Abstract
Background
Most trials have assessed intracranial atherosclerotic disease (ICAD) severity based on angiographic stenosis. However, anatomic stenosis might not accurately identify the actual state of functional post-stenotic flow limitation.
Objective
To investigate whether angiographic stenosis correlates with physiologic distal flow limitation, measured as trans-stenotic pressure gradients, in ICAD patients.
Methods
In patients referred for endovascular treatment of anterior circulation symptomatic ICAD who failed maximal medical therapy (MMT) per SAMMPRIS (Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis) criteria, angiographic luminal diameters and percentages of stenosis were correlated with trans-stenotic pressure gradients, calculated as distal/proximal pressure ratios (DPPR) and proximal minus distal pressure gradients (PDPG), by way of Spearman correlation coefficients.
Results
Nine patients (3 men, 6 women) were evaluated. Atherosclerotic lesions’ locations included internal carotid artery in 5 subjects (2 cavernous, 3 supraclinoid) and proximal middle cerebral artery (M1) in 4 patients. Mean percentage of stenosis was 80 ± 8% (range 75%-94%). Minimal lumen diameter at the most stenotic ICAD site ranged from 0.2 to 0.9 mm (0.59 ± 0.41 mm). DPPR ranged from 0.38 to 0.63 (0.56 ± 0.14). PDPG ranged from 35 to 57 mm Hg (50 ± 8 mm Hg). Spearman coefficients showed no correlation between DPPR or PDPG and angiographic minimal luminal diameters or percentages of stenosis. There were no procedural complications related to trans-stenotic pressure measurements.
CONCLUSION
Angiographic stenosis does not reflect the physiologic severity of distal flow limitation in patients with ICAD. Hemodynamic assessment using trans-stenotic pressure ratios and gradients may serve as a more reliable predictive biomarker for MMT failure and response to revascularization.
Keywords: Intracranial atherosclerotic disease, Flow limitation, Arterial stenosis, Trans-stenotic pressure, Percutaneous transluminal angioplasty, Stenting, Medical therapy
ABBREVIATIONS
- ASTIN/SIR
American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
- CT
computed tomography
- DPRR
distal/proximal pressure ratio
- DSA
digital subtraction angiography
- FFR
fractional flow reserve
- ICA
internal carotid artery
- ICAD
intracranial atherosclerotic disease
- MCA
middle cerebral artery
- MMT
maximal medical therapy
- MRI
magnetic resonance imaging
- mTICI
modified Thrombolysis in Cerebral Ischemia
- PDPG
proximal minus distal pressure gradient
- PTAS
percutaneous transluminal angioplasty and stenting
- SAMMPRIS
Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis
- SD
standard deviation
- TIA
transient ischemic attack
- VISSIT
Vitesse Intracranial Stent Study for Ischemic Stroke Therapy
- WEAVE
Wingspan Stent System Post Market Surveillance Study
Intracranial atherosclerotic disease (ICAD) predisposes individuals to recurrent strokes and transient ischemic attacks (TIAs). It is responsible for approximately 8% of ischemic strokes,1 but recent studies have shown it to be under-reported.2,3 A systematic analysis of 339 consecutive autopsies of patients with stroke showed that intracranial plaques and stenosis occur in 62.2% and 43.2% of patients with brain infarction.2 Suri et al3 reported a U.S. prevalence of ICAD (≥50%) for 65 to 90 yr old to be 8% for whites and 12% for African-Americans. It is recognized as one of the leading causes of stroke in Asian populations.4 The 1-yr recurrence rate of ischemic stroke is 14% and 15% in ICAD patients with >50% stenosis on monotherapy with aspirin and warfarin, respectively, and around 12% in ICAD patients with >70% stenosis on maximal medical therapy (MMT).5 Two randomized trials, the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS)5 and the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT),6 compared stroke recurrence in patients under MMT alone vs MMT plus percutaneous transluminal angioplasty and stenting (PTAS). In both trials, intracranial stenting was associated with a higher risk of stroke, particularly within 30 d of the intervention. This lack of benefit of PTAS compared to MMT alone is attributable to the higher than expected rate of periprocedural complications with PTAS. Since then, neurointerventionalists have been searching for ways to improve patient selection criteria and enhance the technical feasibility of endovascular procedures in order to maximize benefits and reduce complications, such as performing a submaximal angioplasty7 and/or using new stent technologies.
Recently, research efforts have focused on intracranial “atherosclerosis” evaluation instead of the traditional “stenosis” angiographic assessment. Impaired distal blood flow, poor collaterals, and plaque morphology constitute promising markers for risk stratification of patients with symptomatic ICAD.8-13 Thus, proper patient selection could help the success of intervention.
Revascularization of stenoses inducing parenchymal ischemia may improve patients’ functional status and outcome. However, the benefit of endovascular recanalization is less clear for stenoses that do not induce ischemia. Therefore, it can be argued that grading of the stenosis by digital subtraction angiography (DSA) does not accurately identify the state of distal flow limitation, which inadvertently confers an increased risk of recurrent stroke. This is quite problematic since most trials have based their inclusion criteria on the degree of angiographic stenosis. Here, we postulate that functional severity of distal flow limitation (not angiographic severity/stenosis) predicts failure to MMT and, consequently, should guide the type of therapeutic intervention.
As an objective measurement of distal flow limitation, we quantified luminal pressures at proximal and distal ICAD stenotic sites. Herein, we aim to report our preliminary experience with trans-stenotic pressure measurements and the relationship of these measurements with ICAD severity as conventionally assessed by DSA.
METHODS
Patient Selection
After approval from our local Institutional Review Board, we conducted a retrospective analysis of all procedures in which intracranial trans-stenotic pressures were measured using a pressure-sensing microwire. Patients were consented for the off-label use of the device. The consent for the retrospective analysis was waived. The pressure measurements were performed as part of a multimodal clinical assessment of ICAD severity in patients referred for endovascular treatment of anterior circulation symptomatic ICAD who have failed MMT per the SAMMPRIS criteria (defined as recurrent strokes or TIAs despite antiplatelet therapy, intensive management of vascular risk factors, and lifestyle modification). Angioplasty and stenting were considered for patients who satisfied the criteria above and met in addition the following: (1) degree of intracranial atherosclerotic stenosis ≥70% measured by DSA; (2) lesion with proximal vessel diameter of 2.0 to 2.5 mm; (3) lesion length ≤15 mm; (4) distal hypoperfusion with a modified Thrombolysis in Cerebral Ischemia Scale (mTICI) score of 1-2a7; (5) poor collaterals with an American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASTIN/SIR) Collateral Flow score of <3; and (6) failed MMT, as defined above. The clinical symptoms should relate to the area affected by ICAD with a stenosis at least >50%. Exclusion criteria included a major stroke in the territory documented and complete occlusion.
Procedure
All procedures were performed under monitored anesthesia care.14 A 7F sheath was used to access the right femoral artery. Then, a 6F Envoy guide catheter (Codman Neuro, West Chester, Pennsylvania) was used to navigate the internal carotid artery (ICA) on the symptomatic side. Several views were obtained during the initial DSA to assess degree of stenosis, length of stenosis, collaterals, and tortuosity of the intracranial arteries. For all intracranial stenting, the senior author deployed a Rebel balloon-mounted cardiac stent (Boston Scientific, San Jose, California) navigated using a Synchro soft 0.014” microwire (Stryker, Fremont, California).
ICAD distal pressures were measured using 0.014” diameter pressure-sensing microwires approved for use in the coronary and peripheral arterial systems by the FDA (COMET wire, Boston Scientific, Fremont, California; or Verrata Plus wire, Philips Medical Systems, Eindhoven, The Netherlands). These are suitable for use as an interventional guidewire during coronary angioplasty and stent placement. ICAD proximal pressures were measured using a 6F multipurpose guide catheter positioned in the proximal ICA and connected to a fluid-filled transducer. The microwire was advanced to the tip of the guide catheter, and the 2 pressures were electronically equalized. The pressure-sensing microwire was positioned distal to the ICAD by either direct advancement across the stenosis or by exchange technique using a microcatheter. Both distal and proximal pressures were measured simultaneously, and the mean DPPR and mean proximal minus mean distal pressure gradient (PDPG) were calculated. As previously described, angiographic ICAD severity (degree of stenosis) was evaluated by measuring the minimal lumen diameter at the most stenotic site compared to the reference vessel diameter using a digital caliper on initial DSA (before pressure measurements). Stenosis measurements were performed independently by 2 neurointerventionalists, and the values were averaged.
Statistical Analysis
Statistical analyses and graphic display of data were performed using GraphPad software version 7.03 (GraphPad Software, San Diego, California). Correlation between 2 groups was assessed using the Spearman correlation coefficient (R). All values are reported as mean ± standard deviation (SD). In all cases, a P value of less than .05 was considered to indicate statistical significance.
RESULTS
Proximal and distal ICAD pressures were measured in 9 patients (3 men and 6 women) with a mean age of 56 yr (range 26-84 yr, Table). The clinical presentation was recurrent stroke in 4 patients and recurrent TIA in 3 patients. One patient presented with ongoing neurological deficit after placement of a flow diverter which improved with elevation of the mean arterial pressure; the patient was found to have significant stenosis at the edge of the flow diverter stent. Finally, one patient had an incidentally discovered intracranial stenosis in the setting of subarachnoid hemorrhage. The atherosclerotic lesion was located in the ICA in 5 subjects (2 cavernous, 3 supraclinoid) and the proximal middle cerebral artery (MCA, M1 segment) in 4 subjects. The mean quantified percentage of stenosis at the interrogated ICAD was 83.3 ± 5.1% (range 78%-94%). The minimal lumen diameter at the most stenotic ICAD site ranged from 0.2 to 0.9 mm (0.45 ± 0.2 mm, mean ± SD). There were no procedural complications related to trans-stenotic pressure measurement.
TABLE.
Characteristics of the Sample and Trans-stenotic Measurements
| N | Age (years) | Sex | Presentation | Location of stenosis | Visual stenosis (%) | Reference diameter (mm) | Minimal lumen diameter (mm) | QA stenosis (%) | Proximal pressure (mm Hg) | Distal pressure (mm Hg) | PDPG (mm Hg) | DPPR (mm Hg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | W | Stroke, moyamoya | R cavernous ICA | 99 | 2 | 0.25 | 87.5 | 128 | 71 | 57 | 0.55 |
| 2 | 84 | W | Stroke, recurrent | R cavernous ICA | 80 | 3.15 | 0.6 | 80.8 | 103 | 50 | 53 | 0.48 |
| 3 | 26 | W | SAH | L supraclinoid ICA | 75 | 2.65 | 0.5 | 81.2 | 120 | 67 | 53 | 0.56 |
| 4 | 63 | M | TIA | L MCA M1 | 99 | 3.45 | 0.2 | 94.2 | 114 | 58 | 56 | 0.51 |
| 5 | 61 | W | Stroke, multivessel ICAD | R supraclinoid ICA | 70 | 3.8 | 0.85 | 77.6 | 141 | 85 | 56 | 0.60 |
| 6 | 64 | M | Stroke, stenosis after MT | L MCA M1 | 70 | 2.5 | 0.5 | 79.8 | 103 | 65 | 38 | 0.63 |
| 7 | 54 | W | FDD edge stenosis | L MCA M1 | 90 | 2.45 | 0.45 | 81.4 | 94 | 36 | 58 | 0.38 |
| 8 | 27 | W | TIA | L supraclinoid ICA | 95 | 1.825 | 0.25 | 86.3 | 69 | 34 | 35 | 0.49 |
| 9a | 72 | M | TIA | L MCA M1 | 95 | 2.35 | 0.45 | 80.9 | 110 | 65 | 45 | 0.59 |
| Pooled | 56 (26-84) | 85.9 ± 12.2 | 2.7 ± 0.7 | 0.45 ± 0.2 | 83.3 ± 5.1 | 109.1 ± 20.7 | 59 ± 16.6 | 50.1 ± 8.6 | 0.53 ± 0.08 |
DPPR: distal/proximal pressure ratio; FDD: flow-diversion device; ICA: internal carotid artery; ICAD: intracranial atherosclerotic disease; L: left; M: man; MCA: middle cerebral artery; MT: mechanical thrombectomy; PDPG: proximal minus distal pressure gradient; QA: quantified; R: right; SAH: subarachnoid hemorrhage; TIA: transient ischemic attack; W: woman.
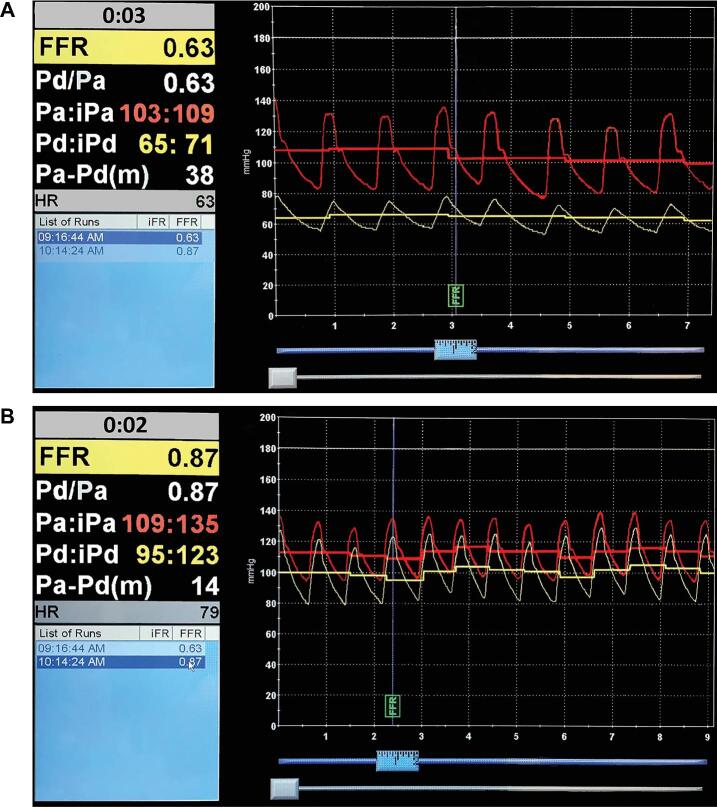
aCase 9 is depicted in Figure 1.
A representative recording of intracranial trans-stenotic pressure measurement is depicted in Figure 1. In this case, the mean pressure proximal to the MCA stenosis was 110 mm Hg, and the mean distal pressure was 65 mm Hg (Figure 1A). The DPPR was 0.59, and the PDPG was 45 mm Hg. After treatment of the ICAD with a balloon expandable stent, the pressure difference was abolished (Figure 1B). In our sample, trans-stenotic DPPR ranged from 0.38 to 0.63 (0.53 ± 0.08, mean ± SD). The PDPG ranged from 35 to 58 mm Hg (50.1 ± 8.6 mm Hg, mean ± SD). All patients underwent angioplasty and stenting except the patient with incidental ICAD in the setting of subarachnoid hemorrhage.
FIGURE 1.
A representative recording of intracranial trans-stenotic pressure measurements. In this case, the mean pressure proximal to the MCA stenosis was 110 mm Hg, and the mean distal pressure was 65 mm Hg A. The distal/proximal pressure ratio (DPPR) was 0.59, and the proximal-distal pressure gradient (PDPG) was 45 mm Hg. After treatment of the ICAD with a balloon expandable stent, the pressure difference was abolished B.
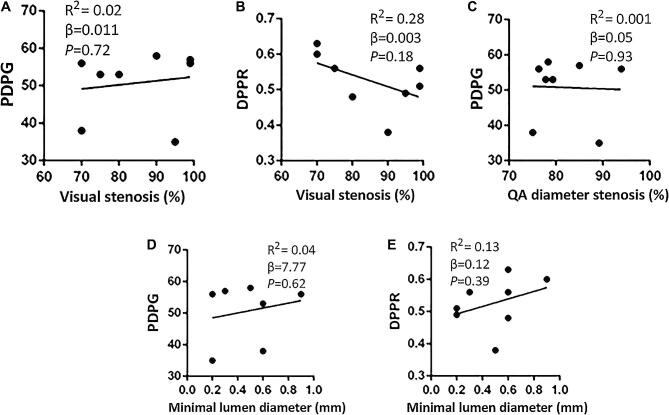
The degree of stenosis assessed by DSA was not correlated with physiologic measures of stenosis severity (Figure 2). Neither luminal diameter nor percentage of the stenosis (visual or quantified) was correlated with DPPR or PDPG. The anatomic severity of the stenosis measured by angiography was not indicative of the post-stenotic perfusion state. Thus, we believe that the traditional assumption “the narrower the lumen diameter, the worse the ischemia” may not always be accurate, and one must consider the functionality of the stenosis.
FIGURE 2.
Scatter plots showing nonsignificant correlation between visual percentage of stenosis and PDPG A, visual percentage of stenosis and DPPR B, quantified (QA) percentage of diameter stenosis and PDPG C, minimal lumen diameter and PDPG D, and minimal lumen diameter and DPPR E. The angiographic severity of stenosis was not indicative of functional impairment.
DISCUSSION
ICAD can cause stroke by 1 of 3 different mechanisms: artery-to-artery embolism, flow-dependent/hypoperfusion, and branch-atheromatous disease.12 The SAMMPRIS5 trial excluded patients with worsening deficits during the first 24 h preceding stent placement, thus excluding the subpopulation of patients who are perfusion-dependent. This subpopulation may be at the highest risk of stroke and could benefit from prompt restoration of flow by endovascular intervention. In fact, recent studies have reported an increased risk of recurrent strokes in ICAD patients with impaired distal flow8 and absent or poor collaterals,15 showing that rapid filling of collateral vessels can be relatively protective.15 While aggressive medical therapy can stabilize the plaque and reduce recurrent emboli, it is unlikely, but not impossible, to improve blood flow significantly and prevent neurological deterioration.12 Antiplatelet therapy can reduce the clot burden and improve viscosity, which could help with blood flow. However, this is unlikely to have the same effect as recanalizing a functional stenosis. This subset of population that are flow dependent, or have poor cerebral perfusion, may have failed MMT in previous trials, while the subsets with artery-to-artery emboli benefited from MMT.
Furthermore, one potential cause for the lack of benefit with stenting in previous clinical trials is the fact that stenosis grading on DSA may not adequately identify the presence of flow limitation in the cerebral circulation, conferring an increased risk for recurrent stroke. In this study, we showed that conventional stenosis measurements on DSA do not provide reliable information regarding distal flow in the territory at risk. This conclusion is based on the weak correlation we found between angiographic markers of ICAD severity (minimal lumen diameter, percentage of stenosis) and the physiologic impact of ICAD as assessed by directly measured trans-stenotic pressure ratios and gradients. Since inclusion/selection criteria in all previous clinical trials relied on the angiographic severity of stenosis, our findings raise doubts about their conclusions and suggest potential benefit of stenting in selected ICAD patients.
For more than a decade, the interventional cardiology literature has demonstrated that fractional flow reserve (FFR, measured as the pressure in the diseased vessel distal to the stenosis divided by the aortic pressure) is a stronger predictor of ischemia than percentage of stenosis alone, and an FFR < 0.75 has been shown to predict a hemodynamically significant stenosis with 95% diagnostic accuracy.16 Pilot studies performed in China have demonstrated the technical feasibility of extrapolating this technique to the intracranial circulation, with similar results to ours.10,17 The DPPR in our study was 0.63 or less in all patients. Based on this observation, we hypothesize that a DPPR of 0.6 or less may be a predictor of high risk for recurrent ischemia in patients with ICAD and, consequently, can serve as a biomarker for intervention and stenting. In the coronary circulation, stenosis evaluation by physiologic assessment using microwire-mounted sensors has an important clinical role and is routinely performed. Initially, it consisted of measurement of the coronary flow velocity response to maximal pharmacologic vasodilation, but this was superseded by trans-stenotic pressure measurements during vasodilation (referred to as FFR). Numerous studies have demonstrated the significant benefit of using invasive measurement of trans-stenotic pressures to guide percutaneous coronary revascularization. Trans-stenotic pressure measurements have several favorable features as a diagnostic tool. The supplies and equipments needed are mobile and relatively inexpensive, pressure measurements can be performed quickly, data analysis requires no specialized expertise, and the results are available in real time. Moreover, measurements can be quickly repeated after angioplasty/stenting of the stenosis to quantify the actual hemodynamic effect of the intervention. Currently, several novel imaging approaches to assess cerebral hemodynamics and stroke risk are being developed, including visualization of ischemic changes in the watershed areas on magnetic resonance imaging (MRI), quantitative MR angiography, and computational fluid dynamic analysis of DSA and computed tomography (CT) angiograms.8 Although perfusion studies and collateral circulation grading can help with the assessment of these patients, a live direct pressure gradient measurement can be more advantageous. However, other studies using MR perfusion have also shown that a Tmax > 6 s, no stenosis, was associated with recurrent ischemic events.18-20
Results from the Wingspan Stent System Post Market Surveillance Study (WEAVE) registry of symptomatic ICAD showed a relatively lower risk of periprocedural complications with stenting (≈5% at 30 d) compared with the SAMMPRIS study. This higher complication rate in SAMMPRIS and VISIT is likely due to (1) the variability in operator experience, (2) the non-weighted higher periprocedural complication rates at low volume centers and (3) lack of consideration of anatomic locations of vascular lesions.21 The authors of WEAVE attributed this finding to the increased operator experience with the device and, perhaps, to the submaximal reperfusion usually achieved by angioplasty strategy alone. Thus, patients with symptomatic ICAD and impaired perfusion may be a distinctive group with a high recurrence risk. Studies evaluating the safety of revascularization intervention against the risk of persistent neurological deterioration if medically treated are urgently needed in this group of patients.12
Limitations
Limitations of our study include its retrospective design, the small number of patients in the sample, and the lack of a control group. For these reasons, the current study presents a proof of concept only that validates previous findings. Future studies should include a larger sample and must have a prospective design to assess whether PDPR can reliably predict stroke recurrence or response to MMT. The indication to intervene remains controversial. In this study, the cases were discussed in multidisciplinary meetings. Patients who have failed MMT and continue to have symptoms that fit the area affected by the stenosis were scheduled for intervention. The pressure measurements did not affect the clinical course as it was for research purposes only. The findings of low cerebral blood flow on the pressure measuring device without stroked parenchyma (on MRI) is suggestive of ischemia. Future studies could assess the blood flow using less invasive monitoring such as computational flow dynamics in addition to MR perfusion. Another method of assessing stenosis would be the use of intravascular ultrasound as performed by Meyers et al.22 The second limitation related to flow measurement with the device can be related to the change in pressure or flow after navigation passes the stenosis with the microwire of the microcatheter. From our standpoint, it is unlikely since the smallest stenosis measures 0.2 mm, whereas the wire diameter measures 0.03 mm. We did not observe any changes in the diameter while navigating the stenosis, but we cannot rule out occult changes. Finally, our small sample is confounded by the presence of stenosis post-pipeline, which has a separate physiology than ICAD; however, it was used as a “positive control” stenosis, since it was a symptomatic stenosis.
CONCLUSION
Angiographic stenosis does not reflect the physiologic severity of distal flow limitation in patients with ICAD. Hemodynamic assessment using trans-stenotic pressure ratios and gradients may serve as a more reliable predictive biomarker for MMT failure and response to revascularization, especially in patients with functional stenosis inducing distal ischemia. Further studies investigating the safety of reperfusion intervention in these patients are needed, with the ultimate goal of reducing the risk of progressive neurological deterioration.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14-20. [DOI] [PubMed] [Google Scholar]
- 2. Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39(4):1142-1147. [DOI] [PubMed] [Google Scholar]
- 3. Suri MFK, Qiao Y, Ma XYet al.. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population the atherosclerosis risk in communities study. Stroke. 2016;47(5):1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396-2399. [DOI] [PubMed] [Google Scholar]
- 5. Chimowitz MI, Lynn MJ, Derdeyn CPet al.. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaidat OO, Fitzsimmons BF, Woodward BKet al.. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015;313(12):1240-1248. [DOI] [PubMed] [Google Scholar]
- 7. Peng G, Zhang J, Jia Bet al.. Submaximal primary angioplasty for symptomatic intracranial atherosclerosis: peri-procedural complications and long-term outcomes. Neuroradiology. 2019;61(1):97-102. [DOI] [PubMed] [Google Scholar]
- 8. Amin-Hanjani S, Pandey DK, Rose-Finnell Let al.. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 2016;73(2):178-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke. 2014;45(2):645-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miao Z, Liebeskind DS, Lo Wet al.. Fractional flow assessment for the evaluation of intracranial atherosclerosis: a feasibility study. Interv Neurol. 2016;5(1-2):65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44(1):287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dakay K, Yaghi S. Symptomatic intracranial atherosclerosis with impaired distal perfusion: a case study. Stroke. 2018;49(1):e10-e13. [DOI] [PubMed] [Google Scholar]
- 13. Yaghi S, Rostanski SK, Boehme AKet al.. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol. 2016;73(5):572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chamczuk AJ, Ogilvy CS, Snyder KVet al.. Elective stenting for intracranial stenosis under conscious sedation. Neurosurgery. 2010;67(5):1189-1193; discussion 1194. [DOI] [PubMed] [Google Scholar]
- 15. Liebeskind DS, Cotsonis GA, Saver JLet al.. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69(6):963-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pijls NH, Tanaka N, Fearon WF. Functional assessment of coronary stenoses: can we live without it? Eur Heart J. 2013;34(18):1335-1344. [DOI] [PubMed] [Google Scholar]
- 17. Han YF, Liu WH, Chen XLet al.. Severity assessment of intracranial large artery stenosis by pressure gradient measurements: a feasibility study. Catheter Cardiovasc Interv. 2016;88(2):255-261. [DOI] [PubMed] [Google Scholar]
- 18. Sacchetti DC, Cutting SM, McTaggart RAet al.. Perfusion imaging and recurrent cerebrovascular events in intracranial atherosclerotic disease or carotid occlusion. Int J Stroke. 2018;13(6):592-599. [DOI] [PubMed] [Google Scholar]
- 19. Yaghi S, Grory BM, Prabhakaran Set al.. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. J Neuroimaging. 2019;29(5):640-644. [DOI] [PubMed] [Google Scholar]
- 20. Yaghi S, Khatri P, Prabhakaran Set al.. What threshold defines penumbral brain tissue in patients with symptomatic anterior circulation intracranial stenosis: an exploratory analysis. J Neuroimaging. 2019;29(2):203-205. [DOI] [PubMed] [Google Scholar]
- 21. Yu W, Jiang WJ. Stenting for intracranial stenosis: potential future for the prevention of disabling or fatal stroke. Stroke Vasc Neurol. 2018;3(3):140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyers PM, Schumacher HC, Gray WAet al.. Intravascular ultrasound of symptomatic intracranial stenosis demonstrates atherosclerotic plaque with intraplaque hemorrhage: a case report. J Neuroimaging. 2009;19(3):266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]