Abstract
Cough is an adverse effect that may hinder the delivery of drugs into the lungs. Chemical or mechanical stimulants activate the transient receptor potential in some airway afferent nerves (C‐fibres or A‐fibres) to trigger cough. Types of inhaler device and drug, dose, excipients and formulation characteristics, including pH, tonicity, aerosol output and particle size may trigger cough by stimulating the cough receptors. Release of inflammatory mediators may increase the sensitivity of the cough receptors to stimulants. The cough‐provoking effect of aerosols is enhanced by bronchoconstriction in diseased airways and reduces drug deposition in the target pulmonary regions. In this article, we review the factors by which inhalation products may cause cough.
Keywords: adverse effect, aerosol, cough, inhalation, pharmaceutical products
Abbreviations
- ASIC
acid‐sensing ion channel
- COPD
chronic obstructive pulmonary disease
- DPI
dry powder inhaler
- FEV1
forced expiratory volume in 1 s
- IPF
idiopathic pulmonary fibrosis
- LAMA
long‐acting muscarinic receptor antagonist
- MDI
metered‐dose inhaler
- PAH
pulmonary arterial hypertension
- pMDI
pressurised metered‐dose inhaler
- RAR
rapidly adapting receptor
- SAR
slowly adapting receptor
- TI
Technosphere insulin
- TIP
tobramycin inhalation powder
- TIS
tobramycin inhalation solution
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankyrin 1
- TRPM8
transient receptor potential melastatin 8
- TRPV1
transient receptor potential vanilloid 1
1. INTRODUCTION
Cough is the most important airway defence mechanism, but it is also a major symptom of respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD) (CDC, 2010). Cough helps to remove potentially harmful substances from entering the respiratory system and clears excessive secretions from the airways. It begins with a brief inspiration (inspiratory phase) followed by expiration against a closed glottis (compression phase), which leads to increased intrapulmonary pressure such that the opening of the glottis evokes a rapid expiratory airflow (expulsive phase) (Mazzone & Undem, 2016). Pleural pressure is increased during coughing and is dissipated amongst the flow‐limiting segments of the lungs, which are the movable sections of bronchi 1–2 cm in length that narrow to limit airflow (Smaldone et al., 1993; Smaldone & Bergofsky, 1976; Smaldone & Messina, 1985). The local airflow limitation effects outweigh the usual particle deposition mechanisms such as inertial impaction, gravitational sedimentation and increases particle deposition in and near these airway segments (Smaldone, Itoh, Swift, & Wagner, 1979; Smaldone & Messina, 1985). The overall result is an increase in particle deposition in the central airways (Smaldone & Messina, 1985). Thus, coughing can change the distribution of particle deposits in the lungs. The increased pleural pressure and decreased transmural pressure in the flow‐limiting segment increase the linear velocity of the airflow through the airways to expel foreign particles (Smaldone et al., 1979; Smaldone & Bergofsky, 1976). However, since mucociliary clearance in the central airways is impaired in obstructive airway diseases, coughing in these patients will increase the extent and duration of particle exposure in the central lung (Smaldone et al., 1993; Smaldone & Messina, 1985). In other words, the particles accumulate in the central airways because of their higher deposition and lower clearance caused by coughing and the disease state. Expiration reflex is a variation of cough triggered by mechanical probing of the glottis, laryngeal vocal folds and tracheal mucosa without an initial inspiratory effort (Mazzone & Undem, 2016). While objects detected in the glottis or larynx are removed by the expiratory reflex, those in the deeper parts of the airways may require an inspiratory effort to generate a high airflow velocity for removal by coughing (Mazzone & Undem, 2016).
Acute and subacute coughs (<8 weeks of duration) are usually resulted from upper respiratory tract infections caused by viruses or bacteria (Irwin, French, Chang, Altman, & Panel, 2018). These coughs are often resolved once the infection is cleared from the airways. However, chronic cough, also known as cough hypersensitivity syndrome, is a condition with a long‐standing hypersensitivity or dysregulation of the “vagal” nervous system (Chung, 2011; Morice, Faruqi, Wright, Thompson, & Bland, 2011). The four most common background disorders for chronic coughs are asthma, eosinophilic bronchitis, chronic rhinosinusitis and oesophageal reflux disease (Chung & Pavord, 2008). Some patients cough excessively due to airway nerve hypersensitivity, without any known pathological complications (Buday, Kovacikova, Ruzinak, & Plevkova, 2017). Cough in chronic respiratory tract diseases is an unpleasant experience and may represent an exaggerated reflex.
Pharmaceutical products are formulated as inhalation aerosols for faster onset of action in the treatment of respiratory diseases. Inhaled therapeutic agents can be delivered as liquid or dry powder aerosols using one of the three delivery platforms:‐ nebulisers, pressurised metered‐dose inhalers (pMDIs) and dry powder inhalers (DPIs). Over the years, there have been clinical reports of cough induced by inhaled pharmaceutical aerosols. For example, in the famous EAGER trial assessing the safety of tobramycin inhalation powder (TIP) and solution, cough was reported for both forms of the aerosol (Konstan, Flume, et al., 2011). In another study, cough was reported in cystic fibrosis (CF) subjects after administration of colistin sulfate powder, while nebulised colistin sulfometate did not cause coughs in healthy subjects (Le Brun et al., 2002). As such, multiple factors are involved in coughs induced by inhaled therapeutics. This article aims to systemically review the literature on clinical studies of inhaled therapeutics to unravel the cause of cough stimulation. This would provide insight on how cough‐associated adverse events may be minimised. An understanding of the cough physiology and different types of airways stimulant are necessary before investigating the impact of inhalation products on evoking cough.
2. COUGH, AIRWAY SENSORY NERVES AND COUGH RECEPTORS
Cough receptors are located in the very peripheral ends of axons of neurons involved in the sensory innervation of the airways that travel to the central nervous system via the vagus nerve (Mazzone & Undem, 2016). The nuclei of these afferent nerves are in the jugular or nodose ganglia. If the receptors are activated with sufficient intensity an action potential would then be generated and travel along the vagus nerve and to the synapses in the medulla where the cough reflex is processed (Bonvini & Belvisi, 2017). The cough reflex arc (Figure 1) consists of afferent, central and efferent pathways. The afferent impulses travel to the brainstem and pons, which co‐ordinate the autonomic functions of breathing. The central pathway, which coordinates coughing, is situated in the upper brain stem and pons (Polverino et al., 2012). The efferent pathway sends signals from the cough centre to the muscles, abdominal wall and diaphragm via the phrenic, parasympathetic travelling in the vagus and spinal motor nerves (Bonvini & Belvisi, 2017). The CNS interprets signals from the vagal afferents and elicits changes in the breathing rate and depth, and autonomic outflow to airway smooth muscles. However, the urge of coughing and feeling of dyspnoea may result if certain types of afferent nerves in the airways are activated. If the sensory nervous system becomes dysregulated as in illness (e.g. rhinitis, bronchitis, asthma and COPD), it may cause bronchospasm, the urge of coughing and dyspnoea (Mazzone & Undem, 2016).
FIGURE 1.
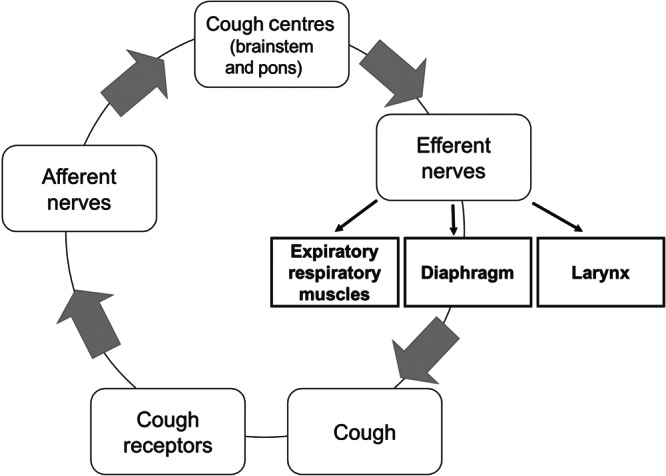
The cough reflex arc
Vagal nerve afferents are generally classified by their conduction velocities (Canning et al., 2014; Mazzone & Undem, 2016). C‐fibres are the slowest, followed by B‐fibres (Table 1). A‐fibres are the fastest, which are further classified as Aδ, Aγ and Aβ, ranging from the slowest to the fastest. However, since Aγ fibres are efferent autonomic nerves, the ranking of vagal sensory nerves is in the order of increasing conduction velocity, which is C‐fibres followed by Aδ and Aβ fibres. C‐fibres respond to potentially damaging mechanical forces, as well as inflammatory mediators and acidic chemicals (Mazzone & Undem, 2016). There are two types of C‐fibres, pulmonary and bronchial (Coleridge & Coleridge, 1977; Coleridge & Coleridge, 1994). Pulmonary C‐fibres respond to chemical stimulants with a short latency when the chemical stimulant is delivered through via the right atrial into the pulmonary circulation (Coleridge & Coleridge, 1977; Kubin, Kimura, & Davies, 1991). On the other hand, bronchial C‐fibres are in the large airways and respond to chemical stimulants also with a short latency when the chemical stimulant is injected directly into the systemic circulation. Nodose A‐fibres generally respond to low threshold mechanical force. The Aδ sensory fibres lead to cough and serve almost exclusively the larynx, trachea and bronchi (Canning et al., 2004). They do not respond to tissue distention, airway smooth muscle contraction and inflammatory mediators (Canning et al., 2004). However, they are sensitive to mechanical stimulation of, and sudden acidity in the epithelium. If the pH decreases gradually, the acid‐sensing mechanisms adapt and the signal does not occur (Mazzone & Undem, 2016). Aβ fibres respond to the lung distention and deflation during breathing. The mechanosensitive Aβ fibres are divided into rapidly adapting receptors (RARs) and slowly adapting receptors (SARs). RARs are activated by lung deflation, bronchospasm and changes in dynamic lung compliance. The conduction velocities of the afferents from SARs' are faster than those from RARs and have a different distribution in the airways. SARs also respond to the mechanical forces that occur during tidal breathing (Figure 2 and Table 1) (Mazzone & Undem, 2016).
TABLE 1.
Characteristics of airway vagal sensory neurons
| Airway vagal neurons | Subtype | Subtype | Speed | Response as cough | Mechanical stimulation | Tissue stretch | Bronchoconstriction | Capsaicin | Acid | ATP |
|---|---|---|---|---|---|---|---|---|---|---|
| A‐fibres | Aδ | Intermediate | √ a | √ a | √ b | √ | ||||
| Aγ | ||||||||||
| Aβ a | RARs | Fastest | √ | √ | √ | Unknown | √ | |||
| SARs | √ | √ | √ | Unknown | √ | |||||
| B‐fibres | Intermediate | |||||||||
| C‐fibres | Pulmonary or bronchial | Slowest | √ b | √ | √ | √ a |
Abbreviations: RARs, rapidly adapting receptors; SARs, slowly adapting receptors.
cell bodies located in nodose ganglia only.
cell bodies located jugular only.
FIGURE 2.
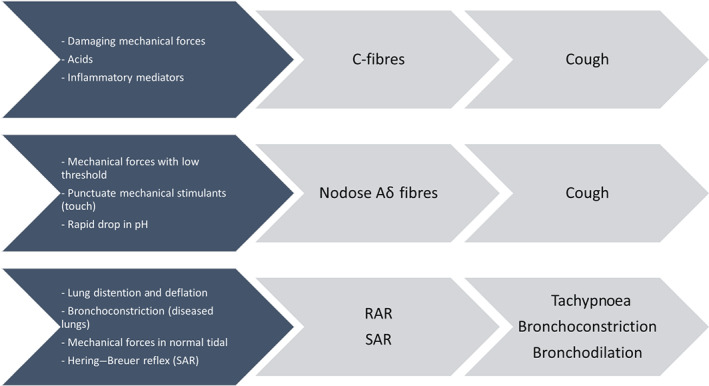
Common stimulants of airway sensory vagal fibres. RAR, rapidly adapting receptor; SAR, slowly adapting receptor
Transient receptor potential (TRP) channels are a group of ion channels consisting of 28 members that are expressed in many types of cells (Patapoutian, Tate, & Woolf, 2009). TRP vanilloid 1 (TRPV1), TRP ankyrin 1 (TRPA1) and TRP melastatin 8 (TRPM8) are the most important TRP channels related to cough (Materazzi, Nassini, Gatti, Trevisani, & Geppetti, 2009; Mazzone & Undem, 2016). Capsaicin strongly activates most vagal afferent C‐fibres through the TRPV1 receptors, which are also sensitive to acid and heat (Kollarik & Undem, 2004). TRPA1 is expressed in C‐fibre afferent neurons and co‐localised with TRPV1. Therefore, inhalation of TRPV1 or TRPA1 agonists will cause coughing. TRPA1 can be activated by natural molecules (e.g. allyl isothiocyanate, allicin and cannabinol) and environmental irritants (e.g. acrolein, hypochlorite, and hydrogen peroxide). TRPM8 is a receptor for menthol and cold, but it constitutes only about 15% of the total bronchopulmonary C‐fibres (Bonvini & Belvisi, 2017; Mazzone & Undem, 2016).
Inhalation of acidic aerosols leads to coughing in human and laboratory animals because they can activate all C‐fibres in the airways through TRPV1 receptors (Allott, Evans, & Marshall, 1980; Canning, Farmer, & Mori, 2006; Forsberg, Karlsson, Theodorsson, Lundberg, & Persson, 1988; Lowry, Wood, & Higenbottam, 1988). A‐fibres can also be activated by acidic stimuli and acid‐sensing ion channels (ASICs) also contribute to acid‐induced action potential. Other endogenous compounds that can activate action potentials in these sensory fibres are bradykinin, histamine (secondary to ATP release), 5‐hydroxytryptamine (5‐HT; serotonin), adenosine and prostaglandins (PGs). In this latter respect inhalation of PGD2 and PGF2α are known to cause cough (Mazzone & Undem, 2016).
Considering patients that inhale therapeutic drugs via aerosols, it is has been shown that the stimulus threshold for coughing is significantly lower in patients with pulmonary diseases (Wong & Morice, 1999). Further, those patients with chronic cough the urge to cough is increase. These conditions indicate that the affected airways have a higher basal vagal afferent activity and sensory hypersensitivity (Dicpinigaitis, Bhat, Rhoton, Tibb, & Negassa, 2011; Hilton et al., 2015). Autacoids (e.g. chemokines, cytokines, purines and eicosanoids) and ATP present in inflammatory airway diseases can stimulate the C‐fibres in the respiratory tract to induce coughing. ATP receptor antagonists can decrease coughing frequency through inhibiting the stimulation of airway nodose C‐fibres by ATP (Mazzone & Undem, 2016). For example AF‐219 (gefapixant), a P2X3 receptor antagonist acts by blocking ATP receptors P2X3 and P2X2/3, was found to reduce the frequency of coughs in a Phase 2 clinical trial (Abdulqawi et al., 2015). Touch‐sensitive airway mechanosensitive afferent receptors respond vigorously to light punctate mechanical stimuli. These cough receptors are located on many derived A‐fibres in large airways (e.g. larynx, trachea and main bronchi), whose cell bodies are located in the nodose ganglia (Bonvini & Belvisi, 2017).
Airway inflammation increases the release of various neurotrophic factors that can interact with these receptors at the nerve terminals and leads to gene expression changes in the cell bodies located in distal vagal ganglia (Lieu, Myers, Meeker, & Undem, 2012; Zhang, Lin, Wiggers, Snow, & Lee, 2008). For example, allergic inflammation may cause phenotypic changes in SAR and RAR neurons to express TRPV1 ion channels, which are normally absent. These nerves consequently become sensitive to capsaicin and other endogenous TRPV1‐activating stimuli. Airway inflammation may also up‐regulate the functional TRPV1 channels in tracheal Aδ sensory neurons that normally do not express these them. Nodose ganglia located C‐fibre neurons may develop responsiveness to neurokinins under similar circumstances. Due to these phenotypic changes, stimuli that are normally inert may potentially cause coughing, bronchospasm and dyspnoea (Moore, Undem, & Weinreich, 2000). Large‐diameter RAR/SAR neurons as well as tracheal Aδ neurons start to express neuropeptides, such as substance P and other neurokinins, and express and transport peptides such as calcitonin gene‐related peptide (CGRP) (Mazzone & Undem, 2016). Peripheral tissue inflammation or neuropathic injury may increase excessive neuronal activity in the central nerves that result in central sensitisation (Mazzone & Undem, 2016).
3. DISEASE STATUS AND COUGH TO INHALED AGENTS
The cough patterns induced by aerosol products in healthy and diseased people are different (Cipolla & Chan, 2013). For example, nebulised antibiotics cause more severe coughing and wheezing in non‐cystic fibrosis bronchiectasis patients compared with cystic fibrosis patients (Cipolla & Chan, 2013). Patients are pretreated with bronchodilators to reduce the frequency and severity of these adverse events (Elkins et al., 2006). Drug deposition profile may be affected by the extent of bronchoconstriction, changes in bronchial blood flow, the presence of excess mucus or oedema. The consequences of reduced airway calibre leads to increased drug deposition in the larger central airways, provoking cough and leads to insufficient delivery of the drug to the target site (Richards et al., 1988; Satia, Badri, Al‐Sheklly, Smith, & Woodcock, 2016).
Asthma is one of the most common causes of chronic cough in non‐smoking adults (Morice, 2004). Moreover, cough as a symptom is more common in patients with severe asthma. Moderate‐to‐severe asthmatic patients suffer from persistent cough and mucus hypersecretion (de Marco et al., 2006). Cough impacts the quality of life of some asthmatic patients and is a major contributor to poor asthma control (Purokivi, Koskela, & Kontra, 2013). The incident rate of cough is significantly higher in asthmatic patients than in healthy subjects (Bonvini & Belvisi, 2017). Cough sensitivity to capsaicin and citrate does not increase in asthma, whereas cough in response to hypertonicity is more pronounced in asthmatic patients (Koskela, Purokivi, Kontra, Taivainen, & Tukiainen, 2008). Exercise causes a loss of respiratory heat and water and this can induce bronchospasm as well as cough in asthmatic patients (Basoglu et al., 2005; Chen & Horton, 1977; O'Byrne et al., 1982; Strauss, McFadden, Ingram, Deal, & Jaeger, 1978).
Similarly, COPD patients cough is due to TRPV1 receptor activation or substantial airway inflammation and subsequent release of inflammatory mediators (e.g. tussive agents such as tachykinins and cough reflex stimulants such as prostaglandins) (Choudry, Fuller, & Pride, 1989; Joos, De Swert, Schelfhout, & Pauwels, 2003). The muscarinic antagonist tiotropium, which is used as a bronchodilator in COPD and more recently in asthma, has been shown in a guinea pig model of cough to inhibit TRPV1‐mediated neuronal activity (Birrell et al., 2014). Acrolein and crotonaldehyde in cigarette smoke can also induce cough through stimulating TRPA1 receptors. This suggests a potential role for TRPA1 receptors in coughs induced by cigarette smoke, which is closely linked to COPD development (Mazzone & Undem, 2016). Furthermore, COPD coughs may be induced by increased protease levels stimulating TRPV4 and TRPA1 receptors (Grace et al., 2014), as well as through mechanical stimulation by the excess mucus in the airways (Hogg, 2004).
The frequency of cough in idiopathic pulmonary fibrosis (IPF) is even higher than in asthma or COPD and is a marker of poor prognosis (Ryerson et al., 2011). TRPV1 and ATP (P2X3) receptors may be involved in the generation of cough in idiopathic pulmonary fibrosis patients. Oral inhalation of sodium cromoglicate (cromoglicic acid) can reduce the cough frequency of idiopathic pulmonary fibrosis patients by 31% (Birring et al., 2017). Thus inflammation and mechanical distortion of the lungs may affect nerve fibres and increase cough reflex sensitivity to mechanical stimulation of the chest wall (Bonvini & Belvisi, 2017).
Increased cough response is also observed in patients with viral respiratory infections (Empey, Laitinen, Jacobs, Gold, & Nadel, 1976). Viral infection exposes the sensory nerves by damaging the airway epithelial cells, rendering them to have increased responsiveness to mechanical and chemical stimuli (Empey et al., 1976). Cough may also be induced by the production of inflammation‐associated mediators that sensitise sensory nerve endings (Fuller & Jackson, 1990). Depending on the respiratory disease status of the patient, cough can be induced by mechanical or chemical stimulation of various ion channels, which activates sensory nerves and the cough reflex.
Understanding of the cough pathophysiology has the potential to design drugs that target the receptors of interest and provide improved quality of life to chronic lung disease patients. In addition, the drug delivery aspects should be considered as changes to airway physiology can impact aerosol deposition and clearance in the lungs (Labiris & Dolovich, 2003). The deposition and distribution patterns of aerosols are affected in the airways of patients with respiratory diseases, such as cystic fibrosis and bronchiectasis. These diseases change the architecture of the lungs (Houtmeyers, Gosselink, Gayan‐Ramirez, & Decramer, 1999; Smaldone et al., 1993). This reduces the clearance of deposited aerosolised drugs and how the secretions are cleared from the lungs by cough (Isawa et al., 1990).
4. COUGH CHALLENGE TESTS
Cough challenge relies on the delivery of tussive agents in aerosols from an inhalation device and the subsequent recording of the number of induced coughs. These agents can be administered as liquid droplets using jet or ultrasonic nebulisers (through a mouthpiece or face mask) or powders using breath‐actuated dry powder inhalers. Nebulisers are often used with a dosimeter to control the inspiratory flow rate, as it can affect the cough response. The dosimeter generates a burst of compressed air that initiates a fixed duration of nebulisation. In each challenge, the agent is delivered in increasing concentrations. The concentration that induces at least two coughs per inhalation is used. Commonly used tussive agents include capsaicin, citric acid and mannitol (Koskela, Lake, Wong, & Brannan, 2018; Midgren, Hansson, Karlsson, Simonsson, & Persson, 1992; Nurmi, Lätti, Brannan, & Koskela, 2019).
Capsaicin has been used extensively for cough challenge as it induces cough in a dose‐dependent and reproducible manner (Midgren et al., 1992). Capsaicin dissolved in alcohol is serially diluted in isotonic saline to give different concentrations between 0.15 and 305 μg·ml−1. Nebulised capsaicin aerosol is thought to induce cough through activating C‐fibres via TRPV1 in the airways (Fuller, Dixon, & Barnes, 1985). Furthermore, oral administration of capsaicin can also desensitise the cough reflex by acting on TRPV1 receptors in the gastrointestinal system, known as central reflex desensitisation (Ternesten‐Hasseus, Johansson, & Millqvist, 2015). Citric acid is another commonly used tussive agent that induces cough through C‐fibre activation (Canning et al., 2004; Nurmi et al., 2019). In addition, nebulised citric acid aerosols have been reported to stimulate RARs within the larynx and the upper airways (Morice, Kastelik, & Thompson, 2001). The traditional cough provocation tests using capsaicin or citric acid usually show a wide range of responses. The cough sensitivity achieved by this method poorly correlates with the symptoms (Buday et al., 2017).
Mannitol challenge test is a regulatory approved bronchial provocation test that uses dry powder mannitol with a handheld dry powder inhaler. Similar to hypertonic saline, mannitol provides an osmotic stimulus to airways and provokes cough (Anderson et al., 1997; Koskela, Hyvärinen, Brannan, Chan, & Anderson, 2004). The subject inhales mannitol powders with increasing doses of 0, 5, 10, 20, 40, 80, 160, 160 and 160 mg, with a maximum cumulative dose of 635 mg. Inhaled mannitol was initially developed as a bronchial hyper‐responsiveness test for asthma diagnosis (Koskela et al., 2018) and then later found its utility as a cough challenge test. A recent study demonstrated that cough induced by nebulised mannitol (40 mg·ml−1, 220 mOsm·L−1) is dependent on the particle size (Kanth, Alaienia, & Smaldone, 2018). No subjects coughed when inhaling 1.2 μm mannitol aerosols, while 6.5 μm particles caused coughs in 86% of the subjects.
Both liquid and dry powder aerosols containing tussive agents can induce cough. Patients as well as healthy subjects experience coughs upon inhalation, though the severity and number of induced coughs may vary. The likelihood of inducing coughs increases with higher delivered doses of tussive agents. Furthermore, the dose required to induce coughs also depends on the compound. For example, capsaicin is used in the microgram range, whereas mannitol is used in the milligrams range for cough challenge tests (Koskela et al., 2018; Midgren et al., 1992).
5. FACTORS IN INHALATIONAL PRODUCTS CONTRIBUTING TO COUGH
Advance in particle engineering techniques and novel device designs have widened the variety of inhalation formulations (Zhou et al., 2015). Inhalation products are often designed and engineered to minimise systemic side effects or to avoid first‐pass metabolism. On the other hand, large molecules, such as insulin, are inhaled to allow deep lung deposition for systemic delivery. Orally inhaled drugs can deposit in the extra‐thoracic region (mouth, oropharynx and larynx), the central airways and peripheral part of the lung. Even with highly efficient inhalation delivery platforms, some of the dose may still deposit in the extra‐thoracic region and be swallowed. Particle deposition in the deep lung could be enhanced using particles with a small aerodynamic diameter of 0.1–2 μm coupled with a low inspiratory flow (<20 L·min−1). Smaller particles (<0.1 μm) will result in low deposition due to high amounts of them being exhaled. Larger particles will likely deposit in the upper and large airways where the majority of cough and expiration reflex receptors are located (Mazzone & Undem, 2016). Therefore, to avoid coughs, it would be beneficial to design the aerosol delivery so that the particles preferentially deposit in the small airways.
5.1. Particle size
The level of throat irritation and cough depends on the aerodynamic particle size. Fine and ultrafine particles depositing in the alveoli, where there are no cough receptors, will not cause coughing (Brunekreef & Holgate, 2002; Mitsakou et al., 2007). On the other hand, coarse particles (>5 μm) inhaled at high inspiratory flow rates primarily deposit in the extra‐thoracic region where they can induce cough by stimulating cough and expiratory reflex receptors (Zwozdziaka, Gini, & Samek, 2017). Small particles in inhaled formulations <5 μm can minimise upper airway deposition and reduce the risk of coughing. In a recent study, idiopathic pulmonary fibrosis patients received inhaled mannitol aerosols, which is known to cause coughs (Kanth et al., 2018). Small mannitol aerosols (<1.2 μm) did not induce coughs, whereas majority of the subjects coughed when inhaling larger mannitol aerosols (6.5 μm). Hence, adjusting the aerosol distribution can potentially prevent coughing during drug inhalation. For dry powders, the availability of fine particles in an aerosol depends on the dispersibility of the formulation, inhalation flow and anatomical features of the airway of the patient (Yang, Chan, & Chan, 2014). These will become confounding factors in consideration of particle deposition and cough.
5.2. Inhaler devices
Dry powder inhalers, pressurised metered‐dose inhalers and nebulisers are the three major devices for respiratory drug delivery. Dry powder inhalers and pressurised metered‐dose inhalers are small, portable devices for general use, whereas nebulisers are typically used in emergency care or at home for children and infants to deliver high doses of drugs (Backman, Adelmann, Petersson, & Jones, 2014). Nebulisers were found to induce less coughing than pressurised metered‐dose inhalers or dry powder inhalers in patients with chronic cough for the administration of corticosteroids (Kamimura et al., 2012).
The type of inhalation device and the required mode of inhalation affect the drug delivery into the lungs. Some dry powder inhalers need high inhalation flow rates for dispersion and deagglomeration (Dal Negro, 2015). Consequently, the particles may deposit in the upper airways due to inertial impaction and induce cough. Two breath‐actuated dry powder inhalers, Turbohaler® (500‐μg terbutaline) and Diskhaler® (400‐μg salbutamol), were compared in chronic asthma patients (Brown, Lenney, Armstrong, Ning, & Crompton, 1992). Despite differences in the drug, dose and device design, both products showed very similar rates of cough and therapeutic effects.
Pressurised metered‐dose inhalers generate aerosols that travel rapidly and require coordination between actuation and inhalation. The impaction problem can be addressed for pressurised metered‐dose inhalers by using a spacer, which is essentially a chamber into which the dose is aerosolised, from which the patient inhales by breathing tidally (Kwok, Collins, & Chan, 2006). The spacer allows the propellant to evaporate to form smaller particles and decelerates the aerosol cloud, thereby reducing upper airway deposition. Despite this, spacers cannot eliminate coughing completely (Dubus et al., 2003).
Dubus et al. (2003) studied the local side effects in asthmatic children treated with fluticasone propionate, budesonide or beclomethasone dipropionate delivered from pressurised metered‐dose inhalers with small or large volume spacers (Dubus et al., 2003). About 54% of the patients coughed after inhaling each corticosteroid, with 30% of them also coughed after inhaling β2‐agonists. All three corticosteroids showed similar tendency of inducing cough, despite the pressurised metered‐dose inhalers containing different propellants. This suggests that the induced cough was more likely to be dependent on the delivery device rather than the formulation. Coughing was not found to be associated with the severity of asthma but was related to the use of long‐acting β2‐agonist and duration of treatment. The type/volume of the spacer and the use of mouthpiece/face mask were deemed to not affect coughing.
A study compared the efficacy and safety of inhaled budesonide (800 or 1,600 μg) administered via a pressurised metered‐dose inhaler coupled to a 750‐ml spacer and via a Turbohaler in patients with stable asthma. The Turbohaler showed significantly less coughs 5 min after inhalation than the pressurised metered‐dose inhaler with spacer (Engel et al., 1989). This might be due to the absence of excipients in the Turbohaler product as the powder only contained budesonide. Other confounding factors such as the regional deposition in the airways and the dose deposited could also contribute to the difference in the cough.
Incorrect use of the device and/or inhalation technique may also be associated with chronic cough and wheezing (Lavorini et al., 2008). Before studying the rate of cough, the participants in the study must be sufficiently trained in using the device correctly and inhaling properly. Deep inspiration of corticosteroids, from pressurised metered‐dose inhalers, in patients complaining of coughing, induced large, but transient specific airway resistance and bronchoconstriction (Dubus et al., 2003). Although deep inspiration may stimulate SAR and RAR receptors that normally do not evoke cough, these stretch fibres may have changed their phenotype and express TRPV1 receptors due to airway inflammation. The bronchoconstriction may be caused by pulmonary mechanoreceptor activation secondary to the release of ATP as discussed above (Mazzone & Undem, 2016).
5.3. Dose of inhalation products
In most cases, increasing the dose of inhaled drug can increase the chance of cough. It may be due to the increased concentration of the drug at the cough receptors. In a Phase I, single‐dose, dose‐escalating study on inhaled capreomycin powder, capsules loaded with a spray‐dried powder consisting of 25 mg capreomycin and 5 mg of l‐leucine were used with a Cyclohaler. Drug doses of 25, 75, 150, and 300 mg were investigated, which corresponded to inhaling from 1, 3, 6 and 12 capsules. Twenty healthy subjects were sequentially assigned to one of four dosage groups. Coughing was observed in five of the 20 healthy subjects across all doses. The coughs ranged from mild (for 25 and 75 mg) to moderate (for 150 and 300 mg) and subsided within 5 min after inhaling powder from the last capsule (Dharmadhikari et al., 2013). It may be argued from this study that the severity of the cough increased, albeit slightly, with the inhaled drug dose. However, another Phase I clinical study showed a lack of relationship between increases in the dose and reports of cough. Only two cases of cough were reported in 16 healthy volunteers who received 200, 400 and 800 μg of aclidinium or placebo (Jansat et al., 2009). This phenomenon can be explained by the fact that long‐acting muscarinic receptor antagonists including aclidinium and tiotropium, can down‐regulate the cough reflex. These two long‐acting muscarinic receptor antagonists have antitussive effects in anaesthetised rabbits in response to citric acid challenge. These effects may be related to the down‐regulation of TRPV1, ASIC and mechanoreceptors of cough‐related airway sensory afferent neurons (Mutolo, Cinelli, Iovino, Pantaleo, & Bongianni, 2016).
Poorly dispersed powders that deposit in large airways are more likely to initiate a cough response through the touch‐sensitive mechanosensitive nerves (Brunaugh & Smyth, 2018). Furthermore, the occurrence of coughing may be related to the amount of powder inhaled per bolus and its subsequent osmotic effect, which is dependent on the extent of dispersion and deposition (Velkov, Abdul Rahim, Zhou, Chan, & Li, 2015). Minimising the use of excipients in the aerosol formulation may reduce the chance of coughing. Budesonide formulation without any excipients as a dry powder (lower powder dose) caused less cough than when using a metered‐dose inhaler (MDI) that contained propellants and lubricants (Engel et al., 1989). However, different deposition in the airways from these two products could be a confounding factor to the difference in cough. Although cough is generally associated with the inhaled dose, the same dose of tobramycin inhalation solution (TIS) delivered using the same nebuliser showed different cough profiles, probably due to patients' former exposure (hence acclimatised) to inhaled aerosols (Konstan, Flume, et al., 2011). However, individual variation in the upper airway anatomical features and deposition in the large airways may also contribute to the difference observed (Martin, Mathur, Marshall, & Douglas, 1997; Vinchurkar et al., 2012).
5.4. Dry versus wet aerosols
Direct comparison of powder and liquid aerosols in cough production is difficult as device‐ or patient‐related factors may also be involved. The EAGER trial assessed the safety of tobramycin inhalation powder™ and tobramycin inhalation solution (TOBI®) (Konstan, Flume, et al., 2011). tobramycin inhalation powder (112 mg) and tobramycin inhalation solution (300 mg per 5 ml) were delivered using a Novartis T‐326 Inhaler and a PARI LC PLUS® nebuliser respectively. Cough was frequently reported for both forms of aerosol (48% and 31% for tobramycin inhalation powder and tobramycin inhalation solution, respectively). The higher cough incidents in tobramycin inhalation powder‐treated patients across all age groups (Geller et al., 2014) may be due to the faster administration, that is, more airway receptors were activated within a shorter time frame. Furthermore, the open‐label design of this study as well as prior tobramycin inhalation solution use in some patients may have had an impact on the tendency to report cough as an adverse event. Nonetheless, cough severity was mostly mild and moderate, with <4% and 1% of tobramycin inhalation powder and tobramycin inhalation solution‐treated patients discontinuing the treatment due to coughing respectively. In another study, mild and self‐limited coughs were observed in 20% of tobramycin inhalation powder subjects and none in tobramycin inhalation solution (Geller et al., 2007). Tobramycin inhalation solution subjects (85%) were previously exposed to this treatment as part of their usual therapy, so it is difficult to directly compare the two types of aerosol formulations. Furthermore, 92% of tobramycin inhalation powder subjects reported coughing prior to tobramycin inhalation powder administration, which implies that this adverse event might be disease or patient related. Other studies have also reported coughing as a common adverse event after tobramycin inhalation solution treatment in cystic fibrosis (Greenwood et al., 2017; Hubert et al., 2009; Lenney et al., 2011; Mazurek et al., 2014) and bronchiectasis (Barker et al., 2000; Scheinberg & Shore, 2005) patients. Nebulised colistimethate sodium also caused coughing in a small proportion of cystic fibrosis subjects (Greenwood et al., 2017). Although tobramycin inhalation powder induced coughs, the post‐inhalation cough rates reduces over time with repeated dosing, probably due to acclimatisation of the airways and cough receptors.
Nebulised colistin sulfometate in normal saline (160 mg) did not cause coughing in healthy subjects (Le Brun et al., 2002). However, moderate‐to‐severe coughs were induced by colistin sulfate powder (25 mg) (Le Brun et al., 2002) and high incidence of cough with colistimethate sodium powder (125 mg) (Schuster et al., 2013) in cystic fibrosis subjects after inhalation of the powders. The varied cough profiles could be due multiple factors, including differences in molecular forms of colistin, aerosol performance (inhaler and formulation), and deposition in the respiratory tract. Nebulised colistin sulfomethate produced no cough and was better tolerated than colistin sulfate, which induced throat irritation and severe coughing (Le Brun et al., 2002; Westerman et al., 2004; Westerman et al., 2007). However, it is still not possible to pinpoint whether the difference observed is solely due to the chemical nature of the drugs and/or differences in the solution properties (pH ~7.4 and 5, osmolality 366 and 306 mOsm·kg−1, for the colistin sulfomethate and colistin sulfate solutions, respectively) (Westerman et al., 2004).
As mentioned previously, Aδ fibres and C‐fibres are sensitive to changes in pH and osmolarity (Mazzone & Undem, 2016). Inhaled solid particles, if they are soluble, may increase the osmolality in the airways more than droplets as the latter have higher water content. Moreover, the pH and osmolarity of a liquid formulation can be easily controlled, thus minimising the potential to induce coughs.
A major difference between inhaled powder and liquid formulations is that the dispersion and deposition of solid particles are affected by the patient's inhalation profile (Yang et al., 2014). The T‐326 Inhaler used for delivering tobramycin inhalation powder is a low‐resistance inhaler, so high inspiratory flows can be achieved easily. This may result in the impaction of particles in the oropharynx and central airways, consequently causing cough (an expiratory reflex), though expiratory reflex can be present in the tracheobronchial tree (Widdicombe, 1995). The optimal inhalation profile for aerosol generation is formulation dependent for a given inhaler. tobramycin inhalation powder particles are porous and dispersible with a small aerodynamic diameter. They only require slow and deep inhalation for effective dispersion. For other dry powder formulations, a high inspiratory flow rate may be needed to generate a sufficiently large fraction of fine particles to reach the peripheral airways. Particle deposition in the lungs of healthy subjects for tobramycin inhalation powder delivered using the T‐326 Inhaler was three times higher than that for tobramycin inhalation solution with the PARI LC PLUS nebuliser (Challoner et al., 2001; Newhouse et al., 2003). Therefore, the distribution of drugs in the lungs achieved by inhaled solid and liquid aerosols may be different due to aerosol output, size distribution and inter‐patient differences in the inhalation profiles. This may explain varying reports in cough‐related adverse events between studies.
5.5. Salt form versus free base of the drugs
Most of the studies (Barker et al., 2000; Greenwood et al., 2017; Konstan, Flume, et al., 2011; Lenney et al., 2011; Mazurek et al., 2014) on inhaled tobramycin mentioned above used the sulfate salt. Interestingly, inhaled tobramycin free base showed a low incidence of cough. Only two out of eight subjects coughed during one of the four visits (Hoppentocht et al., 2016). This may be due to the lower oropharyngeal deposition from the relatively low flow rate (34 L·min−1) produced through the Cyclops inhaler and/or the lower powder dose required with the free base of the drug compared with that with its sulfate form. This effect of different salt forms of a drug on coughing was also apparent when 25 mg colistin sulfate from a dry powder inhaler was less tolerable than 160‐mg nebulised colistin sulfomethate, with the former inducing moderate‐to‐severe cough (Le Brun et al., 2002). However, the observed trend was confounded by the different types of inhaler (dry powder inhaler vs. nebuliser), formulation (solid vs. liquid), and inhalation pattern. There are no studies so far that study the effect of the physical form of a drug on cough alone, with all other variables controlled.
5.6. Active ingredients
Drugs may cause coughs by various mechanisms, such as stimulating cough receptors by changing the local acidity, osmolarity, and/or ATP release (Dubus et al., 2003; Koskela et al., 2018; Midgren et al., 1992; Nurmi et al., 2019; Williamson, Matusiewicz, Brown, Greening, & Crompton, 1995). They may also down‐regulate the cough response. Some drugs provoke more coughs than others, which highlights the importance of thorough clinical safety assessment.
5.6.1. Corticosteroids
Corticosteroids are important in the treatment of asthma and COPD owing to their anti‐inflammatory effects. However, their role in the alleviation of chronic coughs has not been confirmed (Johnstone, Chang, Fong, Bowman, & Yang, 2013). A clinical study showed that budesonide and beclomethasone pressurised metered‐dose inhalers showed equal tendency in causing coughs in 34% of the asthmatic patients (Williamson et al., 1995). High prevalence of coughs (53.7%) was observed in asthmatic children receiving inhaled beclomethasone dipropionate, budesonide or fluticasone propionate (Dubus et al., 2003) and was closely associated with therapy duration and combination therapy with long‐acting β2‐agonist. Coughing was more common in patients using higher daily doses of inhaled corticosteroid (>1,500 μg·day−1) from pressurised metered‐dose inhalers, although the difference in prevalence compared with that at lower doses was not statistically significant (Williamson et al., 1995). Using the pressurised metered‐dose inhalers with a spacer did not prevent coughs. The cause of coughing by the corticosteroid pressurised metered‐dose inhalers is yet unknown. An early study noted that a beclomethasone pressurised metered‐dose inhaler caused coughs in asthma patients, whereas a triamcinolone pressurised metered‐dose inhaler did not (Shim & Williams, 1987b). However, besides their different drugs and doses, those two pressurised metered‐dose inhalers contained different propellants and excipients, so it was difficult to identify the reason/s for the different cough responses. It may or may not be due to the drug. Interplay between the various formulation and aerosolisation parameters may also be possible.
5.6.2. Doxorubicin
Cough was the most frequent adverse event in a Phase I study of inhaled doxorubicin (Otterson et al., 2007). Increased cough was observed in 50% of the patients with metastatic tumours to the lung. The irritant nature of the drug and acidic alcoholic formulation at pH 3 may contribute to the high rate of cough.
5.6.3. Antibiotics
Inhaled antibiotics are commonly used for treating lung infections in patients with cystic fibrosis and bronchiectasis (Barker et al., 2000; Geller et al., 2007; Geller, Weers, & Heuerding, 2011). Coughs after inhaling antibiotics have been reported as mild and transient (Table 2) (Barker et al., 2000; Conole & Keating, 2014; Konstan, Geller, et al., 2011). Although the incidence of cough was high after the administration of antibiotics, the coughs reduced after 28 days of treatment (Conole & Keating, 2014). This might be due to an alleviation of the inflammation and infection in the lungs, which strongly provoke cough. Cough prevalence was reduced in the 28‐day treatment Cycles 4 to 6 (21–22%) compared with Cycle 1 (31.4%) in patients receiving inhaled tobramycin (Sommerwerck et al., 2016). In patients with cystic fibrosis, the rationale for the 28‐day on/28‐day off cycle of inhaled antibiotic administration depends on the peak increase in lung function after 28 days of continuous antibiotic administration. The 28‐day off period reduces the likelihood of the emergence of antibiotic‐resistant organisms (Table 2). In a study by Geller et al. (2007), cough was only observed in patients receiving tobramycin inhalation powder (20%) (Geller et al., 2007). None in the tobramycin inhalation solution group coughed. As mentioned in Section 5.2, tobramycin inhalation solution patients had prior exposure to inhaled antibiotics treatment. Thus, it is difficult to conclude that the reduced cough incidence was associated with the improvement in patients' symptoms because it could be due to adaptation to the therapy (Geller et al., 2007). Tobramycin has anti‐inflammatory effects beyond its antimicrobial activity (Gziut et al., 2013). Other antibiotics, such as macrolides, doxycycline, moxifloxacin and polymyxin B, can also reduce inflammation in the lungs (Huckle, Fairclough, & Todd, 2018; Lin et al., 2017). Thus, besides treating the underlying infection, antibiotics may decrease the stimulation of C‐fibres and consequently reduce cough by reducing inflammation in the airways. The fact that they can also provoke cough points to other molecular and/or formulation factors.
TABLE 2.
Reported cough cases after administration of some antibiotic inhalation products in clinical studies
| Drug | Formulation | Dose | Regimen | Delivery platform | Subjects | Reported coughs | Reference |
|---|---|---|---|---|---|---|---|
| Tobramycin | Powder | 112 mg (4 capsules) | Twice daily | T‐326 dry powder inhaler | Cystic fibrosis |
• 48% (powder) • 31% (solution) • Higher cough incidents in all age groups receiving powder |
(Geller et al., 2014; Konstan et al., 2011) |
| Solution | 300 mg in 5‐ml saline | Twice daily | PARI LC PLUS® air jet nebuliser | ||||
| Tobramycin | Powder | 112 mg (4 capsules) | Twice daily | T‐326 dry powder inhaler | Cystic fibrosis |
• 20% (powder) • 0% (solution) • Increased cough incident with increased powder dose |
(Geller, Konstan, Smith, Noonberg, & Conrad, 2007) |
| Solution | 300 mg in 5‐ml saline | Twice daily | PARI LC PLUS air jet nebuliser | ||||
| Tobramycin | Solution | 300 mg in 5‐ml saline | Twice daily for 28 days | PARI LC PLUS air jet nebuliser | Bronchiectasis |
• 41% (tobramycin solution) • 24% (placebo) |
(Barker et al., 2000) |
| Placebo (1.25 mg of quinine sulfate in saline) | |||||||
| Tobramycin (radiolabelled) | Solution | 300 mg in 5‐ml saline | Single dose | eFlow rapid or PARI LC PLUS air jet nebuliser | Cystic fibrosis |
• 50% (eFlow rapid) • 17% (PARI LC PLUS) |
(Lenney, Edenborough, Kho, & Kovarik, 2011) |
| Tobramycin | Solution | 300 mg in 5‐ml saline | Twice daily, 14‐day on/off cycle; 3 cycles | PARI LC PLUS air jet nebuliser | Severe bronchiectasis | • 44% (27%: thought to be treatment related) | (Scheinberg & Shore, 2005) |
| Tobramycin | Solution | 300 mg in 5‐ml saline | Twice daily, 28‐day on/off cycle; 1 + 6 cycles | PARI LC PLUS air jet nebuliser | Cystic fibrosis |
• 6.4% (300 mg per 4 ml) • 6.0% (300 mg per 5 ml) |
(Mazurek et al., 2014) |
| 300 mg in 4‐ml saline | |||||||
| Tobramycin | Solution | 300 mg in 5‐ml saline | Phase 1: twice daily for 14 days + once a day on Day 15 using one type of nebuliser | eFlow rapid or PARI LC PLUS air jet nebuliser | Cystic fibrosis |
• 12% (eFlow) • 0% (PARI LC PLUS) |
(Hubert et al., 2009) |
| Phase 2: twice daily for 14 days + once a day on Day 15 using other type of nebuliser | |||||||
| Tobramycin | Powder | 112 mg (4 × 28‐mg capsules) |
Group A Phase 1: tobramycin solution, twice daily for 28‐day on/off Phase 2: tobramycin powder, twice daily for 28‐day on/off |
T‐326 dry powder inhaler or patients' personal nebuliser | Cystic fibrosis |
• Group A: not reported • Group B: comparable • Group C: post‐inhalation rates reduced from 55% during Phase 1 down to 37% in Phase 2 |
(Greenwood et al., 2017) |
| Solution | 300 mg in 3‐ml saline |
Group B Phase 1: colistin solution, twice daily for 56 days Phase 2: tobramycin powder, twice daily for 28‐day on/off |
|||||
| Colistimethate sodium | Solution | 1 or 2 million units |
Group C Phases 1 and 2: tobramycin powder, twice daily for 28‐day on/off |
||||
| Tobramycin | Powder | 112 mg (4 × 28‐mg capsules) | Twice daily, 28‐day on/off cycle; 3 cycles | T‐326 dry powder inhaler | Cystic fibrosis |
Cycle 1 • 13% (tobramycin powder) • 27% (placebo) Cycles 2 and 3 • 26% (tobramycin powder) • 24% (placebo) |
(Konstan, Geller, et al., 2011) |
| Placebo: 112 mg of excipients | Cycle 1: tobramycin powder or placebo | ||||||
| Cycles 2 and 3: open‐label tobramycin powder | |||||||
| Colistin | Powder | 25 mg | A single dose at each visit | In‐house built dry powder inhaler or Ventstream® jet nebuliser | Cystic fibrosis |
• Moderate to severe (powder) • No coughs reported (solution) |
(Le Brun et al., 2002) |
| Solution | 160 mg in 6‐ml saline | ||||||
| Colistin sulfometate | Solution | 160 mg in 6‐ml saline | A single dose | Ventstream® jet nebuliser | Cystic fibrosis |
• Severe cough (solistin sulfate) • No coughs reported (colistin sulfometate) |
(Westerman, Le Brun, Touw, Frijlink, & Heijerman, 2004) |
| Colistin sulfate | 100 mg in 6‐ml saline | ||||||
| Both contained an equivalent amount of colistin (67 mg in 6 ml) | |||||||
| Colistimethate sodium | Powder | 125 mg | Twice daily, 24 weeks | Turbospin dry powder inhaler or PARI LC PLUS air jet nebuliser | Cystic fibrosis |
• 75% (colistimethate sodium powder) • 43% (tobramycin solution) |
(Schuster, Haliburn, Doring, & Goldman, 2013) |
| Tobramycin | Solution | 300 mg in 5‐ml saline | Twice daily, three 28‐day courses |
5.6.4. β2‐Adrenoceptor agonists
Indacaterol is formulated as a once‐daily inhaled long‐acting β2‐agonist for COPD treatment. A 12‐week, double‐blind study tested inhaled indacaterol (150 μg from a dry powder inhaler) in patients with moderate‐to‐severe COPD (Feldman et al., 2010). The overall rates of cough in indacaterol‐treated and placebo groups were comparable. No association between coughing and bronchospasm was found for indacaterol. Inhalation of salbutamol and terbutaline from dry powder inhalers caused coughing in one third of patients with chronic asthma in another study (Brown et al., 1992). Pretreatment with inhaled salbutamol did not prevent saline hypertonicity‐induced coughs in healthy volunteers (Koskela et al., 2008).
β2‐Adrenoceptors (β2‐receptors) have been proposed to be involved in the cough reflex. However, they have different effects in an animal cough models (Wex & Bouyssou, 2015). Dose‐dependent inhibition of coughs was observed in naïve and ovalbumin‐sensitised guinea pigs, whereas formoterol and salmeterol tended to reduce coughs (Wex & Bouyssou, 2015). Interestingly, indacaterol demonstrated pro‐tussive properties with increased coughing in both animal groups. Although these data cannot be translated directly to β2‐receptors in humans, it shows that drug molecules can have different effects on cough receptors.
5.6.5. Antimuscarinic drugs
The tolerability of inhaled aclidinium bromide, a long‐acting antimuscarinic drug, has been assessed in humans (Jansat et al., 2009). Healthy subjects received 200, 400 or 800 μg of aclidinium bromide or placebo from a dry powder inhaler for 5 days, with ≥7‐day washout periods. The frequency of adverse events was comparable between the treated and placebo groups. Only two participants reported mild and probably treatment‐related coughs. Aclidinium and tiotropium may down‐regulate the cough reflex and interact with TRPV1 receptors, ASICs and mechanoreceptors in the airway nerves (Mutolo et al., 2016).
5.6.6. Insulin
Despite successful efficacy of inhaled insulin, the rate of reported cough was high compared with conventional subcutaneous insulin in type 2 diabetes patients and healthy volunteers. One of the reasons for withdrawing inhaled insulin powder (Exubera®) was the persistent reports of respiratory adverse effects, including cough. The high prevalence of cough may be related to the complex formulation containing sodium citrate, mannitol, glycine and sodium hydroxide, which are cough inducers. The rate of coughing was much less with Technosphere® insulin (TI) in Afrezza®, another inhaled insulin dry powder inhaler product. This powder contains fumaryl diketopiperazine as an excipient (Angelo, Rousseau, Grant, Leone‐Bay, & Richardson, 2009). The rate of mild‐to‐moderate coughs after the administration of Exubera was much higher than that for subcutaneous insulin injection (27% vs 5%, respectively) (Quattrin, Belanger, Bohannon, Schwartz, & Exubera Phase, 2004). On the other hand, Technosphere® insulin showed similar cough incidents as the placebo powder (23.7% vs. 19.9%, respectively) (Rosenstock et al., 2015), suggesting that the coughs were due to the excipients rather than insulin. The coughs triggered by inhaled insulin, whether due to the excipients or insulin itself, are mild and will gradually subside with treatment (Ceglia, Lau, & Pittas, 2006). This could be due to acclimatisation to the inhaled formulation. The coughs are not associated with changes in pulmonary function (Ceglia et al., 2006), despite the pathological association of diabetes with modest pulmonary function impairment (van den Borst, Gosker, Zeegers, & Schols, 2010).
5.6.7. Mannitol
Mannitol has been identified to trigger cough and used to identify subjects with chronic cough and diagnose asthma (see Section 4) (Koskela et al., 2018; Minasian, Wallis, Metcalfe, & Bush, 2008). Inhaled mannitol or other hyperosmolar agents can stimulate the release of bronchoconstricting mediators in the airways and provoke cough (Lowry et al., 1988). Mannitol also increases mucociliary clearance in the airways and assists mucus removal in cystic fibrosis patients. Mannitol decreases the viscosity of phlegm, hydrates the airway surface liquid and increases mucociliary activity (Flume et al., 2015). The rate of cough caused by inhaled mannitol powder was much higher in asthmatic (85.3%) (Brannan et al., 2005) compared with cystic fibrosis patients (25.4%) (Bilton et al., 2011), despite the lower administered doses.
5.6.8. Treprostinil
Treprostinil is a tricyclic benzindene prostacyclin analogue used for the treatment of pulmonary arterial hypertension (PAH) (McLaughlin et al., 2010). Pulmonary arterial hypertension patients suffer from reduced prostacyclin synthase activity, which leads to inadequate prostacyclin I2 production. The prostacyclin deficiency causes vascular proliferation, vasoconstriction and platelet aggregation. Activation of prostacyclin receptors in the lung can reverse these effects (Kingman et al., 2017). In a randomised clinical trial, inhaled treprostinil via an Opti‐Neb nebuliser in addition to oral therapy, 54% of patients coughed in contrast to the 29% in the placebo group (McLaughlin et al., 2010). Inhalation of treprostinil in dog and guinea pigs also caused coughing (Corboz et al., 2017). The pro‐tussive effect may be attributed to the drug being acidic.
5.7. Excipients
Coughing has been reported for placebo formulations in some clinical trials, including the study on treprostinil (McLaughlin et al., 2010) and tobramycin (Konstan, Geller, et al., 2011). This indicates that excipients can also cause cough. Powder formulations containing engineered particles can contain complex excipients, which trigger cough as described for insulin (Section 5.6.6).
Oleic acid and fluorocarbon propellants from pressurised metered‐dose inhalers can cause coughs in asthmatic patients (Shim & Williams, 1987a; Shim & Williams, 1987b). Aerosols containing the above excipients with and without beclomethasone caused coughs 31 and 19 times respectively (Shim & Williams, 1987a). Since the reduction in forced expiratory volume in 1 s (FEV1) was similar in both groups (22%), the bronchoconstriction was caused by the excipients rather than the drug. Inhaled triamcinolone acetonide was better tolerated than beclomethasone in another study (Shim & Williams, 1987a). It was proposed that the excipients, particularly oleic acid, caused cough and bronchoconstriction. Sterling and Batten (1969) demonstrated that sorbitol triolates and lecithin can cause cough and bronchoconstriction in asthmatic patients (13% and 21% reduction in airway conductance, respectively). In healthy subjects, they decreased airway conductance by 5.3% and 9.7%, respectively, but were unlikely to be clinically significant.
Some preservatives used in inhalation products in the past, including phenol, can cause airway irritation, coughing and bronchoconstriction. Sodium bisulfite and EDTA are still permitted in various pharmaceutical inhalation products to enhance chemical stability, but they can both cause bronchoconstriction (Pilcer & Amighi, 2010).
6. CONCLUSION
Despite great improvement in inhalation drug delivery systems, cough is still a limiting adverse effect affecting patients' compliance and treatment efficacy as it will reduce the delivered dose. Cough is an important symptom of many respiratory diseases, so it may be difficult to distinguish it from the adverse effects caused by the inhalation product. The underlying disease that causes cough and bronchoconstriction may also affect particle deposition and inhalation effort by the patient. Airway acclimatisation and the individual's response to cough stimulants are also important factors. Since cough can be due to so many confounding factors via direct or indirect effect of the stimulants, one cannot simply make general statements such as powder aerosols being more likely to cause cough than nebulised formulations, or vice versa, even for the same drug molecule. Well‐controlled clinical studies specifically designed to address cough would be necessary to identify the underlying cause for cough encountered in patients during aerosol treatment.
In the airways, chemicals may stimulate Aδ fibres or C‐fibres by changing the acidity, osmolarity or by directly activating the receptors. The cough receptors in diseased lungs are highly sensitised and are easier to stimulate than those in healthy people (Adcock, 2009; Lalloo, Lim, DuBois, Barnes, & Chung, 1998). Large particles may stimulate the punctate‐sensitive cough receptors in large airways and trigger the expiratory reflex. Controlling the particles size of the formulation to <5 μm is necessary for minimising cough so that the particles deposit in the deep lung, where cough receptors are absent. However, such an approach would need to take into account the site of therapeutic action. Besides particle size, the form of the aerosol, the dose, solid state of the drug, type of excipients, concentration, and pH and tonicity of the formulation should be considered to avoid causing cough. Higher doses of drugs and/or excipients can increase the risk of cough if the inhaled compounds can activate the cough receptors. Airflow characteristics through the inhaler device must be optimised to achieve the maximum aerosol performance and minimum airway irritation due to unwanted particle deposition. At the same time, the inhaler should be sufficiently simple for patients to operate correctly. Figure 3 summarises these factors in the design and use of inhalation products to avoid causing coughs.
FIGURE 3.
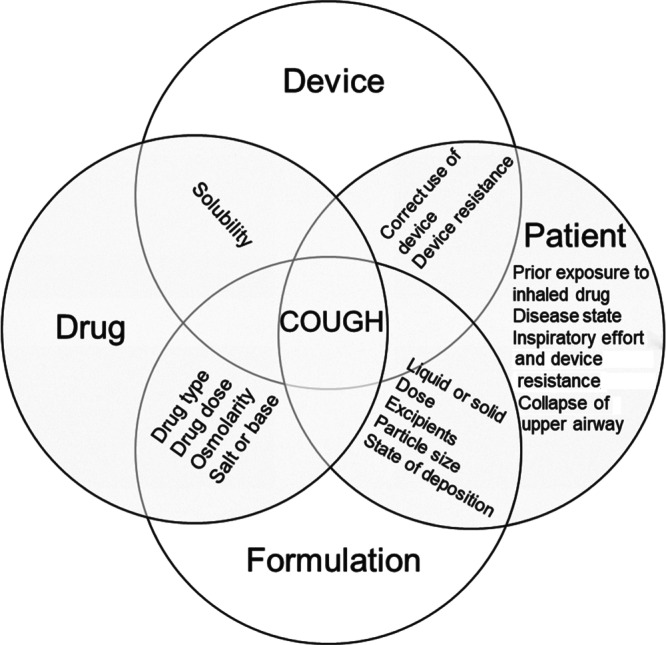
Factors that influence cough in pulmonary drug delivery
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
J.D.B. receives a 10% portion of royalties for the sale of Aridol™/Osmohaler™ that are paid to his prior employer, Royal Prince Alfred Hospital. He holds a minimum number of shares in the manufacturer Pharmaxis Ltd. In the past, he has acted as a consultant to Pharmaxis Ltd and the North American distributor of Aridol, Methapharm Pty Ltd.
Chang RYK, Kwok PCL, Ghassabian S, Brannan JD, Koskela HO, Chan H‐K. Cough as an adverse effect on inhalation pharmaceutical products. Br J Pharmacol. 2020;177:4096–4112. 10.1111/bph.15197
Rachel Yoon Kyung Chang, Philip Chi Lip Kwok and Sussan Ghassabian contributed equally to this work.
REFERENCES
- Abdulqawi, R. , Dockry, R. , Holt, K. , Layton, G. , McCarthy, B. G. , Ford, A. P. , & Smith, J. A. (2015). P2X3 receptor antagonist (AF‐219) in refractory chronic cough: A randomised, double‐blind, placebo‐controlled phase 2 study. Lancet, 385, 1,198–1,205. [DOI] [PubMed] [Google Scholar]
- Adcock, J. J. (2009). TRPV1 receptors in sensitisation of cough and pain reflexes. Pulmonary Pharmacology & Therapeutics, 22, 65–70. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Collaborators, C. G. T. P. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott, C. P. , Evans, D. P. , & Marshall, P. W. (1980). A model of irritant‐induced bronchoconstriction in the spontaneously breathing guinea‐pig. British Journal of Pharmacology, 71, 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, S. D. , Brannan, J. , Spring, J. , Spalding, N. , Rodwell, L. T. , Chan, K. I. M. , … Clark, A. R. (1997). A new method for bronchial‐provocation testing in asthmatic subjects using a dry powder of mannitol. American Journal of Respiratory and Critical Care Medicine, 156, 758–765. [DOI] [PubMed] [Google Scholar]
- Angelo, R. , Rousseau, K. , Grant, M. , Leone‐Bay, A. , & Richardson, P. (2009). Technosphere® insulin: Defining the role of Technosphere particles at the cellular level. Journal of Diabetes Science and Technology, 3, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman, P. , Adelmann, H. , Petersson, G. , & Jones, C. B. (2014). Advances in inhaled technologies: Understanding the therapeutic challenge, predicting clinical performance, and designing the optimal inhaled product. Clinical Pharmacology and Therapeutics, 95, 509–520. [DOI] [PubMed] [Google Scholar]
- Barker, A. F. , Couch, L. , Fiel, S. B. , Gotfried, M. H. , Ilowite, J. , Meyer, K. C. , … Quan, J. (2000). Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. American Journal of Respiratory and Critical Care Medicine, 162, 481–485. [DOI] [PubMed] [Google Scholar]
- Basoglu, O. K. , Pelleg, A. , Essilfie‐Quaye, S. , Brindicci, C. , Barnes, P. J. , & Kharitonov, S. A. (2005). Effects of aerosolized adenosine 5′‐triphosphate vs adenosine 5′‐monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest, 128, 1905–1909. [DOI] [PubMed] [Google Scholar]
- Bilton, D. , Robinson, P. , Cooper, P. , Gallagher, C. G. , Kolbe, J. , Fox, H. , … Investigators, C. F. S. (2011). Inhaled dry powder mannitol in cystic fibrosis: An efficacy and safety study. The European Respiratory Journal, 38, 1,071–1,080. [DOI] [PubMed] [Google Scholar]
- Birrell, M. A. , Bonvini, S. J. , Dubuis, E. , Maher, S. A. , Wortley, M. A. , Grace, M. S. , … Belvisi, M. G. (2014). Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: A beneficial off‐target effect? The Journal of Allergy and Clinical Immunology, 133, 679, e679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birring, S. S. , Wijsenbeek, M. S. , Agrawal, S. , van den Berg, J. W. K. , Stone, H. , Maher, T. M. , … Morice, A. H. (2017). A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: A randomised, double‐blind, proof‐of‐concept, phase 2 trial. The Lancet Respiratory Medicine, 5, 806–815. [DOI] [PubMed] [Google Scholar]
- Bonvini, S. J. , & Belvisi, M. G. (2017). Cough and airway disease: The role of ion channels. Pulmonary Pharmacology & Therapeutics, 47, 21–28. [DOI] [PubMed] [Google Scholar]
- Brannan, J. D. , Anderson, S. D. , Perry, C. P. , Freed‐Martens, R. , Lassig, A. R. , Charlton, B. , & Aridol Study G . (2005). The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: A phase 3 comparison study with hypertonic (4.5%) saline. Respiratory Research, 6, 144 10.1186/1465-9921-6-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P. H. , Lenney, J. , Armstrong, S. , Ning, A. C. , & Crompton, G. K. (1992). Breath‐actuated inhalers in chronic asthma: Comparison of Diskhaler and Turbohaler for delivery of beta‐agonists. The European Respiratory Journal, 5, 1,143–1,145. [PubMed] [Google Scholar]
- Brunaugh, A. D. , & Smyth, H. D. C. (2018). Formulation techniques for high dose dry powders. International Journal of Pharmaceutics, 547, 489–498. [DOI] [PubMed] [Google Scholar]
- Brunekreef, B. , & Holgate, S. T. (2002). Air pollution and health. Lancet, 360, 1,233–1,242. [DOI] [PubMed] [Google Scholar]
- Buday, T. , Kovacikova, L. , Ruzinak, R. , & Plevkova, J. (2017). TRPV4 antagonist GSK2193874 does not modulate cough response to osmotic stimuli. Respiratory Physiology & Neurobiology, 236, 1–4. [DOI] [PubMed] [Google Scholar]
- Canning, B. J. , Chang, A. B. , Bolser, D. C. , Smith, J. A. , Mazzone, S. B. , & McGarvey, L. (2014). Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest, 146, 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning, B. J. , Farmer, D. G. , & Mori, N. (2006). Mechanistic studies of acid‐evoked coughing in anesthetized guinea pigs. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291, R454–R463. [DOI] [PubMed] [Google Scholar]
- Canning, B. J. , Mazzone, S. B. , Meeker, S. N. , Mori, N. , Reynolds, S. M. , & Undem, B. J. (2004). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea‐pigs. The Journal of Physiology, 557, 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . (2010). National ambulatory medical care survey: 2010 Summary tables .
- Ceglia, L. , Lau, J. , & Pittas, A. G. (2006). Meta‐analysis: Efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus. Annals of Internal Medicine, 145, 665–675. [DOI] [PubMed] [Google Scholar]
- Challoner P, Flora M, Hirst P, Klimowicz M, Newman S, Schaeffler B, … Wallis S (2001) Gamma scintigraphy lung deposition comparison of TOBI in the Pari LC Plus nebulizer and the Aerodose inhaler. American Journal of Respiratory and Critical Care Medicine, 163, A83. [Google Scholar]
- Chen, W. , & Horton, D. (1977). Heat and water loss from the airways and exercise‐induced asthma. Respiration, 34, 305–313. [DOI] [PubMed] [Google Scholar]
- Choudry, N. B. , Fuller, R. W. , & Pride, N. B. (1989). Sensitivity of the human cough reflex: Effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. The American Review of Respiratory Disease, 140, 137–141. [DOI] [PubMed] [Google Scholar]
- Chung, K. F. (2011). Chronic ‘cough hypersensitivity syndrome’: A more precise label for chronic cough. Pulmonary Pharmacology & Therapeutics, 24, 267–271. [DOI] [PubMed] [Google Scholar]
- Chung, K. F. , & Pavord, I. D. (2008). Prevalence, pathogenesis, and causes of chronic cough. Lancet, 371, 1,364–1,374. [DOI] [PubMed] [Google Scholar]
- Cipolla, D. , & Chan, H. K. (2013). Inhaled antibiotics to treat lung infection. Pharmaceutical Patent Analyst, 2, 647–663. [DOI] [PubMed] [Google Scholar]
- Coleridge, H. M. , & Coleridge, J. C. (1977). Impulse activity in afferent vagal C‐fibres with endings in the intrapulmonary airways of dogs. Respiration Physiology, 29, 125–142. [DOI] [PubMed] [Google Scholar]
- Coleridge, H. M. , & Coleridge, J. C. (1994). Pulmonary reflexes: Neural mechanisms of pulmonary defense. Annual Review of Physiology, 56, 69–91. [DOI] [PubMed] [Google Scholar]
- Conole, D. , & Keating, G. M. (2014). Colistimethate sodium dry powder for inhalation: A review of its use in the treatment of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Drugs, 74, 377–387. [DOI] [PubMed] [Google Scholar]
- Corboz, M. R. , Li, Z. , Malinin, V. , Plaunt, A. J. , Konicek, D. M. , Leifer, F. G. , … Chapman, R. W. (2017). Preclinical pharmacology and pharmacokinetics of inhaled hexadecyl‐treprostinil (C16TR), a pulmonary vasodilator prodrug. The Journal of Pharmacology and Experimental Therapeutics, 363, 348–357. [DOI] [PubMed] [Google Scholar]
- Dal Negro, R. W. (2015). Dry powder inhalers and the right things to remember: A concept review. Multidisciplinary Respiratory Medicine, 10, 13 10.1186/s40248-015-0012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco, R. , Marcon, A. , Jarvis, D. , Accordini, S. , Almar, E. , Bugiani, M. , … European Community Respiratory Health Survey Therapy G . (2006). Prognostic factors of asthma severity: A 9‐year international prospective cohort study. The Journal of Allergy and Clinical Immunology, 117, 1249–1256. [DOI] [PubMed] [Google Scholar]
- Dharmadhikari, A. S. , Kabadi, M. , Gerety, B. , Hickey, A. J. , Fourie, P. B. , & Nardell, E. (2013). Phase I, single‐dose, dose‐escalating study of inhaled dry powder capreomycin: A new approach to therapy of drug‐resistant tuberculosis. Antimicrobial Agents and Chemotherapy, 57, 2613–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicpinigaitis, P. V. , Bhat, R. , Rhoton, W. A. , Tibb, A. S. , & Negassa, A. (2011). Effect of viral upper respiratory tract infection on the urge‐to‐cough sensation. Respiratory Medicine, 105, 615–618. [DOI] [PubMed] [Google Scholar]
- Dubus, J. C. , Mely, L. , Huiart, L. , Marguet, C. , Le Roux, P. , & Reseau de Recherche Clinique en Pneumologie P . (2003). Cough after inhalation of corticosteroids delivered from spacer devices in children with asthma. Fundamental & Clinical Pharmacology, 17, 627–631. [DOI] [PubMed] [Google Scholar]
- Elkins, M. R. , Robinson, M. , Rose, B. R. , Harbour, C. , Moriarty, C. P. , Marks, G. B. , … Bye, P. T. (2006). A controlled trial of long‐term inhaled hypertonic saline in patients with cystic fibrosis. The New England Journal of Medicine, 354, 229–240. [DOI] [PubMed] [Google Scholar]
- Empey, D. W. , Laitinen, L. A. , Jacobs, L. , Gold, W. M. , & Nadel, J. A. (1976). Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. The American Review of Respiratory Disease, 113, 131–139. [DOI] [PubMed] [Google Scholar]
- Engel, T. , Heinig, J. H. , Malling, H. J. , Scharling, B. , Nikander, K. , & Madsen, F. (1989). Clinical comparison of inhaled budesonide delivered either via pressurized metered dose inhaler or Turbuhaler®. Allergy, 44, 220–225. [DOI] [PubMed] [Google Scholar]
- Feldman, D. N. , Fakorede, F. , Minutello, R. M. , Bergman, G. , Moussa, I. , & Wong, S. C. (2010). Efficacy of high‐dose clopidogrel treatment (600 mg) less than two hours before percutaneous coronary intervention in patients with non‐ST‐segment elevation acute coronary syndromes. The American Journal of Cardiology, 105, 323–332. [DOI] [PubMed] [Google Scholar]
- Flume, P. A. , Aitken, M. L. , Bilton, D. , Agent, P. , Charlton, B. , Forster, E. , … Button, B. M. (2015). Optimising inhaled mannitol for cystic fibrosis in an adult population. Breathe (Sheffield, England), 11, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg, K. , Karlsson, J. A. , Theodorsson, E. , Lundberg, J. M. , & Persson, C. G. (1988). Cough and bronchoconstriction mediated by capsaicin‐sensitive sensory neurons in the guinea‐pig. Pulmonary Pharmacology, 1, 33–39. [DOI] [PubMed] [Google Scholar]
- Fuller, R. W. , Dixon, C. M. , & Barnes, P. J. (1985). Bronchoconstrictor response to inhaled capsaicin in humans. Journal of Applied Physiology, 58, 1,080–1,084. [DOI] [PubMed] [Google Scholar]
- Fuller, R. W. , & Jackson, D. M. (1990). Physiology and treatment of cough. Thorax, 45, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller, D. E. , Konstan, M. W. , Smith, J. , Noonberg, S. B. , & Conrad, C. (2007). Novel tobramycin inhalation powder in cystic fibrosis subjects: Pharmacokinetics and safety. Pediatric Pulmonology, 42, 307–313. [DOI] [PubMed] [Google Scholar]
- Geller, D. E. , Nasr, S. Z. , Piggott, S. , He, E. , Angyalosi, G. , & Higgins, M. (2014). Tobramycin inhalation powder in cystic fibrosis patients: Response by age group. Respiratory Care, 59, 388–398. [DOI] [PubMed] [Google Scholar]
- Geller, D. E. , Weers, J. , & Heuerding, S. (2011). Development of an inhaled dry‐powder formulation of tobramycin using PulmoSphere™ technology. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 24, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, M. S. , Lieu, T. , Darby, B. , Abogadie, F. C. , Veldhuis, N. , Bunnett, N. W. , & McIntyre, P. (2014). The tyrosine kinase inhibitor bafetinib inhibits PAR2‐induced activation of TRPV4 channels in vitro and pain in vivo . British Journal of Pharmacology, 171, 3,881–3,894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, J. , Schwarz, C. , Sommerwerck, U. , Nash, E. F. , Tamm, M. , Cao, W. , … Hamed, K. (2017). Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: A crossover study in cystic fibrosis patients with pulmonary Pseudomonas aeruginosa infection. Therapeutic Advances in Respiratory Disease, 11, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gziut, M. , MacGregor, H. J. , Nevell, T. , Mason, T. , Laight, D. , & Shute, J. K. (2013). Anti‐inflammatory effects of tobramycin and a copper–tobramycin complex with superoxide dismutase‐like activity. British Journal of Pharmacology, 168, 1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton, E. , Marsden, P. , Thurston, A. , Kennedy, S. , Decalmer, S. , & Smith, J. A. (2015). Clinical features of the urge‐to‐cough in patients with chronic cough. Respiratory Medicine, 109, 701–707. [DOI] [PubMed] [Google Scholar]
- Hogg, J. (2004). Peripheral lung remodelling in asthma and chronic obstructive pulmonary disease. The European Respiratory Journal, 24, 893–894. [DOI] [PubMed] [Google Scholar]
- Hoppentocht, M. , Akkerman, O. W. , Hagedoorn, P. , Alffenaar, J. W. , van der Werf, T. S. , Kerstjens, H. A. , … de Boer, A. H. (2016). Tolerability and pharmacokinetic evaluation of inhaled dry powder tobramycin free base in non‐cystic fibrosis bronchiectasis patients. PLoS ONE, 11, e0149768 10.1371/journal.pone.0149768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtmeyers, E. , Gosselink, R. , Gayan‐Ramirez, G. , & Decramer, M. (1999). Regulation of mucociliary clearance in health and disease. The European Respiratory Journal, 13, 1,177–1,188. [DOI] [PubMed] [Google Scholar]
- Hubert, D. , Leroy, S. , Nove‐Josserand, R. , Murris‐Espin, M. , Mely, L. , Dominique, S. , … Kovarik, J. M. (2009). Pharmacokinetics and safety of tobramycin administered by the PARI eFlow® rapid nebulizer in cystic fibrosis. Journal of Cystic Fibrosis, 8, 332–337. [DOI] [PubMed] [Google Scholar]
- Huckle, A. W. , Fairclough, L. C. , & Todd, I. (2018). Prophylactic antibiotic use in COPD and the potential anti‐inflammatory activities of antibiotics. Respiratory Care, 63, 609–619. [DOI] [PubMed] [Google Scholar]
- Irwin, R. S. , French, C. L. , Chang, A. B. , Altman, K. W. , & Panel, C. E. C. (2018). Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest, 153, 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isawa, T. , Teshima, T. , Hirano, T. , Anazawa, Y. , Miki, M. , Konno, K. , & Motomiya, M. (1990). Mucociliary clearance and transport in bronchiectasis: Global and regional assessment. Journal of Nuclear Medicine, 31, 543–548. [PubMed] [Google Scholar]
- Jansat, J. M. , Lamarca, R. , de Miquel, G. , Schrodter, A. , Miletzki, B. , & Gurniak, M. (2009). Safety and pharmacokinetics of multiple doses of aclidinium bromide, a novel long‐acting muscarinic antagonist for the treatment of chronic obstructive pulmonary disease, in healthy participants. Journal of Clinical Pharmacology, 49, 1,239–1,246. [DOI] [PubMed] [Google Scholar]
- Johnstone, K. J. , Chang, A. B. , Fong, K. M. , Bowman, R. V. , & Yang, I. A. (2013). Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database of Systematic Reviews, CD009305 10.1002/14651858.CD009305.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos, G. F. , De Swert, K. O. , Schelfhout, V. , & Pauwels, R. A. (2003). The role of neural inflammation in asthma and chronic obstructive pulmonary disease. Annals of the new York Academy of Sciences, 992, 218–230. [DOI] [PubMed] [Google Scholar]
- Kamimura, M. , Izumi, S. , Hamamoto, Y. , Morita, A. , Toyota, E. , Kobayashi, N. , & Kudo, K. (2012). Superiority of nebulized corticosteroids over dry powder inhalers in certain patients with cough variant asthma or cough‐predominant asthma. Allergology International, 61, 411–417. 10.2332/allergolint.11-OA-0357 [DOI] [PubMed] [Google Scholar]
- Kanth, P. M. , Alaienia, C. , & Smaldone, G. C. (2018). Nebulized mannitol, particle distribution, and cough in idiopathic pulmonary fibrosis. Respiratory Care, 63, 1,407–1,412. [DOI] [PubMed] [Google Scholar]
- Kingman, M. , Archer‐Chicko, C. , Bartlett, M. , Beckmann, J. , Hohsfield, R. , & Lombardi, S. (2017). Management of prostacyclin side effects in adult patients with pulmonary arterial hypertension. Pulmonary Circulation, 7, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik, M. , & Undem, B. J. (2004). Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild‐type and TRPV1−/− mice. The Journal of Physiology, 555, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan, M. W. , Flume, P. A. , Kappler, M. , Chiron, R. , Higgins, M. , Brockhaus, F. , … Investigators, E. S. (2011). Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. Journal of Cystic Fibrosis, 10, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan, M. W. , Geller, D. E. , Minic, P. , Brockhaus, F. , Zhang, J. , & Angyalosi, G. (2011). Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatric Pulmonology, 46, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela, H. O. , Hyvärinen, L. , Brannan, J. D. , Chan, H.‐K. , & Anderson, S. D. (2004). Coughing during mannitol challenge is associated with asthma. Chest, 125, 1985–1992. [DOI] [PubMed] [Google Scholar]
- Koskela, H. O. , Lake, C. , Wong, K. , & Brannan, J. D. (2018). Cough sensitivity to mannitol inhalation challenge identifies subjects with chronic cough. The European Respiratory Journal, 51, 1800294 10.1183/13993003.00294-2018 [DOI] [PubMed] [Google Scholar]
- Koskela, H. O. , Purokivi, M. K. , Kontra, K. M. , Taivainen, A. H. , & Tukiainen, H. O. (2008). Hypertonic saline cough provocation test with salbutamol pre‐treatment: Evidence for sensorineural dysfunction in asthma. Clinical and Experimental Allergy, 38, 1,100–1,107. [DOI] [PubMed] [Google Scholar]
- Kubin, L. , Kimura, H. , & Davies, R. O. (1991). The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. The Journal of Physiology, 435, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, P. C. L. , Collins, R. , & Chan, H.‐K. (2006). Effect of spacers on the electrostatic charge properties of metered dose inhaler aerosols. Journal of Aerosol Science, 37, 1,671–1,682. [Google Scholar]
- Labiris, N. R. , & Dolovich, M. B. (2003). Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. British Journal of Clinical Pharmacology, 56, 588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalloo, U. G. , Lim, S. , DuBois, R. , Barnes, P. J. , & Chung, K. F. (1998). Increased sensitivity of the cough reflex in progressive systemic sclerosis patients with interstitial lung disease. The European Respiratory Journal, 11, 702. [PubMed] [Google Scholar]
- Lavorini, F. , Magnan, A. , Dubus, J. C. , Voshaar, T. , Corbetta, L. , Broeders, M. , … Crompton, G. K. (2008). Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respiratory Medicine, 102, 593–604. [DOI] [PubMed] [Google Scholar]
- Le Brun, P. P. , de Boer, A. H. , Mannes, G. P. , de Fraiture, D. M. , Brimicombe, R. W. , Touw, D. J. , … Heijerman, H. G. (2002). Dry powder inhalation of antibiotics in cystic fibrosis therapy: Part 2. Inhalation of a novel colistin dry powder formulation: A feasibility study in healthy volunteers and patients. European Journal of Pharmaceutics and Biopharmaceutics, 54, 25–32. [DOI] [PubMed] [Google Scholar]
- Lenney, W. , Edenborough, F. , Kho, P. , & Kovarik, J. M. (2011). Lung deposition of inhaled tobramycin with eFlow rapid/LC Plus jet nebuliser in healthy and cystic fibrosis subjects. Journal of Cystic Fibrosis, 10, 9–14. [DOI] [PubMed] [Google Scholar]
- Lieu, T. M. , Myers, A. C. , Meeker, S. , & Undem, B. J. (2012). TRPV1 induction in airway vagal low‐threshold mechanosensory neurons by allergen challenge and neurotrophic factors. American Journal of Physiology. Lung Cellular and Molecular Physiology, 302, L941–L948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. W. , Zhou, Q. , Onufrak, N. J. , Wirth, V. , Chen, K. , Wang, J. , … Li, J. (2017). Aerosolized polymyxin B for treatment of respiratory tract infections: Determination of pharmacokinetic–pharmacodynamic indices for aerosolized polymyxin B against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrobial Agents and Chemotherapy, 61, e00211‐17. 10.1128/AAC.00211-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry, R. H. , Wood, A. M. , & Higenbottam, T. W. (1988). Effects of pH and osmolarity on aerosol‐induced cough in normal volunteers. Clinical Science, 74, 373–376. [DOI] [PubMed] [Google Scholar]
- Martin, S. E. , Mathur, R. , Marshall, I. , & Douglas, N. J. (1997). The effect of age, sex, obesity and posture on upper airway size. The European Respiratory Journal, 10, 2087–2090. [DOI] [PubMed] [Google Scholar]
- Materazzi, S. , Nassini, R. , Gatti, R. , Trevisani, M. , & Geppetti, P. (2009). Cough sensors. II. Transient receptor potential membrane receptors on cough sensors In Chung K. F. & Widdicombe J. (Eds.), Pharmacology and Therapeutics of Cough (pp. 49–61). Berlin, Heidelberg: Springer Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- Mazurek, H. , Chiron, R. , Kucerova, T. , Geidel, C. , Bolbas, K. , Chuchalin, A. , … Antipkin, Y. G. (2014). Long‐term efficacy and safety of aerosolized tobramycin 300 mg/4 ml in cystic fibrosis. Pediatric Pulmonology, 49, 1,076–1,089. [DOI] [PubMed] [Google Scholar]
- Mazzone, S. B. , & Undem, B. J. (2016). Vagal afferent innervation of the airways in health and disease. Physiological Reviews, 96, 975–1,024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, V. V. , Benza, R. L. , Rubin, L. J. , Channick, R. N. , Voswinckel, R. , Tapson, V. F. , … Seeger, W. (2010). Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. Journal of the American College of Cardiology, 55, 1915–1922. [DOI] [PubMed] [Google Scholar]
- Midgren, B. , Hansson, L. , Karlsson, J. A. , Simonsson, B. G. , & Persson, C. G. (1992). Capsaicin‐induced cough in humans. The American Review of Respiratory Disease, 146, 347–351. [DOI] [PubMed] [Google Scholar]
- Minasian, C. , Wallis, C. , Metcalfe, C. , & Bush, A. (2008). Bronchial provocation testing with dry powder mannitol in children with cystic fibrosis. Pediatric Pulmonology, 43, 1,078–1,084. [DOI] [PubMed] [Google Scholar]
- Mitsakou, C. , Housiadas, C. , Eleftheriadis, K. , Vratolis, S. , Helmis, C. , & Asimakopoulos, D. (2007). Lung deposition of fine and ultrafine particles outdoors and indoors during a cooking event and a no activity period. Indoor Air, 17, 143–152. [DOI] [PubMed] [Google Scholar]
- Moore, K. A. , Undem, B. J. , & Weinreich, D. (2000). Antigen inhalation unmasks NK‐2 tachykinin receptor‐mediated responses in vagal afferents. American Journal of Respiratory and Critical Care Medicine, 161, 232–236. [DOI] [PubMed] [Google Scholar]
- Morice, A. H. (2004). The diagnosis and management of chronic cough. The European Respiratory Journal, 24, 481 10.1183/09031936.04.00027804 [DOI] [PubMed] [Google Scholar]
- Morice, A. H. , Faruqi, S. , Wright, C. E. , Thompson, R. , & Bland, J. M. (2011). Cough hypersensitivity syndrome: A distinct clinical entity. Lung, 189, 73–79. [DOI] [PubMed] [Google Scholar]
- Morice, A. H. , Kastelik, J. A. , & Thompson, R. (2001). Cough challenge in the assessment of cough reflex. British Journal of Clinical Pharmacology, 52, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutolo, D. , Cinelli, E. , Iovino, L. , Pantaleo, T. , & Bongianni, F. (2016). Downregulation of the cough reflex by aclidinium and tiotropium in awake and anesthetized rabbits. Pulmonary Pharmacology & Therapeutics, 38, 1–9. [DOI] [PubMed] [Google Scholar]
- Newhouse, M. T. , Hirst, P. H. , Duddu, S. P. , Walter, Y. H. , Tarara, T. E. , Clark, A. R. , & Weers, J. G. (2003). Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest, 124, 360–366. [DOI] [PubMed] [Google Scholar]
- Nurmi, H. M. , Lätti, A. M. , Brannan, J. D. , & Koskela, H. O. (2019). Comparison of mannitol and citric acid cough provocation tests. Respiratory Medicine, 158, 14–20. [DOI] [PubMed] [Google Scholar]
- O'Byrne, P. M. , Ryan, G. , Morris, M. , McCormack, D. , Jones, N. L. , Morse, J. L. C. , & Hargreave, F. E. (1982). Asthma induced by cold air and its relation to nonspecific bronchial responsiveness to methacholine. American Journal of Respiratory and Critical Care Medicine, 125, 281–285. [DOI] [PubMed] [Google Scholar]
- Otterson, G. A. , Villalona‐Calero, M. A. , Sharma, S. , Kris, M. G. , Imondi, A. , Gerber, M. , … Murren, J. R. (2007). Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clinical Cancer Research, 13, 1,246–1,252. [DOI] [PubMed] [Google Scholar]
- Patapoutian, A. , Tate, S. , & Woolf, C. J. (2009). Transient receptor potential channels: Targeting pain at the source. Nature Reviews. Drug Discovery, 8, 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcer, G. , & Amighi, K. (2010). Formulation strategy and use of excipients in pulmonary drug delivery. International Journal of Pharmaceutics, 392, 1–19. [DOI] [PubMed] [Google Scholar]
- Polverino, M. , Polverino, F. , Fasolino, M. , Andò, F. , Alfieri, A. , & De Blasio, F. (2012). Anatomy and neuro‐pathophysiology of the cough reflex arc. Multidiscip Respir Med, 7, 5 10.1186/2049-6958-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purokivi, M. , Koskela, H. , & Kontra, K. (2013). Determinants of asthma control and quality of life in stable asthma: Evaluation of two new cough provocation tests. The Clinical Respiratory Journal, 7, 253–260. [DOI] [PubMed] [Google Scholar]
- Quattrin, T. , Belanger, A. , Bohannon, N. J. , Schwartz, S. L. , & Exubera Phase, I. I. I. S. G. (2004). Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: Results of a 6‐month, randomized, comparative trial. Diabetes Care, 27, 2,622–2,627. [DOI] [PubMed] [Google Scholar]
- Richards, R. , Haas, A. , Simpson, S. , Britten, A. , Renwick, A. , & Holgate, S. (1988). Effect of methacholine induced bronchoconstriction on the pulmonary distribution and plasma pharmacokinetics of inhaled sodium cromoglycate in subjects with normal and hyperreactive airways. Thorax, 43, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock, J. , Franco, D. , Korpachev, V. , Shumel, B. , Ma, Y. , Baughman, R. , … Affinity 2 Study G . (2015). Inhaled technosphere insulin versus inhaled technosphere placebo in insulin‐naive subjects with type 2 diabetes inadequately controlled on oral antidiabetes agents. Diabetes Care, 38, 2,274–2,281. [DOI] [PubMed] [Google Scholar]
- Ryerson, C. J. , Abbritti, M. , Ley, B. , Elicker, B. M. , Jones, K. D. , & Collard, H. R. (2011). Cough predicts prognosis in idiopathic pulmonary fibrosis. Respirology, 16, 969–975. [DOI] [PubMed] [Google Scholar]
- Satia, I. , Badri, H. , Al‐Sheklly, B. , Smith, J. A. , & Woodcock, A. A. (2016). Towards understanding and managing chronic cough. Clinical Medicine, 16, s92–s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg, P. , & Shore, E. (2005). A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest, 127, 1,420–1,426. [DOI] [PubMed] [Google Scholar]
- Schuster, A. , Haliburn, C. , Doring, G. , & Goldman, M. H. (2013). Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax, 68, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, C. , & Williams, M. H. Jr. (1987a). Cough and wheezing from beclomethasone aerosol. Chest, 91, 207–209. [DOI] [PubMed] [Google Scholar]
- Shim, C. S. , & Williams, M. H. Jr. (1987b). Cough and wheezing from beclomethasone dipropionate aerosol are absent after triamcinolone acetonide. Annals of Internal Medicine, 106, 700–703. [DOI] [PubMed] [Google Scholar]
- Smaldone, G. C. , & Bergofsky, E. H. (1976). Delineation of flow‐limiting segment and predicted airway resistance by movable catheter. Journal of Applied Physiology, 40, 943–952. [DOI] [PubMed] [Google Scholar]
- Smaldone, G. C. , Foster, W. M. , O'Riordan, T. G. , Messina, M. S. , Perry, R. J. , & Langenback, E. G. (1993). Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest, 103, 1,390–1,396. [DOI] [PubMed] [Google Scholar]
- Smaldone, G. C. , Itoh, H. , Swift, D. L. , & Wagner, H. N. Jr. (1979). Effect of flow‐limiting segments and cough on particle deposition and mucociliary clearance in the lung. The American Review of Respiratory Disease, 120, 747–758. [DOI] [PubMed] [Google Scholar]
- Smaldone, G. C. , & Messina, M. S. (1985). Flow limitation, cough, and patterns of aerosol deposition in humans. Journal of Applied Physiology, 59, 515–520. [DOI] [PubMed] [Google Scholar]
- Sommerwerck, U. , Virella‐Lowell, I. , Angyalosi, G. , Viegas, A. , Cao, W. , & Debonnett, L. (2016). Long‐term safety of tobramycin inhalation powder in patients with cystic fibrosis: Phase IV (ETOILES) study. Current Medical Research and Opinion, 32, 1789–1795. [DOI] [PubMed] [Google Scholar]
- Sterling, G. M. , & Batten, J. C. (1969). Effect of aerosol propellants and surfactants on airway resistance. Thorax, 24, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, R. H. , McFadden, E. , Ingram, R. , Deal, E. , & Jaeger, J. (1978). Influence of heat and humidity on the airway obstruction induced by exercise in asthma. The Journal of Clinical Investigation, 61, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternesten‐Hasseus, E. , Johansson, E. L. , & Millqvist, E. (2015). Cough reduction using capsaicin. Respiratory Medicine, 109, 27–37. [DOI] [PubMed] [Google Scholar]
- van den Borst, B. , Gosker, H. R. , Zeegers, M. P. , & Schols, A. M. (2010). Pulmonary function in diabetes: A metaanalysis. Chest, 138, 393–406. [DOI] [PubMed] [Google Scholar]
- Velkov, T. , Abdul Rahim, N. , Zhou, Q. T. , Chan, H.‐K. , & Li, J. (2015). Inhaled anti‐infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Advanced Drug Delivery Reviews, 85, 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinchurkar, S. , Backer, L. D. , Vos, W. , Holsbeke, C. V. , Backer, J. D. , & Backer, W. D. (2012). A case series on lung deposition analysis of inhaled medication using functional imaging based computational fluid dynamics in asthmatic patients: Effect of upper airway morphology and comparison with in vivo data. Inhalation Toxicology, 24, 81–88. [DOI] [PubMed] [Google Scholar]
- Westerman, E. M. , De Boer, A. H. , Le Brun, P. P. H. , Touw, D. J. , Roldaan, A. C. , Frijlink, H. W. , & Heijerman, H. G. M. (2007). Dry powder inhalation of colistin in cystic fibrosis patients: A single dose pilot study. Journal of Cystic Fibrosis, 6, 284–292. [DOI] [PubMed] [Google Scholar]
- Westerman, E. M. , Le Brun, P. P. H. , Touw, D. J. , Frijlink, H. W. , & Heijerman, H. G. M. (2004). Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study. Journal of Cystic Fibrosis, 3, 23–28. [DOI] [PubMed] [Google Scholar]
- Wex, E. , & Bouyssou, T. (2015). Olodaterol attenuates citric acid‐induced cough in naive and ovalbumin‐sensitized and challenged guinea pigs. PLoS ONE, 10, e0119953 10.1371/journal.pone.0119953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe, J. G. (1995). Neurophysiology of the cough reflex. The European Respiratory Journal, 8, 1193. [DOI] [PubMed] [Google Scholar]
- Williamson, I. J. , Matusiewicz, S. P. , Brown, P. H. , Greening, A. P. , & Crompton, G. K. (1995). Frequency of voice problems and cough in patients using pressurized aerosol inhaled steroid preparations. The European Respiratory Journal, 8, 590–592. [PubMed] [Google Scholar]
- Wong, C. H. , & Morice, A. H. (1999). Cough threshold in patients with chronic obstructive pulmonary disease. Thorax, 54, 62–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. Y. , Chan, J. G. Y. , & Chan, H.‐K. (2014). Pulmonary drug delivery by powder aerosols. Journal of Controlled Release, 193, 228–240. [DOI] [PubMed] [Google Scholar]
- Zhang, G. , Lin, R.‐L. , Wiggers, M. , Snow, D. M. , & Lee, L.‐Y. (2008). Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. The Journal of Physiology, 586, 5,771–5,786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. T. , Leung, S. S. , Tang, P. , Parumasivam, T. , Loh, Z. H. , & Chan, H. K. (2015). Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced Drug Delivery Reviews, 85, 83–99. [DOI] [PubMed] [Google Scholar]
- Zwozdziaka, A. , Gini, M. , & Samek, L. (2017). Implications of the aerosol size distribution modal structure of trace and major elements on human exposure, inhaled dose and relevance to the PM2.5 and PM10 metrics in a European pollution hotspot urban area. Journal of Aerosol Science, 103, 38–52. [Google Scholar]


