Vitamin D refers to a group of lipid‐soluble secosteroid compounds. Its two major forms are vitamin D2 (ergocalciferol), produced by plants and fungi, and vitamin D3 (cholecalciferol), synthesised in animals (Figure 1). Vitamin D is best known for its roles in skeletal health as well as calcium and phosphate homeostasis. Its clinical importance in bone health is illustrated by diseases associated with severe vitamin D deficiency, rickets, and osteomalacia. These are diseases of bone matrix demineralisation in children and adults, respectively (Holick, 2007).
FIGURE 1.
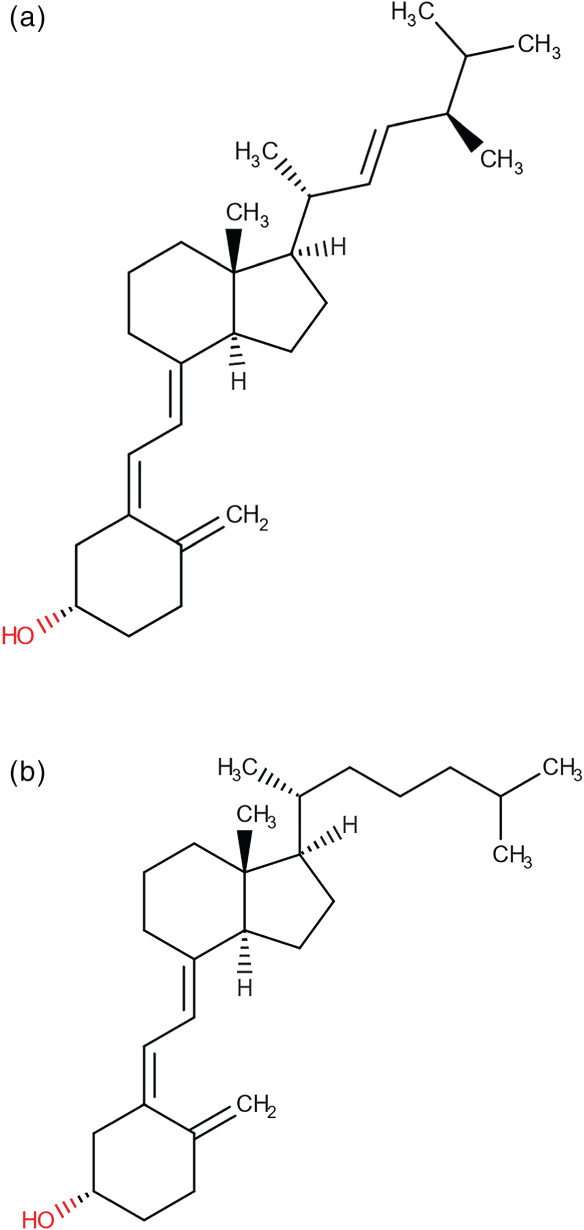
Structural formulae of vitamin D2 (ergocalciferol) (a) and vitamin D3 (cholecalciferol) (b), respectively (Wishart et al., 2018)
The extra‐skeletal actions of vitamin D include immunomodulatory effects in both innate and adaptive immune cells. Vitamin D deficiency increases the risk of developing a number of autoimmune diseases, including multiple sclerosis (MS), type 1 diabetes mellitus, and Crohn's disease (Ananthakrishnan et al., 2012; Gorham et al., 2012; Munger, Levin, Hollis, Howard, & Ascherio, 2006). Based on the strong inverse association of vitamin D levels and risk of MS, it has been proposed that vitamin D supplementation could have a therapeutic role in this disease. Here, we review the known effects of vitamin D on immune cells and provide a summary of recently completed and ongoing studies of vitamin D supplementation for the treatment and/or prevention of MS.
2. MULTIPLE SCLEROSIS
2.1. What is MS?
MS is a chronic autoimmune demyelinating disease of the CNS and is a leading cause of disability affecting young adults (Wallin et al., 2019). The MS disease course exists on a spectrum. The most common phenotype is relapsing–remitting MS. Relapsing–remitting MS is defined by relapses (attacks). During a relapse, focal or multifocal neurological symptoms develop over hours to days. Symptoms persist for days to weeks and then improve, either fully or partially. Relapsing–remitting MS accounts for at least 85% of all diagnoses (Weinshenker, 1994); 5–15% of patients have a phenotype referred to as primary progressive MS. Primary progressive MS is characterised by a gradual progressive course from symptom onset. In both relapsing–remitting and primary progressive MS, disability accumulates over time. The mean age of onset in relapsing–remitting MS is 28.5 years (Scalfari et al., 2010). The female‐to‐male ratio is 2–3:1 in relapsing–remitting MS, but 1:1 in primary progressive MS (Koch‐Henriksen & Sørensen, 2010; Ribbons et al., 2015).
MRI is a key diagnostic and prognostic tool in MS. Typical features on MRI have become an integral part of the diagnostic criteria of MS, and development of new MRI lesions is a key measure of disease activity in trials and clinical practice (Barkhof, Calabresi, Miller, & Reingold, 2009). MRI‐visible MS lesions are hyperintense on T2‐weighted sequences and typically involve the white matter regions of the brain and spinal cord. In MS, the target of the autoimmune attack is principally the myelin sheath of CNS axons, though a target antigen remains elusive after many decades of research (Wootla, Eriguchi, & Rodriguez, 2012).
2.2. MS pathology
Both adaptive and innate arms of the immune system have been implicated in MS pathogenesis. The innate immune response is rapid and non‐antigen specific, whereas the adaptive immune system elicits an antigen‐specific response and carries an antigen‐specific memory. Innate immune cells include myeloid lineage‐derived cells such as monocytes, macrophages, and dendritic cells (DCs), as well as lymphoid‐derived NK cells. Monocytes, macrophages, and DCs recognise pathogens through pattern recognition receptors. Pathogen detection leads to up‐regulation of co‐stimulatory molecules such as CD80 and CD86 on their surface. These co‐stimulatory molecules are required for activation of T cells during antigen presentation, the key interaction between innate and adaptive immune cells in the immune response.
Adaptive immune cells include B and T lymphocytes. B cells are responsible for antibody production (when terminally differentiated into plasma cells) and also have roles in antigen presentation and vcytokine production. T lymphocytes can be divided into two main types: CD4+ T cells and CD8+ cytotoxic T lymphocytes. These cells express antigen receptors that bind to specific epitopes. CD4+ T cells can differentiate into several functionally distinct subsets, including T helper (Th) 1 and Th2 and Th17 subsets, depending on the cytokines they are exposed to during maturation. A small number of both T and B cells are so‐called regulatory lymphocytes. They suppress T cell responses and promote a tolerogenic immune state—important in preventing autoimmunity and limiting the extent of any immune response to avoid severe self‐injury (Sakaguchi, 2011).
Pathological studies of actively demyelinating MS lesions have identified inflammatory infiltrates characterised by a large number of activated macrophages, as well as CD4+ and CD8+ T cells, B cells, activated microglia, and scant plasma cells (Lucchinetti et al., 2000). Some plaques exhibit complement and immunoglobulin deposition and oligodendrocyte apoptosis, implying both innate (complement) and adaptive (B cell production of immunoglobulin) immune system involvement. Axonal injury is common but widely believed to be secondary to the inflammatory demyelination. CNS myelin can regenerate to some extent, whereas CNS axons in humans cannot; thus, any axonal injury is likely to be a significant contributor to irreversible injury and, ultimately, disability. Though MS is most often thought of as a demyelinating disease affecting white matter, it also involves demyelinating lesions in the cerebral cortex, deep nuclei and spinal cord grey matter (Lucchinetti et al., 2011).
2.3. MS risk factors
Both genetic and environmental risk factors have been implicated in the development of MS (International Multiple Sclerosis Genetics Consortium, 2019; van der Mei et al., 2016). The presence of a latitudinal gradient of MS prevalence and incidence, increasing with distance away from the equator, is a key environmental risk factor. A recent meta‐analysis revealed that the latitudinal variation of MS prevalence has increased over time (Simpson et al., 2019). The key candidates to explain these findings are UVB exposure and vitamin D status. Infection with the Epstein–Barr virus (EBV) in adolescence (as symptomatic glandular fever) and smoking are other known risk factors for MS (Hedström, Olsson, & Alfredsson, 2016; Levin, Munger, O'Reilly, Falk, & Ascherio, 2010). Childhood and adolescent obesity is weakly linked with MS risk (Jacobs, Noyce, Giovannoni, & Dobson, 2020; Langer‐Gould, Brara, Beaber, & Koebnick, 2013).
3. VITAMIN D METABOLISM AND PHYSIOLOGICAL FUNCTIONS
In humans, the three major sources of vitamin D are UVB‐mediated production in the skin, dietary intake of vitamin D‐rich foods, and pharmacological supplementation. Dietary intake is generally insufficient to meet requirements. Synthesis via UVB (290‐ to 315‐nm wavelength) exposure is the predominant source of vitamin D. Whole‐body UVB exposure of one minimal erythemal dose, the dose of UV radiation required to cause mild reddening of skin, produces an amount equivalent to ingestion of 10,000‐IU vitamin D (Holick, 1995). Both intrinsic and extrinsic factors can influence the degree of vitamin D3 production, including age, area of exposed skin, skin pigmentation, geographical latitude, and season (Holick, 1995). Vitamin D‐rich foods are few but include fatty fish, in particular salmon, cod liver oil, and shiitake mushrooms (Holick, 2007). Supplementation and fortification of foods (e.g., margarine and milk) are often used when UVB exposure and diet are inadequate to achieve vitamin D sufficiency, particularly in more polar regions.
UVB‐mediated synthesis begins with the precursor 7‐dehydrocholesterol, found in the epidermis. UVB exposure transforms 7‐dehydrocholesterol to previtamin D3, which is then converted to vitamin D3 via temperature‐dependent isomerisation (Figure 2) (Tian, Chen, Matsuoka, Wortsman, & Holick, 1993). There is an upper limit to the amount of vitamin D3 produced, following which previtamin D3 and excess vitamin D3 are photoconverted to inactive metabolites including lumisterol, tachysterol, suprasterol I, and suprasterol II (Webb, DeCosta, & Holick, 1989). Thus, vitamin D toxicity from UVB exposure does not occur unless a concomitant medical condition such as primary hyperparathyroidism is present.
FIGURE 2.
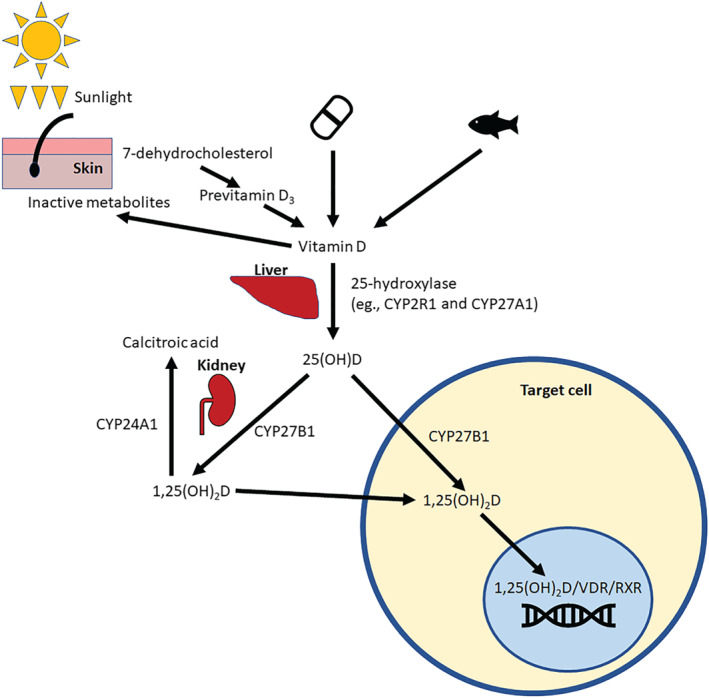
Metabolism and mechanism of action of vitamin D. Vitamin D is either synthesised in the skin during UVB exposure or obtained through intake from supplementation or dietary sources. Vitamin D is then metabolised first to 25(OH)D by the liver and then to 1,25(OH)2D, which is its active form, by the kidney. The kidney can also metabolise 1,25(OH)2D to the inactive calcitroic acid by the action of CYP24A1. Many target cells have the metabolic enzymes required to convert vitamin D to its active form. In the target cell, 1,25(OH)2D binds to vitamin D receptor (VDR) and retinoid X receptor (RXR). This complex then binds to the genome at vitamin D response elements to modulate gene expression
Vitamin D binds to vitamin D‐binding protein in the circulation and, to a lesser extent, lipoproteins (Haddad, Matsuoka, Hollis, Hu, & Wortsman, 1993). Vitamin D is inactive and must undergo two steps to be transformed into its active metabolite. It is first carried to the liver where 25‐hydroxylases, which include CYP2R1, CYP27A1, and CYP3A4, add a hydroxy group to form 25‐hydroxyvitamin D (25(OH)D) (Zhu & DeLuca, 2012). 25(OH)D re‐enters the circulation and is carried to the kidneys. It is transported into renal tubular cells where 1‐α‐hydroxylase (CYP27B1) hydroxylates 25(OH)D to 1,25‐dihydroxyvitamin D (1,25(OH)2D), also known as calcitriol, the active form of vitamin D (Miller & Portale, 2000). 24‐α‐hydroxylase (CYP24A1) is also present in renal tubular cells and inactivates both 1,25(OH)2D and 25(OH)D to calcitroic acid (Makin, Lohnes, Byford, Ray, & Jones, 1989). CYP24A1 is induced by calcitriol in an autoregulatory loop protecting against calcitriol toxicity. The 25(OH)D3 metabolite has a half‐life of 2–4 weeks, in contrast to the much shorter half‐life of 1,25(OH)2D3 of 4–6 h (Gray et al., 1978; Jones et al., 2014). The half‐life of 25(OH)D2 is shorter than that of 25(OH)D3. 25(OH)D3 provides the most accurate measure of vitamin D stores and is the form of vitamin D routinely measured to assess vitamin D status.
Calcitriol (1,25(OH)2D) is carried in the circulation by vitamin D‐binding protein and acts on target cells in an endocrine fashion. It passes into the cell nucleus and binds the vitamin D receptor (VDR). The 1,25(OH)2D‐VDR complex heterodimerises with retinoid X receptor and binds to vitamin D response elements of the DNA, and together with other proteins acts as a transcription factor to alter gene expression. The most common vitamin D response element is the DR3‐type element which consists of two hexameric repeated motifs separated by three nucleotides (Heikkinen et al., 2011).
Many cell types express VDR, including cells of the innate and adaptive immune systems, and can therefore respond to vitamin D (Provvedini, Tsoukas, Deftos, & Manolagas, 1983). Additionally, multiple cell types express the enzymes required for vitamin D metabolism, including CYP27B1 and CYP24A1, allowing them to respond to vitamin D in an autocrine or paracrine fashion. These include T and B lymphocytes, DCs, monocytes, and neural cells (including neurons and microglia) (Baeke et al., 2010; Chen et al., 2007; Hewison et al., 2003; Kreutz et al., 1993; Landel, Stephan, Cui, Eyles, & Feron, 2018). Responses to vitamin D vary by cell type and are dependent on factors such as activation state and presence of environmental signals.
4. VITAMIN D AND ITS EFFECTS ON IMMUNE CELLS
4.1. Immune cell phenotype and function
Immune cells express VDR and the enzymic machinery to metabolise vitamin D to varying degrees. As such, vitamin D can modulate the functions of both innate and adaptive immune cells. Exposure to vitamin D, in vitro, enhances the innate immune system's capacity to eliminate pathogens (Liu, Stenger, Tang, & Modlin, 2007; Xu, Soruri, Gieseler, & Peters, 1993) and promotes tolerance induction through its interactions with adaptive immune cells (Penna & Adorini, 2000; Piemonti et al., 2000; Unger, Laban, Kleijwegt, van der Slik, & Roep, 2009). In adaptive immune cells, vitamin D promotes differentiation towards regulatory B and T cells, increases production of anti‐inflammatory cytokines, such as IL‐10 and TGF‐β, and decreases pro‐inflammatory cytokines (Heine et al., 2008; Jeffery et al., 2009; Lysandropoulos et al., 2011) (the in vitro effects of vitamin D are summarised in Table S1). Overall, these effects facilitate pathogen clearance and minimise excessive inflammation and consequent tissue damage.
By contrast, relatively few studies have assessed the effects of vitamin D supplementation on peripheral blood immune cells of healthy adults in vivo (Table 1). The limited evidence, however, also supports an anti‐inflammatory effect of vitamin D supplementation, with an increase in regulatory T cell (Treg) proportions and IL‐10‐producing cells together with a decrease in IFN‐γ‐ and IL‐17‐secreting CD4+ T cells (Drozdenko et al., 2014; Prietl et al., 2014). Another consideration is that while peripheral blood is accessible, change may be occurring at other sites. Bak et al. (2018) observed a decrease in CD103 + DCs, which are known to induce Treg differentiation, in colonic mucosa after vitamin D supplementation. This was interpreted as tolerogenic, the DCs migrating to mesenteric lymph nodes where they interact with adaptive immune cells. Further study of other tissue may yield novel understanding of vitamin D–immune system interactions.
TABLE 1.
Vitamin D supplementation studies in relatively healthy adults and effects on the immune system
| Study | Study design | Participants | Interventions | Intervention duration | Baseline serum 25(OH)D, mean (nmol·L−1) | Post‐intervention serum 25(OH)D, mean (nmol·L−1) | Findings | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Monocytes and dendritic cells | B cells | T cells | Cytokines | |||||||
| Prietl et al., 2010 | Interventional study | Healthy adults. N = 50 | D3 140,000 IU at baseline and at 4 weeks. | 8 weeks | 59.6 | 145 | ↑ % Treg | |||
| Bock et al., 2011 Prietl et al., 2014 | Double‐blind RCT | Healthy adults. N = 59 | D3 140,000 IU monthly (N = 30). Placebo (N = 29). | 12 weeks |
Vitamin D group: 63.6 Placebo: 64.5 |
Vitamin D group: 138 Placebo: 52.8 |
No change in % DC (peripheral) and absolute monocyte and myeloid DC counts. | No change in % CD19+ B cell. | ↑ % Treg only in vitamin D group. No change to Treg suppressive capacity. | |
| Allen et al., 2012 | Interventional study | Healthy adults. N = 4 | D3 5,000 IU daily for 10 weeks, then either 10,000 or 5,000 IU daily for 5 weeks. | 15 weeks | 38 | 180 | ↓ % Th17 | ↑ IL‐10 level (culture supernatant) | ||
| Drozdenko, Heine, & Worm, 2014 | Interventional study | Healthy adults. N = 43 | D3 escalating dose (2,000–8,000 IU daily) (N = 25). Control (N = 18). | 12 weeks |
Vitamin D: 40.1 Placebo: 49.1 |
Vitamin D group: 159 Placebo: 30 |
↑ % CD38+ B cells in vitamin D group. No change to serum Ig concentrations. | ↓ % IFN‐γ+ and IL‐17+ CD4+ T cells. | ||
| Konijeti et al., 2016 | Double‐blind RCT | Pre‐ or stage 1 hypertensive participants. N = 38 | D3 low (400 IU daily; N = 18) or high (4,000 IU daily; N = 20) dose. | 2 months |
Low dose: 39.3 High dose: 41.8 |
Low dose: 53.7 High dose: 66.2 |
↓ CD4+ T cell activation in high‐dose group. | |||
| Bak et al., 2018 | Interventional study | Healthy adults. N = 10 | D3 200,000‐IU loading dose, then 20,000 IU daily. | 15 days | 52 a | 200 a | ↓ % CD103+ DCs from colonic biopsies. | ↑ IL‐10, TGF‐β, and TNF‐α mRNA expression | ||
Abbreviations: DC, dendritic cell; RCT, randomised‐controlled trial; Th, T helper cell; Treg, regulatory T cell.
Medians provided in article instead of means.
4.2. Changes to the immune transcriptome
The sites at which VDR binds to the genome are enriched at regions of strong enhancer and active promoter elements, which are important for gene regulation (Ramagopalan et al., 2010; Tuoresmäki, Väisänen, Neme, Heikkinen, & Carlberg, 2014). Hence, the changes to gene expression after vitamin D treatment have been the focus of investigation in an attempt to gain insight into its mechanisms. Investigation of the transcriptome, the set of all RNA transcripts of a cell or group of cells, has identified up to 3,650 differentially expressed genes following vitamin D treatment in a single immune cell line (Neme, Seuter, & Carlberg, 2017). Several primary vitamin D target genes encode for transcription factors which can in turn modulate transcription of secondary target genes of vitamin D (Nurminen et al., 2015). Pathway analyses of differentially expressed genes identify enrichment in immune and metabolic functions, though these differ between cell types. The mechanisms that lead to these changes have also been examined, such as the alterations to chromatin accessibility by vitamin D (the molecular modulations by vitamin D are summarised in Table S2).
While there have been extensive in vitro studies of vitamin D‐induced effects in immune cells, it is unclear whether similar changes are seen in vivo. As in vitro studies use supraphysiological concentrations of calcitriol, they may not reflect in vivo effects in tissues. A small number of vitamin D supplementation studies in healthy adults included assessment of immune cell gene expression in vivo, with mixed findings (Table S3). Two studies treated participants for up to 6 months with cholecalciferol doses between 400 and 10,000 IU daily (Hossein‐nezhad, Spira, & Holick, 2013; Shirvani, Kalajian, Song, & Holick, 2019). These identified up to 1,289 differentially expressed genes and suggested a dose‐dependent response with more genes modulated by a higher dose. The longest and largest study included 305 participants supplemented with either 2,000 or 4,000 IU daily for 12 months (Berlanga‐Taylor et al., 2018). This did not identify any differentially expressed genes after supplementation. These mixed findings could suggest that the modulatory effect of vitamin D supplementation has a time‐dependent course with diminishing effect over time due to negative regulatory and other effects. However, methodological differences between these few studies limit any firm conclusions. These differences include varying doses, peripheral blood cells assessed, and statistical approaches.
5. THE ROLE OF VITAMIN D IN MS RISK AND DISEASE ACTIVITY
Latitudinal variation of MS prevalence and incidence implicates environmental factors. The most probable candidates are UVB radiation exposure and vitamin D status. Their potential contribution to MS risk and disease activity is an area of intensive study as vitamin D supplementation could be a safe and effective pharmacological intervention for primary prevention and treatment of MS. Evidence linking vitamin D to MS causation and disease activity will be reviewed in this section.
5.1. Vitamin D deficiency is a risk factor for MS
Low serum vitamin D status has been linked with increased MS risk. Munger et al. (2006) conducted a prospective nested case–control study from a sample of over 7 million US military personnel. They assessed serum vitamin D status prior to MS diagnosis and observed a 41% reduction in subsequent MS diagnosis for every 50 nmol·L−1 increase in serum 25(OH)D3 in whites. Among blacks, a smaller reduction of 34% was seen. In the Ausimmune Study, a multicentre Australian incidence study of cases with a first CNS demyelinating event, a 50 nmol·L−1 increase of serum 25(OH)D3 reduced the risk of a first demyelinating event (FDE) by 31%, independent of prior sun exposure (Lucas et al., 2011). A prospective study of women in the Finnish Maternity Cohort showed that a 50 nmol·L−1 higher level in serum 25(OH)D3 sampled during first trimester of pregnancy reduced their later MS risk by 39% (Munger et al., 2017). Interestingly, there is also some evidence that lower vitamin D levels in the mother during gestation and of the neonate increase MS risk in the offspring (Mirzaei et al., 2011; Munger et al., 2016; Nielsen et al., 2017). A Swedish study used databases of individuals with prospectively collected blood samples tested for serum 25(OH)D3 and showed that levels of at least 75 nmol·L−1 were associated with lower MS risk with an odds ratio of 0.39 (Salzer et al., 2012). Based on these findings, higher vitamin D levels in many prospective cohort studies are strongly associated with lower subsequent MS risk.
The Nurses' Health Study and Nurses' Health Study II studied the association between vitamin D intake and MS risk. They prospectively followed two large cohorts of female nurses in the United States and found that total vitamin D intake and supplementation were inversely associated with risk of later developing MS (Munger et al., 2004). The relative risks were 0.67 for total intake (highest vs. lowest quintile) and 0.59 for supplementation of ≥400 IU daily versus no supplementation. Fatty fish and cod liver oil are dietary sources relatively high in vitamin D. Their consumption has also been associated with reduced risk of MS (Bäärnhielm, Olsson, & Alfredsson, 2014). These studies suggest a role for vitamin D consumption and supplementation in reducing MS risk.
Application of Mendelian randomisation has further strengthened the evidence base for a causal relationship between vitamin D and MS risk. Mendelian randomisation uses genetic variants associated with a proposed risk factor as an instrumental variable to assess their relationship with a given outcome. A significant benefit of this technique over observational studies is its ability to overcome confounding by unknown factors. Using genetic variants or single nucleotide polymorphisms associated with 25(OH)D3 level, four studies have shown a protective effect against developing MS in the presence of variants associated with higher vitamin D levels (Gianfrancesco et al., 2017; Jacobs et al., 2020; Mokry et al., 2015; Rhead et al., 2016).
Collectively, these studies suggest that vitamin D deficiency significantly increases the risk of MS and that repletion of vitamin D status through, for instance, dietary intake, could minimise this risk. These observations also pose the question of whether vitamin D supplementation could be used as a therapeutic agent to prevent MS.
5.2. Vitamin D deficiency and MS disease activity
Studies have also examined the relationship between vitamin D status and various measures of MS disease activity such as risk of relapse after an initial attack, annualised relapse rates, disability progression, and change to MRI lesion load.
In an Italian retrospective study of 100 clinically isolated syndrome (CIS; first demyelinating attack) patients, low serum vitamin D was associated with conversion to clinically definite MS (CDMS), most marked among subjects in the lowest 10th percentile of vitamin D level at CIS (serum 25(OH)D3 of <59.3 nmol·L−1) (Martinelli et al., 2014). Ascherio et al. (2014) reported on the BENEFIT study, a randomised‐controlled trial of IFN‐β1b in CIS patients. Participants had vitamin D status assessed at baseline and over up to 24 months. Using the average serum 25(OH)D3 level in the first 12 months of CIS, a 50 nmol·L−1 increment was associated with a 56% reduction in hazard of converting to MS over the subsequent 4 years. Higher serum vitamin D level was associated with fewer new active MRI lesions, less T2 lesion volume accumulation and brain volume loss, fewer relapses, and less change in disability.
Simpson et al. (2010) performed a prospective cohort study of 145 relapsing–remitting MS patients who had biannual review and 25(OH)D3 measurements, with mean follow‐up duration of 2.3 years. They found a reduction in relapse risk by 12% for every 10 nmol·L−1 increase in serum 25(OH)D3. A similar association was identified in another prospective study of 73 relapsing–remitting MS patients (Runia, Hop, de Rijke, Buljevac, & Hintzen, 2012) and in patients with paediatric‐onset MS (Mowry et al., 2010). Recent Mendelian randomisation studies are also supportive of an association between vitamin D status and relapse rate (Graves et al., 2020). Collectively, these studies suggest an association between reduced acute inflammatory activity (relapses) in MS patients and higher serum 25(OH)D3 levels. Further support for this association was observed in two prospective studies showing that higher vitamin D levels were associated with fewer new MRI lesions (Fitzgerald et al., 2015; Mowry et al., 2012).
In summary, MS risk and disease activity are strongly associated with lower vitamin D levels. Exposures throughout life, from time in the intrauterine environment through to birth, and into early adulthood, appear to influence disease susceptibility. Vitamin D is recognised to have immunomodulatory effects. However, the specific mechanisms by which vitamin D could affect MS risk and disease activity are not well understood.
6. MOLECULAR EVIDENCE FOR A ROLE OF VITAMIN D IN MS RISK
The epidemiological body‐of‐work linking vitamin D deficiency and MS risk, and the in vitro actions of vitamin D on immune cells, strongly suggest a role for vitamin D in MS pathogenesis. However, it is currently unclear what specific mechanisms or interactions between vitamin D and the immune system result in this elevated risk. A number of molecular observations support the relationship between vitamin D and MS risk (summarised in Table S4 and briefly described below).
First, a number of MS risk genes are known to be modulated by vitamin D. From genome wide association studies, over 200 MS risk single nucleotide variants have been identified from which more than 500 susceptibility genes have been proposed (International Multiple Sclerosis Genetics Consortium, 2019). The majority of these risk variants are located in non‐coding regions of the genome which may have regulatory effects on gene expression. Expression quantitative trait locus analyses of these candidate MS risk variants have implicated both adaptive (T and B cells) and innate immune (NK, DC, monocyte, and microglia) cells in MS pathogenesis (Gresle et al., 2020; International Multiple Sclerosis Genetics Consortium, 2019). Several genes have been shown to be modulated by vitamin D treatment in various immune cells, and a number have also been shown to be altered at the protein level. One example is the HLA‐DRB1 gene whose variant, DRB1*15:01, is the strongest genetic risk factor for MS with odds ratio of 3 (International Multiple Sclerosis Genetics Consortium, 2019; Ramagopalan et al., 2009). This variant has a functional vitamin D response element in its promoter. Calcitriol treatment of lymphoblastoid cells homozygous for HLA‐DRB1*15 showed an increase in HLA‐DRB1 cell surface expression (Ramagopalan et al., 2009). This interaction between two significant MS risk factors is hypothesis‐generating. There could be an effect of vitamin D on antigen presentation and thus adaptive immune system activation, with potential effects on CD4+ T cell maturation and central tolerance. The combination of a state of vitamin D deficiency and down‐regulated expression of HLA‐DRB1*15 could allow myelin‐specific autoreactive T cells to escape negative selection which then culminates in later development of MS. A number of other MS risk genes have also been shown to be vitamin D target genes, such as IL2RA which encodes CD25, a subunit of the high‐affinity IL‐2 receptor expressed on Treg cells and effector T cells (Berge et al., 2016). Of interest, CD25 has been the target of daclizumab, a monoclonal antibody previously approved for MS treatment, but now withdrawn from the market due to safety concerns (Cohan, Lucassen, Romba, & Linch, 2019).
Second, VDR binding is enriched near MS risk loci indicating that the genes associated with these loci are potentially regulated by vitamin D (Booth et al., 2016; Ramagopalan et al., 2010). These MS risk variants may also alter vitamin D responsiveness of respective genes. Third, several enzymes involved in vitamin D metabolism have genetic variants in or near their genes associated with MS susceptibility, in particular CYP27B1, CYP24A1, and CYP2R1 (International Multiple Sclerosis Genetics Consortium, 2019). Changes to enzyme function or expression may then lead to altered vitamin D metabolism with downstream effects. MS patients have been reported to have a lower increase in serum 25(OH)D level following supplementation than that in controls (Bhargava et al., 2016). Altered vitamin D metabolism provides a potential explanation for this observation.
Unfortunately, in vivo confirmation for most of the above observations is lacking, and hence, the biological relevance of these observations remain unclear.
7. VITAMIN D SUPPLEMENTATION AND POTENTIAL AS AN MS THERAPY
7.1. Immunological and molecular changes in MS following supplementation with vitamin D
Interventional studies, including randomised‐controlled trials, have investigated immunological alterations following vitamin D supplementation in people with MS (Table 2). Safety was excellent at all vitamin D dosages, even greater than 10,000 IU daily (Burton et al., 2010; Golan et al., 2013; Smolders et al., 2010); safety is discussed in more detail in Section 7.3. Several studies found a reduced T cell proliferative response following supplementation (Burton et al., 2010; Kimball et al., 2011; Mosayebi et al., 2011). The studies showed reduced Th1 and Th17 cells, increased IL‐10‐producing cells, as well as reduction in effector memory T cells (Smolders et al., 2010; Sotirchos et al., 2016). However, this is not a consistent finding. Other studies did not identify significant T cell subset changes (Mrad et al., 2017; O'Connell et al., 2017). The SOLAR study was a randomised‐controlled trial of vitamin D3 supplementation (6,670 IU daily for 4 weeks and then 14,007 IU daily for 44 weeks) in relapsing–remitting MS patients treated with IFN‐β (Muris et al., 2016; Rolf, Muris, Theunissen, et al., 2018). Fifty‐three participants from this study had peripheral blood mononuclear cells collected at baseline and 48 weeks. Reduced IL‐4+ Th cell proportion and CD25 expression on Treg cells were observed in the placebo group. The investigators hypothesised that vitamin D may have a role in maintenance of immune homeostasis.
TABLE 2.
Vitamin D supplementation studies in multiple sclerosis and clinically isolated syndrome, and immunomodulatory effects
| Study | Study design | Participants | Interventions | Intervention duration | Baseline serum 25(OH)D, mean (nmol·L−1) | Post‐intervention serum 25(OH)D, mean (nmol·L−1) | Findings | ||
|---|---|---|---|---|---|---|---|---|---|
| B cells | T cells | Cytokines | |||||||
| Mahon, Gordon, Cruz, Cosman, & Cantorna, 2003 | Double‐blind RCT | MS (N = 39). DMT status not specified. | D3 1,000 IU daily (N = 17). Placebo (N = 22). All received calcium 800 mg daily. | 6 months |
Vitamin D: 43 Placebo: ~38 |
Vitamin D: 70 Placebo: ~43 |
↑ serum TGF‐β1 in vitamin D group. No significant change in PBMC TNF‐α, IL‐2, IFN‐γ, and IL‐13 mRNA expression. | ||
| Burton et al., 2010; Kimball et al., 2011 | Open‐label RCT | MS (N = 49). DMT: IFN‐β (24), glatiramer (4), none (21). | D3 escalating doses up to 40,000 IU daily for 28 weeks, then 10,000 IU daily for 12 weeks, then down‐titration (N = 25). Control (N = 24). All participants also took calcium 1,200 mg daily. Controls could take up to cholecalciferol 4,000 IU daily. | 12 months |
Vitamin D: 73 Control: 83 |
Vitamin D: 179 Control: 83 |
↓ T cell proliferative responses in vitamin D group. | Cytokine levels below detection sensitivities of assays. | |
| Smolders et al., 2010; Knippenberg et al., 2011; Peelen et al., 2013 | Interventional study | RRMS (N = 14–15). All on IFN‐β. | D3 20,000 IU daily. | 12 weeks | 50 a | 380 a | No significant change in B cell numbers or subsets, and plasma Ig. | ↑ % CD4+ IL‐10+ T cells. ↓ IFN‐γ+:IL4+ CD4+ T cell ratio. No change in CD8+ T cell cytokine subsets. Non‐significant ↑ in Treg function. | Non‐significant trend of decrease in BAFF levels. |
| Mosayebi Ghazavi, Ghasami, Jand, &Kokhaei, 2011 | RCT | RRMS (N = 62). All on IFN‐β‐1a. | D3 300,000 IU monthly (N = 28). Placebo (N = 34). | 6 months |
Vitamin D: ~25 Placebo: ~25 |
Vitamin D group: ~140 Placebo: ~25 |
↓ T cell proliferation response. | ↑ IL‐10 and TGF‐β in culture supernatant. | |
| Golan et al., 2013 | Double‐blind randomised trial | RRMS (N = 45). All on IFN‐β. | D3 4,370 IU (N = 24) or 800 IU (N = 21) daily. | 12 months |
High dose: 48.2 Low dose: 48 |
High dose: 122.6 Low dose: 68 |
↑ serum IL‐17 in low‐dose group. | ||
| Røsjø et al., 2015 | Double‐blind RCT | RRMS (N = 68). DMT: IFN‐β (32), glatiramer (2), natalizumab (1), none (33). | D3 20,000 IU weekly (N = 36). Placebo (N = 32). | 96 weeks |
Vitamin D: 56 Placebo: 57 |
Vitamin D: 123 Placebo: 63 |
No significant differences in change to 11 serum markers of inflammation. | ||
| Åivo, Hänninen, Ilonen, & Soilu‐Hänninen, 2015 | RCT | RRMS (N = 59). All on IFN‐β‐1b. | D3 20,000 IU weekly (N = 30). Placebo (N = 29). | 12 months |
Vitamin D: 54 Placebo: 55 |
Vitamin D: 109 Placebo: 51 |
↑ serum TGF‐β/LAP levels. No significant change of other measured cytokines, including IL‐10 and IFN‐γ. | ||
| Ashtari, Toghianifar, Zarkesh‐Esfahani, & Mansourian, 2015; Toghianifar, Ashtari, Zarkesh‐Esfahani, & Mansourian, 2015 | Double‐blind RCT | RRMS (N = 94). All on IFN‐β. | D3 50,000 IU every 5 days (N = 47). Placebo (N = 47). | 3 months |
Vitamin D: 70.7 a Placebo: 99 a |
Vitamin D: 211 a Placebo: 71.7 a |
Significant positive correlations of vitamin D treatment with log of IL‐10 and IL‐17, but changes in serum IL‐10 and IL‐17 not significant. | ||
| Sotirchos et al., 2016 | Double‐blind randomised trial | RRMS (N = 40). DMT: IFN‐β (12), glatiramer (10), natalizumab (11), fingolimod (4), none (2), other (1). | D3 10,400 IU (N = 19) or 800 IU (N = 21) daily. | 6 months |
High dose: 67.8 Low dose: 69.8 |
High dose: 155 Low dose: 87 |
↓ % IL‐17+ CD4+, CD161+ CD4+, effector memory CD4+, and CD85j+ CD8+ T cells, and ↑ % central memory and naïve CD4+ T cells in high‐dose group. | No change in 51 measured serum cytokine levels. | |
| Muris et al., 2016; Rolf, Muris, Theunissen, et al., 2018 | RCT | RRMS (N = 53). All on IFN‐β‐1a. | D3 6,670 IU daily for 4 weeks, then 14,007 IU daily (N = 30). Placebo (N = 23). | 48 weeks |
Vitamin D: 60 Placebo: 54 |
Vitamin D: 231 Placebo: 60 |
No change in % regulatory B cell. | ↓ % IL4+ CD3+ CD8− T cells in placebo group. ↓ CD25 expression on Treg in placebo group. No change in % Treg. | ↑ IL‐5 and TGF‐β (culture supernatant) in placebo group. |
| Mrad, El Ayoubi, Esmerian, Kazan, & Khoury, 2017 | Interventional study | RRMS (N = 46). All on IFN‐β. | D3 10,000 IU weekly if serum 25(OH)D3 <62.5 nmol·L−1 at baseline (N = 21). | 3 months |
Low vitamin D status group: 39.8 High vitamin D status group: 146 |
Low vitamin D status group: 129 High vitamin D status group: 155 |
No change in T cell subsets. | IFN‐γ level (culture supernatant) significantly higher in low vitamin D group. | |
| Rolf, Smolders, van den Ouweland, Hupperts, & Damoiseaux, 2019 | Double‐blind RCT | RRMS (N = 27). DMT: IFN‐β (15), glatiramer (2), dimethyl fumarate (5), teriflunomide (2), none (3) | D3 4,000 IU daily (N = 12). Placebo (N = 15). | 16 weeks |
Vitamin D: 76 a Placebo: 78 a |
Vitamin D: 135 a Placebo: 81 a |
↓ TNF‐α levels (culture supernatant) following supplementation. | ||
| O'Connell et al., 2017 | Double‐blind RCT | CIS (N = 29). No DMT. Healthy controls (N = 38). | D3 10,000 IU (N = 25) or 5,000 IU (N = 23) daily. Placebo (N = 19). | 24 weeks |
CIS: 53 Control: 52 |
CIS 10,000 IU: 168 CIS 5,000 IU: 129 CIS placebo: 71 Control 10,000 IU: 188 Control 5,000 IU: 144 Control placebo: 54 |
No change in % IFN‐γ+ or IL‐17+ CD4+ T cells. | No change in IL‐10, IL‐17, and IFN‐γ (culture supernatant). | |
Abbreviations: BAFF, B cell activating factor; CIS, clinically isolated syndrome; DMT, disease‐modifying therapy for multiple sclerosis; MS, multiple sclerosis; RCT, randomised‐controlled trial; RRMS, relapsing–remitting multiple sclerosis.
Medians provided in article instead of means.
Some studies demonstrated increases in anti‐inflammatory cytokines IL‐10 and TGF‐β, following vitamin D supplementation, but again, this is not consistent across studies (Åivo et al., 2015; Mahon et al., 2003; Mosayebi et al., 2011; Sotirchos et al., 2016). Reasons for these varied findings may relate to differences in design, including vitamin D dosage, study duration, and sample size. Participants' baseline vitamin D status could influence the ability to elicit change following supplementation. A hypothesis is that people who are vitamin D deficient (<50 nmol·L−1) could be more likely to have immunological changes after supplementation. Use of MS disease‐modifying therapies, which are immunomodulatory in nature, could also alter immunological response to vitamin D. Another possibility is that changes could principally occur in immune system compartments not sampled. Despite the inconsistent findings across studies, overall, they are suggestive of an anti‐inflammatory and regulatory response induced by vitamin D in MS.
Several studies of vitamin D supplementation in people with MS have also found a decrease in EBV antibodies (i.e., anti‐EBNA IgG levels) post‐supplementation (Disanto et al., 2013; Rolf, Muris, Mathias, et al., 2018; Røsjø et al., 2017). These observations are of interest as they link two significant MS environmental risk factors. The mechanisms by which vitamin D mediates this effect and their importance in MS pathogenesis are unclear and remain open to further study.
A few studies have examined the effect of vitamin D supplementation on selected gene expression levels using quantitative PCR. Four studies from the same group focussed on mRNA expression of genes encoding pro‐ and anti‐inflammatory cytokines (Table S5) (Farsani, Behmanesh, & Sahraian, 2015; Naghavi Gargari, Behmanesh, Shirvani Farsani, Pahlevan Kakhki, & Azimi, 2015; Shirvani‐Farsani, Behmanesh, Mohammadi, & Naser Moghadasi, 2015; Shirvani‐Farsani et al., 2017). However, a number of limitations include use of only one housekeeping gene for normalisation and the paucity of details provided in regard to their MS cohort, such as the MS therapies used. Control group characteristics and outcomes were not reported in much detail, and comparisons of responses between MS and non‐MS groups are difficult to evaluate. A study by another group assessed expression of IL‐6, IL‐17A, and IL‐10 but again suffers the same drawbacks (Hashemi et al., 2018). A randomised‐controlled trial of vitamin D supplementation included relapsing–remitting MS patients on IFN‐β and did not identify any significant change in IL2RA mRNA expression in PBMCs after 48 weeks (Rolf, Muris, Theunissen, et al., 2018). As yet, studies assessing how vitamin D supplementation modulates the transcriptome in MS patients using next‐generating sequencing techniques or in immune cell subsets are lacking. Any differences in responses between cell types, and between MS and healthy controls, remain to be determined.
Metabolomics, a method for profiling metabolites in body tissue, was used to assess plasma obtained from MS or healthy participants before and after 5,000 IU daily vitamin D supplementation for 90 days (Bhargava, Fitzgerald, Calabresi, & Mowry, 2017). Following supplementation, there was a reduction in metabolites involved in oxidative stress and lipid metabolism. Importantly, these changes were attenuated in MS patients compared to controls, suggesting that there could be an impaired response to vitamin D among MS patients. If confirmed, another consideration is whether higher supplementation doses in MS patients could overcome this impaired response.
7.2. Vitamin D supplementation and clinical outcomes in MS
In humans, vitamin D levels are usually an excellent surrogate for UVB exposure in the prior 2 months. Due to the relationship between UVB and vitamin D, a direct pharmacological effect of vitamin D can ultimately only be confirmed or refuted by randomised‐controlled trials.
The epidemiologic, immunological, and molecular evidence for a beneficial role of vitamin D in MS is further supported by studies in animal models of neuroinflammatory disease. In the experimental autoimmune encephalomyelitis mouse model, vitamin D supplementation prevents disability and inflammatory demyelination (Cantorna, Hayes, & DeLuca, 1996; Sloka, Zhornitsky, Silva, Metz, & Yong, 2015). Vitamin D also appears to enhance remyelination through promotion of oligodendrocyte progenitor cell differentiation in rat and mouse models of demyelination (Gomez‐Pinedo et al., 2020; Shirazi et al., 2017). As such, a number of human randomised‐controlled trials aimed to evaluate the therapeutic role of vitamin D in MS. Unfortunately, the majority of these studies used a design of randomisation to vitamin D or placebo on a background of use of IFN‐β or other MS therapy in all trial participants, severely limiting their power (Table 3).
TABLE 3.
Vitamin D supplementation randomised‐controlled trials in multiple sclerosis and clinically isolated syndrome, and clinical outcomes
| Study | Participants | Interventions | Intervention duration | Baseline serum 25(OH)D, mean (nmol·L−1) | Post‐intervention serum 25(OH)D, mean (nmol·L−1) | Findings | ||
|---|---|---|---|---|---|---|---|---|
| Relapse | Progression | MRI | ||||||
| Burton et al., 2010 | MS (N = 49): RRMS (45), SPMS (4). DMT: IFN‐β (24), glatiramer (4), none (21). | D3 escalating doses up to 40,000 IU daily for 28 weeks, then 10,000 IU daily for 12 weeks, then down‐titration (N = 25). Control (N = 24). All participants also took calcium 1,200 mg daily. Controls could take up to D3 4,000 IU daily. | 12 months |
Vitamin D: 73 Control: 83 |
Vitamin D: 179 Control: 83 |
Treatment group ARR 0.44 pre to 0.26 post (↓ 41%), NS. | Treatment group EDSS 1.46 pre to 1.15 post, NS. | |
| Stein et al., 2011 | RRMS (N = 23). DMT: IFN‐β (14), glatiramer (5), none (4). | D2 high dose (titrate to serum 25(OH)D 130–175 nM; N = 11) or low dose (1,000 IU daily; N = 12). | 6 months |
High dose: 59 a Low dose: 53.5 a |
High dose: 120 a Low dose: 69 a |
36.5% of high‐dose group had relapse versus 0% of low‐dose group. * | Higher median EDSS post‐treatment in high‐dose group (3 vs. 2). * | No significant differences in changes in no. of gadolinium‐enhancing or T2 lesions. |
| Kampman, Steffensen, Mellgren, & Jørgensen, 2012 | RRMS (N = 68). DMT: IFN‐β (31), glatiramer (2), natalizumab (1), none (34). | D3 20,000 IU weekly (N = 35). Placebo (N = 33). | 96 weeks |
Vitamin D: 56 Placebo: 57 |
Vitamin D: 123 Placebo: 62 |
Treatment group ARR 0.11 pre to 0.14 post, NS. | Treatment group EDSS 2.61 pre to 2.77 post. No significant differences in changes in EDSS, MSFC composites, grip strength, and fatigue. | |
| Soilu‐Hänninen et al., 2012 | RRMS (N = 66). All on IFN‐β‐1b. | D3 20,000 IU weekly (N = 34). Placebo (N = 32). | 12 months |
Vitamin D: 54 Placebo: 56 |
Vitamin D: 110 Placebo: 50 |
Treatment group ARR 0.49 pre to 0.26 post, NS. | Treatment group EDSS 2 pre to 1.8 post. No significant differences in changes in EDSS, timed 10‐foot tandem walk, and timed 25‐foot walk. | ↓ T1‐enhancing lesions in treatment group at 12 months, 0.1 versus 0.7 in placebo group. * Change in T2 burden of disease, 83‐mm3 treatment group versus 287‐mm3 placebo group; NS. |
| Mosayebi et al., 2011 | RRMS (N = 62). All on IFN‐β‐1a. | D3 300,000 IU monthly (N = 28). Placebo (N = 34). | 6 months |
Vitamin D: ~25 Placebo: ~25 |
Vitamin D group: ~140 Placebo: ~25 |
Treatment group EDSS 2.1 pre to 2.31 post, NS. | No significant difference in no. of gadolinium‐enhancing lesions. | |
| Shaygannejad, Janghorbani, Ashtari, & Dehghan, 2012 | RRMS (N = 50). DMT: IFN‐β (43), other (2), none (5). | Calcitriol 0.25 μg for 2 weeks, then 0.5 μg daily (N = 25). Placebo (N = 25). | 12 months | Not reported | Not reported | ↓ ARR in both groups, NS. | EDSS stable in vitamin D group, ↑ in placebo group. * EDSS at 12 months between groups, NS. | |
| Golan et al., 2013 | RRMS (N = 45). All on IFN‐β. | D3 4,370 IU (N = 24) or 800 IU (N = 21) daily. | 12 months |
High dose: 48.2 Low dose: 48 |
High dose: 122.6 Low dose: 68 |
No significant change in ARR. | No significant change in EDSS. | |
| Achiron et al., 2015 | MS (N = 158). 107 (67.7%) on DMT. | Alfacalcidol 1 μg daily (N = 80). Placebo (N = 78). | 6 months | Not reported | Not reported | No. of relapses during study period lower in Treatment (8) than Placebo group (25).* Proportion of participants relapse‐free higher in Treatment (89.5%) than Placebo group (67.1%).* | No significant difference in EDSS. | |
| Camu et al., 2019 | RRMS (N = 129). All on IFN‐β‐1a. | D3 100,000 IU 2‐weekly (N = 63). Placebo (N = 66). | 96 weeks |
Vitamin D: 49.19 Placebo: 48.25 |
Vitamin D: 156.92 Placebo: 57.23 |
ARR not significantly different. Of subjects completing follow‐up: 60.5% ↓ in relapse risk. * | Of completers: Lower progression of EDSS in treatment group (−0.06 vs. 0.32). * | Of completers: 50.6% ↓ in new T1 lesions in treatment group. * ↓ volume of T1 hypointense lesions. * No significant changes to no. of enhancing or T2 lesions. |
| Hupperts et al., 2019 | RRMS (N = 229). All on IFN‐β‐1a. | D3 6,670 IU daily for 4 weeks, then 14,007 IU daily (N = 113). Placebo (N = 116). | 48 weeks |
Vitamin D: 53 a Placebo: 54 a |
Vitamin D: 215 a Placebo: 49 a |
ARR 0.28 versus 0.41 in treatment versus placebo; NS. | No difference in risk of EDSS progression. | 32% ↓ in no. of enhancing or new/enlarging T2 lesions in treatment group. * ↓ mean percentage change from baseline in total T2 lesion volume (3.57% vitamin D vs. 6.07% placebo group). * |
| Dörr et al., 2020 | RRMS or CIS (N = 53). All on IFN‐β‐1b. | D3 20,400 IU (N = 28) or 400 IU (N = 25) every alternate day. | 18 months |
High dose: 47 Low dose: 44.5 |
High dose: 163 Low dose: 55.8 |
No difference in cumulative number of relapses. | No difference in disability progression. | No significant difference in new T2 lesions or lesion volume, no. of new enhancing lesions, or percentage brain volume change. |
| Derakhshandi et al., 2013 | CIS with optic neuritis (N = 30). None on DMT. | D3 50,000 IU weekly (N = 15). Placebo (N = 15). | 12 months |
Vitamin D: 34.3 Placebo: 41.1 |
Not reported. In the vitamin D group, target level was 250 nmol·L−1. Dose adjusted once this was reached. | 68.4% relative risk reduction of relapse/conversion to MS. * | Lower incidence rates of black holes, new enhancing and new T2 lesions in vitamin D group. * | |
| O'Connell et al., 2017 | CIS (N = 29). No DMT. | D3 10,000 IU (N = 12) or 5,000 IU (N = 10) daily. Placebo (N = 7). | 24 weeks | 53 |
10,000 IU: 168 5,000 IU: 129 Placebo: 71 |
Only 1 patient experienced relapse during study (5,000 IU group). | No significant difference in EDSS. | No significant differences in new T2 or enhancing lesions. |
| Etemadifar & Janghorbani, 2015 | Pregnant women with MS (N = 15). | D3 50,000 IU weekly (N = 6). Control (N = 9). | ~24–28 weeks; from 12 to 16 weeks of gestation until delivery. |
Vitamin D: 38.3 Control: 45.8 |
Vitamin D: 84.3 b Control: 36.5 b |
Vitamin D group had 0 relapses while control group had 5 and 4 relapses in pregnancy and post‐partum, respectively. | Control group mean EDSS 1.7 versus vitamin D group 1.1 at 6 months after delivery. * | |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; ARR, annualised relapse rate; CIS, clinically isolated syndrome; DMT, disease‐modifying therapy for multiple sclerosis; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; MSFC, multiple sclerosis functional composite; NS, non‐significant; RCT, randomised‐controlled trial; RRMS, relapsing–remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Medians provided in article instead of means.
Serum vitamin D results reported for 6 months post‐delivery.
P < 0.05, significant effect.
Nonetheless, three such studies have reported beneficial effects on MRI measures of disease activity (Camu et al., 2019; Hupperts et al., 2019; Soilu‐Hänninen et al., 2012). The largest of these included 229 relapsing–remitting MS participants and randomised them to either placebo or vitamin D supplementation (14,007 IU daily after initial 4‐week up‐titration) for at least 48 weeks (Hupperts et al., 2019). The investigators found a significant reduction in inflammatory gadolinium‐enhancing or new T2 lesions on MRI in their treatment arm as well as a smaller mean percentage change in total T2 lesion volume at 48 weeks compared to baseline. Their primary outcome of proportion of patients with no relapses, disability progression, or new MRI lesions was not met. However, three other studies did not identify any amelioration of MRI disease activity, though a limitation of these, as well as other randomised‐controlled trials, is their small sample size and low statistical power (Dörr et al., 2020; Mosayebi et al., 2011; Stein et al., 2011).
In a recently published study, Camu et al. (2019) recruited 129 relapsing–remitting MS patients on IFN‐β‐1a and randomised them to either vitamin D3 100,000 IU or placebo every 2 weeks and monitored them over a 2‐year period. Their primary endpoint of significant change in annualised relapse rate was not met in the intention‐to‐treat population. In those that completed the 96‐week study period, a significant reduction in annualised relapse rate was found in the treatment arm, in addition to reduction in new T1 hypointense lesion formation and disability progression as measured with the Expanded Disability Status Scale (EDSS). Approximately 30% of subjects dropped out of each arm, the most common reason being a switch in MS treatment (Camu et al., 2019).
Two recent meta‐analyses have examined vitamin D supplementation and its effects on clinical outcomes of MS (McLaughlin et al., 2018; Zheng, He, Liu, Zhu, & Jin, 2018). Both concluded that there was no significant evidence of a clinical benefit from vitamin D supplementation. The relatively small sample sizes of available studies limit the power to detect significant differences between treatment arms. The add‐on design to MS therapies results in marked amelioration of disease activity in all subjects and thus greatly reduces the power of these studies. Study duration of 1 year may be insufficient to assess changes in relapse rate and, particularly, confirmed disability progression. An additional consideration is that beneficial clinical effects could be greatest in patients who are vitamin D deficient (<50 nmol·L−1) at baseline. A recent Cochrane Review did not identify any benefit of vitamin D but cautioned that their assessment was based on studies which they graded as providing very low‐quality evidence (Jagannath et al., 2018).
A limited number of studies have assessed whether vitamin D can prevent conversion from CIS to MS. Derakhshandi et al. (2013) randomised 30 patients with optic neuritis to take either placebo or 50,000‐IU vitamin D3 weekly for 12 months. Their primary endpoint was conversion to MS, and their secondary endpoints were changes to measures of MRI lesion load. They reported a 68.4% risk reduction in conversion to MS in their vitamin D treatment group as well as reductions of new MRI lesions. O'Connell et al. (2017) studied the immunological effects of vitamin D supplementation in 29 patients with CIS and 38 healthy controls. They randomised participants to take placebo, 5,000 IU, or 10,000 IU of vitamin D3 daily. The primary endpoint of difference in T cell subset frequencies was not met. There was also no benefit in clinical and radiological disease activity with vitamin D supplementation, though their study was limited by the short duration of 24 weeks.
Despite trends of benefit on clinical or paraclinical measures of MS disease activity in vitamin D randomised‐controlled studies, firm conclusions cannot yet be drawn and further study is warranted. Well‐designed and sufficiently powered trials are ongoing. These include the VIDAMS study (NCT01490502). Another important question is whether vitamin D supplementation can prevent MS. The Australia and New Zealand‐based PREVANZ study (ACTRN12612001160820) and the French D‐LAY‐MS study (NCT01817166) of vitamin D supplementation in CIS will hopefully shed additional light. Both PREVANZ and D‐LAY‐MS are recruiting patients with a recent attack consistent with CIS and randomising them to placebo or vitamin D supplementation at various doses (1,000, 5,000, or 10,000 IU daily in PREVANZ and 100,000 IU fortnightly in D‐LAY‐MS). Primary outcomes are risk of recurrent disease activity in PREVANZ and conversion to MS in D‐LAY‐MS. Most pertinently, these trials use their intervention as monotherapy, with participants not on any immunomodulatory therapies before or during the trial. These therefore avoid the confounding which occurs when vitamin D is given as an add‐on with concurrent MS‐specific therapy and increases the power of these studies to detect a treatment benefit.
7.3. Vitamin D supplementation and safety
Safety signals were excellent from the vitamin D randomised‐controlled trials in MS. Patients with contraindications for high‐dose vitamin D supplementation, such as primary hyperparathyroidism, renal dysfunction, and granulomatous disease, were generally excluded. In the largest of these which supplemented their vitamin D arm with 14,007 IU daily for 44 out of the 48‐week study duration, median serum 25(OH)D level increased to 215 nmol·L−1 at the end of the study (Hupperts et al., 2019). Vitamin D supplementation was well tolerated, and no patients developed hypercalcaemia.
Several observational studies have reported an increased risk of mortality at both low and high serum vitamin D levels to suggest a J‐curve relationship between serum vitamin D level and mortality, although this is not a consistent finding (Melamed, Michos, Post, & Astor, 2008; Michaëlsson et al., 2010). However, a harmful effect on mortality has not been borne out by vitamin D supplementation studies. A recent large randomised‐controlled trial of vitamin D supplementation (2,000 IU daily) with 25,871 participants and median intervention period of 5.3 years did not find any significant association with all‐cause mortality but did suggest a protective effect against cancer mortality (Manson et al., 2019). There were no significant safety concerns detected in regard to supplementation. A reduced risk of cancer mortality was also echoed by two meta‐analyses (Keum, Lee, Greenwood, Manson, & Giovannucci, 2019; Zhang et al., 2019). Based on the available evidence, vitamin D supplementation is overall safe in the absence of any disturbed calcium–vitamin D metabolism. Given that vitamin D toxicity can occur at extremely high doses (often >50,000 IU·day−1) over prolonged duration, it is prudent to aim for vitamin D repletion (between 75 and 120 nmol·L−1) among MS patients, pending further evidence of benefits at higher levels.
8. CONCLUSION
Since its discovery and use as treatment for rickets, vitamin D is now recognised to have extra‐skeletal effects, including immunomodulatory effects. Overall, vitamin D induces a tolerogenic immune phenotype, with promotion of Treg phenotype differentiation and increased production of the anti‐inflammatory cytokine IL‐10. The strongest evidence for this comes from in vitro studies but is also supported by in vivo experiments. Vitamin D deficiency is associated with increased risk for a number of autoimmune diseases including MS, with these observations being compatible with the immune effects reported for vitamin D. The involvement of autoimmune disease risk genes in vitamin D metabolism and enrichment of VDR binding in proximity to these genes further suggest a role for vitamin D in disease pathogenesis.
However, the specific mechanism by which vitamin D alters MS and other autoimmune disease risk remains to be identified. Vitamin D's immunoregulatory capacity as a therapeutic to potentially prevent, or ameliorate, MS is not yet proven. To better understand the in vivo actions of vitamin D, examination of immune cell subsets rather than whole blood particularly with transcriptomic analysis may yield novel cell‐specific insights. Whether patients with MS have significant differences in vitamin D processing and response when compared to healthy individuals is still unanswered. The use of established and well‐accepted bioinformatic pipelines will allow for improved comparability of results between transcriptomic studies. In regard to its therapeutic potential, pre‐existing supplementation studies for MS have failed to demonstrate a definitive benefit in disease activity measures. These studies were underpowered, though the larger studies suggest a beneficial effect on MRI measures of MS disease activity. Two randomised, placebo‐controlled monotherapy trials are studying prevention of MS (recurrent disease activity) in patients after a first attack. Results are eagerly awaited.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019a, b; Alexander, Kelly et al., 2019).
CONFLICTS OF INTEREST
Monash University receives compensation for H.B.'s consulting activities including Steering Committees, Presentations, and Advisory Boards from Merck, Roche, Biogen, and Novartis. H.B. receives funding for steering committee activities from IQVIA and Oxford PharmaGenesis and grant support from MS Research Australia and the NHMRC (Australia).
Supporting information
Table S1 Vitamin D and its in vitro immunomodulatory effects on immune cells
Table S2 Vitamin D and its in vitro molecular effects on immune cells
Table S3 Vitamin D supplementation studies in healthy adults and effects on the transcriptome
Table S4 Molecular evidence in support of a role of vitamin D in multiple sclerosis pathogenesis
Table S5 Vitamin D supplementation studies in multiple sclerosis and effects on gene expression
ACKNOWLEDGEMENTS
This work was supported by a Multiple Sclerosis Research Australia Postgraduate Scholarship and an Australian Government Research Training Program (RTP) Scholarship.
Yeh WZ, Gresle M, Jokubaitis V, Stankovich J, van der Walt A, Butzkueven H. Immunoregulatory effects and therapeutic potential of vitamin D in multiple sclerosis. Br J Pharmacol. 2020;177:4113–4133. 10.1111/bph.15201
REFERENCES
- Achiron, A. , Givon, U. , Magalashvili, D. , Dolev, M. , Liraz Zaltzman, S. , Kalron, A. , … Barak, Y. (2015). Effect of Alfacalcidol on multiple sclerosis‐related fatigue: A randomized, double‐blind placebo‐controlled study. Multiple Sclerosis Journal, 21, 767–775. 10.1177/1352458514554053 [DOI] [PubMed] [Google Scholar]
- Åivo, J. , Hänninen, A. , Ilonen, J. , & Soilu‐Hänninen, M. (2015). Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF‐beta. Journal of Neuroimmunology, 280, 12–15. 10.1016/j.jneuroim.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, A. C. , Kelly, S. , Basdeo, S. A. , Kinsella, K. , Mulready, K. J. , Mills, K. H. , … Fletcher, J. M. (2012). A pilot study of the immunological effects of high‐dose vitamin D in healthy volunteers. Multiple Sclerosis Journal, 18, 1797–1800. 10.1177/1352458512442992 [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan, A. N. , Khalili, H. , Higuchi, L. M. , Bao, Y. , Korzenik, J. R. , Giovannucci, E. L. , … Chan, A. T. (2012). Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology, 142, 482–489. 10.1053/j.gastro.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio, A. , Munger, K. L. , White, R. , Köchert, K. , Simon, K. C. , Polman, C. H. , … Pohl, C. (2014). Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurology, 71, 306–314. 10.1001/jamaneurol.2013.5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari, F. , Toghianifar, N. , Zarkesh‐Esfahani, S. H. , & Mansourian, M. (2015). Short‐term effect of high‐dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: A randomized, double‐blind, placebo‐controlled clinical trial. Neuroimmunomodulation, 22, 400–404. 10.1159/000439278 [DOI] [PubMed] [Google Scholar]
- Bäärnhielm, M. , Olsson, T. , & Alfredsson, L. (2014). Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Multiple Sclerosis Journal, 20, 726–732. 10.1177/1352458513509508 [DOI] [PubMed] [Google Scholar]
- Baeke, F. , Korf, H. , Overbergh, L. , van Etten, E. , Verstuyf, A. , Gysemans, C. , & Mathieu, C. (2010). Human T lymphocytes are direct targets of 1,25‐dihydroxyvitamin D3 in the immune system. The Journal of Steroid Biochemistry and Molecular Biology, 121, 221–227. 10.1016/j.jsbmb.2010.03.037 [DOI] [PubMed] [Google Scholar]
- Bak, N. F. , Bendix, M. , Hald, S. , Reinert, L. , Magnusson, M. K. , & Agnholt, J. (2018). High‐dose vitamin D3 supplementation decreases the number of colonic CD103+ dendritic cells in healthy subjects. European Journal of Nutrition, 57, 2607–2619. 10.1007/s00394-017-1531-y [DOI] [PubMed] [Google Scholar]
- Barkhof, F. , Calabresi, P. A. , Miller, D. H. , & Reingold, S. C. (2009). Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nature Reviews Neurology (London), 5, 256–266. [DOI] [PubMed] [Google Scholar]
- Berge, T. , Leikfoss, I. S. , Brorson, I. S. , Bos, S. D. , Page, C. M. , Gustavsen, M. W. , … Spurkland, A. (2016). The multiple sclerosis susceptibility genes TAGAP and IL2RA are regulated by vitamin D in CD4+ T cells. Genes and Immunity, 17, 118–127. 10.1038/gene.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga‐Taylor, A. J. , Plant, K. , Dahl, A. , Lau, E. , Hill, M. , Sims, D. , … Knight, J. C. (2018). Genomic response to vitamin D supplementation in the setting of a randomized, placebo‐controlled trial. eBioMedicine, 31, 133–142. 10.1016/j.ebiom.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava, P. , Fitzgerald, K. C. , Calabresi, P. A. , & Mowry, E. M. (2017). Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight, 2(19), e95302 10.1172/jci.insight.95302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava, P. , Steele, S. U. , Waubant, E. , Revirajan, N. R. , Marcus, J. , Dembele, M. , … Mowry, E. M. (2016). Multiple sclerosis patients have a diminished serologic response to vitamin D supplementation compared to healthy controls. Multiple Sclerosis (Houndmills, Basingstoke, England), 22, 753–760. 10.1177/1352458515600248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, G. , Prietl, B. , Mader, J. K. , Höller, E. , Wolf, M. , Pilz, S. , … Pieber, T. R. (2011). The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: A randomized controlled trial. Diabetes/Metabolism Research and Reviews, 27, 942–945. 10.1002/dmrr.1276 [DOI] [PubMed] [Google Scholar]
- Booth, D. R. , Ding, N. , Parnell, G. P. , Shahijanian, F. , Coulter, S. , Schibeci, S. D. , … Liddle, C. (2016). Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude‐dependent autoimmune diseases. Genes and Immunity, 17, 213–219. 10.1038/gene.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, J. M. , Kimball, S. , Vieth, R. , Bar‐Or, A. , Dosch, H.‐M. , Cheung, R. , … O'Connor, P. (2010). A phase I/II dose‐escalation trial of vitamin D3 and calcium in multiple sclerosis(e–Pub ahead of print) (LOE Classification). Neurology, 74, 1852–1859. 10.1212/WNL.0b013e3181e1cec2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camu, W. , Lehert, P. , Pierrot‐Deseilligny, C. , Hautecoeur, P. , Besserve, A. , Jean Deleglise, A.‐S. , … Souberbielle, J. C. (2019). Cholecalciferol in relapsing‐remitting MS: A randomized clinical trial (CHOLINE). Neurology‐Neuroimmunology Neuroinflammation, 6, e597 10.1212/NXI.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna, M. T. , Hayes, C. E. , & DeLuca, H. F. (1996). 1,25‐Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America, 93, 7861–7864. 10.1073/pnas.93.15.7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Sims, G. P. , Chen, X. X. , Gu, Y. Y. , Chen, S. , & Lipsky, P. E. (2007). Modulatory effects of 1,25‐dihydroxyvitamin D3 on human B cell differentiation. Journal of Immunology, 179, 1634–1647. [DOI] [PubMed] [Google Scholar]
- Cohan, S. , Lucassen, E. , Romba, M. , & Linch, S. (2019). Daclizumab: Mechanisms of action, therapeutic efficacy, adverse events and its uncovering the potential role of innate immune system recruitment as a treatment strategy for relapsing multiple sclerosis. Biomedicine, 7, 1–19. 10.3390/biomedicines7010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshandi, H. , Etemadifar, M. , Feizi, A. , Abtahi, S.‐H. , Minagar, A. , Abtahi, M.‐A. , … Tabrizi, N. (2013). Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: A double blind, randomized, placebo‐controlled pilot clinical trial. Acta Neurologica Belgica, 113, 257–263. [DOI] [PubMed] [Google Scholar]
- Disanto, G. , Handel, A. E. , Damoiseaux, J. , Hupperts, R. , Giovannoni, G. , Smolders, J. , & Ramagopalan, S. V. (2013). Vitamin D supplementation and antibodies against the Epstein‐Barr virus in multiple sclerosis patients. Multiple Sclerosis Journal, 19, 1679–1680. 10.1177/1352458513494494 [DOI] [PubMed] [Google Scholar]
- Dörr, J. , Bäcker‐Koduah, P. , Wernecke, K.‐D. , Becker, E. , Hoffmann, F. , Faiss, J. , … Piper, S. K. (2020). High‐dose vitamin D supplementation in multiple sclerosis—Results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Multiple Sclerosis Journal–Experimental, Translational and Clinical, 6, 2055217320903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdenko, G. , Heine, G. , & Worm, M. (2014). Oral vitamin D increases the frequencies of CD38+ human B cells and ameliorates IL‐17‐producing T cells. Experimental Dermatology, 23, 107–112. [DOI] [PubMed] [Google Scholar]
- Etemadifar, M. , & Janghorbani, M. (2015). Efficacy of high‐dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: Preliminary findings of a randomized‐controlled trial. Iranian Journal of Neurology, 14, 67–73. [PMC free article] [PubMed] [Google Scholar]
- Farsani, Z. S. , Behmanesh, M. , & Sahraian, M. A. (2015). Interleukin‐10 but not transforming growth factor‐β1 gene expression is up‐regulated by vitamin D treatment in multiple sclerosis patients. Journal of the Neurological Sciences, 350, 18–23. 10.1016/j.jns.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Fitzgerald, K. C. , Munger, K. L. , Köchert, K. , Arnason, B. G. W. , Comi, G. , Cook, S. , … Ascherio, A. (2015). Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta‐1b. JAMA Neurology, 72, 1458–1465. 10.1001/jamaneurol.2015.2742 [DOI] [PubMed] [Google Scholar]
- Gianfrancesco, M. A. , Stridh, P. , Rhead, B. , Shao, X. , Xu, E. , Graves, J. S. , … For the Network of Pediatric Multiple Sclerosis Centers . (2017). Evidence for a causal relationship between low vitamin D, high BMI, and pediatric‐onset MS. Neurology, 88, 1623–1629. 10.1212/WNL.0000000000003849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan, D. , Halhal, B. , Glass‐Marmor, L. , Staun‐Ram, E. , Rozenberg, O. , Lavi, I. , … Miller, A. (2013). Vitamin D supplementation for patients with multiple sclerosis treated with interferon‐beta: A randomized controlled trial assessing the effect on flu‐like symptoms and immunomodulatory properties. BMC Neurology, 13(60), 1–10. 10.1186/1471-2377-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pinedo, U. , Cuevas, J. A. , Benito‐Martín, M. S. , Moreno‐Jiménez, L. , Esteban‐Garcia, N. , Torre‐Fuentes, L. , … Matías‐Guiu, J. (2020). Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain and Behavior: A Cognitive Neuroscience Perspective, 10, e01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham, E. D. , Garland, C. F. , Burgi, A. A. , Mohr, S. B. , Zeng, K. , Hofflich, H. , … Ricordi, C. (2012). Lower prediagnostic serum 25‐hydroxyvitamin D concentration is associated with higher risk of insulin‐requiring diabetes: A nested case–control study. Diabetologia, 55, 3224–3227. 10.1007/s00125-012-2709-8 [DOI] [PubMed] [Google Scholar]
- Graves, J. S. , Barcellos, L. F. , Krupp, L. , Belman, A. , Shao, X. , Quach, H. , … Benson, L. (2020). Vitamin D genes influence MS relapses in children. Multiple Sclerosis Journal, 13524585198458426(8), 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, R. W. , Caldas, A. E. , Wilz, D. R. , Lemann, J. , Smith, G. A. , & Deluca, H. F. (1978). Metabolism and excretion of 3H‐1,2 5‐(OH)2‐vitamin D3 in healthy adults. The Journal of Clinical Endocrinology and Metabolism, 46, 756–765. 10.1210/jcem-46-5-756 [DOI] [PubMed] [Google Scholar]
- Gresle, M. M. , Jordan, M. A. , Stankovich, J. , Spelman, T. , Johnson, L. J. , Laverick, L. , … Butzkueven, H. (2020). Multiple sclerosis risk variants regulate gene expression in innate and adaptive immune cells. Life Science Alliance, 3, e202000650 10.26508/lsa.202000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad, J. G. , Matsuoka, L. Y. , Hollis, B. W. , Hu, Y. Z. , & Wortsman, J. (1993). Human plasma transport of vitamin D after its endogenous synthesis. The Journal of Clinical Investigation, 91, 2552–2555. 10.1172/JCI116492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi, R. , Morshedi, M. , Asghari Jafarabadi, M. , Altafi, D. , Saeed Hosseini‐Asl, S. , & Rafie‐Arefhosseini, S. (2018). Anti‐inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurology Genetics, 4, e278 10.1212/NXG.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedström, A. , Olsson, T. , & Alfredsson, L. (2016). Smoking is a major preventable risk factor for multiple sclerosis. Multiple Sclerosis Journal, 22, 1021–1026. 10.1177/1352458515609794 [DOI] [PubMed] [Google Scholar]
- Heikkinen, S. , Väisänen, S. , Pehkonen, P. , Seuter, S. , Benes, V. , & Carlberg, C. (2011). Nuclear hormone 1α,25‐dihydroxyvitamin D3 elicits a genome‐wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Research, 39, 9181–9193. 10.1093/nar/gkr654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine, G. , Niesner, U. , Chang, H.‐D. , Steinmeyer, A. , Zügel, U. , Zuberbier, T. , … Worm, M. (2008). 1,25‐dihydroxyvitamin D3 promotes IL‐10 production in human B cells. European Journal of Immunology, 38, 2210–2218. 10.1002/eji.200838216 [DOI] [PubMed] [Google Scholar]
- Hewison, M. , Freeman, L. , Hughes, S. V. , Evans, K. N. , Bland, R. , Eliopoulos, A. G. , … Chakraverty, R. (2003). Differential regulation of vitamin D receptor and its ligand in human monocyte‐derived dendritic cells. Journal of Immunology, 170, 5382–5390. 10.4049/jimmunol.170.11.5382 [DOI] [PubMed] [Google Scholar]
- Holick, M. F. (1995). Environmental factors that influence the cutaneous production of vitamin D. The American Journal of Clinical Nutrition, 61, 638S–645S. 10.1093/ajcn/61.3.638S [DOI] [PubMed] [Google Scholar]
- Holick, M. F. (2007). Vitamin D deficiency. The New England Journal of Medicine, 357, 266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Hossein‐nezhad, A. , Spira, A. , & Holick, M. F. (2013). Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double‐blind clinical trial. PLoS ONE, 8, e58725 10.1371/journal.pone.0058725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupperts, R. , Smolders, J. , Vieth, R. , Holmøy, T. , Marhardt, K. , Schluep, M. , … SOLAR Study Group . (2019). Randomized trial of daily high‐dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β‐1a. Neurology, 93, e1906–e1916. 10.1212/WNL.0000000000008445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium . (2019). Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science, 365, eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, B. M. , Noyce, A. J. , Giovannoni, G. , & Dobson, R. (2020). BMI and low vitamin D are causal factors for multiple sclerosis: A Mendelian randomization study. Neurology‐Neuroimmunology Neuroinflammation, 7, e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath, V. A. , Filippini, G. , Di Pietrantonj, C. , Asokan, G. V. , Robak, E. W. , Whamond, L. , & Robinson, S. A. (2018). Vitamin D for the management of multiple sclerosis. Cochrane Database of Systematic Reviews, 9, CD008422 10.1002/14651858.CD008422.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery, L. E. , Burke, F. , Mura, M. , Zheng, Y. , Qureshi, O. S. , Hewison, M. , … Sansom, D. M. (2009). 1,25‐dihydroxyvitamin D3 and interleukin‐2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA‐4 and FoxP3. Journal of Immunology (Baltimore, Md.: 1950), 183, 5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. S. , Assar, S. , Harnpanich, D. , Bouillon, R. , Lambrechts, D. , Prentice, A. , & Schoenmakers, I. (2014). 25(OH)D2 half‐life is shorter than 25(OH)D3 half‐life and is influenced by DBP concentration and genotype. The Journal of Clinical Endocrinology and Metabolism, 99, 3373–3381. 10.1210/jc.2014-1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman, M. T. , Steffensen, L. H. , Mellgren, S. I. , & Jørgensen, L. (2012). Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double‐blind randomised controlled trial. Multiple Sclerosis Journal, 18, 1144–1151. 10.1177/1352458511434607 [DOI] [PubMed] [Google Scholar]
- Keum, N. , Lee, D. H. , Greenwood, D. C. , Manson, J. E. , & Giovannucci, E. (2019). Vitamin D supplementation and total cancer incidence and mortality: A meta‐analysis of randomized controlled trials. Annals of Oncology, 30, 733–743. 10.1093/annonc/mdz059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, S. , Vieth, R. , Dosch, H.‐M. , Bar‐Or, A. , Cheung, R. , Gagne, D. , … Burton, J. M. (2011). Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. The Journal of Clinical Endocrinology and Metabolism, 96, 2826–2834. 10.1210/jc.2011-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippenberg, S. , Smolders, J. , Thewissen, M. , Peelen, E. , Tervaert, J. W. C. , Hupperts, R. , & Damoiseaux, J. (2011). Effect of vitamin D3 supplementation on peripheral B cell differentiation and isotype switching in patients with multiple sclerosis. Multiple Sclerosis Journal, 17, 1418–1423. 10.1177/1352458511412655 [DOI] [PubMed] [Google Scholar]
- Koch‐Henriksen, N. , & Sørensen, P. S. (2010). The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurology, 9, 520–532. 10.1016/S1474-4422(10)70064-8 [DOI] [PubMed] [Google Scholar]
- Konijeti, G. G. , Arora, P. , Boylan, M. R. , Song, Y. , Huang, S. , Harrell, F. , … Chan, A. T. (2016). Vitamin D supplementation modulates T cell–mediated immunity in humans: Results from a randomized control trial. The Journal of Clinical Endocrinology and Metabolism, 101, 533–538. 10.1210/jc.2015-3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz, M. , Andreesen, R. , Krause, S. W. , Szabo, A. , Ritz, E. , & Reichel, H. (1993). 1,25‐dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood, 82, 1300–1307. [PubMed] [Google Scholar]
- Landel, V. , Stephan, D. , Cui, X. , Eyles, D. , & Feron, F. (2018). Differential expression of vitamin D‐associated enzymes and receptors in brain cell subtypes. The Journal of Steroid Biochemistry and Molecular Biology, 177, 129–134. 10.1016/j.jsbmb.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Langer‐Gould, A. , Brara, S. M. , Beaber, B. E. , & Koebnick, C. (2013). Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology, 80, 548–552. 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, L. I. , Munger, K. L. , O'Reilly, E. J. , Falk, K. I. , & Ascherio, A. (2010). Primary infection with the Epstein‐Barr virus and risk of multiple sclerosis. Annals of Neurology, 67, 824–830. 10.1002/ana.21978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. T. , Stenger, S. , Tang, D. H. , & Modlin, R. L. (2007). Cutting edge: Vitamin D‐mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. Journal of Immunology, 179, 2060–2063. [DOI] [PubMed] [Google Scholar]
- Lucas, R. M. , Ponsonby, A.‐L. , Dear, K. , Valery, P. C. , Pender, M. P. , Taylor, B. V. , … McMichael, A. J. (2011). Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology, 76, 540–548. 10.1212/WNL.0b013e31820af93d [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C. , Brück, W. , Parisi, J. , Scheithauer, B. , Rodriguez, M. , & Lassmann, H. (2000). Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Annals of Neurology, 47, 707–717. [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C. F. , Popescu, B. F. G. , Bunyan, R. F. , Moll, N. M. , Roemer, S. F. , Lassmann, H. , … Ransohoff, R. M. (2011). Inflammatory cortical demyelination in early multiple sclerosis. The New England Journal of Medicine, 365, 2188–2197. 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysandropoulos, A. P. , Jaquiéry, E. , Jilek, S. , Pantaleo, G. , Schluep, M. , & Du Pasquier, R. A. (2011). Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. Journal of Neuroimmunology, 233, 240–244. [DOI] [PubMed] [Google Scholar]
- Mahon, B. D. , Gordon, S. A. , Cruz, J. , Cosman, F. , & Cantorna, M. T. (2003). Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. Journal of Neuroimmunology, 134, 128–132. 10.1016/S0165-5728(02)00396-X [DOI] [PubMed] [Google Scholar]
- Makin, G. , Lohnes, D. , Byford, V. , Ray, R. , & Jones, G. (1989). Target cell metabolism of 1,25‐dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24‐oxidation. The Biochemical Journal, 262, 173–180. 10.1042/bj2620173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson, J. E. , Cook, N. R. , Lee, I.‐M. , Christen, W. , Bassuk, S. S. , Mora, S. , … Buring, J. E. (2019). Vitamin D supplements and prevention of cancer and cardiovascular disease. The New England Journal of Medicine, 380, 33–44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli, V. , Dalla Costa, G. , Colombo, B. , Dalla Libera, D. , Rubinacci, A. , Filippi, M. , … Comi, G. (2014). Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Multiple Sclerosis Journal, 20, 147–155. 10.1177/1352458513494959 [DOI] [PubMed] [Google Scholar]
- McLaughlin, L. , Clarke, L. , Khalilidehkordi, E. , Butzkueven, H. , Taylor, B. , & Broadley, S. A. (2018). Vitamin D for the treatment of multiple sclerosis: A meta‐analysis. Journal of Neurology, 265, 2893–2905. [DOI] [PubMed] [Google Scholar]
- van der Mei, I. , Lucas, R. , Taylor, B. , Valery, P. , Dwyer, T. , Kilpatrick, T. , … Ponsonby, A. L. (2016). Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Multiple Sclerosis Journal, 22, 461–469. 10.1177/1352458515594040 [DOI] [PubMed] [Google Scholar]
- Melamed, M. L. , Michos, E. D. , Post, W. , & Astor, B. (2008). 25‐Hydroxyvitamin D levels and the risk of mortality in the general population. Archives of Internal Medicine, 168, 1629–1637. 10.1001/archinte.168.15.1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaëlsson, K. , Baron, J. A. , Snellman, G. , Gedeborg, R. , Byberg, L. , Sundström, J. , … Melhus, H. (2010). Plasma vitamin D and mortality in older men: A community‐based prospective cohort study. The American Journal of Clinical Nutrition, 92, 841–848. 10.3945/ajcn.2010.29749 [DOI] [PubMed] [Google Scholar]
- Miller, W. L. , & Portale, A. A. (2000). Vitamin D 1α‐hydroxylase. Trends in Endocrinology and Metabolism, 11, 315–319. 10.1016/S1043-2760(00)00287-3 [DOI] [PubMed] [Google Scholar]
- Mirzaei, F. , Michels, K. B. , Munger, K. , O'Reilly, E. , Chitnis, T. , Forman, M. R. , … Ascherio, A. (2011). Gestational vitamin D and the risk of multiple sclerosis in the offspring. Annals of Neurology, 70, 30–40. 10.1002/ana.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry, L. E. , Ross, S. , Ahmad, O. S. , Forgetta, V. , Smith, G. D. , Leong, A. , … Richards, J. B. (2015). Vitamin D and risk of multiple sclerosis: A Mendelian randomization study. PLoS Medicine, 12, e1001866 10.1371/journal.pmed.1001866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosayebi, G. , Ghazavi, A. , Ghasami, K. , Jand, Y. , & Kokhaei, P. (2011). Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunological Investigations, 40, 627–639. 10.3109/08820139.2011.573041 [DOI] [PubMed] [Google Scholar]
- Mowry, E. M. , Krupp, L. B. , Milazzo, M. , Chabas, D. , Strober, J. B. , Belman, A. L. , … Waubant, E. (2010). Vitamin D status is associated with relapse rate in pediatric‐onset multiple sclerosis. Annals of Neurology, 67, 618–624. 10.1002/ana.21972 [DOI] [PubMed] [Google Scholar]
- Mowry, E. M. , Waubant, E. , McCulloch, C. E. , Okuda, D. T. , Evangelista, A. A. , Lincoln, R. R. , … Pelletier, D. (2012). Vitamin D status predicts new brain MRI activity in multiple sclerosis. Annals of Neurology, 72, 234–240. 10.1002/ana.23591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrad, M. F. , El Ayoubi, N. K. , Esmerian, M. O. , Kazan, J. M. , & Khoury, S. J. (2017). Effect of vitamin D replacement on immunological biomarkers in patients with multiple sclerosis. Clinical Immunology, 181, 9–15. 10.1016/j.clim.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Munger, K. L. , Åivo, J. , Hongell, K. , Soilu‐Hänninen, M. , Surcel, H.‐M. , & Ascherio, A. (2016). Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurology, 73, 515–519. 10.1001/jamaneurol.2015.4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, K. L. , Hongell, K. , Åivo, J. , Soilu‐Hänninen, M. , Surcel, H.‐M. , & Ascherio, A. (2017). 25‐Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology, 89, 1578–1583. 10.1212/WNL.0000000000004489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, K. L. , Levin, L. I. , Hollis, B. W. , Howard, N. S. , & Ascherio, A. (2006). Serum 25‐hydroxyvitamin D levels and risk of multiple sclerosis. JAMA, 296, 2832–2838. 10.1001/jama.296.23.2832 [DOI] [PubMed] [Google Scholar]
- Munger, K. L. , Zhang, S. M. , O'Reilly, E. , Hernán, M. A. , Olek, M. J. , Willett, W. C. , & Ascherio, A. (2004). Vitamin D intake and incidence of multiple sclerosis. Neurology, 62, 60–65. 10.1212/01.WNL.0000101723.79681.38 [DOI] [PubMed] [Google Scholar]
- Muris, A.‐H. , Smolders, J. , Rolf, L. , Thewissen, M. , Hupperts, R. , & Damoiseaux, J. (2016). Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. Journal of Neuroimmunology, 300, 47–56. [DOI] [PubMed] [Google Scholar]
- Naghavi Gargari, B. , Behmanesh, M. , Shirvani Farsani, Z. , Pahlevan Kakhki, M. , & Azimi, A. R. (2015). Vitamin D supplementation up‐regulates IL‐6 and IL‐17A gene expression in multiple sclerosis patients. International Immunopharmacology, 28, 414–419. 10.1016/j.intimp.2015.06.033 [DOI] [PubMed] [Google Scholar]
- Neme, A. , Seuter, S. , & Carlberg, C. (2017). Selective regulation of biological processes by vitamin D based on the spatio‐temporal cistrome of its receptor. Biochimica et Biophysica Acta (BBA)‐Gene Regulatory Mechanisms, 1860, 952–961. 10.1016/j.bbagrm.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Nielsen, N. M. , Munger, K. L. , Koch‐Henriksen, N. , Hougaard, D. M. , Magyari, M. , Jørgensen, K. T. , … Ascherio, A. (2017). Neonatal vitamin D status and risk of multiple sclerosis. Neurology, 88, 44–51. 10.1212/WNL.0000000000003454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen, V. , Neme, A. , Ryynänen, J. , Heikkinen, S. , Seuter, S. , & Carlberg, C. (2015). The transcriptional regulator BCL6 participates in the secondary gene regulatory response to vitamin D. Biochimica et Biophysica Acta (BBA)‐Gene Regulatory Mechanisms, 1849, 300–308. [DOI] [PubMed] [Google Scholar]
- O'Connell, K. , Sulaimani, J. , Basdeo, S. A. , Kinsella, K. , Jordan, S. , Kenny, O. , … Mulready, K. (2017). Effects of vitamin D3 in clinically isolated syndrome and healthy control participants: A double‐blind randomised controlled trial. Multiple Sclerosis Journal–Experimental, Translational and Clinical, 3, 2055217317727296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen, E. , Thewissen, M. , Knippenberg, S. , Smolders, J. , Muris, A.‐H. , Menheere, P. , … Damoiseaux, J. (2013). Fraction of IL‐10+ and IL‐17+ CD8 T cells is increased in MS patients in remission and during a relapse, but is not influenced by immune modulators. Journal of Neuroimmunology, 258, 77–84. 10.1016/j.jneuroim.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Penna, G. , & Adorini, L. (2000). 1α,25‐Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. Journal of Immunology, 164, 2405–2411. 10.4049/jimmunol.164.5.2405 [DOI] [PubMed] [Google Scholar]
- Piemonti, L. , Monti, P. , Sironi, M. , Fraticelli, P. , Leone, B. E. , Dal Cin, E. , … Di Carlo, V. (2000). Vitamin D3 affects differentiation, maturation, and function of human monocyte‐derived dendritic cells. Journal of Immunology, 164, 4443–4451. 10.4049/jimmunol.164.9.4443 [DOI] [PubMed] [Google Scholar]
- Prietl, B. , Pilz, S. , Wolf, M. , Tomaschitz, A. , Obermayer‐Pietsch, B. , Graninger, W. , & Pieber, T. R. (2010). Vitamin D supplementation and regulatory T cells in apparently healthy subjects: Vitamin D treatment for autoimmune diseases? The Israel Medical Association Journal: IMAJ, 12, 136–139. [PubMed] [Google Scholar]
- Prietl, B. , Treiber, G. , Mader, J. K. , Hoeller, E. , Wolf, M. , Pilz, S. , … Pieber, T. R. (2014). High‐dose cholecalciferol supplementation significantly increases peripheral CD4+ Tregs in healthy adults without negatively affecting the frequency of other immune cells. European Journal of Nutrition, 53, 751–759. 10.1007/s00394-013-0579-6 [DOI] [PubMed] [Google Scholar]
- Provvedini, D. M. , Tsoukas, C. D. , Deftos, L. J. , & Manolagas, S. C. (1983). 1,25‐dihydroxyvitamin D3 receptors in human leukocytes. Science, 221, 1181–1183. 10.1126/science.6310748 [DOI] [PubMed] [Google Scholar]
- Ramagopalan, S. V. , Heger, A. , Berlanga, A. J. , Maugeri, N. J. , Lincoln, M. R. , Burrell, A. , … Knight, J. C. (2010). A ChIP‐seq defined genome‐wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Research, 20, 1352–1360. 10.1101/gr.107920.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan, S. V. , Maugeri, N. J. , Handunnetthi, L. , Lincoln, M. R. , Orton, S.‐M. , Dyment, D. A. , … Knight, J. C. (2009). Expression of the multiple sclerosis‐associated MHC class II allele HLA‐DRB1*1501 is regulated by vitamin D. PLoS Genetics, 5, e1000369 10.1371/journal.pgen.1000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhead, B. , Bäärnhielm, M. , Gianfrancesco, M. , Mok, A. , Shao, X. , Quach, H. , … Barcellos, L. F. (2016). Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurology Genetics, 2, e97 10.1212/NXG.0000000000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons, K. A. , McElduff, P. , Boz, C. , Trojano, M. , Izquierdo, G. , Duquette, P. , … Lechner‐Scott, J. (2015). Male sex is independently associated with faster disability accumulation in relapse‐onset MS but not in primary progressive MS. PLoS ONE, 10, e0122686 10.1371/journal.pone.0122686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf, L. , Muris, A.‐H. , Mathias, A. , Du Pasquier, R. , Koneczny, I. , Disanto, G. , … Hupperts, R. (2018). Exploring the effect of vitamin D3 supplementation on the anti‐EBV antibody response in relapsing‐remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England), 24, 1280–1287. 10.1177/1352458517722646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf, L. , Muris, A.‐H. , Theunissen, R. , Hupperts, R. , Damoiseaux, J. , & Smolders, J. (2018). Vitamin D3 supplementation and the IL‐2/IL‐2R pathway in multiple sclerosis: Attenuation of progressive disturbances? Journal of Neuroimmunology, 314, 50–57. [DOI] [PubMed] [Google Scholar]
- Rolf, L. , Smolders, J. , van den Ouweland, J. , Hupperts, R. , & Damoiseaux, J. (2019). Correlation of different cellular assays to analyze T cell‐related cytokine profiles in vitamin D3‐supplemented patients with multiple sclerosis. Molecular Immunology, 105, 198–204. 10.1016/j.molimm.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Røsjø, E. , Lossius, A. , Abdelmagid, N. , Lindstrøm, J. C. , Kampman, M. T. , Jørgensen, L. , … Holmøy, T. (2017). Effect of high‐dose vitamin D3 supplementation on antibody responses against Epstein–Barr virus in relapsing‐remitting multiple sclerosis. Multiple Sclerosis Journal, 23, 395–402. 10.1177/1352458516654310 [DOI] [PubMed] [Google Scholar]
- Røsjø, E. , Steffensen, L. H. , Jørgensen, L. , Lindstrøm, J. C. , Šaltytė Benth, J. , Michelsen, A. E. , … Holmøy, T. (2015). Vitamin D supplementation and systemic inflammation in relapsing‐remitting multiple sclerosis. Journal of Neurology, 262, 2713–2721. 10.1007/s00415-015-7902-5 [DOI] [PubMed] [Google Scholar]
- Runia, T. F. , Hop, W. C. J. , de Rijke, Y. B. , Buljevac, D. , & Hintzen, R. Q. (2012). Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology, 79, 261–266. 10.1212/WNL.0b013e31825fdec7 [DOI] [PubMed] [Google Scholar]
- Sakaguchi, S. (2011). Regulatory T cells: History and perspective In Kassiotis G. & Liston A. (Eds.), Regulatory T cells: Methods and protocols (pp. 3–17). Totowa, NJ: Humana Press. [Google Scholar]
- Salzer, J. , Hallmans, G. , Nystrom, M. , Stenlund, H. , Wadell, G. , & Sundstrom, P. (2012). Vitamin D as a protective factor in multiple sclerosis. Neurology, 79, 2140–2145. 10.1212/WNL.0b013e3182752ea8 [DOI] [PubMed] [Google Scholar]
- Scalfari, A. , Neuhaus, A. , Degenhardt, A. , Rice, G. P. , Muraro, P. A. , Daumer, M. , & Ebers, G. C. (2010). The natural history of multiple sclerosis, a geographically based study 10: Relapses and long‐term disability. Brain, 133, 1914–1929. 10.1093/brain/awq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaygannejad, V. , Janghorbani, M. , Ashtari, F. , & Dehghan, H. (2012). Effects of adjunct low‐dose vitamin D on relapsing‐remitting multiple sclerosis progression: Preliminary findings of a randomized placebo‐controlled trial. Multiple Sclerosis International, 2012, 1–7. 10.1155/2012/452541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi, H. A. , Rasouli, J. , Ciric, B. , Wei, D. , Rostami, A. , & Zhang, G.‐X. (2017). 1,25‐Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Experimental and Molecular Pathology, 102, 515–521. 10.1016/j.yexmp.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvani, A. , Kalajian, T. A. , Song, A. , & Holick, M. F. (2019). Disassociation of vitamin D's calcemic activity and non‐calcemic genomic activity and individual responsiveness: A randomized controlled double‐blind clinical trial. Scientific Reports, 9, 17685 10.1038/s41598-019-53864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvani‐Farsani, Z. , Behmanesh, M. , Mohammadi, S. M. , & Naser Moghadasi, A. (2015). Vitamin D levels in multiple sclerosis patients: Association with TGF‐β2, TGF‐βRI, and TGF‐βRII expression. Life Sciences, 134, 63–67. 10.1016/j.lfs.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Shirvani‐Farsani, Z. , Kakhki, M. P. , Gargari, B. N. , Doosti, R. , Moghadasi, A. N. , Azimi, A. R. , & Behmanesh, M. (2017). The expression of VDR mRNA but not NF‐κB surprisingly decreased after vitamin D treatment in multiple sclerosis patients. Neuroscience Letters, 653, 258–263. 10.1016/j.neulet.2017.05.050 [DOI] [PubMed] [Google Scholar]
- Simpson, S. , Taylor, B. , Blizzard, L. , Ponsonby, A.‐L. , Pittas, F. , Tremlett, H. , … van der Mei, I. (2010). Higher 25‐hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Annals of Neurology, 68, 193–203. 10.1002/ana.22043 [DOI] [PubMed] [Google Scholar]
- Simpson, S. Jr. , Wang, W. , Otahal, P. , Blizzard, L. , van der Mei, I. A. F. , & Taylor, B. V. (2019). Latitude continues to be significantly associated with the prevalence of multiple sclerosis: An updated meta‐analysis. Journal of Neurology, Neurosurgery & Psychiatry, 90, 1193–1200. [DOI] [PubMed] [Google Scholar]
- Sloka, S. , Zhornitsky, S. , Silva, C. , Metz, L. M. , & Yong, V. W. (2015). 1,25‐Dihydroxyvitamin D3 protects against immune‐mediated killing of neurons in culture and in experimental autoimmune encephalomyelitis. PLoS ONE, 10, e0144084 10.1371/journal.pone.0144084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders, J. , Peelen, E. , Thewissen, M. , Tervaert, J. W. C. , Menheere, P. , Hupperts, R. , & Damoiseaux, J. (2010). Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS ONE, 5, e15235 10.1371/journal.pone.0015235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu‐Hänninen, M. , Åivo, J. , Lindström, B.‐M. , Elovaara, I. , Sumelahti, M.‐L. , Färkkilä, M. , … Filippi, M. (2012). A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β‐1b in patients with multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 83, 565–571. 10.1136/jnnp-2011-301876 [DOI] [PubMed] [Google Scholar]
- Sotirchos, E. S. , Bhargava, P. , Eckstein, C. , Van Haren, K. , Baynes, M. , Ntranos, A. , … Calabresi, P. A. (2016). Safety and immunologic effects of high‐ vs low‐dose cholecalciferol in multiple sclerosis. Neurology, 86, 382–390. 10.1212/WNL.0000000000002316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. S. , Liu, Y. , Gray, O. M. , Baker, J. E. , Kolbe, S. C. , Ditchfield, M. R. , … Kilpatrick, T. J. (2011). A randomized trial of high‐dose vitamin D2 in relapsing‐remitting multiple sclerosis. Neurology, 77, 1611–1618. 10.1212/WNL.0b013e3182343274 [DOI] [PubMed] [Google Scholar]
- Tian, X. Q. , Chen, T. C. , Matsuoka, L. Y. , Wortsman, J. , & Holick, M. F. (1993). Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. The Journal of Biological Chemistry, 268, 14888–14892. [PubMed] [Google Scholar]
- Toghianifar, N. , Ashtari, F. , Zarkesh‐Esfahani, S. H. , & Mansourian, M. (2015). Effect of high dose vitamin D intake on interleukin‐17 levels in multiple sclerosis: A randomized, double‐blind, placebo‐controlled clinical trial. Journal of Neuroimmunology, 285, 125–128. [DOI] [PubMed] [Google Scholar]
- Tuoresmäki, P. , Väisänen, S. , Neme, A. , Heikkinen, S. , & Carlberg, C. (2014). Patterns of genome‐wide VDR locations. PLoS ONE, 9, e96105 10.1371/journal.pone.0096105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger, W. W. J. , Laban, S. , Kleijwegt, F. S. , van der Slik, A. R. , & Roep, B. O. (2009). Induction of Treg by monocyte‐derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD‐L1. European Journal of Immunology, 39, 3147–3159. 10.1002/eji.200839103 [DOI] [PubMed] [Google Scholar]
- Wallin, M. T. , Culpepper, W. J. , Nichols, E. , Bhutta, Z. A. , Gebrehiwot, T. T. , Hay, S. I. , … Murray, C. J. L. (2019). Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology, 18, 269–285. 10.1016/S1474-4422(18)30443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, A. R. , DeCosta, B. R. , & Holick, M. F. (1989). Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. The Journal of Clinical Endocrinology and Metabolism, 68, 882–887. 10.1210/jcem-68-5-882 [DOI] [PubMed] [Google Scholar]
- Weinshenker, B. G. (1994). Natural history of multiple sclerosis. Annals of Neurology, 36, S6–S11. [DOI] [PubMed] [Google Scholar]
- Wishart, D. S. , Feunang, Y. D. , Guo, A. C. , Lo, E. J. , Marcu, A. , Grant, J. R. , … Wilson, M. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research, 46, D1074–D1082. 10.1093/nar/gkx1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootla, B. , Eriguchi, M. , & Rodriguez, M. (2012). Is multiple sclerosis an autoimmune disease? Autoimmune diseases, 2012, 969657 10.1155/2012/969657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootla Bharath, Eriguchi Makoto, Rodriguez Moses (2012). Is Multiple Sclerosis an Autoimmune Disease?. Autoimmune Diseases, 2012, 1–12. 10.1155/2012/969657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Soruri, A. , Gieseler, R. K. H. , & Peters, J. H. (1993). 1,25‐Dihydroxyvitamin D3 exerts opposing effects to IL‐4 on MHC class‐II antigen expression, accessory activity, and phagocytosis of human monocytes. Scandinavian Journal of Immunology, 38, 535–540. 10.1111/j.1365-3083.1993.tb03237.x [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Fang, F. , Tang, J. , Jia, L. , Feng, Y. , Xu, P. , & Faramand, A. (2019). Association between vitamin D supplementation and mortality: Systematic review and meta‐analysis. The BMJ, 366, l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, C. , He, L. , Liu, L. , Zhu, J. , & Jin, T. (2018). The efficacy of vitamin D in multiple sclerosis: A meta‐analysis. Multiple Sclerosis and Related Disorders, 23, 56–61. 10.1016/j.msard.2018.05.008 [DOI] [PubMed] [Google Scholar]
- Zhu, J. , & DeLuca, H. F. (2012). Vitamin D 25‐hydroxylase—Four decades of searching, are we there yet? Archives of Biochemistry and Biophysics, 523, 30–36. 10.1016/j.abb.2012.01.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Vitamin D and its in vitro immunomodulatory effects on immune cells
Table S2 Vitamin D and its in vitro molecular effects on immune cells
Table S3 Vitamin D supplementation studies in healthy adults and effects on the transcriptome
Table S4 Molecular evidence in support of a role of vitamin D in multiple sclerosis pathogenesis
Table S5 Vitamin D supplementation studies in multiple sclerosis and effects on gene expression


