Abstract
Ocular myasthenia gravis (OMG) is an autoimmune disease of the neuromuscular junction and commonly associated with other immune diseases. We describe a 16-year-old female who presented to our clinic with 1-month complaints of diplopia and strabismus, visual acuity deterioration, and ocular irritation. Her examination showed crossed diplopia and alternating exotropia of 25 prism diopters, severe blepharitis, conjunctival hyperemia, corneal pannus, epithelial irregularities, and subepithelial opacities. Workup included pediatric neurologic examination, laboratory tests, imaging, and electrophysiological tests. Diagnoses of OMG and blepharitis with ocular surface disease were made. Topical treatment included eyelid hygiene, tea tree oil scrubbing, topical steroids, and tacrolimus ointment. Systemic treatment included corticosteroids, pyridostigmine, azathioprine, intravenous immunoglobulins, amitriptyline, and doxycycline. Both diseases were refractory to intensive immunosuppressive treatment and had simultaneous relapses and an intertwined course. Our hypothesis is that a shared immune mechanism may be the cause of both OMG and ocular surface disease in our patient.
Keywords: Myasthenia gravis, Blepharitis, Tacrolimus, Strabismus
Introduction
Myasthenia gravis (MG) is an autoimmune disease in which antibodies bind to receptors and molecules on the postsynaptic membrane of the neuromuscular junction; the acetylcholine receptors (AChRs) are most commonly involved. Other antibodies include anti-muscle-specific kinase (anti-MuSK) and anti-lipoprotein receptor-related protein 4 (anti-LRP4). Therefore, muscle weakness is the hallmark of the disease. The incidence is estimated at 0.3–2.8/100,000 and the prevalence at 700,000 worldwide [1].
Extraocular involvement is generally the first clinical sign in MG; this is usually asymmetric, in contrast to the presentation in other skeletal muscles. Uni- or bilateral ptosis is the most common sign. Diplopia is also common and may mimic motor cranial nerve palsy, extraocular muscle palsy, internuclear ophthalmoplegia, and supranuclear motility disturbances. Muscle weakness usually fluctuates and increases with muscle activity. The pupil contains muscarinic receptors that are not involved in the disease and are therefore spared. Visual acuity (VA) and visual field are normal and there is no pain or proptosis [2, 3]. Only in 15% of patients is the disease confined to the extraocular muscles – ocular MG (OMG). OMG may progress to other muscles and become general MG (GMG). However, in 90% of patients who have pure ocular disease for 2 years, the symptom that remains is eye muscle weakness which does not spread to other muscles [2]. Ocular irritation may present in some patients with OMG. According to recent studies, dry eye disease may develop in up to 21% of patients; the main reason is lagophthalmos and reduced blinking due to orbicularis muscle weakness [4].
Blepharitis is an inflammation of the eyelids and a common cause for ocular irritation, burning sensation, tearing, red eyes, photophobia, and blurred vision [5, 6, 7]. We describe an adolescent female who presented with both OMG and blepharitis.
Case Report
A 16-year-old female was referred to our institute due to new-onset exotropia and complaints of double vision, in addition to foreign body sensation and deterioration in VA for 1 month prior to presentation. Her ophthalmic history was unremarkable. She had been treated with valproic acid for epilepsy by the age of 3 years.
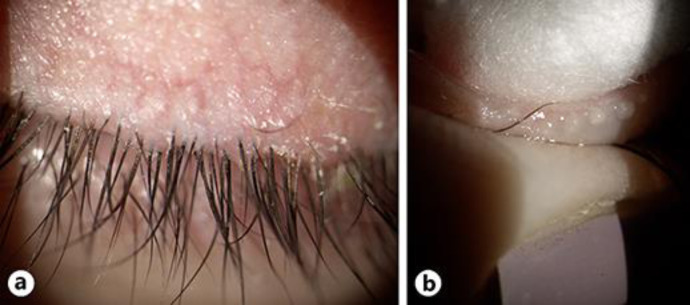
On examination, VA was 20/30 in both eyes by Snellen chart. The Prism Cover Test revealed 25 prism diopter intermittent alternating exotropia, and the Worth Four Dot Test showed a crossed diplopia at near. In addition, bilateral 2-mm lagophthalmos and bilateral ptosis (margin reflex distance 1 of 1) were seen. Slit lamp examination showed, in both eyes, advanced blepharitis, severe conjunctival hyperemia, severe superficial punctate keratopathy, diffuse subepithelial opacities, and an inferior corneal pannus (Fig. 1, 2).
Fig. 1.
a Slit lamp examination (left eye) showed conjunctival hyperemia, corneal pannus and vascularization, and subepithelial opacities. b Fluorescein dye (left eye) showed epithelial irregularities.
Fig. 2.
a Anterior blepharitis including ciliary dandruff, collarette, and eyelid margin telangiectasia. b Clogged meibomian glands were expressed in the clinic with a cotton tip applicator.
Both Schirmer's 1 test and the basic secretion test showed a result of 35 mm after 5 min. Tear breakup time was 5 s in both eyes. The visual field test and optical coherence tomography were normal.
Pediatric neurologic evaluation was unremarkable. Concentric needle stimulation single-fiber electromyography (SFEMG) showed a prominent increase in neuromuscular jitter. Head MRI showed no pathology. Titers of anti-AChR and anti-MuSK were not elevated. Chest X-ray did not show a thymus. Thyroid function tests were normal (TSH, T3, fT4, anti-TPO) and laboratory tests for rheumatologic diseases including Sjögren syndrome and systemic lupus erythematosus were all negative (e.g., antinuclear antibodies, anti-dsDNA, anti-SSA, anti-SSB, C3, C4, antiphospholipid antibodies). Based on these findings, in cooperation with the pediatric neurology team, our patient was diagnosed with OMG.
Treatment was initiated simultaneously for OMG and blepharitis with ocular surface disease. Treatment for OMG included systemic corticosteroids 35 mg/day and pyridostigmine 60 mg/day, both with gradual increases in dosage. Topical treatment for ocular surface disease was initiated, with intensive lubrication up to once an hour, and topical steroids twice a day. Treatment for blepharitis included warm compresses and eyelid hygiene.
From this point, the course of blepharitis with keratoconjunctivitis was intertwined with the course of OMG. After 2 months of treatment with oral corticosteroids, the dose was increased to 60 mg/day due to unsatisfactory results. Tacrolimus 0.03% ointment was added to the treatment and topical steroidal treatment was tapered down. Lubrication was continued and a 2-month course of 100 mg doxycycline p.o. treatment for blepharitis was added to the treatment regimen.
Three months later, VA had improved to 20/25 using Snellen chart, despite persistent double vision, and lagophthalmos had completely resolved. She had no complaints of ocular irritation. The topical treatment was reduced to tacrolimus ointment 0.03% once daily, with artificial tears every 1–2 h. Due to lack of improvement of exotropia and diplopia under high-dose steroid treatment, systemic treatment was modified to amifampridine 120 mg/day with a 5-day course of intravenous immunoglobulins. Corticosteroid was gradually stopped and changed to azathioprine 75 mg/day. Despite this new regimen, on her recent visit 4 months after treatment alteration, a new left adduction deficit appeared (Fig. 3). In addition, after using azathioprine for 10 months, another sign of deterioration appeared. Left adduction deficit remained, and the exotropia was increased to 40 PD for both near and distance. After 1 year of follow-up, the serologic test for anti-AChR and anti-MuSK was repeated and was again negative.
Fig. 3.

Left eye adduction deficit.
When systemic corticosteroid treatment was gradually stopped and after a quiescence period, the ocular surface symptoms relapsed. Repeated examination revealed conjunctival irritation, corneal epithelium irregularities, a tear breakup time of 4 s, meibomian gland dysfunction, ciliary fibrin collarets, and eyelid margin vessel dilation. This deterioration required intensive treatment, which was altered to tacrolimus ointment 0.1%, topical steroids q.i.d. and p.o. doxycycline 100 mg/day, with frequent lubrication and eyelid scrubbing with a tea tree oil-containing agent.
Both the patient's ocular surface disease and her extraocular muscle involvement were refractory to intensive treatment and demonstrated a partial response to a high dosage of immunosuppressive therapy. Both diseases tended to relapse simultaneously after improvement or a stable clinical status when dose reduction was attempted.
Discussion
In the patient described, signs and symptoms were confined to the extraocular muscles. Her clinical signs and symptoms tended to worsen at night, as is typical for this condition. This is because repetitive use of the extraocular muscles leads to their fatigability. Our patient reported worsening of the diplopia and the ptosis throughout the day and in times of tiredness. Orbicularis oculi may also be involved in OMG. GMG can involve bulbar, limb, and respiratory muscles [3, 8]. The diagnosis of GMG/OMG is clinical, supported by a combination of laboratory, imaging, and electrophysiology tests. In highly suspected cases, clinical tests (sleep test, ice-pack test, edrophonium test) can confirm the diagnosis [3, 8]. Chest radiography is an important step in the workup for MG. Up to 10% of individuals with MG are found to have a thymoma on chest imaging. Immunologic testing for MG includes circulating autoantibodies for anti-AChR, anti-MuSK, and anti-LRP4. After standard testing, 10–15% of patients with MG may remain seronegative. Serologic tests were negative in two different exams, 1 year apart in the presented case. This finding is consistent with the diagnosis of OMG where up to 50% of patients are seronegative [2]. In antibody-negative cases, neurophysiological tests and a characteristic response to therapy secure the diagnosis [2, 3]. Using a specialized concentric needle, SFEMG records individual muscle fiber action potential generated by the same motor neuron. It is the most sensitive diagnostic test for detecting abnormal neuromuscular transmission. In up to 99% of MG patients, an abnormal jitter is observed on SFEMG [3, 8]. Though a more established diagnosis is based on SFEMG in two different muscles, our patient was diagnosed with OMG by the pediatric neurology team based on the clinical findings and a single SFEMG test. There is no cure for GMG or OMG. The objective of therapy is full or nearly full pharmacologic remission. Treatment includes supportive, symptomatic, and immunoactive approaches. Mortality rates are as low as 0.06–0.89 per million person-years. This is consequent to improvements in intensive respiratory care and the introduction of immunosuppressive treatments [1]. Acetylcholine esterase inhibitors increase the duration of the neurotransmitter at the neuromuscular junction. Pyridostigmine is considered the first-line treatment for symptomatic relief. Most individuals with GMG need immunosuppressive medication to achieve pharmacologic remission and maintain good quality of life. Corticosteroid treatment is the first immunosuppressive therapy used for MG. The clinical response may start within 2 weeks and achieves maximal improvement after 6–24 months. Low-dose corticosteroid therapy has been shown to reduce the risk of the conversion of OMG to GMG [2, 3, 9]. Therefore, low-dose corticosteroid treatment is recommended for persons with OMG who have risk factors for the involvement of other skeletal muscles, such as detectable AChR antibodies, an enlarged thymus, and additional electrophysiological test results that suggest the involvement of nonocular muscles.
Azathioprine is a steroid-sparing agent. Accordingly, it is used when steroid therapy does not achieve pharmacologic remission or to minimize steroid adverse effects. This cytotoxic antimetabolite agent inhibits purine synthesis and thus inhibits DNA and RNA synthesis and cell proliferation; the effect on B and T cells is particularly strong. The combination of prednisone or prednisolone with azathioprine is considered the first-line treatment by some experts [2]. Other steroid-sparing agents such as methotrexate and mycophenolate can be used in addition for corticosteroids. Cyclosporine, cyclophosphamide, intravenous immunoglobulin, and plasmapheresis can be considered for MG. Thymectomy is recommended for patients with MG and thymoma. However, there is no strong evidence that surgery prevents progression to other sites or that it results in remission in OMG [1, 2, 4].
Ocular irritation is experienced by some patients with OMG. The main reason is dry eye disease, which can be observed in up to 21% of OMG patients [4]. The suggested cause of dry eye disease is lagophthalmos and difficulty blinking due to weakness of the orbicularis muscle. In our patient, her lagophthalmos resolved after the initial systemic treatment. However, her ocular irritation improved but later relapsed despite resolution of lagophthalmos.
Dry eye may also be caused by Sjögren syndrome. In our patient, serology for antinuclear antibodies, anti-Ro/anti-La, was not elevated, and there were no signs and symptoms of xerostomia. In addition to Schirmer's 1 test result, our conclusion was that the source of the ocular surface disease was a high evaporation rate due to severe blepharitis.
Blepharitis is a common cause of ocular irritation, burning sensation, tearing, red eyes, and visual symptoms such as photophobia and blurred vision [5]. Signs of chronic blepharitis include meibomian gland dysfunction, which results in alteration of tear film composition [5, 10], hypertrophy of the eyelid margins, scars, and changes in eyelashes – madarosis (loss of eyelashes), poliosis (loss of eyelash pigment), and trichiasis (misdirected eyelashes) [5, 6]. Meibomian gland dysfunction may also lead to hordeolum and chalazion formation [11]. Demodex mites can cause both anterior and posterior blepharitis. However, Demodex mites can infest healthy, asymptomatic individuals; hence, their contribution to blepharitis is inconclusive [12]. Blepharitis is diagnosed clinically. There is no specific test or gold standard measure. In all forms of blepharitis, the tear film may show a high evaporation rate and a tear breakup time of <10 s, which is considered abnormal. There is no cure for blepharitis, and treatment is aimed for symptomatic relief. Eyelid hygiene is still the mainstay of treatment, with warm compresses and lash scrubbing. Topical antibiotics such as azithromycin and systemic antibiotics such as doxycycline and azithromycin can provide symptomatic relief. This is due to their exertion of an anti-inflammatory effect and their eradicating bacteria from the eyelid margin. Oral antibiotics are used for persistent cases due to their anti-inflammatory properties [13]. Tea tree oil is the most effective treatment for Demodex mites [12]. Tacrolimus ointment can improve symptoms in refractory cases [14]. None of the treatments investigated in a Cochrane review of 34 studies showed strong evidence for curing chronic blepharitis. Lid hygiene was found to provide symptomatic relief, and topical antibiotics were shown to help eradicate the bacteria from the eyelid margins. The effect of topical steroids and oral antibiotics was inconclusive [7]. In most cases, the treatment is longstanding, and a tailored approach for each patient is needed, with long-term follow-up [5, 13].
Our patient had both anterior and posterior chronic blepharitis. Her symptoms included red eye, foreign body sensation, tearing, and a burning sensation. She also reported photophobia and loss of vision. Her clinical examination revealed dandruff on the eyelashes, meibomian gland dysfunction, and telangiectatic vascular changes of the eyelid margin. Her ocular surface examination showed conjunctival hyperemia, superficial punctate keratopathy, corneal neovascularization, and subepithelial opacities. Her tear breakup time was abnormal, <10 s at the time of diagnosis, and did not improve despite aggressive therapy. According to both Schirmer's 1 and basic secretion tests, her aqueous gland function was normal and her symptoms were not due to dry eye disease. She was treated with frequent lubrication, oral antibiotics (doxycycline), eyelid hygiene, scrubbing, and warm compresses. After a quiescence period, the ocular surface disease deteriorated severely. This prompted the addition to her treatment of topical steroid drops, topical tacrolimus ointment, and scrubbing with an agent containing tea tree oil.
Our patient had both OMG and ocular surface disease due to blepharitis. According to the intertwined course of both diseases, a shared immune cause is suspected. Her systemic treatment included high-dose corticosteroids, amifampridine, azathioprine, and intravenous immunoglobulins. Despite the intensive regimen, only minor relief was achieved. The blepharitis and ocular surface symptoms were also treated intensively, by a combination of topical corticosteroid drops, tacrolimus ointment, frequent lubrication, and oral doxycycline, with symptomatic relief. However, when systemic corticosteroid treatment was tapered down, a relapse in the ocular surface disease occurred, which required increased topical treatment.
Even though literature is lacking regarding ocular surface disease associated with OMG, there is some evidence of corneal damage, corneal nerve alterations, ocular surface changes, dry eye, and reduced corneal sensitivity in MG and other immunologic diseases [15].
Conclusion
We describe an adolescent with OMG and severe ocular surface disease. Despite intensive topical and systemic immunomodulatory treatments, both diseases were refractory to therapy. We suggest that a severe immune response may be the underlying cause for both OMG and blepharitis in our patient.
Statement of Ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient's parents for publication of the case, including images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work received no financial support.
Author Contributions
Dr. Arnon wrote and reviewed the manuscript and examined the patient. Dr. Mostovoy is the head of the pediatric ophthalmic institute of our medical center, examined and treated the patient, and reviewed the manuscript. Dr. Rozen-Knisbacher examined the patient and reviewed the manuscript. Prof. Pikkel reviewed the manuscript. Dr. Yahalomi reviewed the manuscript. All authors read, edited, and approved this case report.
References
- 1.Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of Myasthenia Gravis. Neurol Clin. 2018 May;36((2)):311–37. doi: 10.1016/j.ncl.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilhus NE. Myasthenia Gravis. In: Longo DL, editor. N Engl J Med. (26) 29;375. 2016. Dec, pp. 2570–81. [DOI] [PubMed] [Google Scholar]
- 3.Smith SV, Lee AG. Update on Ocular Myasthenia Gravis. Neurol Clin. 2017 Feb;35((1)):115–23. doi: 10.1016/j.ncl.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Roh HS, Lee SY, Yoon JS. Comparison of clinical manifestations between patients with ocular myasthenia gravis and generalized myasthenia gravis. Korean J Ophthalmol. 2011 Feb;25((1)):1–7. doi: 10.3341/kjo.2011.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhardt M. (St LUHN, Rammohan G (St LUHN. Blepharitis. In StatPearls. [PubMed] [Google Scholar]
- 6.Bernardes TF, Bonfioli AA, Blepharitis Seminars in Ophthalmology. Informa Healthcare. 2010;Volume 25:pp. 79–83. doi: 10.3109/08820538.2010.488562. [DOI] [PubMed] [Google Scholar]
- 7.Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012 May;((5)):CD005556. doi: 10.1002/14651858.CD005556.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014 Oct;62((10)):985–91. doi: 10.4103/0301-4738.145987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benatar M, Mcdermott MP, Sanders DB, Wolfe GI, Barohn RJ, Nowak RJ, et al. Muscle Study Group (MSG) Efficacy of prednisone for the treatment of ocular myasthenia (EPITOME): A randomized, controlled trial. Muscle Nerve. 2016 Mar;53((3)):363–9. doi: 10.1002/mus.24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putnam CM. Diagnosis and management of blepharitis: An optometrist's perspective. Clinical Optometry. Dove Medical Press Ltd. 2016;Vol. 8:p. 71–8. doi: 10.2147/OPTO.S84795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsley K, Nichols JJ, Dickersin K, Non-surgical interventions for acute internal hordeolum Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2017;Vol. 2017 doi: 10.1002/14651858.CD007742.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: Clinical perspectives. Clinical Optometry. Dove Medical Press Ltd. 2018;Vol. 10:p. 57–63. doi: 10.2147/OPTO.S142708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thode AR, Latkany RA. Current and Emerging Therapeutic Strategies for the Treatment of Meibomian Gland Dysfunction (MGD) Drugs. 2015 Jul;75((11)):1177–85. doi: 10.1007/s40265-015-0432-8. [DOI] [PubMed] [Google Scholar]
- 14.Sakassegawa-Naves FE, Ricci HM, Moscovici BK, Miyamoto DA, Chiacchio BB, Holzchuh R, et al. Tacrolimus Ointment for Refractory Posterior Blepharitis. Curr Eye Res. 2017 Nov;42((11)):1440–4. doi: 10.1080/02713683.2017.1339805. [DOI] [PubMed] [Google Scholar]
- 15.Erkan Turan K, Kocabeyoglu S, Bekircan-Kurt CE, Bezci F, Erdem-Ozdamar S, Irkec M. Ocular surface alterations and in vivo confocal microscopic characteristics of corneas in patients with myasthenia gravis. Eur J Ophthalmol. 2018 Sep;28((5)):541–6. doi: 10.1177/1120672117753688. [DOI] [PubMed] [Google Scholar]