Abstract
Pregravid obesity results in several complications during pregnancy and is a major determinant of fetal outcomes and offspring health. Specifically, pregravid maternal obesity and in utero exposure to high fat diet have been shown to have detrimental effects on fetal programming, predisposing the offspring to adverse cardiometabolic, endocrine, and neurodevelopmental outcomes. More recently, a deeper appreciation for the modulation of offspring immunity and infectious disease-related outcomes by maternal pregravid obesity has emerged. This review will describe currently available animal models for studying the impact of maternal pregravid obesity on fetal immunity and review the data from clinical and animal model studies. We also examine the burden of pregravid obesity on the maternal-fetal interface and the link between placental and systemic inflammation. Finally, we discuss future studies needed to identify key mechanistic underpinnings that link maternal inflammatory changes and fetal cellular reprogramming events.
Introduction
Almost 36% of women of childbearing age in the US are categorized as obese (BMI > 30) [1]. Several studies have linked high maternal BMI before/during pregnancy with a higher incidence of several obstetric and post-partum complications, including gestational diabetes [2, 3], gestational hypertension, preeclampsia (PE) [4, 5], higher need for caesarean delivery [6], microbial infections (urinary and genital tract infection, and sepsis) [7–11], chorioamnionitis [12, 13], and post-partum surgical site infections [14–17]. Pregravid (pre-pregnancy) obesity also poses significant risks to the fetus including early pregnancy loss, preterm delivery [18, 19], stillbirth [20], and delivery of large-for-gestational-age infants [21].
In line with the developmental origins of health and disease (DOHaD) hypothesis, epidemiologic studies have linked pregravid obesity with detrimental cardiometabolic, neurocognitive, and behavioral outcomes in the offspring [22–28]. More recent studies have also revealed a significant impact on the fetal immune system indicated by higher incidence of bacterial and viral infections in neonates born to mothers with obesity that require admission to the neonatal intensive care unit [29–31]. Moreover, the higher incidence of pregravid obesity has been implicated in the rising incidence of allergic diseases (childhood wheeze and atopy) [32] and asthma [33–35] during early life [36, 37], childhood [38–40], and adolescence [41]. We aim to provide an overview of the impact of pregravid obesity on 1) maternal and offspring immunity, 2) the maternal-fetal interface, and 3) cellular and molecular mechanisms that mediate these changes.
Modeling maternal obesity.
Ex vivo studies using human samples provide the ideal system to study the impact of maternal obesity on fetal immunity [42, 43]. In clinical studies, obesity is defined as a body mass index (BMI) that exceeds 30kg/m2. Clinical studies have the advantage of collecting longitudinal data on fat mass, gestational weight gain, and post-partum weight retention/loss as well as identifying the areas of fat deposition that provide a comprehensive picture of the obesogenic perinatal environment [44]. However, studies involving human samples can also be challenging to control and interpret. Firstly, it is not clear if the experimental readouts in human studies are direct consequences of obesity or mediated by comorbidities associated with obesity. Although large epidemiological studies are successful in establishing associations between maternal BMI and adverse offspring outcomes, they do not provide precise mechanisms of action. Finally, it is not feasible to study the impact of maternal obesity on immune cells in maternal/fetal tissue compartments. Nevertheless, the placenta, cord blood, and the microbiota provide practical avenues to study fetal reprogramming.
The use of animal models obviates some aforementioned hurdles and allows a more mechanistic approach to identify molecular underpinnings of observed outcomes [45]. One major challenge in animal models is establishing the duration of nutritional insult, which is critical in mimicking outcomes observed in human subjects. In this section, we briefly describe the more frequently used animal models for studying the impact of maternal obesity on both the maternal and fetal immune systems. Extensive details of these models have been reviewed in [45–47]. Rodent models are often used to study developmental reprogramming, owing to short gestation, genetic homogeneity, ease of genetic manipulation, and access to multiple tissues [48]. These studies have linked maternal overnutrition using high fat (HFD) or western diet (WD) to poor fetal growth, metabolic syndrome, and behavioral outcomes in the offspring [49–51]. Rat models using Sprague-Dawley and Wistar strains have also been used to study the effects of nutritional status during pregnancy on short term and long-term offspring health [52–54]. In contrast to mouse strains, rats are outbred thereby providing greater genetic heterogeneity and offer the advantage of having larger placentas and fetuses. Finally, rodent models have been developed to study the impact of maternal adiposity on fetal health, independent of dietary fat intake [55]. However, several key differences exist between rodent and human immune systems. First, unlike humans, splenic hematopoiesis persists in rodents for several weeks after birth [56]. Secondly, T and B cell development is not complete at birth and several subsets of T lymphocytes are absent in 10 days old pups, suggesting an immature adaptive immune system at birth [57].
These limitations can be in part overcome by the use of larger animal models. For example, sheep are precocial species like humans and have been used to study fetal growth and metabolic consequences in the context of maternal obesity [58, 59]. Furthermore, sheep models of maternal pregravid obesity have demonstrated increased offspring predisposition to cardiac [60] and hepatic dysfunction [61]. However, sheep have a distinct placentation compared to humans and hence, may not be ideal for studying immune development at the maternal-fetal interface.
Non-human primates (NHPs) are closely related to humans and have been used extensively to study fetal-placental adaptations. NHPs are born with a fully functional immune system that shares significant homology with that of humans, making them a particularly attractive alternative for in utero immune reprogramming studies. Research groups have leveraged baboons [62], rhesus macaques [63–65], and Japanese macaques [66] to model adiposity and inflammation both on the maternal and fetal fronts. Although rhesus macaques have a bidiscoid placenta [67], baboons have very similar placental structure as humans [68].
Early immune development: a window of opportunity for in utero reprogramming
The profile of the immune system and the magnitude of its responses vary significantly with gestational and post birth age. Here, we provide a brief overview of the ontogeny of the fetal immune system, highlighting striking differences in its responses from that of adults (Figure 1). Greater details can be obtained from these additional reviews [69–71]. Fetal hematopoiesis occurs in distinct spatial and temporal sites: the extraembryonic yolk sac, the fetal liver, thymus, and bone marrow. Primitive erythroid macrophage, and megakaryocyte lineage cells appear in blood islands of the yolk sac as early as day 18 of gestation [72]. By gestational week 3–4, hematopoietic cells appear in the fetal liver, reaching roughly 60% of the liver cells between weeks 7 and 15. While neutrophils are present in the human fetal liver parenchyma as early as gestational week 5 [73], monocytes appear in circulation by week 6 following seeding of newly formed self-renewing HSCs in the fetal liver [73, 74]. Monocyte progenitors then colonize various organs and compartmentalize into specialized tissue-resident macrophages that can persist for life [73]. The frequencies of monocytes and dendritic cells (DCs) increase over gestation. However, neutrophils are the most prominent immune cell subtype at birth.
Figure 1:

Overview of the ontogeny of the human immune system and composition of circulating immune cells at birth.
Fetal lymphoid organs (spleen, thymus, and lymph nodes) appear between 8–12 weeks. Mature B-lymphocytes can be detected in the fetal liver at 8 weeks of gestation, then in circulation by week 12 [75], and the primary follicles of the lymph nodes at week 17 [76]. Peripheral and marginal zone B cells then undergo somatic hypermutation forming a diverse B cell receptor repertoire [77]. By contrast, T cell progenitors begin migrating to the thymus at gestational week 8–9 and circulating mature fetal α T lymphocytes are detected at 14 weeks of gestation, several months after detection of subpopulations of innate lymphoid cell subsets. The fetal T cell repertoire continues to diversify significantly during the second and third trimester [77]. Finally, by week 20, human HSCs migrate from the liver to the bone marrow, which takes over as the primary production site for red blood cells, lymphoid progenitors, mature B cells, and myeloid cells. Absolute numbers of major lymphoid subsets stabilize at gestational week 20–26 and are comparable to the numbers seen in newborns [78].
Neonatal environment instructs distinct immune functions
Both intrinsic and extrinsic cellular factors instruct the neonatal immune response to be poised towards a predominantly Th2 phenotype. Cord blood and neonatal serum levels of anti-inflammatory cytokines (IL-4, IL-10, IL-13, and TGF-b) are higher than those observed in adults, supporting a Th2 biased response [79]. Additionally, cord blood contains a higher concentration of immunosuppressive adenosine, which inhibits neutrophil activation [80] and TLR mediated production of Th1 cytokines [81]. However, fetal skin and the mucosal layers produce high levels of antimicrobial proteins and peptides (APPs) such as defensins, lactoferrin, lysozyme, and cathelicidins, which limit infection [82–84]. Moreover, serum levels of hepcidin, which reduce free iron, are high at birth thereby limiting bacterial growth and reducing the risk for neonatal sepsis [85, 86].
Innate Immunity
At birth, neutrophils exhibit quantitative and qualitative differences compared to their adult counterparts [87]. Cord blood and infant neutrophils are poorly phagocytic [88], less chemotactic [89], produce less APP and reactive oxygen species (ROS) in response to pathogenic encounters [90, 91], and exhibit impaired ability to form neutrophil extracellular traps (NETs) compared to their adult counterparts [92]. Monocytes in newborns are primarily classical (CD16low) and produce lower levels of pro-inflammatory cytokines (TNF-α) and Th1 promoting cytokines (IL-12, IFNα), higher levels of IL-10 and IL-1RA [93], but comparable or higher levels of Th-17 promoting cytokines (IL-6, IL-23) compared to adult monocytes [94].
Newborns have fewer conventional DCs (myeloid; mDCs) at birth [95], which progressively increase over the first five years of life [96]. Like monocytes, they are less responsive to TLR stimulation than adult DCs, with significant impairment in IL-12 production [97, 98]. Furthermore, quantitative differences in surface expression of costimulatory molecules CD80 and HLA-DR also exist [99]. Neonatal mDCs also are highly susceptible to external factors such as regulatory B cell suppression [100]. On the other hand, numbers and responses of circulating plasmacytoid dendritic cells (pDCs) are comparable in newborns and adults [101]; however, both neonatal mDCs and pDCs demonstrate reduced ability to generate a polyfunctional response to TLR stimulation. [102].
These age-associated differences in myeloid cell responses have been attributed to cell-intrinsic factors such as: (a) high levels of intracellular cAMP in neonatal cells, which suppress Th1 but enhance Th2 cytokine production [103]; (b) changes in chromatin accessibility that regulate binding of transcription factors such as IRF3 and subsequent cytokine expression [104]; and (c) alterations in histone modifications (trimethylation on H3K4) within promoters of inflammatory genes marking activation [105]. Human cord blood is also highly enriched with immunosuppressive CD71+ erythroid cells [106] that regulate T cell activation and cytokine release from myeloid cells [107]. Frequencies of these cells correlate with gestational age and with gestational age-dependent susceptibility to neonatal infections [108]. Finally, newborn blood is also enriched with myeloid-derived suppressor cells (MDSCs) that play a critical role in controlling inflammation.
Effector functions of innate lymphocytes such as invariant Natural Killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells [109], and gamma delta (γδ) T cells [110] appear to be lower in early life. Natural Killer (NK) cell responses such as degranulation and release of lytic factors are reduced early in neonatal/infant cells compared to adults, explaining increased infant susceptibility to viral infections [111]. This age-associated immaturity in NK cell and innate lymphoid cells has been attributed to TGF-β mediated suppression [112].
Adaptive immunity
The adaptive immune system of the newborn is fully developed but lacks antigenic experience. Specifically, newborns and infants have fewer effector memory T cells (CD45RO+CD45RA-) and B cells (CD27+) [113, 114]. These populations increase with age and antigenic experience. In a newborn, the majority of αβ T lymphocytes are recent thymic emigrants (RTE) [115] and exhibit a distinct epigenetic program compared to adult mature T cells [116, 117]. More often, newborn T cells are epigenetically poised to mount Th2-dominant responses (IL-4 and IL-13) [118–120] and the cytokine profile of neonatal APCs (IL-6 and IL-23) support their robust ability to mount Th-17 responses [121]. Over time, the newborn’s immune system undergoes a gradual shift from Th2 to Th1 responses characterized by the production of IL-12p70 by DCs, promoting cellular immunity and macrophage activation [69].
Neonatal T cells can generate limited Th1 responses characterized by lower IFNγ production by T cells and Th1 polarizing cytokines by APCs [122]. The magnitude of Th1 responses is determined by the quality of signals present at the time of T cell priming and antigen presentation [123]. Similarly, multiple pathogens including CMV, HIV, and T. cruzi can induce effector CD8 T lymphocytes in the neonate [124, 125], albeit with reduced cytotoxicity, and reduced production of antimicrobial peptides and ROS [126]. Additionally, fetal T cells are prone to a more rapid onset of functional exhaustion [127]. Finally, newborns and infants have higher frequencies of regulatory T cells [128] but with reduced capacity to suppress DC function [129]. As described for T cells, neonatal B cells generate reduced primary antibody responses to vaccines and infections due to dampened BCR signaling, decreased plasma cell differentiation and germinal center responses, and lower DC activation signals [130].
Evidence of fetal immune reprogramming by pregravid obesity
Recent studies have documented that the adverse impact of maternal pregravid obesity on fetal cellular developmental processes extend beyond the cardiovascular and central nervous system and have a significant impact on phenotypic and functional aspects of offspring immunity (Figure 2). Clinical studies are limited to the profiling of cord blood, which serves as an excellent surrogate for a snapshot of the fetal immune system at birth [98]. Animal models, on the other hand, facilitate long-term studies and the study of various tissue-resident cells.
Figure 2:
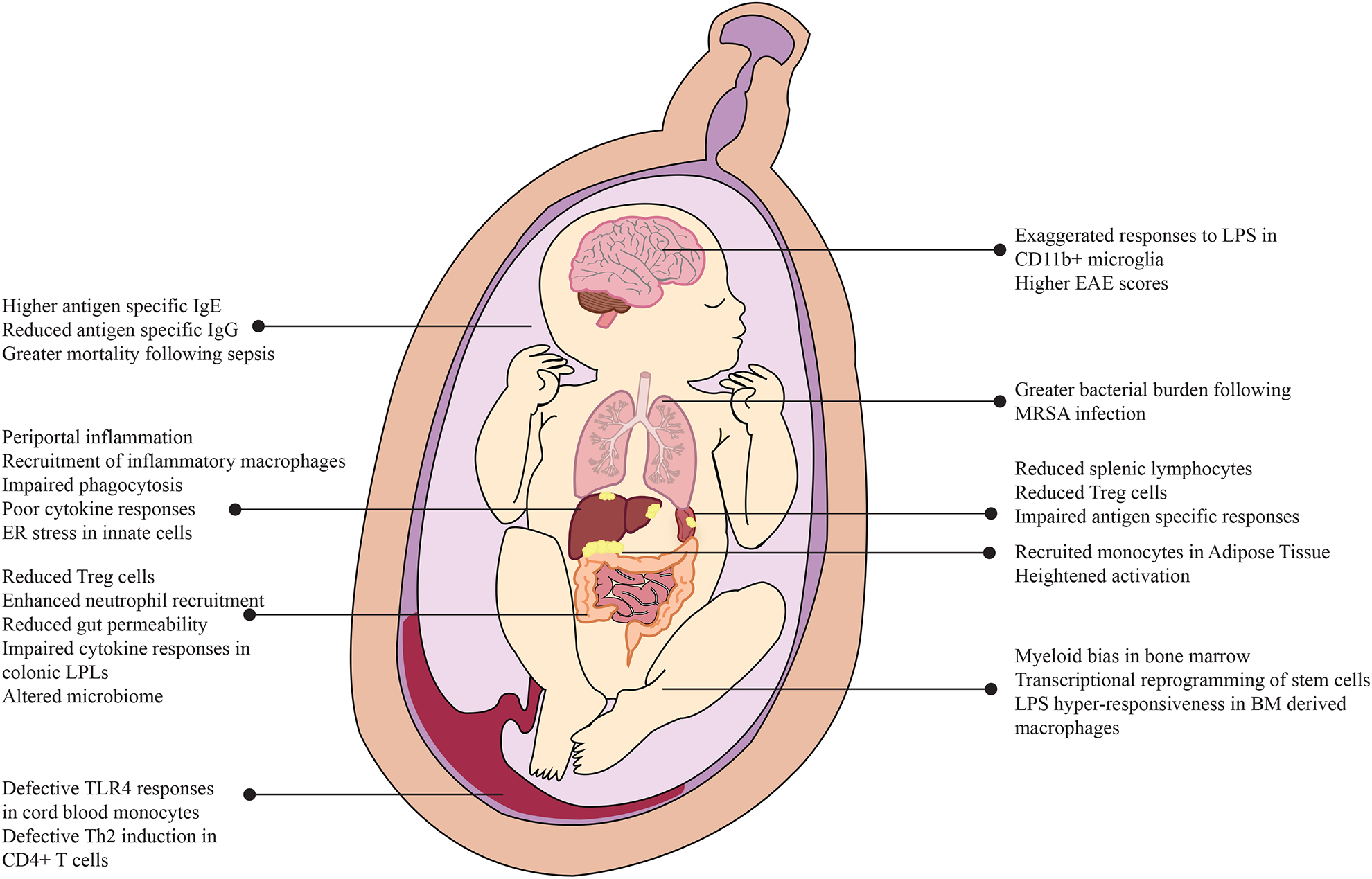
Pleiotropic impact of high pregravid BMI on circulating and tissue resident immune cells as well as lung, adipose tissue, liver, gut, and brain.
Impact of Maternal obesity on phenotypic and functional changes in cord blood cells
There is increasing evidence from human studies linking pregravid maternal BMI and changes in immune cell frequency and phenotype in cord blood [42, 131, 132]. One recent study reported a drop in eosinophils and CD4+ T cells in the cord blood [42] with pregravid obesity after controlling for several maternal factors (race, maternal infection, gestational diabetes, gestational hypertension, and preeclampsia). A second retrospective study reported that pregravid maternal BMI positively correlated with frequencies of NK T cells and regulatory CD8+ T cells and negatively correlated with that of CD34+ stem cells in cord blood samples [131]. More recent work reported higher numbers of immunosuppressive nucleated RBCs [132] in cord blood of babies born to mothers with obesity.
From a functional standpoint, maternal BMI is negatively correlated with cord blood CD4+ T cell IL-4 production [42]. Additionally, cord blood monocytes are hypo-responsive to ex vivo stimulation with TLR agonists in the absence of any differences in the relative frequencies of classical and non-classical monocytes [42] or differences in surface expression of CD14, CD16 or TLR4 [43]. This phenotype was consistent when purified monocytes were used, suggesting a cell-intrinsic defect [43]. A more recent study reported that cord blood monocyte-derived macrophages (MODM) from babies born to mothers with obesity showed a basal anti-inflammatory phenotype and exhibited unbalanced responses to M1 and M2 polarization stimuli [133]. These in vitro findings are line with recent clinical data indicating increased incidences of severe bacterial infections [134] and sepsis [135] necessitating NICU admission in neonates born to mothers with BMI >30 [29, 136] and highlight the importance of cord blood monocytes as critical early pathogen sensors and phagocytes in the neonate. Surprisingly, pregravid maternal BMI did not impact resting gene expression in cord blood monocytes, suggesting a significant role for epigenetic poising. Indeed, dampened monocyte responses to LPS were mirrored at the transcriptional level and was partially explained by loss of DNA cytosine methylation at promoters of inflammatory genes such as PPARG, FOLR2, and ITGAX [43].
Poor ex vivo immune responses in the offspring could potentially be tied to an altered inflammatory intrauterine environment. Very high maternal BMI (>35) has been associated with elevated systemic levels of inflammatory mediators, notably TNFα and CRP in the cord plasma [137, 138]. In addition to these canonical inflammatory markers, maternal BMI is also a strong indicator of cord blood levels of malondialdehyde (MDA) and nitric oxide (NO), contributing to increased oxidative stress in the newborn [139]. In agreement with this hypothesis, transcriptional studies in cord blood have demonstrated elevated expression of genes involved in the cellular response to oxidative stress and inflammatory signaling with high maternal BMI, independent of offspring adiposity [140]. Gene expression studies in NHP models have extended these findings and reported altered expression of several markers of vascular inflammation and altered endothelial cell function in offspring of dams with high pregravid BMI [141]. Additionally, PBMC isolated from offspring of obese and lean baboons (based on pre-pregnancy BMI) revealed widespread transcriptional changes in genes inherent to antigen presentation pathways, complement and coagulation pathways, leukocyte trans-endothelial migration, signaling pathways (BCR signaling, MAPK, and VEGF signaling) [62]. These observations provide evidence of fetal reprogramming events that persist during early childhood.
Maternal obesity and alterations in offspring lymphoid tissue resident cells
Studies using a variety of animal models have assessed the impact of pregravid maternal obesity on tissue-resident immune cells in the offspring (Figure 2). Some of the earliest studies using mice demonstrated that the pups born to dams fed a HFD during gestation had an altered immune system regardless of postnatal diet [142, 143]. One key study concluded that gestational HFD exposure results in fewer splenic lymphocytes and thinner thymic cortex [143]. More importantly, pups born to dams fed a HFD diet during gestation exhibit impaired antigen-specific immune responses as evidenced by: (a) higher mortality rates in an E. coli sepsis model; (b) increased bacterial burden; (c) larger abscesses following Methicillin-resistant Staphylococcus aureus (MRSA) infection [143]; and exacerbated respiratory disease following infection with respiratory syncytial virus (RSV) [144, 145]. Furthermore, in utero HFD exposure resulted in reduced production of IL-6 and TNFα by splenocytes, but exacerbated IL-17, IL-6, and TNFα production by colonic lamina propria lymphocytes (LPLs) [143]. Both in the spleen and the colon, in utero HFD exposure resulted in reduced frequencies of regulatory T cells [143]. In line with that observation, pups born to obese dams were also more likely to develop signs of experimental autoimmune encephalitis (EAE) [143]. Finally, offspring with HFD exposure both during gestation and post weaning produced higher antigen-specific (ovalbumin) IgE and lower IgG following in vivo immunization, potentially linking maternal pregravid obesity to increased risk of allergic responses and autoimmunity in the offspring [142].
Maternal obesity and liver, adipose tissue, and gut homeostasis in the offspring
Several lines of evidence suggest that maternal obesity significantly reprograms the liver of the offspring, contributing to metabolic disease. Exposure to an obesogenic diet both during gestation and post weaning contributes to higher frequencies of Kupffer cells in the offspring liver. Furthermore, Kupffer cells in the offspring of HFD-fed dams were less phagocytic and produced higher levels of ROS [146]. Gene expression studies in infant rhesus macaques exposed to HFD during gestation provide substantial evidence of oxidative stress in fetal livers during the third trimester as evidenced by elevated expression of metabolic enzymes and lipotoxicity [63]. Furthermore, cellular changes in fetal livers are observed as early as 8–12 weeks of gestation, with increased recruitment of pro-inflammatory macrophages in fetuses of dams exposed to WSD [147]. Similarly, in utero exposure to WSD in mice resulted in reprogramming of bone marrow derived macrophages to be hyper-responsive to LPS, as evidenced by elevated Il1b, Tnf, Il6, and Il10 transcript levels [147].
More recent work has mechanistically linked liver inflammation in the offspring of mothers with obesity to alterations in the gut microbiome. Specifically, mice that received a fecal transplant from two-week old human babies born to mothers with obesity exhibit histological evidence of peri-portal inflammation, impaired macrophage phagocytosis, and dampened cytokine production, all hallmarks of non-alcoholic fatty liver disease (NAFLD) [148]. Furthermore, hepatic gene expression signatures in recipient mice suggest ER stress and reprogramming of innate immune cells [148]. Similar phenotypes have also been reported in adipose tissues, where offspring of obese dams had higher CD68, CCR2, and TNFA transcript levels, indicative of a shift towards a more inflammatory phenotype potentially due to increased infiltration of macrophages [149]. Finally, reduced expression of Glut4 gene in the sub-cutaneous fat of these offspring suggests potential metabolic reprogramming [149].
In addition to the liver and adipose depots, exposure to HFD during gestation alters inflammatory responses in the fetal gut. Specifically, rodent studies have reported enhanced susceptibility to DSS induced colitis in the offspring that was accompanied by up regulation of NF-κB signaling and expression of Il1b, Il6, and Il17 genes [150]. This phenotype was associated with increased neutrophil infiltration in the gut, paralleled with higher CCL2 transcripts [150]. A sheep model of maternal obesity reported similar changes in the fetal gut as early as the second trimester, with increased expression of monocyte/macrophage genes TNF, IL1B, IL6, IL8, CCL2, CD68, ITGAM (encoding CD11b), and CD14 in fetal large intestine [151]. Furthermore, both mRNA and protein levels of TGF-β were elevated in gut tissue obtained from offspring of obese ewes. TGF-β plays a critical role in maintaining immune homeostasis and regulating interactions between the microbiota and lymphocytes in the gut [152]. Moreover, increased levels of TGF-β could potentially predispose the offspring to inflammatory conditions such as IBD [153]. In line with these observations, mice exposed to gut microbes from two-week-old infants born to mothers with obesity had impaired gut barrier indicated by reduced gene expression of tight junction proteins Tjp1 and Ocln and increased intestinal permeability [148]. In addition, infants born to mothers with obesity show dysbiosis in their gut microbiome [154, 155] for up to 2 years [156].
Finally, mouse models have recently revealed that the impact of maternal obesity extends to microglia in the offspring brain. Specifically, CD11b+ cells from offspring born to obese dams exhibit exaggerated TNFα responses to LPS [157]. This observation is in line with an increased incidence of autism, and Attention Deficit/Hyperactivity Disorder (ADHD) in offspring of mothers with obesity [158–161].
Pregravid obesity and changes in offspring stem cells
The overall fitness of hematopoietic stem cell (HSC) populations is extremely sensitive to an obesogenic environment. Using a combination of colony formation assays, flow cytometry, and gene expression experiments, studies showed that maternal HFD restricts physiological expansion of fetal HSCs, compromises repopulation of precursor cells in fetal liver, and biases cellular differentiation towards the myeloid branch [162]. This bias towards myeloid lineage and dysregulated hematopoiesis was shown to be dependent on toll-like receptor 4 (TLR4) signaling [163]. Interestingly, metabolic rewiring and increased energy demand of stem cells have been recently reported in mesenchymal stem cells isolated from human cord blood samples obtained from babies born to mothers with obesity [164].
Pregravid obesity and inflammation at the maternal-fetal interface
A successful pregnancy involves complex interactions between the immune cells residing at the maternal-fetal interface [165]. Structurally, the interface between the uterine mucosa and extraembryonic tissue comprise the “maternal-fetal interface” or the placenta. At term, this interface includes the maternal portion called the decidua (or decidua basalis), which is primarily the uterine mucosal layer after it has undergone implantation-associated differentiation process called decidualization. The fetal portion of the placenta is called the villous chorion, which allows for the transfer of nutrients from maternal blood to fetal circulation. During the first trimester, the placenta enables the establishment of pregnancy, providing physical support and immunologic tolerance to the developing embryo [166]. As gestation progresses, the placenta takes over the roles of facilitating nutrients, waste, and gas exchange, and produces hormones and other factors that support fetal growth. Finally, closer to parturition, the cells in the placenta promote contraction of the uterus, the expulsion of the baby and rejection of the placenta [167]. In this section, we briefly summarize the impact of pregravid obesity on placental structure and function as well as the role of these changes in reprogramming the fetal immune system (Figure 3).
Figure 3:

Impact of obesity on maternal systemic immunity and adaptations at the maternal-fetal interface.
Data from animal model studies show that consumption of HFD increased placental glucose, amino acid, and fatty acid nutrient transport in dams [53, 168–171]. Evidence for altered trophoblastic invasion and vessel remodeling have been reported in a rat model of HFD induced obesity [172, 173]. Furthermore, placentas of rhesus macaque dams fed a HFD exhibited reduced blood flow, increased calcification and risk for infarction [66]. A reduction in placental microvessel density has been observed as early as mid-gestation in a mouse model of diet-induced obesity [174]. In obese human subjects, significant structural changes in the placenta have been observed, ranging from alterations in placental maturity, vessel density, muscularity in blood vessels [175] and non-branching angiogenesis in term placentas [176].
Inflammation in the placenta has a bidirectional effect on both the maternal and fetal immune systems [177]. Transcriptional studies using human term placenta have reported changes in genes regulating inflammation, lipid storage and transport [178], insulin resistance, as well as angiogenesis in both the decidua [179] and villi [180, 181] with pregravid obesity. Similarly, RNA-Seq analysis of villous tissue revealed down-regulation of several genes involved in inflammation (IL1R2, IL2RB, C3, TNFSF10), immune tolerance (HLAG), cell matrix communication, nutrient transport, and retinoic acid metabolism with pregravid obesity [181]. While the source of placental inflammation is not clear, it has been linked to the high levels of oxidative stress and lipotoxicity in the decidua of mothers with obesity [180]. In the next section, we describe the consequences of pregravid obesity on the immune landscape at the maternal-fetal interface.
Obesity associated adaptations in the placenta: impact on the microbiome and immune cell landscape
A combination of 16S rDNA and whole-genome shotgun metagenomic analyses has showed that the placenta harbors a low abundance but metabolically rich microbiome [182] that varies from the decidua to basal plates to the fetal membranes [183]. This microbial community is disrupted with preterm labor [184], preeclampsia [185] and gestational weight gain [186], and pregravid obesity [181]. Some of these studies were carried out using placenta samples of preterm births; therefore, our understanding of the impact of pregravid obesity on microbial communities within the term placenta remains limited.
Technical advances in single cell RNA sequencing has allowed for high throughput profiling of placenta-resident immune cells in an unbiased manner in the early placental bed [166, 187]. These studies indicate the presence of NK cells, macrophages, and T-cells as well as differences in origin, phenotype, and function of immune cells within the decidua and villous compartments. For example, the decidual immune cells, which are of maternal origin, are primarily composed of NK cells, macrophages, and T cells. Immune cells in the villi, which are of fetal origin, are primarily composed of macrophages called “Hofbauer cells” [166]. Both diversity and phenotypes of placenta-resident immune cells have been shown to considerably shift over the course of gestation [188–190]. For instance, studies have shown a shift in placental cytokine milieu (decreased IL-35 and IL-9) and concurrent drop in CD11c+ myleloid cells and regulatory T cell populations over the course of gestation [188].
Local changes in frequencies, phenotype, and functions of immune cells in the decidua and villi have been attributed in the pathogenesis of several obstetric complications such as preterm labor [191], preeclampsia [192–194] and chorioamnionitis [195]. Given the increased frequencies of these pathologies with high pre-pregnancy BMI, it is widely believed that alterations in placental immune cells could be the drivers. Indeed, first trimester placentas from women with obesity show reduced uterine NK cell numbers, which normally comprise ~70% of the leukocyte population in early human decidua and play a central role in uterine artery remodeling and altered expression of genes in pathways associated with matrix remodeling and growth factor signaling [196]. Furthermore, first trimester uNK cells from women with obesity exhibited an imbalance in expression of NK cell receptors KIR2DL1 (inhibitory receptor) and KIR2DS1 (activating receptor), ultimately favoring HLA-C2 directed activation [197]. Finally, leptin levels have been linked to altered expression of CD56 and CD16 on circulating NK cells during pregnancy [198]. Therefore, it is possible that obesity-associated increase in leptin levels could also modulate phenotype and function of uterine NKs, however, this hypothesis has yet to be tested.
Macrophages comprise 20% of the decidual leukocyte population. These cells are distinct in their origin but are functionally similar to their fetal counterpart called the “Hofbauer cells”. Decidual macrophages play important roles in maintaining immune tolerance [199], protecting the fetus against infectious agents [200], spinal artery remodeling [201], and clearance of apoptotic bodies from the placental vasculature [202]. Although phenotypic and functional changes in the decidual macrophage populations over the course of gestation remain ill defined, these cells are extremely heterogeneous [203]. In humans, pregravid obesity results in an increased accumulation of macrophages (CD68+) in the placenta [175, 204]. In line with these observations, higher transcript levels of pro-inflammatory genes IL6, CCL2, IL8, and TLR4 are detected in a baboon model of HFD-induced obesity [66, 151, 205]. Similarly, chorionic villi macrophages obtained from placentas of diet-induced obese dams generate exacerbated responses to LPS (higher TNFα, IL-6, and IL-1β) both at the gene and protein levels, suggesting functional reprogramming of fetal macrophages as well [157]. A more recent study demonstrated reduced frequency of M1-like macrophages (HLA-DR+ CD163-) in the decidua with pregravid obesity [206], potentially a compensatory mechanism against obesity-associated inflammation.
Pregravid-obesity induced changes in systemic maternal immunity
Given that a number of immune cells in the placental bed (NK cells, T cells, and subsets of macrophages) are recruited from peripheral blood during gestation, BMI-associated alteration in inflammatory pathways in the placenta could potentially be linked to systemic changes. Indeed, several studies have linked alterations in frequencies and phenotypes of immune cells in the placenta with circulating cytokines and markers of inflammation. In this section, we briefly describe pregnancy-associated alterations in the maternal circulating immune system and disruptions triggered by an obesogenic environment.
Recent proteomic and immunological studies in peripheral blood have suggested the presence of an “immunological clock of pregnancy” characterized by enhanced immune activation with gestation [207, 208]. Changes in the maternal adaptive immune include decreased Th1/Th17 and increased Th2/Treg responses [207, 209, 210] as well as increased surface expression of CD11a, CD11b, CD49d, CD14, CD64, and CD54 on monocytes during third trimester [211–213]. Moreover, the production of pro-inflammatory cytokines IL-1β, IL-12, IL-6 and oxygen free radicals following stimulation with LPS or bacteria [207, 214, 215] as well as responses to viral particles by NK cells [216], monocytes [207] and plasmacytoid dendritic (pDC) cells [217] were enhanced with gestation. While the mechanisms regulating these changes are still unknown, several studies have hypothesized a role for placental microparticles [218], fetal DNA [219], and/or maternal metabolic and cytokine changes [208, 220]. However, it should be noted that these studies were largely carried out using peripheral blood mononuclear cells (PBMC) and therefore intrinsic changes within specific immune cell subsets remain largely ill defined.
Obesity exacerbates inflammatory changes normally associated with pregnancy [221] (Figure 3). While most studies have consistently reported increased IL-6 levels with higher pregravid BMI in pregnant women [9, 175, 222–229], others have reported no associations at all [230–232]. These inconsistencies arise due to sample size limitations, variability in exclusion criteria, racial differences in cohorts, and the precise time of sample collection (first, second, third trimester, at term, during labor or post-partum). On the other hand, all studies report a significant increase in CRP, a canonical marker of inflammation produced by the liver, during various stages of pregnancy with pregravid BMI [9, 181, 204, 223–225, 229, 232–234]. Pregravid obesity also impacts circulating levels of GM-CSF and FGF-2 [181]. In contrast, several studies found no association between high pregravid BMI and circulating TNFα [204, 230, 231, 233, 235, 236] IL-1β [175, 181, 225, 230, 237], or anti-inflammatory IL-10 [181, 223, 228, 229] during pregnancy.
Profiles of circulating chemokines have also been shown to vary with pregnancy [238, 239]. Significant shifts in chemokine profiles, notably MCP1 at the maternal-fetal interface, have been shown to trigger labor [240] and in some cases even initiate obstetric complications [239]. Pregravid obesity has been shown to elevate circulating innate immune cell recruiting factors CCL2 at various stages of pregnancy [181, 228, 231, 241] and CXCL8 at term [181]. There is emerging evidence of some cytokines such as IL-6 bidirectionally crossing placental barriers [242], however, whether similar patterns exist for other hormone/cytokines is still unclear.
Glucose tolerance and insulin resistance, often observed during pregnancy, are exacerbated with high pregravid BMI. Women with obesity exhibit significantly high levels of insulin at term, which vary linearly with BMI [181]. Similarly, other mediators associated with insulin resistance are also impacted by obesity. For example, leptin, a pro-inflammatory mediator that modulates appetite, implantation, and immune responses during pregnancy increases with gestation [243] and drops immediately after delivery [244, 245]. During pregnancy [246], serum leptin levels positively correlate with BMI and fat mass and remain significantly higher in obese mothers at term [181, 246, 247]. In contrast, while some studies report a positive association between pregravid BMI and circulating resistin levels [248, 249], others report no relationship [250, 251]. Taken together, these studies highlight the systemic impact of high BMI on chronic inflammation, immune activation, glucose tolerance, and insulin resistance.
Linking maternal obesity and fetal immune reprogramming – role of epigenetic mechanisms
The final section in this review summarizes mechanisms that link the maternal obesogenic environment with immune dysregulation observed in the offspring (Figure 4). Given the strong association between maternal pregravid obesity and evidence of systemic inflammation as well as in the liver, adipose tissue, and placenta, one can hypothesize that the fetus is exposed to a heightened inflammatory milieu. The placenta also harbors cells (immune and non-immune) that respond to conditions of oxidative stress and nutrient deficits by secreting various immune mediators, hormones, and growth factors into the fetal circulation. Newborns of mothers with obesity have relatively higher levels of leptin, and IL-6 in cord blood compared to newborns of lean mothers [224]. However, no studies to date have systematically measured maternal and cord blood cytokine levels in matched mother-baby pairs to assess potential transplacental transfer of inflammatory factors with the exception of IL-6 [242, 252].
Figure 4:

Mechanisms linking obesity associated changes on the maternal front and adverse immunological outcomes in the fetus and offspring.
Maternal lipids are also known to cross the placenta and contribute to fetal cholesterol synthesis, particularly during early gestation [253]. Fetal exposure to excess lipids, particularly saturated fatty acids, can activate pro-inflammatory pathways impacting nutrient metabolism, mitochondrial function, hematopoietic stem cell fate, and immune cell plasticity. Excess nutrients such as lipids can rewire cellular metabolism through epigenetic mechanisms, triggering transcriptional adaptations that ultimately alter immune cell function. Evidence of transcriptional reprogramming has been reported as early as the blastocyst stage in rats, with up-regulation of NF-κB regulated Ccl4 and Ccl5 in embryos of obese dams [254]. Pregravid maternal obesity has also been shown to alter expression of mitochondrial and lipid metabolism genes in human infant umbilical vein endothelial cells [255]. Stable changes in gene expression can be regulated via epigenetic mechanisms such as DNA methylation, and chromatin remodeling, and post-transcriptional regulation via microRNAs, which have been hypothesized as central to the fetal origins of adult health and disease [256].
DNA methylation patterns are established mainly during embryogenesis/early postnatal life. Within the immune system, DNA methylation play critical roles in hematopoiesis and can vary within cells of a particular lineage depending on their source (fetal liver, cord blood, bone marrow vs. peripheral blood) [257]. Additionally, methylation patterns overlapping enhancers and promoters of lineage-determining factors define differentiation trajectories of immune cells during development [257]. High maternal BMI is inversely correlated with methylation levels within the promoter of inflammatory genes in cord blood mononuclear cells [258]. Patterns of global loss in methylation seen in cord blood leukocytes from offspring born to mothers with obesity remained consistent up to three years of age [259]. Recent studies carried out using targeted bisulfite sequencing in purified cord blood monocytes demonstrated that pregravid maternal obesity is associated with DNA hypomethylation, particularly at promoters of transcription factors such as PPARG that are associated with a dampened transcriptional responses to LPS [43].
Maternal pregravid obesity is also associated with reduced abundance of the repressive histone mark H3K27me3 and increase expression of Zfp423, a key regulator committing cells to adipogenic lineage [260] within fetal adipose tissue in rodent models. Furthermore, altered expression of Wnt genes in livers of offspring born to dams on HFD show hyper-acetylation of Histone H4 and H3K9 [261]. Additionally, studies in Japanese macaques have demonstrated elevated acetylation at H3K9, H3K14, and H3K18 in fetal hepatic tissue exposed to HFD in utero [262]. Studies in animal models have suggested the potential role for histone deacetylases, mainly sirtuins in these epigenetic changes. Reduced SIRT1 activity in the fetal livers of macaque fetuses exposed to HFD during gestation were linked to altered expression of metabolic regulators PPARs and fatty acid synthases [263] as well as impaired liver mitochondrial SIRT3 expression [264]. Moreover, increased expression of the repressive methylation (H3K9Me3) was noted on TLR4 and LBP gene promoters in splenocytes of HFD-fed dams offspring contributing to poor responses to LPS stimulation [143]. These epigenetic “tags” could function as mechanisms of cellular memory within the fetal compartment in response to sustained maternal inflammation.
The precise series of events by which maternal environment/inflammation alter fetal epigenome are still unclear. However, it has been hypothesized that excess lipid exposure in utero [265] could impact cellular metabolism in immune precursor cells [162] thereby impacting epigenetic profiles of mature fetal immune cells. Additionally, inter-placental transfer of maternal inflammatory factors (including maternal cytokines and metabolic hormones) could establish immunological tolerance in fetal cells. Leptin, which functions as the nutritional signal in the fetus, is produced by the placenta and released into circulation. Some studies have reported elevated leptin levels in cord blood with higher maternal BMI [42, 224] where it is negatively associated with leptin promoter methylation [266]. Furthermore, a more recent study has demonstrated hypermethylation of LEPR (leptin receptor) promoter in the villi of mothers with higher BMI [267]. Whether these changes alter gene expression or surface expression of leptin receptor within cord blood immune cells would be an exciting avenue for future research, given the poignant role for leptin in both nutrient sensing and regulating immune responses.
Whether these epigenetic changes contribute to the adverse outcomes seen in offspring of obese mothers or if these cellular adaptations to sustained in utero exposure to inflammation is still up for debate. Future work in animal models will have to test if changes in fetal cells can be linked to specific changes in offspring immune function. Nevertheless, these studies demonstrate the importance of epigenetics in intergenerational health and provide a potential mechanism by which maternal obesity could program fetal immune function.
CONCLUDING REMARKS
Despite the recent progress made assessing the specific burden of maternal obesity on several compartments of fetal immunity, the specific sequences of events that link maternal inflammation with fetal outcomes is still unclear. Several other questions still remain: Is there a specific window during gestation when the fetus is most susceptible to maternal obesogenic environment? Are there cell extrinsic factors (cytokines, hormones, defective cell to cell communication) that contribute to dysregulated immune responses in the fetus? Are there factors that cross the placental barrier skewing fetal immune cells to a refractory state or is it just an outcome of poor immune development? Are these adverse outcomes in the offspring reversible with maternal pre-pregnancy weight loss interventions or therapies? While large human cohort-based studies are able to identify specific defects in immune responses, the genomic and epigenomic determinants and more importantly causality can only be tested in well-designed animal models. These models can to a large extent overcome the complexity of homeostatic gestational immune adaptations both in the blood and in the placenta, and genetic and environmental confounders in human subjects. Deep profiling of immune cells using a combination of single cell technologies will expedite and provide a more accurate assessment of the immunological outcomes to specific perturbations. Elucidating these molecular underpinnings will be crucial in designing new effective therapeutic strategies or leveraging existing therapies for amelioration of maternal obesity-associated changes in offspring immunity.
ACKNOWLEDGEMENTS
We thank Selene Bich Nguyen for generating illustrations presented in this manuscript. This work was by the National Institutes of Health (R03AI112808, 1K23HD06952, and 1R01AI142841).
Abbreviations
- BMI
Body Mass Index
- WD
Western Diet
- HFD
High Fat Diet
- PE
Preeclampsia
- GH
Gestational Hypertension
- GD
Gestational Diabetes
- NHP
Non Human Primate
- HSC
Hematopoietic Stem Cel
- (p/m) DC
(plasmacytoid/myeloid) Dendritic Cell
- NK
Natural Killer
- MODM
Monocyte-Derived Macrophage
- TLR
Toll-like Receptor
- NLR
NOD-like Receptor
- APP
Antimicrobial Proteins and Peptides
- PRR
Pattern Recognition Receptor
- MDSC
Myeloid-Derived Suppressor Cell
- APC
Antigen Presenting Cell
- ROS
Reactive Oxygen Species
- CRP
C-reactive protein
- LPS
Lipopolysaccharide
- HLA
Human Leukocyte Antigen
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM (2013) Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- 2.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM (2007) Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30, 2070–6. [DOI] [PubMed] [Google Scholar]
- 3.Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O (2009) Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 10, 194–203. [DOI] [PubMed] [Google Scholar]
- 4.Salihu HM, De La Cruz C, Rahman S, August EM (2012) Does maternal obesity cause preeclampsia? A systematic review of the evidence. Minerva Ginecol 64, 259–80. [PubMed] [Google Scholar]
- 5.Wang Z, Wang P, Liu H, He X, Zhang J, Yan H, Xu D, Wang B (2013) Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes Rev 14, 508–21. [DOI] [PubMed] [Google Scholar]
- 6.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM (2007) Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev 8, 385–94. [DOI] [PubMed] [Google Scholar]
- 7.Robinson HE, O’Connell CM, Joseph KS, McLeod NL (2005) Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 106, 1357–64. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton RD, Kahn JM, Evans LE, Critchlow CW, Gardella CM (2005) Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet Gynecol 106, 1246–52. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Hauguel-de Mouzon S (2011) Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 19, 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLean M, Hines R, Polinkovsky M, Stuebe A, Thorp J, Strauss R (2012) Type of skin incision and wound complications in the obese parturient. Am J Perinatol 29, 301–6. [DOI] [PubMed] [Google Scholar]
- 11.Magann EF, Doherty DA, Sandlin AT, Chauhan SP, Morrison JC (2013) The effects of an increasing gradient of maternal obesity on pregnancy outcomes. Aust N Z J Obstet Gynaecol 53, 250–7. [DOI] [PubMed] [Google Scholar]
- 12.Korkmaz L, Bastug O, Kurtoglu S (2016) Maternal Obesity and its Short- and Long-Term Maternal and Infantile Effects. J Clin Res Pediatr Endocrinol 8, 114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadley EE, Discacciati A, Costantine MM, Munn MB, Pacheco LD, Saade GR, Chiossi G (2019) Maternal obesity is associated with chorioamnionitis and earlier indicated preterm delivery among expectantly managed women with preterm premature rupture of membranes. J Matern Fetal Neonatal Med 32, 271–278. [DOI] [PubMed] [Google Scholar]
- 14.Salim R, Braverman M, Teitler N, Berkovic I, Suliman A, Shalev E (2012) Risk factors for infection following cesarean delivery: an interventional study. J Matern Fetal Neonatal Med 25, 2708–12. [DOI] [PubMed] [Google Scholar]
- 15.Anderson V, Chaboyer W, Gillespie B (2013) The relationship between obesity and surgical site infections in women undergoing caesarean sections: an integrative review. Midwifery 29, 1331–8. [DOI] [PubMed] [Google Scholar]
- 16.Paiva LV, Nomura RM, Dias MC, Zugaib M (2012) Maternal obesity in high-risk pregnancies and postpartum infectious complications. Rev Assoc Med Bras (1992) 58, 453–8. [PubMed] [Google Scholar]
- 17.Meenakshi, Srivastava R, Sharma NR, Kushwaha KP, Aditya V (2012) Obstetric behavior and pregnancy outcome in overweight and obese women: maternal and fetal complications and risks in relation to maternal overweight and obesity. J Obstet Gynaecol India 62, 276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, Granath F (2013) Maternal obesity and risk of preterm delivery. JAMA 309, 2362–70. [DOI] [PubMed] [Google Scholar]
- 19.Aune D, Saugstad OD, Henriksen T, Tonstad S (2014) Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 311, 1536–46. [DOI] [PubMed] [Google Scholar]
- 20.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, Curtis KM (2007) Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol 197, 223–8. [DOI] [PubMed] [Google Scholar]
- 21.Norman JE and Reynolds RM (2011) The consequences of obesity and excess weight gain in pregnancy. Proc Nutr Soc 70, 450–6. [DOI] [PubMed] [Google Scholar]
- 22.Gaillard R, Felix JF, Duijts L, Jaddoe VW (2014) Childhood consequences of maternal obesity and excessive weight gain during pregnancy. Acta Obstet Gynecol Scand 93, 1085–9. [DOI] [PubMed] [Google Scholar]
- 23.Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW (2015) Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond) 39, 677–85. [DOI] [PubMed] [Google Scholar]
- 24.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, Jaddoe VW (2014) Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 63, 683–91. [DOI] [PubMed] [Google Scholar]
- 25.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB (2009) Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 90, 1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X (2013) Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One 8, e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tie HT, Xia YY, Zeng YS, Zhang Y, Dai CL, Guo JJ, Zhao Y (2014) Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: a meta-analysis. Arch Gynecol Obstet 289, 247–57. [DOI] [PubMed] [Google Scholar]
- 28.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, Broekman BF (2017) Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 5, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastogi S, Rojas M, Rastogi D, Haberman S (2015) Neonatal morbidities among full-term infants born to obese mothers. J Matern Fetal Neonatal Med 28, 829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suk D, Kwak T, Khawar N, Vanhorn S, Salafia CM, Gudavalli MB, Narula P (2016) Increasing maternal body mass index during pregnancy increases neonatal intensive care unit admission in near and full-term infants. J Matern Fetal Neonatal Med 29, 3249–53. [DOI] [PubMed] [Google Scholar]
- 31.Yang Z, Phung H, Freebairn L, Sexton R, Raulli A, Kelly P (2018) Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. [DOI] [PubMed] [Google Scholar]
- 32.Rajappan A, Pearce A, Inskip HM, Baird J, Crozier SR, Cooper C, Godfrey KM, Roberts G, Lucas JSA, Pike KC, Southampton Women’s Survey Study, G. (2017) Maternal body mass index: Relation with infant respiratory symptoms and infections. Pediatr Pulmonol 52, 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pike KC, Inskip HM, Robinson SM, Cooper C, Godfrey KM, Roberts G, Lucas JS, Southampton Women’s Survey Study, G. (2013) The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax 68, 372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerra S, Sartini C, Mendez M, Morales E, Guxens M, Basterrechea M, Arranz L, Sunyer J (2013) Maternal prepregnancy obesity is an independent risk factor for frequent wheezing in infants by age 14 months. Paediatr Perinat Epidemiol 27, 100–8. [DOI] [PubMed] [Google Scholar]
- 35.Watson PE and McDonald BW (2013) Subcutaneous body fat in pregnant New Zealand women: association with wheeze in their infants at 18 months. Matern Child Health J 17, 959–67. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Story RE, Pongracic JA, Hong X, Arguelles L, Wang G, Kuptsova-Clarkson N, Pearson C, Ortiz K, Bonzagni A, Apollon S, Fu L, Bauchner H, Wang X (2010) Maternal Pre-Pregnancy Obesity and Recurrent Wheezing in Early Childhood. Pediatr Allergy Immunol Pulmonol 23, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumas O, Varraso R, Gillman MW, Field AE, Camargo CA Jr. (2016) Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 71, 1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haberg SE, Stigum H, London SJ, Nystad W, Nafstad P (2009) Maternal obesity in pregnancy and respiratory health in early childhood. Paediatr Perinat Epidemiol 23, 352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Postma DS, Oldenwening M, de Jongste JC, Smit HA (2010) Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond) 34, 606–13. [DOI] [PubMed] [Google Scholar]
- 40.Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B (2011) Maternal obesity during pregnancy as a risk for early-life asthma. J Allergy Clin Immunol 128, 1107–9 e1–2. [DOI] [PubMed] [Google Scholar]
- 41.Patel SP, Rodriguez A, Little MP, Elliott P, Pekkanen J, Hartikainen AL, Pouta A, Laitinen J, Harju T, Canoy D, Jarvelin MR (2012) Associations between pre-pregnancy obesity and asthma symptoms in adolescents. J Epidemiol Community Health 66, 809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I (2015) Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol 26, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sureshchandra S, Wilson RM, Rais M, Marshall NE, Purnell JQ, Thornburg KL, Messaoudi I (2017) Maternal Pregravid Obesity Remodels the DNA Methylation Landscape of Cord Blood Monocytes Disrupting Their Inflammatory Program. J Immunol 199, 2729–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y (2010) Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 34 Suppl 2, S4–17. [DOI] [PubMed] [Google Scholar]
- 45.Williams L, Seki Y, Vuguin PM, Charron MJ (2014) Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta 1842, 507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfaradhi MZ and Ozanne SE (2011) Developmental programming in response to maternal overnutrition. Front Genet 2, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathanielsz PW, Poston L, Taylor PD (2007) In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Obstet Gynecol Clin North Am 34, 201–12, vii–viii. [DOI] [PubMed] [Google Scholar]
- 48.Li M, Sloboda DM, Vickers MH (2011) Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res 2011, 592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA (2009) Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr 102, 514–9. [DOI] [PubMed] [Google Scholar]
- 50.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–92. [DOI] [PubMed] [Google Scholar]
- 51.Bouanane S, Merzouk H, Benkalfat NB, Soulimane N, Merzouk SA, Gresti J, Tessier C, Narce M (2010) Hepatic and very low-density lipoprotein fatty acids in obese offspring of overfed dams. Metabolism 59, 1701–9. [DOI] [PubMed] [Google Scholar]
- 52.Dunn GA and Bale TL (2009) Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T (2009) High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J 23, 271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW (2009) Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4, e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM (2008) Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol 294, R528–38. [DOI] [PubMed] [Google Scholar]
- 56.Holladay SD and Smialowicz RJ (2000) Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 108 Suppl 3, 463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ladics GS, Smith C, Bunn TL, Dietert RR, Anderson PK, Wiescinski CM, Holsapple MP (2000) Characterization of an approach to developmental immunotoxicology assessment in the rat using SRBC as the antigen. Toxicol Method 10, 283–311. [Google Scholar]
- 58.George LA, Uthlaut AB, Long NM, Zhang L, Ma Y, Smith DT, Nathanielsz PW, Ford SP (2010) Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol 8, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long NM, Rule DC, Tuersunjiang N, Nathanielsz PW, Ford SP (2015) Maternal obesity in sheep increases fatty acid synthesis, upregulates nutrient transporters, and increases adiposity in adult male offspring after a feeding challenge. PLoS One 10, e0122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y, Yan X, Zhao JX, Zhu MJ, McCormick RJ, Ford SP, Nathanielsz PW, Ren J, Du M (2010) Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab 299, E968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, Walker SK, Morrison JL, Zhang S, Muhlhausler BS, Martin-Gronert MS, McMillen IC (2013) Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J 27, 3786–96. [DOI] [PubMed] [Google Scholar]
- 62.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ Jr., Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE (2009) Feto-placental adaptations to maternal obesity in the baboon. Placenta 30, 752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL (2009) Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119, 323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL (2010) Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151, 1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL (2010) Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 30, 3826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL (2011) Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grigsby PL (2016) Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin Reprod Med 34, 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL (2003) The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res 11, 75–80. [DOI] [PubMed] [Google Scholar]
- 69.Kollmann TR, Levy O, Montgomery RR, Goriely S (2012) Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37, 771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dowling DJ and Levy O (2014) Ontogeny of early life immunity. Trends Immunol 35, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O (2017) Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 46, 350–363. [DOI] [PubMed] [Google Scholar]
- 72.Fukuda T (1973) Fetal hemopoiesis. I. Electron microscopic studies on human yolk sac hemopoiesis. Virchows Arch B Cell Pathol 14, 197–213. [PubMed] [Google Scholar]
- 73.De Kleer I, Willems F, Lambrecht B, Goriely S (2014) Ontogeny of myeloid cells. Front Immunol 5, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikkola HK and Orkin SH (2006) The journey of developing hematopoietic stem cells. Development 133, 3733–44. [DOI] [PubMed] [Google Scholar]
- 75.Thilaganathan B, Nicolaides KH, Mansur CA, Levinsky RJ, Morgan G (1993) Fetal B lymphocyte subpopulations in normal pregnancies. Fetal Diagn Ther 8, 15–21. [DOI] [PubMed] [Google Scholar]
- 76.Pahal GS, Jauniaux E, Kinnon C, Thrasher AJ, Rodeck CH (2000) Normal development of human fetal hematopoiesis between eight and seventeen weeks’ gestation. American Journal of Obstetrics and Gynecology 183, 1029–1034. [DOI] [PubMed] [Google Scholar]
- 77.Rechavi E, Lev A, Lee YN, Simon AJ, Yinon Y, Lipitz S, Amariglio N, Weisz B, Notarangelo LD, Somech R (2015) Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med 7, 276ra25. [DOI] [PubMed] [Google Scholar]
- 78.Rainaut M, Pagniez M, Hercend T, Daffos F, Forestier F (1987) Characterization of Mononuclear Cell Subpopulations in Normal Fetal Peripheral-Blood. Hum Immunol 18, 331–337. [DOI] [PubMed] [Google Scholar]
- 79.Belderbos ME, Levy O, Meyaard L, Bont L (2013) Plasma-mediated immune suppression: a neonatal perspective. Pediatr Allergy Immunol 24, 102–13. [DOI] [PubMed] [Google Scholar]
- 80.Hasko G and Cronstein B (2013) Regulation of inflammation by adenosine. Front Immunol 4, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pettengill M, Robson S, Tresenriter M, Millan JL, Usheva A, Bingham T, Belderbos M, Bergelson I, Burl S, Kampmann B, Gelinas L, Kollmann T, Bont L, Levy O (2013) Soluble ecto-5’-nucleotidase (5’-NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem 288, 27315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR (2007) Innate immune defences in the human uterus during pregnancy. Placenta 28, 1099–106. [DOI] [PubMed] [Google Scholar]
- 83.Wiesner J and Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1, 440–64. [DOI] [PubMed] [Google Scholar]
- 84.Dorschner RA, Lin KH, Murakami M, Gallo RL (2003) Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res 53, 566–72. [DOI] [PubMed] [Google Scholar]
- 85.Bullen J, Griffiths E, Rogers H, Ward G (2000) Sepsis: the critical role of iron. Microbes Infect 2, 409–15. [DOI] [PubMed] [Google Scholar]
- 86.Johnson EE and Wessling-Resnick M (2012) Iron metabolism and the innate immune response to infection. Microbes Infect 14, 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carr R (2000) Neutrophil production and function in newborn infants. Br J Haematol 110, 18–28. [DOI] [PubMed] [Google Scholar]
- 88.Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M (2011) Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 11, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prosser A, Hibbert J, Strunk T, Kok CH, Simmer K, Richmond P, Burgner D, Currie A (2013) Phagocytosis of neonatal pathogens by peripheral blood neutrophils and monocytes from newborn preterm and term infants. Pediatr Res 74, 503–10. [DOI] [PubMed] [Google Scholar]
- 90.Levy O (2002) Impaired innate immunity at birth: deficiency of bactericidal/permeability-increasing protein (BPI) in the neutrophils of newborns. Pediatr Res 51, 667–9. [DOI] [PubMed] [Google Scholar]
- 91.Strunk T, Doherty D, Richmond P, Simmer K, Charles A, Levy O, Liyanage K, Smith T, Currie A, Burgner D (2009) Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch Dis Child Fetal Neonatal Ed 94, F230–1. [DOI] [PubMed] [Google Scholar]
- 92.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, Chandler NB, Rodesch CK, Albertine KH, Petti CA, Weyrich AS, Zimmerman GA (2009) Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 113, 6419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geiger R, Ellemunter H, Fink FM, Falk M, Tilg H (1996) Circulating interleukin-1 receptor antagonist levels in neonates. Eur J Pediatr 155, 811–4. [DOI] [PubMed] [Google Scholar]
- 94.Pedraza-Sanchez S, Hise AG, Ramachandra L, Arechavaleta-Velasco F, King CL (2013) Reduced frequency of a CD14+ CD16+ monocyte subset with high Toll-like receptor 4 expression in cord blood compared to adult blood contributes to lipopolysaccharide hyporesponsiveness in newborns. Clin Vaccine Immunol 20, 962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG (2006) Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun 74, 1106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES 3rd, Kollmann TR (2010) Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One 5, e15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M (2004) A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med 199, 1011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levy O (2005) Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res 11, 113–6. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S (2010) Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One 5, e10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lo-Man R (2011) Regulatory B cells control dendritic cell functions. Immunotherapy 3, 19–20. [DOI] [PubMed] [Google Scholar]
- 101.Zhang X, Lepelley A, Azria E, Lebon P, Roguet G, Schwartz O, Launay O, Leclerc C, Lo-Man R (2013) Neonatal plasmacytoid dendritic cells (pDCs) display subset variation but can elicit potent anti-viral innate responses. PLoS One 8, e52003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB (2009) Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 183, 7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu JC, Khodadadi H, Malik A, Davidson B, Salles E, Bhatia J, Hale VL, Baban B (2018) Innate Immunity of Neonates and Infants. Front Immunol 9, 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aksoy E, Albarani V, Nguyen M, Laes JF, Ruelle JL, De Wit D, Willems F, Goldman M, Goriely S (2007) Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood 109, 2887–93. [DOI] [PubMed] [Google Scholar]
- 105.Bermick JR, Lambrecht NJ, denDekker AD, Kunkel SL, Lukacs NW, Hogaboam CM, Schaller MA (2016) Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics 8, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS (2013) Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller D, Romero R, Unkel R, Xu Y, Vadillo-Ortega F, Hassan SS, Gomez-Lopez N (2018) CD71+ erythroid cells from neonates born to women with preterm labor regulate cytokine and cellular responses. J Leukoc Biol 103, 761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perrone S, Vezzosi P, Longini M, Marzocchi B, Tanganelli D, Testa M, Santilli T, Buonocore G, Gruppo di Studio di Ematologia Neonatale della Societa Italiana di, N. (2005) Nucleated red blood cell count in term and preterm newborns: reference values at birth. Arch Dis Child Fetal Neonatal Ed 90, F174–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakazawa T, Agematsu K, Yabuhara A (1997) Later development of Fas ligand-mediated cytotoxicity as compared with granule-mediated cytotoxicity during the maturation of natural killer cells. Immunology 92, 180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, Marchant A, Vermijlen D (2015) Effector Vgamma9Vdelta2 T cells dominate the human fetal gammadelta T-cell repertoire. Proc Natl Acad Sci U S A 112, E556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C (2011) Natural killer cell responses to infections in early life. J Innate Immun 3, 280–8. [DOI] [PubMed] [Google Scholar]
- 112.Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, Farr AR, Vidal SM, Laouar Y (2012) TGF-beta is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol 13, 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adkins B, Leclerc C, Marshall-Clarke S (2004) Neonatal adaptive immunity comes of age. Nat Rev Immunol 4, 553–64. [DOI] [PubMed] [Google Scholar]
- 114.Adkins B (2007) Heterogeneity in the CD4 T Cell Compartment and the Variability of Neonatal Immune Responsiveness. Curr Immunol Rev 3, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB (2009) Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med 206, 275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berkley AM, Hendricks DW, Simmons KB, Fink PJ (2013) Recent thymic emigrants and mature naive T cells exhibit differential DNA methylation at key cytokine loci. J Immunol 190, 6180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fink PJ (2013) The biology of recent thymic emigrants. Annu Rev Immunol 31, 31–50. [DOI] [PubMed] [Google Scholar]
- 118.Zaghouani H, Hoeman CM, Adkins B (2009) Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol 30, 585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hebel K, Weinert S, Kuropka B, Knolle J, Kosak B, Jorch G, Arens C, Krause E, Braun-Dullaeus RC, Brunner-Weinzierl MC (2014) CD4+ T cells from human neonates and infants are poised spontaneously to run a nonclassical IL-4 program. J Immunol 192, 5160–70. [DOI] [PubMed] [Google Scholar]
- 120.Adkins B (2000) Development of neonatal Th1/Th2 function. Int Rev Immunol 19, 157–71. [DOI] [PubMed] [Google Scholar]
- 121.Debock I and Flamand V (2014) Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol 5, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Mozeleski B, Lemoine S, Deriaud E, Lim A, Zhivaki D, Azria E, Le Ray C, Roguet G, Launay O, Vanet A, Leclerc C, Lo-Man R (2014) CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med 6, 238ra72. [DOI] [PubMed] [Google Scholar]
- 123.Delespesse G, Yang LP, Ohshima Y, Demeure C, Shu U, Byun DG, Sarfati M (1998) Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine 16, 1415–9. [DOI] [PubMed] [Google Scholar]
- 124.Hermann E, Truyens C, Alonso-Vega C, Even J, Rodriguez P, Berthe A, Gonzalez-Merino E, Torrico F, Carlier Y (2002) Human fetuses are able to mount an adultlike CD8 T-cell response. Blood 100, 2153–8. [PubMed] [Google Scholar]
- 125.Muenchhoff M, Prendergast AJ, Goulder PJ (2014) Immunity to HIV in Early Life. Front Immunol 5, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Galindo-Albarran AO, Lopez-Portales OH, Gutierrez-Reyna DY, Rodriguez-Jorge O, Sanchez-Villanueva JA, Ramirez-Pliego O, Bergon A, Loriod B, Holota H, Imbert J, Hernandez-Mendoza A, Ferrier P, Carrillo-de Santa Pau E, Valencia A, Spicuglia S, Santana MA (2016) CD8(+) T Cells from Human Neonates Are Biased toward an Innate Immune Response. Cell Rep 17, 2151–2160. [DOI] [PubMed] [Google Scholar]
- 127.Huygens A, Lecomte S, Tackoen M, Olislagers V, Delmarcelle Y, Burny W, Van Rysselberge M, Liesnard C, Larsen M, Appay V, Donner C, Marchant A (2015) Functional Exhaustion Limits CD4+ and CD8+ T-Cell Responses to Congenital Cytomegalovirus Infection. J Infect Dis 212, 484–94. [DOI] [PubMed] [Google Scholar]
- 128.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W (2010) “Default” generation of neonatal regulatory T cells. J Immunol 185, 71–8. [DOI] [PubMed] [Google Scholar]
- 129.Rueda CM, Moreno-Fernandez ME, Jackson CM, Kallapur SG, Jobe AH, Chougnet CA (2015) Neonatal regulatory T cells have reduced capacity to suppress dendritic cell function. Eur J Immunol 45, 2582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siegrist CA and Aspinall R (2009) B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9, 185–94. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalez-Espinosa LO, Montiel-Cervantes LA, Guerra-Marquez A, Penaflor-Juarez K, Reyes-Maldonado E, Vela-Ojeda J (2016) Maternal obesity associated with increase in natural killer T cells and CD8+ regulatory T cells in cord blood units. Transfusion 56, 1075–81. [DOI] [PubMed] [Google Scholar]
- 132.Ibrahim MH, Moustafa AN, Saedii AAF, Hassan EE (2017) Cord blood erythropoietin and cord blood nucleated red blood cells for prediction of adverse neonatal outcome associated with maternal obesity in term pregnancy: prospective cohort study. J Matern Fetal Neonatal Med 30, 2237–2242. [DOI] [PubMed] [Google Scholar]
- 133.Cifuentes-Zuniga F, Arroyo-Jousse V, Soto-Carrasco G, Casanello P, Uauy R, Krause BJ, Castro-Rodriguez JA (2017) IL-10 expression in macrophages from neonates born from obese mothers is suppressed by IL-4 and LPS/INFgamma. J Cell Physiol 232, 3693–3701. [DOI] [PubMed] [Google Scholar]
- 134.Kleweis SM, Cahill AG, Odibo AO, Tuuli MG (2015) Maternal Obesity and Rectovaginal Group B Streptococcus Colonization at Term. Infect Dis Obstet Gynecol 2015, 586767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polnaszek BE, Raghuraman N, Lopez JD, Frolova AL, Wesevich V, Tuuli MG, Cahill AG (2018) Neonatal Morbidity in the Offspring of Obese Women Without Hypertension or Diabetes. Obstet Gynecol 132, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA (2013) The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004–2011. BJOG 120, 932–9. [DOI] [PubMed] [Google Scholar]
- 137.Dosch NC, Guslits EF, Weber MB, Murray SE, Ha B, Coe CL, Auger AP, Kling PJ (2016) Maternal Obesity Affects Inflammatory and Iron Indices in Umbilical Cord Blood. J Pediatr 172, 20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCloskey K, Ponsonby AL, Collier F, Allen K, Tang MLK, Carlin JB, Saffery R, Skilton MR, Cheung M, Ranganathan S, Dwyer T, Burgner D, Vuillermin P (2018) The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes 13, 46–53. [DOI] [PubMed] [Google Scholar]
- 139.Gallardo JM, Gomez-Lopez J, Medina-Bravo P, Juarez-Sanchez F, Contreras-Ramos A, Galicia-Esquivel M, Sanchez-Urbina R, Klunder-Klunder M (2015) Maternal obesity increases oxidative stress in the newborn. Obesity (Silver Spring) 23, 1650–4. [DOI] [PubMed] [Google Scholar]
- 140.Edlow AG, Hui L, Wick HC, Fried I, Bianchi DW (2016) Assessing the fetal effects of maternal obesity via transcriptomic analysis of cord blood: a prospective case-control study. BJOG 123, 180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fan L, Lindsley SR, Comstock SM, Takahashi DL, Evans AE, He GW, Thornburg KL, Grove KL (2013) Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes (Lond) 37, 254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Odaka Y, Nakano M, Tanaka T, Kaburagi T, Yoshino H, Sato-Mito N, Sato K (2010) The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring) 18, 1688–94. [DOI] [PubMed] [Google Scholar]
- 143.Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK (2013) Parental dietary fat intake alters offspring microbiome and immunity. J Immunol 191, 3200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferolla FM, Hijano DR, Acosta PL, Rodriguez A, Duenas K, Sancilio A, Barboza E, Caria A, Gago GF, Almeida RE, Castro L, Pozzolo C, Martinez MV, Grimaldi LA, Rebec B, Calvo M, Henrichsen J, Nocito C, Gonzalez M, Barbero G, Losada JV, Caballero MT, Zurankovas V, Raggio M, Schavlovsky G, Kobylarz A, Wimmenauer V, Bugna J, Williams JV, Sastre G, Flamenco E, Perez AR, Ferrero F, Libster R, Grijalva CG, Polack FP (2013) Macronutrients during pregnancy and life-threatening respiratory syncytial virus infections in children. Am J Respir Crit Care Med 187, 983–90. [DOI] [PubMed] [Google Scholar]
- 145.Griffiths PS, Walton C, Samsell L, Perez MK, Piedimonte G (2016) Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr Res 79, 278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson AM, Pombo J, Maragkoudaki X, Butt A, Saraswati R, Novelli M, Fusai G, Poston L, Taylor PD, Oben JA (2013) Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 58, 128–38. [DOI] [PubMed] [Google Scholar]
- 147.Friedman JE, Dobrinskikh E, Alfonso-Garcia A, Fast A, Janssen RC, Soderborg TK, Anderson AL, Reisz JA, D’Alessandro A, Frank DN, Robertson CE, de la Houssaye BA, Johnson LK, Orlicky DJ, Wang XX, Levi M, Potma EO, El Kasmi KC, Jonscher KR (2018) Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol Commun 2, 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D’Alessandro A, Barbour LA, El Kasmi KC, Friedman JE (2018) The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 9, 4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murabayashi N, Sugiyama T, Zhang L, Kamimoto Y, Umekawa T, Ma N, Sagawa N (2013) Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur J Obstet Gynecol Reprod Biol 169, 39–44. [DOI] [PubMed] [Google Scholar]
- 150.Bibi S, Kang Y, Du M, Zhu MJ (2017) Maternal high-fat diet consumption enhances offspring susceptibility to DSS-induced colitis in mice. Obesity (Silver Spring) 25, 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ (2011) Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep. Inflamm Bowel Dis 17, 1513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bauche D and Marie JC (2017) Transforming growth factor beta: a master regulator of the gut microbiota and immune cell interactions. Clin Transl Immunology 6, e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yan X, Huang Y, Zhao JX, Long NM, Uthlaut AB, Zhu MJ, Ford SP, Nathanielsz PW, Du M (2011) Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol Reprod 85, 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Collado MC, Isolauri E, Laitinen K, Salminen S (2010) Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr 92, 1023–30. [DOI] [PubMed] [Google Scholar]
- 155.Mueller NT, Shin H, Pizoni A, Werlang IC, Matte U, Goldani MZ, Goldani HA, Dominguez-Bello MG (2016) Birth mode-dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep 6, 23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM (2014) Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One 9, e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Edlow AG, Glass RM, Smith CJ, Tran PK, James K, Bilbo S (2018) Placental Macrophages: A Window Into Fetal Microglial Function in Maternal Obesity. Int J Dev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gardner RM, Lee BK, Magnusson C, Rai D, Frisell T, Karlsson H, Idring S, Dalman C (2015) Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol 44, 870–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jo H, Schieve LA, Sharma AJ, Hinkle SN, Li R, Lind JN (2015) Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 135, e1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li YM, Ou JJ, Liu L, Zhang D, Zhao JP, Tang SY (2016) Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J Autism Dev Disord 46, 95–102. [DOI] [PubMed] [Google Scholar]
- 161.Rodriguez A (2010) Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry 51, 134–43. [DOI] [PubMed] [Google Scholar]
- 162.Kamimae-Lanning AN, Krasnow SM, Goloviznina NA, Zhu X, Roth-Carter QR, Levasseur PR, Jeng S, McWeeney SK, Kurre P, Marks DL (2015) Maternal high-fat diet and obesity compromise fetal hematopoiesis. Mol Metab 4, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu A, Chen M, Kumar R, Stefanovic-Racic M, O’Doherty RM, Ding Y, Jahnen-Dechent W, Borghesi L (2018) Bone marrow lympho-myeloid malfunction in obesity requires precursor cell-autonomous TLR4. Nat Commun 9, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Iaffaldano L, Nardelli C, D’Alessio F, D’Argenio V, Nunziato M, Mauriello L, Procaccini C, Maruotti GM, Martinelli P, Matarese G, Pastore L, Del Vecchio L, Labruna G, Sacchetti L (2018) Altered Bioenergetic Profile in Umbilical Cord and Amniotic Mesenchymal Stem Cells from Newborns of Obese Women. Stem Cells Dev 27, 199–206. [DOI] [PubMed] [Google Scholar]
- 165.Maltepe E and Fisher SJ (2015) Placenta: the forgotten organ. Annu Rev Cell Dev Biol 31, 523–52. [DOI] [PubMed] [Google Scholar]
- 166.Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, Kustagi M, Tuschl T, Williams Z (2018) A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 4, eaau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK (2006) Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 11, 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rosario FJ, Kanai Y, Powell TL, Jansson T (2015) Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring) 23, 1663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jansson N, Rosario FJ, Gaccioli F, Lager S, Jones HN, Roos S, Jansson T, Powell TL (2013) Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J Clin Endocrinol Metab 98, 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL (2016) Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta 40, 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T (2015) Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol 212, 227 e1–7. [DOI] [PubMed] [Google Scholar]
- 172.Hayes EK, Tessier DR, Percival ME, Holloway AC, Petrik JJ, Gruslin A, Raha S (2014) Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod Sci 21, 648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lenartowicz M, Kennedy C, Hayes H, McArdle HJ (2015) Transcriptional regulation of copper metabolism genes in the liver of fetal and neonatal control and iron-deficient rats. Biometals 28, 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stuart TJ, O’Neill K, Condon D, Sasson I, Sen P, Xia Y, Simmons RA (2018) Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol Reprod 98, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roberts KA, Riley SC, Reynolds RM, Barr S, Evans M, Statham A, Hor K, Jabbour HN, Norman JE, Denison FC (2011) Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–54. [DOI] [PubMed] [Google Scholar]
- 176.Dubova EA, Pavlov KA, Borovkova EI, Bayramova MA, Makarov IO, Shchegolev AI (2011) Vascular endothelial growth factor and its receptors in the placenta of pregnant women with obesity. Bull Exp Biol Med 151, 253–8. [DOI] [PubMed] [Google Scholar]
- 177.Mor G and Cardenas I (2010) The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 63, 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hirschmugl B, Desoye G, Catalano P, Klymiuk I, Scharnagl H, Payr S, Kitzinger E, Schliefsteiner C, Lang U, Wadsack C, Hauguel-de Mouzon S (2017) Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes (Lond) 41, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Altmae S, Segura MT, Esteban FJ, Bartel S, Brandi P, Irmler M, Beckers J, Demmelmair H, Lopez-Sabater C, Koletzko B, Krauss-Etschmann S, Campoy C (2017) Maternal Pre-Pregnancy Obesity Is Associated with Altered Placental Transcriptome. PLoS One 12, e0169223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K (2014) Maternal obesity is associated with a lipotoxic placental environment. Placenta 35, 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sureshchandra S, Marshall NE, Wilson RM, Barr T, Rais M, Purnell JQ, Thornburg KL, Messaoudi I (2018) Inflammatory Determinants of Pregravid Obesity in Placenta and Peripheral Blood. Front Physiol 9, 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Sci Transl Med 6, 237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Parnell LA, Briggs CM, Cao B, Delannoy-Bruno O, Schrieffer AE, Mysorekar IU (2017) Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep 7, 11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, Sweeney EL, Knox CL, Lambers DS, Jobe AH, Chougnet CA, Kallapur SG, Aagaard KM (2016) The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol 214, 627 e1–627 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH (2015) Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res 41, 662–9. [DOI] [PubMed] [Google Scholar]
- 186.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K (2015) The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol 212, 653 e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polanski K, Goncalves A, Gardner L, Holmqvist S, Henriksson J, Zou A, Sharkey AM, Millar B, Innes B, Wood L, Wilbrey-Clark A, Payne RP, Ivarsson MA, Lisgo S, Filby A, Rowitch DH, Bulmer JN, Wright GJ, Stubbington MJT, Haniffa M, Moffett A, Teichmann SA (2018) Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lewis EL, Sierra LJ, Barila GO, Brown AG, Porrett PM, Elovitz MA (2018) Placental immune state shifts with gestational age. Am J Reprod Immunol 79, e12848. [DOI] [PubMed] [Google Scholar]
- 189.Mor G, Aldo P, Alvero AB (2017) The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 17, 469–482. [DOI] [PubMed] [Google Scholar]
- 190.Ander SE, Diamond MS, Coyne CB (2019) Immune responses at the maternal-fetal interface. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S (2016) Inflammation and preterm birth. J Leukoc Biol 99, 67–78. [DOI] [PubMed] [Google Scholar]
- 192.Geldenhuys J, Rossouw TM, Lombaard HA, Ehlers MM, Kock MM (2018) Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front Immunol 9, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boucas AP, de Souza BM, Bauer AC, Crispim D (2017) Role of Innate Immunity in Preeclampsia: A Systematic Review. Reprod Sci 24, 1362–1370. [DOI] [PubMed] [Google Scholar]
- 194.Faas MM and De Vos P (2018) Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 69, 125–133. [DOI] [PubMed] [Google Scholar]
- 195.Ben Amara A, Gorvel L, Baulan K, Derain-Court J, Buffat C, Verollet C, Textoris J, Ghigo E, Bretelle F, Maridonneau-Parini I, Mege JL (2013) Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J Immunol 191, 5501–14. [DOI] [PubMed] [Google Scholar]
- 196.Perdu S, Castellana B, Kim Y, Chan K, DeLuca L, Beristain AG (2016) Maternal obesity drives functional alterations in uterine NK cells. JCI Insight 1, e85560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Castellana B, Perdu S, Kim Y, Chan K, Atif J, Marziali M, Beristain AG (2018) Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunol Cell Biol 96, 805–819. [DOI] [PubMed] [Google Scholar]
- 198.Orlova EG and Shirshev SV (2009) Leptin as an immunocorrecting agent during normal pregnancy. Bull Exp Biol Med 148, 75–8. [DOI] [PubMed] [Google Scholar]
- 199.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J (2008) Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 3, e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Singh U, Nicholson G, Urban BC, Sargent IL, Kishore U, Bernal AL (2005) Immunological properties of human decidual macrophages--a possible role in intrauterine immunity. Reproduction 129, 631–7. [DOI] [PubMed] [Google Scholar]
- 201.Nagamatsu T and Schust DJ (2010) The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci 17, 209–18. [DOI] [PubMed] [Google Scholar]
- 202.Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G (2004) Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol 51, 275–82. [DOI] [PubMed] [Google Scholar]
- 203.Houser BL (2012) Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med 85, 105–18. [PMC free article] [PubMed] [Google Scholar]
- 204.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S (2008) Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang X, Li M, Haghiac M, Catalano PM, O’Tierney-Ginn P, Hauguel-de Mouzon S (2016) Causal relationship between obesity-related traits and TLR4-driven responses at the maternal-fetal interface. Diabetologia 59, 2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Laskewitz A, van Benthem KL, Kieffer TEC, Faas MM, Verkaik-Schakel RN, Plosch T, Scherjon SA, Prins JR (2019) The influence of maternal obesity on macrophage subsets in the human decidua. Cell Immunol 336, 75–82. [DOI] [PubMed] [Google Scholar]
- 207.Aghaeepour N, Ganio EA, McIlwain D, Tsai AS, Tingle M, Van Gassen S, Gaudilliere DK, Baca Q, McNeil L, Okada R, Ghaemi MS, Furman D, Wong RJ, Winn VD, Druzin ML, El-Sayed YY, Quaintance C, Gibbs R, Darmstadt GL, Shaw GM, Stevenson DK, Tibshirani R, Nolan GP, Lewis DB, Angst MS, Gaudilliere B (2017) An immune clock of human pregnancy. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aghaeepour N, Lehallier B, Baca Q, Ganio EA, Wong RJ, Ghaemi MS, Culos A, El-Sayed YY, Blumenfeld YJ, Druzin ML, Winn VD, Gibbs RS, Tibshirani R, Shaw GM, Stevenson DK, Gaudilliere B, Angst MS (2018) A proteomic clock of human pregnancy. Am J Obstet Gynecol 218, 347 e1–347 e14. [DOI] [PubMed] [Google Scholar]
- 209.Luppi P, Haluszczak C, Trucco M, Deloia JA (2002) Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol 47, 72–81. [DOI] [PubMed] [Google Scholar]
- 210.Polese B, Gridelet V, Araklioti E, Martens H, Perrier d’Hauterive S, Geenen V (2014) The Endocrine Milieu and CD4 T-Lymphocyte Polarization during Pregnancy. Front Endocrinol (Lausanne) 5, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Faas MM, Schuiling GA, Linton EA, Sargent IL, Redman CW (2000) Activation of peripheral leukocytes in rat pregnancy and experimental preeclampsia. Am J Obstet Gynecol 182, 351–7. [DOI] [PubMed] [Google Scholar]
- 212.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R (2001) Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 185, 1118–23. [DOI] [PubMed] [Google Scholar]
- 213.Sacks GP, Studena K, Sargent K, Redman CW (1998) Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 179, 80–6. [DOI] [PubMed] [Google Scholar]
- 214.Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA (2002) Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol 72, 874–84. [PubMed] [Google Scholar]
- 215.Sacks GP, Redman CW, Sargent IL (2003) Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol 131, 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kay AW, Fukuyama J, Aziz N, Dekker CL, Mackey S, Swan GE, Davis MM, Holmes S, Blish CA (2014) Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci U S A 111, 14506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Le Gars M, Kay AW, Bayless NL, Aziz N, Dekker CL, Swan GE, Davis MM, Blish CA (2016) Increased Proinflammatory Responses of Monocytes and Plasmacytoid Dendritic Cells to Influenza A Virus Infection During Pregnancy. J Infect Dis 214, 1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL (2012) Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 33 Suppl, S48–54. [DOI] [PubMed] [Google Scholar]
- 219.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA (1996) Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A 93, 705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL (2001) Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta 22, 550–9. [DOI] [PubMed] [Google Scholar]
- 221.Pendeloski KPT, Ono E, Torloni MR, Mattar R, Daher S (2017) Maternal obesity and inflammatory mediators: A controversial association. Am J Reprod Immunol 77. [DOI] [PubMed] [Google Scholar]
- 222.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N (2002) Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87, 4231–7. [DOI] [PubMed] [Google Scholar]
- 223.Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR (2007) Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab 92, 969–75. [DOI] [PubMed] [Google Scholar]
- 224.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S (2009) Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Christian LM and Porter K (2014) Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine 70, 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Holtan SG, Chen Y, Kaimal R, Creedon DJ, Enninga EA, Nevala WK, Markovic SN (2015) Growth modeling of the maternal cytokine milieu throughout normal pregnancy: macrophage-derived chemokine decreases as inflammation/counterregulation increases. J Immunol Res 2015, 952571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, Hougaard DM, Thorsen P (2008) Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol 77, 152–60. [DOI] [PubMed] [Google Scholar]
- 228.Friis CM, Paasche Roland MC, Godang K, Ueland T, Tanbo T, Bollerslev J, Henriksen T (2013) Adiposity-related inflammation: effects of pregnancy. Obesity (Silver Spring) 21, E124–30. [DOI] [PubMed] [Google Scholar]
- 229.Sen S, Iyer C, Meydani SN (2014) Obesity during pregnancy alters maternal oxidant balance and micronutrient status. J Perinatol 34, 105–11. [DOI] [PubMed] [Google Scholar]
- 230.Farah N, Hogan AE, O’Connor N, Kennelly MM, O’Shea D, Turner MJ (2012) Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine 60, 96–9. [DOI] [PubMed] [Google Scholar]
- 231.Aye IL, Lager S, Ramirez VI, Gaccioli F, Dudley DJ, Jansson T, Powell TL (2014) Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod 90, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kac G, Vaz JS, Schlussel MM, Moura AS (2011) C-reactive protein and hormones but not IL-6 are associated to body mass index in first trimester of pregnancy. Arch Gynecol Obstet 284, 567–73. [DOI] [PubMed] [Google Scholar]
- 233.Sen S, Iyer C, Klebenov D, Histed A, Aviles JA, Meydani SN (2013) Obesity impairs cell-mediated immunity during the second trimester of pregnancy. Am J Obstet Gynecol 208, 139 e1–8. [DOI] [PubMed] [Google Scholar]
- 234.Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS (2003) Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88, 4823–31. [DOI] [PubMed] [Google Scholar]
- 235.Walsh JM, McGowan CA, Byrne JA, Rath A, McAuliffe FM (2013) The association between TNF-alpha and insulin resistance in euglycemic women. Cytokine 64, 208–12. [DOI] [PubMed] [Google Scholar]
- 236.Founds SA, Powers RW, Patrick TE, Ren D, Harger GF, Markovic N, Roberts JM (2008) A comparison of circulating TNF-alpha in obese and lean women with and without preeclampsia. Hypertens Pregnancy 27, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ferraro ZM, Qiu Q, Gruslin A, Adamo KB (2012) Characterization of the insulin-like growth factor axis in term pregnancies complicated by maternal obesity. Hum Reprod 27, 2467–75. [DOI] [PubMed] [Google Scholar]
- 238.Segerer S, Kammerer U, Kapp M, Dietl J, Rieger L (2009) Upregulation of chemokine and cytokine production during pregnancy. Gynecol Obstet Invest 67, 145–50. [DOI] [PubMed] [Google Scholar]
- 239.Du MR, Wang SC, Li DJ (2014) The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell Mol Immunol 11, 438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bardou M, Hadi T, Mace G, Pesant M, Debermont J, Barrichon M, Wendremaire M, Laurent N, Sagot P, Lirussi F (2014) Systemic increase in human maternal circulating CD14+CD16- MCP-1+ monocytes as a marker of labor. Am J Obstet Gynecol 210, 70 e1–9. [DOI] [PubMed] [Google Scholar]
- 241.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, Dammann O (2009) Maternal obesity and markers of inflammation in pregnancy. Cytokine 47, 61–4. [DOI] [PubMed] [Google Scholar]
- 242.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE (2004) Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 103, 546–50. [DOI] [PubMed] [Google Scholar]
- 243.Briana DD and Malamitsi-Puchner A (2009) Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci 16, 921–37. [DOI] [PubMed] [Google Scholar]
- 244.Schubring C, Englaro P, Siebler T, Blum WF, Demirakca T, Kratzsch J, Kiess W (1998) Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Horm Res 50, 276–83. [DOI] [PubMed] [Google Scholar]
- 245.van der Wijden CL, Delemarre-van der Waal HA, van Mechelen W, van Poppel MN (2013) The concurrent validity between leptin, BMI and skin folds during pregnancy and the year after. Nutr Diabetes 3, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Franco-Sena AB, Rebelo F, Pinto T, Farias DR, Silveira GE, Mendes RH, Henriques VT, Kac G (2016) The effect of leptin concentrations and other maternal characteristics on gestational weight gain is different according to pre-gestational BMI: results from a prospective cohort. BJOG 123, 1804–13. [DOI] [PubMed] [Google Scholar]
- 247.Ozias MK, Li S, Hull HR, Brooks WM, Carlson SE (2015) Relationship of circulating adipokines to body composition in pregnant women. Adipocyte 4, 44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Palik E, Baranyi E, Melczer Z, Audikovszky M, Szocs A, Winkler G, Cseh K (2007) Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance. Diabetes Res Clin Pract 76, 351–7. [DOI] [PubMed] [Google Scholar]
- 249.Kusminski CM, McTernan PG, Kumar S (2005) Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond) 109, 243–56. [DOI] [PubMed] [Google Scholar]
- 250.Rea R and Donnelly R (2004) Resistin: an adipocyte-derived hormone. Has it a role in diabetes and obesity? Diabetes Obes Metab 6, 163–70. [DOI] [PubMed] [Google Scholar]
- 251.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A (2004) Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 27, 2450–7. [DOI] [PubMed] [Google Scholar]
- 252.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A (2005) Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol 106, 802–7. [DOI] [PubMed] [Google Scholar]
- 253.Herrera E, Amusquivar E, Lopez-Soldado I, Ortega H (2006) Maternal lipid metabolism and placental lipid transfer. Horm Res 65 Suppl 3, 59–64. [DOI] [PubMed] [Google Scholar]
- 254.Shankar K, Zhong Y, Kang P, Lau F, Blackburn ML, Chen JR, Borengasser SJ, Ronis MJ, Badger TM (2011) Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 152, 4158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Costa SM, Isganaitis E, Matthews TJ, Hughes K, Daher G, Dreyfuss JM, da Silva GA, Patti ME (2016) Maternal obesity programs mitochondrial and lipid metabolism gene expression in infant umbilical vein endothelial cells. Int J Obes (Lond) 40, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Calkins K, Roy D, Molchan L, Bradley L, Grogan T, Elashoff D, Walker V (2015) Predictive value of cord blood bilirubin for hyperbilirubinemia in neonates at risk for maternal-fetal blood group incompatibility and hemolytic disease of the newborn. J Neonatal Perinatal Med 8, 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Farlik M, Halbritter F, Muller F, Choudry FA, Ebert P, Klughammer J, Farrow S, Santoro A, Ciaurro V, Mathur A, Uppal R, Stunnenberg HG, Ouwehand WH, Laurenti E, Lengauer T, Frontini M, Bock C (2016) DNA Methylation Dynamics of Human Hematopoietic Stem Cell Differentiation. Cell Stem Cell 19, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu X, Chen Q, Tsai HJ, Wang G, Hong X, Zhou Y, Zhang C, Liu C, Liu R, Wang H, Zhang S, Yu Y, Mestan KK, Pearson C, Otlans P, Zuckerman B, Wang X (2014) Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 55, 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Herbstman JB, Wang S, Perera FP, Lederman SA, Vishnevetsky J, Rundle AG, Hoepner LA, Qu L, Tang D (2013) Predictors and consequences of global DNA methylation in cord blood and at three years. PLoS One 8, e72824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M (2013) Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 62, 3727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yang KF, Cai W, Xu JL, Shi W (2012) Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol 49, 107–14. [DOI] [PubMed] [Google Scholar]
- 262.Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH (2008) Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 41, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Suter MA, Chen A, Burdine MS, Choudhury M, Harris RA, Lane RH, Friedman JE, Grove KL, Tackett AJ, Aagaard KM (2012) A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J 26, 5106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Borengasser SJ, Lau F, Kang P, Blackburn ML, Ronis MJ, Badger TM, Shankar K (2011) Maternal obesity during gestation impairs fatty acid oxidation and mitochondrial SIRT3 expression in rat offspring at weaning. PLoS One 6, e24068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE (2010) Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 299, R711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kadakia R, Zheng Y, Zhang Z, Zhang W, Hou L, Josefson JL (2017) Maternal pre-pregnancy BMI downregulates neonatal cord blood LEP methylation. Pediatr Obes 12 Suppl 1, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nogues P, Dos Santos E, Jammes H, Berveiller P, Arnould L, Vialard F, Dieudonne MN (2019) Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin Epigenetics 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]


