Abstract
Sertoli-Leydig cell ovarian tumors (SLCT) are rare ovarian tumors of the sex cord-stroma subset. Their incidence peaks in the second to third decade of life. Most SCLT are diagnosed at an early stage and have a good prognosis. Fertility-sparing surgery may thus be offered. Adjuvant chemotherapy may be indicated according to prognostic factors. However, outcome in relapsing SLCT is poor. There is no evidence supporting a best treatment option upon relapse, but most publications combine radical surgery, chemotherapy, and rarely radiotherapy. Two years after left adnexectomy for FIGO IA SLCT, a now 22-year-old patient presented with peritoneal recurrence without involvement of the remaining ovary and uterus. Since there is no evidence of a survival benefit in the literature of macroscopically healthy contralateral ovary ablation in relapse and hormonal replacement therapy is contraindicative, we consented to endocrine-sparing surgery with conservation of the contralateral ovary, followed by 3 cycles of BEP chemotherapy regimen. Our patient is disease-free 16 months after relapse diagnosis. Since recurrence of SLCT has a very poor prognosis and hormonal treatment is contraindicated, endocrine-sparing surgery for young patients with a normal contralateral ovary might be a legitimate option. This is one of the first reported cases of conservative surgery in SLCT recurrence, we therefore aimed to illustrate its management in a young patient with considerations of contraception, fertility- and then endocrine-sparing surgery, and quality of life.
Keywords: Gynecological cancer, Recurrence, Fertility-sparing surgery, Endocrine-sparing surgery, Sertoli-Leydig cell tumor
Introduction
Sertoli-Leydig cell tumors (SLCT) represent a rare and heterogeneous subgroup of sex-cord ovarian neoplasms, accounting for <0.2% of all ovarian tumors. Since most SLCT are unilateral, diagnosed at an early stage, most prevalent in patients in their second or third decade, and harbor a good prognosis, fertility-sparing surgery is an accepted option [1, 2, 3, 4]. Tumors with poor differentiation, the presence of heterologous elements, retiform patterns, and advanced stages at diagnosis are more prone to relapse, and adjuvant chemotherapy is therefore indicated [3].
Approximately 20% of all SLCT will relapse, 95% within 5 years [5, 6]. Available data report a very poor prognosis upon relapse, despite combination treatments such as radical surgery, chemotherapy, and occasionally radiotherapy [5, 7]. There are no prospective studies to support the management of relapsed SLCT and to select potentially aggressive therapeutic options in the face of the poor prognosis.
Whenever possible, surgery remains a cornerstone of SLCT treatment upon relapse. Available guidelines recommend radical surgery [8] with two subsequent major consequences: first, it terminates any family planning, something which may occasionally be neglected due to the poor prognosis; second, contralateral ovariectomy exposes the young women to estrogen depletion, while hormonal replacement therapy is contraindicated because of tumor hormonal sensitivity [9]. “Endocrine-sparing” surgery may be an option, in order to preserve quality of life.
We hereby present the case of a patient with unique SLCT peritoneal relapse treated with ovarian-sparing surgery in order to prevent endocrine depletion symptoms. To our knowledge, this is the second case of conservative surgery upon SLCT relapse.
Case Presentation
A 20-year-old nulligravida presented to Sion Hospital with nausea and abdominal pain that had evolved over several weeks. She had no significant personal medical history. A cousin had been diagnosed with cervical rhabdomyosarcoma at 13 years and was now, 6 years later, free of disease.
On pelvic ultrasound, we detected a 14 × 10 cm left polycystic ovarian mass with a solid component and a normal contralateral ovary and uterus. A detailed analysis of hormonal levels and tumor markers showed slightly elevated CA-125 (49 kU/L), low levels of estradiol (<18 pmol/L), and normal CA-15.3, CA-19.9, LDH, AMH, testosterone, beta-HCG, and AFP. The patient underwent left laparoscopic adnexectomy with peritoneal washing on May 30th, 2016. There was no rupture or tumor leakage before or during surgery (Fig. 1).
Fig. 1.
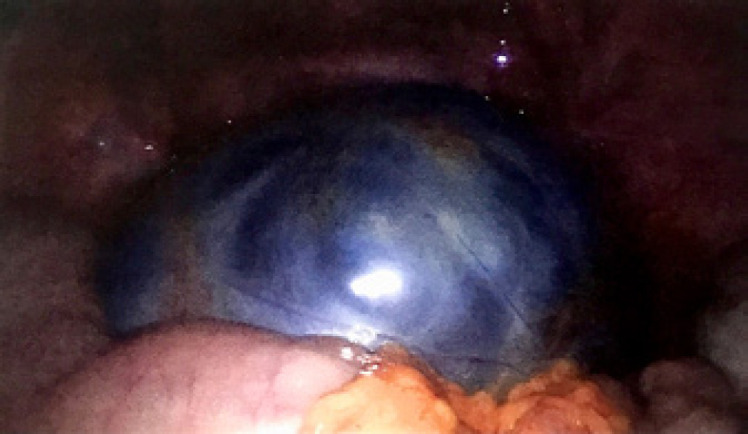
Laparoscopic aspect of the Sertoli-Leydig cell tumor of the left adnexa at initial surgery.
Histopathological and immunochemistry studies revealed a moderately differentiated SLCT with heterologous elements (intestinal mucinous glands and a low-grade neuroendocrine contingent). Peritoneal washing was negative for malignant cells. Subsequently, on June 6th, 2016, our patient underwent full peritoneal staging including infracolic omentectomy, appendectomy, endometrial and multiple peritoneal biopsies. The definitive FIGO stage was IA with no additional tumor retrieved during this restaging surgery.
The multidisciplinary tumor board recommended fertility preservation counseling and genetic testing for DICER-1 mutations, which the patient refused. Despite the presence of heterologous elements, adjuvant chemotherapy was not proposed. Our patient had an uneventful gynecological follow-up with vaginal ultrasound every 3 months until July 2018. During this time, the patient used oral contraceptives.
The patient presented again end of November 2018 with pain and an increased abdominal circumference. An abdominal CT scan and MRI raised suspicion of an SLCT relapse (unique 17 × 14 cm abdominopelvic polycystic mass with a solid component; Fig. 2). CA-125 levels were slightly over the normal range (41 kU/L) and the AMH levels were low (8 pmol/L). All other tumor markers were within normal ranges (CA-19.9, CA-15.3, inhibin B, HCG, AFP, testosterone, and LDH). Due to the rapid progression of her severe abdominal pain, emergency surgical exploration was performed on December 27th, 2018 (Fig. 3). Midline laparotomy confirmed a left-side pelvic peritoneal relapse with a polycystic mass. The tumor was resected during an extensive pelvic and abdominal peritonectomy. The right ovary and the uterus appeared macroscopically normal and were conserved. Full peritoneal exploration confirmed a unique lesion, with no other upper abdominal secondary lesions. A copper intrauterine device was inserted postoperatively.
Fig. 2.
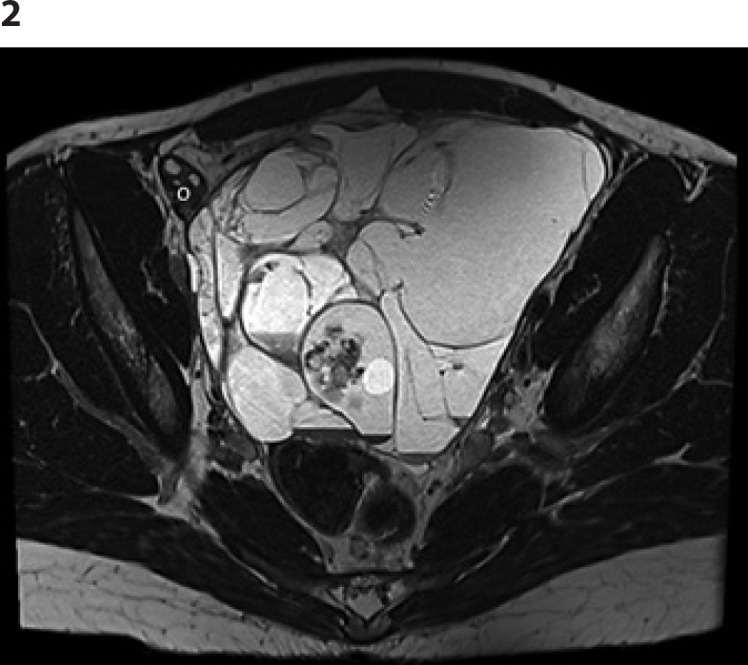
Pelvic MRI showing the peritoneal relapse (multiloculated lesion). O, remaining ovary.
Fig. 3.
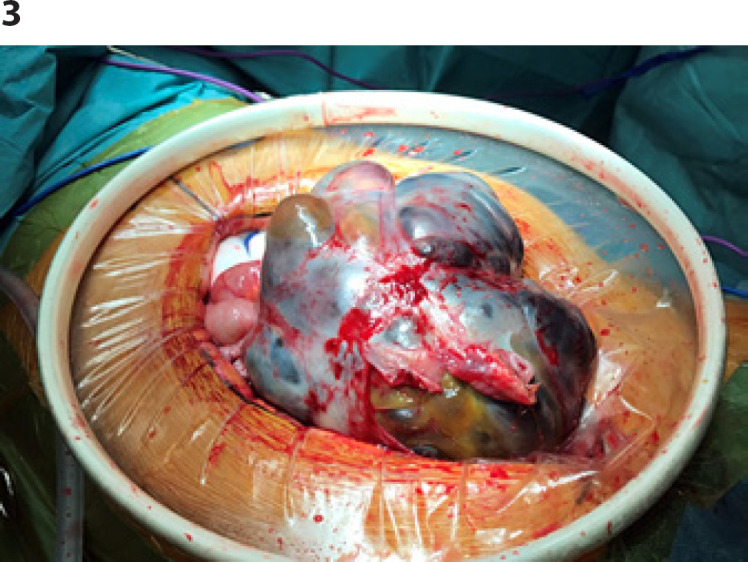
Intraoperative photography of the relapse.
Histopathological analysis showed an undifferentiated SLCT with retiform pattern and negative peritoneal washing. The patient underwent 3 cycles of BEP chemotherapy between January 28th, 2019, and March 11th, 2019. Her menstruation resumed in August 2019, and the patient was free of disease at her last follow-up in April 2020. No DICER-1 mutation was identified.
Discussion
SLCT are part of the sex cord-stroma subset of ovarian tumors and account for <0.2% of all ovarian tumors. They are characterized by a varying proliferation of Sertoli and Leydig cells that may secrete testosterone causing virilization or defeminization in up to 85% of all patients [3]. Tumors of the retiform type or those presenting heterologous elements are rarely secretant [8]. In morphological ambiguous cases, immunomarkers such as inhibin alpha, calretinin, and FOXL2 help discriminating among sex cord-stroma subset tumors. Recent research has shown that many patients with ovarian SLCT carry DICER-1 germline mutations which are also associated with other rare tumors such as rhabdomyosarcomas, pleuropulmonary blastomas, and nephromas [10].
Most SLCT are diagnosed in young patients at an early stage, they are well differentiated and have an excellent prognosis. Fertility-sparing surgery with preservation of the uterus and contralateral ovary are therefore recommended for stage IA [3, 11]. According to the last ESMO guidelines (2018), fertility-sparing surgery may be an option for selected cases even in advanced stages. Some authors propose a completion of surgery after childbearing [4]. Spontaneous pregnancies have been reported in women treated for SLCT [5, 12, 13].
For patients at advanced stages or with recurrence, chemotherapy is part of the treatment. The most commonly used regimen is BEP or EP (for patients >40 years). Another option is carboplatin and paclitaxel, similarly to the treatment for epithelial ovarian tumors.
A fifth of all SLCT relapse cases and most recurrences appear within 3 years. In Young and Scully's series from 1985 [3], only 9 of 22 patients with poorly differentiated tumors were alive without relapse after a mean follow-up of 7 years. A review of all published retrospective series calculated relapse rates of 5–8% for stage IA tumors and of approximately 30% for stage IC [5]. The mortality rate in case of relapse was around 70%. Risk factors for recurrence include advanced FIGO stage, poor differentiation, the presence of heterologous elements, and a retiform pattern. Only 4 out of 47 patients (8.5%) experienced unique contralateral ovarian recurrence after fertility-sparing surgery during early stages (IA to IC1).
In Gouy et al.'s institutional series [5], 5 out of 13 patients staged IA to IC1 relapsed after fertility-sparing surgery (unilateral adnexectomy with or without contralateral cystectomy). Four of the 5 patients underwent chemotherapy and 3 underwent radical surgery. Two patients died 3 and 83 months after treatment, respectively, and the other 3 were alive with disease.
Most patients with recurrence have systemic relapses at multiple locations. The peritoneum is the most commonly involved site [12]. Other frequent locations include the upper abdomen (55–70%) and the pelvis (30–45%) [10]. Very few patients experience localized relapse, which probably explains the extremely poor prognosis of relapse despite extensive surgery and chemotherapy. In Gouy et al.'s series [5], only 2 patients experienced localized relapse. The first was 4 years old at diagnosis, relapsed at 10 years in the uterus, and underwent hysterectomy and remaining adnexectomy. The second patient was 15 years old, experienced a contralateral ovarian relapse after 18 months, and underwent adnexectomy with uterine conservation. Both patients were alive with disease despite radical surgeries and, for one of them, chemotherapy. In Brown et al.'s series [14], 28% of all SLCT recurred (9 patients). Six out of the 9 patients had an abdominopelvic recurrence (4 isolated and 2 multifocal), but no data on the type of surgery or systemic treatments are available.
The prognosis of patients with SLCT relapse is reportedly poor despite extensive surgery. Very few data are available about the oncologic safety of ovarian conservation upon relapse. Most reported surgery was radical, including adnexectomy and hysterectomy. Currently, there is no evidence of a survival benefit of adnexectomy in patients with relapse and a macroscopically normal ovary. The prognosis seems to be dictated by the aggressive nature of the disease rather than by the removal or conservation of an apparently healthy remaining ovary.
SLCT harbor hormone receptors, and consequently, hormonal replacement therapy is contraindicated [9]. Young patients undergoing radical surgery with bilateral salpingo-oophorectomy are consequently facing estrogen depletion and menopausal symptoms with a strong impact on their quality of life. Where possible, conservation of the normal remaining ovary (endocrine-sparing surgery) might improve quality of life of very young women who experience tumor recurrence.
To the best of our knowledge, we present here the second case of fertility-sparing surgery in a young patient with relapsed SLCT. The first case was a 20-year-old patient with FIGO IA SLCT and fertility-sparing surgery with relapse in the prevesical peritoneum 27 months after initial diagnosis [15]. She underwent fertility-sparing surgery with mass excision and adjuvant chemotherapy. The patient showed no evidence of disease after 50 months of follow-up.
Conclusion
The outcome of patients with SLCT relapse is very poor. There is no strong evidence to support the best treatment option upon relapse, but most of the published literature combines radical surgery and chemotherapy. For the few patients with a localized relapse and a normal contralateral ovary, an endocrine-sparing surgery is an option, as hormonal replacement therapies are contraindicated. The prognosis upon relapse does not seem to be linked to the conservation of a healthy ovary or uterus, but rather to the tumor aggressivity itself. For these patients, the remaining ovary and uterus could allow a better quality of life or preserve fertility depending on their survival and future advances in the treatment of these tumors.
Statement of Ethics
The patient has given her written informed consent to publish the case (including publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors received no funding.
Author Contributions
Concept: D.E.H.; data collection and literature search: S.J.S., D.E.H.; writing of the manuscript: all authors.
References
- 1.Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. J Clin Oncol. 2007;25((20)):2944–51. doi: 10.1200/JCO.2007.11.1005. [DOI] [PubMed] [Google Scholar]
- 2.Gui T, Cao D, Shen K, Yang J, Zhang Y, Yu Q, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol. 2012 Nov;127((2)):384–9. doi: 10.1016/j.ygyno.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 3.Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985 Aug;9((8)):543–69. doi: 10.1097/00000478-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Morice P, Denschlag D, Rodolakis A, Reed N, Schneider A, Kesic V, et al. Recommendations of the Fertility Task Force of the European Society of Gynecologic Oncology about the conservative management of ovarian malignant tumors. Int J Gynecol Cancer. 2011 Jul;21((5)):951–63. doi: 10.1097/IGC.0b013e31821bec6b. [DOI] [PubMed] [Google Scholar]
- 5.Gouy S, Arfi A, Maulard A, Pautier P, Bentivegna E, Leary A, et al. Results from a monocentric long-term analysis of 23 patients with ovarian Sertoli-Leydig cell tumors. Oncologist. 2019;24((5)):702–9. doi: 10.1634/theoncologist.2017-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litta P, Saccardi C, Conte L, Codroma A, Angioni S, Mioni R. Sertoli-Leydig cell tumors: current status of surgical management: literature review and proposal of treatment. Gynecol Endocrinol. 2013 May;29((5)):412–7. doi: 10.3109/09513590.2012.754878. [DOI] [PubMed] [Google Scholar]
- 7.Akman L, Ertas IE, Gokcu M, Terek MC, Sanci M, Sanli UA, et al. Ovarian Sertoli-Leydig cell tumors: a multicenter long-term clinicopathological analysis of 27 patients. J Cancer Res Ther. 2016;12((1)):290–4. doi: 10.4103/0973-1482.158037. [DOI] [PubMed] [Google Scholar]
- 8.Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct 1;29((Suppl 4)):iv1. doi: 10.1093/annonc/mdy001. [DOI] [PubMed] [Google Scholar]
- 9.Rousset-Jablonski C, Selle F, Adda-Herzog E, Planchamp F, Selleret L, Pomel C, et al. Fertility preservation, contraception and menopause hormone therapy in women treated for rare ovarian tumours: guidelines from the French national network dedicated to rare gynaecological cancers. Eur J Cancer. 2019 Jul;116:35–44. doi: 10.1016/j.ejca.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Nam SM, Kim JW, Eoh KJ, Kim HM, Lee JY, Nam EJ, et al. A novel clinicopathological analysis of early stage ovarian Sertoli-Leydig cell tumors at a single institution. Obstet Gynecol Sci. 2017 Jan;60((1)):39–45. doi: 10.5468/ogs.2017.60.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray-Coquard I, Brown J, Harter P, Provencher DM, Fong PC, Maenpaa J, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int J Gynecol Cancer. 2014 Nov;24((9 Suppl 3)):S42–7. doi: 10.1097/IGC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 12.Tomao F, Di Pinto A, Sassu CM, Bardhi E, Di Donato V, Muzii L, et al. Fertility preservation in ovarian tumours. Ecancermedicalscience. 2018;12:885. doi: 10.3332/ecancer.2018.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Chen R, Hua K, Zhang Y. A retrospective study of reproductive outcomes after fertility-sparing surgery and postoperative adjuvant chemotherapy in malignant ovarian germ cell tumors and sex cord-stromal tumors. J Ovarian Res. 2017 Jul;10((1)):52. doi: 10.1186/s13048-017-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown J, Sood AK, Deavers MT, Milojevic L, Gershenson DM. Patterns of metastasis in sex cord-stromal tumors of the ovary: can routine staging lymphadenectomy be omitted? Gynecol Oncol. 2009 Apr;113((1)):86–90. doi: 10.1016/j.ygyno.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Ghalleb M, Bouzaiene H, Sghaier S, Bouaziz H, Hechiche M, Hassouna JB, et al. Fertility sparing surgery for ovarian sex cord stromal tumors: a nine case series. Pan Afr Med J. 2018;31:221. doi: 10.11604/pamj.2018.31.221.15531. [DOI] [PMC free article] [PubMed] [Google Scholar]


