Abstract
Background and Purpose
Extracts from the cannabis plant can dramatically improve the health of children suffering from refractory epilepsies such as Dravet syndrome. These extracts typically contain cannabidiol (CBD), a phytocannabinoid with well‐documented anticonvulsant effects, but may also contain Δ9‐tetrahydrocannabinol (Δ9‐THC). It is unclear whether the presence of Δ9‐THC modulates the anticonvulsant efficacy of CBD. Here, we utilized the Scn1a +/− mouse model of Dravet syndrome to examine this question.
Experimental Approach
Scn1a +/− mice recapitulate core features of Dravet syndrome, including hyperthermia‐induced seizures, early onset spontaneous seizures and sudden death. We assessed the effects on CBD and Δ9‐THC alone, and in combination on hyperthermia‐induced seizures, spontaneous seizures and premature mortality.
Key Results
Administered alone, CBD (100 mg·kg−1 i.p.) was anticonvulsant against hyperthermia‐induced seizures as were low (0.1 and 0.3 mg·kg−1 i.p.) but not higher doses of Δ9‐THC. A subthreshold dose of CBD (12 mg·kg−1) enhanced the anticonvulsant effects of Δ9‐THC (0.1 mg·kg−1). Sub‐chronic oral administration of Δ9‐THC or CBD alone did not affect spontaneous seizure frequency or mortality while, surprisingly, their co‐administration increased the severity of spontaneous seizures and overall mortality.
Conclusion and Implications
Low doses of Δ9‐THC are anticonvulsant against hyperthermia‐induced seizures in Scn1a +/− mice, effects that are enhanced by a sub‐anticonvulsant dose of CBD. However, proconvulsant effects and increased premature mortality are observed when CBD and Δ9‐THC are sub‐chronically dosed in combination. The possible explanations and implications of this are discussed.
Abbreviations
- CBD
cannabidiol
- GTCS
generalized tonic–clonic seizure
- Δ9‐THC
Δ9‐tetrahydrocannabinol
What is already known
Cannabis extracts are reported to reduce seizures and improve the health of Dravet syndrome patients.
Extracts often contain Δ9‐THC in addition to CBD, which has well‐documented anticonvulsant effects.
What this study adds
The anticonvulsant potential of Δ9‐THC, alone and in combination with CBD, for Dravet syndrome was assessed.
What is the clinical significance
Low doses of Δ9‐THC are anticonvulsant against thermally induced seizures in Scn1a +/− mice.
Combination Δ9‐THC and CBD treatment has augmented anticonvulsant and proconvulsant effects depending on seizure type.
1. INTRODUCTION
Cannabis extracts have been used for millennia to treat epilepsy. These positive therapeutic effects are increasingly validated by well‐publicized cases of cannabis extracts dramatically improving the health of children with refractory epilepsies (Perucca, 2018). Epidyolex™, a purified preparation of the phytocannabinoid cannabidiol (CBD), was recently approved in the United States and Europe for the treatment of intractable childhood epilepsies following a series of successful Phase III clinical trials (Devinsky et al., 2017; Devinsky et al., 2018; Greenwood et al., 2018; Thiele et al., 2018). Despite the availability of Epidyolex™, many childhood epilepsy patients continue to be treated with unregulated medicinal cannabis products, often in the form of artisanal oils extracted from the cannabis plant (Huntsman et al., 2019; McCoy et al., 2018; Suraev et al., 2018; Tzadok et al., 2016; Wang et al., 2020).
These artisanal oils contain a wide variety of different phytocannabinoids at different concentrations. A common profile is one involving CBD together with low concentrations of Δ9‐tetrahydrocannabinol (Δ9‐THC) (Suraev et al., 2018). While CBD has clear anticonvulsant properties, it is unclear whether the presence of Δ9‐THC might confer additional anticonvulsant efficacy or, conversely, interfere with these therapeutic effects of CBD. Pharmacological interactions between CBD and Δ9‐THC are well documented and many medicinal cannabis products contain both phytocannabinoids (Boggs, Nguyen, Morgenson, Taffe, & Ranganathan, 2018; Boggs, Peckham, Boggs, & Ranganathan, 2016; Silveira et al., 2017). CBD and Δ9‐THC can interact synergistically to improve therapeutic outcomes in some animal models of disease (Casey, Atwal, & Vaughan, 2017; Samarut, Nixon, Kundap, Drapeau, & Ellis, 2019) and CBD has sometimes been found to reduce sedative, anxiogenic, and other adverse side effects of Δ9‐THC (Freeman et al., 2019; Todd & Arnold, 2016; Vann et al., 2008). In contrast, in some human (Arkell et al., 2019) and animal studies (Klein et al., 2011) CBD has been found to potentiate THC‐induced adverse effects. Whether CBD and Δ9‐THC interact to affect therapeutic outcomes within the context of epilepsy remains largely unexplored.
In previous studies involving conventional rodent seizure models, Δ9‐THC exhibited anticonvulsant effects (Chesher & Jackson, 1974; Colasanti, Lindamood, & Craig, 1982; Consroe & Wolkin, 1977; Lindamood & Colasanti, 1980; Sofia, Solomon, & Barry, 1976; Wallace, Wiley, Martin, & DeLorenzo, 2001). However, Δ9‐THC has also exhibited proconvulsant effects in mice and rats in some studies (Chan, Sills, Braun, Haseman, & Bucher, 1996; Sofia et al., 1976; Whalley et al., 2019). There is currently a lack of knowledge concerning the effects of Δ9‐THC in refractory epilepsies such as Dravet syndrome, a rare paediatric encephalopathy that is resistant to pharmacological therapies (Dravet, 2011; Dravet & Oguni, 2013; Shmuely, Sisodiya, Gunning, Sander, & Thijs, 2016). Here, we examined this issue using a mouse model of Dravet syndrome. Over 80% of Dravet syndrome patients have a de novo mutation in the SCN1A gene, which encodes the voltage‐gated sodium channel Nav1.1 (Marini et al., 2011). Heterozygous deletion of Scn1a in mice recapitulates the core features of Dravet syndrome, such as susceptibility to thermally induced seizures, development of early onset spontaneous seizures and premature mortality (Anderson, Absalom, et al., 2019; Anderson, Low, Banister, McGregor, & Arnold, 2019; Hawkins et al., 2017). The Scn1a +/− mouse model also has predictive validity, as first‐line treatments clobazam and valproic acid, as well as CBD are anticonvulsant in these mice (Anderson, Absalom, et al., 2019; Hawkins et al., 2017; Kaplan, Stella, Catterall, & Westenbroek, 2017).
In the current study, we first examined the anticonvulsant potential of Δ9‐THC and then confirmed the anticonvulsant effects of CBD in Scn1a +/− mice. We then sought to explore whether the combined administration of Δ9‐THC and CBD would lead to greater anticonvulsant effects than either phytocannabinoid administered alone.
2. METHODS
2.1. Overview of anticonvulsant screening pipeline for Scn1a +/− mice
The effects of CBD and Δ9‐THC treatments, alone and in combination, were examined on hyperthermia‐induced seizures, spontaneous seizures and mortality in Scn1a +/− mice. For hyperthermia‐induced seizure experiments, the cannabinoids were administered acutely via intraperitoneal (i.p.) injection at postnatal day 14–16 (P14–16) and mice were then subjected to a hyperthermia‐induced seizure as described in detail below (Figure 1a). Immediately following this procedure mice were euthanised and plasma and brain samples were collected from a cohort of mice.
FIGURE 1.
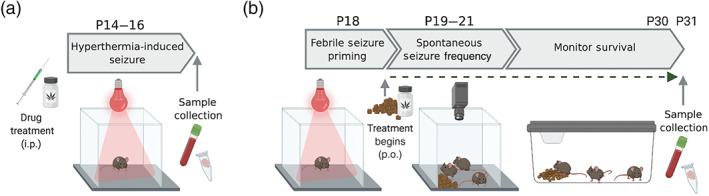
Schematic overview of the anticonvulsant screening pipeline in Scn1a +/− mice. (a) Hyperthermia‐induced seizure experiments. (b) Spontaneous seizure and survival experimental design. Figure created using BioRender.com
For spontaneous seizure and survival experiments, Scn1a +/− mice were subjected to a hyperthermia‐induced seizure at P18 (Figure 1b). Cannabinoid treatment commenced immediately after this via oral delivery through supplementation in mouse chow and continued until P31. The mice were monitored for spontaneous seizure frequency at P19–21, before being returned to their home cages where survival was monitored to P30. On P31, plasma and brain samples were collected from a cohort of mice. Detailed protocols for these experiments are presented below.
2.2. Drugs
For acute administration, solutions of CBD and Δ9‐THC (THC Pharm; Frankfurt, Germany) were freshly prepared each day in ethanol:Tween‐80:saline (1:1:18), a commonly used vehicle in many cannabinoid pharmacological studies in rodents. All doses were completely soluble in this vehicle except for the 100 mg·kg−1 dose of CBD, which was a milky suspension. Cannabinoids were administered as an intraperitoneal injection in a volume of 10 ml·kg−1.
For spontaneous seizure and survival experiments, CBD and Δ9‐THC were administered sub‐chronically through supplementation in chow. This was done to avoid the stress associated with repeated intraperitoneal injections or repeated oral gavage, which might exacerbate seizures in Scn1a +/− mice. Drugs were prepared in a variety of formulations in‐house using R&M Standard Diet irradiated powder (Specialty Feeds; Glen Forrest, Australia). The chow was formulated to provide target plasma concentrations (see Section 3) with the daily oral dose for each mouse based on the assumption of mice consuming 3.5–4 g chow·day−1.
The formulations used across different experiments were as follows with daily dosage estimates shown in parentheses: 3,500 mg CBD·kg−1 chow (500 mg CBD·kg−1·day−1), 7,000 mg CBD·kg−1 chow (1,000 mg CBD·kg−1·day−1), 70 mg Δ9‐THC·kg−1 chow (10 mg Δ9‐THC·kg−1·day−1), 200 mg Δ9‐THC·kg−1 chow (28.5 mg Δ9‐THC·kg−1·day−1), 35 mg Δ9‐THC + 900 mg CBD·kg−1 chow (5 mg Δ9‐THC + 130 mg CBD·kg−1·day−1) and 70 mg Δ9‐THC + 3,500 mg CBD·kg−1 chow (10 mg Δ9‐THC + 500 mg CBD·kg−1·day−1).
2.3. Animals
All animal care and experimental procedures were approved by the University of Sydney Animal Ethics Committee (protocols 2016/1035 and 2017/1292), and all procedures were in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Scn1a +/− mice were purchased from The Jackson Laboratory (stock 37107‐JAX; Bar Harbor, MA, USA) and maintained as a congenic line on the 129S6/SvEvTac background (129.Scn1a +/−). For experiments, F1 mice were generated by breeding heterozygous 129.Scn1a +/− mice with wild‐type C57BL/6J mice (Jackson Laboratory stock 000664; Animal Resources Centre; Canning Vale, Australia) mice. The Scn1a genotype was determined as previously described (Miller, Hawkins, McCollom, & Kearney, 2014). Mice were group‐housed in a specific pathogen‐free mouse facility under standard laboratory conditions (12 h light/12 h dark cycle) with ad libitum access to food and water.
2.4. Hyperthermia‐induced seizures
Seizures in children with Dravet syndrome are often provoked by fever. Scn1a +/− mice replicate this phenotype by exhibiting a generalized tonic–clonic seizure (GTCS) in response to elevated body temperature. Hyperthermia‐induced seizure experiments were conducted on male and female Scn1a +/− mice at P14–16 as previously described (Anderson, Absalom, et al., 2019; Hawkins et al., 2017) (Figure 1a). Briefly, mice were randomly injected intraperitoneally with vehicle, CBD (12–100 mg·kg−1) and/or Δ9‐THC (0.01–10 mg·kg−1) by a researcher blinded to treatment before being returned to their home cage. Fifteen minutes prior to the target experimental (post‐dose) time point, a RET‐3 rectal temperature probe was inserted and mice acclimated to the temperature probe for 5 min. Mouse core body temperature was then elevated 0.5°C every 2 min until onset of the first clonic convulsion with loss of posture or until 42.5°C was reached. Mice that reached 42.5°C were held at temperature for 3 min and were considered seizure‐free if no seizure occurred during the hold. Following the hyperthermia‐induced seizure protocol, a cohort of mice (n = 5–11 per treatment) were anaesthetized with isoflurane and whole blood was collected via cardiac puncture and brains were harvested. Plasma was isolated by centrifugation (9,000 g for 10 min, 4°C). Plasma and brain samples were stored at −80°C until assayed.
Experimental time points were 30 min for Δ9‐THC and 60 min for CBD, these were based on previously determined time‐to‐peak plasma and brain concentrations (Deiana et al., 2012; Spiro, Wong, Boucher, & Arnold, 2012). Matched vehicle controls were run for each experimental time point. No statistical difference was identified between the two time points, so all vehicle‐treated mice were combined into one group for the combination hyperthermia‐induced seizure experiments.
Seizure threshold temperatures were compared across conditions using Mantel–Cox log‐rank test from GraphPad Prism 7.0 (La Jolla, USA), and P < 0.05 was considered statistically significant.
2.5. Frequency and severity of spontaneous seizures
Dravet syndrome typically presents with febrile seizures that later progress to spontaneous afebrile GTCS. We prime Scn1a +/− mice with a hyperthermia‐induced seizure to model this seizure progression. Male and female Scn1a +/− mice were exposed to a single hyperthermia‐induced seizure event at P18 as described previously (Anderson, Absalom, et al., 2019; Hawkins et al., 2017) (Figure 1b). Following the thermally induced seizure, mice were randomly assigned to drug treatment groups (untreated, CBD, Δ9‐THC, or CBD + Δ9‐THC), with n = 16–27 per treatment. Drugs were administered orally through supplementation in chow as described above. Continuous video recordings from 12:00 on P19 through 24:00 on P21 were made using a day/night camera with an IR lens (Samsung SCB5003, Tamron 13F604IRSQ). The number of spontaneous GTCS during this 60 h recording session was quantified offline by an observer blinded to treatment. The severity of each spontaneous GTCS was scored via assessment of whether there was progression to full hindlimb extension (hindlimbs at 180° angle to the torso), which is the most severe stage of GTCS. Statistical comparisons were made with GraphPad Prism using Fisher's exact test (proportion of mice seizure‐free and hindlimb seizures) or one‐way ANOVA followed by Bonferroni's post hoc (seizure frequency) and P < 0.05 was considered statistically significant. Post hoc analyses were conducted only when F in ANOVA achieved P < 0.05 and there was no significant variance in homogeneity. Sample sizes subjected to statistical analysis had at least 5 samples per group (n = 5), where n = number or independent values.
2.6. Survival analysis
Mice continued drug treatment to P30 to monitor survival. Scn1a +/− mice have a mortality rate of approximately 50% to P30. This recapitulates the “Sudden Death in Epilepsy” (SUDEP) phenomenon in children with Dravet syndrome, where up to 21% die before reaching adulthood (Shmuely et al., 2016). To examine whether treatment with Δ9‐THC and/or CBD affected this outcome, we continue to administer drug treatment in lab chow following testing for spontaneous seizures until P30 while monitoring survival of the mice. Plasma and brain samples were isolated as described above on P31 within 30 min of lights on and stored at −80°C until assayed. Statistical comparisons of survival were made with GraphPad Prism using log‐rank Mantel–Cox and P < 0.05 was considered statistically significant. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.7. Analytical chemistry
Cannabinoid concentrations in plasma and brain were assayed by LC–MS/MS based on previously described methods with modifications (Anderson, Absalom, et al., 2019; Suraev et al., 2018). Analytical standards were purchased from Cerilliant Corporation (Round Rock, TX, USA). Plasma samples (50 μl) were spiked with internal standard (2 μg·ml−1 diazepam, 5 μl), and extraction was achieved by vortex‐mixing with 4× volume of acetonitrile. The organic layer was isolated by centrifugation (4,000 g for 10 min, 4°C) and evaporated to dryness with N2(g). Samples were reconstituted in methanol and 0.1% formic acid in water (150:250, v/v) for solid–liquid extraction with methyl tert‐butyl ether using Biotage Isolute SLE+ columns (Uppsala, Sweden). Samples were evaporated to dryness with N2(g) and reconstituted in acetonitrile and 0.1% formic acid in water (1:1.5, v/v) for analysis.
Brain samples were prepared by homogenizing a half‐brain in 5× volume of acetonitrile. Homogenates were centrifuged (20,000 g for 30 min, 4°C) and brain supernatants (500 μl) were spiked with internal standard (10 μg·ml−1 diazepam, 15 μl). Extraction was achieved by vortex‐mixing with a 3× volume of ice‐cold acetonitrile. The organic layer was isolated by centrifugation (20,000 g for 15 min, 4°C). Supernatants were filtered through Amicon Ultracel‐3K (Merck‐Millipore; Burlington, USA) filtration devices. Filtrates were evaporated to dryness with N2(g). Samples were reconstituted in acetonitrile (90 μl) and 0.1% formic acid in water (300 μl) for solid–liquid extraction with methyl tert‐butyl ether using Biotage Isolute SLE+ columns. Samples were evaporated to dryness with N2(g) and reconstituted in acetonitrile and 0.1% formic acid in water (1:1, v/v) for analysis.
Samples were assayed by LC–MS/MS using a Shimadzu Nexera ultra‐HPLC coupled to a Shimadzu 8030 triple quadrupole mass spectrometer (Shimadzu Corp.; Kyoto, Japan). The mass spectrometer was operated in positive electrospray ionization mode with multiple reaction monitoring with the following mass transition pairs: m/z 315.3 > 193.15, 315.3 > 135.1 (CBD); 315.15 > 193.15, 315.15 > 259.2 (Δ9‐THC) and 284.6 > 257.0, 284.6 > 220.0 (diazepam). Quantification was achieved by comparing experimental samples to standards prepared with known amounts of drug. Limit of quantification for Δ9‐THC was 0.1 ng·ml−1 and 0.005 ng·mg−1 brain in plasma and brain samples, respectively.
CBD and Δ9‐THC concentrations were measured numerical values, which were not influenced by observer bias, so blinding was not considered necessary. Statistical comparisons of plasma and brain concentrations were made with GraphPad Prism using Student's t‐test or one‐way ANOVA followed by Tukey's post hoc, and P < 0.05 was considered statistically significant. Post hoc analyses were conducted only in F in ANOVA achieved P < 0.05 and there was no significant variance in homogeneity. Sample sizes subjected to statistical analysis had at least 5 samples per group (n = 5), where n = number or independent values.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Mathie, et al., 2019).
3. RESULTS
3.1. Experiment 1: Δ9‐THC treatment
3.1.1. Effect of Δ9‐THC on hyperthermia‐induced seizures
We evaluated the dose effect of a single intraperitoneal injection of Δ9‐THC against hyperthermia‐induced seizures in Scn1a +/− mice. Low‐dose Δ9‐THC was anticonvulsant against hyperthermia‐induced seizures (Figure 2a), with treatment at 0.1 and 0.3 mg·kg−1 Δ9‐THC significantly increasing the GTCS temperature threshold.
FIGURE 2.
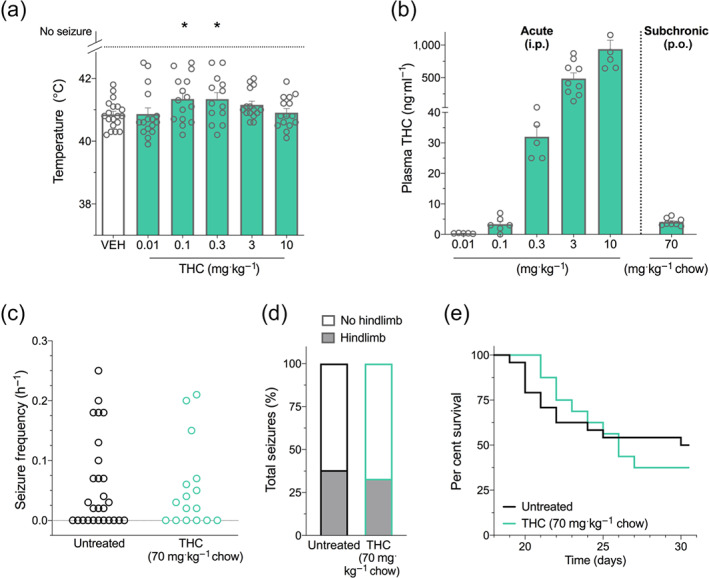
Δ9‐THC treatment in Scn1a +/− mice. (a) Threshold temperature of individual mice for generalized tonic–clonic seizure (GTCS) induced by hyperthermia following acute intraperitoneal treatment with vehicle (VEH) or varying doses of THC (green bars). THC (0.1 and 0.3 mg·kg−1) significantly increased the temperature threshold for hyperthermia‐induced seizures. The average temperatures of seizure induction are depicted by the bars, and error bars represent SEM, with n = 13–19 per group (* P < 0.05; log‐rank Mantel–Cox). (b) Plasma concentrations of THC from individual experimental animals. THC plasma concentrations measured following acute intraperitoneal (i.p.) administration of THC in Scn1a +/− mice used in hyperthermia‐induced seizure experiments or following subchronic oral (p.o.) administration of 70 mg THC·kg−1 chow. The average plasma THC concentrations are depicted by the bars, and error bars represent SEM, with n = 5–9 per group. (c) GTCS frequency of individual untreated and THC‐treated mice. Drug treatment administered orally through supplementation in chow was initiated following the induction of a single thermally induced seizure. Unprovoked, spontaneous GTCSs were quantified over a 60 h recording period. THC treatment had no effect on incidence or frequency of seizures, with n = 16–27 per group (Fisher's exact text and one‐way ANOVA followed by Bonferroni's post hoc, respectively). (d) Proportion of spontaneous GTCS with (grey bars) or without (white bars) full tonic hindlimb extension is depicted. Seizure severity was not affected by THC treatment (Fisher's exact test). Total number of spontaneous GTCS was 71 (untreated) and 45 (THC). (e) Survival curves comparing untreated and THC‐treated mice. Treatment began at postnatal day 18 (P18), and survival was monitored until P30. Survival of Scn1a +/− mice was not affected by THC treatment, with n = 16–27 per group (log‐rank Mantel–Cox)
3.1.2. Plasma Δ9‐THC concentrations
The plasma Δ9‐THC concentrations found in Δ9‐THC‐treated Scn1a +/− mice in hyperthermia‐induced seizure experiments are shown in Figure 2b. The 0.1 mg·kg−1 dose of Δ9‐THC resulted in a plasma concentration of 3 ± 1 ng·ml−1. This approximates peak plasma Δ9‐THC concentrations measured in childhood epilepsy patients taking CBD‐dominant cannabis oil preparations (range: 0.8–3.6 ng·ml−1) (Huntsman et al., 2019; Wang et al., 2020). In order to assess the effect of Δ9‐THC on spontaneous seizures and mortality of Scn1a +/− mice, Δ9‐THC was administered sub‐chronically through supplementation in chow. We formulated mouse chow with 70 mg Δ9‐THC·kg−1 chow, which resulted in similar mean plasma concentrations (4 ± 1 ng·ml−1) to that achieved with acute administration of 0.1 mg·kg−1 Δ9‐THC, a dose that had anticonvulsant effects against hyperthermia‐induced seizures (Figure 2b).
3.1.3. Spontaneous seizures during sub‐chronic Δ9‐THC treatment
Treatment with Δ9‐THC delivered orally through supplementation in chow had no effect on the proportion of mice that experienced spontaneous seizures and did not affect spontaneous seizure frequency (Figure 2c). Additionally, Δ9‐THC treatment did not affect the severity of spontaneous seizures as gauged by the proportion of seizures that advanced to tonic hindlimb extension (Figure 2d).
3.1.4. Survival with sub‐chronic Δ9‐THC treatment
Additionally, Dravet syndrome patients have poor long‐term survival and Scn1a +/− mice also exhibit premature mortality (Hawkins et al., 2017: Shmuely et al., 2016). Survival of Scn1a +/− mice was not affected by Δ9‐THC treatment (Figure 2e).
3.2. Experiment 2: CBD treatment
3.2.1. Effects of CBD on hyperthermia‐induced seizures
Replicating previous results, CBD was anticonvulsant against hyperthermia‐induced seizures at 100 mg·kg−1 i.p. but not 12 and 25 mg·kg−1 (Figure 3a) (Anderson, Absalom, et al., 2019; Kaplan et al., 2017).
FIGURE 3.
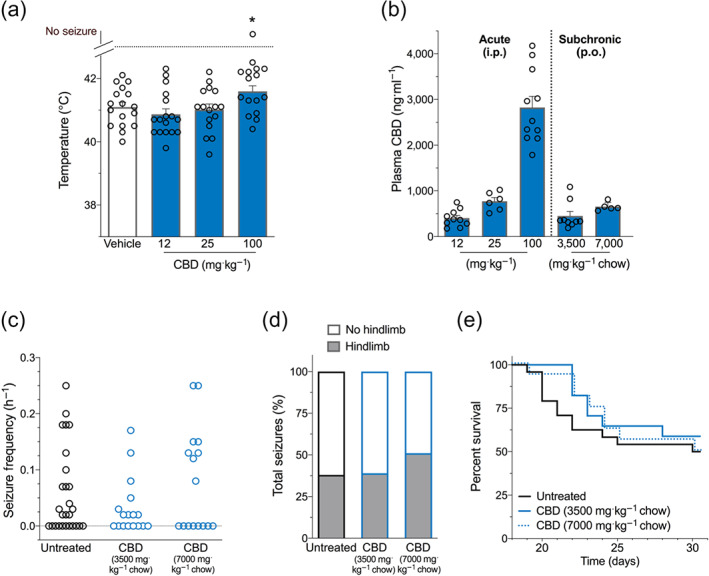
CBD treatment in Scn1a +/− mice. (a) Threshold temperature of individual mice for generalized tonic–clonic seizure (GTCS) induced by hyperthermia following acute intraperitoneal treatment with varying doses of CBD (blue bars). CBD (100 mg·kg−1) significantly increased the temperature threshold for hyperthermia‐induced seizures. The average temperatures of seizure induction are depicted by the bars, and error bars represent SEM, with n = 16–17 per group (* P < 0.05; log‐rank Mantel–Cox). (b) Plasma concentrations of CBD from individual experimental animals. CBD plasma concentrations measured following acute intraperitoneal (i.p.) administration of CBD in Scn1a +/− mice used in hyperthermia‐induced seizure experiments or following subchronic oral (p.o.) administration of 3,500 mg CBD or 7,000 mg CBD·kg−1 chow. The average plasma CBD concentrations are depicted by the bars and error bars represent SEM, with n = 5–11 per group. (c) Generalized tonic–clonic seizure (GTCS) frequency of individual untreated and CBD‐treated mice. Drug treatment administered orally through supplementation in chow was initiated following the induction of a single thermally induced seizure. Unprovoked, spontaneous GTCSs were quantified over a 60 h recording period. Neither CBD treatment had any effect on incidence or frequency of seizures, with n = 16–27 per group (Fisher's exact text and one‐way ANOVA followed by Bonferroni's post hoc, respectively). (d) Proportion of spontaneous GTCS with (grey bars) or without (white bars) full tonic hindlimb extension is depicted. Seizure severity was not affected by either CBD treatment (Fisher's exact test). Total number of spontaneous GTCS was 71 (untreated), 28 (3,500 mg CBD·kg−1 chow), and 63 (7,000 mg CBD·kg−1 chow). (e) Survival curves comparing untreated and CBD‐treated mice. Treatment began at postnatal day 18 (P18), and survival was monitored until P30. Survival of Scn1a +/− mice was not affected by CBD treatments, with n = 16–27 per group (log‐rank Mantel–Cox) (Note that untreated mice are replotted from Figure 2 for clarity.)
3.2.2. Plasma CBD concentrations
Plasma concentrations of CBD in hyperthermia‐induced seizure experimental animals treated acutely with CBD are shown in Figure 3b. The 12 and 25 mg·kg−1 doses of CBD resulted in plasma concentrations of 402 ± 59 and 771 ± 82 ng·ml−1, respectively, consistent with CBD plasma concentrations observed in patients with intractable epilepsy, who are treated with Epidyolex (mean: 450 ng·ml−1, range: 100–800 ng·ml−1) (Geffrey, Pollack, Bruno, & Thiele, 2015). Plasma concentrations were 2,826 ± 242 ng·ml−1 following the 100 mg·kg−1 CBD dose.
We then formulated mouse chow containing CBD to assess its effect on spontaneous seizures and survival of Scn1a +/− mice. The two doses of sub‐chronic, oral CBD used (3,500 mg·kg−1 chow and 7,000 mg·kg−1 chow) resulted in steady‐state plasma concentrations of 450 ± 94 and 645 ± 49 ng·ml−1, respectively (Figure 3b), mirroring plasma concentrations observed following acute administration of 12 and 25 mg·kg−1 CBD and consistent with CBD plasma concentrations observed in patients (Geffrey et al., 2015). Since mice have a taste aversion to chow containing higher amounts of CBD, we were unable to achieve plasma concentrations equivalent to the 100 mg·kg−1 acute intraperitoneal dose.
3.2.3. Spontaneous seizures during sub‐chronic CBD treatment
CBD treatment delivered sub‐chronically had no effect on the proportion of Scn1a +/− mice exhibiting spontaneous seizures at a dose of either 3,500 mg CBD·kg−1 chow or 7,000 mg CBD·kg−1 chow. There was no effect of either dose on seizure frequency (Figure 3c). Additionally, neither CBD treatment modified the severity of spontaneous seizures with the proportion of hindlimb seizures observed with CBD treatment not differing from untreated controls (Figure 3d).
3.2.4. Survival with sub‐chronic CBD treatment
Both CBD chow formulations had no effect on survival of Scn1a +/− mice compared to untreated controls (Figure 3e).
3.3. Experiment 3: Combination Δ9‐THC and CBD treatment
3.3.1. Effects of combination treatment on hyperthermia‐induced seizures
Co‐administration of an anticonvulsant dose of Δ9‐THC (0.1 mg·kg−1) and a subthreshold dose of CBD (12 mg·kg−1) significantly increased the temperature threshold for hyperthermia‐induced seizures compared to either treatment alone (Figure 4a). When Δ9‐THC (0.1 mg·kg−1) was co‐administered with 25 or 100 mg·kg−1 CBD, no additional anticonvulsant effects were observed relative to equivalent monotherapies (Figure S1).
FIGURE 4.
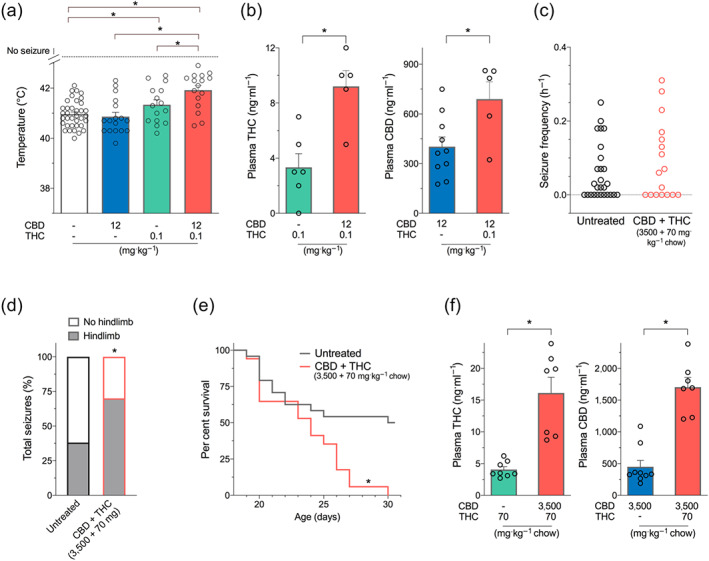
Combination Δ9‐THC and CBD treatment in Scn1a +/− mice. (a) Threshold temperature of individual mice for generalized tonic–clonic seizure (GTCS) induced by hyperthermia following acute treatment with CBD (blue bar) and THC (green bar) administered individually or in combination (salmon bar). Cannabinoids were administered as intraperitoneal injections. Co‐treatment with CBD and THC (12 and 0.1 mg·kg−1, respectively) and THC (0.1 mg·kg−1) resulted in a significantly improved response to thermal seizure induction compared to vehicle. Combination CBD and THC treatment was significantly more effective than either treatment alone, with n = 15–36 per group (* P < 0.05, log‐rank Mantel–Cox). (Note that vehicle, CBD, and THC are replotted from Figures 2 and 3 for clarity.) (b) Plasma concentrations of THC (left panel) and CBD (right panel) from individual hyperthermia‐induced seizure experimental animals. Combination treatment with CBD and THC (12 + 0.1 mg·kg−1) resulted in significantly higher plasma concentrations of CBD and THC (* P < 0.05, Student's t test). Error bars represent SEM, with n = 5–10 per group. (c) Spontaneous GTCS frequency of individual untreated and CBD and THC co‐treated mice. Drug treatment was administered orally through supplementation in chow following the induction of a single thermally induced seizure. Unprovoked, spontaneous GTCSs were quantified. Co‐treatment with CBD and THC had no effect on incidence or frequency of seizures, with n = 17–27 per group (Fisher's exact text and one‐way ANOVA followed by Bonferroni's post hoc, respectively). (d) Proportion of spontaneous GTCS with or without full tonic hindlimb extension is depicted. Combination treatment with CBD and THC increased the severity of GTCS in Scn1a +/− mice. The proportion of spontaneous GTCS with tonic hindlimb extension was significantly greater in combination‐treated mice (salmon bar) compared to untreated controls (* P < 0.05, Fisher's exact test). Total number of spontaneous GTCS was 71 (untreated) and 54 (CBD + THC). (e) Survival curves comparing untreated and cannabinoid‐treated mice. Co‐treatment with CBD and THC resulted in significantly worse survival (salmon line) of Scn1a +/− mice (* P < 0.05, log‐rank Mantel–Cox). (f) Plasma concentrations of THC (left panel) and CBD (right panel) from individual experimental animals following sub‐chronic oral administration of cannabinoids in Scn1a +/− mice used in spontaneous seizure and survival experiments. (Note that CBD + THC [3,500 + 70 mg] concentrations were assayed in a separate cohort of treated wild‐type mice since there was no survival of experimental animals.) Combination treatment with CBD and THC (3,500 + 70 mg) resulted in significantly higher plasma levels of CBD and THC (* P < 0.05, one‐way ANOVA followed by Tukey's post hoc). Error bars represent SEM, with n = 5–9 per group (Note that untreated, CBD [3,500 mg·kg−1 chow], and THC [70 mg·kg−1 chow] are replotted from Figures 2 and 3 for clarity.)
3.3.2. Plasma concentrations during acute combination treatment
Acute combination treatment resulted in a pharmacokinetic interaction with significantly higher plasma CBD and Δ9‐THC concentrations than respective monotherapies observed in hyperthermia‐induced seizure experimental mice (Figure 4b).
3.3.3. Spontaneous seizures during sub‐chronic combination treatment
Mice treated with a combination of CBD and Δ9‐THC (70 mg Δ9‐THC + 3,500 mg CBD·kg−1 chow) did not differ from untreated controls in the proportion of mice having spontaneous seizures (co‐treatment: 10/17 mice [59%] compared to untreated: 16/26 mice [59%]). There was also no difference between groups in the seizure frequency of those mice that exhibited seizures (Figure 4c). However, co‐treatment with CBD and Δ9‐THC resulted in significantly increased severity of the spontaneous seizure with the proportion of seizures that advanced to the most severe stage of tonic hindlimb extension being higher in the CBD and Δ9‐THC co‐treated mice than untreated controls (Figure 4d).
3.3.4. Mortality with sub‐chronic combination treatment
The increased severity of seizures in the CBD and Δ9‐THC combination‐treated mice was associated with very poor survival. None of the Scn1a +/− mice treated with combination CBD and Δ9‐THC survived to P30 compared to 50% survival in untreated controls (Figure 4e).
3.3.5. Pharmacokinetic interaction between CBD and Δ9‐THC following sub‐chronic treatment
Plasma concentrations of Δ9‐THC and CBD following combination treatment (70 mg Δ9‐THC + 3,500 mg CBD·kg−1 chow) were significantly higher than with matched monotherapies (70 mg Δ9‐THC·kg−1 chow alone and 3,500 mg CBD·kg−1 chow alone) (Figure 4f). Brain concentrations of CBD and Δ9‐THC tended to be higher following combination treatment, reflecting the higher plasma concentrations observed with the combination. Indeed, brain‐plasma ratios of both CBD and Δ9‐THC tended to remain the same across all treatments (Table S1).
3.4. Experiment 4: Higher dose of Δ9‐THC treatment
Given that Δ9‐THC and CBD combination treatment resulted in plasma Δ9‐THC levels four times higher than the equivalent monotherapy, we hypothesized that these higher concentrations of Δ9‐THC may be proconvulsant in Scn1a +/− mice, leading to the poor survival and increased seizure severity effect following co‐treatment. To examine this hypothesis, we made a high‐dose formulation of Δ9‐THC (200 mg Δ9‐THC·kg−1 chow) that achieved equivalent plasma and brain Δ9‐THC concentrations to the 3,500 mg CBD + 70 mg Δ9‐THC·kg−1 chow co‐treatment associated with poor survival (Figure 5a and Table S1). Sub‐chronic administration of this Δ9‐THC dose did not affect spontaneous seizure frequency or severity (Figure 5b,c) or have any differential effect on survival of Scn1a +/− mice (Figure 5d).
FIGURE 5.
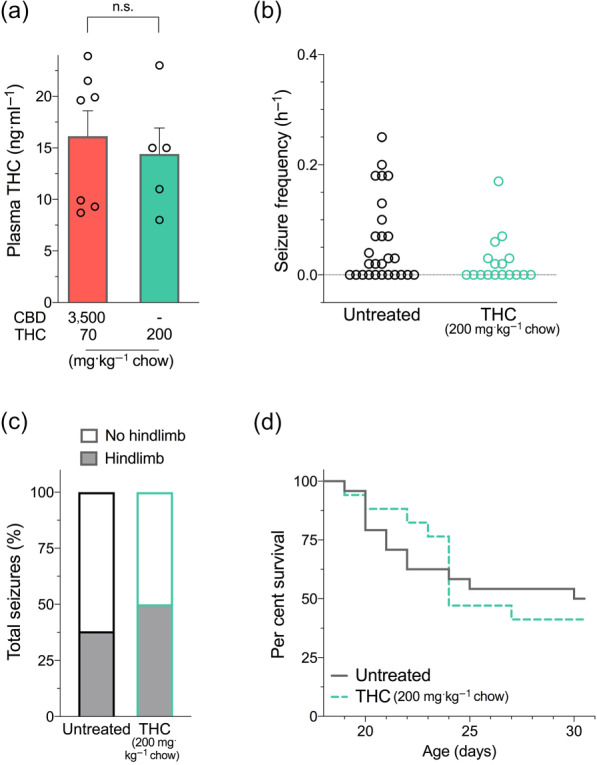
Higher dose of Δ9‐THC treatment in Scn1a +/− mice. (a) Plasma THC concentrations of individual mice measured following sub‐chronic treatment with a high dose of THC [200 mg·kg−1 chow] compared to the toxic CBD + THC co‐treatment [3,500 + 70 mg·kg−1 chow]. (n.s., non significant; unpaired Student's t‐test). (Note that CBD + THC [3,500 + 70 mg] is replotted from Figure 4 for clarity.) Error bars represent SEM, with n = 5–7 per group. (b) Spontaneous GTCS frequency of individual untreated and THC‐treated mice. THC treatment had no effect on incidence or frequency of seizures, with n = 17–27 per group (Fisher's exact test and one‐way ANOVA followed by Bonferroni's post hoc, respectively). (c) Proportion of spontaneous GTCS with or without full tonic hindlimb extension is depicted. The proportion of spontaneous GTCS with tonic hindlimb extension was not different following THC treatment compared to untreated controls (Fisher's exact test). Total number of spontaneous GTCS was 71 (untreated) and 12 (THC). (d) Survival curves comparing untreated and THC‐treated mice. THC had no effect on survival of Scn1a +/− mice, with n = 17–27 per group (log‐rank Mantel–Cox.)
3.5. Experiment 5: Combined lower dose of Δ9‐THC and CBD treatment
To further probe the interaction between CBD and Δ9‐THC, a chow was prepared with lower doses of both drugs (35 mg Δ9‐THC + 900 mg CBD·kg−1 chow). Plasma concentrations achieved with this new formulation provided a similar plasma exposure to those of the monotherapies (70 mg Δ9‐THC·kg−1 chow alone and 3,500 mg CBD·kg−1 chow alone) (Figure 6a). Treatment with this lower dose combination formulation had no effect on the proportion of mice with seizures (71%) compared to untreated controls (59%) (Figure 6b). Seizure frequency also did not differ between groups (Figure 6b). However, similar to the higher Δ9‐THC and CBD combination dose, the severity of the seizures as gauged by the proportion of seizures progressing to tonic hindlimb extension was also significantly higher in the lower dose of CBD and Δ9‐THC co‐treatment group than the untreated controls (Figure 6c). Again, survival of the lower dose of CBD and Δ9‐THC co‐treated Scn1a +/− mice was significantly worse than untreated controls (Figure 6d).
FIGURE 6.
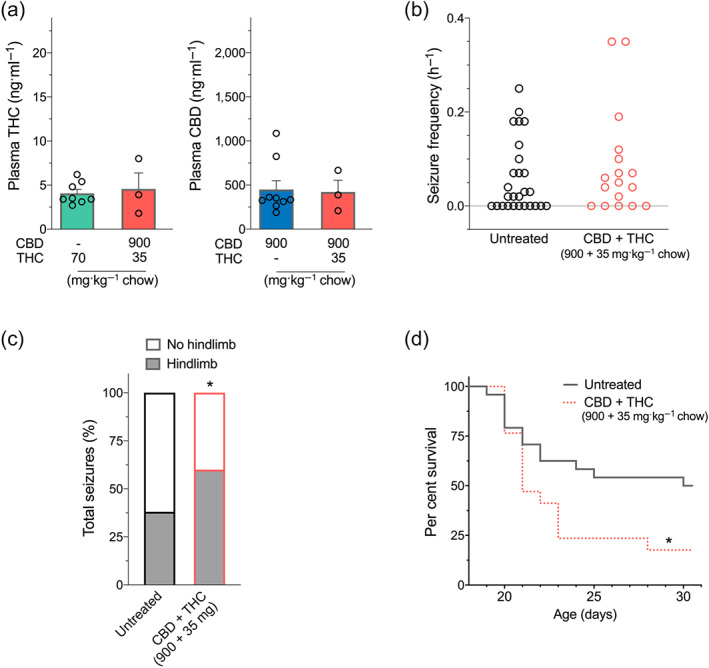
Combined lower dose of Δ9‐THC and CBD treatment in Scn1a +/− mice. (a) Plasma concentrations of THC (left panel) and CBD (right panel) from individual experimental animals following sub‐chronic oral administration of cannabinoids in Scn1a +/− mice used in spontaneous seizure and survival experiments. (Note that THC [70 mg·kg−1 chow] and CBD [3,500 mg·kg−1 chow] are replotted from Figures 2 and 3 for clarity.) Error bars represent SEM, with n = 3–9 per group. Since only three CBD + THC (900 + 35 mg)‐treated mice survived, no statistical comparisons were made between groups. (b) Spontaneous generalized tonic–clonic seizure (GTCS) frequency of individual untreated and CBD and THC co‐treated mice. Drug treatment was administered orally through supplementation in chow following the induction of a single thermally induced seizure. Unprovoked, spontaneous GTCSs were quantified. Co‐treatment with CBD and THC had no effect on incidence or frequency of seizures, with n = 17–27 per group (Fisher's exact text and one‐way ANOVA followed by Bonferroni's post hoc, respectively). (Note that untreated mice are replotted from Figure 2 for clarity.) (c) Proportion of spontaneous GTCS with or without full tonic hindlimb extension is depicted. Combination treatment with the lower dose of CBD and THC (900 + 35 mg·kg−1 chow) increased the severity of GTCS in Scn1a +/− mice. The proportion of spontaneous GTCS with tonic hindlimb extension was significantly greater in combination‐treated mice (salmon bar) compared to untreated controls (* P < 0.05, Fisher's exact test). Total number of spontaneous GTCS was 71 (untreated) and 73 (CBD + THC). (d) Survival curves comparing untreated and cannabinoid‐treated mice. Co‐treatment with CBD and THC resulted in significantly worse survival (salmon dashed line) of Scn1a +/− mice (P < 0.05, log‐rank Mantel–Cox) (Note that untreated mice are replotted from Figure 2 for clarity.)
4. DISCUSSION
The current series of experiments aimed to assess whether the presence of Δ9‐THC influenced the anticonvulsant effects of CBD in the Scn1a +/− mouse model of Dravet syndrome. The results provide evidence of a complex interaction between CBD and Δ9‐THC, whereby low doses of Δ9‐THC alone and a higher dose of CBD alone have intrinsic anticonvulsant effects against hyperthermia‐induced seizures. Moreover, the anticonvulsant effect of low‐dose Δ9‐THC was enhanced by a subthreshold dose of CBD suggesting a positive pharmacological interaction between these two major phytocannabinoids. However, Δ9‐THC or CBD exposure alone or in combination did not affect the frequency of spontaneous GTCSs following sub‐chronic, oral administration. Moreover, co‐administration of Δ9‐THC and CBD, using two different dose combinations, increased the severity of spontaneous seizures and reduced the lifespan of Scn1a +/− mice.
4.1. Anticonvulsant properties of low doses of Δ9‐THC in Scn1a +/− mice
We examined a broad range of Δ9‐THC doses against hyperthermia‐induced seizures in the Scn1a +/− mice, with the low doses employed yielding plasma concentrations consistent with those found in childhood epilepsy patients utilizing medicinal cannabis products (Huntsman et al., 2019; Wang et al., 2020). The low doses (0.1 and 0.3 mg·kg−1) of Δ9‐THC that exhibited anticonvulsant effects did not produce any overt sedation and, based on allometric scaling, appeared well below the intoxicating doses of Δ9‐THC in humans. The anticonvulsant effect of Δ9‐THC in Scn1a +/− mice is consistent with other studies showing anticonvulsant properties of Δ9‐THC in conventional seizure models, such as the maximal electroshock (MES) and audiogenic seizure models (Chesher & Jackson, 1974; Colasanti et al., 1982; Consroe & Wolkin, 1977; Lindamood & Colasanti, 1980; Sofia et al., 1976; Wallace et al., 2001). However, in contrast to the low anticonvulsant doses of Δ9‐THC reported here, higher doses of Δ9‐THC were typically required in these other seizure models (ED50 values: 7–43.8 mg·kg−1). It is noteworthy that Δ9‐THC achieved a similar anticonvulsant effect to CBD on thermally induced seizures but at a dose 3 orders of magnitude below the effective dose of CBD (100 mg·kg−1 i.p.). Collectively, these data suggest that the Scn1a +/− mouse model of a drug‐resistant childhood epilepsy has exceptional sensitivity to the anticonvulsant effects of Δ9‐THC.
In assessing the effects of Δ9‐THC on spontaneous seizures and premature mortality in Scn1a +/− mice, we administered Δ9‐THC at a dose designed to achieve plasma levels equivalent to those observed in childhood epilepsy patients. Accordingly, Δ9‐THC at a dose of 70 mg·kg−1 chow achieved plasma concentrations matching those in patients using medicinal cannabis products (4 ng·ml−1) and those observed following the 0.1 mg·kg−1 intraperitoneal dose that was anticonvulsant in Scn1a +/− mice against hyperthermia‐induced seizures (Huntsman et al., 2019; Wang et al., 2020). However, sub‐chronic treatment with Δ9‐THC at this dose had no effect on spontaneous seizure frequency, seizure severity or the survival of Scn1a +/− mice.
4.2. Enhanced anticonvulsant effect of combined CBD and Δ9‐THC on thermally induced seizures
Despite the purified CBD preparation Epidyolex™ being approved in Europe and the United States for the treatment of intractable childhood epilepsies, many families of childhood epilepsy patients continue to utilize CBD‐dominant medicinal cannabis products due to easier access, lesser cost or the perception that whole plant preparations may be more effective than purified single molecule products. Often these CBD‐dominant products also contain Δ9‐THC (Huntsman et al., 2019; McCoy et al., 2018; Suraev et al., 2018; Tzadok et al., 2016; Wang et al., 2020). We, therefore, examined acute CBD and Δ9‐THC co‐treatment effects on hyperthermia‐induced seizures in Scn1a +/− mice and discovered that a subthreshold dose of CBD (12 mg·kg−1) combined with 0.1 mg·kg−1 THC resulted in a greater anticonvulsant effect than either phytocannabinoid alone.
This result is consistent with studies reporting synergistic effects of acute CBD and Δ9‐THC co‐treatment in other animal models of disease including neuropathic pain and epilepsy (Casey et al., 2017; Samarut et al., 2019). Notably, Varvel et al. (2006) showed that CBD potentiated the antinociceptive effects of a low 0.3 mg·kg−1 Δ9‐THC dose in mice (Varvel et al., 2006). Since a pharmacokinetic interaction resulting in increased plasma concentrations of CBD and Δ9‐THC was observed following co‐treatment, the enhanced anticonvulsant effect of CBD and Δ9‐THC co‐administration might be explained by the increased plasma concentrations of both drugs.
In addition, cooperative pharmacodynamic action(s) on relevant neuronal circuits might subserve this effect. A converging interaction on cannabinoid CB1 receptors seems unlikely given evidence that CBD is a negative allosteric modulator, which attenuates the effects of Δ9‐THC on the CB1 receptor (Laprairie, Bagher, Kelly, & Denovan‐Wright, 2015). It is more likely that CBD and Δ9‐THC achieve greater anticonvulsant effects by modulating complementary targets that regulate neuronal excitability. For example, the actions of Δ9‐THC on presynaptically located CB1 receptors to reduce glutamate release might simultaneously combine with the anticonvulsant effects of CBD on various targets including GPR55, transient receptor potential vanilloid type 1 receptor (TRPV1) and GABA receptors (Anderson, Absalom, et al., 2019; Gray & Whalley, 2020; Kaplan et al., 2017).
4.3. Proconvulsant effect of combined CBD and Δ9‐THC in Scn1a +/− mice
We then examined whether combination CBD and Δ9‐THC treatment might also reduce spontaneous seizures and prolong lifespan of Scn1a +/− mice. We first formulated mouse chow with CBD and Δ9‐THC doses that, when given individually, yielded equivalent plasma concentrations to the 12 mg·kg−1 CBD and 0.1 mg·kg−1 Δ9‐THC intraperitoneal doses that were successfully used in the hyperthermia‐induced seizure experiments. Surprisingly, this formulation of CBD and Δ9‐THC not only failed to reduce spontaneous seizures but also promoted greater seizure severity, as gauged by a greater proportion of seizures progressing to full hindlimb extension. Moreover, the combination treatment dramatically reduced the lifespan of Scn1a +/− mice.
We hypothesized that this detrimental outcome might simply be explained by a significant pharmacokinetic interaction between CBD and Δ9‐THC, as plasma and brain concentrations of both Δ9‐THC and CBD were dramatically increased. We surmised that the high plasma and brain concentrations of Δ9‐THC might induce proconvulsant effects of Δ9‐THC, which has been demonstrated in mice and rats in other epilepsy models (Chan et al., 1996; Sofia et al., 1976). However, control experiments, involving a high‐dose formulation of Δ9‐THC alone (200 mg Δ9‐THC·kg−1 chow), which achieved equivalent plasma Δ9‐THC concentrations to those observed with CBD co‐administration, did not affect spontaneous seizures or mortality. Therefore, the proconvulsant effect observed with the combination seems unlikely to be attributable to a pharmacokinetic interaction that elevates Δ9‐THC concentrations.
A new combination chow was formulated with lower cannabinoid doses (35 mg Δ9‐THC + 900 mg CBD·kg−1 chow) to account for the pharmacokinetic interaction and produce plasma concentrations equivalent to those observed in our acute hyperthermia‐induced seizure experiments. Again, however, this lower CBD and Δ9‐THC dose not only failed to reduce spontaneous seizure frequency but also worsened seizure severity and reduced the lifespan of the Scn1a +/− mice. Taken together, these results demonstrate an unexpected proconvulsant effect when CBD and Δ9‐THC are used in combination sub‐chronically.
4.4. Divergent convulsant effects of CBD and Δ9‐THC in Scn1a +/− mice
While on the surface it is difficult to reconcile the augmented anticonvulsant effects of CBD and Δ9‐THC co‐administration on hyperthermia‐induced seizures with the proconvulsant effects observed on spontaneous seizure severity and premature mortality, pharmacological studies suggest distinct neurochemical influences on these different phenotype measures in the Scn1a +/− mouse model of Dravet syndrome. For example, stiripentol is anticonvulsant against hyperthermia‐induced seizures yet has no effect on spontaneous seizures or survival; whereas, the novel sodium channel blocker GS967 does not affect thermally induced seizures but reduces spontaneous seizures and improves lifespan in Scn1a +/− mice (Anderson, Hawkins, Thompson, Kearney, & George, 2017; Hawkins et al., 2017). Moreover, lamotrigine, which is contraindicated in the treatment of Dravet syndrome, had proconvulsant effects on hyperthermia‐induced and spontaneous seizures but resulted in 100% survival of Scn1a +/− mice (Hawkins et al., 2017). Our results with CBD and Δ9‐THC co‐administration further reaffirm that drugs may have differential effects on the phenotypes of Scn1a +/− mice dependent on their class and mode(s) of action.
The increased proportion of severe hindlimb seizures with CBD and Δ9‐THC co‐treatment is consistent with exacerbation of neuronal hyperexcitability in Scn1a +/− mice, which could compromise brain circuits that maintain cardiorespiratory function and lead to death. Future studies, however, will be required to properly elucidate the mechanism(s) involved in the detrimental effects of Δ9‐THC and CBD combinations, which may involve both pharmacodynamic and pharmacokinetic components. Our study also demonstrated a pharmacokinetic interaction between CBD and Δ9‐THC, with significantly increased concentrations of both phytocannabinoids in the brain and plasma of mice following co‐treatment. Since both CBD and Δ9‐THC are metabolized by cytochrome P450 enzymes (Stout & Cimino, 2014), a pharmacokinetic interaction is not surprising. Indeed, animal and human studies have reported that CBD and Δ9‐THC co‐administration enhances plasma concentrations of Δ9‐THC (Arkell et al., 2019; Britch, Wiley, Yu, Clowers, & Craft, 2017; Greene, Wiley, Yu, Clowers, & Craft, 2018; Hlozek et al., 2017; Klein et al., 2011; Varvel et al., 2006), although here we report novel data of the converse scenario where co‐administered Δ9‐THC increases plasma concentrations of CBD.
4.5. Translation and clinical implications
Whether our findings in mice readily translate to humans is unclear. At face value, our results suggest that the co‐administration of CBD and Δ9‐THC might be better utilized as an acute rescue therapy for febrile seizures but that ongoing chronic use of CBD and Δ9‐THC co‐formulations might be problematic for Dravet syndrome patients. An important caveat in translating our findings is that we sought to match plasma CBD levels in our mice with those in childhood epilepsy patients taking Epidyolex™ (mean: 450 ng·ml−1, range: 100–800 ng·ml−1) (Geffrey et al., 2015). These CBD concentrations are notably in excess of those reported in Dravet syndrome patients using artisanal medicinal cannabis products (14.8–124.7 ng·ml−1) (Huntsman et al., 2019). Future studies might attempt to more closely mirror the CBD plasma concentrations in such patients. Another important limitation is interspecies translation. Cannabinoid‐induced convulsions may be species specific as demonstrated in a recent study in which prolonged exposure to Δ9‐THC and CBD containing cannabis extracts (at a ratio of 1.08:1) induced spontaneous seizure activity in rats but not in dogs. This is consistent with the view that the toxicity of cannabinoids may diminish as one ascends the phylogenetic tree (Whalley et al., 2019). Indeed, most studies report that medicinal cannabis products are generally well tolerated with perceived efficacy and no serious adverse events in childhood epilepsy patients (Huntsman et al., 2019; McCoy et al., 2018; Suraev et al., 2018; Tzadok et al., 2016; Wang et al., 2020).
5. CONCLUSION
To a certain extent, our results resonate with reports of efficacy of artisanal medicinal cannabis products exerting seizure control in childhood epilepsy patients, with CBD and Δ9‐THC working together to inhibit febrile seizures. On the other hand, the unique adverse impact of combined CBD and Δ9‐THC on spontaneous seizure severity and lifespan provides some cause for contemplation. These results suggest that continuing close surveillance and collection of safety data in childhood epilepsy patients using medicinal cannabis products is imperative.
AUTHOR CONTRIBUTIONS
L.L.A., I.S.M. and J.C.A. contributed to the conception and design of the study. L.L.A. and I.K.L. contributed to the acquisition and analyses of data. All authors contributed to drafting the manuscript or figures.
CONFLICT OF INTEREST
Associate Professor Jonathon Arnold is Deputy Academic Director of the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded research centre at the University of Sydney. He has served as an expert witness in various medicolegal cases involving cannabis and in 2018 was a temporary advisor to the World Health Organization (WHO) on their review of cannabis and the cannabinoids. His research is funded by the Lambert Initiative and the Australian National Health and Medical Research Council (NHMRC). A/Prof Arnold, Dr Anderson, and Prof McGregor hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554). Prof McGregor is Academic Director of the Lambert Initiative for Cannabinoid Therapeutics. He has served as an expert witness in various medicolegal cases involving cannabis use, has received honoraria from Janssen, is currently a consultant to Kinoxis Therapeutics, and has received research funding and fellowships from the NHMRC and Australian Research Council (ARC).
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1. Dose–response of CBD combination treatment with THC against hyperthermia‐induced seizures in Scn1a +/− mice. Temperature threshold of individual mice for generalized tonic–clonic seizure (GTCS) induced by hyperthermia following acute treatment with THC (0.1 mg/kg) or CBD (25 or 100 mg/kg) administered individually or as a combination. CBD (25 mg/kg) had no effect on GTCS threshold. All other treatments resulted in significantly improved response to thermal seizure induction compared to vehicle (green, open symbols; p < 0.05). The average temperatures of seizure induction are depicted by the bars and error bars represent SEM, with n = 15–36 per treatment group.
Table S1. Cannabinoid concentrations in plasma and brain samples.
ACKNOWLEDGEMENTS
This work was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded centre for medicinal cannabis research at the University of Sydney and an Australian National Health and Medical Research Council (NHMRC) Project Grant (J.C.A. and I.S.M.). The authors gratefully acknowledge Barry and Joy Lambert for their continued support. In addition, we thank Katelyn Lambert for inspiring our work on novel cannabinoid therapies for childhood epilepsy.
Anderson LL, Low IK, McGregor IS, Arnold JC. Interactions between cannabidiol and Δ9‐tetrahydrocannabinol in modulating seizure susceptibility and survival in a mouse model of Dravet syndrome. Br J Pharmacol. 2020;177:4261–4274. 10.1111/bph.15181
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H., Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Sharman, J. L. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, L. L. , Absalom, N. L. , Abelev, S. V. , Low, I. K. , Doohan, P. T. , Martin, L. J. , … Arnold, J. C. (2019). Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia, 60(11), 2224–2234. 10.1111/epi.16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, L. L. , Hawkins, N. A. , Thompson, C. H. , Kearney, J. A. , & George, A. L. (2017). Unexpected efficacy of a novel sodium channel modulator in Dravet syndrome. Scientific Reports, 7, 1682–1690. 10.1038/s41598-017-01851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, L. L. , Low, I. K. , Banister, S. D. , McGregor, I. S. , & Arnold, J. C. (2019). Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. Journal of Natural Products, 82(11), 3047–3055. 10.1021/acs.jnatprod.9b00600 [DOI] [PubMed] [Google Scholar]
- Arkell, T. R. , Lintzeris, N. , Kevin, R. C. , Ramaekers, J. G. , Vandry, R. , Irwin, C. , … McGregor, I. S. (2019). Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)‐induced impairment of driving and cognition. Psychopharmacology, 236(9), 2713–2724. 10.1007/s00213-019-05246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs, D. L. , Nguyen, J. D. , Morgenson, D. , Taffe, M. A. , & Ranganathan, M. (2018). Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9‐tetrahydrocannabinol. Neuropsychopharmacology, 43(1), 142–154. 10.1038/npp.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs, D. L. , Peckham, A. , Boggs, A. A. , & Ranganathan, M. (2016). Delta‐9‐tetrahydrocannabinol and cannabidiol: Separating the chemicals from the “weed,” a pharmacodynamic discussion. Mental Health Clinician, 6(6), 277–284. 10.9740/mhc.2016.11.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britch, S. C. , Wiley, J. L. , Yu, Z. , Clowers, B. H. , & Craft, R. M. (2017). Cannabidiol‐Δ9‐tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug and Alcohol Dependence, 175, 187–197. 10.1016/j.drugalcdep.2017.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, S. L. , Atwal, N. , & Vaughan, C. W. (2017). Cannabis constituent synergy in a mouse neuropathic pain model. Pain, 158(12), 2452–2460. 10.1097/j.pain.0000000000001051 [DOI] [PubMed] [Google Scholar]
- Chan, P. C. , Sills, R. C. , Braun, A. G. , Haseman, J. K. , & Bucher, J. R. (1996). Toxicity and carcinogenicity of Δ9‐tetrahydrocannabinol in Fischer rats and B6C3F1 mice. Fundamental and Applied Toxicology, 30(1), 109–117. 10.1006/faat.1996.0048 [DOI] [PubMed] [Google Scholar]
- Chesher, G. B. , & Jackson, D. M. (1974). Anticonvulsant effects of cannabinoids in mice: Drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacologia, 37(3), 255–264. 10.1007/bf00421539 [DOI] [PubMed] [Google Scholar]
- Colasanti, B. K. , Lindamood, C. 3rd , & Craig, C. R. (1982). Effects of marihuana cannabinoids on seizure activity in cobalt‐epileptic rats. Pharmacology Biochemistry and Behavior, 16(4), 573–578. 10.1016/0091-3057(82)90418-x [DOI] [PubMed] [Google Scholar]
- Consroe, P. , & Wolkin, A. (1977). Cannabidiol–antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. Journal of Pharmacology and Experimental Therapeutics, 201(1), 26–32. [PubMed] [Google Scholar]
- Deiana, S. , Watanabe, A. , Yamasaki, Y. , Amada, N. , Arthur, M. , Fleming, S. , … Riedel, G. (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9‐tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology, 219, 859–873. 10.1007/s00213-011-2415-0 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Cross, J. H. , Laux, L. , Marsh, E. , Miller, I. , Nabbout, R. , … Wright, S. (2017). Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. New England Journal of Medicine, 376, 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Patel, A. D. , Cross, J. H. , Villanueva, V. , Wirrell, E. C. , Privitera, M. , … Zuberi, S. M. (2018). Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. New England Journal of Medicine, 378(20), 1888–1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- Dravet, C. (2011). The core Dravet syndrome phenotype. Epilepsia, 52, 3–9. 10.1111/j.1528-1167.2011.02994.x [DOI] [PubMed] [Google Scholar]
- Dravet, C. , & Oguni, H. (2013). Dravet syndrome (severe myoclonic epilepsy of infancy). Handbook of Clinical Neurology, 111, 627–633. 10.1016/B978-0-444-52891-9.00065-8 [DOI] [PubMed] [Google Scholar]
- Freeman, A. M. , Petrilli, K. , Lees, R. , Hindocha, C. , Mokrysz, C. , Curran, H. V. , … Freeman, T. P. (2019). How does cannabidiol (CBD) influence the acute effects of delta‐9‐tetrahydrocannabinol (THC) in humans? A systematic review. Neuroscience and Biobehavioral Reviews, 107, 696–712. 10.1016/j.neubiorev.2019.09.036 [DOI] [PubMed] [Google Scholar]
- Geffrey, A. L. , Pollack, S. F. , Bruno, P. L. , & Thiele, E. A. (2015). Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia, 56, 1246–1251. 10.1111/epi.13060 [DOI] [PubMed] [Google Scholar]
- Gray, R. A. , & Whalley, B. J. (2020). The proposed mechanisms of action of CBD in epilepsy. Epileptic Disorders, 22(S1), 10–15. 10.1684/epd.2020.1135 [DOI] [PubMed] [Google Scholar]
- Greene, N. Z. , Wiley, J. L. , Yu, Z. , Clowers, B. H. , & Craft, R. M. (2018). Cannabidiol modulation of antinociceptive tolerance to Δ9‐tetrahydrocannabinol. Psychopharmacology, 235(11), 3289–3302. 10.1007/s00213-018-5036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, S. M. , VanLandingham, K. E. , Cross, J. H. , Zuberi, S. M. , Wirrell, E. C. , Villanueva, V. , … Privitera, M. (2018). Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. New England Journal of Medicine, 378, 1888–1897. 10.1056/nejmoa17146310.1 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, N. A. , Anderson, L. L. , Gertler, T. S. , Laux, L. , George, A. L. , & Kearney, J. A. (2017). Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Annals of Clinical and Translational Neurology, 4, 326–339. 10.1002/acn3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlozek, T. , Uttl, L. , Kaderabek, L. , Balikova, M. , Lhotkova, E. , Horsley, R. R. , … Kuchař, M. (2017). Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European Neuropsychopharmacology, 27(12), 1223–1237. 10.1016/j.euroneuro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- Huntsman, R. J. , Tang‐Wai, R. , Alcorn, J. , Vuong, S. , Acton, B. , Corley, S. , … Major, P. (2019). Dosage related efficacy and tolerability of cannabidiol in children with treatment‐resistant epileptic encephalopathy: Preliminary results of the CARE‐E study. Frontiers in Neurology, 10, 716 10.3389/fneur.2019.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, J. S. , Stella, N. , Catterall, W. A. , & Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences., 114, 11229–11234. 10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, C. , Karanges, E. , Spiro, A. , Wong, A. , Spencer, J. , Huynh, T. , … McGregor, I. S. (2011). Cannabidiol potentiates Δ9‐tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology, 218(2), 443–457. 10.1007/s00213-011-2342-0 [DOI] [PubMed] [Google Scholar]
- Laprairie, R. B. , Bagher, A. M. , Kelly, M. E. , & Denovan‐Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology, 172(20), 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindamood, C. 3rd , & Colasanti, B. K. (1980). Effects of delta 9‐tetrahydrocannabinol and cannabidiol on sodium‐dependent high affinity choline uptake in the rat hippocampus. Journal of Pharmacology and Experimental Therapeutics, 213(2), 216–221. [PubMed] [Google Scholar]
- Marini, C. , Scheffer, I. E. , Nabbout, R. , Suls, A. , De Jonghe, P. , Zara, F. , & Guerrini, R. (2011). The genetics of Dravet syndrome. Epilepsia, 52, 24–29. 10.1111/j.1528-1167.2011.02997.x [DOI] [PubMed] [Google Scholar]
- McCoy, B. , Wang, L. , Zak, M. , Al‐Mehmadi, S. , Kabir, N. , Alhadid, K. , … Sinopoli, K. (2018). A prospective open‐label trial of a CBD/THC cannabis oil in Dravet syndrome. Annals of Clinical and Translational Neurology, 5(9), 1077–1088. 10.1002/acn3.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. R. , Hawkins, N. A. , McCollom, C. E. , & Kearney, J. A. (2014). Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes, Brain and Behavior, 13(2), 163–172. 10.1111/gbb.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca, E. (2018). Cannabinoids in the treatment of epilepsy: Hard evidence at last? Journal of Epilepsy Research, 7, 61–76. 10.14581/jer.17012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarut, E. , Nixon, J. , Kundap, U. P. , Drapeau, P. , & Ellis, L. D. (2019). Single and synergistic effects of cannabidiol and Δ‐9‐tetrahydrocannabinol on zebrafish models of neuro‐hyperactivity. Frontiers in Pharmacology, 10, 226 10.3389/fphar.2019.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuely, S. , Sisodiya, S. M. , Gunning, W. B. , Sander, J. W. , & Thijs, R. D. (2016). Mortality in Dravet syndrome: A review. Epilepsy & Behavior, 64, 69–74. 10.1016/j.yebeh.2016.09.007 [DOI] [PubMed] [Google Scholar]
- Silveira, M. M. , Arnold, J. C. , Laviolette, S. R. , Hillard, C. J. , Celorrio, M. , Aymerich, M. S. , & Adams, W. K. (2017). Seeing through the smoke: Human and animal studies of cannabis use and endocannabinoid signalling in corticolimbic networks. Neuroscience and Biobehavioral Reviews, 76(Pt B), 380–395. 10.1016/j.neubiorev.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia, R. D. , Solomon, T. A. , & Barry, H. 3rd. (1976). Anticonvulsant activity of Δ9‐tetrahydrocannabinol compared with three other drugs. European Journal of Pharmacology, 35(1), 7–16. 10.1016/0014-2999(76)90295-8 [DOI] [PubMed] [Google Scholar]
- Spiro, A. S. , Wong, A. , Boucher, A. A. , & Arnold, J. C. (2012). Enhanced brain disposition and effects of Δ9‐tetrahydrocannabinol in P‐glycoprotein and breast cancer resistance protein knockout mice. PLoS ONE, 7, e35937 10.1371/journal.pone.0035937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout, S. M. , & Cimino, N. M. (2014). Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metabolism Reviews, 46(1), 86–95. 10.3109/03602532.2013.849268 [DOI] [PubMed] [Google Scholar]
- Suraev, A. , Lintzeris, N. , Stuart, J. , Kevin, R. C. , Blackburn, R. , Richards, E. , … McGregor, I. S. (2018). Composition and use of cannabis extracts for childhood epilepsy in the Australian community. Scientific Reports, 8, 10154 10.1038/s41598-018-28127-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele, E. A. , Marsh, E. D. , French, J. A. , Mazurkiewicz‐Beldzinska, M. , Benbadis, S. R. , Joshi, C. , … Wilfong, A. (2018). Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): A randomised, double‐blind, placebo‐controlled phase 3 trial. The Lancet, 391, 1085–1096. 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- Todd, S. M. , & Arnold, J. C. (2016). Neural correlates of interactions between cannabidiol and Δ9‐tetrahydrocannabinol in mice: Implications for medical cannabis. British Journal of Pharmacology, 173(1), 53–65. 10.1111/bph.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzadok, M. , Uliel‐Siboni, S. , Linder, I. , Kramer, U. , Epstein, O. , Menascu, S. , … Ben‐Zeev, B. (2016). CBD‐enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure, 35, 41–44. 10.1016/j.seizure.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Vann, R. E. , Gamage, T. F. , Warner, J. A. , Marshall, E. M. , Taylor, N. L. , Martin, B. R. , & Wiley, J. L. (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9‐tetrahydrocannabinol. Drug and Alcohol Dependence, 94(1–3), 191–198. 10.1016/j.drugalcdep.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel, S. A. , Wiley, J. L. , Yang, R. , Bridgen, D. T. , Long, K. , Lichtman, A. H. , & Martin, B. R. (2006). Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology, 186(2), 226–234. 10.1007/s00213-006-0356-9 [DOI] [PubMed] [Google Scholar]
- Wallace, M. J. , Wiley, J. L. , Martin, B. R. , & DeLorenzo, R. J. (2001). Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. European Journal of Pharmacology, 428(1), 51–57. 10.1016/s0014-2999(01)01243-2 [DOI] [PubMed] [Google Scholar]
- Wang, G. S. , Bourne, D. W. A. , Klawitter, J. , Sempio, C. , Chapman, K. , Knupp, K. , … Bajaj, L. (2020). Disposition of oral delta‐9 tetrahydrocannabinol (THC) in children receiving cannabis extracts for epilepsy. Clinical Toxicology, 58(2), 124–128. 10.1080/15563650.2019.1616093 [DOI] [PubMed] [Google Scholar]
- Whalley, B. J. , Lin, H. , Bell, L. , Hill, T. , Patel, A. , Gray, R. A. , … Stephens, G. J. (2019). Species‐specific susceptibility to cannabis‐induced convulsions. British Journal of Pharmacology, 176(10), 1506–1523. 10.1111/bph.14165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dose–response of CBD combination treatment with THC against hyperthermia‐induced seizures in Scn1a +/− mice. Temperature threshold of individual mice for generalized tonic–clonic seizure (GTCS) induced by hyperthermia following acute treatment with THC (0.1 mg/kg) or CBD (25 or 100 mg/kg) administered individually or as a combination. CBD (25 mg/kg) had no effect on GTCS threshold. All other treatments resulted in significantly improved response to thermal seizure induction compared to vehicle (green, open symbols; p < 0.05). The average temperatures of seizure induction are depicted by the bars and error bars represent SEM, with n = 15–36 per treatment group.
Table S1. Cannabinoid concentrations in plasma and brain samples.


