Abstract
Background and Purpose
Glutamate and metabotropic glutamate (mGlu) receptors on primary sensory neurons are crucial in modulating pain sensitivity. However, it is unclear how inflammation affects mGlu receptor‐mediated nociceptive responses. We therefore investigated the effects of mGlu1/5 receptor agonists on pain‐related behaviour during persistent inflammation and their underlying mechanisms.
Experimental Approach
Effects of a mGlu1/5 receptor agonist on pain‐related behaviour during inflammation was assessed in mice. Intracellular calcium responses, membrane current responses, and protein expression in primary sensory neurons were examined using cultured dorsal root ganglion (DRG) neurons, dissociated from wild‐type and gene knockout mice.
Key Results
Persistent inflammation induced by complete Freund's adjuvant increased the duration of mGlu1/5 receptor‐mediated pain behaviour, which was antagonized by inhibition of nerve growth factor (NGF)–tropomyosin receptor kinase A (TrkA) signalling. Calcium imaging revealed that NGF treatment increased the number of cultured DRG neurons responding to mGlu1/5 receptor activation. Stimulation of mGlu1/5 receptors in NGF‐treated DRG neurons induced inward currents through TRPV1 channels in association with PLC but not with IP3 receptors. NGF treatment also increased the number of neurons responding to a DAG analogue via TRPV1 channel activation. Furthermore, NGF up‐regulated expression of TRPV1 and A‐kinase anchoring protein 5 (AKAP5), resulting in increased AKAP5‐dependent TRPV1 phosphorylation. AKAP5 knockout mice did not exhibit mGlu1/5 receptor‐mediated excitation in NGF‐treated DRG neurons or pain response facilitation under inflammatory conditions.
Conclusions and Implications
NGF augments glutamate‐ and mGlu1/5 receptor‐mediated excitation of nociceptive neurons by AKAP5‐dependent phosphorylation of TRPV1 channels, potentiating hypersensitivity to glutamate in inflamed tissues.
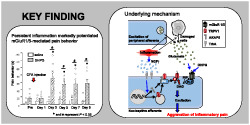
Abbreviations
- 2‐APB
2‐aminoethyl diphenylborate
- 5′‐IRTX
5′‐iodoresiniferatoxin
- A784168
6‐dihydro‐3′‐(trifluoromethyl)‐N‐[4‐[(trifluoromethyl)sulfonyl]phenyl]‐[1(2H),2′‐bipyridine]‐4‐carboxamide
- ACSF
artificial CSF
- AKAP5
A‐kinase anchoring protein 5
- AP18
4‐(4‐chlorophenyl)‐3‐methyl‐3‐buten‐2‐one oxime
- CFA
complete Freund's adjuvant
- CPCCOEt
7‐(hydroxyimino)cyclopropa[b]chromen‐1a‐carboxylate ethyl ester
- CPZ
capsazepine
- DHPG
3,5‐dihydroxyphenylglycine
- DRG
dorsal root ganglion
- IP3
inositol 1,4,5‐trisphosphate
- K252a
(9S,10R,12R)‐2,3,9,10,11,12‐hexahydro‐10‐hydroxy‐9‐methyl‐1‐oxo‐9,12‐epoxy‐1H‐diindolo[1,2,3‐fg:3′,2′,1′‐kl]pyrrolo[3,4‐i][1,6]benzodiazocine‐10‐carboxylic acid methyl ester
- KO
knockout
- MPEP
2‐methyl‐6‐(2‐phenylethynyl)pyridine
- NGF
nerve growth factor
- OAG
1‐oleoyl‐2‐acetyl‐sn‐glycerol
- SOCE
store‐operated calcium entry
- Trk
tropomyosin receptor kinase
- TRP
transient receptor potential
- U73122
1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐1H‐pyrrole‐2,5‐dione
- U73343
1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐2,5‐pyrrolidinedione
What is already known
Peripheral glutamate enhances the sensitivity of sensory afferent fibres to pain stimuli via mGlu receptors.
What does this study add
NGF‐linked signalling facilitates mGlu1/5 receptor‐mediated pain behaviour during inflammation.
NGF increases a subpopulation of nociceptive DRG neurons directly excited by mGlu1/5 receptor activation.
What is the clinical significance
Enhancing mGluR1/5 receptors function in nociceptive neurons may contribute to pain during persistent inflammation.
1. INTRODUCTION
Acute and chronic inflammation decrease the threshold for the activation of primary sensory neurons by thermal or mechanical stimuli, leading to increased pain sensation, such as burning, pricking, and tingling sensations. Inflammation and tissue damage cause the leakage of intracellular contents and the generation and release of various neuroactive agents from noninflammatory and inflammatory cells. Nerve growth factor (NGF) is a peripheral inflammatory mediator that transcriptionally alters primary sensory neuron phenotypes, such as membrane excitability, nociceptive transducer expression, and release of synaptic transmitters and modulators involved in pain and hyperalgesia (Amaya et al., 2004; Chien et al., 2007; Ma & Woolf, 1997; Safieh‐Garabedian, Poole, Allchorne, Winter, & Woolf, 1995). There are two types of NGF receptors, tropomyosin receptor kinase A (TrkA), which predominantly mediates the algesic effects of NGF, and pan‐neurotrophin p75NTR receptors (McMahon, Armanini, Ling, & Phillips, 1994). NGF increases the expression and sensitivity of TRPV1 channels, a polymodal nociceptor, through several signalling pathways responsible for both acute and chronic inflammatory pain (Huang, Zhang, & McNaughton, 2006). However, how NGF‐induced signal transduction alters the responsiveness of sensory neurons to pro‐nociceptive mediators under persistent inflammation is not fully understood.
The principal excitatory neurotransmitter, l‐glutamate, modulates the sensitivity of primary sensory neurons by activating ionotropic and metabotropic receptors in peripheral and central terminals of afferent fibres. deGroot, Zhou, and Carlton (2000) and others (Cao et al., 2007; Guo et al., 2008) showed that antidromic electrical stimulation induces cutaneous primary afferents in spinal dorsal ramus to release glutamate from their peripheral endings, which sensitizes cutaneous afferents from adjacent spinal segments. The application of glutamate receptor antagonists to the receptive field blocks this sensitization (Cao et al., 2007; Li et al., 2018), indicating that peripheral glutamate and its receptors modulate the excitation of nerve endings of primary sensory neurons. Peripheral inflammation and nerve injury also induce the release of glutamate from sensory nerves, glial cells, and damaged cells in peripheral tissues (Lawand, McNearney, & Westlund, 2000; Omote, Kawamata, Kawamata, & Namiki, 1998), suggesting that endogenous glutamate regulates the excitation of nerve terminals in inflammatory and neuropathic pain.
Group I metabotropic glutamate receptors (mGlu1 and mGlu5 receptors), coupled to Gq/11 protein, are abundantly expressed in the cell bodies of dorsal root ganglion (DRG) neurons and unmyelinated afferent fibres in peripheral tissues (Bhave, Karim, Carlton, & Gereau, 2001). A systemically administered agonist of mGlu1/5 receptors increased the sensitivity to noxious heat, leading to heat hyperalgesia, whereas selective antagonists attenuate inflammatory pain (Bhave et al., 2001). We previously showed that transient activation of mGlu5 receptors potentiates TRPV1 channel‐mediated currents and persistently inhibits voltage‐gated calcium currents, thereby modulating pain behaviours in response to noxious heat (Masuoka et al., 2015). Moreover, long‐term (several hours) activation of mGlu1/5 receptors increases the number of DRG neurons expressing functional TRPV1 channels, especially those nociceptive neurons expressing TRPA1 channels (Masuoka et al., 2016). Therefore, mGlu1/5 receptors in the primary afferent fibres are potential therapeutic targets for the prevention and treatment of inflammation‐associated pain disorders. However, it is unclear how inflammation affects the pain responses mediated by peripheral mGlu receptors. Here, we examined the effects of persistent inflammation on pain‐related behaviours induced by activation of mGlu1/5 receptors and explored the underlying mechanisms.
2. METHODS
2.1. Animals
All animal care and experimental procedures were approved by the Ethics Committees of Kanazawa Medical University and Tokushima Bunri University. Animals were treated humanely, in accordance with the Guiding Principles for the Care and Use of Laboratory Animals set by the Japanese Pharmacological Society. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010; McGrath, Drummond, McLachlan, Kilkenny, & Wainwright, 2010) and with the recommendations made by the British Journal of Pharmacology.
Male C57BL/6J mice (RRID:IMSR_JAX:000664) purchased from SLC (Shizuoka, Japan) and CLEA (Tokyo, Japan) were used. Mice were housed in groups of four or less in clear acrylic cages maintained specific pathogen free in a temperature‐controlled room (25 ± 1°C) with a 12‐h light/dark cycle. Some experiments were performed using TRPV1 knockout (TRPV1 KO) mice generated by Caterina et al. (2000) and A‐kinase anchoring protein 5 knockout (AKAP5 KO: B6NCrl.129‐Akap5tm2Gsm/Mmucd#034250‐UCD, RRID:MMRRC_034250‐UCD) mice that were kindly supplied by Dr. Makoto Tominaga (National Institute for Physiological Science) and Dr. Hirofumi Tomita (Hirosaki University), respectively. These knockout (KO) mice had been backcrossed into commercially obtained C57BL/6J mice for more than 10 generations. “Wild‐type mice” in control experiments were normal C57BL/6J mice that had the same genetic background as the KO mice. Heterozygous AKAP5 KO mice were obtained by backcrossing homozygous AKAP5 KO mice with C57BL/6J mice of the same genetic background.
2.2. Behavioural tests
Pain responses were assessed in 8‐week‐old male mice (body weight, 20–25 g). Inflammatory pain was induced by intraplantar injections of 50% complete Freund's adjuvant (CFA) emulsion (Sigma‐Aldrich, St. Louis, MO) made with 0.9% NaCl solution (saline) (10 μl·paw−1) into the left hind paws under isoflurane (Escain®, Mylan Inc., Cecil Township, PA) inhalation. Before or 1–9 days after CFA injection, behavioural tests were performed. Mice were habituated to the experimental room for at least 30 min before the test and were subsequently placed on an elevated mesh platform surrounded by a clear acrylic cage (100‐mm diameter, 250‐mm height) for 30 min. Saline or 3,5‐dihydroxyphenylglycine (DHPG; 1 mM) dissolved in saline was subcutaneously injected into the left footpad (10 μl·paw−1), and then the time the mouse spent licking, shaking, withdrawing, or biting its hind paw was measured for 20 min. The pain behaviour induced by DHPG was only observed once in each mouse, to avoid sensitization or desensitization of the pain response by repeated DHPG injection. To examine inhibitory effects of 7‐(hydroxyimino)cyclopropa[b]chromen‐1a‐carboxylate ethyl ester (CPCCOEt) and 2‐methyl‐6‐(2‐phenylethynyl)pyridine (MPEP) on DHPG‐induced pain behaviour, saline (control) or these drugs dissolved with saline were subcutaneously injected beneath back skin (0.01 ml·g−1) 20 min before intraplantar injection of DHPG. After finishing behavioural observation, the thickness of the footpad was measured with a vernier calliper under isoflurane inhalation. To further examine the contribution of Trk receptors to CFA‐induced pain facilitation, (i) saline (control) or (9S,10R,12R)‐2,3,9,10,11,12‐hexahydro‐10‐hydroxy‐9‐methyl‐1‐oxo‐9,12‐epoxy‐1H‐diindolo[1,2,3‐fg:3′,2′,1′‐kl]pyrrolo[3,4‐i][1,6]benzodiazocine‐10‐carboxylic acid methyl ester (K252a) dissolved in saline, was injected i.p. (0.01 ml·g−1) 30 min before and 1 day after CFA injection, or (ii) MNAC13 (Absolute Antibody, Wilton, UK, Cat#Ab00439) or the control IgG (Sigma, Cat# I5006) dissolved in saline was repeatedly injected into inflamed left hind paw (10 μl·paw−1) 30 min before and 1 and 2 days after CFA injection, under isoflurane inhalation, eliminating pain induced by the intraplantar injection. Three days after CFA injection, DHPG‐induced pain behaviour and thickness of footpad were assessed with same procedure.
To assess the effect of consecutive injection of NGF on DHPG‐induced pain behaviour, saline (control) or NGF (50 μg·ml−1) was injected into left hind paw (10 μl·paw−1) under isoflurane inhalation once a day for 3 days without CFA injection. Twenty‐four hours after the last NGF injection, DHPG‐induced pain behaviour and the footpad thickness were subsequently observed.
2.3. Preparation of primary DRG cultures
Cultures were prepared as described previously (Masuoka et al., 2015, 2016 and 2017). Six‐ to 14‐day‐old mice were anaesthetized with isoflurane. DRGs were rapidly dissected in ice‐cold Ca2+/Mg2+‐free artificial CSF (ACSF; 143.9‐mM NaCl, 3.35‐mM KCl, 21‐mM NaHCO3, 9.9‐mM glucose, and 0.6‐mM NaH2PO4) gassed with a mixture of 95% O2 and 5% CO2 (pH 7.4). DRG neurons were dissociated following treatment with 0.1% type II collagenase (Worthington Biochemical Co., Lakewood, NJ), 0.1% trypsin (Gibco, San Diego, CA), and 0.01% DNase I (Sigma‐Aldrich) in Ca2+/Mg2+‐free ACSF and shaken in a water bath at 37°C for 30 min. Cells were gently triturated in DMEM (Sigma‐Aldrich) containing 10% horse serum (Gibco), 5% fetal calf serum (Gibco), and 1% penicillin–streptomycin (Wako, Osaka, Japan). Dispersed cells were passed through a 100‐μm Cell Strainer (BD Biosciences, San Jose, CA), and the filtered cells were seeded on glass coverslips (13‐mm diameter for calcium imaging and whole‐cell patch clamp recording; 25‐mm diameter for Western blotting) coated with poly‐l‐lysine (Matsunami Glass Ind., Osaka, Japan). Medium with or without mouse NGF 2.5S (100 ng·ml−1; Alomone Labs, Jerusalem, Israel) was added 2 h after seeding; all experiments were performed 72–84 h after the cells were dissociated. To ensure a more uniform neuronal population in DRG culture, cultures made from the same mice at the same time were used in control and comparison groups in each experiment. Each cultured coverslip was used only once for calcium imaging or whole‐cell recording, to prevent drug interaction effects.
2.4. Calcium imaging
Changes in intracellular calcium were measured with a fluorescent calcium indicator, as described previously (Masuoka et al., 2015 and 2016). For microscopic fluorometric measurement, cultured DRG neurons were washed with ACSF (138.6‐mM NaCl, 3.35‐mM KCl, 21‐mM NaHCO3, 9.9‐mM glucose, 0.6‐mM NaH2PO4, 2.5‐mM CaCl2, and 1‐mM MgCl2) and incubated for 45 min in the CO2 incubator (37 ± 2°C) in a solution containing 3‐μM Fura‐2‐acetoxymethyl ester (Fura‐2 AM; Dojindo Laboratories, Kumamoto, Japan) and 0.005% Cremophor® EL (Sigma‐Aldrich). After incubating, the cells were washed in ACSF for 30 min, and culture dishes were placed on the stage of an inverted microscope (ECLIPSE TE 300; Nikon, Tokyo, Japan) equipped with a 20× S‐fluor lens objective. Fluorescence images were recorded and analysed using a video image analysis system (HCimage; Hamamatsu Photonics, Hamamatsu, Japan). Experimental agents were dissolved in ACSF and delivered by continuous perfusion (2 ml·min−1) in the recording chamber (0.8 ml) with a peristaltic pump. Solutions in recording chambers were maintained at 34°C using a thermal controller (TC‐324C, Warner Instruments, Hamden, CT). Image pairs were captured at 10‐s intervals. Fura‐2 fluorescence was recorded at an emission wavelength of 510 nm by exciting Fura‐2 at 340 and 380 nm. The 340‐ to 380‐nm fluorescence ratio (F340/F380) was used as a parameter of intracellular calcium concentration. Change in the ratio induced by drugs shows large variations in individual neurons because of the difference in the efficiency of Fura‐2 uptake and the other experimental conditions. To reduce such variations, the changes in the ratio were normalized to that induced by 50‐mM KCl solution in each neuron.
2.5. Whole‐cell patch clamp recording
Cultured neurons plated on coverslips were transferred to the recording chamber, perfused with ACSF, and maintained at 34°C. Neurons were visually identified using a 60× microscope lens objective (BX51W1; Olympus, Tokyo, Japan). Pipettes for whole‐cell recordings were made from borosilicate glass capillaries (1.5‐mm outer diameter; World Precision Instruments Inc., Sarasota, FL). Patch pipettes (4–6 MΩ) were filled with an internal solution containing 120‐mM KCH3SO3, 5‐mM KCl, 10‐mM K‐EGTA, 5‐mM Na‐HEPES, 3‐mM Mg‐ATP, and 0.4‐mM Na‐GTP (pH 7.4). Series resistance was 8–20 MΩ, which was monitored throughout the recording. Membrane currents were recorded in a whole‐cell configuration using an EPC8 amplifier (HEKA, Lamprecht, Germany) and pCLAMP 9 software (Axon Instruments, Foster City, CA, RRID:SCR_011323), digitized, and stored on a computer disk for off‐line analysis. DHPG‐ and capsaicin‐induced current responses were recorded >5 min after the establishment of whole‐cell configuration. DHPG (100 μM) and capsaicin (0.5 μM) were applied near the recorded neurons via air pressure injection from pipettes using a PV830 pneumatic PicoPump (World Precision Instruments Inc.). Current amplitude induced by DHPG or capsaicin was determined with the basal current subtracted from the peak current after puff application of these drugs. To examine the effects of drugs on DHPG‐induced current amplitude, DHPG was applied by air pressure at 2‐min intervals and perfused drugs into recording chamber for 5 min. DHPG‐induced current amplitudes before and 5 min after the starting drugs perfusion were analysed, and the relative current amplitude (%) during drugs treatment compared with pretreatment was calculated in each neuron. Calcium imaging showed that, as almost all DHPG‐induced responses were observed in capsaicin‐sensitive neurons; the DHPG‐induced currents were selectively recorded from DRG neurons with small soma diameters (15–25 μm), which were more likely to be capsaicin‐sensitive nociceptive neurons (Mohammed, Doran, Grundy, & Nassar, 2017).
2.6. Western blotting and immunoprecipitation
The Immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). The cells were lysed in 0.1–0.5 ml of mammalian cell lysis/extraction reagent (CelLytic™‐M; Sigma‐Aldrich) containing protease inhibitors (protease inhibitor cocktail; Sigma‐Aldrich), phosphatase inhibitor (phosphatase inhibitor cocktail 3; Sigma‐Aldrich), and 1‐mM Na3VO4. The extract was centrifuged at 12,500×g for 15 min at 4°C to remove insoluble material. The protein concentration of the supernatant was determined with the Bio‐Rad protein assay reagent (Bio‐Rad Laboratories, Hercules, CA, RRID:SCR_008426). For Western blot analysis, the cell lysate (10–25 μg of protein) was denatured in 5× sample buffer (625‐mM Tris–HCl, 10% SDS, 25% glycerol, 0.015% bromophenol blue, and 5% 2‐mercaptoethanol, pH 6.8) at 95°C for 5 min and then electrophoretically separated on a polyacrylamide gel (MULTIGEL II Mini 7.5 or 8/16; Cosmo Bio Co., Tokyo, Japan). For co‐immunoprecipitation, samples were diluted to 500 μl with homogenization buffer, incubated with rabbit anti‐TRPV1 antibodies (Alomone Labs, Cat#ACC‐030, RRID:AB_2313819), and pulled down with protein A‐agarose resin (Santa Cruz Biotechnology, Santa Cruz, CA). The resin was washed with homogenization buffer and mixed with 5× sample buffer, and the proteins were resolved by SDS‐PAGE. The proteins were transferred to a PVDF membrane, probed with primary antibodies (1:1,000, rabbit anti‐ mGlu1 receptor [Alomone Labs, Cat#AGC‐006, RRID:AB_2039984]; 1:1,000, rabbit anti‐ mGlu5 receptor [Alomone Labs, Cat#AGC‐007, RRID:AB_2039991]; 1:1,000, rabbit anti‐TRPV1 [Alomone Labs, Cat#ACC‐030, RRID:AB_2313819]; 1:500, rabbit anti‐pTRPV1 [TransGenic Inc., Kumamoto, Japan, Cat#KM112, RRID:AB_1627228]; 1:200, rabbit anti‐AKAP5 [Santa Cruz Biotechnology, Cat#sc‐10765, RRID:AB_2289482]; and 1:3,000, mouse anti‐β‐actin [Sigma‐Aldrich, Cat#A5316, RRID:AB_476743]), and then incubated with secondary antibodies conjugated to HRP (1:3,000, anti‐rabbit and anti‐mouse antibodies; Cell Signaling Technology Inc., Danvers, MA, Cat#7074S and 7076S, RRID:AB_2099233 and AB_330924). Immunoreactive bands were visualized with an enhanced chemiluminescence system (Atto Co., Piscataway, NJ) and laser densitometry (Molecular Dynamics, Sunnyvale, CA). The intensities of bands were analysed utilizing Molecular Dynamic's ImageQuant Software (RRID:SCR_014246). For quantitative analysis of protein expression with Western blot, to avoid variations induced by sample loading and transfer efficiency, immunoreactive band intensity of a target protein was normalized to that of housekeeping protein β‐actin. Quantitative analysis of mGlu1 and mGlu5 receptors was performed using only the monomer band, because dimer bands were not consistently present in all samples.
2.7. Experimental design, and data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). In behavioural tests, mice were equally assigned to two or three groups with simple randomization in each experiment. In vitro experiments, the cultured coverslips dissociated from same mice were randomly assigned to two or more groups. The number of samples in each group was six to eight mice in behavioural test and five to eight samples in in vitro experiments, reported in the literature for similar experiments. The independent values generated from all five to eight samples including outliners were used for statistical analysis. Researchers assessing animal behaviour were blinded to drug treatment or experimental group to avoid subjective bias. Blinding during in vitro experiment was not feasible because an individual experimenter prepared the drug solutions and applied them to samples. To assess the inhibitory effects of drugs on DHPG‐induced current amplitude in whole‐cell patch clamp recording, the current amplitude during drug treatments was normalized to the pretreated current amplitude because the original current showed large variations in individual neurons. Data were analysed with SigmaPlot 13.0 software (Systat Software Inc., San Jose, CA, RRID:SCR_003210). Results are expressed as means ± SEM. The number of the cells, animals, or preparations examined is represented by n. ANOVAs or unpaired Student's t tests were used where assumptions of normality (Shapiro–Wilk test) and equal variance (Brown–Forsythe test) were met and were replaced by the Mann–Whitney test where appropriate. If a significant difference was found by one‐way ANOVA test, then Dunnett's or Tukey's post hoc test was conducted among the groups. Chi‐square tests were used to assess changes in the proportion of DHPG‐ and capsaicin‐sensitive neurons. To compare protein expression between NGF‐untreated and ‐treated cultures, paired Student's t tests were applied, because quantitative Western blot analyses were routinely performed using DRG cultures derived from same mice at the same time. A P value less than 0.05 was considered statistically significant.
2.8Materials
Capsaicin, capsazepine (CPZ), 1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐2,5‐pyrrolidinedione (U73343), 2‐aminoethyl diphenylborinate (2‐APB), 1‐oleoyl‐2‐acetyl‐sn‐glycerol (OAG), and gadolinium(III) chloride were obtained from Sigma‐Aldrich. DHPG, CPCCOEt, MPEP, 1‐[6‐[[(17β)‐3‐methoxyestra‐1,3,5(10)‐trien‐17‐yl]amino]hexyl]‐1H‐pyrrole‐2,5‐dione (U73122), 5′‐iodoresiniferatoxin (5′‐IRTX), and 4‐(4‐chlorophenyl)‐3‐methyl‐3‐buten‐2‐one oxime (AP18) were obtained from Tocris Cookson (Bristol, UK). K252a and 3,6‐dihydro‐3′‐(trifluoromethyl)‐N‐[4‐[(trifluoromethyl)sulfonyl]phenyl]‐[1(2H),2′‐bipyridine]‐4‐carboxamide (A784168) were obtained from Alomone Labs.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Mathie, et al., 2019).
3. RESULTS
3.1. Inflammation enhances mGlu1/5 receptor‐mediated pain behaviour through NGF‐linked signalling
We first examined the effects of DHPG, a selective mGlu1/5 receptor agonist, on the pain‐related behaviours during CFA‐induced inflammation. Unilateral intraplantar injection of CFA immediately elicited oedema and sustainably increased the thickness of footpads 1–9 days after the injections (Figure 1a). DHPG‐induced pain behaviours for inflamed hind paws were determined as described in Section 2 before and 1–9 days after CFA injections (Figure 1b). Before and 1 day after the CFA treatment, intraplantar application of DHPG (1 mM) elicited a brief (~15 s) pain behaviour. From 3 to 9 days after CFA injection, the DHPG‐induced pain behaviour was sporadically induced several times within 20 min after injection, and its durations were significantly prolonged compared with that of the CFA pretreatment group, although saline‐induced pain behaviours tended to increase after CFA treatment. In addition, compared with saline, DHPG significantly increased the duration of pain behaviour 3–9 days after CFA injection. The DHPG‐induced pain response 3 days after CFA treatment was blocked by both the mGlu1 receptor‐specific antagonist CPCCOEt and the mGlu5 receptor‐specific antagonist MPEP (Figure 1c), indicating that the DHPG‐induced pain behaviour was mediated by coordination of both receptors. As the application of neurotrophins, including NGF, induces pain hypersensitivity by activating Trk receptors on the primary sensory neurons (McMahon et al., 1994), we examined whether the Trk inhibitor K252a (Tapley, Lamballe, & Barbacid, 1992) and the anti‐TrkA monoclonal antibody MNAC13, which prevents the NGF–TrkA interaction (Ugolini, Marinelli, Covaceuszach, Cattaneo, & Pavone, 2007), affected DHPG‐induced pain behaviour 3 days after the CFA treatment. Injection of K252a (25 μg·kg−1; i.p.) 30 min before and 1 day after the CFA treatment significantly attenuated the DHPG‐induced pain behaviour but did not alter the CFA‐induced oedema (Figure 1d). MNAC13 (25 μg·ml−1) injected into inflamed footpads 30 min before and 1 and 2 days after CFA treatment significantly attenuated the DHPG‐induced pain behaviour without altering the oedema (Figure 1e), suggesting that NGF–TrkA signalling generated during the inflammatory reaction contributes to the increase in DHPG‐induced pain behaviour. We further examined the effects of intraplantar NGF on DHPG‐induced pain behaviour and the thickness of footpads. As illustrated in Figure 1f, NGF (50 μg·ml−1) was unilaterally injected once a day for 3 days, and the DHPG‐induced pain behaviour and the footpad thickness were subsequently examined 24 h after the last NGF treatment. The repeated NGF treatment significantly prolonged the duration of DHPG‐induced pain behaviour without affecting footpad thickness. This result suggests that NGF‐ and TrkA‐activated signalling augment the mGlu1/5 receptor‐mediated pain behaviour under inflammatory conditions without affecting oedema.
FIGURE 1.
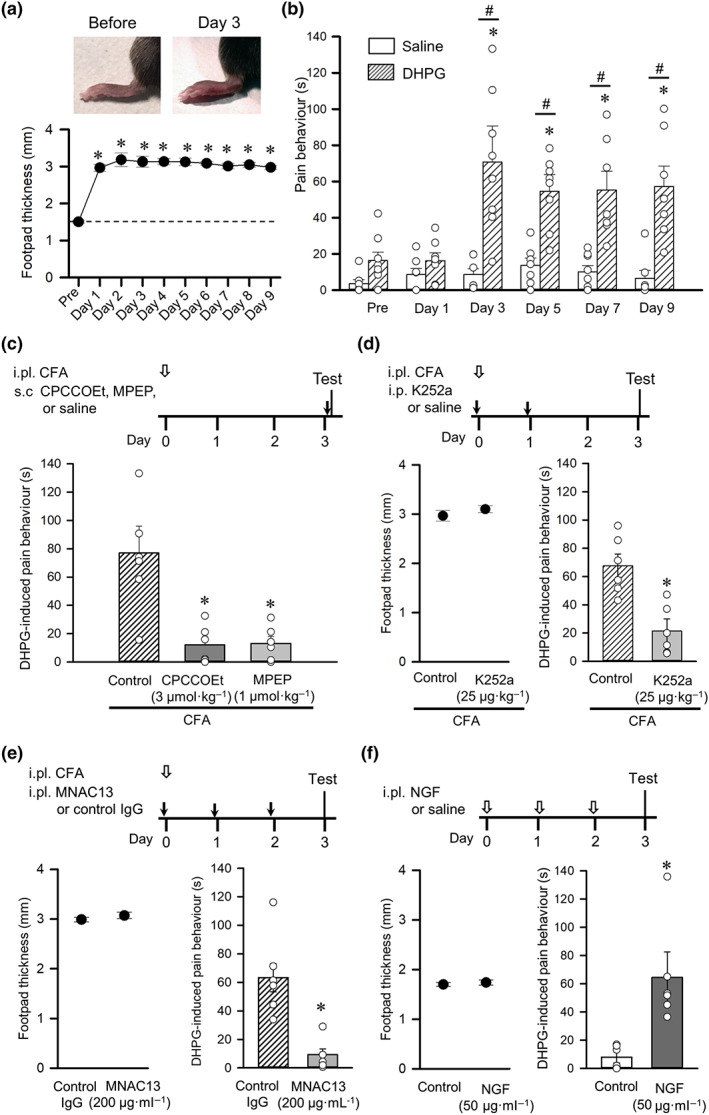
Inflammation facilitates mGlu1/5 receptor agonist‐induced pain behaviour in mice. (a) Time course of footpad thickness after unilateral intraplantar injection of CFA (n = 8). Representative pictures of ipsilateral foot paw before and 3 days after CFA injection (top). *P < 0.05, significantly different from before CFA (Pre); one‐way ANOVA, Dunnett's test. (b) Changes in duration of pain‐related behaviours induced by injection of saline (n = 7) and the mGlu1/5 receptor agonist DHPG (n = 8) into the inflamed footpad. *P < 0.05, significantly different from before CFA (Pre); one‐way ANOVA, Dunnett's test. # P < 0.05, significantly different from the saline group; unpaired Student's t‐test. (c) Timeline of the experimental procedures (top). Intraplantar (i.pl.) injection of CFA at day 0 (open arrow) was followed by tests on day 3 conducted 20 min after subcutaneous (s.c.) injection of the selective mGlu1 receptor antagonist CPCCOEt, the selective mGlu5 receptor antagonist MPEP or saline (control) (black arrow). Effects of CPCCOEt and MPEP on hyperalgesia to DHPG induced by CFA (bottom, n = 6). *P < 0.05, significantly different from control; Kruskal‐Wallis followed by Mann‐Whitney test. (d) Timeline of experimental procedures (top). Tests were conducted 3 days after intraplantar injection of CFA (open arrow). Intraperitoneal (i.p.) injections of the Trk inhibitor K252a or saline (control) were performed at day 0 and day 1 (black arrows). Effect of K252a on foot oedema (bottom left) and on hyperalgesia to DHPG (bottom right) induced by CFA (n = 6). *P < 0.05, significantly different from control; unpaired Student's t‐test. (e) Timeline of experimental procedures (top). Tests were conducted 3 days after intraplantar (i.pl.) injection of CFA (open arrow). Intraplantar injections of MNAC13 or the control IgG into ipsilateral footpads were performed at day 0, day 1, and day2 (black arrows). Effect of MNAC13 on foot oedema (bottom left) and on hyperalgesia to DHPG (bottom right) induced by CFA (n = 6). *P < 0.05, significantly different from control; unpaired Student's t‐test. (f) Timeline of experimental procedures (top). Tests were conducted after repeated intraplantar injections of NGF or saline (control) (open arrows). Effect of NGF on footpad thickness (bottom left) and on DHPG‐induced pain behaviour (bottom right) (n = 6). Each column or closed circle and vertical bar represent mean ± SEM. Open circles indicate values of six to eight mice. *P < 0.05, significantly different from the control group; unpaired Student's t‐test
3.2. NGF increases the proportion of TRPV1‐expressing nociceptive DRG neurons responding to an mGlu1/5 receptor agonist
We then assessed whether NGF alters the sensitivity of nociceptive neurons to a mGlu1/5 receptor agonist by using Ca2+ imaging and Fura‐2 AM‐loaded primary DRG neurons cultured for 3 days. The effect of 100‐μM DHPG or 0.3‐μM capsaicin, an agonist of the polymodal nociceptor TRPV1 channels, was examined in the absence or presence of NGF (100 ng·ml−1). DHPG did not alter intracellular Ca2+ concentrations in control DRG neurons (111 neurons from five primary cultures, Figure 2a–c) but elicited a sharp increase in a subpopulation of NGF‐treated DRG neurons (109 neurons from five primary cultures, Figure 2a,d,e); the percentage of DRG neurons sensitive to DHPG significantly increased from 0.9% to 12.8% with NGF treatment (Figure 2f). Almost all (92.9%) of NGF‐treated DHPG‐sensitive neurons also responded to capsaicin (Figure 2e,f), indicating that they express the polymodal nociceptor TRPV1 channels and mGlu receptors. In fact, NGF significantly increased the proportion of capsaicin‐sensitive neurons (Figure 2g), which is consistent with a previous study (Ji, Samad, Jin, Schmoll, & Woolf, 2002). We then tried to elucidate which subtypes of mGlu receptors contribute to the DHPG‐induced responses in NGF‐treated DRG neurons by using selective mGlu receptor antagonists. DHPG‐induced calcium increases were recorded in the presence of the antagonist from 30 s before DHPG perfusion for 2 min, and the percentage of DHPG‐sensitive neurons in total DRG neurons responding to 50‐mM KCl was calculated in each image. As shown in Figure 2h, the percentage of DRG neurons that exhibited the DHPG‐induced rise of intracellular Ca2+ significantly decreased in the presence of either 30‐μM CPCCOEt or 10‐μM MPEP. In addition, the combined treatment of CPCCOEt and MPEP further reduced the percentage of DHPG‐sensitive DRG neurons treated with NGF. These results indicated that both mGlu1 and mGlu5 receptors mediated the DHPG‐induced increase in intracellular Ca2+. As the use of a Ca2+‐free extracellular solution abolished the DHPG‐induced calcium response (Figure 2h), the mGlu1/5 receptor‐mediated Ca2+ rise is likely to reflect the influx of extracellular Ca2+ rather than the release from intracellular calcium stores, such as from the endoplasmic reticulum.
FIGURE 2.
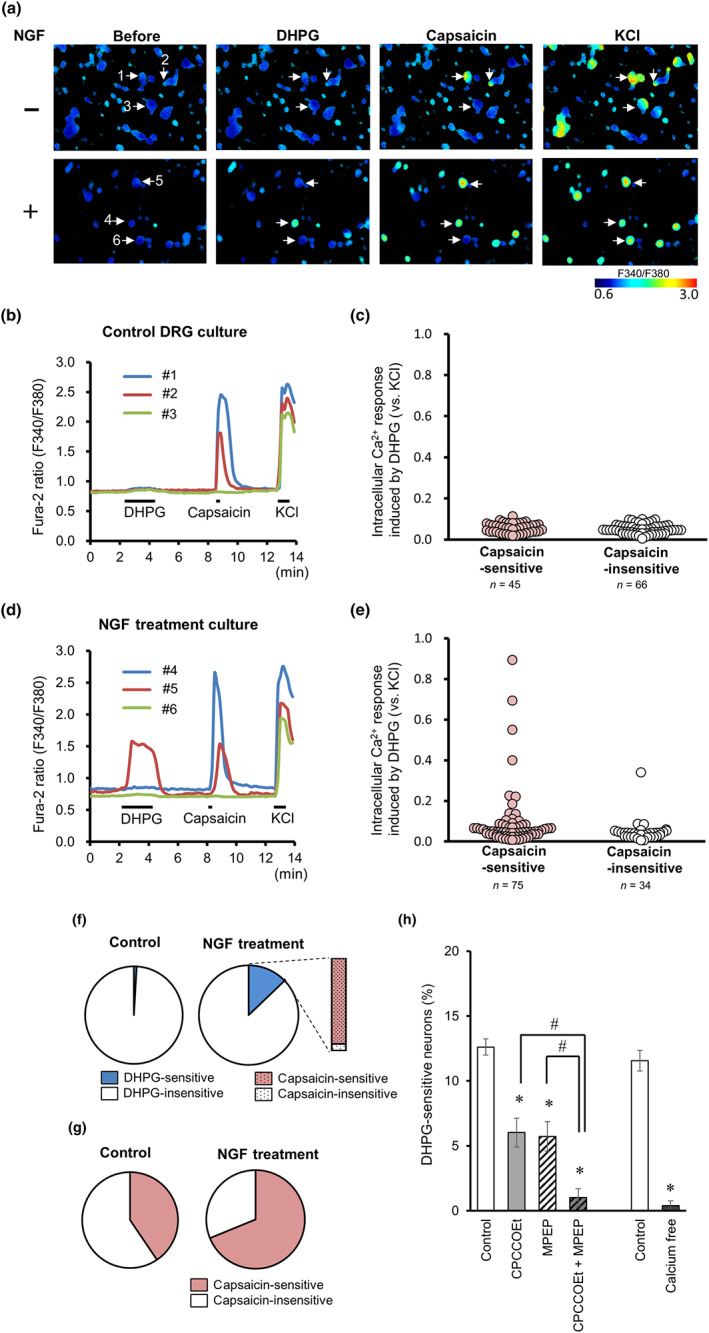
NGF increases the proportion of nociceptive DRG neurons responding to DHPG. (a) Representative images of the F340/F380 ratio before and after perfusion of DHPG (100 μM), capsaicin (0.3 μM), and KCl (50 mM) using Fura‐2 AM dye in the control (upper) and NGF‐treated DRG culture (lower). (b and d) Representative time courses of F340/F380 ratios for Fura‐2 AM in three independent cells numbered in panel (a) (coloured lines) in the control (b) and NGF‐treated (d) cultures. DHPG, capsaicin, and KCl were perfused for 2 min, 30 s, and 1 min, respectively, as indicated by horizontal bars. (c and e) Intracellular calcium elevations induced by DHPG in capsaicin‐sensitive and capsaicin‐insensitive neurons of the control (c) and NGF‐treated (e) DRG cultures. DHPG‐induced responses were normalized with maximal response to KCl. (f) Pie charts represent the proportions of DHPG‐sensitive neurons (blue) in the control (left) and NGF‐treated (right) DRG cultures. The column indicates the proportion of capsaicin‐sensitive neurons in DHPG‐sensitive neurons after NGF treatment. (g) Proportions of capsaicin‐sensitive neurons in the control (left) and NGF‐treated (right) DRG cultures. (h) Percentages of DHPG‐sensitive neurons in NGF‐treated cultures. DHPG‐induced responses were recorded in the presence of CPCCOEt (mGlu1 receptor antagonist) and/or MPEP (mGlu5 receptor antagonist), or in the absence of extracellular calcium (n = 8). Each column and vertical bar represent means ± SEM. *P < 0.05, significantly different from control, # P < 0.05, significantly different from the CPCCOEt group or the MPEP group; one‐way ANOVA, Tukey's test (for comparison of the CPCCOEt and MPEP groups) or unpaired Student's t‐test (for the comparison of the calcium‐free group)
3.3. TRPV1 channels contribute to mGlu1/5 receptor‐mediated inward currents in NGF‐treated DRG neurons
To further explore the subpopulation of DHPG‐ and capsaicin‐sensitive DRG neurons, we measured current responses via whole‐cell patch clump recording. Puff application of 100‐μM DHPG produced an inward current in 27 out of 36 NGF‐treated DRG neurons held at −60 mV (55.3 ± 5.7 pA, n = 27, Figure 3a), while three out of 11 NGF‐untreated neurons showed a smaller inward current (15.9 ± 2.4 pA, n = 3). For pharmacological experiments, the DHPG currents in NGF‐treated neurons were repeatedly induced by puff application at 2‐min intervals and were observed during perfusion of drug solution for 5 min in each neuron. The DHPG response was suppressed by either 30‐μM CPCCOEt or 10‐μM MPEP and further suppressed by treatment with both (Figure 3b). Therefore, activation of both mGlu1 and mGlu5 receptors mediated the DHPG‐induced current in a subpopulation of DRG neurons, as was observed with the DHPG‐induced calcium signals (see Figure 2h). To determine whether PLC signalling contributes to the mGlu1/5 receptor‐mediated current response, we treated DRG neurons with the PLC inhibitor U73122 or the inositol 1,4,5‐trisphosphate (IP3) receptor antagonist 2‐APB. The DHPG‐induced current was partly attenuated by 20‐μM U73122 but not by its inactive analogue U73343 (Figure 3c), whereas 10‐μM 2‐APB had no significant effect (Figure 3d). These results suggest that a PLC, but not IP3 receptor, signalling pathway underlies the DHPG‐induced mGlu1/5 receptor‐mediated current response in DRG neurons.
FIGURE 3.
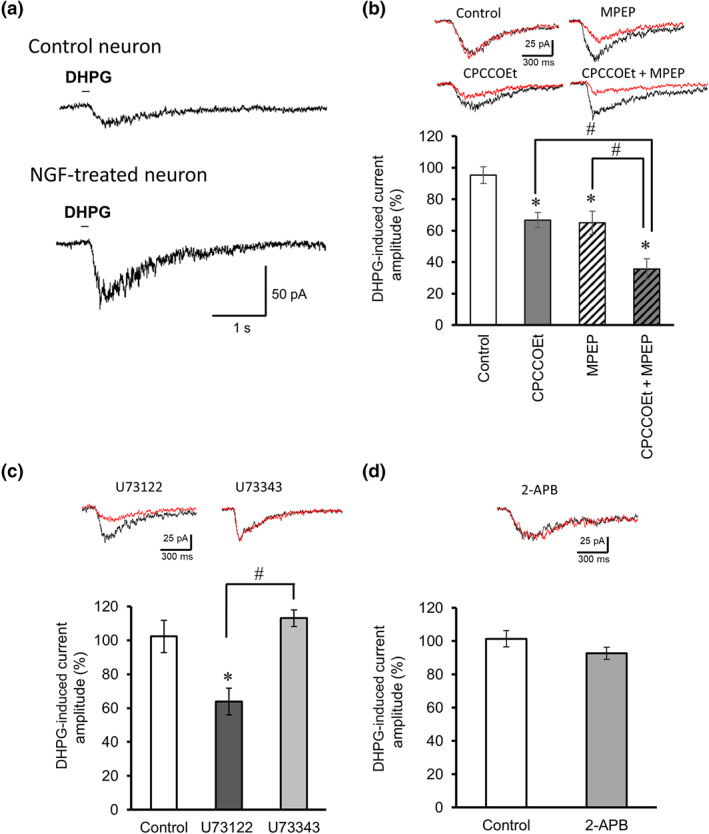
DHPG‐induced current responses in NGF‐treated DRG neurons. (a) Representative current responses induced by puff application of DHPG in the control (upper trace) and NGF‐treated small‐diameter DRG neurons (lower trace). (b) Effects of the selective mGlu1 receptor antagonist CPCCOEt (30 μM) and/or the selective mGlu5 receptor antagonist MPEP (10 μM) on the amplitudes of DHPG‐induced currents (n = 5) in NGF‐treated neurons. The insets show representative currents recorded from before (black) and 5 min after (red) drug perfusion. *P < 0.05, significantly different from control, # P < 0.05, significantly different from the CPCCOEt group or the MPEP group; one‐way ANOVA, Tukey's test. (c) Effects of the PLC inhibitor U73122 and its inactive analogue U73343 on the amplitudes of DHPG‐induced currents (n = 5). *P < 0.05, significantly different from control, # P < 0.05, significantly different from the U73343 group; one‐way ANOVA, Tukey's test. (d) Effect of the IP3 receptor antagonist 2‐APB on the amplitudes of DHPG‐induced currents (n = 5). Each column and vertical bar represents mean ± SEM
We further analysed the properties of the mGlu1/5 receptor‐mediated currents to determine the membrane channels responsible. TRPC1/3 channels are involved in mGlu1 receptor‐mediated excitatory synaptic transmission in Purkinje cells of cerebellar parallel fibres (Hartmann et al., 2008; Kim et al., 2003). However, perfusion of the TRPC1/3 channel blocker gadolinium(III) chloride (10 μM for 5 min) did not affect the DHPG‐induced currents recorded from DRG neurons, indicating that TRPC1 and TRPC3 channels were not involved (Figure 4a). As DRG neurons express other TRP ion channels at high levels (Caterina et al., 1997; Story et al., 2003), we also recorded the responses of DRG neurons treated with TRPV1 channel blockers, 5′‐IRTX and CPZ. As shown in Figure 4a, the DHPG‐induced inward current was suppressed by both 5′‐IRTX (30 and 300 nM) and CPZ (10 and 100 μM) but not by AP18, a blocker of TRPA1 channels. These observations suggest that the DHPG‐induced inward current is mediated by both mGlu1/5 receptors and TRPV1 channels. To further substantiate this suggestion, we recorded DRG neurons taken from TRPV1 KO mice. Of the 13 NGF‐treated small‐diameter (<25 μm) DRG neurons recorded, 12 did not respond to either DHPG or capsaicin (Figure 4c). In only one cell, DHPG elicited a small inward current that was insensitive to the TRPV1 channel blocker 5′‐IRTX (300 nM, data not shown). By contrast, 4/7 NGF‐treated small‐diameter DRG neurons from wild‐type mice were sensitive to both DHPG (84.4 ± 25.1 pA) and capsaicin, and 2/3 DHPG‐insensitive neurons responded to capsaicin (Figure 4b). The DHPG‐ and capsaicin‐induced current amplitudes of TRPV1 KO DRG neurons were significantly smaller than those of wild‐type DRG neurons. We next performed behavioural tests to examine the effect of the TRPV1 channel blocker CPZ on DHPG‐induced pain behaviour under inflammatory conditions. CPZ, applied by i.p. injection to CFA‐treated mice, 30 min before the behavioural test, markedly inhibited the DHPG‐induced pain behaviour (Figure 4d) without altering footpad thickness. Thus, the activation of TRPV1 channels on nociceptive neurons exposed to NGF, contributes to the pain sensation mediated by mGlu1/5 receptor–PLC signalling.
FIGURE 4.
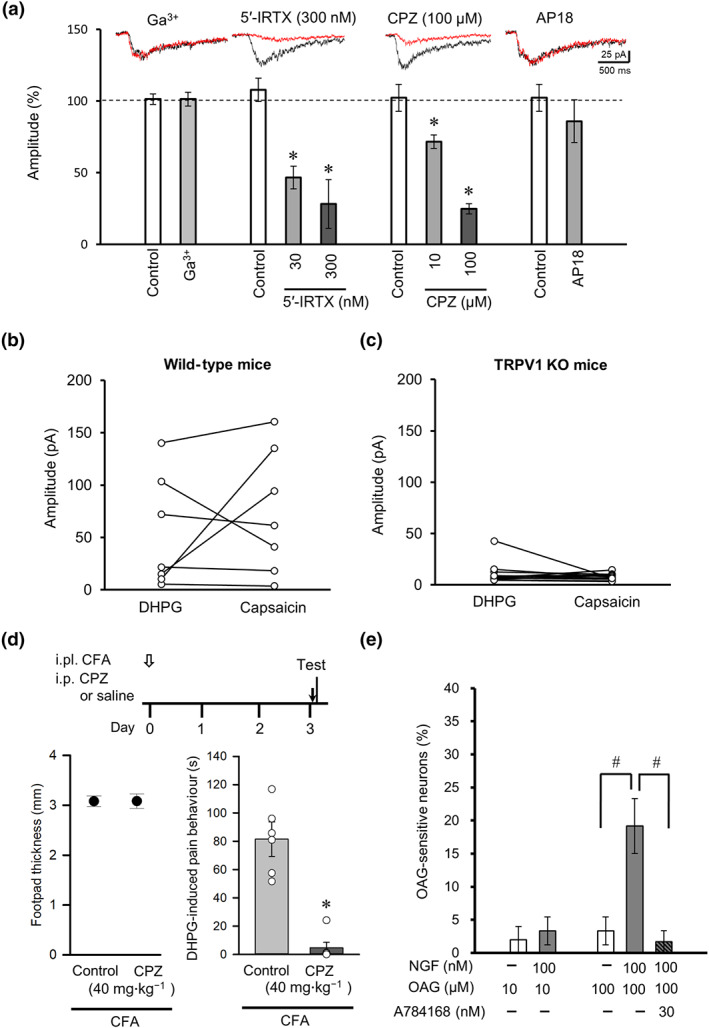
Contribution of TRPV1 to DHPG‐induced currents in NGF‐treated DRG neurons and DHPG‐induced pain behaviour under inflammatory conditions. (a) Effects of TRP channel antagonists on the amplitudes of DHPG‐induced currents (n = 5). The TRPC1/3 antagonist gadolinium(III) (Ga3+), the TRPV1 antagonists 5′‐iodoresiniferatoxin (5′‐IRTX) and capsazepine (CPZ), and the TRPA1 antagonist AP18 were used. The insets show representative currents recorded from before (black) and 5 min after (red) drug perfusion. *P < 0.05, significantly different from control; one‐way ANOVA, Dunnett's test. (b and c) Comparison of maximal current amplitudes induced by puff application of DHPG and capsaicin in small‐diameter DRG neurons dissociated from wild‐type (b) and TRPV1 KO (c) mice. (d) Timeline of experimental procedures (top). Intraplantar injection of CFA at day 0 (open arrow) was followed by tests on day 3 conducted 30 min after intraperitoneal injection of CPZ or saline (control) (black arrow). Effects of CPZ on footpad thickness (bottom left) and DHPG‐induced pain behaviour (bottom right) in CFA‐treated mice. *P < 0.05, significantly different from control; unpaired t‐test. (e) Proportions of neurons responding to the DAG analogue OAG in NGF‐untreated and NGF‐treated DRG neurons (n = 6). Column or closed circle and vertical bar represent mean ± SEM (n=6); open circles indicate the individual values. # P < 0.05, significantly different as indicated; one‐way ANOVA, Tukey's test
3.4. NGF increases the percentage of DRG neurons responding to a DAG analogue
Our results indicated that PLC and TRPV1 channels contributed to the mGlu1/5 receptor‐mediated response in NGF‐treated DRG neurons. As the downstream PLC signalling molecule DAG directly activates TRPV1 channels (Woo et al., 2008), we examined the effects of a membrane‐permeable DAG analogue, OAG, on the TRPV1 channel‐mediated intracellular Ca2+ elevation in cultured DRG neurons with or without NGF treatment. In control DRG neurons (without NGF), OAG (10 and 100 μM) induced responses in <5% of cells, whereas a markedly larger proportion responded when NGF was applied along with 100‐μM OAG (Figure 4e). The increase of OAG‐sensitive neurons in the presence of NGF was completely abolished by the TRPV1 channel antagonist A784168 (30 nM; Figure 4e). These results confirm the involvement of PLC signalling and activation of TRPV1 channels in the nociceptive responses of NGF‐treated DRG neurons.
3.5. NGF increases expression of TRPV1 channels and AKAP5 in DRG neurons and enhances TRPV1 channel phosphorylation at S800
To determine the molecular mechanisms underlying the NGF facilitation of nociceptive responses in DRG neurons, we analysed the expression of mGlu1/5 receptors and TRPV1 channels in DRG neurons cultured with or without NGF. The monomer band of mGlu1 receptor protein (likely isoform c) was predominantly detected at 100 kDa in DRG cultures, and NGF treatment for 3 days did not change its intensity (Figure 5a,b). The protein levels of monomer mGlu5 receptor detected at 150 kDa were not different between cells culture with and without NGF (Figure 5a,c). Interestingly, the expression of TRPV1 was significantly increased in DRG neurons after NGF treatment (Figure 5a,d). We also examined the expression of AKAP5, a scaffolding protein essential for positioning serine–threonine kinases and their target phosphorylation sites, because interactions between TRPV1 channels and AKAP5 enhance pain sensation after inflammation (Fischer, Btesh, & McNaughton, 2013). Interestingly, AKAP5 expression was significantly increased in DRG neurons treated with NGF (Figure 5e,f).
FIGURE 5.
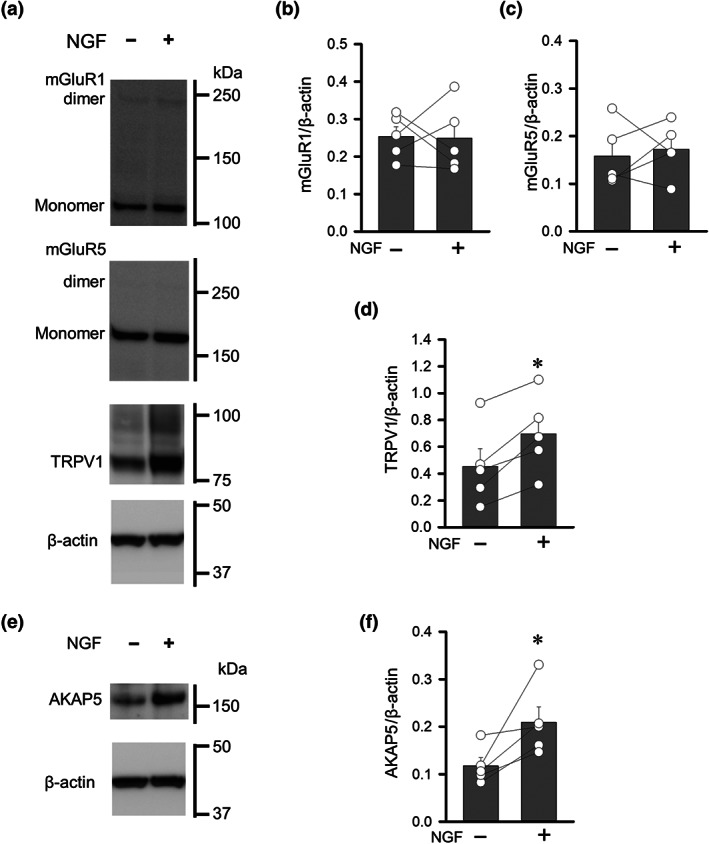
Effects of NGF on the expression of mGlu1/5 receptors, TRPV1 channels and AKAP5 in cultured DRGs. (a) Changes in the expression of mGlu1 receptor, mGlu5 receptor and TRPV1 channel proteins in primary DRG cultures treated with NGF. (b–d) Quantification of mGlu1 receptor (b), mGlu5 receptor (c), and TRPV1 channel protein (d), normalized to total β‐actin. (e) Changes in the AKAP5 protein in primary DRG cultures treated with NGF. (f) Quantification of AKAP5 normalized to total β‐actin. Each column and vertical bar represent mean ± SEM (n=5); open circles indicate the individual values. *P < 0.05, significantly different from the NGF‐untreated group; paired t‐test
Consistent with a previous report that AKAP5 binding to PKC sensitizes TRPV1 channels by phosphorylation at Ser800 (S800) (Bhave et al., 2003), our immunoblotting assay demonstrated that NGF treatment significantly increased the proportion of S800‐phosphorylated TRPV1 (Figure 6a,b). Furthermore, co‐immunoprecipitation assays revealed that although TRPV1 channels did not interact with mGlu1 receptors, there was a detectable interaction with mGlu5 receptors; however, this interaction was not affected by NGF treatment (Figure 6c,d). Thus, the NGF‐induced enhancement of mGlu1/5 receptor responses is not likely to be a result of increased physical coupling of mGlu receptors with TRPV1 channels in DRG neurons.
FIGURE 6.
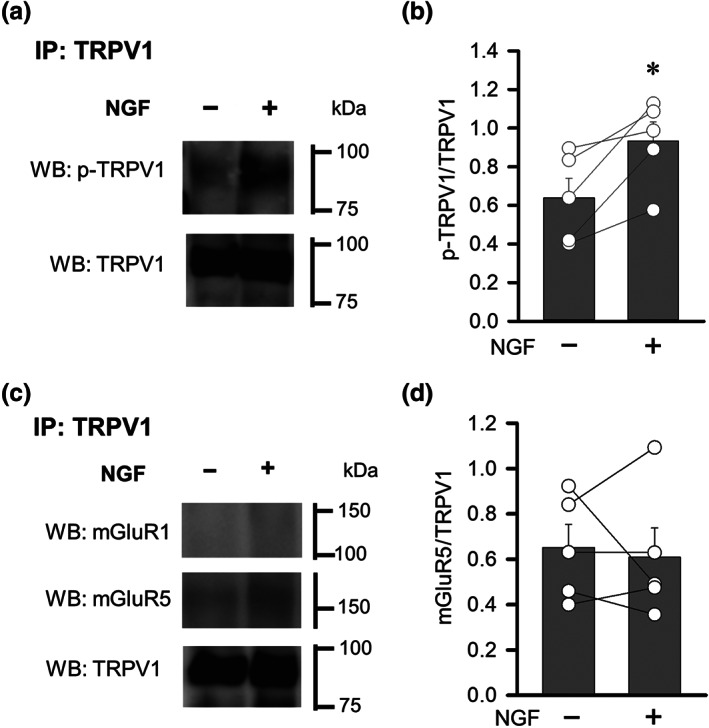
Effects of NGF on the phosphorylation of TRPV1 and on the interaction between TRPV1 and mGlu1/5 receptors in cultured DRGs. (a) Changes in the phosphorylation of TRPV1 at S800 in DRG cultures treated with NGF. Samples were immunoprecipitated with anti‐TRPV1 antibody. (b) Quantification of phosphorylated TRPV1 normalized to total TRPV1. (c) Changes in the interaction of mGlu1/5 receptors and TRPV1 channels in DRG cultures treated with NGF. Samples were immunoprecipitated with anti‐TRPV1 antibody. (d) Quantification of mGlu5 receptor protein normalized to total TRPV1 channel protein. Each column and vertical bar represent mean ± SEM (n =5); open circles indicate the individual values. *P < 0.05, significantly different from the NGF‐untreated group; paired t‐test
3.6. AKAP5 KO mice with inflammation do not display mGlu1/5 receptor‐mediated pain behaviour
To further assess the role of AKAP5 in the nociceptive responses of DRG neurons exposed to NGF, we examined DRG neurons from AKAP5 KO mice. As expected, the expression of AKAP5 protein was attenuated in DRG neurons from heterozygous mice and was undetectable in those from homozygous AKAP5 KO mice (Figure 7a). Unilateral intraplantar injections of CFA increased the footpad thickness in these mice 3 days after treatment (Figure 7b), similar to observed in wild‐type mice (Figure 1a). Interestingly, the CFA‐induced inflammation in AKAP5 KO mice did not facilitate DHPG‐induced pain behaviour (Figure 7b). Moreover, NGF treatment of cultured DRG neurons from AKAP5 KO mice did not significantly alter the proportion that were sensitive to DHPG (Figure 7c). NGF did not affect mGlu1 or mGlu5 receptor protein levels (Figure 8a,c) but increased the expression of TRPV1 protein in cultured DRG neurons from AKAP5 KO mice (Figure 8d,e). Although the amount of S800‐phosphorylated TRPV1 in DRG neurons from AKAP5 KO mice was unaffected by NGF (Figure 8f), a co‐immunoprecipitation assay showed that the proportion of TRPV1 protein that was phosphorylated was significantly reduced by the NGF treatment (Figure 8g,h). These results suggest that AKAP5 contributes to NGF‐induced phosphorylation of TRPV1 channels, and the absence of this abolishes the mGlu1/5 receptor‐mediated excitatory response in NGF‐treated DRG neurons as well as the sensitization of mGlu1/5 receptor‐mediated pain behaviour under inflammatory conditions.
FIGURE 7.
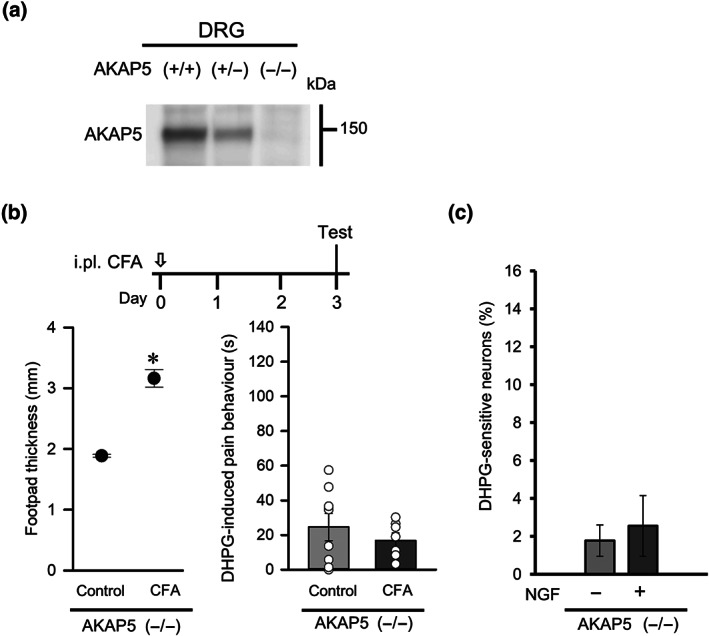
Effect of AKAP5 depletion on the potentiation of DHPG‐induced pain behaviour in mice with inflammation. (a) Expression of AKAP5 protein in the DRGs of AKAP5 KO mice. (b) Timeline of experimental procedures (top). Tests were conducted 3 days after intraplantar (i.pl.) injection of CFA (open arrow). Effects of CFA on footpad thickness (bottom left) and on DHPG‐induced pain behaviour (bottom right) in AKAP5 KO mice (n = 8). (c) Changes in the percentages of DHPG‐sensitive neurons after NGF treatment using DRGs dissociated from AKAP5 KO mice (n = 8). Each column and vertical bar represent mean ± SEM (n =8); open circles indicate the individual values. *P < 0.05, significantly different from the control group; unpaired t‐test
FIGURE 8.
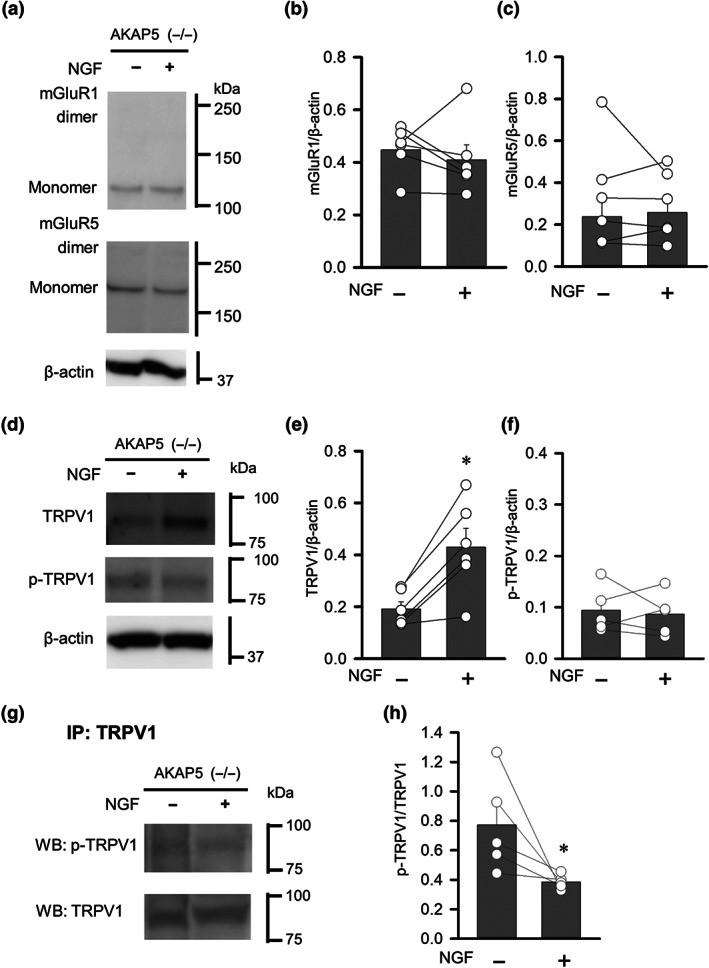
Changes in mGlu1/5 receptors, TRPV1 and phosphorylated TRPV1 induced by NGF treatment of DRGs from AKAP5 KO mice. (a) Changes in mGlu1 and mGlu5 receptor proteins in primary DRG cultures treated with NGF and dissociated from AKAP5 KO mice. (b and c) Quantification of mGlu1 (b) and mGlu5 protein (c) normalized to total β‐actin. (d) Changes in the TRPV1 and S800‐phosphorylated TRPV1 proteins in primary DRG cultures treated with NGF and dissociated from AKAP5 KO mice. (e and f) Quantification of TRPV1 (e) and phosphorylated TRPV1 proteins (f) normalized to total β‐actin. (g) Changes in S800‐phosphorylated TRPV1 protein in primary DRG cultures treated with NGF and dissociated from AKAP5 KO mice. Samples were immunoprecipitated with anti‐TRPV1 antibody. (h) Quantification of phosphorylated TRPV1, normalized to total TRPV1 proteins. Each column and vertical bar represent mean ± SEM (n =5‐6); open circles indicate the individual values. *P < 0.05, significantly different from the NGF‐untreated group; paired t‐test
4. DISCUSSION
This study showed that (i) injection of the mGlu1/5 receptor agonist DHPG into mouse hind paws after CFA‐provoked inflammation enhanced pain behaviour, (ii) this DHPG‐facilitated pain behaviour was suppressed by inhibitors of NGF–TrkA signalling (Figure 1), and (iii) NGF treatment in vitro increased the population of DRG neurons excited by both DHPG and capsaicin, a TRPV1 channel activator (Figure 2). These findings indicate that NGF‐ and TrkA‐activated signalling not only facilitated mGlu1/5 receptor‐mediated pain behaviour under inflammatory conditions but also increased the proportion of nociceptive DRG neurons that responded to activation of mGlu1/5 receptors. The mechanism for this facilitation involves NGF‐mediated induction of TRPV1 channels and its scaffolding protein AKAP5, leading to an increase in AKAP5‐dependent phosphorylation of TRPV1 channels. These findings provide strong support for the notion that glutamate released from the peripheral endings of nociceptive primary afferents and damaged cells increases pain sensation under inflammatory conditions via NGF regulation of TRPV1 channels and AKAP5 expression, as illustrated in Figure 9.
FIGURE 9.
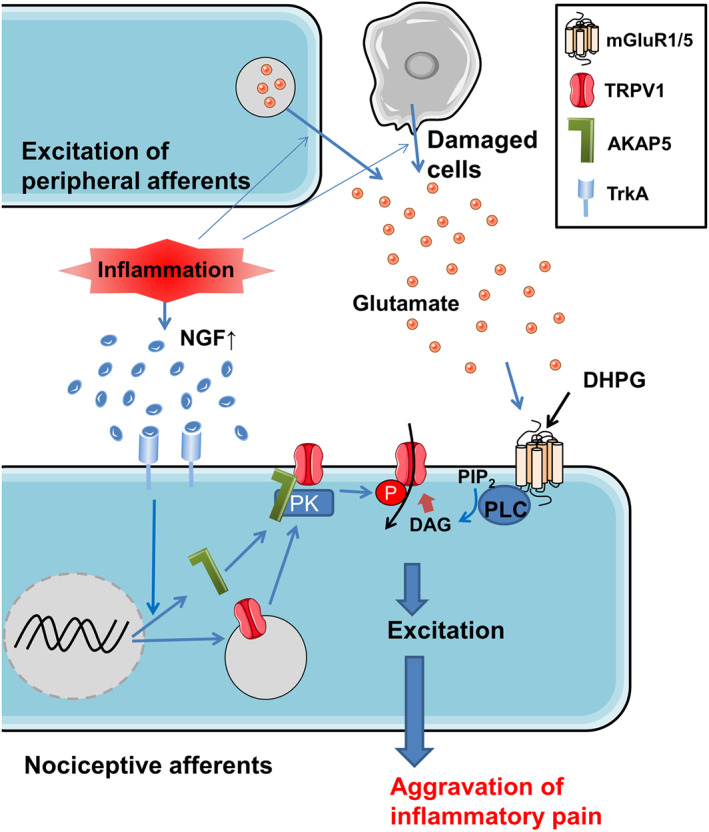
Pathways of facilitation of mGlu1/5 receptor‐mediated pain under inflammatory conditions. The increase of TrkA signalling by NGF in inflamed tissue stimulates the expression of TRPV1 channels and its scaffolding protein AKAP5, leading to an increase in AKAP5‐dependent phosphorylation of TRPV1 protein on S800. The stimulation of mGlu1/5 receptors (mGluR1/5) by glutamate and DHPG generates TRPV1‐mediated inward current via the PLC‐DAG pathway to directly excite the nociceptive neurons. The facilitation of mGlu1/5 receptor‐mediated excitation in nociceptive neurons by endogenous glutamate release exacerbates inflammatory pain
Recent studies have shown that inflammation causes peripheral pain sensitization and that glutamate and NGF are the inflammatory mediators, raising the possibility that these agents may be potential targets of drug therapy for pain sensation associated with various inflammatory disorders (Amaya et al., 2004; Bhave et al., 2001; Prato et al., 2017). Therefore, it is imperative to further elucidate the mechanism(s) underlying this sensitization of inflammation‐provoked pain. Our results are consistent with those from a previous study by Gong, Kung, Magni, Bhargava, and Jasmin (2014), in which glutamate‐ and mGlu receptor‐mediated currents were increased in DRG neurons dissociated from rats with chronic constriction injury to the sciatic nerve. Indeed, the mGlu1/5 receptor agonist DHPG enhanced inflammatory pain behaviours, and the treatment of dissociated DRG neurons with NGF to mimic inflammatory conditions increased the proportion of DHPG‐activated neurons. The study by Bennett (2001) showed that during embryogenesis, the NGF receptor TrkA is highly expressed in DRG neurons, and the expression of TrkA as well as its sensitivity to NGF decline after birth. Accordingly, the physiological significance of NGF–TrkA signalling appears to shift from promoting growth, maintenance, and survival of its target neurons to regulating the capability of the peripheral nervous system to sense pathophysiological conditions, such as inflammatory noxious stimuli. Conceivably, NGF–TrkA signalling is a critical switch enabling glutamate to act as an endogenous algesic agent or pain sensitizer for nociceptive sensory neurons, thereby conveying an alarm signal to the CNS.
Our present experiments showed that the DHPG‐induced Ca2+ signals in DRG neurons were suppressed more by concomitant application of mGlu1 receptor‐specific and mGlu5 receptor‐specific antagonists than by their separate applications (Figure 2h). These data suggest that activation of both mGlu1 and mGlu5 receptors excites a subpopulation of DRG neurons. This is in contrast to a previous suggestion that only mGlu5 receptors were responsible for the DHPG‐induced Ca2+ signals in mouse DRG neurons (Hu, Bhave, & Gereau, 2002). Our data from whole‐cell recordings showed that DHPG induced inward currents by activating TRPV1 channels because the current responses were markedly suppressed by the blockers of these channels and were absent from almost all DRG neurons derived from TRPV1 KO mice (Figure 4a–c). This coupling of TRPV1 channels and mGlu receptors is in marked contrast to what has been reported in the brain, where mGlu1 or mGlu5 receptors were shown to be coupled to canonical TRP channels, such as TRPC1 at glutamatergic synapses on Purkinje neurons (Hartmann et al., 2008; Kim et al., 2003) and TRPC3/7 on striatal cholinergic interneurons (Berg, Sen, & Bayliss, 2007). Furthermore, the mGlu1/5 receptor‐mediated activation of TRPV1 channels in DRG neurons was coupled to PLC–DAG signalling because treatment with OAG increased intracellular Ca2+ levels but not in the presence of a TRPV1 channel antagonist (Figure 4e). These data are consistent with results from a previous study showing that mGlu5 receptors in the central terminals of primary sensory neurons activated TRPV1 channels coupled to PLC‐dependent signalling (Kim et al., 2009). However, mGlu1/5 receptors on hippocampal CA3 interneurons activate TRPV1 channels and produces slow excitation independent of PLC signalling (Eguchi, Hishimoto, Sora, & Mori, 2016). Therefore, mGlu1/5 receptor‐coupled signalling pathways differ depending on neuron type/location. The ubiquitous calcium entry pathway, which operates through store‐operated calcium entry (SOCE) channels such as TRPC1–7, was reported to be activated by GPCRs through PIP2 hydrolysis followed by depletion of calcium stores (Ong, de Souza, & Ambudkar, 2016). The mGlu1/5 receptor‐induced calcium entry pathway observed in the present study might be different from that of the SOCE system.
Growing evidence indicates that glutamate–mGlu receptors–TRPV1 channel signalling is a nociceptive signal transduction pathway associated with inflammation. For example, mGlu5 receptor activation by glutamate in peripheral sensory afferents in normal mice results in hypersensitivity to noxious heat (Masuoka et al., 2015; Szteyn et al., 2015), most likely via the liberation of AKAP5 from its anchorage site in the plasma membrane (in a PLC‐dependent manner) to associate with TRPV1 channels (Szteyn et al., 2015). Moreover, phosphorylation of these channels is likely to contribute to the aggravation of mGlu1/5 receptor‐mediated pain sensation under inflammatory conditions, as our experiments show that AKAP5‐mediated phosphorylation of TRPV1 channels, as well as expression levels of both, was increased in the cultured DRG neurons (Figure 5). Furthermore, mGlu1/5 receptor‐mediated pain behaviour was not potentiated in AKAP5 KO mice with CFA‐induced inflammation (Figure 7). mGlu5 receptor‐activated TRPV1 currents are present in the central terminals of a subset of DRG neurons under healthy normal conditions (Kim et al., 2009). The difference of mGlu receptor ‐mediated activation of TRPV1 channels between the peripheral and central terminals might depend on the amount of NGF supplied in the spinal cord and peripheral tissues (Hoener, Hewitt, Conner, Costello, & Varon, 1996).
NGF rapidly produces hyperalgesia 15–180 min after injection via activation of the PI3K/ERK pathway (Zhuang, Xu, Clapham, & Ji, 2004). This hyperalgesia is attributed to the rapid activation of the TrkA and PI3K‐mediated Src kinase activation, which phosphorylates a tyrosine residue (Y200) on TRPV1 channels, promoting its insertion into the plasma membrane (Zhang, Huang, & McNaughton, 2005). Similarly, we found that persistent NGF treatment of dissociated DRG neurons increased the phosphorylation of TRPV1 on S800, possibly through enhanced expression of AKAP5 (Figures 6b and 8h). This phosphorylation may underlie the marked increase in the number of DRG neurons that exhibited TRPV1‐mediated Ca2+ elevation in response to the DAG analogue (Figure 4e). AKAP5 localizes serine–threonine kinases PKA and PKC for phosphorylation of TRPV1 channels at specific amino acid residues (Jeske et al., 2008). Specifically, PKA phosphorylates S116 on TRPV1 channels, leading to sensitized responses to capsaicin (Bhave et al., 2002), whereas PKC phosphorylates TRPV1 channels at S502 and S800 (Bhave et al., 2003). In addition, DAG directly activates TRPV1 channels in DRG neurons by binding to the same site as capsaicin (Y511) (Woo et al., 2008). Consequently, NGF secreted during inflammation may also sensitize glutamate– mGlu1/5 receptor–TRPV1 channel signalling via phosphorylation of S800 on TRPV1 channels and by up‐regulating the expression of TRPV1 and AKAP5.
In conclusion, we found that the mGlu1/5 receptor agonist‐induced pain behaviour in mice subjected to CFA‐elicited inflammation was markedly potentiated by NGF–TrkA signalling. The underlying mechanism is an increase in the subpopulation of DRG neurons with activated mGlu1/5 receptor signalling. This increase was attributed to functional coupling of mGlu receptors with TRPV1 channels as a result of increased TRPV1 and AKAP5 expression and subsequent phosphorylation of TRPV1 channels. This NGF‐mediated facilitation of mGlu1/5 receptor–TRPV1 channel signalling explains how glutamate is able to act as an endogenous algetic agent and produce spontaneous pain and severe hyperalgesia under inflammatory conditions. Further study of the molecular mechanism in peripheral sensory neurons could facilitate the clinical management of inflammatory pain and furnish attractive targets for the development of novel analgesics.
AUTHOR CONTRIBUTIONS
T.M. designed the study. T.M., Y.Y., J.Y., and K.N. performed research and analysed data. T.M., J.Y., M.T., M.N., and T.I. wrote the paper.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
ACKNOWLEDGEMENTS
We thank Dr. Makoto Tominaga (National Institute for Physiological Science) and Dr. Hirofumi Tomita (Hirosaki University) for providing the TRPV1 knockout mice and the AKAP5 knockout mice, respectively. We also thank Ms. Keiko Tokumaru (Tokushima Bunri University) for her technical and secretarial assistance and Ms. Mika Ikeda (Kanazawa Medical University) for secretarial assistance. Our work was supported by a Grant for Promoted Research from Kanazawa Medical University (S2016‐5 and S2019‐1), Japan Society for the Promotion of Science (JSPS) KAKENHI grants (16K19023, 17K16992, 19K18895, 19K09961, 19K07117, and 20K09814), and grants from the Nakatomi Foundation and the Smoking Research Foundation of Japan.
Masuoka T, Yamashita Y, Yoshida J, et al. Sensitization of glutamate receptor‐mediated pain behaviour via nerve growth factor‐dependent phosphorylation of transient receptor potential V1 under inflammatory conditions. Br J Pharmacol. 2020;177:4223–4241. 10.1111/bph.15176
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya, F. , Shimosato, G. , Nagano, M. , Ueda, M. , Hashimoto, S. , Tanaka, Y. , … Tanaka, M. (2004). NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. European Journal of Neuroscience, 20, 2303–2310. 10.1111/j.1460-9568.2004.03701.x [DOI] [PubMed] [Google Scholar]
- Bennett, D. L. H. (2001). Neurotrophic factors: Important regulators of nociceptive function. The Neuroscientist, 7, 13–17. 10.1177/107385840100700105 [DOI] [PubMed] [Google Scholar]
- Berg, A. P. , Sen, N. , & Bayliss, D. A. (2007). TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. The Journal of Neuroscience, 27, 8845–8856. 10.1523/JNEUROSCI.0551-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave, G. , Hu, H. J. , Glauner, K. S. , Zhu, W. , Wang, H. , Brasier, D. J. , … Gereau, R. D. 4th. (2003). Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proceedings of the National Academy of Sciences of the United States of America, 100, 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave, G. , Karim, F. , Carlton, S. M. , & Gereau, R. W. 4th (2001). Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nature Neuroscience, 4, 417–423. 10.1038/86075 [DOI] [PubMed] [Google Scholar]
- Bhave, G. , Zhu, W. , Wang, H. , Brasier, D. J. , Oxford, G. S. , & Gereau, R. W. 4th. (2002). cAMP‐dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron, 35, 721–731. 10.1016/S0896-6273(02)00802-4 [DOI] [PubMed] [Google Scholar]
- Cao, D. Y. , You, H. J. , Zhao, Y. , Guo, Y. , Wang, H. S. , Arendt‐Nielsen, L. , … Zhang, Q. (2007). Involvement of peripheral ionotropic glutamate receptors in activation of cutaneous branches of spinal dorsal rami following antidromic electrical stimulation of adjacent afferent nerves in rats. Brain Research Bulletin, 72, 10–17. 10.1016/j.brainresbull.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Leffler, A. , Malmberg, A. B. , Martin, W. J. , Trafton, J. , Petersen‐Zeitz, K. R. , … Julius, D. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science, 288, 306–313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Schumacher, M. A. , Tominaga, M. , Rosen, T. A. , Levine, J. D. , & Julius, D. (1997). The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature, 389, 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chien, C. C. , Fu, W. M. , Huang, H. I. , Lai, Y. H. , Tsai, Y. F. , Guo, S. L. , … Ling, Q. D. (2007). Expression of neurotrophic factors in neonatal rats after peripheral inflammation. The Journal of Pain, 8, 161–167. 10.1016/j.jpain.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deGroot, J. , Zhou, S. , & Carlton, S. M. (2000). Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport, 11, 497–502. 10.1097/00001756-200002280-00014 [DOI] [PubMed] [Google Scholar]
- Eguchi, N. , Hishimoto, A. , Sora, I. , & Mori, M. (2016). Slow synaptic transmission mediated by TRPV1 channels in CA3 interneurons of the hippocampus. Neuroscience Letters, 616, 170–176. 10.1016/j.neulet.2015.12.065 [DOI] [PubMed] [Google Scholar]
- Fischer, M. J. , Btesh, J. , & McNaughton, P. A. (2013). Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. The Journal of Neuroscience, 33, 7407–7414. 10.1523/JNEUROSCI.3721-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, K. , Kung, L. H. , Magni, G. , Bhargava, A. , & Jasmin, L. (2014). Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS ONE, 9, e95491 10.1371/journal.pone.0095491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. , Yao, F. R. , Cao, D. Y. , Pickar, J. G. , Zhang, Q. , Wang, H. S. , & Zhao, Y. (2008). Somatostatin inhibits activation of dorsal cutaneous primary afferents induced by antidromic stimulation of primary afferents from an adjacent thoracic segment in the rat. Brain Research, 1229, 61–71. 10.1016/j.brainres.2008.06.111 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Bryant, C. (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, J. , Dragicevic, E. , Adelsberger, H. , Henning, H. A. , Sumser, M. , Abramowitz, J. , … Konnerth, A. (2008). TRPC3 channels are required for synaptic transmission and motor coordination. Neuron, 59, 392–398. 10.1016/j.neuron.2008.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoener, M. C. , Hewitt, E. , Conner, J. M. , Costello, J. W. , & Varon, S. (1996). Nerve growth factor (NGF) content in adult rat brain tissues is several‐fold higher than generally reported and is largely associated with sedimentable fractions. Brain Research, 728, 47–56. 10.1016/0006-8993(96)00386-1 [DOI] [PubMed] [Google Scholar]
- Hu, H. J. , Bhave, G. , & Gereau, R. W. 4th. (2002). Prostaglandin and protein kinase A‐dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: Potential mechanism for thermal hyperalgesia. The Journal of Neuroscience, 22, 7444–7452. 10.1523/JNEUROSCI.22-17-07444.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Zhang, X. , & McNaughton, P. A. (2006). Inflammatory pain: The cellular basis of heat hyperalgesia. Current Neuropharmacology, 4, 197–206. 10.2174/157015906778019554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske, N. A. , Diogenes, A. , Ruparel, N. B. , Fehrenbacher, J. C. , Henry, M. , Akopian, A. N. , & Hargreaves, K. M. (2008). A‐kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain, 138, 604–616. 10.1016/j.pain.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, R. R. , Samad, T. A. , Jin, S. X. , Schmoll, R. , & Woolf, C. J. (2002). p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron, 36, 57–68. 10.1016/S0896-6273(02)00908-X [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. J. , Kim, Y. S. , Yuan, J. P. , Petralia, R. S. , Worley, P. F. , & Linden, D. J. (2003). Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature, 426, 285–291. 10.1038/nature02162 [DOI] [PubMed] [Google Scholar]
- Kim, Y. H. , Park, C. K. , Back, S. K. , Lee, C. J. , Hwang, S. J. , Bae, Y. C. , … Oh, S. B. (2009). Membrane‐delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. The Journal of Neuroscience, 29, 10000–10009. 10.1523/JNEUROSCI.5030-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawand, N. B. , McNearney, T. , & Westlund, K. N. (2000). Amino acid release into the knee joint: Key role in nociception and inflammation. Pain, 86, 69–74. 10.1016/S0304-3959(99)00311-5 [DOI] [PubMed] [Google Scholar]
- Li, J. H. , He, P. Y. , Fan, D. N. , Alemujiang, D. , Huo, F. Q. , Zhao, Y. , & Cao, D. Y. (2018). Peripheral ionotropic glutamate receptors contribute to Fos expression increase in the spinal cord through antidromic electrical stimulation of sensory nerves. Neuroscience Letters, 678, 1–7. 10.1016/j.neulet.2018.04.051 [DOI] [PubMed] [Google Scholar]
- Ma, Q. P. , & Woolf, C. J. (1997). The progressive tactile hyperalgesia induced by peripheral inflammation is nerve growth factor dependent. Neuroreport, 8, 807–810. 10.1097/00001756-199703030-00001 [DOI] [PubMed] [Google Scholar]
- Masuoka, T. , Kudo, M. , Yamashita, Y. , Yoshida, J. , Imaizumi, N. , Muramatsu, I. , … Ishibashi, T. (2017). TRPA1 channels modify TRPV1‐mediated current responses in dorsal root ganglion neurons. Frontiers in Physiology, 8, 272 10.3389/fphys.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka, T. , Kudo, M. , Yoshida, J. , Ishibashi, T. , Muramatsu, I. , Kato, N. , … Nishio, M. (2016). Long‐term activation of group I metabotropic glutamate receptors increases functional TRPV1‐expressing neurons in mouse dorsal root ganglia. Frontiers in Cellular Neuroscience, 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka, T. , Nakamura, T. , Kudo, M. , Yoshida, J. , Takaoka, Y. , Kato, N. , … Nishio, M. (2015). Biphasic modulation by mGlu5 receptors of TRPV1‐mediated intracellular calcium elevation in sensory neurons contributes to heat sensitivity. British Journal of Pharmacology, 172, 1020–1033. 10.1111/bph.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. C. , Drummond, G. B. , McLachlan, E. M. , Kilkenny, C. , & Wainwright, C. L. (2010). Guidelines for reporting experiments involving animals: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1573–1576. 10.1111/j.1476-5381.2010.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, S. B. , Armanini, M. P. , Ling, L. H. , & Phillips, H. S. (1994). Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron, 12, 1161–1171. 10.1016/0896-6273(94)90323-9 [DOI] [PubMed] [Google Scholar]
- Mohammed, Z. A. , Doran, C. , Grundy, D. , & Nassar, M. A. (2017). Veratridine produces distinct calcium response profiles in mouse dorsal root ganglia neurons. Scientific Reports, 7, 45221 10.1038/srep45221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote, K. , Kawamata, T. , Kawamata, M. , & Namiki, A. (1998). Formalin‐induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Research, 787, 161–164. 10.1016/S0006-8993(97)01568-0 [DOI] [PubMed] [Google Scholar]
- Ong, H. L. , de Souza, L. B. , & Ambudkar, I. S. (2016). Role of TRPC channels in store‐operated calcium entry. Advances in Experimental Medicine and Biology, 898, 87–109. 10.1007/978-3-319-26974-0_5 [DOI] [PubMed] [Google Scholar]
- Prato, V. , Taberner, F. J. , Hockley, J. R. F. , Callejo, G. , Arcourt, A. , Tazir, B. , … Lechner, S. G. (2017). Functional and molecular characterization of mechanoinsensitive “silent” nociceptors. Cell Reports, 21, 3102–3115. 10.1016/j.celrep.2017.11.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh‐Garabedian, B. , Poole, S. , Allchorne, A. , Winter, J. , & Woolf, C. J. (1995). Contribution of interleukin‐1β to the inflammation‐induced increase in nerve growth factor levels and inflammatory hyperalgesia. British Journal of Pharmacology, 115, 1265–1275. 10.1111/j.1476-5381.1995.tb15035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story, G. M. , Peier, A. M. , Reeve, A. J. , Eid, S. R. , Mosbacher, J. , Hricik, T. R. , … Patapoutian, A. (2003). ANKTM1, a TRP‐like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell, 112, 819–829. 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- Szteyn, K. , Rowan, M. P. , Gomez, R. , Du, J. , Carlton, S. M. , & Jeske, N. A. (2015). A‐kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain, 156, 2364–2372. 10.1097/j.pain.0000000000000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley, P. , Lamballe, F. , & Barbacid, M. (1992). K252a is a selective inhibitor of the tyrosine protein kinase activity of the Trk family of oncogenes and neurotrophin receptors. Oncogene, 7, 371–381. [PubMed] [Google Scholar]
- Ugolini, G. , Marinelli, S. , Covaceuszach, S. , Cattaneo, A. , & Pavone, F. (2007). The function neutralizing anti‐TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America, 104, 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, D. H. , Jung, S. J. , Zhu, M. H. , Park, C. K. , Kim, Y. H. , Oh, S. B. , & Lee, C. J. (2008). Direct activation of transient receptor potential vanilloid 1 (TRPV1) by diacylglycerol (DAG). Molecular Pain, 4, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Huang, J. , & McNaughton, P. A. (2005). NGF rapidly increases membrane expression of TRPV1 heat‐gated ion channels. The EMBO Journal, 24, 4211–4223. 10.1038/sj.emboj.7600893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, Z. Y. , Xu, H. , Clapham, D. E. , & Ji, R. R. (2004). Phosphatidylinositol 3‐kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. The Journal of Neuroscience, 24, 8300–8309. 10.1523/JNEUROSCI.2893-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]


