Abstract
In atherosclerosis patients, vascular endothelial dysfunction is commonly observed alongside damage of the vascular endothelial glycocalyx, an extracellular matrix bound to and encapsulating the endothelial cells lining the blood vessel wall. Although atherosclerotic risk factors have been reported in severe patients with coronavirus disease 2019 (COVID-19), the exact mechanisms are unclear. The mortality associated with the COVID-19 outbreak is increased by comorbidities, including hypertension, diabetes, obesity, chronic obstructive pulmonary disease (COPD), and cardiovascular disease. Besides, older individuals and smokers have significantly worse outcomes. Interestingly, these comorbidities and risk factors are consistent with the pathophysiology that causes vascular endothelial glycocalyx damage. Moreover, vascular glycocalyx dysfunction causes microvascular leakage, which results in interstitial pulmonary abnormal shadows (multiple patchy shadows with a ground glass inter-pneumonic appearance). This is frequently followed by severe acute respiratory distress syndrome (ARDS), closely related to coagulo-fibrinolytic changes contributing to disseminated intravascular coagulation (DIC) and Kawasaki disease shock syndrome, as well as inducing activation of the coagulation cascade, leading to thromboembolism and multiple organ failure. Notably, SARS-CoV-2, the causative virus of COVID-19, binds to ACE2, which is abundantly present not only in human epithelia of the lung and the small intestine, but also in vascular endothelial cells and arterial smooth muscle cells. Moreover, COVID-19 can induce severe septic shock, and sepsis can easily lead to systemic degradation of the vascular endothelial glycocalyx. In the current review, we propose new concepts and therapeutic goals for COVID-19-related vascular endothelial glycocalyx damage, based on previous vascular endothelial medicine research.
Keywords: Vascular endothelial dysfunction, Systemic inflammatory response, Cytokine storm, ARDS, Kawasaki disease shock syndrome
It has been said that scientists could win the Nobel Prize in physiology or medicine if they could invent a cure for the common cold. Since the common cold is caused by various viruses, which can easily mutate their genes, it has been extremely difficult to develop any specific medicine or vaccine for influenza infection. For this reason, following infection, individuals are advised to wait for recovery by taking coping medications for the symptoms, such as fever, cough/sputum, diarrhea, and headache, as well as getting sufficient nutrition and rest. It is known that 15% of common colds are caused by conventional human coronavirus (HCoV) infections (e.g., HCoV-229E, HCoV-0C43, HCoV-HKU1, and HCoVNL63). Coronavirus disease 2019 (COVID-19) was initially thought to be a slightly stronger viral infection compared to the seasonal common cold or flu. However, it has become clear that the infectious power of severe acute respiratory coronavirus 2 (SARS-CoV-2), the virus causing COVID-19, is remarkable, and as a result has led to life-threatening complications in a significant proportion of patients.
SARS-CoV-2 spread rapidly throughout the world, largely due to asymptomatic viral transfer. According to the COVID-19 dashboard website by the Center for Systemics Science and Engineering at Johns Hopkin University, the number of global deaths due to COVID-19 was 291,487 as of May 12, 2020.
A subset of infected patients go on to develop a more severe form of disease, which is characterized by expanding pulmonary lesions, sepsis, acute respiratory distress syndrome, and respiratory failure [1]. The fight against SARS-CoV-2 is decisively different from that against conventional viral infections. Since many infected people are asymptomatic, SSARS-CoV-2 is easily spread to others indiscriminately, which has led to the formation of huge clusters of severe COVID-19 patients, especially in situations where there are increased numbers of individuals with opportunistic infections, such as in hospitals and nursing homes for the elderly.
There are numerous differences between COVID-19 and the common cold-induced by traditional coronaviruses or flu. For instance, thrombotic complications are emerging as a critical complication in patients with COVID-19 [2]. In line with this, COVID-19 patients often present with special features, such as increasing D-dimer and fibrin degradation levels, prolonged prothrombin time, and the development of disseminated intravascular coagulation (DIC) [3], which have all been associated with poor prognosis in severe COVID-19 patients [4]. Indeed, microvascular thrombosis can induce swollen hands, and toes like frost have been reported in COVID-19 patients.
It is known that patients with chronic obstructive pulmonary disease (COPD) are more likely to develop pneumonia as a result of COVID-19 infection. Furthermore, according to accumulating evidence, high-risk patients with critical COVID-19 are more frequently older (>65 years of age), male, obese, smokers, and have common comorbidities, such as hypertension (57%), obesity (42%), and diabetes (34%) [5]. Thus, some cardiologists speculated that a large number of acute coronary syndrome (ACS) patients might be at an increased risk of mortality from COVID-19, because these risk factors were thought to coincide with traditional coronary risk factors of atherosclerotic diseases. However, as COVID-19 outbreak spread, a significant number of patients without ACS were struck with severe disease in a short period of time, and DIC has been observed in 71.4% of non-survivors [4]. Most of the medical doctors were at a loss to what type of SARS-CoV-2-infected patients should be considered at high risk of critical COVID-19. Recent evidence revealed that severe COVID-19 patients have cardiac arrhythmias, myocardial injury and heart failure, DIC, and pulmonary embolism [6]. Interestingly, these phenomena can be explained centrally with one concept: the vascular endothelial glycocalyx, an extracellular matrix bound to and encapsulating the endothelial cells lining the blood vessel wall.
In the current review, previously unrevealed key components in severe COVID-19 pathophysiology will be outlined, with the aim to accelerate related research for a diagnostic and therapeutic approach in the fight against COVID-19.
Specific features of SARS-CoV-2
SARS-CoV-2 binds to the transmembrane angiotensin-converting enzyme 2 (ACE2) protein to enter type II alveolar epithelial cells, macrophages, and other cell types [7]. The spike protein of SARS-CoV-2 is primed by transmembrane protease, serine-2 (TMPRSS2). The primary symptoms of COVID-19 are cough (67.8%), diarrhea (3.8%), and fever (total 88.7% during hospitalization) [3], which might provide possible routes of infection via the respiratory tract and intestines, as the entry for SARS-CoV-2.
ACE2 is also present on vascular endothelial cells and arterial smooth muscle cells in all organs [8]. As a result, SARS-CoV-2 can directly adhere to vascular endothelial cells, and induce vascular endothelial dysfunction, followed by microvascular leakage, microvascular coagulation, excessive release of inflammatory cytokines, and disruption of cell-cell contact.
The most notable features in COVID-19 infection are asymptomatic pneumonia detected by chest X-ray or computed tomography (CT). Furthermore, multiple ground glass patchy shadows are common radiological findings in SARS-CoV-2 infected patients with mild symptoms. The difficulty in perceiving signs of worsening COVID-19 may make it difficult to notice the rapid deterioration in the condition of patients, which may also increase the number of sudden deaths before hospitalization. It is known that blood oxygen saturation (SpO2) is decreased in COVID-19 patients before symptoms such as shortness of breath and dyspnea; however, an increase in respiratory rate is observed just before the decrease in SpO2. Therefore, if the respiratory rate is 20 times/min or more, patients should be carefully observed for worsening respiratory conditions.
SARS-CoV-2 has a long viral spreading time (median, 20.0 days; interquartile range, 17.0–24.0 days) in survivors [9]; indeed, the longest period of virus excretion was 37 days. Furthermore, in non-survivors SARS-CoV-2 could be detected up until their death [9]. This long viral excretion has contributed significantly to the rapid spread of the disease, and provides the rationale for further isolation of infected patients and optimal antiviral therapeutic strategies.
Life-threatening complications in COVID-19
In Zhongnan Hospital of Wuhan University in Wuhan, China, of 138 hospitalized patients with SARS-CoV-2-infected pneumonia, 36 patients (26.1%) were transferred to the intensive care unit (ICU) because of complications, including ARDS (61.1%), arrhythmia (44.4%), and shock (30.6%) [10]. Compared to the 102 patients not treated in the ICU, patients treated in the ICU were much older and were more likely to have comorbidities [10].
Indeed, it is now understood that the mortality associated with the COVID-19 outbreak is increased by the presence of comorbidities, including hypertension, diabetes, COPD, and cardiovascular disease. Furthermore, elderly individuals (>65 years of age) and smokers have been shown to have significantly worse outcomes. Interestingly, these comorbidities and risk factors are consistent with the pathophysiology that causes damage to vascular endothelial glycocalyx [11], a negative charged brush-like monolayer of endothelial cells [12].
The generation of a cytokine storm induces organ damage, followed by edema, air exchange dysfunction, ARDS, acute cardiac injury, and secondary infection, all of which may lead to death. The presence of a cytokine storm is an important factor that leads to the exacerbation of COVID-19 or even death [13]. Therefore, avoidance of a cytokine storm may be the key to the treatment of COVID-19 patients [14].
Coagulation disorders occur in patients infected with COVID-19, SARS-CoV-1, and MERS-CoV [2]. Regarding COVID-19, DIC has been observed in 71.4% of non-survivors [4]. In line with this, D-dimer levels of 2.0 μg/mL or more (4-fold increase) on admission can predict in-hospital mortality in patients with COVID-19, which indicates that D-dimer could be an early marker to improve the management and stratification of COVID-19 patients [15]. Furthermore, dysregulation of the coagulation cascade and the subsequent formation of intra-alveolar or systemic fibrin clots are prominent findings in coronavirus infections associated with severe respiratory disease. In addition, microvascular endothelial failure and peripheral thrombosis may induce frost-like swollen hands and toes. Therefore, severe COVID-19 patients should be given treatments for coagulation disorders in order to prevent multiple organ failure [2].
COVID-19 and vascular endothelial dysfunction
Underlying cardiovascular disease is associated with an increased risk of in-hospital death among patients hospitalized with COVID-19 [16]. According to a previous report of COVID-19 in Wuhan, 48% of patients had comorbidities, including hypertension (39%), diabetes (19%), and coronary heart disease (8%) [9]. Furthermore, patients with preexisting coronary risk factors and cardiovascular disease had the highest mortality rates (10.5%) following infection with SARS-CoV-2 [17]. Data have shown that SARS-CoV-2-infected patients ≥60 years old have more systemic symptoms and more severe pneumonia than patients aged ≤60 years [18]. In support of this, multivariable regression analysis showed increased odds of in-hospital death with old age [9]. It appears that COVID-19 is more likely to deteriorate due to an increased in comorbidities in elderly, which may lead to immune dysfunction in elderly COVID-19 patients. In other words, microvascular leakage, which acts as a window for SARS-CoV-2 organ invasion, is caused by more advanced vascular endothelial glycocalyx damage in elderly patients [19]. In addition, the vascular endothelial glycocalyx is more easily damaged in elderly people than young, and common comorbidities are known to perturbate the vascular endothelial glycocalyx [20]. The vascular endothelial glycocalyx is systemically damaged under the conditions of old age and multiple comorbidities, which may be a potent mechanism for the development of lethal complications in COVID-19 patients.
Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and SARS-CoV-2 bind to ACE2, which is abundantly present not only on human epithelia of the lung and the small intestine, but also on vascular endothelial cells, arterial smooth muscle cells, cardiomyocytes, cardiofibroblasts, and coronary endothelial cells [21]. The exploitation of ACE2 by coronavirus may impair the renin-angiotensin-aldosterone system (RAAS). Furthermore, ACE2 is highly expressed on failing human hearts and pericytes, which could lead to the development of microvascular dysfunction [22], and explain the greater propensity for ACS [23,24]. Therefore, careful attention must be paid to the exacerbation of COVID-19 in patients with highly expressed ACE2. On the contrary, in severe COVID-19 patients, it is necessary to pay attention to cardiovascular diseases, such as microvascular endothelial dysfunction [Fig. 1], the onset and exacerbation of heart failure, and the onset of ACS.
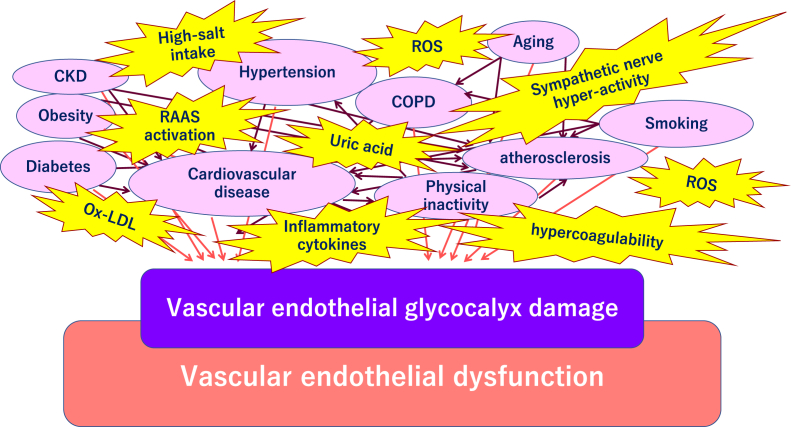
Fig. 1.
Comorbidities related to worsening of COVID-19 and vascular endothelial glycocalyx damage. The vascular endothelial glycocalyx is impaired due to factors such as smoking, physical inactivity, hypertension, diabetes, obesity, and cardiovascular diseases. Severe acute respiratory coronavirus 2 (SARS-CoV-2) can easily infected the increased endothelial glycocalyx-damaged microvasculature that is observed to a greater extent in elderly people compared to young people, and in males more than females. Abbreviations used: ARDS: Acute respiratory distress syndrome; DIC: Disseminated intravascular coagulation; CKD: Chronic kidney disease; ROS: Reactive oxygen species; RAAS: Renin-angiotensin-aldosterone system; COPD: Chronic obstructive pulmonary disease.
ACE2 is a potent cardioprotective and counterregulatory enzyme that degrades angiotensin II to angiotensin-(1–7), thereby attenuating its effects on vasoconstriction, sodium retention, and fibrosis [25]. MasR, an endogenous receptor of angiotensin-(1–7), has emerged as a physiological antagonist that counter-regulates RAAS activation via the ACE2/angiotensin-(1–7)/MasR axis. The angiotensin II/AT1 receptor is critically involved in disease progression leading to non-ischemic, ischemic, and diabetic cardiomyopathy, and to obesity-associated cardiac dysfunction. ACE2 shifts the balance to the cardioprotective ACE2/angiotensin-(1–7)/MasR axis through converting angiotensin II to angiotensin-(1–7) [21]. In a recent paper that reported on 8910 patients with COVID-19, no harmful association of ACE inhibitors or angiotensin-receptor blockers with in-hospital death was confirmed [16].
Vascular endothelial glycocalyx
Glycocalyx is defined as a thick mixture of protein lipids and post-translational sugar structures, which surround all living cells and act as a buffer between the cell and the extracellular matrix [26]. The monolayer of vascular endothelial cells constitutes the inner cellular lining of vasculatures such as arteries, veins, and capillaries. The luminal layer is in direct contact with blood as a vascular protective barrier between blood and organs. The vascular endothelial glycocalyx on the luminal surface of all endothelial cells plays an essential role to regulate coagulation, inflammation, trans-capillary flux, and microvascular permeability [27,28]. The glycocalyx is a complex gel-like layer of sialic acid-containing glycoproteins, membrane-bound proteoglycans (e.g., syndecans and glypicans), and glycosaminoglycan side chains (e.g., heparin sulfate and chondroitin sulfate), and long chains of hyaluronan (HA) [29,30]. The vascular endothelial glycocalyx is stabilized by shear stress [31], which is pivotal for proper nitric oxide (NO) production [32,33]. Glycosaminoglycans are constantly degraded through enzymes, and also synthesized and extruded through vesicles of the Golgi apparatus to maintain homeostatic balance [34]. As shown in Fig. 2, homeostasis is broken down, and vascular endothelial glycocalyx shedding/degradation occurs in conditions of cellular stress, ischemia/reperfusion injury [35], the presence of endotoxins [36], inflammatory mediators [37], atrial natriuretic peptide, excessive reactive oxygen species [38], hyperglycemia [39,40], high-salt intake [41], hypertension [42], familial hypercholesterolemia [43], and oxidized low-density lipoprotein (ox-LDL) [44]. Of note, rosuvastatin administration has been shown to partially restore damaged vascular endothelial cells in patients with heterozygous familial hypercholesterolemia [43]. Moreover, unfavorable lifestyle, including smoking and physical inactivity, also induces glycocalyx degradation. Indeed, it has been previously shown that a smoking cessation program using varenicline or nicotine replacement therapy for 3 months resulted in a decrease of carbon monoxide (CO), oxidative stress, arterial stiffness, and restored the endothelial glycocalyx [45]. Physical inactivity induces systemic low shear stress in the body, and it has been demonstrated that AMP-activated protein kinase regulates glycocalyx impairment due to hyaluronan degradation and macrophage recruitment in response to low shear stress in a mice common carotid artery ligation model [46]. Moreover, the vascular endothelial glycocalyx is perturbed by various unfavorable disease conditions, including dehydration, acute infectious disease [47], trauma [48], sepsis [49], ARDS [50], preeclampsia [51], gestational diabetes mellitus [52], and chronic disease conditions, such as hypertension, diabetes [19,53], chronic kidney disease [54], atherosclerosis [[55], [56], [57], [58], [59], [60]], stroke [61,62], dementia [63], microvascular angina [64], ACS [65], and heart failure [66]. In ApoE knockout mice, an inhibitor of hyaluronan synthesis, 4-metylumbelliferone (4-MU) has been shown to interfere with the protective function of the endothelial glycocalyx, thereby facilitating leukocyte adhesion, subsequent inflammation, and progression of atherosclerosis [56].
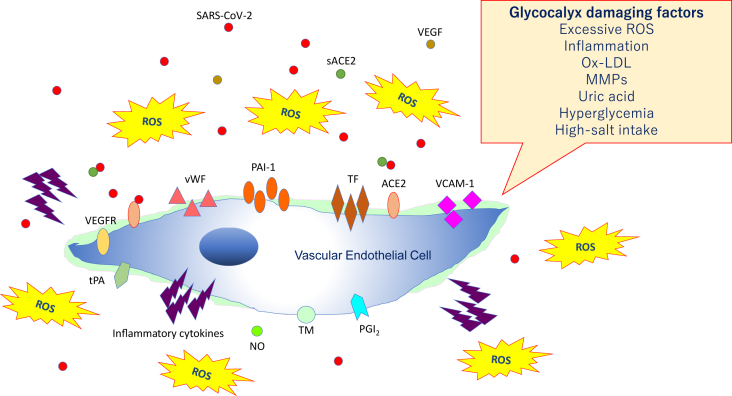
Fig. 2.
Damaged vascular endothelial glycocalyx. Vascular endothelial glycocalyx damage is associated with vascular endothelial dysfunction, which leads to reduced nitric oxide (NO) bioavailability, excessive reactive oxygen species (ROS) production, inflammatory cytokine release, platelet adherence, coagulation, and leukocyte adhesion. Abbreviations used: SARS-CoV-2: Severe acute respiratory coronavirus 2; VEGF: Vascular endothelial growth factor; VEGFR: VEGF receptor; ACE2: Angiotensin-converting enzyme 2, sACE2: Soluble ACE2; PAI-1: Plasminogen activator inhibitor-1; TF: Tissue factor; vWF: von Willebrand factor; ox-LDL: Oxidized low-density lipoprotein; MMPs: Matrix metalloproteases; tPA: Tissue plasminogen activator; PGI2: Prostacyclin; TM: Thrombomodulin.
The vascular endothelial glycocalyx is crucial to endothelial function [67], as it is involved in microvascular reactivity, and modulates the interaction between the endothelium and blood constituents [68]. In addition, the vascular endothelial glycocalyx protects endothelial cells from shear stress caused by blood flow, and serves as a vascular permeability barrier [69]. As shown in Fig. 3, the intact vascular endothelial glycocalyx harbors various cytokines and chemokines, receptors, growth factors, gap junction proteins, and enzymes, including extracellular superoxide dismutase (ecSOD), endothelial nitric oxide synthase (eNOS), ACEs, lipoprotein lipase, xanthine oxidase, and antithrombin III, all of which play a central role in endothelial function and blood/microvascular/tissue interactions [68]. Vascular endothelial dysfunction and vascular failure occur in situations where the endothelial glycocalyx is impaired, which has roles in the development of various cardiovascular diseases [70,71].
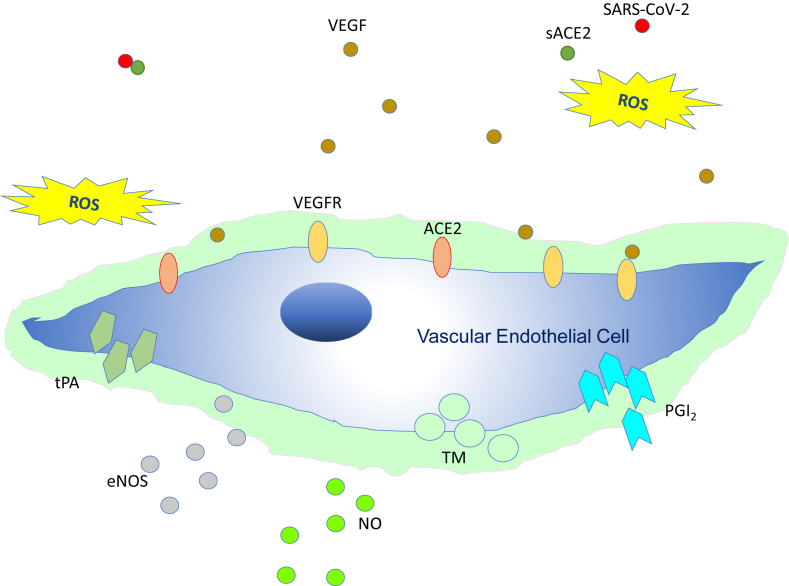
Fig. 3.
Intact vascular endothelial glycocalyx. In a situation where vascular endothelial cells are sufficiently covered with healthy vascular endothelial glycocalyx, even if severe acute respiratory coronavirus 2 (SARS-CoV-2) enters the body, it may be neutralized by the effects of appropriate reactive oxygen species (ROS) and soluble angiotensin-converting enzyme 2 (sACE2); consequently, it may be possible to prevent entry of the virus into the vascular endothelium. Abbreviations used: VEGF: Vascular endothelial growth factor; VEGFR: VEGF receptor; NO: Nitric oxide; eNOS: Endothelial NO synthase; TM: Thrombomodulin; tPA: Tissue plasminogen activator; PGI2: Prostacyclin.
The vascular endothelial glycocalyx has the potential to not only function as a physical cytoprotective barrier for vascular endothelial cells, but also as a mechanism to regulate intracellular cell signaling. IQGAP1, an essential scaffolding protein that binds to vascular endothelial growth factor (VEGF) receptor-2 [72], has roles in many different aspects of cell physiology and interacts with numerous proteins [73]. IQGAP1 modulates the actin cytoskeleton through Rac1 and Cdc42, while cell-cell adhesion through VE-cadherin and β-catenin regulates the mitogen-activated protein kinase pathway and forms a complex with the hyaluronan receptor CD44 to regulate cell migration and proliferation [74]. In vascular endothelial cells, IQGAP1 induces angiogenesis through binding to VEGF receptor-2 and VE-cadherin containing adherens junctions in a ROS-dependent manner [75]. IQGAP is required for the establishment of cell-cell contact, and is presumably necessary to collaborate with the vascular endothelial glycocalyx.
Virus infectious disease and vascular endothelial glycocalyx
Among viral infectious diseases, research on the relationship between dengue fever and vascular endothelial glycocalyx is progressing. Dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) is characterized by vascular leakage and shock. The dengue virus nonstructural protein 1 (NS1) is the only membrane-associated protein that anchors its replication complex to the cellular membrane. Increased circulating levels of vascular endothelial glycocalyx layer components, such as hyaluronic acid, heparin sulfate, claudin-5, and syndecan-1, have been associated with disruption of the vascular endothelial glycocalyx, and the subsequent development of plasma leakage and severe dengue disease [76,77]. Sialic acid has been established as an important determinant of endothelial barrier function in both in vitro and in vivo studies [78,79]. This evidence emphasizes the importance of evaluation and therapy targeted to vascular endothelial glycocalyx in severe conditions induced by viral infections, potentially including COVID-19.
Severe inflammation induces vascular endothelial glycocalyx dysfunction
The vascular endothelial glycocalyx maintains homeostasis of the vasculature, including the control of vascular permeability and microvascular tone, prevention of microvascular thrombosis, and regulation of leukocyte adhesion [80]. During sepsis, the glycocalyx is degraded via inflammatory factors, such as metalloproteinases, heparinase, and hyaluronidase [81].
Systemic damage to the delicate layer of the vascular glycocalyx results in increased protein and water transit to the extra-vascular space. In septic conditions, the vascular endothelial glycocalyx is perturbated and the layer becomes thinner, which induces microvascular excessive permeability and contributes to interstitial edema in various organs [81,82].
The systemic breakdown of the glycocalyx occurs dramatically in fatal disease conditions, such as severe infectious diseases, sepsis, hemorrhagic shock, burn, traumatic brain injury [83], and traumatic endotheliopathy, a syndrome associated with high mortality [84]. Fig. 4 shows a schematic image of severe COVID-19 comorbidity induced by vascular endothelial glycocalyx damage. Patients with underlying diseases have systemic endothelial glycocalyx disorders due to complicated mechanisms. Once these patients are infected with SARS-CoV-2, COVID-19-induced systemic vascular inflammatory endotheliopathy is more likely to develop serious complications such as ARDS, DIC, Kawasaki disease shock syndrome, microvascular thrombosis, and arrhythmias.
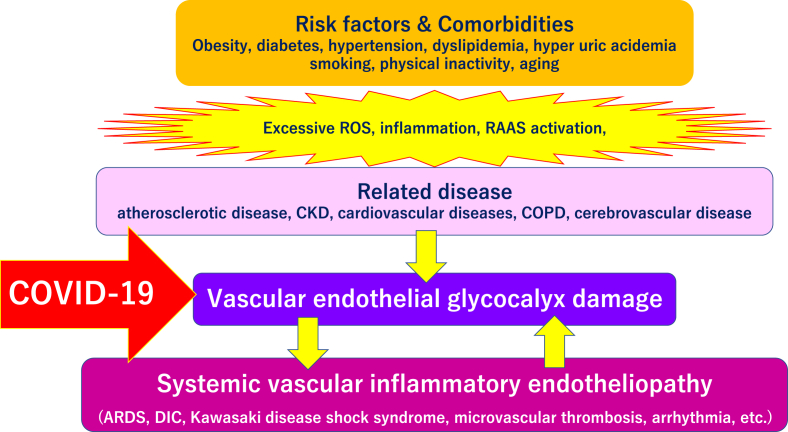
Fig. 4.
Severe COVID-19 comorbidity induced by vascular endothelial glycocalyx damage. The vascular endothelial glycocalyx can be damaged by various factors, including smoking, physical inactivity, hypertension, diabetes, obesity, and cardiovascular diseases. Various lethal conditions in COVID-19 (e.g., acute respiratory distress syndrome [ARDS], disseminated intravascular coagulation [DIC], Kawasaki disease, microvascular thrombosis, and arrhythmias) may be caused by a common mechanism, damage of the vascular endothelial glycocalyx. Abbreviations used: CKD: Chronic kidney disease; ROS: Reactive oxygen species; RAAS: Renin-angiotensin aldosterone system; COPD: Chronic obstructive pulmonary disease.
Arrhythmia and sudden death following vascular endothelial glycocalyx damage
In acute cytokine storm models utilized to examine systemic inflammatory response syndrome (SIRS), intravenous injection of proinflammatory cytokines, including interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF), induces vascular hyperpermeability. It has been suggested that the inhibition of connexin43 (Cx43) hemichannels could counteract TNF-induced SIRS-associated vascular permeability and lethality in mice [85]. Cx43 is an important cardiac gap junction protein, and excessive opening of Cx43 hemichannels are observed in ischemic or inflammatory conditions [86,87]. Inhibition of Cx43 protects against vascular leakage, hypothermia, and mortality in a TNF-induced SIRS mouse model. Furthermore, altered Cx43 expression produces an arrhythmia substrate in the heart, which contributes to the arrhythmias of sudden cardiac death [88]. Interestingly, vascular endothelial glycocalyx degradation disrupts endothelial Cx43 proteins, likely blocking the inter-endothelial molecular transport that maintains endothelial cell and vascular tissue homeostasis to resist disease [89]. Endothelial glycocalyx damage in sepsis induced by severe viral infections like COVID-19 may occur as a result of a change in Cx43 expression in the microvasculature and the heart. SAR-CoV-2 binds to ACE2, which is also abundantly expressed in cardiomyocytes, cardiofibroblasts, and coronary endothelial cells [21]; consequently, the virus has the potential to induce lethal arrhythmia in COVID-19 patients via damaging Cx43 in these cell types. Examination of the blood electrolyte levels is important to predict lethal arrhythmias, because COVID-19 patients tend to have hyponatremia or abnormalities of other electrolytes. Furthermore, all COVID-19 patients should receive an electrocardiogram to check for iatrogenic QT prolongation or other arrhythmias. Because many antiviral drugs can cause cardiac arrhythmia or other cardiovascular disorders, the presence of cardiac toxicity must be closely monitored [90].
Kawasaki disease shock syndrome and vascular endothelial glycocalyx
Kawasaki disease is an acute febrile systemic vasculitis that predominantly occurs in children below 5 years of age. Systemic vasculitis is particularly observed in small- and medium-sized arteries. Because Kawasaki disease shows seasonal, temporal, and regional patterns, an infectious agent is thought to cause or trigger the disease presentation [91]. According to a previous serological test, the development of Kawasaki disease is involved in human coronavirus (HCoV)-229E infection [92]. Although its exact etiopathogenesis is unclear, it is thought to be a complex interplay of genetic factors, infections, and immunity [93]. Though self-limiting in many cases, Kawasaki disease can lead to severe complications, such as coronary artery aneurysms and thrombo-embolic occlusions; thus, early diagnosis and urgent attention tis required to avoid these complications. The presence of coronary aneurysms was significantly and positively correlated with male sex, IVIG resistance, higher neutrophil/lymphocyte ratio, inotrope treatment, cardiac failure, abdominal pain, and neurological symptoms [94].
In both Kawasaki disease and COVID-19, some clinical symptoms such as fever, rash, and eye redness (conjunctival injection) are present in many infected children. The first case of Kawasaki disease with concurrent COVID-19 was reported in April 2020 [95], since then, between April 29 and May 3, 2020, 15 cases were reported by the New York City Health Department, and 64 cases statewide were reported from the New York State Department of Health. Furthermore, an uptick in Kawasaki disease or Kawasaki-like disease was established among children coincident with the COVID-19 outbreaks in the U.K., Italy, and Spain.
Kawasaki disease shock syndrome, a severe subtype of Kawasaki disease, is a RARE complication of Kawasaki disease that can lead to significant sequelae and poor outcome [96]. According to a previous report of 187 consecutive patients with Kawasaki disease, 13 (7%) met the definition for Kawasaki disease shock syndrome [96]. Furthermore, Kawasaki disease shock syndrome has been shown to be characteristic of more severe inflammatory cytokine production, and a tendency to develop IVIG non-responsiveness and coronary abnormalities [97]. Experts have indicate that there may be a small increase in the numbers of children with severe COVID-19 and features consistent with toxic shock syndrome (abdominal pain and gastrointestinal symptoms), which appear to be similar to those of Kawasaki disease shock syndrome.
Surprisingly, circulating endothelial glycocalyx components (syndecan-1 and hyaluronan) were significantly elevated at the acute phase, and serum hyaluronan was determined as the biomarker that is the best predictor of future development of coronary artery lesions in Kawasaki disease [98]. Serum levels of soluble syndecan-1 (sCD138), one of the major core proteins expressed on the vascular endothelial glycocalyx, is considered to reflect vascular endothelial damage and inflammation in Kawasaki disease [99]. Considering the common pathophysiology between Kawasaki disease and COVID-19, it is expected that the knowledge on vascular endothelial glycocalyx-related Kawasaki disease can be applied to research on new therapeutic strategies and biomarkers for predicting deterioration in patients with severe COVID-19.
Vascular endothelial glycocalyx dysfunction induces a severe phenotype in disease
COVID-19 can induce severe septic shock, and sepsis can easily magnitude systemic degradation of the vascular endothelial glycocalyx. Vascular endothelial glycocalyx dysfunction contributes to septic-induced vascular endothelial cell damage leading to altered microvascular permeability. Therefore, vascular endothelial glycocalyx may have a key regulatory role in maintaining the pulmonary vascular barrier and its homeostasis [100]. The recent findings on vascular endothelial glycocalyx are outlined below, in the context of COVID-19-related complications.
-
(1)
Septic shock
Degradation of vascular endothelial glycocalyx represents one of the earliest and most significant sites of injury during sepsis [101]. In mice models, the total volume of vascular endothelial glycocalyx has been shown to be drastically reduced in sepsis. Excessive ROS and proinflammatory cytokines, such as TNF-α and IL-1β, are considered the main actors in endothelial glycocalyx degradation in sepsis [102]. Both mechanisms activate the sheddases heparinase and matrix metalloproteases (MMPs). Furthermore, a thinner and sparser endothelial glycocalyx is associated with vascular permeability and resulting edema, hypovolemia, vasodilation troubles, leukocyte attraction, platelet aggregation, and lung injury [81,103]. The increase in pulmonary vascular permeability as a result of sepsis, manifests acute lung injury and ARDS [104].
-
(2)
Acute respiratory distress syndrome (ARDS)
The main features of ARDS are lung endothelial cell injury, severe inflammatory responses, neutrophil adhesion or infiltration, and interstitial edema. Vascular endothelial glycocalyx and inflammatory responses are crucial for the pathogenesis of ARDS [105]. Pulmonary edema associated with albumin leakage is closely related to degradation of the endothelial glycocalyx [100]. The endothelial glycocalyx not only acts as a physical barrier to prevent albumin exudation, but also as signaling molecules to participate in hemodynamics [100,[106], [107], [108], [109]].
-
(3)
Microvascular thrombosis/DIC
The vascular endothelial glycocalyx is an important regulator of microvascular permeability preventive thrombus formation [110]. Coagulation disorders occur in coronavirus infected patients with COVID-19, SARS-CoV-1, and MERS-CoV [2]. Moreover, DIC has been observed in 71.4% of non-survivors of COVID-19 [4]. Recently the International Society on Thrombosis and Hemostasis (ISTH) DIC Scientific Standardization Committee has proposed a new category termed “sepsis-induced coagulopathy (SIC)” to facilitate earlier diagnosis of DIC, which is hoped to lead to more rapid interventions in these critically ill patients [111].
-
(4)
Multiple organ failure
Systemic ischemia occurs in various life-threatening clinical settings, including cardiac arrest, hemorrhagic shock during trauma, or ST-elevation myocardial infarction complicated by cardiogenic shock [112,113]. During these situations, distortion of the glycocalyx structure and function contributes to the multiorgan dysfunction that follows global ischemia of various etiologies, such as renal, cardiac, pulmonary, and hepatic ischemia/reperfusion injuries [68,114]. Damage of the vascular endothelial glycocalyx in these ischemia/reperfusion injuries is largely mediated by ROS, specifically through the activation of endothelial NADPH oxidase 2 (NOX2) and xanthine oxidase that are bound to glycosaminoglycans anchored at the endothelial surface layer [115,116].
Remaining question: could vascular endothelial glycocalyx damage influence the sex difference in COVID-19?
It has been reported that there is a clear sex difference in severe COVID-19 and the rate of in-hospital mortality. The relationship between COVID-19 severity and male hormones, or the possibility that male smokers are included to a greater extent in these studies has been investigated, although there remain no definitive conclusions. We propose that sex differences in the vascular endothelial glycocalyx could represent a crucial factor for the sex difference of COVID-19 severity and mortality.
In ACS patients, it has been reported that males shed more syndecan-1 than females [65]. Circulating levels of syndecan-4 have been associated with incident myocardial infarction, and the association is stronger in women than in men [117]. These data imply either an increase in the amount of glycocalyx, a denser glycocalyx, or higher protease activity in male endothelial cells [118]. Therefore, the mechanisms underlying sex the differences in atherosclerosis progression and ischemic cardiovascular disease may be explained by the sex difference of the vascular endothelial glycocalyx.
Possible therapeutic targets on COVID-19 associated with the vascular endothelial glycocalyx
Most of the patients in China receive antiviral therapy such as ribavirin, lopinavir/ritonavir, and remdesivir [9,119,120]. Clinical trials using ivermectin, avigan, and remdesivir are ongoing worldwide to clarify their effectiveness on COVID-19. Furthermore, more recent clinical trials have tested the efficacy of inhibition of TMPRSS2 by camostat mesylate, the recombinant form of human soluble ACE2 [121], monoclonal antibodies against IL-6 receptor, and interferon-α 2b for the treatment of patients with COVID-19. Convalescent plasma transfusion has also been reported to be beneficial in the treatment of critically ill patients with COVID-19 [122,123].
Anticoagulant therapy resulted in lower mortality in patients with sepsis-induced coagulopathy, as well as lower mortality in COVID-19 patients with increased levels of D-dimer. However, there were no overall benefits for patients following the administration of low molecular weight heparin for at least 7 days [124]. Thus, hypothesis-driven studies based on the knowledge of the molecular details of virus–cell interaction are still crucial for the identification of therapeutic targets to treat COVID-19 [125].
Degradation of the vascular endothelial glycocalyx significantly increased endothelial cell uptake of nanoparticle vehicles designed for drug delivery compared to the intact glycocalyx [126]. Ultra-small gold nanospheres coated with polyethylene glycol were successfully delivered intravenously in the glycocalyx degradation mouse model [127]. These lines of evidence suggest that vascular endothelial glycocalyx dysfunction induced by SARS-CoV-2 may be targeted for enhanced drug delivery, offering a new therapeutic approach for COVID-19. In particular, the possibility of a therapeutic approach focusing on vascular endothelial glycocalyx is explored in this section as follows:
-
(1)
A disintegrin and metalloprotease 17 (ADAM17)
ADAM17 was initially described to specifically cleave the precursor of TNF-α (pro-TNF-α) [128]. ADAM17 activity is induced in sepsis, and leads to shedding of components of leukocytes and endothelial cell tether machinery, facilitating systemic inflammation [129]. It is already known that ADAM17 can release the ectodomains of a diverse variety of membrane-anchored cytokines, cell adhesion molecules, receptors, ligands, and enzymes. Since ADAM17 leads to shedding of membrane-bound ACE2 and release of the soluble extracellular domain of ACE2 [130], ADAM17 and other proteases to do ACE2 shedding are expected to be valid as treatments for patients with COVID-19 [131]. Of relevance, ADAM17 is co-expressed with syndecan-1 and has been shown to mediate syndecan-1 shedding in lung epithelial cells, which may aggravate endothelial glycocalyx disorders [132,133]. Thus, careful consideration should be given to an ADAM17-related therapy, which is expected to shed membrane-bound ACE2, for COVID-19 patients.
-
(2)
Glycocalyx administration
Vascular endothelial glycocalyx has cardiovascular protective effects. Since it has been shown to protect against myocardial edema in a rat model [134], investigators have expected that intravenous administration of glycocalyx may improve damage to the vascular endothelial glycocalyx [135]. Restoring the vascular endothelial glycocalyx by infusion of the combination of hyaluronan and chondroitin sulfate was confirmed in an animal model [136]. The effectiveness of administration of glycocalyx to restore the vascular endothelial glycocalyx was examined using hyaluronan and chondroitin sulfate [137]. However, a similar effect has not yet been confirmed in COVID-19 patients.
-
(3)
Inhibitors of glycocalyx sheddase
Heparanase inhibitor: A protein heparinase inhibitor, PG545 plays a deleterious role in the development of renal injury and kidney dysfunction, attesting heparinase inhibition as a therapeutic approach for acute kidney disease [138,139].
MMP inhibitors: Matrix metalloprotease (MMP) inhibitors have both pro-adhesion effects, by reducing sheddase activity, and anti-adhesion effects by inhibiting glycocalyx shedding and subsequent exposure of adhesion molecules on the endothelial cell surface [140].
Sulodexide: A heparin sulfate-like compound resistant to degradation by heparase, sulodexide can accelerate endothelial glycocalyx regeneration in vitro and in vivo. Type 2 diabetes is associated with glycocalyx perturbation and increased vascular permeability, which are partially restored following sulodexide administration in these patients [53].
-
(4)
Anti-inflammatory mediators
Numerous anti-inflammatory mediators, such as TNF-α or its receptor inhibitor (etanercept) [37], allopurinol [38], sphingosine-1 phosphate (S1P) [89], and hydrocortisone, have been shown to have protective roles on the vascular endothelial glycocalyx [141]. Since these substances are expected to have anti-inflammatory and anti-oxidative effects, which impair vascular endothelial glycocalyx, they affects not only vascular endothelial cells but also vascular endothelial glycocalyx composition.
The simplest way to achieve protection of the endothelial glycocalyx is to maintain a sufficiently high concentration of plasma proteins [20]. Indeed the early and empiric use of fresh frozen plasma in hemodynamically unstable patients with bleeding has led to a decrease in early hemorrhagic deaths [144,145]. Endothelial dysfunction not only leads to coagulation abnormalities, but also to inflammation and the breakdown of organ-specific endothelial and epithelial barrier integrity [146]. Fresh frozen plasma reduced lung inflammation and injury in a rodent model of hemorrhagic shock that was correlated with restitution of syndecan-1 [147]. Together, these observations suggest that after hemorrhagic shock, TNF-α induces syndecan-1 shedding in an ADAM17-dependent manner, which is inhibited by fresh frozen plasma [146].
-
(6)
Stem cell therapy
Cell-based approaches primarily using mesenchymal stem cells, have demonstrated safety and possible efficacy in patients with ARDS [148]. Intravenous administration of clinical-grade human mesenchymal cells into patients with COPD-19 was also shown to improve functional outcomes [149].
-
(7)
Antioxidant
Shedding of the vascular endothelial glycocalyx is triggered by redox stress encountered during reperfusion, and therefore, should be alleviated by the radical scavenger NO. The cardioprotective effect of NO in post-ischemic reperfusion includes the prevention of coronary vascular leak and interstitial edema, as well as a tendency to forestall both no-reflow and degradation of the endothelial glycocalyx [150]. In theory, antioxidants seem to be a therapeutic option for COVID-19; however, the results of various large-scale clinical trials to date suggest that this would be difficult to induce. The reason being that many antioxidants lose their effectiveness immediately after administration, and may affect the redox regulatory control necessary to maintain homeostasis.
-
(8)
Ivermectin
Ivermectin, an FDA-approved anti-parasitic previously shown to have broad-spectrum antiviral activity in vitro, inhibits the replication of SARS-CoV-2 in vitro [151]. The previous study revealed that ivermectin is a specific inhibitor of importin α/β-mediated nuclear importable to inhibit replication of HIV and dengue virus [152]. It has already reported that ivermectin can improve the prognosis of patients with COVID-19, and ivermectin is currently considered to be one of the drugs with the highest potential.
-
(9)
Antithrombin III
Antithrombin III is a physiological inhibitor of serine proteases (e.g., thrombin, elastase) [153], which inhibits coagulation abnormalities and reduces inflammatory responses [154]. The combination of antithrombin III and hydrocortisone has also been reported to be effective. However, randomized control trials of Antithrombin III are not sufficient, and further trials with prespecified inclusion criteria and good bias protection is needed [154].
-
(10)
Sevoflurane
Sevoflurane is a modulator of the inflammatory response triggered by ischemia-reperfusion lung injury [155,156]. Sevoflurane protects the lung endothelial glycocalyx in an in vivo lung auto-transplant model in pigs, and reduces the expression of leukocyte on the vessels [157]. These data may explain the beneficial outcomes linked to clinical use of volatile anesthetics after ischemia-reperfusion.
-
(11)
Intravenous immunoglobulin (IVIG)
IVIG is the standard treatment for Kawasaki disease. IVIG should be started within 7 days from the onset of fever in high suspicious patients for Kawasaki disease with COVID-19 [158], because coronary artery aneurysms could occur in up to 25% of children with Kawasaki disease without timely treatment [159]. IVIG is used to neutralize bacterial super-antigens and other infectious agents, inhibit the production of proinflammatory cytokines, neutralize pathogenic autoantibodies, enhance regulatory T cells (as well as inhibit other T cells), inhibit differentiation of Th17 cells, and reduce excessive ROS [93]. However, IVIG is ineffective in approximately 15% of children with Kawasaki disease, and insufficient control of monocyte suppression and T-cell activation, especially in terms of the CD8-T cells, are associated with IVIG resistance [160]. Given the pathological treatment of Kawasaki disease, IVIG treatment should be considered as an effective therapeutic option for severe COVID-19.
-
(12)
Tranexamic acid
The serine protease inhibitor, tranexamic acid, may prevent degradation of the glycocalyx. The effect of tranexamic acid administration on stress-related vascular endothelial glycocalyx damage has been examined in human umbilical vein endothelial cells (HUVECs) [161], in which it was shown to prevent vascular endothelial glycocalyx degradation via inhibition of endothelial sheddase activation of ADMA17 and MMP-9 in vitro.
-
(13)
Antihyperglycemic agents
Empagliflozin has been reported to restore the integrity of the endothelial glycocalyx in cultured human abdominal aortic endothelial cells treated with heparinase III-mediated glycocalyx disruption [162]. Although Empagliflozin is known to reduce cardiovascular events, the mechanism is still unclear. Therefore, a clinical study with the treatment of COVID-19 associated with vascular endothelial glycocalyx should be performed.
A traditional anti-diabetic drug, metformin, has been demonstrated to have a protective role in cardiovascular disease. In db/db mice, 2 weeks of metformin administration has been shown to improve obesity and diabetes-induced glycocalyx damage and hydration of the heart and kidney [163].
Summary and future outlook
To summarize, the vascular endothelial glycocalyx could explain the features of critical patients with COVID-19.
-
(1)
The vascular endothelial glycocalyx is perturbed by SARS-CoV-2 infection-induced inflammation, as well as in patients with hypertension, obesity, diabetes, cardiovascular disease, and who are current smokers.
-
(2)
SARS-CoV-2 can more easily infect the endothelial glycocalyx-damaged microvasculature in elderly people compared to young people, and in males mode than females.
-
(3)
Damage to the vascular endothelial glycocalyx leads to a rapid worsening of ARDS, microvascular thrombosis/DIC, Kawasaki disease shock syndrome, and may lead to arrhythmia and sudden death. Circulating levels of glycocalyx (e.g., syndecan-1 and hyaluronan) may be effective biomarkers to detect worsening signs earlier.
The composition of the vascular endothelial glycocalyx affects all aspect of severe COVID-19, including high risks of SARS-CoV-2 infection in the damaged endothelial glycocalyx, perturbed endothelial glycocalyx-induced microvascular leakage, thrombosis formation, excessive inflammatory cytokine release, leukocyte activation, platelet adhesion to the endothelium, and excessive ROS production [Fig. 2].
The COVID-19 pandemic has fundamentally changed our lives. Many cities are, or have been locked down, with people forced to stay at home and avoid contact with others. All unnecessary activities are encouraged to stop, and even educational and labor opportunities have been impacted by this infectious disease. We believe that the world will have to change as opposed to be restored to its previous state. Greater understanding of the virus will allow us to devise ways in which we can collectively survive the next “new-normal” era. Although the endothelial glycocalyx is a classical physical barrier common to many living creatures, this field has been poorly studies thus far. Given the international nature of the virus, we believe that it is necessary to share the latest knowledge from new research areas to offer the novel concept regarding the impact of vascular endothelial glycocalyx on COVID-19 to other researchers.
Conflicts of interest
Author has no support, financial or otherwise, has been received from any organization that may have an interest in the submitted work; and there are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgment
This work was partly supported by JSPS KAKENHI grant number JP19K11371.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Kazory A., Ronco C., McCullough P.A. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent) 2020;33:370–375. doi: 10.1080/08998280.2020.1754700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., China Medical Treatment Expert Group for C Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaoka-Tojo M. Cardiac rehabilitation-mediated molecular mechanisms of cardiovascular protection. Circ J. 2014;78:2624–2626. doi: 10.1253/circj.cj-14-1038. [DOI] [PubMed] [Google Scholar]
- 12.Cosgun Z.C., Fels B., Kusche-Vihrog K. Nanomechanics of the endothelial glycocalyx: from structure to function. Am J Pathol. 2020;190:732–741. doi: 10.1016/j.ajpath.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical Cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 15.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiology Working Group for Ncip Epidemic Response CCfDC and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groen B.B., Hamer H.M., Snijders T., van Kranenburg J., Frijns D., Vink H. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol. 2014;116:998–1005. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 20.Becker B.F., Chappell D., Bruegger D., Annecke T., Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–310. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 21.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bairey Merz C.N., Pepine C.J., Shimokawa H., Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116:856–870. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler P.J., Bhatnagar A. Mechanobiology of the abluminal glycocalyx. Biorheology. 2019;56:101–112. doi: 10.3233/BIR-190212. [DOI] [PubMed] [Google Scholar]
- 27.Betteridge K.B., Arkill K.P., Neal C.R., Harper S.J., Foster R.R., Satchell S.C. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595:5015–5035. doi: 10.1113/JP274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler M.J., Ramnath R., Kadoya H., Desposito D., Riquier-Brison A., Ferguson J.K. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019;95:94–107. doi: 10.1016/j.kint.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curry F.R. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 30.Curry F.E. Layer upon layer: the functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc Res. 2017;113:559–561. doi: 10.1093/cvr/cvx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda A., Shimomura M., Ikeda M., Yamaguchi R., Tanishita K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2287–H2294. doi: 10.1152/ajpheart.00808.2003. [DOI] [PubMed] [Google Scholar]
- 32.Thi M.M., Tarbell J.M., Weinbaum S., Spray D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo A., Dewey C.F., Jr., Garcia-Cardena G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol. 2013;304:C137–C146. doi: 10.1152/ajpcell.00187.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prydz K. Determinants of glycosaminoglycan (GAG) structure. Biomolecules. 2015;5:2003–2022. doi: 10.3390/biom5032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio-Gayosso I., Platts S.H., Duling B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290:H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwdorp M., Meuwese M.C., Vink H., Hoekstra J.B., Kastelein J.J., Stroes E.S. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol. 2005;16:507–511. doi: 10.1097/01.mol.0000181325.08926.9c. [DOI] [PubMed] [Google Scholar]
- 37.Nieuwdorp M., Meuwese M.C., Mooij H.L., van Lieshout M.H., Hayden A., Levi M. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Ko J., Kang H.J., Kim D.A., Kim M.J., Ryu E.S., Lee S. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019;33:13334–13345. doi: 10.1096/fj.201901148R. [DOI] [PubMed] [Google Scholar]
- 39.Zuurbier C.J., Demirci C., Koeman A., Vink H., Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]
- 40.Pahwa R., Nallasamy P., Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J Diabet Complicat. 2016;30:563–572. doi: 10.1016/j.jdiacomp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Rorije N.M.G., Rademaker E., Schrooten E.M., Wouda R.D., Homan Van Der Heide J.J., Van Den Born B.H. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: a randomized cross-over trial. J Hypertens. 2019;37:1254–1261. doi: 10.1097/HJH.0000000000002015. [DOI] [PubMed] [Google Scholar]
- 42.Kumase F., Morizane Y., Mohri S., Takasu I., Ohtsuka A., Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama. 2010;64:277–283. doi: 10.18926/AMO/40502. [DOI] [PubMed] [Google Scholar]
- 43.Meuwese M.C., Mooij H.L., Nieuwdorp M., van Lith B., Marck R., Vink H. Partial recovery of the endothelial glycocalyx upon rosuvastatin therapy in patients with heterozygous familial hypercholesterolemia. J Lipid Res. 2009;50:148–153. doi: 10.1194/jlr.P800025-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Vink H., Constantinescu A.A., Spaan J.A. Oxidized lipoproteins degrade the endothelial surface layer : implications for platelet-endothelial cell adhesion. Circulation. 2000;101:1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 45.Ikonomidis I., Marinou M., Vlastos D., Kourea K., Andreadou I., Liarakos N. Effects of varenicline and nicotine replacement therapy on arterial elasticity, endothelial glycocalyx and oxidative stress during a 3-month smoking cessation program. Atherosclerosis. 2017;262:123–130. doi: 10.1016/j.atherosclerosis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J., Kong X., Wang Z., Gao X., Ge Z., Gu Y. AMP-activated protein kinase regulates glycocalyx impairment and macrophage recruitment in response to low shear stress. FASEB J. 2019;33:7202–7212. doi: 10.1096/fj.201801869RRR. [DOI] [PubMed] [Google Scholar]
- 47.Ostrowski S.R., Gaini S., Pedersen C., Johansson P.I. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: an observational study. J Crit Care. 2015;30:90–96. doi: 10.1016/j.jcrc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Chignalia A.Z., Yetimakman F., Christiaans S.C., Unal S., Bayrakci B., Wagener B.M. The glycocalyx and trauma: a review. Shock. 2016;45:338–348. doi: 10.1097/SHK.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steppan J., Hofer S., Funke B., Brenner T., Henrich M., Martin E. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165:136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt E.P., Overdier K.H., Sun X., Lin L., Liu X., Yang Y. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weissgerber T.L., Garcia-Valencia O., Milic N.M., Codsi E., Cubro H., Nath M.C. Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long D.S., Hou W., Taylor R.S., McCowan L.M. Serum levels of endothelial glycocalyx constituents in women at 20 weeks' gestation who later develop gestational diabetes mellitus compared to matched controls: a pilot study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broekhuizen L.N., Lemkes B.A., Mooij H.L., Meuwese M.C., Verberne H., Holleman F. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padberg J.S., Wiesinger A., di Marco G.S., Reuter S., Grabner A., Kentrup D. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234:335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Voyvodic P.L., Min D., Liu R., Williams E., Chitalia V., Dunn A.K. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014;289:9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagy N., Freudenberger T., Melchior-Becker A., Rock K., Ter Braak M., Jastrow H. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122:2313–2322. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 57.Cancel L.M., Ebong E.E., Mensah S., Hirschberg C., Tarbell J.M. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016;252:136–146. doi: 10.1016/j.atherosclerosis.2016.07.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullick A.E., Tobias P.S., Curtiss L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer J.W. Role of hyaluronan in atherosclerosis: current knowledge and open questions. Matrix Biol. 2019;78–79:324–336. doi: 10.1016/j.matbio.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Grandoch M., Kohlmorgen C., Melchior-Becker A., Feldmann K., Homann S., Muller J. Loss of Biglycan enhances thrombin generation in apolipoprotein E-deficient mice: implications for inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:e41–e50. doi: 10.1161/ATVBAHA.115.306973. [DOI] [PubMed] [Google Scholar]
- 61.Martens R.J., Vink H., van Oostenbrugge R.J., Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013;35:451–454. doi: 10.1159/000348854. [DOI] [PubMed] [Google Scholar]
- 62.DellaValle B., Hasseldam H., Johansen F.F., Iversen H.K., Rungby J., Hempel C. Multiple soluble components of the glycocalyx are increased in patient plasma after ischemic stroke. Stroke. 2019;50:2948–2951. doi: 10.1161/STROKEAHA.119.025953. [DOI] [PubMed] [Google Scholar]
- 63.Nagga K., Hansson O., van Westen D., Minthon L., Wennstrom M. Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. J Alzheimers Dis. 2014;42:1435–1441. doi: 10.3233/JAD-141200. [DOI] [PubMed] [Google Scholar]
- 64.Jaarsma C., Vink H., van Haare J., Bekkers S., van Rooijen B.D., Backes W.H. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int J Cardiol. 2017;248:433–439. doi: 10.1016/j.ijcard.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Miranda C.H., de Carvalho Borges M., Schmidt A., Marin-Neto J.A., Pazin-Filho A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis. 2016;247:184–188. doi: 10.1016/j.atherosclerosis.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 66.Wadowski P.P., Hulsmann M., Schorgenhofer C., Lang I.M., Wurm R., Gremmel T. Sublingual functional capillary rarefaction in chronic heart failure. Eur J Clin Invest. 2018;48:e12869. doi: 10.1111/eci.12869. [DOI] [PubMed] [Google Scholar]
- 67.Bar A., Targosz-Korecka M., Suraj J., Proniewski B., Jasztal A., Marczyk B. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/Low-Density lipoprotein receptor-deficient mice. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abassi Z., Armaly Z., Heyman S.N. Glycocalyx degradation in ischemia-reperfusion injury. Am J Pathol. 2020;190:752–767. doi: 10.1016/j.ajpath.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 69.Yamaoka-Tojo M. Endothelial function for cardiovascular disease prevention and management. Int J Clinic Cardiol. 2017;4:103. [Google Scholar]
- 70.Tarbell J.M., Cancel L.M. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280:97–113. doi: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- 71.Ushiyama A., Kataoka H., Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care. 2016;4:59. doi: 10.1186/s40560-016-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaoka-Tojo M., Ushio-Fukai M., Hilenski L., Dikalov S.I., Chen Y.E., Tojo T. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 73.Brown M.D., Sacks D.B. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Kozlova I., Ruusala A., Voytyuk O., Skandalis S.S., Heldin P. IQGAP1 regulates hyaluronan-mediated fibroblast motility and proliferation. Cell Signal. 2012;24:1856–1862. doi: 10.1016/j.cellsig.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Yamaoka-Tojo M., Tojo T., Kim H.W., Hilenski L., Patrushev N.A., Zhang L. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 76.Tang T.H., Alonso S., Ng L.F., Thein T.L., Pang V.J., Leo Y.S. Increased serum hyaluronic acid and heparan sulfate in dengue fever: association with plasma leakage and disease severity. Sci Rep. 2017;7:46191. doi: 10.1038/srep46191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suwarto S., Sasmono R.T., Sinto R., Ibrahim E., Suryamin M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J Infect Dis. 2017;215:992–999. doi: 10.1093/infdis/jix041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cioffi D.L., Pandey S., Alvarez D.F., Cioffi E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1067–L1077. doi: 10.1152/ajplung.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Espinosa D.A., Beatty P.R., Puerta-Guardo H., Islam M.N., Belisle J.T., Perera R. Increased serum sialic acid is associated with morbidity and mortality in a murine model of dengue disease. J Gen Virol. 2019;100:1515–1522. doi: 10.1099/jgv.0.001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grandoch M., Bollyky P.L., Fischer J.W. Hyaluronan: a master switch between vascular homeostasis and inflammation. Circ Res. 2018;122:1341–1343. doi: 10.1161/CIRCRESAHA.118.312522. [DOI] [PubMed] [Google Scholar]
- 81.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chelazzi C., Villa G., Mancinelli P., De Gaudio A.R., Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19:26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez Rodriguez E., Cardenas J.C., Cox C.S., Kitagawa R.S., Stensballe J., Holcomb J.B. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand J Trauma Resusc Emerg Med. 2018;26:102. doi: 10.1186/s13049-018-0565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johansson P.I., Stensballe J., Ostrowski S.R. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21:25. doi: 10.1186/s13054-017-1605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delvaeye T., De Smet M.A.J., Verwaerde S., Decrock E., Czekaj A., Vandenbroucke R.E. Blocking connexin43 hemichannels protects mice against tumour necrosis factor-induced inflammatory shock. Sci Rep. 2019;9:16623. doi: 10.1038/s41598-019-52900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Retamal M.A., Froger N., Palacios-Prado N., Ezan P., Saez P.J., Saez J.C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Vuyst E., Decrock E., De Bock M., Yamasaki H., Naus C.C., Evans W.H. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poelzing S., Rosenbaum D.S. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 89.Mensah S.A., Cheng M.J., Homayoni H., Plouffe B.D., Coury A.J., Ebong E.E. Regeneration of glycocalyx by heparan sulfate and sphingosine 1-phosphate restores inter-endothelial communication. PloS One. 2017;12 doi: 10.1371/journal.pone.0186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sakabe M., Yoshioka R., Fujiki A. Sick sinus syndrome induced by interferon and ribavirin therapy in a patient with chronic hepatitis C. J Cardiol Cases. 2013;8:173–175. doi: 10.1016/j.jccase.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burns J.C., Cayan D.R., Tong G., Bainto E.V., Turner C.L., Shike H. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–225. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shirato K., Imada Y., Kawase M., Nakagaki K., Matsuyama S., Taguchi F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol. 2014;86:2146–2153. doi: 10.1002/jmv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agarwal S., Agrawal D.K. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol. 2017;13:247–258. doi: 10.1080/1744666X.2017.1232165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gamez-Gonzalez L.B., Moribe-Quintero I., Cisneros-Castolo M., Varela-Ortiz J., Munoz-Ramirez M., Garrido-Garcia M. Kawasaki disease shock syndrome: unique and severe subtype of Kawasaki disease. Pediatr Int. 2018;60:781–790. doi: 10.1111/ped.13614. [DOI] [PubMed] [Google Scholar]
- 95.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 96.Kanegaye J.T., Wilder M.S., Molkara D., Frazer J.R., Pancheri J., Tremoulet A.H. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Zheng Q., Zou L., Wu J., Guo L., Teng L. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-gamma as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17:1. doi: 10.1186/s12969-018-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohnishi Y., Yasudo H., Suzuki Y., Furuta T., Matsuguma C., Azuma Y. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int J Cardiol. 2019;292:236–240. doi: 10.1016/j.ijcard.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 99.Luo L., Feng S., Wu Y., Su Y., Jing F., Yi Q. Serum levels of syndecan-1 in patients with Kawasaki disease. Pediatr Infect Dis J. 2019;38:89–94. doi: 10.1097/INF.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Y., Schmidt E.P. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1:e23494. doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dogne S., Flamion B. Endothelial glycocalyx impairment in disease: focus on hyaluronan shedding. Am J Pathol. 2020;190:768–780. doi: 10.1016/j.ajpath.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 102.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iba T., Levy J.H. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17:283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 104.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang D., Qi B.Y., Zhu W.W., Huang X., Wang X.Z. Crocin alleviates lipopolysaccharide-induced acute respiratory distress syndrome by protecting against glycocalyx damage and suppressing inflammatory signaling pathways. Inflamm Res. 2020;69:267–278. doi: 10.1007/s00011-019-01314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McDonald K.K., Cooper S., Danielzak L., Leask R.L. Glycocalyx degradation induces a proinflammatory phenotype and increased leukocyte adhesion in cultured endothelial cells under flow. PloS One. 2016;11 doi: 10.1371/journal.pone.0167576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marki A., Esko J.D., Pries A.R., Ley K. Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol. 2015;98:503–515. doi: 10.1189/jlb.3MR0115-011R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iba T., Levy J.H., Hirota T., Hiki M., Sato K., Murakami T. Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis. Thromb Res. 2018;171:1–6. doi: 10.1016/j.thromres.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 109.Garsen M., Lenoir O., Rops A.L., Dijkman H.B., Willemsen B., van Kuppevelt T.H. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol. 2016;27:3545–3551. doi: 10.1681/ASN.2015091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butler M.J., Down C.J., Foster R.R., Satchell S.C. The pathological relevance of increased endothelial glycocalyx permeability. Am J Pathol. 2020;190:742–751. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iba T., Levy J.H., Raj A., Warkentin T.E. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8:728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frydland M., Ostrowski S.R., Moller J.E., Hadziselimovic E., Holmvang L., Ravn H.B. Plasma concentration of biomarkers reflecting endothelial cell- and glycocalyx damage are increased in patients with suspected ST-elevation myocardial infarction complicated by cardiogenic shock. Shock. 2018;50:538–544. doi: 10.1097/SHK.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 113.Naumann D.N., Hazeldine J., Midwinter M.J., Hutchings S.D., Harrison P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2018;84:81–88. doi: 10.1097/TA.0000000000001695. [DOI] [PubMed] [Google Scholar]
- 114.Rehm M., Bruegger D., Christ F., Conzen P., Thiel M., Jacob M. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 115.Yu H., Kalogeris T., Korthuis R.J. Reactive species-induced microvascular dysfunction in ischemia/reperfusion. Free Radic Biol Med. 2019;135:182–197. doi: 10.1016/j.freeradbiomed.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Solbu M.D., Kolset S.O., Jenssen T.G., Wilsgaard T., Lochen M.L., Mathiesen E.B. Gender differences in the association of syndecan-4 with myocardial infarction: the population-based Tromso Study. Atherosclerosis. 2018;278:166–173. doi: 10.1016/j.atherosclerosis.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 118.Huxley V.H., Kemp S.S. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol. 2018;1065:307–328. doi: 10.1007/978-3-319-77932-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rizzo P., Vieceli Dalla Sega F., Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol. 2020;115:31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng M.J., Kumar R., Sridhar S., Webster T.J., Ebong E.E. Endothelial glycocalyx conditions influence nanoparticle uptake for passive targeting. Int J Nanomedicine. 2016;11:3305–3315. doi: 10.2147/IJN.S106299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng M.J., Mitra R., Okorafor C.C., Nersesyan A.A., Harding I.C., Bal N.N. Targeted intravenous nanoparticle delivery: role of flow and endothelial glycocalyx integrity. Ann Biomed Eng. 2020;48:1941–1954. doi: 10.1007/s10439-020-02474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 129.Mishra H.K., Ma J., Walcheck B. Ectodomain shedding by ADAM17: its role in neutrophil recruitment and the impairment of this process during sepsis. Front Cell Infect Microbiol. 2017;7:138. doi: 10.3389/fcimb.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Palau V., Riera M., Soler M.J. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol Dial Transplant. 2020;35:1071–1072. doi: 10.1093/ndt/gfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pruessmeyer J., Martin C., Hess F.M., Schwarz N., Schmidt S., Kogel T. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285:555–564. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cesaro A., Abakar-Mahamat A., Brest P., Lassalle S., Selva E., Filippi J. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 134.van den Berg B.M., Vink H., Spaan J.A. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 135.Vink H., Duling B.R. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 136.Henry C.B., Duling B.R. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- 137.Potter D.R., Damiano E.R. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 138.Abassi Z., Hamoud S., Hassan A., Khamaysi I., Nativ O., Heyman S.N. Involvement of heparanase in the pathogenesis of acute kidney injury: nephroprotective effect of PG545. Oncotarget. 2017;8:34191–34204. doi: 10.18632/oncotarget.16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masola V., Zaza G., Gambaro G., Onisto M., Bellin G., Vischini G. A potential new factor involved in the renal epithelial mesenchymal transition (EMT) induced by ischemia/reperfusion (I/R) injury. PloS One. 2016;11 doi: 10.1371/journal.pone.0160074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lipowsky H.H., Sah R., Lescanic A. Relative roles of doxycycline and cation chelation in endothelial glycan shedding and adhesion of leukocytes. Am J Physiol Heart Circ Physiol. 2011;300:H415–H422. doi: 10.1152/ajpheart.00923.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chappell D., Jacob M., Hofmann-Kiefer K., Bruegger D., Rehm M., Conzen P. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 142.Jacob M., Bruegger D., Rehm M., Welsch U., Conzen P., Becker B.F. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104:1223–1231. doi: 10.1097/00000542-200606000-00018. [DOI] [PubMed] [Google Scholar]
- 143.Jacob M., Paul O., Mehringer L., Chappell D., Rehm M., Welsch U. Albumin augmentation improves condition of Guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–965. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 144.Holcomb J.B., del Junco D.J., Fox E.E., Wade C.E., Cohen M.J., Schreiber M.A. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Holcomb J.B., Tilley B.C., Baraniuk S., Fox E.E., Wade C.E., Podbielski J.M. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ban K., Peng Z., Pati S., Witkov R.B., Park P.W., Kozar R.A. Plasma-mediated gut protection after hemorrhagic shock is lessened in syndecan-1-/- mice. Shock. 2015;44:452–457. doi: 10.1097/SHK.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peng Z., Pati S., Potter D., Brown R., Holcomb J.B., Grill R. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40:195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R.M., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55:2000858. doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bruegger D., Rehm M., Jacob M., Chappell D., Stoeckelhuber M., Welsch U. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in Guinea pig hearts. Crit Care. 2008;12:R73. doi: 10.1186/cc6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grundmann S., Fink K., Rabadzhieva L., Bourgeois N., Schwab T., Moser M. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83:715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 154.Afshari A., Wetterslev J., Brok J., Moller A.M. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2008:CD005370. doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- 155.Chappell D., Heindl B., Jacob M., Annecke T., Chen C., Rehm M. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology. 2011;115:483–491. doi: 10.1097/ALN.0b013e3182289988. [DOI] [PubMed] [Google Scholar]
- 156.Annecke T., Chappell D., Chen C., Jacob M., Welsch U., Sommerhoff C.P. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010;104:414–421. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 157.Casanova J., Simon C., Vara E., Sanchez G., Rancan L., Abubakra S. Sevoflurane anesthetic preconditioning protects the lung endothelial glycocalyx from ischemia reperfusion injury in an experimental lung autotransplant model. J Anesth. 2016;30:755–762. doi: 10.1007/s00540-016-2195-0. [DOI] [PubMed] [Google Scholar]
- 158.Harahsheh A.S., Dahdah N., Newburger J.W., Portman M.A., Piram M., Tulloh R. Missed or delayed diagnosis of Kawasaki disease during the 2019 novel coronavirus disease (COVID-19) pandemic. J Pediatr. 2020;222:261–262. doi: 10.1016/j.jpeds.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 160.Matsuguma C., Wakiguchi H., Suzuki Y., Okada S., Furuta T., Ohnishi Y. Dynamics of immunocyte activation during intravenous immunoglobulin treatment in Kawasaki disease. Scand J Rheumatol. 2019;48:491–496. doi: 10.1080/03009742.2019.1604992. [DOI] [PubMed] [Google Scholar]
- 161.Diebel M.E., Martin J.V., Liberati D.M., Diebel L.N. The temporal response and mechanism of action of tranexamic acid in endothelial glycocalyx degradation. J Trauma Acute Care Surg. 2018;84:75–80. doi: 10.1097/TA.0000000000001726. [DOI] [PubMed] [Google Scholar]
- 162.Cooper S., Teoh H., Campeau M.A., Verma S., Leask R.L. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol Cell Biochem. 2019;459:121–130. doi: 10.1007/s11010-019-03555-2. [DOI] [PubMed] [Google Scholar]
- 163.Eskens B.J., Zuurbier C.J., van Haare J., Vink H., van Teeffelen J.W. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc Diabetol. 2013;12:175. doi: 10.1186/1475-2840-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]