Abstract
Background and Purpose
Genetic deletion and pharmacological studies suggest a role for lysophosphatidic acid (LPA1) receptor in fibrosis. We investigated the therapeutic potential in systemic sclerosis (SSc) of a new orally active selective LPA1 receptor antagonist using dermal fibroblasts from patients and an animal model of skin fibrosis.
Experimental Approach
Dermal fibroblast and skin biopsies from systemic sclerosis patients were used. Myofibroblast differentiation, gene expression and cytokine secretion were measured following LPA and/or SAR100842 treatment. Pharmacolgical effect of SAR100842 was assessed in the tight skin 1 (Tsk1) mouse model.
Key Results
SAR100842 is equipotent against various LPA isoforms. Dermal fibroblasts and skin biopsies from patients with systemic sclerosis expressed high levels of LPA1 receptor. The LPA functional response (Ca2+) in systemic sclerosis dermal fibroblasts was fully antagonized with SAR100842. LPA induced myofibroblast differentiation in systemic sclerosis dermal and idiopathic pulmonary fibrosis lung fibroblasts and the secretion of inflammatory markers and activated Wnt markers. Results from systemic sclerosis dermal fibroblasts mirror those obtained in a mouse Tsk1 model of skin fibrosis. Using a therapeutic protocol, SAR100842 consistently reversed dermal thickening, inhibited myofibroblast differentiation and reduced skin collagen content. Inflammatory and Wnt pathway markers were also inhibited by SAR100842 in the skin of Tsk1 mice.
Conclusion and Implications
The effects of SAR100842 on LPA‐induced inflammation and on mechanisms linked to fibrosis like myofibroblast differentiation and Wnt pathway activation indicate that LPA1 receptor activation plays a key role in skin fibrosis. Our results support the therapeutic potential of LPA1 receptor antagonists in systemic sclerosis.
Keywords: fibrosis, LPA1 receptor, lysophosphatidic acid, SAR100842, systemic sclerosis, tight skin mouse

Abbreviations
- IPF
idiopathic pulmonary fibrosis
- LPA
lysophosphatidic acid
- αSMA
α‐smooth muscle actin
- SSc
systemic sclerosis
- Tsk1
tight skin 1
What is already known
LPA1 receptor inhibition has been reported to reduce fibrosis in some animal models
What this study adds
Translational evidence of the therapeutic potential of LPA1 antagonist from systemic sclerosis patients’ dermal fibroblasts
LPA both in human fibroblasts and Tsk1 animal model activates a WNT pathway
What is the clinical significance
Demonstration of LPA1 receptor involvement in systemic sclerosis
SAR100842, a new orally active selective LPA1 receptor antagonist has therapeutic potential in systemic sclerosis.
1. INTRODUCTION
Systemic sclerosis (SSc) is a rare autoimmune disorder characterized by microvasculopathy and fibrosis of skin and internal organs. During the course of the disease, excessive accumulation of extracellular matrix leads to severe dysfunction of affected tissues. The transition of quiescent fibroblasts to activated myofibroblasts expressing α‐smooth muscle actin (αSMA) and secreting increased amounts of collagens are an important step in this process (Abraham, Krieg, Distler, & Distler, 2009). Despite an increasing number of molecular targeted therapies in clinical testing, effective anti‐fibrotic therapies are still not available.
Lysophosphatidic acid (LPA, 1‐oleoyl‐sn‐glycerol‐3‐phosphate and derivatives), a small glycerophospholipid, is present in all eukaryotic tissues and is produced from membrane phospholipids via different enzymatic pathways. LPA is generated at sites of inflammation or cell injury mainly by enzyme systems involving autotaxin (lysophospholipase D) or phospholipases (Van Meeteren & Moolenaar, 2007). Lysophosphatidic acid mediates its biological effects via distinct GPCRs called LPA1 to LPA6 as designated by International Union of Pharmacology (IUPHAR) (Blaho et al., 2019; Kihara, Maceyka, Spiegel, & Chun, 2014). Although there is some crosstalk in their downstream signalling pathways, these receptors differ in their tissue distribution. LPA mediates a variety of cell activities, including mitogenesis, cell differentiation, cell survival, cytoskeletal reorganization, cell migration and extracellular matrix production (Kim, Keys, & Eckhart, 2006).
LPA signalling has been associated with skin, pulmonary and tubulointerstitial fibrosis, suggesting that it may be a potential therapeutic target in fibrotic diseases (Rancoule et al., 2011). LPA is able to increase the production of connective tissue growth factor (CTGF) in mesangial cells, epithelial cells, kidney interstitial cells and keloid fibroblasts. In patients with idiopathic pulmonary fibrosis (IPF), LPA levels are increased in bronchoalveolar lavage fluid. Genetic deletion of Lpar1 blunted bleomycin‐induced lung fibrosis (Tager et al., 2008), reduced fibroblast activation and chemotactic activity, and protected from vascular leakage. Similar results were obtained with selective LPA1/LPA3 receptor antagonists in models of lung fibrosis induced by bleomycin (Swaney et al., 2010) or irradiation (Gan et al., 2011). Interestingly, plasma and serum levels of LPA 20:4 have been found to be elevated in patients with systemic sclerosis (Tokumura et al., 2009) and Lpar1 knockout mice are protected from bleomycin‐induced dermal fibrosis. Similar results were obtained with orally available LPA1/LPA3 receptor antagonist (AM095) using prophylactic protocol (Castelino et al., 2011).
The objective of this study was to investigate in dermal fibroblasts from systemic sclerosis patients the effect of SAR100842, a selective LPA1 receptor antagonist, on different markers of the fibrotic process and on myofibroblast differentiation. In addition, these markers, myofibroblast differentiation and collagen production, were used to evaluate if SAR100842 could reverse fibrosis in the tight skin 1 (Tsk1) mouse model where fibrosis is already present.
We demonstrated that dermal fibroblasts and skin biopsies from systemic sclerosis patients express high levels of LPA1 receptor. Exposure of these fibroblasts to LPA led to myofibroblast differentiation and induction of systemic sclerosis‐associated markers that were inhibited by SAR100842. Pharmacological blockade of LPA1 receptor by SAR100842 improved skin fibrosis in the Tsk1 model together with a reduction of markers of inflammation and WNT signalling activation.
2. METHODS
In general, studies were designed to generate groups of equal size, using randomization and blinded analysis. Statistical analysis was undertaken only for studies where each group size was at least n = 5. In vitro experiments were performed in duplicate or triplicate, and the n designed the number of different experiments or different primary culture (patients) when appropriate.
2.1. Chemicals
SAR100842,2‐(4‐methoxy‐3‐(2‐m‐tolyl‐ethoxy)‐benzoylamino)‐indan‐2‐carboxylic acid, is a novel LPA1 receptor antagonist (Sanofi R&D, France). SAR100842 was synthesized as described previously (WO2009135590). LPA 18:1, oleoyl‐l‐α‐lysophosphatidic acid sodium (18:1 form) (Sigma, St. Louis, USA) was prepared at 10 mM in PBS free of Ca2+ and Mg2+, containing 0.1% fatty acid‐free BSA (Sigma). After sonication for a few minutes, the solution was aliquoted, stored at −20°C, and used within 1 month. PBS free of Ca2+ and Mg2+, containing 0.1% fatty acid‐free BSA (Sigma) was used for subsequent dilutions. A similar preparation method was used for other LPA isoforms (LPA 18:1, LPA 18:3, LPA 20:4, LPA 18:0, and LPA 16:0, all from Echelon, Salt Lake City, USA).
2.2. Cell culture and cell line authentication
Normal human dermal fibroblasts (NHDF, Lonza, Walkersville, USA), dermal fibroblasts from ten patients with systemic sclerosis and lung fibroblasts from five different patients with idiopathic pulmonary fibrosis (University of Giessen, Germany), LL29 (ATCC Manassas, USA, Cat# CCL‐134, RRID:CVCL_2576) and LL97A cells (ATCC Manassas, USA, Cat# CCL‐191, RRID:CVCL_3736) were used. Systemic sclerosis patients fulfilled the ACR/EULAR classification criteria (Van den Hoogen et al., 2013). Clinical characteristics of systemic sclerosis dermal fibroblasts are given in Table S1. Since sex has to be considered as an experimental variable, systemic sclerosis fibroblasts were prepared from forearm biopsies from women and men, following established outgrowth conditions and cultured in F‐12K medium with 10% heat‐inactivated fetal calf serum (FCS), 100 U·ml−1 of penicillin, 100 μg·ml−1 of streptomycin, and 0.3 mg·ml−1 of l‐glutamine. The skin biopsy procedure was approved by the local ethics committee (University of Naples) and patients signed informed consent. Fibroblasts from passages 3–10 were used for the experiments. Lung fibroblast cells were derived from the lung of idiopathic pulmonary fibrosis patients. The diagnosis of idiopathic pulmonary fibrosis was reviewed and validated using current American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines. Patients were included only when current ATS/ERS criteria were met. The study protocol was approved by the ethics committee of the Justus‐Liebig‐University School of Medicine (Nos. 31/93, 84/93, and 29/01). Informed consent was obtained in written form from each subject for the study protocol. Primary lines were established by using an outgrowth from explants according to the methods as previously described (Jordana et al., 1988). In all experiments, cell lines were used at early passages (4–8). Skin biopsies from the forearm for gene expression at baseline and serum samples for SAR100842 activity were obtained from another study enrolling patients to test the safety, biomarkers, and clinical efficacy of SAR100842 in patients with diffuse cutaneous systemic sclerosis (dcSSc) (NCT01651143). All patients provided written informed consent prior to the conduct of any study‐related procedures, and the optional skin biopsy informed consent form (ICF) was obtained from patients who agreed to the collection of skin biopsy.
2.3. RNA extraction and reverse transcription
For RNA isolation, the RNeasy mini kit and QIAshredder DNase digest were used. Cells were lysed in 600 μl of lysis buffer, and RNA was isolated via the QIACube system (all Qiagen, Hilden, Germany, RRID:SCR_018618). For skin biopsies, tissues were grinded beforehand by using a TissueLyser homogenizer (Qiagen, Hilden, Germany) with tubes containing stainless steel beads (Qiagen) and lysis buffer. The lysates were homogenized with QIAshredder columns, and RNA was precipitated by addition of 70% ethanol. Cell membrane residues were removed with RPE and RW1 buffer (Qiagen). DNase digestion was performed to eliminate genomic DNA. The RNA was eluted in RNase‐free water. Reverse transcription was performed using the Superscript VILO cDNA Synthesis Kit and an iCycler. Real‐time PCR was performed according to standard protocols using TaqMan Low Density Micro Fluidic Cards Array and the 7900HT Fast Real‐Time PCR System (RRID:SCR_018060). The amounts of loaded cDNA were normalized using appropriate housekeeping genes as indicated.
2.4. Real‐time PCR
Five hundred nanograms of purified total RNA was reverse transcribed using Superscript VILO cDNA Synthesis Kit (Life Technologies, Carlsbad, USA). A reaction mixture including random primers, MgCl2, dNTPs and Superscript III RT Enzyme was added and reverse transcription was performed in an iCycler (Bio‐Rad Laboratories, Hercules, USA) according to the manufacturer's protocol. For real‐time PCR, 20 μl of cDNA was mixed with 35‐μl RNase‐free and DNase‐free H2O and 55‐μl TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, USA); 100 μl per well was used on TaqMan Low Density Micro Fluidic Cards Array (Applied Biosystems 4342253) containing pre‐designed single TaqMan assays.
The PCR protocol consisted of one step at 50°C for 2 min, one step at 95.4°C for 10 min, and then 40 cycles at 97°C for 30 s and at 59.7°C for 1 min, all performed in the 7900HT Fast Real‐Time PCR System (Applied Biosystems, RRID:SCR_018060). Measurements of A260/A280 and A260/A230 ratios were done with Nanodrop Spectrophotometer (Waltham, MA, USA), and the quality check of the mRNA was performed with an Agilent 2100 Bioanalyser (Agilent Technologies, Waldbronn, Germany, RRID:SCR_018043). The amounts of loaded cDNA were normalized using appropriate housekeeping genes as indicated in the experiments as an endogenous control. For relative quantification, the comparative threshold cycle (Ct) method was used, and the delta Ct comparison was used to compare gene expression in cells and tissues. Individual data (−delta Ct) were presented to comply with BJP guidelines (Curtis et al., 2018). Fold induction was calculated as 2−ΔΔCt for cellular and in vivo experiments and was presented in the Supporting Information.
2.5. Calcium measurement
CHO cells stably expressing human LPA receptors were grown in 96‐well poly‐d‐lysine‐coated black‐wall clear‐bottom plates (BioCoat, BD Biosciences, Franklin Lakes, USA) at 20,000–40,000 cells per well and cultured 48 h in complete medium. Cells were then washed with assay buffer and loaded for 1 h at 37°C with 100 μl of FLIPR Calcium 4 dye (Molecular Devices, Sunnyvale, USA) in HBSS supplemented with 20‐mM HEPES, 2‐mM probenecid and 0.3% fatty acid‐free human serum albumin. LPA1 receptor antagonists (in 25 μl of 1% DMSO) were added to each well and incubated at room temperature for 30 min. LPA (in HBSS and 0.3% fatty acid‐free human serum albumin) was added after 15 s of baseline measurement. The final concentrations of LPA used to test the antagonists corresponded to the LPA EC80 response on each receptor. They were as follows: 150‐nM LPA for LPA1 assays, 200‐nM LPA for LPA3 assays, 500‐nM LPA for LPA2 and 30‐nM LPA for LPA5 assays. Intracellular calcium mobilization was measured using a FLIPR Calcium Assay Kit (Molecular Devices). The antagonistic activity of SAR100842 was evaluated against various LPA isoforms (16:0, 18:0, 18:1, 18:3 or 20:4, at their EC80) in the CHO–LPAR1 cell lines. Inhibition curves were generated by plotting the percentage inhibition of calcium flux versus log10 of the compound concentration. The IC50 was calculated by nonlinear regression using the sigmoidal dose–response (variable slope) equation with the Prism 5 software (GraphPad Prism, RRID:SCR_002798).
When dermal fibroblasts from systemic sclerosis patients were used for calcium experiments, they were plated in 96‐well collagen‐coated black‐wall clear‐bottom plates (BioCoat, BD Biosciences, Franklin Lakes, USA) at 30,000 cells per well and cultured for 48 h in complete medium. Cells were then washed with assay buffer and loaded for 1 h at 37°C with 100 μl of FLIPR Calcium 4 dye (Molecular Devices) in HBSS supplemented with 20‐mM HEPES, 2‐mM probenecid, and 0.3% fatty acid‐free human serum albumin. Cells were then washed with assay buffer and incubated at room temperature for 20 min with 100 μl of assay buffer. LPA (in HBSS and 0.3% fatty acid‐free human serum albumin) was added after 30 s of baseline measurement. Intracellular calcium mobilization was measured using a FLIPR Calcium Assay Kit (Molecular Devices) with FLIPR TETRA (Molecular Devices). SAR100842 (in 25 μl of 1% DMSO) was added 30 s after basal measurement and incubated for 4 min before adding 1‐μM LPA (EC80 of LPA response in these cells). For Gi/Go coupling assays, cells were pre‐incubated overnight with 50 ng·ml−1 of pertussis toxin (Sigma, P7208) before the addition of LPA.
2.6. Quantification of CXCL1, IL‐6 and CCL2
Cytokine measurements were performed with a Luminex assay according to the manufacturer's instructions (Bio‐Plex Reagent Kit, Bio‐Rad Laboratories, Hercules, USA). Cell supernatants were incubated with magnetic beads coupled with the capture antibody for 30 min at room temperature. Then, the biotinylated detection antibody was incubated for 30 min at room temperature, and streptavidin–phycoerythrin conjugate was added for 10 min for detection. Readouts were performed with the Bio‐Plex 200 System (Bio‐Rad Laboratories, RRID:SCR_018026), and cytokine concentrations were calculated using specific standards. The ratio of the specific level of each cytokine in the supernatant after LPA treatment versus its basal level after vehicle treatment was used to represent the data (ratio vs. control).
2.7. Immunoblotting
Systemic sclerosis dermal fibroblasts or lung fibroblasts were suspended in Laemmli buffer to extract cell content and surrounding matrix material. After addition of a reducing agent (DTT) and heating at 95°C for 10 min, samples were frozen at −20°C until immunoblotting. Samples were separated in NuPAGE 3–8% Tris‐Acetate (Novex, Life Technologies) using Tris‐acetate buffer for 1 h and 30 min. They were then transferred onto nitrocellulose membranes for 1 h and 30 min. The membranes were stained with Ponceau to ensure efficacy of transfer and were saturated 1 h with TBS + 0.1% Tween 20 (TTBS) + 5% skimmed milk. Membranes were then incubated overnight at 4°C with TTBS + 1% skimmed milk and monoclonal antibodies against αSMA (1/2,000) (Sigma‐Aldrich Cat# A2547, RRID:AB_476701), cellular fibronectin (1/5,000) (Sigma‐Aldrich Cat# F6140, RRID:AB_476981) and vimentin (1/1,000) (Sigma‐Aldrich Cat# V5255, RRID:AB_477625) used as control. The next day after washing with TTBS, membranes were incubated with an HRP goat anti‐mouse Ig (1/2,500) (Ref. 554002 BD Pharmingen) for 1 h in TTBS + 1% skim milk. After two washes in TTBS and one in TBS all for 15 min, membranes were revealed with 4 ml of ECL (ECL Western Blotting Reagent, Amersham), and luminescence was directly quantified with a G:BOX imaging system (Syngene, Cambridge, UK). The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
2.8. Tsk1 mouse model
All animal care and experimental protocols followed the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee of the Government of Mittelfranken. The animal procedures were conducted in compliance with the German legislation. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. To determine group size, power calculation was based on previous experiments and experience in this model (Soare, Ramming, Avouac, & Distler, 2016). Male Tsk1 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine 04609, USA) and used as described (Maurer et al., 2014). Four groups of mice (n = 7 per group) were used. Tsk1 mice were treated for 5 weeks with (a) imatinib at 50 mg·kg−1 q.i.d. i.p. (positive control) (Maurer et al., 2013), (b) SAR100842 at 30 mg·kg−1 b.i.d. p.o. or (c) vehicle (methyl cellulose 0.5% + Tween80 0.2%). The fourth group consisted of pa/pa control mice of the same genetic background not carrying the Tsk1 mutation receiving the vehicle. Randomization was performed on weight and sex before initiation of treatment and equal number of animals was used for each treatment group. The treatment was initiated in 5‐week‐old mice.
2.8.1. Collagen measurements
The collagen content was quantified using the hydroxyproline assay as described (Palumbo‐Zerr et al., 2015). Tissue samples were digested in 500 μl of 6‐M hydrochloric acid at 120°C for 3 h. After adjusting the pH to 6–7, chloramine T (0.06 M) was added to 250 μl of each sample. Samples were mixed and incubated for 20 min at room temperature. Afterwards, 3.15‐M perchloric acid and 20% p‐dimethylaminobenzaldehyde were added and samples were incubated for 20 min at 60°C. The absorbance was determined at 557 nm with an Epoch microplate spectrophotometer (BioTek Instruments).
2.8.2. Histological analysis, detection of myofibroblasts and skin thickness
For the Tsk1 model, hypodermal thickness was determined by measuring the thickness of the subcutaneous connective tissue beneath the panniculus carnosus. Dermal thickness was analysed at 100‐fold magnification by measuring the maximal distance between the epidermal–dermal junction and the dermal subcutaneous fat junction in three consecutive skin sections. Four different sites in each mouse were measured in a blinded manner.
For quantification of myofibroblasts, skin sections were stained with monoclonal anti‐αSMA antibodies (Sigma‐Aldrich Cat# A2547, RRID:AB_476701) and visualized with 3,3‐diaminobenzidine tetrahydrochloride (DAB). To determine the number of activated fibroblasts (myofibroblasts) as another parameter of skin fibrosis, pictures at ×100 magnification were taken, and spindle‐shaped, αSMA+ cells not part of vascular structures were counted in three randomly chosen HPF per slide. The myofibroblast count was determined in the dermis without the epidermal and the subdermal layers.
The analyses of all histological and immunohistochemical staining were performed by an examiner blinded with regard to the different groups. All slides were analysed twice. In case of a variation of the results >10%, the respective slides were re‐assessed to reach consensus. Pictures were taken with a digital camera on an Imager1 microscope (Carl Zeiss AG, Feldbach, Switzerland), using AxioVision software Release 4.6 (AxioVision Imaging System, RRID:SCR_002677).
2.9. Analysis of the data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
Group size is the number of independent values, and statistical analysis (n > 5) was done using these independent values (i.e. not treating technical replicates as independent values).
When the number of repeated experiments (n) was <5, data were referred as preliminary. This was the case only when cytokines measurements were performed in cell supernatant. The number of experiments was limited to n = 4 as it was only to further confirm gene expression results.
2.9.1 Summary of the statistical methods
For human LPAR expression, statistical analyses were performed to evaluate the difference of expression of LPAR1 versus the other LPA receptors. An ANOVA was performed on factors “Receptor” (repeated) and “Type of donor” (for dermal fibrosis) followed by Dunnett's test.
For Ccl2, Cxcl1, Sfrp4 and Wnt2 2 gene expression in the Tsk1 model, statistical analyses were performed to evaluate treatment (imatinib or SAR100842) effect. An ANOVA followed by Dunnett's test was performed.
For αSMA gene expression and SFRP4 and WNT2 markers in cells, statistical analyses were performed to evaluate the LPA and/or SAR100842 effect versus control and then the LPA + SAR100842 treatment effect versus LPA. A pairwise Student's t‐test or repeated ANOVA followed by Dunnett's test was performed.
For all (−delta Ct) value analyses, the variance heterogeneity was taken into account if necessary. A rank transformation was applied in case of violation of ANOVA assumptions. For analyses performed on rank transformed data, estimates of the differences between groups and associated 95% confidence intervals were calculated with Hodge–Lehmann estimator. The fold changes and associated 95% confidence intervals were presented.
For the quantification of αSMA in western blot or cytokines secretion, data were expressed as ratio versus matched control values. Statistical analyses were performed to evaluate the LPA effect and then for αSMA expression the SAR100842 treatment effect. In the case of two groups, Student's t‐test versus constant 1 was performed. In case of several groups, a repeated ANOVA was performed followed by Dunnett's test. A rank transformation was applied on αSMA expression due to violation of ANOVA assumptions.
For the animal model, application conditions for parametric analysis were evaluated using Shapiro–Wilk test for normality and Levene's test for the homogeneity of variances. Hydroxyproline was analysed using a one‐way ANOVA followed by multiple comparisons. αSMA and skin thickness were analysed using Kruskal–Wallis test followed by multiple comparisons. Multiple comparison P values were corrected using the Bonferroni–Holm method.
Data are expressed as mean ± SD if not otherwise indicated. Samples sizes subjected to statistical analysis when n = 5 at least whene n = number of independent values. For Tsk1 model, 7 animals per grop were analyzed. All fold analyses derived from the different figures are provided with statistical analysis as the Supporting Information. A single P value of less than 0.05 was considered statistically significant. Potential outliers were included in data analysis and presentation unless specified in results. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA, Statistical Analysis System, RRID:SCR_008567) on Windows 7 PC.
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. SAR100842 is a selective LPA1 receptor antagonist and inhibits LPA contained in serum of systemic sclerosis patients
Since LPA exists as different polyunsaturated forms in human fluids, SAR100842 was tested against the most common forms of LPA including LPA 20:4, which is increased in the serum of patients with systemic sclerosis (Tokumura et al., 2009). In CHO cells stably expressing human LPAR1, SAR100842 was more potent on LPA 16:0 and LPA 18:0 than against LPA 18:1, LPA 18:3 and LPA 20:4, tested at concentrations found in the circulation of systemic sclerosis patients (from 0.1 to 10 μM) (Figure 1a and Table 1). SAR100842 was inactive on LPA2, LPA3 and LPA5 receptors up to 10 μM on these receptors (Table S2). In an attempt to characterize the antagonistic activity of SAR100842 against LPA6 receptors, SAR100842 was tested in human endothelial cells expressing endogenous LPAR2 and LPAR6 and using IL‐6 as marker of LPA6 engagement (Table S3). Although SAR100842 at 10 μM diminished by 50% IL‐6 secretion induced by LPA, this assay might not be completely appropriate to precisely determine IC50 on LPA6, as a high concentration of LPA (50 μM) was required to produce IL‐6 secretion (Figure S1). However, according to the IUPHAR/BPS database, endothelial cells are considered to be suitable for measuring LPA6 receptor activity and high LPA concentrations have been previously reported by others to be necessary to activate LPA6 (Yung, Stoddard, & Chun, 2014).
FIGURE 1.

SAR100842 inhibits functionally active forms of lysophosphatidic acid (LPA). (a) The inhibitory effects of SAR100842 on the different LPA isoforms (EC80) were analysed by measuring the LPA1 receptor‐induced calcium response in the CHO–LPAR1 cell lines (n = 6–10). No significant difference was observed in the potency of SAR100842 on LPA 18:1 (blue circle), LPA 18:3 (blue square) or LPA 20:4 (blue triangle). SAR100842 is more potent on LPA unsaturated forms, LPA 16:0 (dark triangle) and LPA 18:0 (dark square). IC50s are provided in Table 1. (b) LPA contained in 0.3% and 1% serum of systemic sclerosis (SSc) donors (n = 6) induced a calcium response in the CHO–hLPAR1 cell line that was limited in control CHO cells. (c) Calcium response induced by LPA contained in 0.3% and 1% serum of systemic sclerosis (SSc) patients was fully inhibited by SAR100842 in the CHO–hLPAR1 cell line (n = 6 patients)
TABLE 1.
SAR100842 IC50 against lysophosphatidic acid (LPA) isoforms in CHO cells expressing recombinant human LPA1 receptor
| LPA isoforms | SAR100842 IC50 (nM) | 95% CI |
|---|---|---|
| LPA 16:0 | 59 | 48–73 |
| LPA 18:0 | 64 | 47–85 |
| LPA 18:1 | 262 | 140–490 |
| LPA 18:3 | 156 | 122–199 |
| LPA 20:4 | 173 | 130–230 |
Note: Data represent the geometric mean + 95% confidence interval (CI) (n = 6–10).
In the absence of cell line expressing solely endogenous human LPA4, SAR100842 was not tested on this receptor. Of note, LPA4 receptor is barely detectable in all the cells and tissues used in this study and therefore not included in the results. In addition, human LPA1, LPA2 and LPA3 belong to the Edg family receptors, while human LPA4, LPA5 and LPA6 belong to the purinergic family of receptors, which are a large distance apart on GPCR phylogenic tree and thus have very different binding sites. This makes the probability to identify mixed LPA1–3/4–6 antagonists more challenging. Interestingly, H2L5765834 has been reported as a selective LPA1,3,5 antagonist, but to our knowledge its effect has not been investigated in fibroblasts or in a fibrotic context (Williams et al., 2009). In summary, these results indicate that SAR100842 is selective for LPA1 receptor with some activity at the LPA6 receptor which needs to be further refined in the future when appropriate cellular models will be available.
Serum LPA can be produced from phospholipid precursors either in membranes of blood cells or in plasma by sequential actions of phospholipases present in plasma or expressed by blood cells. On average, serum LPA concentration is in the micromolar range and is partly bound to albumin. In our experimental conditions, human serum from patients with systemic sclerosis induced up to 1% concentration a specific LPA‐dependent calcium response in CHO cells overexpressing human LPA1 receptor that was limited in control CHO cells (Figure 1b). SAR100842 alone did not cause a significant response in control CHO cells, but completely inhibited the calcium response induced by the LPA in the serum from patients with systemic sclerosis (n = 6 patients) in CHO–LPAR1 cells (Figure 1c).
3.2. LPA1 receptor is highly expressed on systemic sclerosis dermal fibroblasts
Experiments were performed in dermal fibroblasts from systemic sclerosis patients to bring translational value to the study. Experiments were performed on skin fibroblasts from dSSc (diffuse systemic sclerosis) and lSSc (limited systemic sclerosis) patients to confirm that they maintained their inflammatory and fibrotic phenotype in culture. Basal expression of the set of genes involved in inflammatory and fibrotic processes was significantly higher when compared with dermal fibroblast from normal donors (Table S4).
The expression of the different LPA receptors was measured by RT‐PCR in dermal fibroblasts from systemic sclerosis patients (Figure 2a). LPAR1 expression was higher than the expression of other LPA receptor subtypes. Consistently, LPAR1 expression was also elevated compared with other LPA receptors in skin biopsies obtained from systemic sclerosis patients (n = 28) (Figure 2b). Although indicative of the predominant expression of LPA1 receptor, we acknowledge that this does not necessary correlate with its cell surface protein expression levels and would have to be confirmed upon the availability of selective LPA receptors antibodies for FAC studies.
FIGURE 2.

LPA1 receptor is the key driver of lysophosphatidic acid (LPA) responses in dermal fibroblasts from systemic sclerosis (SSc) patients. (a) LPA receptor expression was measured by RT‐PCR in dermal fibroblasts from SSc patients (n = 10). Each black symbol represents data obtained from a different patient, and data were normalized using β2M (β2 microglobulin) as a housekeeping gene. LPAR1 expression was significantly higher compared with the other LPA receptors (P < 0.05). (b) LPA receptor expression was measured by RT‐PCR in skin biopsies from SSc patients (n = 28). Each blue symbol represents data obtained from a different patient, and data were normalized using RPLP0 as a housekeeping gene. LPAR1 expression was significantly higher compared with the other LPA receptors (P < 0.05). (c) SSc dermal fibroblasts were incubated overnight with (grey) or without (black) 50 ng·ml−1 of pertussis toxin (PTX) to inhibit Gi‐coupled responses before stimulation with increasing concentrations of LPA (18:1). LPA‐induced calcium response was inhibited by 90% by PTX (n = 4). (d) Increasing concentration of SAR100842, a selective LPA1 receptor antagonist, fully inhibited LPA (100 nM)‐induced calcium response in dermal fibroblasts from SSc patients (n = 12)
LPA (18:1) induced a concentration‐dependent transient increase in cytosolic calcium with an EC50 of 150 ± 50 nM in systemic sclerosis dermal fibroblasts (Figure 2c). This effect was blocked by pertussis toxin, a Gi/Go protein inhibitor, indicating Gi/Go coupling of the receptor (Figure 2c). Similar Gi pathway activation has been observed in normal human skin fibroblasts (Pietruck, Busch, Virchow, Brockmeyer, & Siffert, 1997). SAR100842 induced a concentration‐dependent inhibition (mean IC50 237 nM with 95% confidence interval [166–339 nM]) of the LPA 18:1‐induced calcium response (n = 12), confirming that LPA1 receptor is the main driver of the LPA response in systemic sclerosis dermal fibroblasts (Figure 2d).
3.3. LPA1 receptor activation induces myofibroblast differentiation in systemic sclerosis and idiopathic pulmonary fibrosis fibroblasts
Experiments were performed in dermal fibroblasts from systemic sclerosis patients to evaluate the effect of LPA on myofibroblast differentiation with αSMA and cellular fibronectin as markers of this differentiation. Heterogeneity was observed in the responses from patients to patients with cells expressing high basal level of αSMA causing a degree of uncertainty in the evaluation of LPA response. To further confirm the effect of LPA on myofibroblast differentiation, we studied its effect on pulmonary fibroblasts from idiopathic pulmonary fibrosis patients, where responses were easier to calibrate. αSMA expression measured in western blot was used as a marker of differentiation. LPA induced a dose‐dependent differentiation of idiopathic pulmonary fibrosis fibroblasts into myofibroblasts (Figure 3a). This effect was blocked by SAR100842 in a concentration‐dependent manner, indicating that LPA1 receptors more likely to be playing a central role of in this differentiation process (Figure 3b,c). The potency of SAR100842 in this assay was in the same range (~100 nM) as that determined on the calcium response in the same fibroblasts or in CHO expressing LPAR1. αSMA gene expression was also measured in the same primary cultures of idiopathic pulmonary fibrosis fibroblasts to confirm this important observation in another technique. LPA‐induced αSMA gene expression was significantly blocked by SAR100842 (Figure 3d).
FIGURE 3.

Lysophosphatidic acid (LPA) induces α‐smooth muscle actin (αSMA) expression in lung fibroblast from idiopathic pulmonary fibrosis (IP) patients. Myofibroblast differentiation was evaluated by Western blot. αSMA was measured as an index of myofibroblast differentiation, while vimentin was used for normalization. (a) Increasing concentrations of LPA in serum‐free medium induced IPF fibroblasts differentiation after 48 h (one blot representative of three experiments performed with three different primary cultures). (b) The effect of LPA was blocked by increasing concentrations of the LPA1 receptor antagonist SAR100842 (one blot representative of six experiments performed with five different primary cultures of IPF lung fibroblasts). (c) Quantitative analysis was performed using vimentin for normalization and the mean of six experiments on αSMA expression. *A two‐tailed Dunnett's test versus LPA for factor treatment was performed. Differences were considered significant when P < 0.05. (d) αSMA gene expression was measured in eight primary cultures of idiopathic pulmonary fibrosis (IPF) fibroblasts. LPA (10 μM) induced a significant increase (at least above twofold) in all primary cultures (cf. the Supporting Information). This effect was fully blocked by SAR100842 at 10 μM in all primary cultures tested (n = 6)
3.4. LPA1 receptor antagonism reverses fibrosis in the Tsk1 model
Bleomycin‐induced skin fibrosis reproduces the inflammatory stages of systemic sclerosis, with collagen deposition, myofibroblast activation and increased dermal thickness. In this model, SAR100842 reversed dermal thickening, significantly inhibited myofibroblast differentiation, and reduced collagen content in mouse skin (Illiano et al., 2013). These results are also in agreement with studies performed in mice lacking Lpar1, which are resistant to bleomycin‐induced dermal fibrosis (Castelino et al., 2011). Studies in the Tsk1 model, which mimics the later, non‐inflammatory stages of systemic sclerosis (Jordan, Chung, & Distler, 2013), indicated that the expression of the different LPA receptors in the skin was similar between Tsk1 and pa control mice with high expression of Lpar1, Lpar2 and Lpar5 (Figure S3). However, hydroxyproline content (Figure 4a), number of skin myofibroblasts (Figure 4b) and hypodermal thickness (Figure 4c,d) were increased in Tsk1 mice compared with pa control mice.
FIGURE 4.

SAR100842 reverses fibrosis and decreases dermal thickening in a model of skin fibrosis. Each group includes seven different animals for which individual data with median +95% CI are presented. In Tsk1 mice, SAR100842 inhibited hydroxyproline content (a), the number of myofibroblasts (b), and hypodermal thickness (c) after 5 weeks of treatment. Hydroxyproline was analysed using a one‐way ANOVA followed by multiple comparisons. The hypodermal thickness was determined by measuring the thickness of the subcutaneous connective tissue beneath the panniculus carnosus at four different sites at the upper back in each mouse. (d) α‐Smooth muscle actin (αSMA) and skin thickness were analysed using Kruskal–Wallis test followed by multiple comparisons. *P < 0.05
Administration of SAR100842 markedly reduced hypodermal thickening (−39%), the number of skin myofibroblasts (−54%) and hydroxyproline content (−33%) in Tsk1 mice compared with vehicle‐treated Tsk1 mice (Figure 4a–d).
3.5. Inhibition of inflammatory markers in systemic sclerosis dermal fibroblasts and Tsk1 mouse skin following SAR100842 treatment signalling
LPA induced CXCL1, CCL2 and IL‐6 protein secretion in the supernatant of cells treated with LPA for 24 h (Figure 5a). These exploratory data (n = 4) were backed up by gene expression in dermal systemic sclerosis fibroblast at physiological concentrations (Figure S1). LPA‐induced protein secretion of IL‐6, CCL2 and CXCL1 was inhibited in a concentration‐dependent manner by SAR100842 with IC50s of 27.1 ± 15.7, 34.3 ± 13.1, and 24.8 ± 5.2 nM, respectively (Figure 5b). To determine LPA1 receptor engagement in the Tsk1 model, IL‐6, CCL2 and CXCL1 skin expression was quantified. After 5 weeks of treatment, SAR100842 inhibited CXCL1 and CCL2 expression (Figure 5c), suggesting that these cytokines could reflect LPA1 involvement in the disease process of this model. IL‐6 expression level in the skin was too low to evaluate the effect of SAR100842.
FIGURE 5.

Inflammatory markers induced by lysophosphatidic acid (LPA) in dermal systemic sclerosis (SSc) fibroblasts are inhibited by SAR100842 in the skin of Tsk1 mice. (a) SSc dermal fibroblasts were incubated for 24 h with LPA (10 μM) in serum‐free medium and CXCL1, CCL2 and IL‐6 secretion were measured by elisa. LPA induced a significant increase of all three cytokines (n = 4, exploratory data) confirming gene expression data. (b) SAR100842 caused a concentration‐dependent inhibition of the LPA‐induced secretion of CXCL1 (black dot), CCL2 (dark grey dot) and IL‐6 (light grey dot) in SSc dermal fibroblasts with an average IC50 around 50 nM (n = 4). (c) Inflammatory markers CXCL1, CCL2 and IL‐6 were measured in Tsk1 mouse skin by RT‐PCR following 5 weeks' treatment with SAR100842 and imatinib. IL‐6 was expressed at very low levels in the skin of Tsk1 mice, but both the expression of CXCL1 and CCL2 were reduced by SAR100842 (n = 7)
3.6. Inhibition of LPA1 receptor interferes with Wnt signalling in systemic sclerosis fibroblasts and Tsk1 skin
Most interestingly, we found that markers of Wnt signalling were up‐regulated in systemic sclerosis dermal fibroblasts after LPA stimulation and inhibited by SAR100842 (Figure 6a). The Wnt pathway has been shown to play a major role in the development of fibrotic diseases (Akhmetshina et al., 2012), and Wnt2 and Sfrp4 expression is up‐regulated in Tsk1 mouse skin (Bayle et al., 2008). LPA‐induced Wnt2 and Sfrp4 expression in systemic sclerosis dermal fibroblasts was inhibited by SAR100842 in a concentration‐dependent manner (Figure 6a,b). Consistently, Wnt2 and Sfrp4 skin gene expression in Tsk1 mouse was inhibited by SAR100842 treatment (Figure 6c).
FIGURE 6.
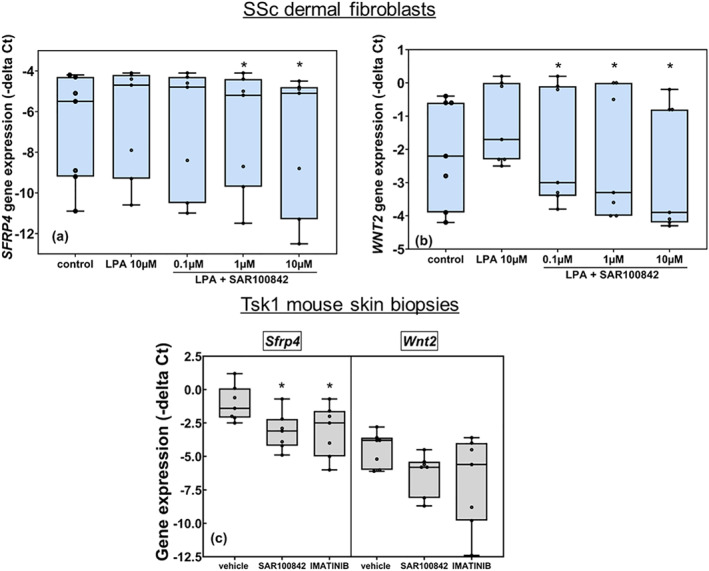
Wnt pathway is involved in LPA1 response in dermal systemic sclerosis (SSc) fibroblasts and in the skin of Tsk1 mice. (a, b) SSc dermal fibroblasts were incubated for 24 h with lysophosphatidic acid (LPA) (10 μM) in serum‐free medium, and expression of markers of the Wnt pathway was measured by RT‐PCR (n = 8). SFRP4 and WNT2 were significantly induced by LPA (10 μM) and inhibited by SAR100842 in a concentration‐dependent manner (*P < 0.05 vs. LPA). The Newman–Keuls test was performed for each level of LPA factor (CT and LPA), and Dunnett's test was performed to compare treatment groups (SAR100842 at 1, 3, and 10 μM). (c) SFRP4 and WNT2 were inhibited in the skin of Tsk1 mice following treatment with SAR100842 (n = 7). Since the normality and the homogeneity of variances hypothesis were not fulfilled, Kruskal–Wallis test on factor treatment is done. The decrease of SFRP4 was significant (*P < 0.05)
4. DISCUSSION
Several lines of evidence suggest that lysophosphatidic acid (LPA) could play a role in the pathogenesis of systemic sclerosis. LPA levels are increased in the serum of systemic sclerosis patients in comparison with healthy subjects (Tokumura et al., 2009). LPA generation is consistently increased in human skin under inflammatory condition (Mazereeuw‐Hautier et al., 2005). Studies performed in Lpar1–ko mice have highlighted the contribution of this mechanism in skin fibrosis, but no study has investigated the role of LPA1 receptor in dermal fibroblasts from systemic sclerosis patients or in less inflammatory systemic sclerosis model like the Tsk1 mouse using therapeutic protocols. Novelty comes from the results obtained on dermal fibroblasts from systemic sclerosis patients, which have not been reported before with such level of detail and from the efficacy of LPA1 receptor blockade in reducing skin fibrosis in a low‐grade inflammatory‐driven systemic sclerosis mouse model.
In the Tsk1 mouse model, a large in‐frame duplication of exons 17–40 of the fibrillin gene leads to the production of a larger form of fibrillin resulting in dermal and hypodermal fibrosis. The altered fibrillin protein facilitates overproduction of matrix proteins, even in the absence of inflammatory infiltrates. Even in the absence of overt inflammatory infiltrates, the Tsk1 model mimics the persistently activated phenotype of systemic sclerosis fibroblasts at later disease stages. As a limitation, major fibrotic changes in this model occur in the hypodermis rather than in the dermis as in human systemic sclerosis. In the Tsk1 mouse model, SAR100842 was able to significantly decrease hypodermal thickening, to inhibit myofibroblast differentiation, and to reduce collagen content in the skin to levels comparable with those obtained after treatment with imatinib. These results are in agreement with studies performed in mice lacking Lpar1, which are resistant to bleomycin‐induced dermal fibrosis or with similar results obtained with other LPA1 receptor antagonists or SAR100842 in bleomycin model of skin fibrosis (Castelino et al., 2011; Illiano et al., 2013). Altogether, this supports the potential therapeutic benefit of LPA1 receptor antagonists as anti‐fibrotic agents.
To confirm the translational value of these in vivo results, experiments were performed with dermal fibroblasts obtained from patients with systemic sclerosis. These cells expressed high levels of LPAR1, and LPA functional response was fully inhibited by SAR100842. SAR100842 was equipotent against different monounsaturated and polyunsaturated forms of LPA including the 20:4 form, which is the most representative form in serum of systemic sclerosis patients (Tokumura et al., 2009). Calcium response of CHO cells overexpressing recombinant LPA1 to serum from systemic sclerosis patients was fully blocked by SAR100842 indicating the contribution of disease‐related LPA in this setting. LPA induced αSMA and fibronectin expression in dermal fibroblasts from systemic sclerosis patients and in lung fibroblasts from idiopathic pulmonary fibrosis patients. Both are known markers of myofibroblast differentiation and are increased in the skin of systemic sclerosis patients. Myofibroblast activation is the hallmark of fibrosis. This effect was fully blocked by SAR100842. In addition, in systemic sclerosis fibroblasts, LPA induced the expression of markers of the Wnt pathway. We focused on WNT2 and SFRP4, known to be overexpressed in the skin of systemic sclerosis patients (Wei et al., 2012). In our experimental settings, LPA induced the expression of WNT2 and SFRP4 in dermal fibroblasts from systemic sclerosis patients and these effects were inhibited by SAR100842 as observed in Tsk1 mice. These new data suggest that some of the anti‐fibrotic effects of LPA1 receptor blockade could be partly mediated via the inhibition of the Wnt signalling pathway.
Consistent with the anti‐fibrotic effects observed in the animal models, changes in the expression of other well‐known inflammatory markers of systemic sclerosis such as IL‐6 and CCL2 were observed after stimulation with LPA. Among chemokines, CCL2 has been identified as the most critical chemokine for tissue fibrosis and inflammation in systemic sclerosis (Denton et al., 1998). In patients with systemic sclerosis, CCL2 levels are increased in serum, along with enhanced CCL2 expression in the epidermis, inflammatory mononuclear cells, and endothelial cells. In systemic sclerosis dermal fibroblasts, SAR100842 also inhibited the secretion of these markers, and LPA1 receptor engagement was also confirmed in the skin of Tsk1 mice, where CXCL1 and CCL2 expression was decreased by SAR100842. Beyond its profibrotic effects and effects on cytoskeleton reorganization, LPA strongly stimulates the motility of human neutrophils, increased the binding of monocytes and neutrophils to human aortic endothelial cells (Idzko et al., 2004), and induced de‐differentiation of vascular smooth muscle cells (Zhou, Niu, & Zhang, 2009). Thus, in addition to fibrosis, LPA could play a role in several main mechanisms involved in the pathogenesis of systemic sclerosis including microvasculopathy and inflammation (Tigyi & Parrill, 2003). As a limitation of our study, the effects of SAR100842 on the specific subtypes of inflammatory cells in vivo could not be analysed and this has been well documented by others (Castelino et al., 2016). Our data on dermal fibroblasts from systemic sclerosis patients are bringing additional translational value to these findings and are the first to show that LPA can activate WNT pathway under fibrotic conditions.
Overall, our unprecedented results obtained with SAR100842 in animal models of skin fibrosis using therapeutics protocols and our data in dermal fibroblasts from systemic sclerosis patients indicate that the inhibition of LPA1 receptor signalling could be a promising therapeutic strategy in both patients with inflammatory as well as non‐inflammatory subtypes of systemic sclerosis. Indeed, a recently performed Phase 2 trial with SAR100842 demonstrated the safety of the approach in these patients (Allanore et al., 2018; Khanna et al., 2015).
AUTHOR CONTRIBUTIONS
L.L. has organized and realized in vitro experiments with systemic sclerosis fibroblasts. B.L. has realized in vitro experiments with systemic sclerosis fibroblast more specifically than calcium experiments. C.D., C.B., and A.D. have been involved in the in live experiment with Tsk1 model including IHC measurements. S.V. has realized the primary culture of systemic sclerosis fibroblasts and been involved in their characterization. R.B. has organized and realized some in vitro experiments with CHO cells. J.P.B. has organized the planning and the design of the pharmacological experiments. M.S. has realized and supervised the original experiments allowing the identification of SAR100842 and is a co‐inventor of the patent on SAR100842. J.P. has synthetized SAR100842 and is at the origin of the patent of the product. H.R. has supervised studies leading to the identification of SAR100842. A.J. has contributed to the design of clinical study with SAR100842. P.J. has contributed to the writing of the publication. J.H.W.D. and O.D. have supervised the pharmacological experiments and have contributed to the overall design of the study and the writing of the publication. S.I. has supervised the in vitro experiments and the translational aspect of the experiments and has contributed to the overall design of the study and the writing of the publication.
CONFLICT OF INTEREST
J.H.W.D. has consultancy relationships with Actelion, Active Biotech, Anamar, ARXX, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, GSK, Inventiva, JB Therapeutics, Medac, Pfizer, RuiYi and UCB. J.H.W.D. has received research funding from Anamar, Active Biotech, Array BioPharma, ARXX, aTyr, Bristol‐Myers Squibb, Bayer, Boehringer Ingelheim, Celgene, Galapagos NV, GlaxoSmithKline, Inventiva, Novartis, Sanofi, RedX, and UCB. J.H.W.D. is stock owner of 4D Science. O.D. has/had consultancy relationship and/or has received research funding from AbbVie, Actelion Pharmaceuticals, Acceleron, Amgen, AnaMar, ARXX, Baecon Discovery, Blade Therapeutics, Bayer, Boehringer Ingelheim, Catenion, Competitive Corpus, Drug Development International Ltd, CSL Behring, ChemomAb, Ergonex, Galapagos NV, Glenmark Pharmaceuticals, GSK, Horizon (Curzion) Pharmaceuticals, Inventiva, Italfarmaco, iQone, iQvia, Kymera Therapeutics, Eli Lilly and Company, medac, Medscape, Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme, Novartis, Pfizer, Roche, Sanofi, Target Bio Science and UCB in the area of potential treatments of scleroderma and its complications. In addition, he has a patent miR‐29 for the treatment of systemic sclerosis issued (US8247389 and EP2331143). S.V. received consultancy fees from Boehringer‐Ingelheim and Thermo‐Fischer, speeking fees and/or educational support from Abbvie, Roche, Pfizer, BMS. L.L., B.L., R.B., M.S., J.P., H.R., A.J., P.J. and S.I. are Sanofi's employees.
The real or perceived potential conflicts listed above are accurately stated.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Table S1. Clinical characterization of patients used for the generation of primary fibroblast cultures (lcSSc = limited SSc / dcSSc=diffuse SSc)
Table S2. SAR100842 selectivity on LPA receptors
Table S3. LPARs expression in endothelial cells
Figure S1. Effect of SAR100842 on IL6 secretion induced by LPA in endothelial cells4
Figure S2. LPARs expression pattern in control dermal fibroblasts
Table S4. Basal gene expression measured by RTPCR in dermal fibroblasts from SSc patients used throughout the study.
Figure S3. Induction of CXCL1. IL6 and CCL2 gene expression by LPA in SSc dermal fibroblasts
Figure S4. Expression of LPA receptors in the skin of Tsk1 and pa control mouse.
Figure S2A: Results (fold change vs LPAR1) from statistical analysis of LPA receptors expression in dermal fibroblasts of SSc donors
Figure S2B: Results (fold change vs LPAR1) from statistical analysis of LPA receptors expression in skin biopsies of SSc donors
Figure S3C: Effect of LPA on the induction of αSMA measured in WB.
Figure S3D: Effect of LPA on the induction of αSMA gene expression.
Figure S5A: Effect of LPA on CCl2. CXCl1 and IL6 secretion.
Figure S5C and S6C: effect of SAR100842 on gene expression in Tsk1 skin
Figure S6A and S6B: Effect of SAR100842 on sfrp4 and wnt2 gene expression in SSc dermal fibroblasts
ACKNOWLEDGEMENTS
Special thanks to Dorothée Tamarelle and Mathieu Boucher for the statistical analysis of data presented in this paper and to Charlotte Saint‐Omer for performing some additional experiments with the different LPA isoforms. This study was supported by Sanofi.
Ledein L, Léger B, Dees C, et al. Translational engagement of lysophosphatidic acid receptor 1 in skin fibrosis: from dermal fibroblasts of patients with scleroderma to tight skin 1 mouse. Br J Pharmacol. 2020;177:4296–4309. 10.1111/bph.15190
REFERENCES
- Abraham, D. J. , Krieg, T. , Distler, J. , & Distler, O. (2009). Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford), 48(Suppl 3), 3–7. [DOI] [PubMed] [Google Scholar]
- Akhmetshina, A. , Palumbo, K. , Dees, C. , Bergmann, C. , Venalis, P. , Zerr, P. , … Distler, J. H. W. (2012). Activation of canonical Wnt signalling is required for TGF‐β‐mediated fibrosis. Nature Communications, 3, 1–12. 10.1038/ncomms1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanore, Y. , Distler, O. , Jagerschmidt, A. , Illiano, S. , Ledein, L. , Boitier, E. , … Khanna, D. (2018). Lysophosphatidic acid receptor 1 antagonist sar100842 for patients with diffuse cutaneous systemic sclerosis. Arthritis & Rheumatology, 70(10), 1634–1643. 10.1002/art.40547 [DOI] [PubMed] [Google Scholar]
- Bayle, J. , Fitch, J. , Jacobsen, K. , Kumar, R. , Lafyatis, R. , & Lemaire, R. (2008). Increased expression of wnt2 and sfrp4 in tsk mouse skin: role of wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. Journal of Investigative Dermatology, 128(4), 871–881. 10.1038/sj.jid.5701101 [DOI] [PubMed] [Google Scholar]
- Blaho, V. , Chun, J. , Frantz, A. , Hla, T. , Jones, D. , Jonnalagadda, D. , … Yung, Y. C. (2019). Lysophospholipid (LPA) receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE, 2019(4), 1–9. 10.2218/gtopdb/F36/2019.4 [DOI] [Google Scholar]
- Castelino, F. , Bain, G. , Pace, V. , Black, K. , George, L. , Probst, C. , … Tager, A. M. (2016). An autotaxin/lysophosphatidic acid/interleukin‐6 amplification loop drives scleroderma fibrosis. Arthritis and Rheumatism, 68(12), 2964–2974. 10.1002/art.39797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino, F. V. , Seiders, J. , Bain, G. , Brooks, S. F. , King, C. D. , Swaney, J. S. , … Tager, A. M. (2011). Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis and Rheumatism, 63, 1405–1415. 10.1002/art.30262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. , Geaogre, C. H. , Giembycz, M. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton, C. P. , Shi‐Wen, X. , Sutton, A. , Abraham, D. J. , Black, C. M. , & Pearson, J. D. (1998). Scleroderma fibroblasts promote migration of mononuclear leucocytes across endothelial cell monolayers. Clinical and Experimental Immunology, 114, 293–300. 10.1046/j.1365-2249.1998.00721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, L. , Xue, J. X. , Li, X. , Liu, D. S. , Ge, Y. , Ni, P. Y. , … Jiang, W. (2011). Blockade of lysophosphatidic acid receptors LPAR1/3 ameliorates lung fibrosis induced by irradiation. Biochemical and Biophysical Research Communications, 409, 7–13. 10.1016/j.bbrc.2011.04.084 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko, M. , Laut, M. , Panther, E. , Sorichter, S. , Durk, T. , Fluhr, J. W. , … Norgauer, J. (2004). Lysophosphatidic acid induces chemotaxis, oxygen radical production, CD11b up‐regulation, Ca2+ mobilization, and actin reorganization in human eosinophils via pertussis toxin‐sensitive G proteins. Journal of Immunology, 172, 4480–4485. 10.4049/jimmunol.172.7.4480 [DOI] [PubMed] [Google Scholar]
- Illiano, S. , Ledein, L. , Bidouard, J. P. , Schaefer, M. , Ruetten, H. , Janiak, P. , … Distler, O. (2013). Protective effect of LPA1 and 3 receptor antagonism in experimental skin fibrosis is linked to LPA activity in dermal fibroblasts of SSC patients. Annals of the Rheumatic Diseases, 72(Suppl 3), A129. [Google Scholar]
- Jordan, S. , Chung, J. , & Distler, O. (2013). Preclinical and translational research to discover potentially effective antifibrotic therapies in systemic sclerosis. Current Opinion in Rheumatology, 25, 679–685. 10.1097/01.bor.0000434598.51526.0e [DOI] [PubMed] [Google Scholar]
- Jordana, M. , Schulman, J. , McSharry, C. , Irving, L. B. , Newhouse, M. T. , Jordana, G. , & Gauldie, J. (1988). Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. The American Review of Respiratory Disease, 137, 579–584. 10.1164/ajrccm/137.3.579 [DOI] [PubMed] [Google Scholar]
- Khanna, D. , Denton, C. , Jagerschmidt, A. , Jasson, M. , Distler, O. , & Allanore, Y. (2015). SAR1000842, an antagonist of lysophophatidic acid receptor 1, as a potential treatment for patients with systemic sclerosis. Annals of the Rheumatic Diseases, 74(Suppl2), 172–173. [Google Scholar]
- Kihara, Y. , Maceyka, M. , Spiegel, S. , & Chun, J. (2014). Lysophospholipid receptor nomenclature review: IUPHAR Review 8. British Journal of Pharmacology, 171, 3575–3594. 10.1111/bph.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Keys, J. R. , & Eckhart, A. D. (2006). Vascular smooth muscle migration and proliferation in response to lysophosphatidic acid (LPA) is mediated by LPA receptors coupling to Gq. Cellular Signalling, 18, 1695–1701. 10.1016/j.cellsig.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Maurer, B. , Distler, A. , Dees, C. , Khan, K. , Denton, C. P. , Abraham, D. , … Distler, O. (2013). Levels of target activation predict antifibrotic responses to tyrosine kinase inhibitors. Annals of the Rheumatic Diseases, 72, 2039–2046. 10.1136/annrheumdis-2013-203729 [DOI] [PubMed] [Google Scholar]
- Maurer, B. , Distler, A. , Suliman, Y. A. , Gay, R. E. , Michel, B. A. , Gay, S. , … Distler, O. (2014). Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Annals of the Rheumatic Diseases, 73, 1880–1887. 10.1136/annrheumdis-2013-203535 [DOI] [PubMed] [Google Scholar]
- Mazereeuw‐Hautier, J. , Gres, S. , Fanguin, M. , Cariven, C. , Fauvel, J. , Perret, B. , … Saulnier‐Blache, J. S. (2005). Production of lysophosphatidic acid in blister fluid: Involvement of a lysophospholipase D activity. The Journal of Investigative Dermatology, 125, 421–427. 10.1111/j.0022-202X.2005.23855.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo‐Zerr, K. , Zerr, P. , Distler, A. , Fliehr, J. , Mancuso, R. , Huang, J. , … Distler, J. H. W. (2015). Orphan nuclear receptor NR4A1 regulates transforming growth factor‐β signaling and fibrosis. Nature Medicine, 21, 150–158. 10.1038/nm.3777 [DOI] [PubMed] [Google Scholar]
- Pietruck, F. , Busch, S. , Virchow, S. , Brockmeyer, N. , & Siffert, W. (1997). Signalling properties of lysophosphatidic acid in primary human skin fibroblasts: Role of pertussis toxin‐sensitive GTP‐binding proteins. Naunyn‐Schmiedeberg's Archives of Pharmacology, 355, 1–7. [DOI] [PubMed] [Google Scholar]
- Rancoule, C. , Pradere, J. P. , Gonzalez, J. , Klein, J. , Valet, P. , Bascands, J. L. , … Saulnier‐Blache, J. S. (2011). Lysophosphatidic acid‐1‐receptor targeting agents for fibrosis. Expert Opinion on Investigational Drugs, 20, 657–667. 10.1517/13543784.2011.566864 [DOI] [PubMed] [Google Scholar]
- Soare, A. , Ramming, A. , Avouac, J. , & Distler, J. (2016). Updates on animal models of systemic sclerosis. Journal of Scleroderma and Related Disorders, 1(3), 266–276. 10.5301/jsrd.5000220 [DOI] [Google Scholar]
- Swaney, J. S. , Chapman, C. , Correa, L. D. , Stebbins, K. J. , Bundey, R. A. , Prodanovich, P. C. , … Lorrain, D. S. (2010). A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. British Journal of Pharmacology, 160, 1699–1713. 10.1111/j.1476-5381.2010.00828.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager, A. M. , LaCamera, P. , Shea, B. S. , Campanella, G. S. , Selman, M. , Zhao, Z. , … Luster, A. D. (2008). The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nature Medicine, 14, 45–54. 10.1038/nm1685 [DOI] [PubMed] [Google Scholar]
- Tigyi, G. , & Parrill, A. L. (2003). Molecular mechanisms of lysophosphatidic acid action. Progress in Lipid Research, 42, 498–526. 10.1016/S0163-7827(03)00035-3 [DOI] [PubMed] [Google Scholar]
- Tokumura, A. , Carbone, L. D. , Yoshioka, Y. , Morishige, J. , Kikuchi, M. , Postlethwaite, A. , & Watsky, M. A. (2009). Elevated serum levels of arachidonoyl‐lysophosphatidic acid and sphingosine 1‐phosphate in systemic sclerosis. International Journal of Medical Sciences, 6, 168–176. 10.7150/ijms.6.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoogen, F. , Khanna, D. , Fransen, J. , Johnson, S. R. , Baron, M. , Tyndall, A. , … Riemekasten, G. (2013). Classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases, 72, 1747–1755. 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- Van Meeteren, L. A. , & Moolenaar, W. H. (2007). Regulation and biological activities of the autotaxin–LPA axis. Progress in Lipid Research, 46, 145–160. 10.1016/j.plipres.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Wei, J. , Fang, F. , Lam, A. P. , Sargent, J. L. , Hamburg, E. , Hinchcliff, M. E. , … Varga, J. (2012). Wnt/β‐catenin signaling is hyperactivated in systemic sclerosis and induces Smad‐dependent fibrotic responses in mesenchymal cells. Arthritis and Rheumatism, 64, 2734–2745. 10.1002/art.34424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. R. , Khandoga, A. L. , Goyal, P. , Fells, J. I. , Perygin, D. H. , Siess, W. , … Fujiwara, Y. (2009). Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. The Journal of Biological Chemistry, 284, 17304–17319. 10.1074/jbc.M109.003194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung, C. , Stoddard, N. C. , & Chun, J. (2014). Lysophospholipids and their receptors: LPA receptor signaling: Pharmacology, physiology, and pathophysiology. Journal of Lipid Research, 55(7), 1192–1214. 10.1194/jlr.R046458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. B. , Niu, J. P. , & Zhang, Z. J. (2009). Receptor‐mediated vascular smooth muscle migration induced by LPA involves p38 mitogen‐activated protein kinase pathway activation. International Journal of Molecular Sciences, 10, 3194–3208. 10.3390/ijms10073194 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characterization of patients used for the generation of primary fibroblast cultures (lcSSc = limited SSc / dcSSc=diffuse SSc)
Table S2. SAR100842 selectivity on LPA receptors
Table S3. LPARs expression in endothelial cells
Figure S1. Effect of SAR100842 on IL6 secretion induced by LPA in endothelial cells4
Figure S2. LPARs expression pattern in control dermal fibroblasts
Table S4. Basal gene expression measured by RTPCR in dermal fibroblasts from SSc patients used throughout the study.
Figure S3. Induction of CXCL1. IL6 and CCL2 gene expression by LPA in SSc dermal fibroblasts
Figure S4. Expression of LPA receptors in the skin of Tsk1 and pa control mouse.
Figure S2A: Results (fold change vs LPAR1) from statistical analysis of LPA receptors expression in dermal fibroblasts of SSc donors
Figure S2B: Results (fold change vs LPAR1) from statistical analysis of LPA receptors expression in skin biopsies of SSc donors
Figure S3C: Effect of LPA on the induction of αSMA measured in WB.
Figure S3D: Effect of LPA on the induction of αSMA gene expression.
Figure S5A: Effect of LPA on CCl2. CXCl1 and IL6 secretion.
Figure S5C and S6C: effect of SAR100842 on gene expression in Tsk1 skin
Figure S6A and S6B: Effect of SAR100842 on sfrp4 and wnt2 gene expression in SSc dermal fibroblasts


