Abstract
Ensuring that people have access to sufficient and nutritious food is necessary for a healthy life and the core tenet of food security. With the global population set to reach 9.8 billion by 2050, and the compounding effects of climate change, the planet is facing challenges that necessitate significant and rapid changes in agricultural practices. In the effort to provide food in terms of calories, the essential contribution of micronutrients (vitamins and minerals) to nutrition is often overlooked. Here, we focus on the importance of thiamine (vitamin B1) in plant health and discuss its impact on human health. Vitamin B1 is an essential dietary component, and deficiencies in this micronutrient underlie several diseases, notably nervous system disorders. The predominant source of dietary vitamin B1 is plant-based foods. Moreover, vitamin B1 is also vital for plants themselves, and its benefits in plant health have received less attention than in the human health sphere. In general, vitamin B1 is well-characterized for its role as a coenzyme in metabolic pathways, particularly those involved in energy production and central metabolism, including carbon assimilation and respiration. Vitamin B1 is also emerging as an important component of plant stress responses, and several noncoenzyme roles of this vitamin are being characterized. We summarize the importance of vitamin B1 in plants from the perspective of food security, including its roles in plant disease resistance, stress tolerance, and crop yield, and review the potential benefits of biofortification of crops with increased vitamin B1 content to improve human health.
Keywords: Biofortification, coenzyme, defense, food security, genetic engineering, metabolism, micronutrient, physiology, plant, thiamine, vitamin, yield, plant defense, plant biochemistry, yield
The autotrophic (i.e. self-sustaining) ability of plants allows them to take elements from the soil and atmosphere and build them into complex macromolecules through electromagnetic energy harvested from the sun. Through this assembly of complex organic compounds driven by photosynthesis, plants produce nutrients that allow them to survive, grow, and reproduce. In their role as producers at the base of the food chain, plants provide the predominant dietary source of the same nutrients to humans, in turn allowing us to also survive, grow, and reproduce. An intermediate step in this transfer might exist through our consumption of meat or fish, but as animals are also heterotrophs, the source of these nutrients and energy can always be traced back to autotrophic plants, bacteria or fungi. The essential macronutrients (carbohydrates, fats, protein, and fiber) originating from plants provide us with energy and the building blocks of growth, whereas inorganic (minerals) and organic (vitamins) micronutrients are also vital to human health.
Although the organic micronutrients essential to health and survival are alike in both plants and animals, they are only classified as vitamins in relation to animals. To be classified as a vitamin, the organism must both need the organic micronutrient and also lack the capacity to synthesize the compound de novo or only be able to produce insufficient quantities, instead relying on dietary consumption of precursors or analogs to meet their needs (1). Nonetheless, for ease of understanding, we will use the term vitamin here also when referring to these compounds in a generic way in plants. The biochemical functions derived from vitamins and their forms are hugely varied and include antioxidant (e.g. ascorbate/vitamin C and tocochromanols/vitamin E), coenzyme (e.g. thiamine/vitamin B1 and riboflavin/vitamin B2), regulator of gene transcription (e.g. retinol/vitamin A), and hormone (e.g. calciferol/vitamin D). Deficiencies in vitamins can cause severe and deadly disorders; for example, of the 500,000 children who develop blindness each year from vitamin A deficiency, half will die within 12 months (World Health Organization (2013) Micronutrient deficiencies, https://www.who.int/nutrition/topics/vad/en/; accessed March 25, 2020). Similarly, in plants, deficiencies in vitamins also impact overall fitness and growth, causing deleterious phenotypes and even lethality (2–7).
For scientists, researching vitamin compounds in plants themselves offers a broad reach, with potential research applications in improving human health. Furthermore, vitamin compounds have important roles in plant disease resistance and yield (8), which are key to increasing agricultural productivity and improving global food security, especially in a changing climate. Understanding how vitamins are synthesized and salvaged in plants has been intensively researched (for reviews, see Refs. 3 and 9), but how they are transported and regulated has received only scant attention, particularly for the B vitamin family (10). Many of the advances in plant vitamin research have been coupled with breakthroughs in genetic modification techniques (11–13) and improved understanding of plant metabolic networks (14–16), which have paved the way for biotechnological exploitation of a plant's natural vitamin physiology. Due to the breadth of this field, this review will predominantly focus on the importance of plant thiamine (vitamin B1) in food security, covering its roles in plant disease resistance, stress tolerance, and crop yield, and continuing onto the potentials of biofortification of crops with increased thiamine content for human consumption.
Vitamins and vitamers
The classification and naming of vitamins is largely anachronistic and has little chemical basis. The term vitamin was coined from the incorrect hypothesis that these “vital” compounds were comprised of “amines” and led to the portmanteau “vitamine” (17). However, non-amine vitamins, such as ascorbate, resulted in the terminal “e” being dropped. Today 13 vitamins are recognized, which are commonly divided into those that are either fat-soluble (vitamins A, D, E, and K) or water-soluble (vitamin C and the vitamin B complex) (Fig. 1). The B vitamin complex itself is comprised of eight vitamins (B1, B2, B3, B5, B6, B7, B9, and B12), which bear no chemical similarities to one another but were primarily grouped due to their similar ability to function as coenzymes. Notably, among the individual vitamins, there are structural analogs that have proven vitamin activity (i.e. can remedy a deficiency in that vitamin) and are described as vitamers. Therefore, each B vitamin is actually a family of compounds (e.g. the vitamer and coenzyme thiamine diphosphate (TDP) within the vitamin B1 family) (Fig. 2). Originally, more compounds were classified as vitamins; however, many of these were removed, having since been found to be nonessential and/or able to be synthesized by the human body. This has resulted in gaps in the current alphabetical (vitamins F–J and L–Z) and numerical (vitamin B4, B10, and B11) nomenclature. These historical relics in the classification system demonstrate the extent to which the field has moved since the discoveries of each vitamin.
Figure 1.
Broad classification of vitamins. Vitamins are divided into two general groups, those that are fat-soluble (A, D, E, and K) and those that are water-soluble (B1, B2, B3, B5, B6, B7, B9, B12, and C).
Figure 2.
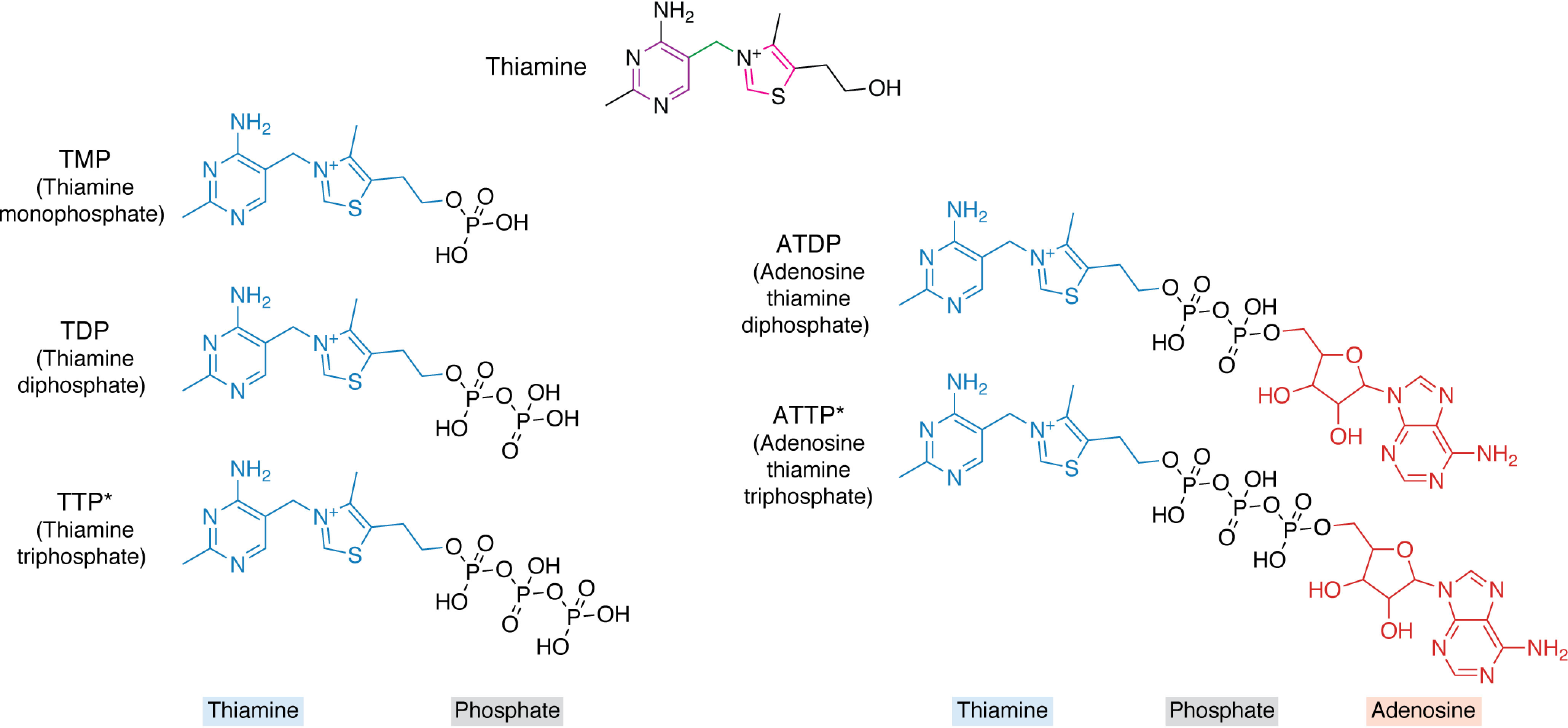
Chemical structures of the vitamin B1 family. The basic unit thiamine (blue) is shown at the top, comprising pyrimidine (purple) and thiazolium (pink) heterocycles linked by a methylene bridge (green). Thiamine derivatives vary in their phosphorylation states (black) and adenosylation states (red) and include TMP, TDP, TTP, ATDP, and ATTP. Those marked with an asterisk have been implicated as signaling molecules.
Thiamine
The earliest written descriptions of thiamine deficiency, now known as beriberi disorder, date back to the 3rd century, but it only became prevalent in the 19th century, when sailors were fed on monotonous unvaried diets (18). Beriberi affects the nervous, cardiovascular, and digestive systems, causing numbness, weakness, atrophy, and eventually death. A paradigm shift, away from the long-held germ theory, led to the acceptance that diseases could also be caused by lack of “accessory food factors” as well as by pathogens (19). It was not until 1901 that beriberi was proposed to be a deficiency syndrome arising from poor diet (19), which could be avoided by consumption of rice bran, which is normally removed during the conversion of brown rice to white, polished rice (17). Many attempts to characterize and isolate the specific rice bran chemical and “anti-beriberi compound” were made, until the final structure was determined in 1936 and named thiamine (20).
Thiamine (chemical formula C12H17N4OS; name 2-[3-[(4-amino-2-methylpyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium-5-yl]ethanol) is an organo-sulfur compound comprising pyrimidine and thiazolium heterocycles linked by a methylene bridge (Fig. 2). Six vitamer forms of thiamine are currently known, varying in their phosphorylation and adenosylation states (Fig. 2) (21–23). Although thiamine biosynthesis has been covered in depth in other reviews (detailing the different pathways that bacteria, fungi, yeasts, and plants utilize (24–26)), we will briefly describe thiamine biosynthesis de novo in plants (specifically Arabidopsis) here. First, the thiazole moiety (hydroxyethylthiazole phosphate, HET-P) and pyrimidine moiety (hydroxymethylpyrimidine pyrophosphate, HMP-PP) are biosynthesized through separate pathways in the chloroplast (5, 27) (Fig. 3). Although primarily based on biochemical evidence from yeast rather than in planta data, HET-P is synthesized by the THI1 protein (27), and an as yet unknown NUDIX hydrolase (28), using NAD+, glycine, and a sulfur atom from a cysteine in the THI1 protein backbone itself (29) (Fig. 3). The backbone sulfur donation renders THI1 catalytically inactive, and the protein is therefore referred to as a “suicide enzyme” due to its single turnover (29), rather than an enzyme per se, which by definition would catalyze multiple turnovers. On the other side, the first step in HMP-PP formation is catalyzed by the THIC enzyme, which rearranges aminoimidazole ribonucleotide to hydroxymethylpyrimidine phosphate (HMP-P) enabled by a 5′-deoxyadenosyl radical and a [4Fe-4S]+ cluster within the enzyme (5, 30). The HMP-P moiety is then further phosphorylated to generate HMP-PP by the TH1 enzyme (31). TH1 is a bifunctional enzyme that also catalyzes the condensation of HMP-PP and HET-P to form thiamine monophosphate (TMP) (31, 32) (Fig. 3). Interestingly, TMP is not directly phosphorylated to TDP; rather, it is first dephosphorylated to thiamine by a phosphatase (Fig. 3). Although this was originally thought to be a phosphatase of broad specificity (33, 34), the elucidation of the role of TH2—a mutant of which was one of the classical thiamine-requiring mutants isolated in 1969 (th2-1)—demonstrated that it is its specific function (35, 36). That TH2 encodes a specific TMP phosphatase was supported in an independent study with the isolation of the Arabidopsis mutant palegreen1 (pale1) (37). Intriguingly, these studies have shown that TH2/PALE1 is localized to the mitochondria and perhaps also the cytosol (36, 37). Thus, as TMP is made in the chloroplast, either it is transported to the site of TH2, or other TMP phosphatases also exist in plastids (Fig. 3). In Arabidopsis, conversion of thiamine into TDP by the thiamine kinase TPK is reported to occur in the cytosol (38) (Fig. 3). To date, thiamine kinases have not been studied in other plant species, so it is not known whether TPKs are exclusive to the cytosol among plantae. However, if the sole localization of TPKs is to the cytoplasm, then as TDP is a polar molecule, it needs to be actively imported into the organelles to furnish enzymes dependent on it as a coenzyme (Fig. 3).
Figure 3.
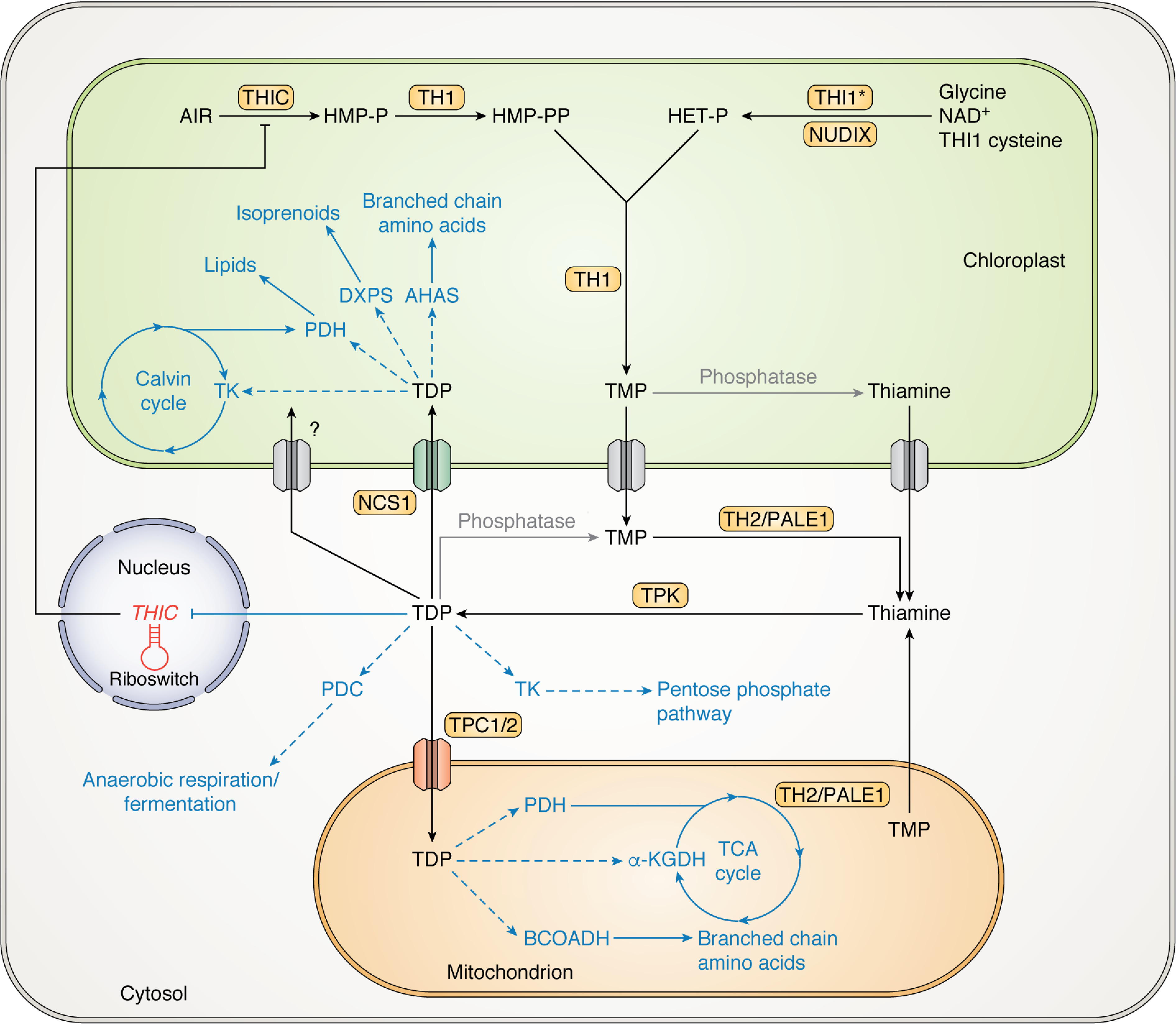
Biosynthesis, transport, and roles of the coenzyme thiamine diphosphate in the model plant Arabidopsis. TDP biosynthesis (black) and its roles in the plant cell (blue dashed arrows, coenzyme activity) are displayed, with currently uncharacterized aspects of the pathways shown in gray. TMP is generated from the condensation of HMP-PP and HET-P, catalyzed via TH1 in the chloroplast. HET-P is synthesized by THI1 and a NUDIX hydrolase using NAD+, glycine, and a sulfur atom from the THI1 backbone, which renders this protein inactive as a catalyst for this reaction after one cycle (*). HMP-PP is formed from aminoimidazole ribonucleotide (AIR) by THIC and the phosphorylation activity of TH1. To generate the coenzyme form, TDP, TMP is first dephosphorylated to thiamine catalyzed by TH2/PALE1 in the cytosol (or mitochondrion) or uncharacterized phosphatases (in the chloroplast or cytosol) before phosphorylation to TDP by TPK in the cytosol. TDP has many roles in the cell, including in a negative feedback loop whereby TDP regulates thiamine biosynthesis through THIC gene expression in the nucleus via a riboswitch. TDP may be transported from the cytosol into the plastid via the nucleotide cation symporter 1 (NCS1), and into the mitochondrion by thiamine phosphate carriers (TPC1 and TPC2), whereas other thiamine vitamer transporters have not yet been characterized, particularly at the chloroplast envelope (gray). Enzymes requiring TDP as a coenzyme (dashed arrows) take key positions in central metabolism; in the chloroplast, TDP is involved in carbon assimilation, acting as a coenzyme for the Calvin cycle enzyme transketolase (TK) and for the TDP-dependent enzymes 2-deoxyxylulose 5-phosphate synthase (DXPS) and acetohydroxyacid synthase (AHAS) for isoprenoid and branched-chain amino acid biosynthesis, respectively. Another TDP-dependent enzyme, pyruvate dehydrogenase (PDH), is involved in lipid biosynthesis in the chloroplast. In the cytosol, TDP-dependent enzymes include TK in the pentose phosphate pathway, important for generating NADPH and pentoses, and also pyruvate decarboxylase (PDC) involved in anaerobic respiration/fermentation. In the mitochondrion, the TDP-dependent enzyme PDH is involved in feeding carbon into the TCA cycle, and α-ketoglutarate dehydrogenase (α-KGDH) is a key enzyme that modulates flux through the TCA cycle, affecting the redox, energy, and nitrogen balance of the cell. Branched-chain oxy-acid dehydrogenase (BCOADH) is a mitochondrial TDP-dependent enzyme complex that is involved in branched-chain amino acid catabolism.
Transporters specific for TDP at the mitochondrial membrane are known and are annotated as thiamine pyrophosphate carriers (TPCs) (39). Arabidopsis has two members (TPC1 and TPC2), which have recently been shown to be vital for plant survival (40) (Fig. 3). Additional transporters involved in thiamine metabolism may be present on the plastid envelope, in particular for import of TDP to furnish the enzymes dependent on it as a coenzyme, but have not been characterized. Interestingly, the nucleotide cation symporter NCS1 (also annotated as PLUTO) (41, 42) is localized to the plastid (Fig. 3) and was recently implicated in transport of the thiamine precursor HMP, as well as TDP when plants are compromised in biosynthesis de novo (40, 43). However, transporters at the plastid membrane that import TDP biosynthesized de novo in the cytosol remain elusive and would be expected to be essential for plant survival. Due to the numerous substrates and suicide enzymes involved, thiamine biosynthesis de novo incurs relatively high energy costs (44, 45). A consequence of this is that TDP appears to only be biosynthesized when required and is under tight control. In plants, one way to regulate TDP abundance is through the only riboswitch known to exist in plants, and which is present in the 3′-UTR (UTR) of the biosynthesis gene THIC (46, 47). When there is an elevated level of free TDP (although this level remains undefined), it can bind to the 3′-UTR of THIC pre-mRNA in the nucleus, modifying the secondary structure in a way that promotes splicing of an intron and forming an unstable mRNA, which lowers the amount of THIC and, in turn, the biosynthesis of the pyrimidine precursor in a negative feedback loop (Figs. 3 and 4). In addition, many components of the TDP biosynthesis machinery in plants are under the control of the genetically encoded circadian clock at the transcriptional level (40, 48) (Fig. 4). Interestingly, the peaks in abundance of the biosynthesis components and known transporters are at distinct times of the day (evening and morning, respectively), which may serve to coordinate supply with the needs of the cell (40). It is also noteworthy that thiamine salvage pathways have undergone investigation in plants (49–51) and may provide roles in maintaining vitamer homeostasis, bypassing the significant energetic costs required in biosynthesis de novo. Such pathways deserve further attention because they may confer an adaptive advantage to plants in times of environmental stress.
Figure 4.
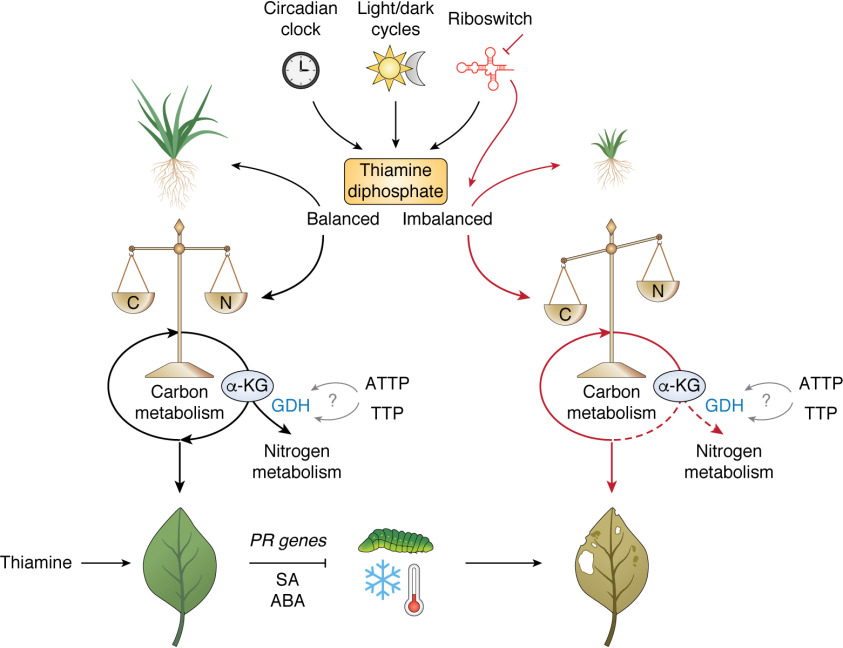
Importance of maintaining thiamine diphosphate balance in plants. TDP levels are regulated by the circadian clock, light/dark cycles, and a riboswitch. Balancing of TDP levels is important and may in turn be integrated into the balance of carbon (C) and nitrogen (N) that yields healthy thriving plants possibly mediated through α-ketoglutarate (α-KG) abundance. α-KG lies at the intersection between carbon and nitrogen metabolism and is metabolized by α-ketoglutarate dehydrogenase (not shown) during carbon metabolism by the TCA cycle or GDH during nitrogen metabolism. ATTP and TTP act as allosteric activators of GDH in mammalian systems, but a similar function in plants has not yet been explored. Application of thiamine primes plants against biotic stress via up-regulation of PR genes and salicyclic acid (SA). Thiamine also positively influences against abiotic stress via abscisic acid (ABA). Inhibition of riboswitch function (red line) leads to TDP imbalance. Inappropriate perturbation of TDP levels impacts carbon/nitrogen balance, plant health, and yield. This figure was made with BioRender.
From published profiles of B1 vitamers, TDP appears to be the most abundant form in both shoots and roots of plants, followed by TMP and thiamine (34, 52), although thiamine is the most abundant form in seeds by far (53). The other analogs of thiamine (Fig. 2) have been reported to exist in several organisms, but it is thought that they have messenger activity rather than acting as potential vitamer forms that could contribute to TDP biosynthesis. Among these is thiamine triphosphate (TTP) (Fig. 2), which is implicated in nerve cell biochemistry in animals, and much work has been done to elucidate its function therein (21, 54). Interestingly, it is thought that TTP may function as an allosteric effector of metabolic enzymes (55). More specifically, it is thought that TTP binds distal to the active site of enzymes, such as pyridoxal kinase, malate dehydrogenase, and glutamate dehydrogenase, where in the case of the latter, it acts as a positive effector of activity (55). TTP has also recently been characterized by our group in plants (115). In plants, the abundance of TTP increases during the light period, and its synthesis may be coupled to the proton motive force and TDP levels, as has been shown in bacteria (56). It is possible that TTP may play a role in plant metabolic homeostasis (see below), similar to its role in animal cells, but this remains to be demonstrated. Notably, homologs for the three target proteins in humans mentioned above are also present in plants. Another vitamer form is adenosine thiamine triphosphate (ATTP) (Fig. 2), which has been characterized mainly in bacteria, where it appears under nutritional stress (22), but its precise function remains elusive. ATTP has been detected in root material of Arabidopsis (22), but it could not be detected in a recent study of these compounds from our group and awaits further characterization in plants (115). It has also been reported that adenosine thiamine diphosphate exists (57) (Fig. 2), but it has not undergone rigorous characterization.
The role of thiamine in plant metabolism, crop yield, and plant health
Thiamine and its vitamers have many roles in plant cells, the best-characterized of which is in the form of TDP, a coenzyme for many central metabolic enzymes (Fig. 3). Such enzymes include those involved in both photosynthesis (transketolase, TK) and respiration (pyruvate dehydrogenase, PDH; α-ketoglutarate dehydrogenase, α-KGDH) in the Calvin and TCA cycles of chloroplasts and mitochondria, respectively. The abundance of TDP in plant cells and organelles can affect flux through these key metabolic pathways. Indeed, α-KGDH exhibits one of the highest flux control coefficients of plant TCA cycle enzymes (58), and low TDP has been attributed to reduced flux through the TCA cycle via modulation of α-KGDH activity (59). Conversely, elevated levels of TDP are attributed to increased flux and a general increase in respiration rate (48). Moreover, TDP modulation has been demonstrated to directly alter plant metabolite abundance, in particular several photosynthetic pigments (40). However, it is not yet clear whether this remodeling is adaptive or responsive (i.e. are thiamine vitamer levels actively controlled by the cell to modulate, redistribute, or promote fluxes?) (54, 59). If this is the case, then cellular thiamine vitamers could play an exciting role in the active coordination of carbon catabolism (respiration) and anabolism (photosynthesis) and exert control over the carbon budget in plant cells and even organelles.
Indeed, the apparent homeostasis of TDP in plants is beginning to appear increasingly important (Fig. 4). In particular, organellular levels of the coenzyme TDP appear to be rate-limiting, resulting in flux remodeling through central metabolism (59). As mentioned above, intracellular TDP concentrations are modulated by a riboswitch, diel (i.e. daily) rhythms in the biosynthesis and organellular transport machinery, and the circadian clock (40, 48) (Fig. 4). So many layers of functional redundancy in control can serve to show the importance of TDP abundance to plant function and may even play a role in diel metabolite rhythms. The latter occur as a function of the light-dark cycle iterations that plants need to endure, being sessile, by reconfiguring metabolism on a daily basis (60). Indeed, loss of one of the TDP control systems—as demonstrated through a defective riboswitch—prevented plants from adapting to variable photoperiods and had dramatic negative effects on plant health and growth (48, 61) (Fig. 4). Interestingly, although in a mechanistically different context, overexpression of TK appears to result in the hoarding of cellular TDP reserves, depriving other enzymes of the coenzyme and leading to stunted growth and chlorophyll deficiencies (62). Therefore, tight regulation of cellular TDP concentrations appears to be key to metabolic homeostasis, photoperiod adaptation, and plant health (40, 61). Nonetheless, it can also present an opportunity for exploitation; might slight modifications and fine-tuning of TDP allow us to modulate processes that it is involved in, such as photosynthesis or carbon assimilation? As TDP concentrations affect metabolite abundances, can we use this relationship to increase plant sugars, amino acids, and lipid reserves that could improve crop yields?
To avoid a Malthusian crisis (i.e. Thomas Robert Malthus's theory on population), growing demands are expected to necessitate an increase in food production by 40–70% (63). Moreover, the compounding effects of climate change and the impact of agricultural land use on biodiversity, soil erosion, and water use emphasize the need to change agricultural practices imminently. Increasing plant productivity and crop yield has been a strong focus of plant scientists for several decades. Efforts in enhancing crop productivity and yields have largely concentrated on increasing photosynthetic carbon assimilation in the Calvin cycle and transfer of this carbon into storage sinks, such as starchy cereal grains or tubers (64, 65). Efforts have also been made to reduce the respiratory losses of plants and their maintenance costs, allowing more resources to be directed into biosynthesis and growth (66). In the context of thiamine, it has recently been proposed that reengineering more stable and nonsuicide versions of thiamine biosynthesis enzymes offers the potential to reduce the energetic costs of thiamine biosynthesis and puts forward ideas of how it could impact crop yield potential (67). Indeed, as thiamine has roles across key metabolic processes in plants, including both carbon assimilation (photosynthesis) and respiration (TCA cycle), and with its coordinated and wide-reaching responses throughout central plant metabolism, it presents an attractive target in the efforts to increase crop yield. However, plant macromolecules are not made of carbon alone, and the provision of carbon skeletons by the TCA cycle into biosynthetic molecules, such as amino acids for proteins, demonstrates the need to balance and coordinate carbon and nitrogen assimilation for optimal yield increases. The metabolite α-ketoglutarate (α-KG) (or 2-oxoglutarate) lies at the intersection of the main carbon and nitrogen metabolic pathways (Fig. 4). Although coordination of carbon and nitrogen metabolism in plants has not fully been elucidated, the abundance of α-KG appears to be a master regulator in the maintenance of this balance, at least in bacteria (68, 69). Funneling of α-KG either through the TCA cycle, by the TDP-dependent α-KGDH, or into nitrogen assimilation, by glutamate dehydrogenase (GDH), is integrated into the energy status of the cell. Intriguingly, in mammalian cells, the noncoenzyme thiamine vitamers TTP and ATTP have been demonstrated to be able to allosterically activate GDH and thereby may also have a role in the fate of α-KG (54, 55) (Fig. 4). In bacteria, TTP appears transiently in amino acid–starved cultures supplemented with glucose and is proposed to have a signal function (70, 71); however, it is not known what this function is. Although recently our group has characterized TTP in Arabidopsis, it is also not known yet what its physiological role is. ATTP, on the other hand, is present under general starvation conditions in bacteria (i.e. cultures devoid of amino acids and carbon), and it has also been proposed to be a signal molecule, but its function also remains to be elucidated (71). An early report claims to have detected ATTP in Arabidopsis (22), but it could not be detected under the conditions used in our recent study. Notwithstanding, the specific roles and potential influence of these thiamine derivatives on the nitrogen and carbon status in plants could represent a novel research area that could offer significant biotechnological opportunities.
Thiamine is also implicated in abiotic and biotic plant stress responses, with up-regulation of thiamine biosynthesis under stress conditions. In terms of abiotic stress, salt, temperature, and osmotic and oxidative stress have been shown to up-regulate thiamine biosynthetic genes in plants (72). In particular, THIC and THI1 transcript abundance is rapidly induced in Arabidopsis by these stresses and appears to be an early stress response mediated by abscisic acid (73). In this latter study, up-regulation of these thiamine biosynthesis genes under stress is thought to provide more TDP to furnish metabolic enzymes dependent on it as a coenzyme, which may in turn support abiotic defense responses. Recently, it was proposed that thiamine compounds may act as antioxidants themselves (74), but there is currently no direct evidence for this, and it remains to be demonstrated in the future. On the other hand, exogenous thiamine supplementation has been shown to be beneficial in improving plant pathogen resistance. Thiamine-treated crops display increased resistance to biotic infections via up-regulation of pathogenesis-related (PR) genes, effects that last for up to 15 days after treatment and prime the plant against infection via whole-plant systemic-acquired resistance mediated by salicylic acid production (75, 76) (Fig. 4). This response is widespread across many crops, with increases in wheat, pea, barley, oat, and millet resistance to herbivory and fungal infections after thiamine application (77–79). Conversely, rice plants with diminished thiamine contents had increased susceptibility to bacterial blight infections (80). Furthermore, and also of significance for plant health, thiamine has recently been implicated in both root nodule and the arbuscular mycorrhizal (an endophytic fungus) symbioses (81, 82). The impact of thiamine on these symbioses could have profound effects on nutrient uptake, particularly nitrogen and phosphorus, and thereby influence plant growth and development. Therefore, thiamine is emerging as an important molecule for plant resistance and adaptation under abiotic and biotic stress, which directly impacts crop yields. Increasing thiamine content of plants may thus have the potential to improve crop defense, yield reliability, and plant metabolic robustness in a changing climate and is discussed further in the next section.
Thiamine biofortification in crops for human health
As a water-soluble vitamin, thiamine cannot be stored by the body and is readily excreted. Therefore, consistent consumption of thiamine is necessary to avoid deficiencies, as depletion in the body can occur within just 2–3 weeks (83). As mentioned above, clinical manifestations of thiamine deficiency can take the form of beriberi disease. Notably, the disease presents itself in two types: wet beriberi affects the cardiovascular system and results in symptoms ranging from a shortness of breath to rapid heart rates and palpitations; dry beriberi affects the nervous system, causing numbness, difficulty moving, amnesia (Korsakoff syndrome), and brain lesions (Wernicke encephalopathy) (84). These extreme forms of thiamine deficiency can ultimately cause death (84). Clinical deficiencies appear to have become less frequent and are thought to be largely limited to populations adhering to sustenance diets high in carbohydrate content (37, 83). Although subclinical deficiencies are less extreme, they still have significant negative effects on human metabolism and health and are thought to be more widespread (84); 16% of diets from the elderly in the United States were found to contain insufficient thiamine (85, 86), and in the United Kingdom, 21% of randomly selected patients admitted to an emergency department were found to have thiamine deficiencies (87). These can be due to poor diets or medical conditions such as alcoholism (88), vomiting, and diarrhea, which all limit the absorbance of thiamine (83, 84). Deficiencies in micronutrients, thiamine included, are commonly described as “hidden hunger” due to their chronic manifestation, which is often invisible until serious or fatal, compounded by a lack of sufficient methods to efficiently perform diagnoses (84, 89). This differs from hunger per se, which is used to refer to reduced intake of food in terms of calories. Hidden hunger is derived from a lack of food that is nutritious (i.e. in terms of micronutrients, stemming from a lack of access to a varied diet). Hidden hunger is becoming more prevalent due to the burgeoning population, especially over the last 5–6 decades, many of whom are poverty-stricken, in addition to the global dependence on just a few high-calorie but low-in-micronutrient content crops, and the global drift to consuming highly processed foods (89).
Of the staple crops that constitute most of humanity's diet, many are low in thiamine abundance (3, 90). Indeed, based on the documented thiamine content of the world's most consumed staple crops, excessive quantities of rice, corn, wheat, potatoes, and the orphan food crop, cassava, would need to be consumed to meet the recommended daily allowance (Table 1). For example, 5.5–7.5 kg of boiled white rice would need to be consumed daily to provide sufficient dietary thiamine (Table 1). Further significant losses of thiamine result from refining and polishing of cereal; during the processing of brown rice to white rice, the thiamine-rich bran and germ components of the grain are removed, and in the production of white flour, the germ and aleurone layers are discarded, leaving the endosperm, which is relatively thiamine-poor (3, 28, 92) (Table 1). Moreover, processing and cooking can reduce thiamine content even further (Table 1); for example, baked bread made from white wheat flour contains 15–48% less thiamine than its dough, due to heat degradation and yeast-driven conversion of thiamine to the more heat-labile TDP (93). Preserving food in tins or cans can also reduce thiamine content due to the alkaline pH from sulfate preservatives causing degradation, and boiling of food results in the leeching of thiamine (Table 1) (World Health Organization (2013) Micronutrient deficiencies, https://www.who.int/nutrition/topics/vad/en/; accessed March 25, 2020). To counteract these processing steps, it is common to reintroduce thiamine to food after processing via fortification and enrichment with synthetic forms of the vitamin (Table 1), which is even mandated by some governments (84). However, the costs of supplementation can be prohibitive, and crops still make up the main source of thiamine in most diets. Notably, the provision of vitamin pills as an alternative is short-term, may not be sustainable, requires continuous financing, and in many cases cannot be delivered to those in most need. Therefore, there remains significant and wide-reaching public health benefits from the production of food crops with increased thiamine contents.
Table 1.
Minimum daily consumption (kg) of each of the five most consumed staple crops required to meet the recommended daily amounts (RDA) of thiamine for males, females, and lactating females
Amounts were calculated from the United States Department of Agriculture Food Data Central using recommended daily allowances of thiamine from the World Health Organization. Each crop can be identified using the United States Department of Agriculture National Database Number (NDB number). Amounts were calculated using mg of thiamine per kg of each crop divided by the recommended daily allowance of thiamine (mg).
| Crop (USDA NDB number) (91) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potato |
Rice |
Corn |
Wheat |
Cassava |
|||||||||
| Potatoes, white, flesh and skin, raw (11354) | Potatoes, white, flesh and skin, baked (11357) | Rice, white, medium-grain, raw, unenriched (20450) | Rice, white, medium-grain, cooked, unenriched (20451) | Rice, brown, medium-grain, raw (20040) | Rice, brown, medium-grain, cooked (20041) | Corn, raw (787790) | Corn, cooked (788178) | Wheat flour, white, all-purpose, unenriched (169761) | Flour, whole wheat, unenriched (790085) | Wheat flour, white, all-purpose, enriched, bleached (1 68894) | Cassava, raw (169985) | Cassava, cooked (787178) | |
| Thiamine content (mg/kg crop) | 0.71 | 0.48 | 0.7 | 0.2 | 4.13 | 1.02 | 1.55 | 0.35 | 1.2 | 5.04 | 7.85 | 0.87 | 0.7 |
| Males (RDA 1.2 mg) (kg) | 1.69 | 2.50 | 1.71 | 6.00 | 0.29 | 1.18 | 0.77 | 3.43 | 1.00 | 0.24 | 0.15 | 1.38 | 1.71 |
| Females (RDA 1.1 mg) (kg) | 1.55 | 2.29 | 1.57 | 5.50 | 0.27 | 1.08 | 0.71 | 3.14 | 0.92 | 0.22 | 0.14 | 1.26 | 1.57 |
| Lactating females (RDA 1.5 mg) (kg) | 2.11 | 3.13 | 2.14 | 7.50 | 0.36 | 1.47 | 0.97 | 4.29 | 1.25 | 0.30 | 0.19 | 1.72 | 2.14 |
Biofortification is one mechanism to provide a solution, as it offers a one-time investment to improve crop micronutrients that avoids changes in diet with minimal (if any) impact on agricultural practices or the costly fortification of food during processing. Biofortification can be achieved through breeding strategies if the trait exists (e.g. higher thiamine content in a related variety) and can be exploited in this manner, although breeding strategies can be limited by their lengthy generation times and only exploit beneficial cisgenes that can be introduced if sexual compatibility allows. As an alternative, introduction of novel genes and rapid modification of crop phenotypes can be achieved through genetic engineering. However, the success of genetic modification relies on how well-characterized the metabolism and physiology of the plant is. Fortunately, the understanding of thiamine biosynthesis, transport, salvage, regulation, and metabolism in plants has progressed significantly in recent years, as described above, and has been matched with advances in bioengineering techniques.
Readily available and improved genomic toolkits, such as the publication of crop genomes and quantitative trait locus (QTL) mapping, have enabled the identification and sequencing of genes behind phenotypic variation. Through these methods, it is increasingly possible to identify the alleles that convey advantageous traits; QTL mapping has successfully identified several loci that correlate with increased thiamine content in modern wheat varieties (94). Similarly, analysis of thiamine content across cassava accessions identified cultivars with a 170% increase in thiamine content (95), which could be introduced to other cultivars by introgressive hybridization and backcrossing. Undomesticated relatives of crop plants also offer large pools of genetic variation to exploit, and targeted breeding programs are being pursued to cross modern potato varieties with wild cultivars that have over 2-fold increases in their thiamine content (91). Understanding the molecular and physiological basis of these differences in thiamine content may also serve to help direct metabolic engineering efforts in other crops.
Modulation of riboswitch control of THIC expression was an obvious target to test for an increase in thiamine content upon its discovery. However, in Arabidopsis, only a modest increase in thiamine content was observed in plants with a nonfunctional riboswitch (48) or upon overexpression of THIC by placing its expression under the constitutive cauliflower mosaic virus 35S promoter (48, 96). Moreover, these approaches led to modified B1 vitamer profiles, and the plants suffered from stunted growth, chlorosis (i.e. loss of green color), and delayed development (48) (Fig. 4). Furthermore, central metabolism was perturbed, and in the case of the nonfunctional riboswitch lines, plants could not acclimate to an abrupt change in photoperiod (61), implying that these lines would not perform well if faced with an environmental challenge. This rendered the manipulation of THIC alone as an undesirable target for rational vitamin B1 biofortification of plants. However, we have previously proposed that a balance of precursors was required to appropriately increase thiamine content (92). This was supported by experimentation in Arabidopsis, where supplementation with both thiamine precursors, HET and HMP (Fig. 3), led to an 8-fold increase in thiamine vitamer content, but no change was observed if each was supplemented alone (92). Importantly, there was no obvious impact on growth or phenotype, and there was no significant alteration of the coenzyme form, TDP, which could negatively influence metabolic homeostasis (92). Later studies simulated this work by overexpression of both THIC and THI1 in either Arabidopsis or rice (97, 98). Indeed, thiamine vitamer content increased by 3.4- and 5-fold in Arabidopsis leaves (97) and rice leaves (98), respectively. However, whereas this increased thiamine content could also be observed in the rice unpolished grain, it was not found in the polished grain, suggesting that the elevated thiamine content was derived from the rice bran or embryo and had not reached the endosperm. Therefore, whereas it appears that both the pyrimidine and thiazole branches of thiamine biosynthesis are necessary targets to modify in tandem to maximize thiamine abundance in plants (28, 92), further strategies need to be explored to increase thiamine content in the endosperm of rice (i.e. the part left after polishing of the grain). As mentioned above, with current technologies, mining of the thousands of rice varieties available may reveal cultivars with increased thiamine contents (99), the molecular basis of which could be used to increase endosperm thiamine content in this food crop through engineering or breeding. To date, a set of cassava cultures have been examined for their diversity in vitamin B1 content (95), as well as a small set of rice and potato cultivars (91, 100–102). However, only up to 3-fold variation was observed in the varieties analyzed in these studies; thus, further genetic resources remain to be examined for their thiamine content.
It is essential to note that metabolic engineering of individual pathways is futile unless the metabolic system offers the capacity to support these changes; overexpression of the thiamine biosynthesis pathways alone will not elevate thiamine abundance if the supply of upstream precursors is not also balanced. For example, HET production needs to be matched with NAD+ biosynthesis, and expression changes in the suicide enzyme THI1 need to be matched with changes in energy production to meet the associated protein synthesis costs. Similarly, HMP production relies on purine biosynthesis and the need to furnish the THIC enzyme with an iron-sulfur cluster and SAM, which should not create a dearth in supply for other enzymes. To avoid simply moving a metabolic bottleneck to elsewhere in the system and to successfully realize biotechnological aims, a network approach may prove useful for rational engineering. Advances in computational modeling of the plant metabolic network (e.g. by flux balance analysis) are identifying suites of genes required to achieve successful biotechnological outcomes (103, 104), and similar approaches could be useful in identifying targets to achieve vitamin B1 biofortification in plants and their edible organs. An attempt at this approach has already been made, and preliminary testing was carried out using cassava as a model with the convenience of altering gene expression using virus-induced genomic silencing (105), but the desired outcome of effectively increasing thiamine content was not achieved (106), and this approach would need refinement if it were to be explored further. In particular, some of the targets identified (purine metabolism) are part of essential metabolic pathways and, when silenced completely, appeared to cause deleterious effects. The use of weak promoters or targets with altered desired activity may provide a solution.
Conventional methods of plant genetic modification previously employed cloning genes into the tumor-inducing plasmid of Agrobacterium tumefaciens, exploiting its DNA transmission capabilities to insert novel genes into plant genomes (107). However, the introduction of CRISPR/Cas9 technologies for genetic engineering (108) has enabled targeted insertion of transgenes at specific locations throughout plant genomes (11, 13) and without additional insertion of bacterial flanking DNA (109). Recently, these techniques were successful in inserting a carotenoid biosynthesis cassette into rice, which yielded processed rice grains with high carotenoid (vitamin A) content and had no deleterious effects on crop yield or physiology (109). Once appropriate genes are identified for thiamine enhancement, a similar approach could be used. Although these advances have improved genetic engineering outcomes and show success in vitamin biofortification attempts, the remaining issues with public perception, regulation, and cost still hinder biofortification efforts achieved in this way.
Plants are able to uptake thiamine from their surroundings, as demonstrated by the better growth of thiamine biosynthesis mutants in soil compared with that in culture and supported by experiments in vitro where thiamine supplementation rescues the severe phenotypes of stunted growth and chlorosis (5). However, soil pH, mineral content, and pesticide use are likely to affect thiamine distribution in soil (79). Interestingly in this context, root exudates (i.e. secretions) of crop plants are known to contain thiamine, but it is not clear if this is from the plants themselves or secreted from microbes associated with plant roots (110). Plant growth–promoting rhizobacteria (PGPR) (i.e. bacteria that inhabit the area around plant roots) have been demonstrated to secrete thiamine into the rhizosphere, and direct exchange of vitamins between PGPR and plants has been reported (110). It is possible to hypothesize that plants could become, or already are, involved in symbiotic exchange of thiamine with rhizobacteria and maybe also with mycorrhizal fungi (i.e. fungi that inhabit the area around plant roots) (81, 82, 110, 111). Considering that between 2 and 12% of total plant maintenance energy demands have solely been attributed to thiamine biosynthesis enzyme turnover (THIC and THI1), out-sourcing thiamine anabolism might confer significant benefits to the plant (45, 67). Moreover, application of exogenous thiamine is known to decrease expression of the THIC gene, as demonstrated in Arabidopsis (5) and cassava cultivars (95), thus switching off thiamine biosynthesis de novo. Therefore, the perception of an alternative thiamine source by plants may have the same effect (i.e. switch off the expensive, energy-draining biosynthesis de novo pathway within the plant itself). In turn, it is possible to envisage how this situation would also benefit the associated bacteria, as they would receive more carbon exudates from larger and healthier plants, keeping in mind that 12–40% of photosynthetic carbon is released by plants as exudates to support soil biota (112). In a different view, there is also evidence for the exploitation of plants by microbes. For example, root colonization of oil palm seedlings with the endophytic (i.e. the life cycle is completed within plants) fungus Hendersonula toruloidea resulted in increased endogenous expression of thiamine biosynthesis genes and a 2-fold increase in thiamine vitamers in the plant as a response to colonization (113). Although the mechanism behind this is unknown and will certainly be interesting to elucidate, it suggests that nonbeneficial (i.e. pathogenic) microbes could exploit plants by increasing thiamine content for their own purposes. Although the wider impacts on crop yield and health need to be considered, farming practices that promote microbial interactions (i.e. fewer pesticides) have been shown to yield spinach crops with higher thiamine contents than alternatives grown conventionally (114). Thus, application of beneficial thiamine-synthesizing bacteria to agricultural fields may offer a method to improve thiamine content of crops but has not been explored. Nonetheless, thinking beyond biofortification sensu stricto may provide novel ways to also manipulate crop thiamine content.
Conclusion
Thiamine and its vitamers appear to have vital roles in plant health and metabolism, both through a role as an essential enzymatic coenzyme and as molecules for plant stress resistance. The full extent of the effect of thiamine modulation on plant metabolism and defense responses is still not fully understood, but it is clear that thiamine is essential for plant health and survival. Here we present an emerging idea that thiamine vitamers may be involved in the coordination of carbon metabolism in plants and even in the balance between carbon and nitrogen assimilation. Further experimental validation will be necessary to properly characterize the importance of thiamine in balancing plant energy and anabolism and any possible involvement in crop yield. Additionally, the intrinsic role of TDP across several central plant metabolic processes and in different cellular organelles presents it as a molecule that, if modified, could be used to create coordinated and wide-reaching responses throughout plant metabolism. Modulation of thiamine abundance through biotechnological methods may be used to increase crop yields or increase plant tolerance to changing environmental conditions or biotic stresses. Similarly, these novel technologies and improved understanding in both thiamine and plant metabolism have created opportunities to address thiamine dietary deficiencies through biofortification. Indeed, engineering thiamine enhancement may not only assist in improving nutritional quality but also provide insight into the role of thiamine in central metabolic processes. Thiamine in plants is therefore highly important to both public health and food security. Finally, although we have focused on thiamine here, we would also like to mention again that the other B vitamins are also vital and likely to have similar important contributions to plant central metabolism, while also being targets for nutritional enhancement. Moreover, there is even likely to be cross-talk between the B vitamins, as key pathways and even some individual enzymes use several B vitamins as coenzymes. The ongoing investigation of all vitamers within a B vitamin family and engineering for nutritional needs will help to decipher these communication pathways. It can be envisioned that perception and even signaling of vitamin B status is transmitted to trigger a specific outcome and provides a fertile area of research for the future with the potential for great impact in terms of fundamental understanding and access to sustainable nutritious food.
Funding and additional information—Work on thiamine in the Fitzpatrick laboratory has been supported by Swiss National Science Foundation Grants 31003A-140911, 31003A-141117/1, and 31003A_162555/1; the University of Geneva; and the VELUX Foundation.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- α-KG
- α-ketoglutarate (α-KG)
- α-KGDH
- α-ketoglutarate dehydrogenase (α-KGDH)
- ATDP
- adenosine thiamine diphosphate
- ATTP
- adenosine thiamine triphosphate
- GDH
- glutamate dehydrogenase
- HET-P
- hydroxyethylthiazole phosphate
- HMP-P
- hydroxymethylpyrimidine phosphate
- HMP-PP
- hydroxymethylpyrimidine pyrophosphate
- QTL
- quantitative trait locus
- PGPR
- plant growth-promoting rhizobacteria
- TCA
- tricarboxylic acid
- TMP
- thiamine monophosphate
- TDP
- thiamine diphosphate
- TK
- transketolase
- TPC
- thiamine pyrophosphate carrier
- TTP
- thiamine triphosphate.
References
- 1. Bender D. A. (2003) Nutritional Biochemistry of the Vitamins, Cambridge University Press, Cambridge, UK [Google Scholar]
- 2. Ajjawi I., Tsegaye Y., and Shintani D. (2007) Determination of the genetic, molecular, and biochemical basis of the Arabidopsis thaliana thiamin auxotroph th1. Arch. Biochem. Biophys. 459, 107–114 10.1016/j.abb.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick T. B., Basset G. J., Borel P., Carrari F., DellaPenna D., Fraser P. D., Hellmann H., Osorio S., Rothan C., Valpuesta V., Caris-Veyrat C., and Fernie A. R. (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell 24, 395–414 10.1105/tpc.111.093120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishikawa T., Machida C., Yoshioka Y., Kitano H., and Machida Y. (2003) The GLOBULAR ARREST1 gene, which is involved in the biosynthesis of folates, is essential for embryogenesis in Arabidopsis thaliana. Plant J. 33, 235–244 10.1046/j.1365-313x.2003.01621.x [DOI] [PubMed] [Google Scholar]
- 5. Raschke M., Bürkle L., Müller N., Nunes-Nesi A., Fernie A. R., Arigoni D., Amrhein N., and Fitzpatrick T. B. (2007) Vitamin B1 biosynthesis in plants requires the essential iron sulfur cluster protein THIC. Proc. Natl. Acad. Sci. U. S. A. 104, 19637–19642 10.1073/pnas.0709597104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneider T., Dinkins R., Robinson K., Shellhammer J., and Meinke D. W. (1989) An embryo-lethal mutant of Arabidopsis thaliana is a biotin auxotroph. Dev. Biol. 131, 161–167 10.1016/S0012-1606(89)80047-8 [DOI] [PubMed] [Google Scholar]
- 7. Tambasco-Studart M., Titiz O., Raschle T., Forster G., Amrhein N., and Fitzpatrick T. B. (2005) Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. U. S. A. 102, 13687–13692 10.1073/pnas.0506228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boubakri H., Gargouri M., Mliki A., Brini F., Chong J., and Jbara M. (2016) Vitamins for enhancing plant resistance. Planta 244, 529–543 10.1007/s00425-016-2552-0 [DOI] [PubMed] [Google Scholar]
- 9. Smith A. G., Croft M. T., Moulin M., and Webb M. E. (2007) Plants need their vitamins too. Curr. Opin. Plant Biol. 10, 266–275 10.1016/j.pbi.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 10. Gerdes S., Lerma-Ortiz C., Frelin O., Seaver S. M., Henry C. S., de Crécy-Lagard V., and Hanson A. D. (2012) Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J. Exp. Bot. 63, 5379–5395 10.1093/jxb/ers208 [DOI] [PubMed] [Google Scholar]
- 11. Shan Q., Wang Y., Li J., Zhang Y., Chen K., Liang Z., Zhang K., Liu J., Xi J. J., Qiu J.-L., and Gao C. (2013) Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688 10.1038/nbt.2650 [DOI] [PubMed] [Google Scholar]
- 12. Jiang L., Wang W., Lian T., and Zhang C. (2017) Manipulation of metabolic pathways to develop vitamin-enriched crops for human health. Front. Plant Sci. 8, 937 10.3389/fpls.2017.00937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y., Malzahn A. A., Sretenovic S., and Qi Y. (2019) The emerging and uncultivated potential of CRISPR technology in plant science. Nat. Plants 5, 778–794 10.1038/s41477-019-0461-5 [DOI] [PubMed] [Google Scholar]
- 14. de Oliveira Dal'Molin C. G., Quek L.-E., Palfreyman R. W., Brumbley S. M., and Nielsen L. K. (2010) AraGEM, a genome-scale reconstruction of the primary metabolic network in Arabidopsis. Plant Physiol. 152, 579–589 10.1104/pp.109.148817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saha R., Suthers P. F., and Maranas C. D. (2011) Zea mays iRS1563: a comprehensive genome-scale metabolic reconstruction of maize metabolism. PLoS ONE 6, e21784 10.1371/journal.pone.0021784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poolman M. G., Kundu S., Shaw R., and Fell D. A. (2013) Responses to light intensity in a genome-scale model of rice metabolism. Plant Physiol. 162, 1060–1072 10.1104/pp.113.216762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Funk C. (1912) The etiology of the deficiency diseases. J. State Med. 20, 341 [Google Scholar]
- 18. Itokawa Y. (1976) Kanehiro Takaki (1849–1920). J. Nutr. 106, 581–588 10.1093/jn/106.5.581 [DOI] [PubMed] [Google Scholar]
- 19. Braun R. (2011) Accessory food factors: understanding the catalytic function. J. Hist. Biol. 44, 483–504 10.1007/s10739-010-9255-3 [DOI] [PubMed] [Google Scholar]
- 20. Williams R. R., and Cline J. K. (1936) Synthesis of vitamin B1. J. Am. Chem. Soc. 58, 1504–1505 10.1021/ja01299a505 [DOI] [Google Scholar]
- 21. Bettendorff L., Lakaye B., Kohn G., and Wins P. (2014) Thiamine triphosphate: a ubiquitous molecule in search of a physiological role. Metab. Brain Dis. 29, 1069–1082 10.1007/s11011-014-9509-4 [DOI] [PubMed] [Google Scholar]
- 22. Bettendorff L., Wirtzfeld B., Makarchikov A. F., Mazzucchelli G., Frédérich M., Gigliobianco T., Gangolf M., De Pauw E., Angenot L., and Wins P. (2007) Discovery of a natural thiamine adenine nucleotide. Nat. Chem. Biol. 3, 211–212 10.1038/nchembio867 [DOI] [PubMed] [Google Scholar]
- 23. Makarchikov A. F., Lakaye B., Gulyai I. E., Czerniecki J., Coumans B., Wins P., Grisar T., and Bettendorff L. (2003) Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals. Cell. Mol. Life Sci. 60, 1477–1488 10.1007/s00018-003-3098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begley T. P., Ealick S. E., and McLafferty F. W. (2012) Thiamin biosynthesis: still yielding fascinating biological chemistry. Biochem. Soc. Trans. 40, 555–560 10.1042/BST20120084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzpatrick T. B., and Thore S. (2014) Complex behavior: from cannibalism to suicide in the vitamin B1 biosynthesis world. Curr. Opin. Struct. Biol. 29, 34–43 10.1016/j.sbi.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 26. Kowalska E., and Kozik A. (2008) The genes and enzymes involved in the biosynthesis of thiamin and thiamin diphosphate in yeasts. Cell. Mol. Biol. Lett. 13, 271–282 10.2478/s11658-007-0055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Machado C. R., de Oliveira R. L., Boiteux S., Praekelt U. M., Meacock P. A., and Menck C. F. (1996) Thi1, a thiamine biosynthetic gene in Arabidopsis thaliana, complements bacterial defects in DNA repair. Plant Mol. Biol. 31, 585–593 10.1007/BF00042231 [DOI] [PubMed] [Google Scholar]
- 28. Goyer A. (2017) Thiamin biofortification of crops. Curr. Opin. Biotechnol. 44, 1–7 10.1016/j.copbio.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 29. Chatterjee A., Abeydeera N. D., Bale S., Pai P. J., Dorrestein P. C., Russell D. H., Ealick S. E., and Begley T. P. (2011) Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature 478, 542–546 10.1038/nature10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coquille S., Roux C., Mehta A., Begley T. P., Fitzpatrick T. B., and Thore S. (2013) High-resolution crystal structure of the eukaryotic HMP-P synthase (THIC) from Arabidopsis thaliana. J. Struct. Biol. 184, 438–444 10.1016/j.jsb.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rapala-Kozik M., Olczak M., Ostrowska K., Starosta A., and Kozik A. (2007) Molecular characterization of the thi3 gene involved in thiamine biosynthesis in Zea mays: cDNA sequence and enzymatic and structural properties of the recombinant bifunctional protein with 4-amino-5-hydroxymethyl-2-methylpyrimidine (phosphate) kinase and thiamine monophosphate synthase activities. Biochem. J. 408, 149–159 10.1042/BJ20070677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Komeda Y., Tanaka M., and Nishimune T. (1988) A th-1 mutant of Arabidopsis thaliana is defective for a thiamin-phosphate-synthesizing enzyme: thiamin phosphate pyrophosphorylase. Plant Physiol. 88, 248–250 10.1104/pp.88.2.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goyer A. (2010) Thiamine in plants: aspects of its metabolism and functions. Phytochemistry 71, 1615–1624 10.1016/j.phytochem.2010.06.022 [DOI] [PubMed] [Google Scholar]
- 34. Rapala-Kozik M., Gołda A., and Kujda M. (2009) Enzymes that control the thiamine diphosphate pool in plant tissues. Properties of thiamine pyrophosphokinase and thiamine-(di)phosphate phosphatase purified from Zea mays seedlings. Plant Physiol. Biochem. 47, 237–242 10.1016/j.plaphy.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 35. Li S. L., and Rédei G. P. (1969) Thiamine mutants of the crucifer, Arabidopsis. Biochem. Genet. 3, 163–170 10.1007/BF00520351 [DOI] [PubMed] [Google Scholar]
- 36. Mimura M., Zallot R., Niehaus T. D., Hasnain G., Gidda S. K., Nguyen T. N., Anderson E. M., Mullen R. T., Brown G., Yakunin A. F., de Crécy-Lagard V., Gregory J. F., McCarty D. R., and Hanson A. D. (2016) Arabidopsis TH2 encodes the orphan enzyme thiamin monophosphate phosphatase. Plant Cell 28, 2683–2696 10.1105/tpc.16.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsieh W. Y., Liao J. C., Wang H. T., Hung T. H., Tseng C. C., Chung T. Y., and Hsieh M. H. (2017) The Arabidopsis thiamin-deficient mutant pale green1 lacks thiamin monophosphate phosphatase of the vitamin B1 biosynthesis pathway. Plant J. 91, 145–157 10.1111/tpj.13552 [DOI] [PubMed] [Google Scholar]
- 38. Ajjawi I., Rodriguez Milla M. A., Cushman J., and Shintani D. K. (2007) Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Mol. Biol. 65, 151–162 10.1007/s11103-007-9205-4 [DOI] [PubMed] [Google Scholar]
- 39. Frelin O., Agrimi G., Laera V. L., Castegna A., Richardson L. G., Mullen R. T., Lerma-Ortiz C., Palmieri F., and Hanson A. D. (2012) Identification of mitochondrial thiamin diphosphate carriers from Arabidopsis and maize. Funct. Integr. Genomics 12, 317–326 10.1007/s10142-012-0273-4 [DOI] [PubMed] [Google Scholar]
- 40. Noordally Z., Trichtinger C., Dalvit I., Hofmann M., Zamboni N., Pourcel L., Gas E., Gisler A., and Fitzpatrick T. B. (2020) The coenzyme thiamine diphosphate displays a nuclear rhythm independent of the circadian clock. Commun. Biol. 3, 209 10.1038/s42003-020-0927-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Witz S., Panwar P., Schober M., Deppe J., Pasha F. A., Lemieux M. J., and Möhlmann T. (2014) Structure-function relationship of a plant NCS1 member—homology modeling and mutagenesis identified residues critical for substrate specificity of PLUTO, a nucleobase transporter from Arabidopsis. PLoS ONE 9, e91343 10.1371/journal.pone.0091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mourad G. S., Tippmann-Crosby J., Hunt K. A., Gicheru Y., Bade K., Mansfield T. A., and Schultes N. P. (2012) Genetic and molecular characterization reveals a unique nucleobase cation symporter 1 in Arabidopsis. FEBS Lett. 586, 1370–1378 10.1016/j.febslet.2012.03.058 [DOI] [PubMed] [Google Scholar]
- 43. Beaudoin G. A. W., Johnson T. S., and Hanson A. D. (2018) The PLUTO plastidial nucleobase transporter also transports the thiamin precursor hydroxymethylpyrimidine. Biosci. Rep. 38, BSR20180048 10.1042/bsr20180048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guan J.-C., Hasnain G., Garrett T. J., Chase C. D., Gregory J., Hanson A. D., and McCarty D. R. (2014) Divisions of labor in the thiamin biosynthetic pathway among organs of maize. Front. Plant Sci. 5, 370 10.3389/fpls.2014.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hanson A. D., Amthor J. S., Sun J., Niehaus T. D., Gregory J. F., Bruner S. D., and Ding Y. (2018) Redesigning thiamin synthesis: prospects and potential payoffs. Plant Sci. 273, 92–99 10.1016/j.plantsci.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 46. Bocobza S., Adato A., Mandel T., Shapira M., Nudler E., and Aharoni A. (2007) Riboswitch-dependent gene regulation and its evolution in the plant kingdom. Genes Dev. 21, 2874–2879 10.1101/gad.443907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wachter A., Tunc-Ozdemir M., Grove B. C., Green P. J., Shintani D. K., and Breaker R. R. (2007) Riboswitch control of gene expression in plants by splicing and alternative 3' end processing of mRNAs. Plant Cell 19, 3437–3450 10.1105/tpc.107.053645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bocobza S. E., Malitsky S., Araújo W. L., Nunes-Nesi A., Meir S., Shapira M., Fernie A. R., and Aharoni A. (2013) Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell 25, 288–307 10.1105/tpc.112.106385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zallot R., Yazdani M., Goyer A., Ziemak M. J., Guan J. C., McCarty D. R., de Crécy-Lagard V., Gerdes S., Garrett T. J., Benach J., Hunt J. F., Shintani D. K., and Hanson A. D. (2014) Salvage of the thiamin pyrimidine moiety by plant TenA proteins lacking an active-site cysteine. Biochem. J. 463, 145–155 10.1042/BJ20140522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yazdani M., Zallot R., Tunc-Ozdemir M., de Crécy-Lagard V., Shintani D. K., and Hanson A. D. (2013) Identification of the thiamin salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry 94, 68–73 10.1016/j.phytochem.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 51. Pribat A., Blaby I. K., Lara-Núñez A., Jeanguenin L., Fouquet R., Frelin O., Gregory J. F. R., Philmus B., Begley T. P., de Crécy-Lagard V., and Hanson A. D. (2011) A 5-formyltetrahydrofolate cycloligase paralog from all domains of life: comparative genomic and experimental evidence for a cryptic role in thiamin metabolism. Funct. Integr. Genomics 11, 467–478 10.1007/s10142-011-0224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martinis J., Gas-Pascual E., Szydlowski N., Crèvecoeur M., Gisler A., Bürkle L., and Fitzpatrick T. B. (2016) Long-distance transport of thiamine (vitamin B1) is concomitant with that of polyamines. Plant Physiol. 171, 542–553 10.1104/pp.16.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Golda A., Szyniarowski P., Ostrowska K., Kozik A., and Rapala-Kozik M. (2004) Thiamine binding and metabolism in germinating seeds of selected cereals and legumes. Plant Physiol. Biochem. 42, 187–195 10.1016/j.plaphy.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 54. Aleshin V. A., Mezhenska O. A., Parkhomenko Y. M., Kaehne T., and Bunik V. I. (2020) Thiamine mono- and diphosphate phosphatases in bovine brain synaptosomes. Biochemistry (Mosc.) 85, 378–386 10.1134/S000629792003013X [DOI] [PubMed] [Google Scholar]
- 55. Mkrtchyan G., Aleshin V., Parkhomenko Y., Kaehne T., Di Salvo M. L., Parroni A., Contestabile R., Vovk A., Bettendorff L., and Bunik V. (2015) Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis. Sci. Rep. 5, 12583 10.1038/srep12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gigliobianco T., Gangolf M., Lakaye B., Pirson B., von Ballmoos C., Wins P., and Bettendorff L. (2013) An alternative role of FoF1-ATP synthase in Escherichia coli: synthesis of thiamine triphosphate. Sci. Rep. 3, 1071 10.1038/srep01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frédérich M., Delvaux D., Gigliobianco T., Gangolf M., Dive G., Mazzucchelli G., Elias B., De Pauw E., Angenot L., Wins P., and Bettendorff L. (2009) Thiaminylated adenine nucleotides. Chemical synthesis, structural characterization and natural occurrence. FEBS J. 276, 3256–3268 10.1111/j.1742-4658.2009.07040.x [DOI] [PubMed] [Google Scholar]
- 58. Araújo W. L., Nunes-Nesi A., Nikoloski Z., Sweetlove L. J., and Fernie A. R. (2012) Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 35, 1–21 10.1111/j.1365-3040.2011.02332.x [DOI] [PubMed] [Google Scholar]
- 59. Joshi J., Folz J. S., Gregory J. F. R., McCarty D. R., Fiehn O., and Hanson A. D. (2019) Rethinking the PDH bypass and GABA shunt as thiamin-deficiency workarounds. Plant Physiol. 181, 389–393 10.1104/pp.19.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim J. A., Kim H.-S., Choi S.-H., Jang J.-Y., Jeong M.-J., and Lee S. I. (2017) The importance of the circadian clock in regulating plant metabolism. Int. J. Mol. Sci. 18, 2680 10.3390/ijms18122680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rosado-Souza L., Proost S., Moulin M., Bergmann S., Bocobza S. E., Aharoni A., Fitzpatrick T. B., Mutwil M., Fernie A. R., and Obata T. (2019) Appropriate thiamin pyrophosphate levels are required for acclimation to changes in photoperiod. Plant Physiol. 180, 185–197 10.1104/pp.18.01346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khozaei M., Fisk S., Lawson T., Gibon Y., Sulpice R., Stitt M., Lefebvre S. C., and Raines C. A. (2015) Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 27, 432–447 10.1105/tpc.114.131011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tilman D., Balzer C., Hill J., and Befort B. L. (2011) Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. U. S. A. 108, 20260–20264 10.1073/pnas.1116437108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith M. R., Rao I. M., and Merchant A. (2018) Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 9, 1889 10.3389/fpls.2018.01889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simkin A. J., López-Calcagno P. E., and Raines C. A. (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70, 1119–1140 10.1093/jxb/ery445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amthor J. S., Bar-Even A., Hanson A. D., Millar A. H., Stitt M., Sweetlove L. J., and Tyerman S. D. (2019) Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 31, 297–314 10.1105/tpc.18.00743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun J., Sigler C. L., Beaudoin G. A. W., Joshi J., Patterson J. A., Cho K. H., Ralat M. A., Gregory J. F., Clark D. G., Deng Z., Colquhoun T. A., and Hanson A. D. (2019) Parts-prospecting for a high-efficiency thiamin thiazole biosynthesis pathway. Plant Physiol. 179, 958–968 10.1104/pp.18.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huergo L. F., and Dixon R. (2015) The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. 79, 419–435 10.1128/MMBR.00038-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Araújo W. L., Martins A. O., Fernie A. R., and Tohge T. (2014) 2-oxoglutarate: linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front. Plant Sci. 5, 552 10.3389/fpls.2014.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lakaye B., Wirtzfeld B., Wins P., Grisar T., and Bettendorff L. (2004) Thiamine triphosphate, a new signal required for optimal growth of Escherichia coli during amino acid starvation. J. Biol. Chem. 279, 17142–17147 10.1074/jbc.M313569200 [DOI] [PubMed] [Google Scholar]
- 71. Gigliobianco T., Lakaye B., Wins P., El Moualij B., Zorzi W., and Bettendorff L. (2010) Adenosine thiamine triphosphate accumulates in Escherichia coli cells in response to specific conditions of metabolic stress. BMC Microbiol. 10, 148 10.1186/1471-2180-10-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tunc-Ozdemir M., Miller G., Song L., Kim J., Sodek A., Koussevitzky S., Misra A. N., Mittler R., and Shintani D. (2009) Thiamin confers enhanced tolerance to oxidative stress in Arabidopsis. Plant Physiol. 151, 421–432 10.1104/pp.109.140046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rapala-Kozik M., Wolak N., Kujda M., and Banas A. K. (2012) The upregulation of thiamine (vitamin B-1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 12, 2 10.1186/1471-2229-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rosado-Souza L., Fernie A. R., and Aarabi F. (2020) Ascorbate and thiamin: metabolic modulators in plant acclimation responses. Plants 9, 101 10.3390/plants9010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahn I. P., Kim S., Lee Y. H., and Suh S. C. (2007) Vitamin B1-induced priming is dependent on hydrogen peroxide and the NPR1 gene in Arabidopsis. Plant Physiol. 143, 838–848 10.1104/pp.106.092627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Westman S. M., Kloth K. J., Hanson J., Ohlsson A. B., and Albrectsen B. R. (2019) Defence priming in Arabidopsis—a meta-analysis. Sci. Rep. 9, 13309 10.1038/s41598-019-49811-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hamada A. M., and Jonsson L. M. V. (2013) Thiamine treatments alleviate aphid infestations in barley and pea. Phytochemistry 94, 135–141 10.1016/j.phytochem.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 78. Hamada A. M., Fatehi J., and Jonsson L. M. V. (2018) Seed treatments with thiamine reduce the performance of generalist and specialist aphids on crop plants. Bull. Entomol. Res. 108, 84–92 10.1017/S0007485317000529 [DOI] [PubMed] [Google Scholar]
- 79. Pushpalatha H. G., Sudisha J., Geetha N. P., Amruthesh K. N., and Shetty H. (2011) Thiamine seed treatment enhances LOX expression, promotes growth and induces downy mildew disease resistance in pearl millet. Biol. Plant 55, 522–527 10.1007/s10535-011-0118-3 [DOI] [Google Scholar]
- 80. Bahuguna R. N., Joshi R., Shukla A., Pandey M., and Kumar J. (2012) Thiamine primed defense provides reliable alternative to systemic fungicide carbendazim against sheath blight disease in rice (Oryza sativa L.). Plant Physiol. Biochem. 57, 159–167 10.1016/j.plaphy.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 81. Nagae M., Parniske M., Kawaguchi M., and Takeda N. (2016) The thiamine biosynthesis gene THI1 promotes nodule growth and seed maturation. Plant Physiol. 172, 2033–2043 10.1104/pp.16.01254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nagae M., Parniske M., Kawaguchi M., and Takeda N. (2016) The relationship between thiamine and two symbioses: root nodule symbiosis and arbuscular mycorrhiza. Plant Signal. Behav. 11, e1265723 10.1080/15592324.2016.1265723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. World Health Organization (1999) Thiamine Deficiency and Its Prevention and Control in Major Emergencies, World Health Organization, Geneva [Google Scholar]
- 84. Whitfield K. C., Bourassa M. W., Adamolekun B., Bergeron G., Bettendorff L., Brown K. H., Cox L., Fattal-Valevski A., Fischer P. R., Frank E. L., Hiffler L., Hlaing L. M., Jefferds M. E., Kapner H., Kounnavong S., et al. (2018) Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 1430, 3–43 10.1111/nyas.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilkinson T. J., Hanger H. C., Elmslie J., George P. M., and Sainsbury R. (1997) The response to treatment of subclinical thiamine deficiency in the elderly. Am. J. Clin. Nutr. 66, 925–928 10.1093/ajcn/66.4.925 [DOI] [PubMed] [Google Scholar]
- 86. Lee D. C., Chu J., Satz W., and Silbergleit R. (2000) Low plasma thiamine levels in elder patients admitted through the emergency department. Acad. Emerg. Med. 7, 1156–1159 10.1111/j.1553-2712.2000.tb01268.x [DOI] [PubMed] [Google Scholar]
- 87. Jamieson C. P., Obeid O. A., and Powell-Tuck J. (1999) The thiamin, riboflavin and pyridoxine status of patients on emergency admission to hospital. Clin. Nutr. 18, 87–91 10.1016/S0261-5614(99)80057-0 [DOI] [PubMed] [Google Scholar]
- 88. Hoyumpa A. M. (1980) Mechanisms of thiamin deficiency in chronic alcoholism. Am. J. Clin. Nutr. 33, 2750–2761 10.1093/ajcn/33.12.2750 [DOI] [PubMed] [Google Scholar]
- 89. Gödecke T., Stein A. J., and Qaim M. (2018) The global burden of chronic and hidden hunger: trends and determinants. Glob. Food Sec. 17, 21–29 10.1016/j.gfs.2018.03.004 [DOI] [Google Scholar]
- 90. Strobbe S., and Van Der Straeten D. (2018) Toward eradication of B-vitamin deficiencies: considerations for crop biofortification. Front. Plant Sci. 9, 443 10.3389/fpls.2018.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goyer A., and Sweek K. (2011) Genetic diversity of thiamin and folate in primitive cultivated and wild potato species. J. Agric. Food Chem. 59, 13072–13080 10.1021/jf203736e [DOI] [PubMed] [Google Scholar]
- 92. Pourcel L., Moulin M., and Fitzpatrick T. B. (2013) Examining strategies to facilitate vitamin B1 biofortification of plants by genetic engineering. Front. Plant Sci. 4, 160 10.3389/fpls.2013.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Batifoulier F., Verny M.-A., Chanliaud E., Rémésy C., and Demigné C. (2005) Effect of different breadmaking methods on thiamine, riboflavin and pyridoxine contents of wheat bread. J. Cereal Sci. 42, 101–108 10.1016/j.jcs.2005.03.003 [DOI] [Google Scholar]
- 94. Li J., Liu J., Wen W., Zhang P., Wan Y., Xia X., Zhang Y., and He Z. (2018) Genome-wide association mapping of vitamins B1 and B2 in common wheat. Crop J. 6, 263–270 10.1016/j.cj.2017.08.002 [DOI] [Google Scholar]
- 95. Mangel N., Fudge J. B., Fitzpatrick T. B., Gruissem W., and Vanderschuren H. (2017) Vitamin B1 diversity and characterization of biosynthesis genes in cassava. J. Exp. Bot. 68, 3351–3363 10.1093/jxb/erx196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kong D., Zhu Y., Wu H., Cheng X., Liang H., and Ling H. Q. (2008) AtTHIC, a gene involved in thiamine biosynthesis in Arabidopsis thaliana. Cell Res. 18, 566–576 10.1038/cr.2008.35 [DOI] [PubMed] [Google Scholar]
- 97. Dong W., Stockwell V. O., and Goyer A. (2015) Enhancement of thiamin content in Arabidopsis thaliana by metabolic engineering. Plant Cell Physiol. 56, 2285–2296 10.1093/pcp/pcv148 [DOI] [PubMed] [Google Scholar]
- 98. Dong W., Thomas N., Ronald P. C., and Goyer A. (2016) Overexpression of thiamin biosynthesis genes in rice increases leaf and unpolished grain thiamin content but not resistance to Xanthomonas oryzae pv. oryzae. Front. Plant Sci. 7, 616 10.3389/fpls.2016.00616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Minhas A. P., Tuli R., and Puri S. (2018) Pathway editing targets for thiamine biofortification in rice grains. Front. Plant Sci. 9, 975 10.3389/fpls.2018.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goyer A., and Haynes K. G. (2011) Vitamin B1 content in potato: effect of genotype, tuber enlargement, and storage, and estimation of stability and broad-sense heritability. Am. J. Potato Res. 88, 374–385 10.1007/s12230-011-9203-6 [DOI] [Google Scholar]
- 101. Kennedy G., and Burlingame B. (2003) Analysis of food composition data on rice from a plant genetic resources perspective. Food Chem. 80, 589–596 10.1016/S0308-8146(02)00507-1 [DOI] [Google Scholar]
- 102. Villareal C. P., and Juliano B. O. (1989) Variability in contents of thiamine and riboflavin in brown rice, crude oil in brown rice and bran-polish, and silicon in hull of IR rices. Plant Foods Hum. Nutr. 39, 287–297 10.1007/BF01091939 [DOI] [PubMed] [Google Scholar]
- 103. Sweetlove L. J., and Ratcliffe R. G. (2011) Flux-balance modeling of plant metabolism. Front. Plant Sci. 2, 38 10.3389/fpls.2011.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gomes de Oliveira Dal'Molin C., and Nielsen L. K. (2018) Plant genome-scale reconstruction: from single cell to multi-tissue modelling and omics analyses. Curr. Opin. Biotechnol. 49, 42–48 10.1016/j.copbio.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 105. Lentz E. M., Kuon J.-E., Alder A., Mangel N., Zainuddin I. M., McCallum E. J., Anjanappa R. B., Gruissem W., and Vanderschuren H. (2018) Cassava geminivirus agroclones for virus-induced gene silencing in cassava leaves and roots. Plant Methods 14, 73 10.1186/s13007-018-0340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mangel N. (2016) Natural variation, molecular determinants and genetic engineering of vitamin B1 and vitamin B6 biosynthesis in cassava and rice, Ph.D. thesis, Thesis 23811, ETH Zurich [Google Scholar]
- 107. Nester E. W. (2015) Agrobacterium: nature's genetic engineer. Front. Plant Sci. 5, 730 10.3389/fpls.2014.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Doudna J. A., and Charpentier E. (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- 109. Dong O. X., Yu S., Jain R., Zhang N., Duong P. Q., Butler C., Li Y., Lipzen A., Martin J. A., Barry K. W., Schmutz J., Tian L., and Ronald P. C. (2020) Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR-Cas9. Nat. Commun. 11, 1178 10.1038/s41467-020-14981-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Palacios O. A., Bashan Y., and de-Bashan L. E. (2014) Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biol. Fertil. Soils 50, 415–432 10.1007/s00374-013-0894-3 [DOI] [Google Scholar]
- 111. Derylo M., and Skorupska A. (1993) Enhancement of symbiotic nitrogen fixation by vitamin-secreting fluorescent Pseudomonas. Plant Soil 154, 211–217 10.1007/BF00012526 [DOI] [Google Scholar]
- 112. Badri D. V., and Vivanco J. M. (2009) Regulation and function of root exudates. Plant Cell Environ. 32, 666–681 10.1111/j.1365-3040.2009.01926.x [DOI] [PubMed] [Google Scholar]
- 113. Kamarudin A. N., Lai K. S., Lamasudin D. U., Idris A. S., and Balia Yusof Z. N. (2017) Enhancement of thiamine biosynthesis in oil palm seedlings by colonization of endophytic fungus Hendersonia toruloidea. Front. Plant Sci. 8, 1799 10.3389/fpls.2017.01799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ku Y.-S., Rehman H. M., and Lam H.-M. (2019) Possible roles of rhizospheric and endophytic microbes to provide a safe and affordable means of crop biofortification. Agronomy 9, 764 10.3390/agronomy9110764 [DOI] [Google Scholar]
- 115. Hofmann M., Loubéry S., and Fitzpatrick T. B. (2020) On the nature of triphosphorylated thiamine (vitamin B1) derivatives in Arabidopsis. Plant Direct, in press [DOI] [PMC free article] [PubMed] [Google Scholar]