Abstract
We show that commercially sourced n-channel silicon field-effect transistors (nFETs) operating above their threshold voltage with closed loop feedback to maintain a constant channel current allow a pH readout resolution of (7.2±0.3)×10−3 at a bandwidth of 10 Hz, or ≈3-fold better than the open loop operation commonly employed by integrated ion-sensitive field-effect transistors (ISFETs). We leveraged the improved nFET performance to measure the change in solution pH arising from the activity of a pathological form of the kinase Cdk5, an enzyme implicated in Alzheimer’s disease, and showed quantitative agreement with previous measurements. The improved pH resolution was realized while the devices were operated in a remote sensing configuration with the pH sensing element off-chip and connected electrically to the FET gate terminal. We compared these results with those measured by using a custom-built dual-gate 2D field-effect transistor (dg2DFET) fabricated with 2D semi-conducting MoS2 channels and a signal amplification of 8. Under identical solution conditions the nFET performance approached the dg2DFETs pH resolution of (3.9±0.7)×10−3. Finally, using the nFETs, we demonstrated the effectiveness of a custom polypeptide, p5, as a therapeutic agent in restoring the function of Cdk5. We expect that the straight-forward modifications to commercially sourced nFETs demonstrated here will lower the barrier to widespread adoption of these remote-gate devices and enable sensitive bioanalytical measurements for high throughput screening in drug discovery and precision medicine applications.
Keywords: Field-effect transistor (FET), Complementary metal oxide semi-conductor (CMOS), 2D MoS2, Biosensor, Super-Nernstian, pH, Enzyme activity, Enzyme therapeutics
Introduction
Field-effect transistors (FETs) have a long history as sensitive and label-free bioanalytical tools.1,2 Since that time FETs have been adapted for numerous applications that include measurements of protein-ligand interactions,3 monitoring of ocean acidification,4 low-cost DNA sequencing,5 enzyme measurements,6-8 and the detection of ionic action potentials in nerve and other neuronal systems.9,10 In most cases, the FET-based sensors are fabricated by leveraging nanomanufacturing processes that underpin the silicon-based complementary metal-oxide semiconductor (CMOS) industry. More recently, the emergence of 2D semi-conducting materials has resulted in new FET-based chemical sensors,11,12 and novel device geometries such as dual-gate FETs, which provide ≈100-fold higher sensitivity than silicon devices, while simultaneously improving the signal-to-noise ratio (SNR) of the measurements.8,13
The sensitivity and resolution of pH measurements are important metrics of device function and are used, in particular, to validate the performance of new sensor designs and FET structures.8,14,15 Traditional ion sensitive field-effect transistor (ISFET) technology has been optimized to return a pH sensitivity that approaches the Nernst value of 59.5 mV when the solution pH changes by 1 at room temperature.16 Efforts to improve the sensitivity of pH measurements have led to the exploration of dual-gate FETs for signal readout, which leverage the asymmetric capacitive coupling between the top- and back-gate with the device channel to amplify small pH signals measured remotely by using commercial sensing elements. Recent measurements based on this dual-gate FET approach have demonstrated the amplification of pH signals by 2-fold with silicon devices15 and 37-fold (sensitivity of 2.25 V)17 to 75-fold (sensitivity of 4.4 V)8 higher than the Nernst sensitivity by using novel channel materials and gate structures. However, the improvement in sensitivity does not always result in better pH resolution for silicon devices because the noise level increases with the signal, as seen from recent theoretical18 and experimental studies.15 As a result, the typical pH resolution of commercial meters is limited to 0.1, while that of silicon ISFETs range from 50×10−3 for commercial devices4 to as low as 15×10−3 for silicon nanowire sensors.19 On the other hand, dual-gate FETs fabricated with a 2D MoS2 channel and with a room temperature ionic liquid top-gate dielectric have been demonstrated with a pH resolution as small as 92×10−6 with a 10 Hz bandwidth.8 In particular, these devices, which were operated in a low-noise regime of the atomically thin channel, exhibit a linear scaling in pH resolution improvement with device gain.
In order to drive adoption of high-resolution FET measurements within bioanalytical applications, we show how techniques developed for dual-gate FETs can be applied to commercially sourced silicon FETs. The techniques allow silicon devices to achieve pH readout resolution that exceeds most ISFET results and is on par with a solid-state version of recently published dual-gate 2D FETs (dg2DFETs).8 Importantly, the improved performance of both nFETs and dg2DFETs was achieved in a remote sensing configuration where the pH sensitive surface is located off-chip and connected electrically to the FETs. The advantage of the remote sensing approach is the separation of signal transduction from the sensing surface. This allows reuse of the electronic components, minimizes parasitic noise sources, and enables measurements from a myriad of commercial and custom-built sensing elements (e.g., glass electrodes, conductive thin film membranes, etc.). Finally, this setup also differs from most ISFET studies where the gate dielectric also serves as the pH sensing membrane.
We illustrate high-resolution pH measurements by comparing closed-loop transduction of the signal from a commercial nFET to the open loop operation of the same device. The improved performance observed in the closed-loop configuration establishes that this approach can allow the use of readily obtained commercially packaged transistors for laboratory grade bioanalytical measurements. Furthermore, the closed-loop transduction approach can be applied to a wide range of sensor technologies that could be based on other stand-alone transistors such as junction FETs (JFETs) and bipolar junction transistors (BJTs) and even to integrated sensors such as ISFETs. It is expected that this readout approach will improve the performance of sensor systems based on any of these transistors. The technique is demonstrated through measurements of the activity and the effect of customized polypeptide therapeutics on the kinase Cdk5, which is implicated in Alzheimer’s disease and numerous other debilitating neurological disorders.
Experimental
n-Channel Silicon Field-Effect Transistors:
Commercially sourced (ALD110900PAL; Advanced Linear Devices, Sunnyvale, CA)† silicon n-channel field-effect transistors (nFETs) were soldered onto a printed circuit board (PCB) prior to measurements using a commercial probe station. Electrical characterization of the nFETs was performed using a semiconductor parameter analyzer (4155C; Agilent. Santa Clara, CA). Time-series measurements with the nFETs were performed similarly to the measurements done with dg2DFET as described below.
2D Dual-Gate Transistor Fabrication:
A detailed description of the device fabrication process and monolayer characterization for dual-gate 2D field-effect transistors (dg2DFETs) was provided in our previous work.8,13,20 Briefly, monolayer MoS2 was first transferred onto an oxidized Si substrate (SiO2 with a thickness of 70 nm) by using a gold-mediated exfoliation technique.21 The thickness of the transferred material was confirmed with Raman spectroscopy.13 Optical lithography was used to first pattern the source (S) and drain (D) contacts followed by electron-beam metal deposition (80 nm Au on 2 nm Ti) and lift-off in acetone. A second optical lithography step was then used to define and etch a 5 μm × 5 μm channel for each FET. The devices were then annealed under forming gas (5 % H2, 95 % Ar) for 24 hours to minimize organic contamination and improve the contact resistance.13 This was immediately followed by the atomic layer deposition (ALD) of a 20 nm top-gate (TG) Al2O3 dielectric. Finally, another optical lithography step was used to pattern the top-gate metal, followed by electron-beam metal deposition (100 nm Au on 10 nm Ti) and lift-off in acetone.
Remote Biological Activity Measurements:
The enzyme and pH calibration measurements were performed with the nFETs and dg2DFETs were performed by connecting a pH sensor to the top-gate metal contact with a shielded coaxial cable. This remote sensing approach allows electronic components to be separated from the biological components and thereby reused. In the present work, a glass combination microelectrode (MI-4156; Microelectrodes, Bedford, NH) capable of measurement volumes as small as 50 μL was used as the pH sensor, although the techniques described here are compatible with other sensing and bioanalytical surfaces that can be electrically connected to the top-gate metal contact.
Time-Series Field-Effect Transistor Measurements and PID Control:
Time-series measurements were performed by operating the nFETs and dg2DFETs under proportional-integral-derivative (PID) control (HF2LI; Zurich Instruments, Zurich, Switzerland). The channel current, ID, was maintained at a constant value by continuously varying the back-gate voltage (VBG) in the case of the dg2DFETs or by adding the controller output to the signal from the pH sensor (VpH) for the nFETs in response to changes in the top-gate potential.
The PID control system was implemented by first amplifying the channel current, ID, with a current preamplifier (DLPCA-200; FEMTO, Berlin, Germany) at a gain of either 106 V/A (dg2DFET) or 103 V/A (nFET). The output of the current preamplifier was then filtered through a 4-pole Bessel filter with a cutoff frequency of 5 kHz and sampled with a frequency of 25 kHz by using a 14-bit analog-to-digital converter (HF2LI; Zurich Instruments, Zurich, Switzerland). The digital PID controller (KP=553.5 mV, KI=9.22×103 s−1 and KD=10.4 μs) was operated with a bandwidth of 1 kHz to maintain the channel current set-point. Because most biological processes are slow and do not require high bandwidth measurements, the controller output was further filtered by using a low-pass filter with a cutoff frequency of 10 Hz prior to being recorded.
Sensitivity and Resolution of pH Measurements:
The pH sensitivity and resolution were established as described in our previous work.8 Briefly, a histogram from the raw VPID time-series data was computed for each measured pH. A sum of two Gaussian distribution functions was then fit to the histograms to obtain the peak positions and standard deviations of the reference potential and the measured pH signal. The difference in the peak positions between the pH and reference potentials (ΔVPID) was used to determine the pH sensitivity of the device. The measurement uncertainty (σPID) was then obtained by propagating the error when determining ΔVPID. For the nFETs, the pH resolution, ΔpH=(k×σPID)/VNernst, is reported with expanded uncertainty (k=2), where VNernst is the Nernst potential at room temperature. For the dg2DFETs, ΔpH=(k×σPID)/(α×VNernst), where α is the device gain.
Kinase Measurement Reagents:
The activity of Cdk5/p25 was estimated by measuring the phosphorylation of histone H1 as reported previously.8 All measurements were performed with 18.5 nM of Cdk5/p25 (C0745; Sigma Aldrich, MO) in 1× kinase buffer to match physiological conditions using a volume of 50 μL. The substrate protein histone H1 (10223549001; Sigma Aldrich, MO) was suspended in deionized water at a stock concentration of 2 mg/mL and further diluted as described in the in the Results section. Substrate phosphorylation was initiated with a mixture of dithiothreitol (DTT) and adenosine triphosphate (ATP) with final concentrations of 250 μM and 5 mM respectively. The measurements were buffered using 5× kinase buffer, prepared by suspending 25 mM β-glycerol (G9422; Sigma Aldrich, MO), 50 mM MgCl2 (5980; Millipore, MA), 5 mM EGTA (E0396; Sigma Aldrich, MO), 2.4 mM EDTA (1002264786; Sigma Aldrich, MO), 1.25 mM MOPS (M1254; Sigma Aldrich, MO) in deionized water (DIW). The kinase buffer was then diluted in DIW to form the 1× kinase buffer used in the assays.
Results and Discussion
We electrically characterized nFET and dg2DFET devices to evaluate their performance and to determine the optimal operating conditions for biosensing applications. The devices were calibrated with standard pH buffer solutions to determine the gain, α, of the dg2DFET, and the noise performance, sensitivity, and resolution of both the nFETs and the dg2DFETs. Finally, both device types were used to measure the activity of the kinase Cdk5, an enzyme implicated in Alzheimer’s disease, and to evaluate the effectiveness of a custom polypeptide, p5, as a therapeutic agent in modulating Cdk5 function.
Silicon n-Channel FET Performance.
The nFETs (Fig. 1a; right) were first characterized electrically using the configuration shown in the schematic in Fig. 1a (left). The transfer characteristics of the device were determined by recording the drain current (ID) as a function of the gate potential (VG), while the drain voltage (VD) was held constant. Fig. 1b shows the typical transfer characteristics (ID—VG) for the nFETs. The device exhibits up to ≈8 orders of magnitude change in ID when switching from an off-state to an on-state with a steep sub-threshold slope of ≈100 mV per decade at ≈300 K and gate leakage current (IG) of ≈1 pA. Fig. 1b (inset) shows the transconductance (gm), obtained by taking the numerical derivative of the transfer curve. The peak transconductance of the nFETs (gm,peak) was found to be ≈78 μS at a voltage (Vgm,max) of 0.385 V.
Figure 1:
Electrical characterization of a commercially sourced silicon n-channel field-effect transistor (nFET) for biosensing applications. (a) left: Schematic of the electrical characterization setup of the nFET. A constant voltage, VD, is applied to the drain contact while the source is grounded. The transfer characteristics of the device are obtained by sweeping the top-gate voltage, VG, to electrostatically control the channel current (ID). right: An image of the packaged nFET used in this work. (b) The transfer curve of the nFET is shown by measuring ID as a function of a sweep of VG. The sub-threshold slope was found to be 100 mV per decade. (inset) The device transconductance as a function of VG. The voltage at peak transconductance determines the point of maximum sensitivity and is used to optimally bias the device for biosensing.
pH Sensitivity Using n-Channel Silicon Transistors.
The pH sensitivity of the nFETs was measured by using commercial standard pH buffer solutions over a range of 4 to 10. When operating the device in an open loop, shown schematically in Fig. 2a, found the pH response to be linear when the device was operated about gm,peak as seen in Fig. 2b (blue). This behavior is expected when operating the device in the linear regime of the transfer curve (see Fig. 1). Under these conditions, we found the pH sensitivity, δID/δpH≈6 μA (R2=0.992) when VD=0.1 V, yielding a transimpedance gain of 9.9×103 V/A, assuming VNernst of 59.5 mV at room temperature (300 K). The pH response was drastically different, as expected, when the device was operated about its threshold voltage (VT=0 V) as seen from Fig. 2b (pink and inset). In this case, the pH response was linear under acidic conditions (pH < 7), when the pH sensor returned a positive voltage, thereby driving the FET into the inversion regime. Under more basic conditions (pH > 7), the sensor potential was negative causing the nFET to operate in the sub-threshold regime where the current decreases exponentially at increasingly negative gate voltages. The net result is a pH response that is highly non-linear over the measured pH range as has been observed by others in the literature.22 Therefore, it may be advantageous to operate the nFET in the linear regime, particularly when operating over a wide pH range. Additionally, the larger ID in the linear regime over its value at VT results in lower relative noise and improved pH resolution as will be discussed in greater detail in later sections.
Figure 2:
Calibration of pH measured with a commercially sourced n-channel silicon field-effect transistor (nFET). (a) The nFET was operated in open loop by directly measuring the changes in the drain current (ID) due to changes in the gate potential (VTG) applied to the top-gate contact (G). A constant offset potential (Vo) was summed with the potential (VpH) generated by a glass pH microelectrode prior to being applied to the gate contact. (b) The change in ID as a function of measuring standard pH buffer solutions ranging from pH 4 to pH 10 with a glass pH microelectrode. Setting Vo to operate the device at peak transconductance (gm,peak) resulted in a linear response over the measured pH range (blue). Operating the device about the threshold voltage (VT) of 0 V resulted in a highly non-linear pH response (pink and inset). (c) Constant current mode operation of the FET was performed by using a proportional-integral-derivative (PID) controller to monitor ID and continually adjust the PID voltage (VPID). VPID was summed with VpH prior to being applied to the gate contact. (d) The change in VPID as a function of measuring standard pH buffer solutions ranging from pH 4 to pH 10 using a glass pH microelectrode. The PID set-point was set to a value of ID to allow device operation either at gm,peak (blue) or at the threshold voltage of 0 V (pink). Note that the error bars are smaller than the open circles that represent the data points. The error bars in (b) and (d) represent the expanded uncertainty (k=2) of the measurand.
The sensitivity and performance of the commercial nFET improved considerably when operated under closed-loop PID control as shown schematically in Fig. 2c. In this configuration, the controller continually adds a control voltage (VPID) to VpH, thereby maintaining ID at a constant value. A key advantage of this approach is that because the device will always operate at the same point in its transfer curve (see Fig. 1b), its performance remains consistent across a wide range of measured pH values. This is clearly seen in Fig. 2d, where the device exhibits a linear pH response both when the PID controller was set up to hold the nFET at gm,peak (blue) and when the device was operating at its VT of 0 V (pink). Furthermore, the pH sensitivity in both cases, dVPID/dpH≈56 mV (R2=0.997), was obtained from a linear regression of the curves in Fig. 2d and approached the Nernst value of 59.5 mV at room temperature. Finally, the remote sensing configuration used here allows the PID output to be summed with the sensor signal, allowing the controller to operate at a higher bandwidth and allowing better noise suppression, thereby improving pH resolution. Please note, since the Si-FET operates in a single-gate configuration, there is no intrinsic sensor gain in this case (i.e., amplification of the pH sensor output). The optimal conditions for the measurements were obtained when the devices were operated in closed-loop control, by maintaining a constant channel current. Furthermore, to minimize measurement noise, the current value was chosen to be at a level above that at the threshold voltage of the FET.
Dual-Gate 2D FET Performance.
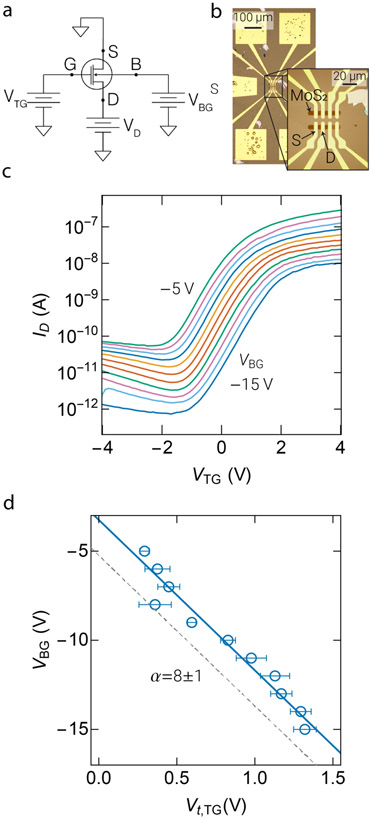
We compared the performance of the nFETs with that of dg2DFETs that we fabricated from atomically thin MoS2 films. An optical image of a representative dg2DFET device is shown in Fig. 3b. The dg2DFETs were electrically characterized following fabrication by using the setup shown schematically in Fig. 3a. The transfer characteristics of the device were measured by recording the drain current (ID) as a function of the top-gate potential (VTG) with the drain voltage (VD) held constant (Fig. 3c). The measurements were repeated for different VBG to determine the signal amplification (a) due to the asymmetric capacitance of the top and back gates.8 The devices exhibited a dynamic range of up to ≈5 orders of magnitude in ID and a subthreshold slope of ≈800 mV per decade, consistent with expected behavior for a 20 nm high-k Al2O3 gate dielectric.
Figure 3:
Electrical characterization of dual-gated monolayer MoS2 field-effect transistors (FETs) for biological sensing applications. (a) Measurement schematic for characterizing a dual-gated 2D-FET for remote biosensing. The MoS2 2D semi-conducting channel spans the source (S) and drain (D) contacts. While the source contact is grounded, a constant potential (VD) is applied to the drain contact driving a current across the 2D channel. The channel conduction is electrostatically controlled by a voltage applied to the silicon substrate, which forms the global back-gate (B) or to the metal top-gate (G). (b) Top view optical image of an array of 2D MoS2 FETs. (c) Transfer characteristics of a dual-gated 2D FET showing drain current (ID) as function of the top-gate voltage (VTG) while stepping back-gate voltage (VBG). (d) The change in VBG as function of top-gate threshold voltage (Vt,TG) is shown. A linear regression to the data (n=5) is used to determine the signal amplification (α) of VTG at the back-gate. The error bars report the standard error defined as the standard deviation of the population mean.
For each curve in Fig. 3c, the top-gate threshold voltage (Vt,LG) was determined from a linear extrapolation of ID(VTG) at the voltage corresponding to peak transconductance to ID=0.23 Fig. 3d plots the back-gate voltage (VBG) against the top-gate threshold voltage (Vt,TG). This allowed the determination of the device gain from the expression α=dVBG/dVt,TG.8. The value of α for devices measured as part of this work was then determined numerically from a linear regression to the data in Fig. 3d, resulting in α=8±1, or ≈4 times larger than state-of-the-art dual-gate silicon devices.15 The measured value of α is in good agreement with theoretical predictions for devices with a 20 nm Al2O3 top-gate dielectric and 70 nm SiO2 bottom oxide.
It is important to note that the all solid state, oxide gate dielectric dg2DFETs presented here do not operate in the quantum capacitance limited regime where the dual-gated ionic liquid devices we previously reported operated.8 In the inversion regime where the dg2DFETs reported here operate, the top-and back-gate capacitances (CTG≈0.4 μF/cm2 and CBG≈0.05 μF/cm2) are more than an order of magnitude smaller than the quantum capacitance (CQ≈4 μF/cm2) of the 2D channel.24,25 These relatively smaller gate capacitances allow the effects of CQ to be ignored which gives rise to the simplified expression for the device gain, α=dVBG/dVt,LG=CTG/CBG (see Ref. 8 for a detailed derivation).
The closed-loop control techniques employed to operate the single gate Si-FET are similar, but not identical, to those used in dg2DFET case. For both devices, the channel current is maintained at a constant level by the PID controller. The subtle differences between the control circuits for the two devices arise from the way the PID controller output is connected to maintain a constant channel current as can be seen from Fig. 2c for the nFET and Fig. 4a for the dg2DFET. In the case of the dg2DFETs, the output of the PID controller is used to adjust the potential of the back gate (VBG) as seen from Fig. 4a. On the other hand, for the single gate nFET operation, the output of the controller is summed with the sensor signal as seen from Fig. 2c to account for the lack of a second gate.
Figure 4:
Electrical calibration and pH sensitivity measurements of dual-gate 2D field-effect transistors (dg2DFETs) when operated in a constant current mode. (a) Schematic representation of constant current mode operation of dg2DFETs.8 A proportional-integral-derivative (PID) controller was used to maintain the channel current (ID) at a constant value. Control of ID was achieved by continually adjusting the back-gate voltage (VBG) in response to changes in the top-gate potential (VTG) applied either using a waveform generator or from the output of a pH sensor (Vsig). A DC offset voltage (Vo) was summed with Vsig to determine the optimal operation region of the dg2DFET. (b) The time-variant response of VBG under PID control is shown when the top-gate is biased with a 1 Hz AC sine wave signal with a peak-to-peak amplitude of 200 mV. (c) Response of VBG when measuring standard buffer solutions from pH 4 to 10. The error bars represent the expanded uncertainty (k=2) of the measurement. Note that the error bars are smaller than the open circles that represent the data points. (inset) Time-series data, relative to a reference potential value, show the response of VBG when measuring standard buffer solutions from pH 4 to 10.
pH Sensitivity of Dual-Gate 2D FETs.
The dg2DFETs were operated in a constant current mode as described in the Experimental section and shown schematically in Fig. 4a. A PID controller was used to maintain ID at a preset value by continuously varying VBG in response to changes in VTG. The controller current set-point (50 nA) and a DC offset voltage applied to the top gate (Vo=+0.5 V) were optimized to operate the device in the linear and low-noise region of the transconductance curves in Fig. 3c. The performance of the device was then validated by applying a sine wave at a frequency of 1 Hz and peak-to-peak amplitude of 200 mV to the top-gate and measuring the response of VBG (Fig. 4b) as regulated by the PID controller. The device gain, α, was then obtained from the ratio of the amplitudes of VBG to VTG to yield α=5.8±0.1, consistent with the data in Fig. 3d.
The pH sensitivity of the dg2DFETs was measured by remotely connecting a pH sensor to the top-gate metal contact with a shielded cable. A switch was used to alternatively either ground the top-gate or connect it to the pH sensor. Time-series measurements of the system response, under PID control, to commercial standard buffer solutions from pH 4 to pH 10 are shown in Fig. 4c (inset). The time-series data were analyzed as described in the Experimental section to yield the pH response curve in Fig. 4c with a sensitivity, dVBG/dpH= 236.3 mV (R2=0.998).
Comparison of pH Resolution of n-Channel Silicon and Dual-Gate MoS2 Transistors.
The pH resolution (ΔpH) of nFETs and dg2DFETs at a bandwidth of 10 Hz were determined when measuring phosphate buffered saline (PBS) and are summarized in Table 1. Following a procedure we developed previously,8 a switch was used to alternatively either connect the gate terminal to the PBS solution or to ground. This method allowed the measurements of time-series of nFETs operated in an open loop as seen in Fig. 5a (inset). A histogram of the time-series was then used to determine the mean value of ID for each measured pH solution and the expanded uncertainty (k=2) as described in the Experimental section. As seen from Fig. 5a, linear regression of the measured pH data yielded a sensitivity, dID/dpH≈4 μA (R2=0.974) when VD=0.1 V, similar to the value obtained from the data in Fig. 2b. By inverting the curve and propagating the uncertainty in ID, we determined ΔpH with an expanded uncertainty (k=2) to be (22±2) × 10−3 at a bandwidth of 10 Hz in the open loop configuration.
Table 1:
The smallest detectable pH change (ΔpH) of phosphate buffered saline solution measured with a bandwidth of 10 Hz. The values were determined from the error bars reported in Fig. 5.
| Nominal pH | 6.72 | 6.81 | 6.88 | 6.96 | 7.05 |
|---|---|---|---|---|---|
| ΔpH nFETopen-loop | 0.018 | 0.027 | 0.024 | 0.019 | 0.025 |
| ΔpH nFETPID | 0.006 | 0.007 | 0.008 | 0.007 | 0.008 |
| ΔpH dg2DFETPID | 0.003 | 0.003 | 0.006 | 0.005 | 0.002 |
Figure 5:
A comparison of pH sensitivity and resolution between a commercially sourced n-channel silicon field-effect transistor (nFET) operating open loop, operating under closed-loop proportional-integral-derivative (PID) control, and a 2D dual-gated MoS2 transistor (dg2DFET) operating under closed-loop PID control. All measurements were performed with a pH sensitive glass microelectrode. (a) The change in the nFET channel current (ΔID) when operating the device in open loop, as a function of phosphate buffered saline (PBS) solutions adjusted to different pH values. The pH sensitivity in this case was dID/dpH≈4 μA (R2=0.974) when the drain voltage, VD, was 0.1 V (b) The change in the PID control voltage (ΔVPID) as a function of solution pH when operating the nFET devices with PID control. In this case, the pH sensitivity was dVPID/dpH=58.7 mV (R2=0.988). (c) The change in the back-gate voltage, ΔVBG, as a function of solution pH when operating dg2DFETs under PID control. The pH sensitivity of the measurements, dVBG/dpH was found to be 384 mV (R2=0.999) (insets) Underlying time-series data from nFETs and dg2DFETs that were analyzed to obtain the plots in panels (a), (b) and (c). The error bars in all cases represent the expanded uncertainty (k=2) of the measured quantity shown on the y-axis of the plot. Note that in (b) and (c) the error bars are smaller than the open circles that represent the data points.
The measurements were repeated when operating the nFET under PID control. The time-series of the PID controller output (Fig. 5b, inset) were analyzed identically to the open loop data to yield a pH sensitivity, dVBG/dpH=58.7 mV (R2=0.988) as seen from Fig. 5b, consistent with the expected value of VNernst at room temperature. The error bars at each measured pH value, which represent the expanded uncertainty (k=2) in the PID output voltage, are a direct measure of ΔpH. We found that, on average, ΔpH=(7.2±0.3)×10−3 at a bandwidth of 10 Hz or an ≈3-fold improved over nFETs operating in open loop.
Both modes of nFET operation described above were compared with pH measurements performed by using dg2DFETs in a closed-loop configuration. Fig. 5c (inset) shows time-series measurements of the pH sensitivity of PBS buffers acquired by dg2DFETs when they were operated using the PID control scheme in Fig. 4a. An analysis of the pH time-series (see Experimental section for details) yielded a sensitivity, dVBG/dpH=384 mV (R2=0.999) as seen from Fig. 5c, which represents an ≈6.5-fold amplification of VNernst at room temperature. The error bars in the figure estimate the expanded uncertainty (k=2) of the ΔVBG at each pH value. This in turn allowed the estimation of the pH resolution from the expression ΔpH=ΔVBG/(α×VNernst)=(3.9±0.7)×10−3 at a bandwidth of 10 Hz, or ≈2-fold better than the nFET devices operating under PID control.
As seen from Fig. 6a (blue and pink) and Table 1, the pH resolution of nFETs can be substantially improved by operating them under PID control. Under this mode of operation, the low intrinsic noise and the high channel current of the nFETs allow their performance to approach that of the custom-built dg2DFETs (Fig. 6a, green). To better understand the improvement in nFET performance under PID control, we measured the power spectral density (PSD) of the channel current noise scaled by the channel current, SID/ID2, as seen in Fig. 6b. Under open loop operation (Fig. 6b, blue), the devices exhibit 1/f noise scaling as observed by others in the literature.15,26 The root mean squared noise figure of merit (FOMrms) was then determined from the expression to be 61×10−6 in the open loop case for a bandwidth of 10 Hz. The PID controller greatly suppresses 1/f noise as seen from Fig. 6b (pink). When operating under PID control, we found FOMrms to be only 4.3×10−6, or an order of magnitude lower than the open loop case. This reduction directly results in the improved ΔpH seen in Fig. 6a (pink triangles vs. blue circles).
Figure 6:
(a) A comparison of the pH resolution (ΔpH) as a function of pH when operating the n-channel silicon field-effect transistor (nFET) in open loop (blue), the nFET under PID control (pink) and the 2D dual-gated MoS2 transistor (dg2DFET) under PID control (green). (b) Power spectral density (PSD) of the channel current, ID, of nFETs under open loop operation (blue) and under PID control (pink). (c) Power spectral density (PSD) of the channel current, ID, of dg2DFETs s under open loop operation (blue) and under PID control (pink).
An improvement in the channel current noise is also observed for the dg2DFETs when operating under PID control, in comparison with the open loop case, as seen from Fig. 6c. At a bandwidth of 10 Hz, FOMrms decreased from 139×10−6 for open loop operation to 42×10−6 when operating under PID control. The higher noise in the dg2DFETs, compared with the nFETs, is offset by a sensor gain of α≈8, which improves their overall pH resolution seen in Fig. 6a (nFETPID; pink triangles and dg2DFETPID; green diamonds). Furthermore, as noted earlier, the remote sensing configuration and the use of commercial devices for signal transduction implies that the performance of this system can be continually improved by substituting the existing commercial FETs with other lower noise devices, which in turn can improve ΔpH.
While the pH resolution of the nFETs can approach that of the dg2DFETs with a moderate internal gain, their performance is expected to fall short of high gain devices such as ionic liquid gated dual-gate FETs.8 The highly asymmetric gate geometries of the ionic liquid gated FETs allows the realization of α>150 while operating in a low-noise regime similar to the dg2DFETs. The combination of high gain and low noise allows those devices to resolve pH values as small as 92×10−6, which is an order of magnitude below the resolution attainable by the dg2DFETs (Fig. 6a). While this improved resolution is critical in certain bioanalytical applications, we show below that the improvements in the operation modes of nFETs demonstrated here can be leveraged to measure both enzymatic activity and the effect of therapeutics on enzyme function at physiological concentrations.
Cdk5-p25 Pathological Activity and Neurodegeneration.
The cyclin-dependent kinase 5 (Cdk5) is of fundamental importance for neuronal development, memory and pain signaling.27-30 Its physiological activators, the proteins p35 and p39, trigger the Cdk5-mediated phosphorylation of neuronal proteins and organelles that are essential for the normal function of the human nervous system.31-33 Numerous factors, which include environment, lifestyle and genetics, result in an increased uptake of intracellular Ca2+ that activates the protease calpain, truncating p35 into the fragments p10 and p25. The latter is a pathological activator of Cdk5, leading to its hyperactivation. Multiple cascading effects within the cell cycle can be traced back to the hyperactivation of Cdk5 resulting in the formation of β-amyloid plaques and intracellular neurofibrillary tangles – the well-known indicators of neurodegenerative diseases such as Alzheimer’s disease (AD).34-40
Therapeutic approaches targeting Cdk5-related pathologies have focused on inhibitors such as aminothizole and roscovitine, which bind to the ATP-docking pocket and prevent Cdk5-mediated hyperphosphorylation.41-44 However, targeting the ATP-binding pocket also causes non-specific interactions with other ATP-mediated cellular reactions, often causing serious side-effects. This has led to the pursuit of alternative approaches, such as the use of cholinesterase inhibitors45 or antioxidants,46,47 which have thus far not resulted in safe therapeutics.
In past work, we have shown a novel approach to inhibit the Cdk5-p25 pathology,37 for example using the 24 amino acid, p5, obtained through the repeated truncation of p35. Importantly, these polypeptides act as selective inhibitors of Cdk5 pathological hyperactivity in both in vivo and in vitro experiments.37,48,49 A variant derived from p5, TFP5, which was designed to cross the blood-brain barrier 50 showed a drastic decrease in pathology by allowing the rescue of cortical neurons in transgenic 5XFAD AD model mice in vivo.40,50
In ongoing work, we have used computer simulations to determine the molecular basis of p5-based inhibition mechanisms – a critical step towards developing safe therapeutics for the regulation of Cdk5/p25 hyperactivity.51 This in turn could also lead to new molecules that are more selective and therefore safer. The FET-based measurements developed here can play a central role in this development cycle by enabling the rapid testing of candidate molecules. As a first step towards this goal, we demonstrate the ability of nFETs to measure the activity of Cdk5/p25 and the effect of p5 on re-regulation of this enzyme under physiological conditions.
Enzymatic Activity of the Pathological Cdk5-P25 Complex.
Fig. 7a shows a molecular representation (protein data bank structure: 1UNL52) of the Cdk5-mediated phosphorylation (top) and the phosphorylation reaction scheme (bottom). In the presence of an activator protein (e.g., the pathological p25), Cdk5 catalyzes the transfer of a single phosphate group from ATP to a serine or threonine residue in a substrate protein (e.g., histone H1). The reaction also releases a single proton, thereby causing the surrounding medium to become slightly more acidic and decreasing its pH. When using the nFETs, the change in pH resulted in a change in VPID relative to a control sample with no enzyme, for concentrations of histone ([H1]) ranging from 9 μM to 25 μM as shown in Fig. 7b. All measurements were performed at a physiological concentration of Cdk5/p25 of 18.5 nM. For all measurements, a control sample with no Cdk5/p25 showed no change in the enzymatic activity, and therefore no change in the recorded electrical signal. The volumes of solution in each measurement was 50 μL, which is consistent with experiments that use ISFETs for biomedical applications.
Figure 7:
Measurements of the activity of the proline directed kinase, Cdk5 and the effect of the custom designed therapeutic polypeptide, p5, on modulating its activity. (a) (top) The molecular structure of the pathological Cdk5/p25 complex when phosphorylating a substrate protein, histone H1, during adenosine triphosphate (ATP) hydrolysis. (bottom) The reaction scheme of Cdk5-mediated phosphorylation of serine or threonine residues in histone. Upon hydrolysis of ATP, a single proton is released causing a slight acidification of the surrounding medium. (b) The change in the measured gate voltage (ΔVPID) of a n-channel silicon field-effect transistor (nFET) upon Cdk5-mediated phosphorylation as a function of the substrate protein, histone H1, concentration ([H1]). The solid line shows the fit of a Langmuir adsorption model to the data, which returned an activity coefficient, ka=(8.2±1.3) μM (c) Molecular representation of p5 interactions with the Cdk5/p25 complex that result in a decrease in its activity. (d) ΔVPID as function of p5 concentration ([p5]) shows decreasing Cdk5 activity. The concentration of Cdk5/p25 and histone H1 were held a fixed value for each measurement in the plot. The solid line plots an exponential function to illustrate the trend in the data. The error bars in (b) and (d) represent the expanded uncertainty (k=2) in ΔVPID.
As expected, the change in VPID increased monotonically with increasing histone concentration. A simple model of the form , where ka is the activity and γ is a scaling constant, was fit to the data in Fig. 7b. The value of ka was then estimated to be (8.2±1.3) μM when measured with the nFETs. This value was consistent with ka=(9.1±0.9) μM for measurements performed using dg2DFET-based sensors (Fig. S1) under identical solution conditions, and also in agreement with our previous measurements using a γ-32P-ATP assay (ka=12.1±2.3 μM) as seen from Fig. S2.8 In each case, the error bars of the estimated quantity represent the standard error of the measurement.
We leveraged the high time-resolution of our technique to measure the kinetics of the Cdk5/p25 enzymatic reaction for histone concentrations of 9.1 μM, 12.7 μM and 18.2 μM as seen from Fig. S3. In each case, the reaction was initiated upon the addition of ATP. After ≈1 minute, we observed a distinct change in the voltage signal that was indicative of histone phosphorylation. A control sample with no Cdk5/p25 showed no change in signal upon the addition of ATP. A nonlinear regression of the form β(1 − e−k1t), where β is a scaling constant, was used to estimate the enzyme rate constant k1 to be (0.35±0.1) min−1, consistent with literature values.53
Inhibition of Cdk5/p25 activity with p5.
Upon the addition of the 24 amino acid polypeptide p5, we observed a strong reduction in Cdk5/p25 activity. A plausible structure of the complex formed between p5 and Cdk5/p25, derived from our previous computer simulations,51 is shown in Fig. 7c. However, the molecular and regulatory mechanism of p5 action on Cdk5/p25 activity is still under investigation. As in Fig. 7b, the measurements were performed with a Cdk5/p25 concentration of 18.5 nM and a histone concentration of 25.4 μM. Fig. 7d shows the change in VPID when the p5 concentration ([p5]) was increased from 0.25 μM to 1.2 μM. From the figure, we can clearly see the effect of p5-based inhibition. Furthermore, the measurements agree with previous results of the interaction of p5 with the Cdk5/p25 complex obtained using a γ-32P-ATP assay.37 In particular, the sharp decrease in Cdk5/p25 activity past [p5]=0.7 μM. This decrease is indicative of a specific threshold for p5 inhibition and will be studied further in future work.
Conclusions
We show that the operation of commercially sourced nFETs can be optimized by utilizing closed-loop control to maintain a constant channel current at a level above the threshold voltage of the device. In this mode of operation, we demonstrate that single-gate nFETs can achieve a pH resolution of (7.2±0.3)×10−3, ≈3-fold better than traditional ISFETs, and on par with solid-state dg2DFETs which have an intrinsic gain (α=8). Furthermore, the improved performance is attained when the devices are operated in a remote configuration, with the pH sensing element located off-chip and connected electrically to the FET. This remote sensing approach is in contrast to conventional ISFETs which use integrated pH sensing membranes that form the gate dielectric. Our design greatly increases the versatility of nFETs in sensing applications, allowing them to be rapidly and easily interfaced with different biochemical sensors. It also allows the easy integration of other transduction elements into the biosensing setup that can be customized to meet the sensitivity, resolution and other performance needs of individual applications. Finally, the techniques outlined here can also be used to benefit applications that require integrated sensing, for example by improving the performance of traditional ISFETs.
The improved performance of the nFETs, operated in a remote configuration, was shown to be adequate to measure the activity of a pathological form of the proline directed kinase, Cdk5, which has been implicated to cause numerous neurodegenerative conditions including Alzheimer’s disease. By using nFET-based measurements, we confirmed the effectiveness of a custom polypeptide, p5, in re-regulating Cdk5 function. Together the measurements demonstrate the feasibility of performing sensitive bioanalytical measurements with commercially available FETs, thereby drastically lowering the barrier to the adoption of this sensing technology.
Supplementary Material
Acknowledgements
S.T.L. acknowledges support by the National Institute of Standards and Technology (NIST) grant 70NANB16H170. J.B.K. and N.B.G. acknowledge support by NIST grant 70NAHB15H023. A.C. acknowledges support by the NIST grant 70NANB17H259. Research performed in part at the NIST Center for Nanoscale Science and Technology nanofabrication facility.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identifications are not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- 1.Bergveld P, IEEE Trans Biomed Eng, 1970, 17, 70–71. [DOI] [PubMed] [Google Scholar]
- 2.Bergveld P, Sensors & Actuators: B. Chemical, 2003, 88, 1–20. [Google Scholar]
- 3.Duan X, Li Y, Rajan NK, Routenberg DA, Modis Y and Reed MA, Nature Nanotech, 2012, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KS, Jannasch HW, Coletti LJ, Elrod VA, Martz TR, Takeshita Y, Carlson RJ and Connery JG, Anal. Chem, 2016, 88, 3249–3256. [DOI] [PubMed] [Google Scholar]
- 5.Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, Leamon JH, Johnson K, Milgrew MJ, Edwards M, Hoon J, Simons JF, Marran D, Myers JW, Davidson JF, Branting A, Nobile JR, Puc BP, Light D, Clark TA, Huber M, Branciforte JT, Stoner IB, Cawley SE, Lyons M, Fu Y, Homer N, Sedova M, Miao X, Reed B, Sabina J, Feierstein E, Schorn M, Alanjary M, Dimalanta E, Dressman D, Kasinskas R, Sokolsky T, Fidanza JA, Namsaraev E, McKernan KJ, Williams A, Roth GT and Bustillo J, Nature, 2011, 475, 348–352. [DOI] [PubMed] [Google Scholar]
- 6.Mu L, Droujinine IA, Rajan NK, Sawtelle SD and Reed MA, Nano Lett., 2014, 14, 5315–5322. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla N, Di Lorenzo M, Pula G and Estrela P, Sci Rep, 2015, 5, 8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le ST, Guros NB, Bruce RC, Cardone A, Amin ND, Zhang S, Klauda JB, Pant HC, Richter CA and Balijepalli A, Nanoscale, 2019, 11, 15622–15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patolsky F, Timko BP, Yu G, Fang Y, Greytak AB, Zheng G and Lieber CM, Science, 2006, 313, 1100–1104. [DOI] [PubMed] [Google Scholar]
- 10.Bakkum DJ, Frey U, Radivojevic M, Russell TL, Müller J, Fiscella M, Takahashi H and Hierlemann A, Nat. Commun, 2013, 4, 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar D, Liu W, Xie X, Anselmo AC, Mitragotri S and Banerjee K, ACS Nano, 2014, 8, 3992–4003. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Vo R, Hsu H-H, Deng P, Zhang Y and Jiang X, Nano Lett., 2019, acs.nanolett.9b02939. [DOI] [PubMed] [Google Scholar]
- 13.Guros NB, Le ST, Zhang S, Sperling BA, Klauda JB, Richter CA and Balijepalli A, ACS Appl. Mater. Interfaces, 2019, 11, 16683–16692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zafar S, Khater M, Jain V and Ning T, Appl. Phys. Lett, 2015, 106, 063701–5. [Google Scholar]
- 15.Wu T, Alharbi A, You K-D, Kisslinger K, Stach EA and Shahrjerdi D, ACS Nano, 2017, 11, 7142–7147. [DOI] [PubMed] [Google Scholar]
- 16.Zafar S, D'Emic C, Afzali A, Fletcher B, Zhu Y and Ning T, Nanotechnology, 2011, 22, 405501. [DOI] [PubMed] [Google Scholar]
- 17.Spijkman M, Smits ECP, Cillessen JFM, Biscarini F, Blom PWM and de Leeuw DM, Appl. Phys. Lett, 2011, 98, 043502. [Google Scholar]
- 18.Go J, Nair PR and Alam MA, Journal of Applied Physics, 2012, 112, 34516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu L, Droujinine IA, Lee J, Wipf M, Davis P, Adams C, Hannant J and Reed MA, Anal. Chem, 2017, 89, 11325–11331. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Le ST, Richter CA and Hacker CA, Appl. Phys. Lett, 2019, 115, 073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai SB, Madhvapathy SR, Amani M, Kiriya D, Hettick M, Tosun M, Zhou Y, Dubey M, Ager III JW, Chrzan D and Javey A, Adv. Mater, 2016, 28, 4053–4058. [DOI] [PubMed] [Google Scholar]
- 22.Zafar S, Lu M and Jagtiani A, Sci Rep, 2017, 7, 41430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Conde A, Sánchez F, Liou JJ and Cerdeira A, Microelectron. Reliab, 2002, 42, 583–596. [Google Scholar]
- 24.Chu L, Schmidt H, Pu J, Wang S, Özyilmaz B, Takenobu T and Eda G, Sci Rep, 2014, 4, 7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon Y, Ganapathi K and Salahuddin S, Nano Lett., 2011, 11, 3768–3773. [DOI] [PubMed] [Google Scholar]
- 26.Tarasov A, Fu W, Knopfmacher O, Brunner J, Calame M and Schönenberger C, Appl. Phys. Lett, 2011, 98, 012114. [Google Scholar]
- 27.Shah K and Rossie S, Mol. Neurobiol, 2018, 55, 3426–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortés N, Guzmán-Martínez L, Andrade V, Gonzalez A and Maccioni RB, Journal of Alzheimer's Disease, 68, 843–855. [DOI] [PubMed] [Google Scholar]
- 29.Shupp A, Casimiro MC and Pestell RG, Oncotarget, 2017, 8, 17373–17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utreras E, Futatsugi A, Pareek TK and Kulkarni AB, Drug Discov Today Ther Strateg, 2009, 6, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolic M, Dudek H, Kwon YT, Ramos YF and Tsai LH, Genes Dev., 1996, 10, 816–825. [DOI] [PubMed] [Google Scholar]
- 32.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna HC Pant RO Brady LJ MartinKulkarni AB, P Natl Acad Sci USA, 1996, 93, 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA and Robinson PJ, Nature Cell Biology, 2003, 5, 701–710. [DOI] [PubMed] [Google Scholar]
- 34.Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB and McNeish JD, P Natl Acad Sci USA, 2000, 97, 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM and Tsai LH, Nature, 2000, 405, 360–364. [DOI] [PubMed] [Google Scholar]
- 36.Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau L-F and Duff K, Neuron, 2003, 38, 555–565. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y-L, Amin ND, Hu Y-F, Rudrabhatla P, Shukla V, Kanungo J, Kesavapany S, Grant P, Albers W and Pant HC, Journal of Biological Chemistry, 2010, 285, 34202–34212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De La Monte SM, Ganju N, Feroz N, Luong T, Banerjee K, Cannon J and Wands JR, J. Alzheimers Dis, 2000, 2, 261–281. [DOI] [PubMed] [Google Scholar]
- 39.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P and Tsai LH, Nature, 1999, 402, 615–622. [DOI] [PubMed] [Google Scholar]
- 40.Shukla V, Zheng Y-L, Mishra SK, Amin ND, Steiner J, Grant P, Kesavapany S and Pant HC, FASEB J., 2013, 27, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helal CJ, Sanner MA, Cooper CB, Gant T, Adam M, Lucas JC, Kang Z, Kupchinsky S, Ahlijanian MK, Tate B, Menniti FS, Kelly K and Peterson M, Bioorganic & Medicinal Chemistry Letters, 2004, 14, 5521–5525. [DOI] [PubMed] [Google Scholar]
- 42.Helal CJ, Kang Z, Lucas JC, Gant T, Ahlijanian MK, Schachter JB, Richter KEG, Cook JM, Menniti FS, Kelly K, Mente S, Pandit J and Hosea N, Bioorganic & Medicinal Chemistry Letters, 2009, 19, 5703–5707. [DOI] [PubMed] [Google Scholar]
- 43.Knockaert M, Wieking K, Schmitt S, Leost M, Grant KM, Mottram JC, Kunick C and Meijer L, J. Biol. Chem, 2002, 277, 25493–25501. [DOI] [PubMed] [Google Scholar]
- 44.Cicenas J, Kalyan K, Sorokinas A, Stankunas E, Levy J, Meskinyte I, Stankevicius V, Kaupinis A and Valius M, Ann Transl Med, 2015, 3, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Li Y, Liu R, Pang X, Li C, Yang R, He Y, Lian W, Liu A-L and Du G-H, Journal of chemical information and modeling, 2015, 55, 149–164. [DOI] [PubMed] [Google Scholar]
- 46.Persson T, Popescu BO and Cedazo-Minguez A, Oxid Med Cell Longev, 2014, 2014, 427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finley JW and Gao S, J. Agric. Food Chem, 2017, 65, 1005–1020. [DOI] [PubMed] [Google Scholar]
- 48.Zheng YL, Kesavapany S, Gravell M, Hamilton RS, Schubert M, Amin N, Albers W, Grant P and Pant HC, Embo Journal, 2005, 24, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Asad ABMA, Rajkumar R, Cheong WF, Wenk MR, Dawe GS, Chuang K-H, Pant HC and Kesavapany S, J. Neurosci, 2013, 33, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binukumar BK, Zheng Y-L, Shukla V, Amin ND, Grant P and Pant HC, J. Alzheimers Dis, 2014, 39, 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardone A, Brady M, Sriram R, Pant HC and Hassan SA, J. Comput. Aided Mol. Des, 2016, 30, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mapelli M, Massimiliano L, Crovace C, Seeliger MA, Tsai L-H, Meijer L and Musacchio A, J. Med. Chem, 2005, 48, 671–679. [DOI] [PubMed] [Google Scholar]
- 53.Hashiguchi M, Saito T, Hisanaga S-I and Hashiguchi T, J. Biol. Chem, 2002, 277, 44525–44530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.