Abstract
Pigment dispersion syndrome and pigmentary glaucoma are two conditions characterized by pigment dispersion originating from the posterior part of the iris and its accumulation on the trabecular meshwork, corneal endothelium, and anterior surface of the lens. The pigment on the trabecular meshwork can cause chronic inflammation with a secondary reduction of its function and an increase in intraocular pressure. The case presented represents a typical example of pigmentary glaucoma in a myopic patient in which all the signs, symptoms, and complications typical of these pathologies were present. We report and describe an 8-year-long follow-up period with clinical and instrumental examinations.
Keywords: Pigment dispersion syndrome, Pigmentary glaucoma, Myopia, Retinal detachment
Introduction
Pigment dispersion syndrome (PDS) and pigmentary glaucoma (PG) are two bilateral pathologies characterized by an excessive dispersion of pigment from the iris, due to the scraping of the iris against the anterior lens zonulas and its accumulation in various structures of the anterior chamber of the eye. Typically, there is pigment accumulation in the trabecular meshwork resulting in a reduction of aqueous humor outflow and an increase in intraocular pressure (IOP) [1]. PG is a secondary open-angle glaucoma with visual field damage, glaucomatous optic neuropathy, and an increase in IOP. Both these diseases are characterized by a classic objective triad consisting of mild-peripheral iris transillumination defects, pigmentation of the trabecular meshwork, and pigmentation of a central elongated area of corneal endothelium (Krukenberg spindle) [2]. PDS and PG have a strong male predominance (male-to-female ratio is 2:1–5:1) and occur mostly during the third-fourth decade of life; PG represents 1–1.5% of cases of glaucoma in occidental countries.
The rate of conversion of PDS to PG is estimated to be between 35 and 50%. The pathophysiologic mechanism underlying the development of these two pathologies is the “reverse pupillary block” that consists of an accentuated posterior curvature of the medium-peripheral iris causing mechanical rubbing of the posterior pigmented iris surface against lens zonula. PDS and PG are frequently associated with moderate-severe myopia (80% of cases) because of the increased space behind the iris and the greater length of the eye.
The mechanism through which a PDS converts into a PG is not clear; it is supposed that it is a heterogeneous phenomenon resulting from a combination of environmental and genetic factors. Risk factors that could induce a high percentage of conversion in PG are male sex, black race, moderate-severe myopia, baseline IOP, massive dispersion of pigment, accommodation, pupil dilation, and Krukenberg spindles. Surprisingly, family history of glaucoma seems not to be involved in a higher risk of progression. Mutations in genes codifying for the embryological development of the anterior segment of the eye and for the normal anatomy of trabecular meshwork have been found in patients affected by PG; however, the genetics of these pathologies is still under study and large-scale GWAS analyses may be useful to provide insight into genetic risks for conversion [3].
These patients also have a higher risk of lattice degeneration and retinal breaks, with an incidence of 20–33.3 and 12%, respectively; both these conditions predispose to retinal detachment, which occurs in about half of the cases, probably due to the fact that the pigment epithelium of the iris and the retinal pigment epithelium share the same embryological derivation [4, 5]. An increased incidence of nonsenile nuclear cataract in patients with PDS is also described in the literature [6].
Diagnosis of PDS and PG is performed with a thorough clinical inspection involving slit lamp examination of the anterior chamber (focusing on chamber depth, corneal pigment deposits, and iris defects), IOP measurement, ocular fundus assessment, and gonioscopy; the latter examination typically reveals an open chamber angle with a hyperpigmentation of the trabecular meshwork [7]. In order to complete diagnosis and to establish the clinical stage of the disease other instrumental tests are usually performed, such as optical coherence tomography (OCT), useful to evaluate the optic nerve and the retinal nerve fiber layer (RNFL), ultrasound biomicroscopy, essential to study the relationship between the structures in the anterior chamber, and visual field analysis [8, 9, 10]. Medical therapy and surgical and parasurgical treatments are identical to those performed for primary open-angle glaucoma [11].
Case Report
A 39-year-old man presented to our clinic in October 2011 with the diagnosis of bilateral nuclear cataract. He complained of progressive visual reduction in both eyes. He had anamnesis positive for moderate myopia and PDS and was treated with bilateral administration of topical prostaglandin analog eye drops (Bimatoprost 0.1 mg) in order to reduce IOP in both eyes. The first examination revealed a best corrected visual acuity (BCVA) of 20/70 in the right eye (RE) and 20/40 in the left eye (LE) with a correction of −5 Dsph in RE and −4.50 Dsph = −0.50 Dcyl × 70° (TABO) in LE, respectively. The biometry revealed an axial length of 27.58 mm of the RE and 27.17 mm of the LE. The IOP was 14 mm Hg in RE and 14 mm Hg in LE. The slit lamp examination of the anterior chamber showed the following in both eyes: corneal Krukenberg spindle, initial defects of the mid-peripheral iris, and presence of pigmental dispersion on the surface of the lens with a nuclear cataract. Gonioscopy examination, performed with a Goldmann three-mirror lens, revealed an open angle with a uniform increase of trabecular meshwork pigmentation in its entire circumference. The fundus examination revealed cup-disc ratio of 0.6 in both eyes, normal foveal light reflex, and small peripheral areas of degeneration for which laser treatment was not required.
A visual field examination (Octopus, 24-2 fast threshold) and an OCT RNFL and ganglion cell complex (GCC) analysis were performed between February and March 2012. The first visual field showed nonspecific defects of the superior hemisphere in LE, and the OCT revealed a peripapillary RNFL thinning with a GCC alteration in LE and a mild reduction of RNFL thickness in the superonasal sector in RE.
Because of the good IOP values and the results of the OCT examination, it was decided to suppress the IOP therapy in LE and to replace Bimatoprost eye drops in RE with a fixed combination of brinzolamide 1% plus timolol 0.5% in an ophthalmic suspension 2 times a day.
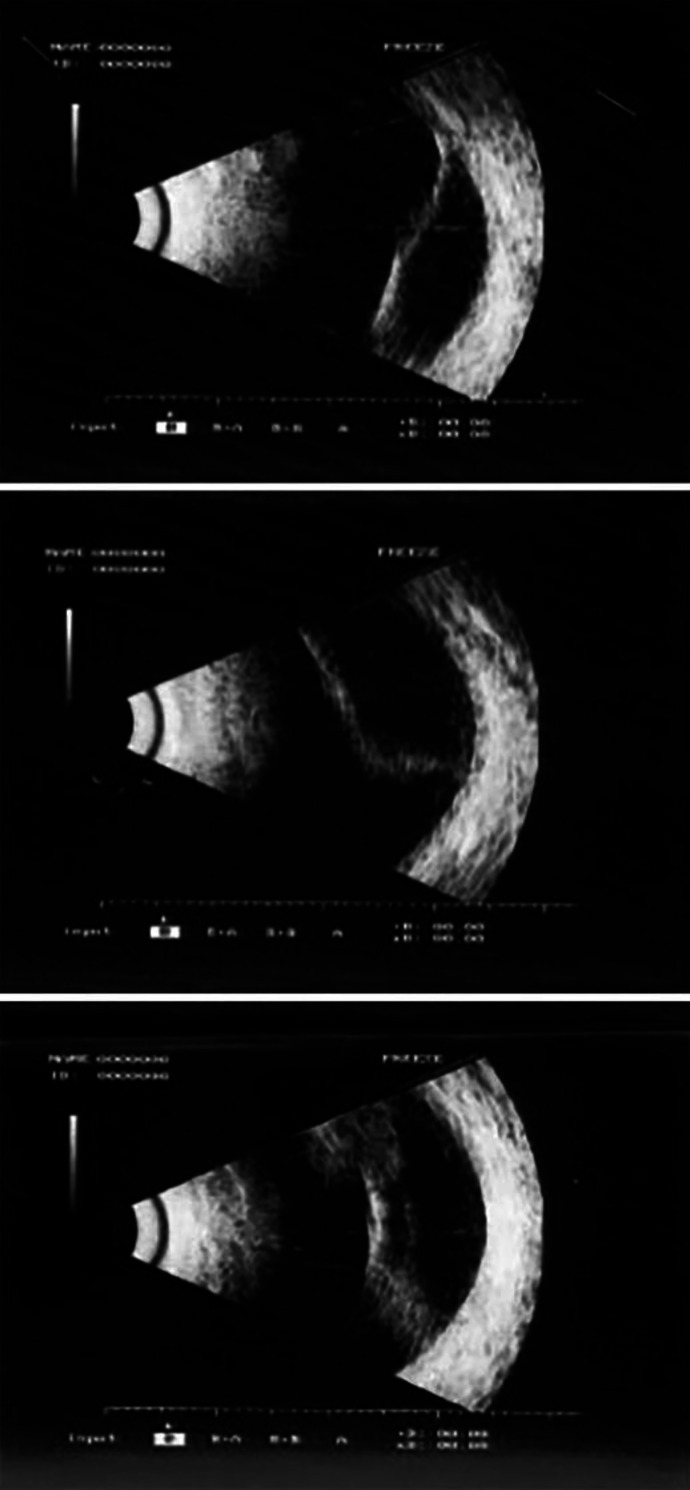
In September 2012 and November 2012, the patient underwent two interventions of phacoemulsification of the cataract in RE and in LE, respectively, with intraocular lens implantation inside the capsular bag, which eventually resulted in a complete visual recovery of 20/20 in both eyes. Six months after the cataract surgery (March 2013), the patient presented a retinal detachment in the superonasal quadrant of RE with no involvement of the posterior pole and the macular area (Fig. 1), and an ab externo encircling scleral buckle surgery was performed in order to solve the retinal detachment.
Fig. 1.
Ultrasound images of retinal detachment in the right eye.
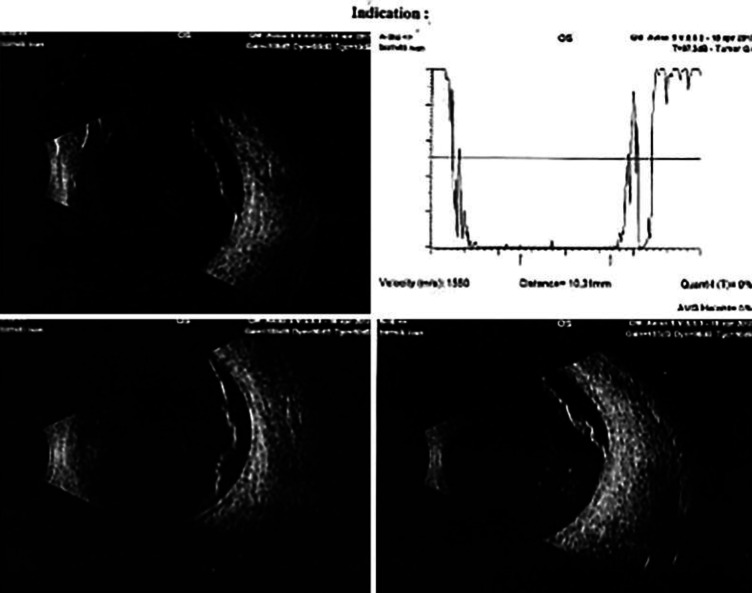
In the following years, the patient was subjected to routine ophthalmological visits with complete clinical ocular examination, IOP measurements, visual field analysis, and OCT evaluations. During one of these visits, a small area of palisade degeneration with a microhole in the retinal periphery of LE was found at 5 h and therefore promptly treated with a barrage laser in order to prevent any further retinal complications. However, despite this, in April 2017, the patient had a retinal detachment of the superior quadrants in LE due to a horseshoe tear at 11 h and a microhole at 12 h (Fig. 2), for which he was treated with pneumoretinopexy combined with cryopexy of the retinal tears. In order to avoid IOP spikes, IOP-lowering therapy was started again in LE. In May 2017, the patient had a second retinal detachment of the inferior sectors in LE, which was treated with encircling scleral buckle retinal surgery.
Fig. 2.
Ultrasound images of retinal detachment in the left eye.
During the last follow-up visit, the patient presented a BCVA of 20/20 in both eyes with a correction of −1.50 Dsph = −1.00 Dcyl × 100° in RE and −1.50 Dsph = −0.75 Dcyl × 75° in LE. The IOP was 17 mm Hg in RE and 16 mm Hg in LE. Slit lamp examination showed bilateral pseudophakia, and the fundus examination presented bilateral outcomes of encircling scleral buckle surgery with retinal adherence in all retinal quadrants of both eyes.
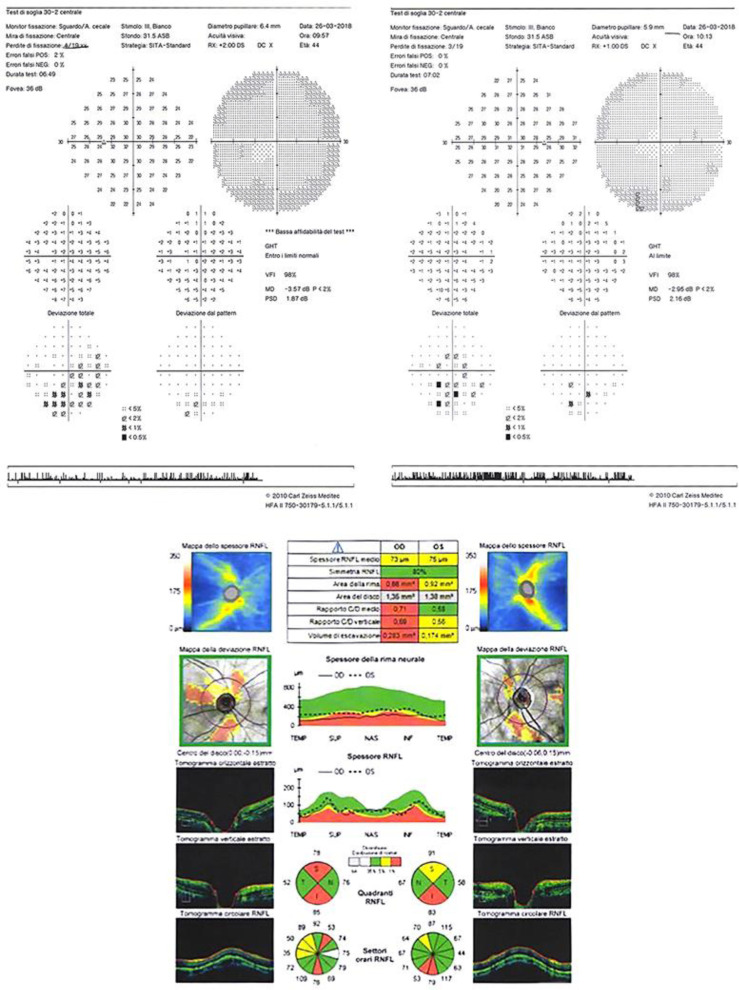
The visual field analysis performed in March 2018 showed a generalized mean defect without any typical localized glaucomatous defect in either eye (Fig. 3). On the contrary, OCT RNFL and GCC analysis highlighted a reduction of GCC thickness and average RNFL in both eyes (Fig. 3).
Fig. 3.
Top: visual field 30-2 analysis (performed in March 2018) of right eye (right) and left eye (left). Bottom: OCT RNFL analysis of right eye (right) and left eye (left).
Discussion/Conclusion
The case report described is a real-life textbook example of a moderate myopic patient suffering from PG where all the possible related alterations and complications of this pathology have occurred despite their low incidence.
It is well known how the pigment deposit originated by PG and PDS is due to both the rubbing of the posterior iris against the lens zonules and the spontaneous release of pigment caused by a hereditary developmental abnormality of the iris [1].
In the general population, the incidence of retinal alterations is approximately 1 in 10,000 and both eyes are affected in only 7% of cases; in these patients retinal changes occur in 33% of cases for lattice degeneration, in 10% of cases for retinal breaks, and in 6% of cases for retinal detachment. In the case presented, all these causes of retinal changes occurred simultaneously in both eyes of the patient.
The retinal involvement, characterized by dystrophy of the pigmented epithelium of the retina, confirms the hypothesis that PDS and PG are two pathologies caused by developmental defects not only of the anterior segment but of all the structures of the eye [4].
This association between alterations of both the pigment epithelium of the iris and retinal pigment epithelium has an important implication, especially for the therapeutic management of these patients. As we know, miotic drugs are frequently used in PG in order to reduce the friction between the iris and the lens and increase the outflow of the aqueous, but the use of this type of drug should be avoided in patients presenting peripherical retinal alterations at the time of diagnosis in order to prevent lesion progression. Therefore, for the treatment of our patient we chose topical prostaglandins to lower the IOP and enhance the uveoscleral outflow.
Moreover, it is interesting to note how the complications (such as retinal detachment) of moderate-severe myopia and PG are identical, and this fact is reflected by the strong similarity between the outcomes of the instrumental examinations (such as visual field or OCT analysis). Indeed, although our patient presented a reduction of thickness of OCT RNFL, the visual field did not present any typical defect of glaucoma, and this occurrence could probably have been caused by retinal changes due to both myopia and glaucoma rather than glaucoma alone [12, 13].
In conclusion, in the presence of PG, a careful analysis of clinical and instrumental characteristics both in the diagnosis and in the follow-up of the patient should be performed in order to prevent severe complications which could affect both lens transparency and retinal integrity.
Statement of Ethics
This research complies with the guidelines for human studies and has been conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The patient gave written informed consent to publish his case (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded with the support of PRIN No. 20152EKS4Y_002: “Experimental and clinical evidence on the therapeutic role of nerve growth factor in neurodegenerative ocular diseases.”
Author Contributions
Dr. M. Di Pippo and Dr. A. Perdicchi had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition, analysis, and interpretation of the data: all authors. Study supervision: Dr. A. Perdicchi.
References
- 1.Scuderi G, Contestabile MT, Scuderi L, Librando A, Fenicia V, Rahimi S. Correction to: Pigment dispersion syndrome and pigmentary glaucoma: a review and update. Int Ophthalmol. 2019 Jul;39((7)):1663. doi: 10.1007/s10792-019-01097-6. [DOI] [PubMed] [Google Scholar]
- 2.Klingenstein A, Kernt M, Seidensticker F, et al. Anterior-segment morphology and corneal biomechanical characteristics in pigmentary glaucoma. Clin Ophthalmol. 201, 4(8):119–126. doi: 10.2147/OPTH.S53088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lascaratos G, Shah A, Garway-Heath DF. The genetics of pigment dispersion syndrome and pigmentary glaucoma. Surv Ophthalmol. 2013 Mar-Apr;58((2)):164–75. doi: 10.1016/j.survophthal.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Scuderi G, Papale A, Nucci C, Cerulli L. Retinal involvement in pigment dispersion syndrome. Int Ophthalmol. 1995-1996-1996;19((6)):375–8. doi: 10.1007/BF00130858. [DOI] [PubMed] [Google Scholar]
- 5.Scuderi GL, Ricci F, Nucci C, Galasso MJ, Cerulli L. Electro-oculography in pigment dispersion syndrome. Ophthalmic Res. 1998;30((1)):23–9. doi: 10.1159/000055450. [DOI] [PubMed] [Google Scholar]
- 6.Mosaed S, Haider A, Kim D, Zhang Z. Association of Pigmentary Glaucoma and Nonsenile Nuclear Cataracts. J Glaucoma. 2016 Jul;25((7)):547–50. doi: 10.1097/IJG.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 7.Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma—a major review. Clin Exp Ophthalmol. 2008 Dec;36((9)):868–82. doi: 10.1111/j.1442-9071.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 8.Perdicchi A, Abdolrahimzadeh S, Cutini A, Ciarnella A, Scuderi GL. Evaluation of the progression of visual field damage in patients suffering from early manifest glaucoma. Clin Ophthalmol. 2016 Aug;10:1647–51. doi: 10.2147/OPTH.S113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scuderi GL, Cesareo M, Perdicchi A, Recupero SM. Standard automated perimetry and algorithms for monitoring glaucoma progression. Prog Brain Res. 2008;173:77–99. doi: 10.1016/S0079-6123(08)01107-2. [DOI] [PubMed] [Google Scholar]
- 10.Di Staso S, Agnifili L, Di Staso F, Climastone H, Ciancaglini M, Scuderi GL. Diagnostic capability of optic nerve head rim width and retinal nerve fiber thickness in open-angle glaucoma. Eur J Ophthalmol. 2018 Jul;28((4)):459–64. doi: 10.1177/1120672117750057. [DOI] [PubMed] [Google Scholar]
- 11.Scuderi GL, Pasquale N. Laser therapies for glaucoma: new frontiers. Prog Brain Res. 2008;173:225–36. doi: 10.1016/S0079-6123(08)01116-3. [DOI] [PubMed] [Google Scholar]
- 12.Abdolrahimzadeh S, Parisi F, Plateroti AM, Evangelista F, Fenicia V, Scuderi G, et al. Visual acuity, and macular and peripapillary thickness in high myopia. Curr Eye Res. 2017 Nov;42((11)):1468–73. doi: 10.1080/02713683.2017.1347692. [DOI] [PubMed] [Google Scholar]
- 13.Perdicchi A, de Paula A, Sordi E, Scuderi G. Cluster analysis of computerized visual field and optical coherence tomography-ganglion cell complex defects in high intraocular pressure patients or early stage glaucoma. Eur J Ophthalmol. 2019 Apr;•••:1120672119841774. doi: 10.1177/1120672119841774. [DOI] [PubMed] [Google Scholar]