Abstract
Background
This study examines vector density, the prevailing knowledge, awareness, attitudes and practice (KAAP) of community members regarding dengue disease and their willingness to pay (WTP) for vector control in Dhaka, Bangladesh.
Methods
A population-based, cross-sectional study design was followed: (i) an entomological survey was carried out in 727 randomly selected households in 12 wards, representing four urban ecological zones and (ii) a survey of 330 household heads was conducted to study their KAAP. The χ2 test and multinomial logistic regression (MLR) were applied to investigate factors associated with WTP and other variables.
Results
The Stegomyia indices significantly vary among the urban zones, revealing that the paved and built areas with concentrated public/commercial services have the highest mosquito density. Most respondents (93.9%) knew about dengue and its severity (90.3%); however, many of them were unaware (79.3%) about the types of mosquitoes causing dengue. MLR modelling reveals that average spending per month for mosquito control, household income and knowledge about the effects of land use and seasonality on dengue were significantly associated with the WTP for controlling the dengue vector.
Conclusions
Concerted efforts should be made to increase awareness about dengue transmission and develop community-based sustainable dengue vector control programmes involving both the public and private sectors.
Keywords: attitude and practice, dengue, knowledge and awareness, vector control, vector density, willingness to pay
Introduction
Dengue is one of the major vector-borne diseases in the world.1 The spread of the dengue vector (Aedes aegypti and Aedes albopictus) has caused dengue fever (DF) and severe dengue to become endemic in more than 128 countries.2 Estimates suggest 96 million new apparent infections take place every year, with 294 million unapparent infections.3 To tackle such an invasive disease, a vaccine for dengue, Dengvaxia (CYD-TDV), was developed by Sanofi Pasteur (Lyon, France) and licensed in 2015. The vaccine has obtained approval in 20 countries. However, results of phase 3 trials have been mixed, with contradictions for subpopulations. In particular, recent serostatus results revealed that subpopulations with no prior dengue virus (DENV) infection had a higher risk of severe dengue with the vaccination.4 Despite some progress being made in recent years on vaccine development, none are universally available for public health use for all at-risk individuals because of safety concerns. Hence prevention and control of DENV transmission relies primarily on effective vector control.2,5
The spread of the vector and DENV are accelerated by numerous factors at various scales (household, neighbourhood, urban zone, city, region etc.), including urbanization, climate change, changes in land use, population growth, erratic water supply due to increased population, problems in the water supply system, droughts, and increased trade and mobility. The altered neighbourhood environment has become the preferred habitat and breeding site for the major vector, Aedes mosquitoes.6 Recognizing that a vector control strategy is the major intervention for controlling dengue, and that the urban ecological zones may be associated with vector abundance and density, research on detecting and explaining these complex relationships is essential.
Integrated urban ecological zones (IUEZs) are distinct areas of a city characterized by unique land- and waterscapes, and habitats that maintain a distinct ecological character in respect to biological and cultural diversity.7 Numerous studies have revealed that better knowledge and awareness about the vector and the disease among city dwellers are associated with a reduction in vector breeding,8,9 thus it is crucial to investigate the state of knowledge, awareness, attitudes and practice (KAAP) and their associated attributes. With a general failure of public agencies in vector control in many developing countries, the focus has recently shifted to private citizens’ efforts and their willingness to pay (WTP) for vector control.10
To our knowledge, no systematic study has yet been conducted in Bangladesh that encompasses the aspects of vector density by UEZ, inhabitants’ KAAP regarding dengue and its control, and their WTP for vector control. An International Development Research Centre (IDRC) project on dengue in Bangladesh, initiated in 2010–11 to investigate the problem from a socio-economic perspective, published its results previously.11 The present study was carried out during 2013–14 under the same project, but with a different and expanded scope, and the following objectives: (i) examine whether there are any significant differences among the IUEZs (see Supplementary Table 1) in terms of Stegomyia indices (house index, Breteau index, container index, pupae per person index [PPI]); (ii) examine the current status of KAAP regarding dengue, its transmission, IUEZ differentials, and their explanatory factors; (iii) map the status of vector control measures undertaken by city dwellers and IUEZ differentials and identify the explanatory factors; and (iv) determine the status of WTP for vector control and IUEZ differentials, and identify the underlying influencing factors that explain dwellers’ WTP for vector control.
Materials and methods
Selection of the study area for the entomological survey
The Dhaka City Corporation (DCC) area was chosen as the study area considering its socio-economic, political and demographic importance as the capital of Bangladesh and its high vulnerability to dengue epidemics.12 The city core covers 126.3 km2 and is divided into 90 wards–a ward refers to an administrative subunit representing at least one local community. With >12 million people,11 Dhaka is one of the most densely populated cities in the world. Dengue outbreaks take place most years, with varying annual fatalities and hospitalization rates: since the 2000 outbreak, >49 000 people have suffered from DF and severe dengue, with 316 fatalities.13
Delineation of IUEZs
A RapidEye image (RapidEye, Berlin, Germany) for the city of Dhaka (23°40′09″N to 23°54′05″N and 90°19′45″E to 90°30′35″E) for 2013 was procured for identification of current land cover. Supervised classification with six training samples and four test samples for each of the categories of land cover was performed. Land use data provided by the Dhaka City authority were overlaid with the classified land cover map. The land use classes were then integrated with the RapidEye classified image through a spatial join operation in Arcmap 10.1 (ESRI, Redlands, CA, USA). The average percentage of each land cover/use type for each of the 90 wards was determined by overlaying the ward boundaries on the land cover map. The city was then classified into four IUEZs: (i) residential, vegetation and bare soil (RVBS); (ii) residential and water-bodies (RAWB); (iii) paved/built area, services and commercial activities (PASC); and (iv) commercial, residential and industrial areas (CRIA) using the function agnes from the R package cluster14 (see Supplementary Table 1).
Entomological data collection
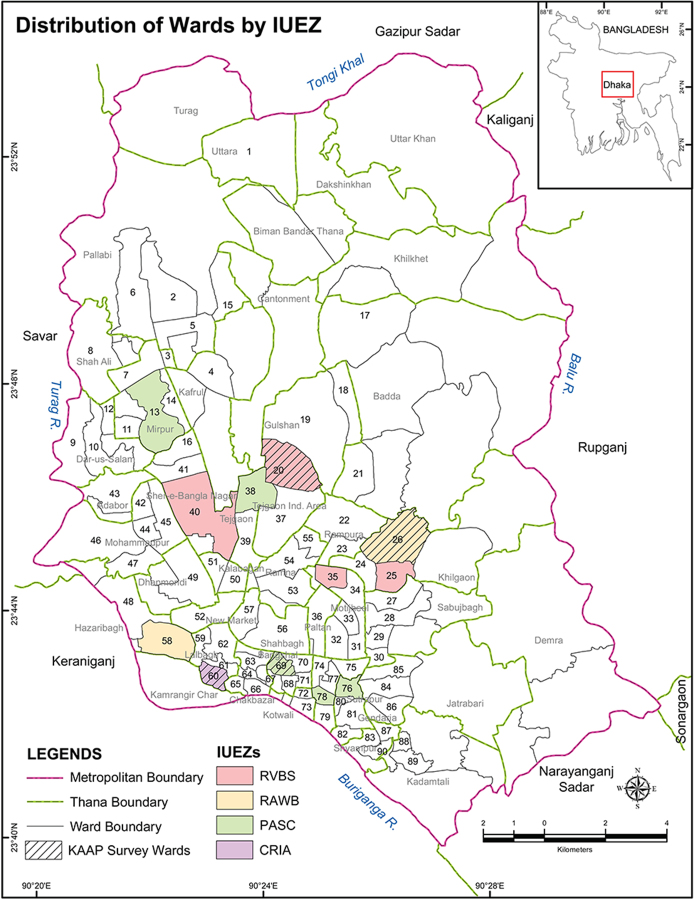
Using a probability proportional sampling procedure by IUEZ, a total of 12 wards (13.3%) were randomly chosen, resulting in 4 wards (20, 25, 35 and 40) in the RVBS, 2 wards (26 and 58) in the RAWB, 5 wards (13, 38, 69, 76 and 78) in the PASC and 1 ward (60) in the CRIA (Figure 1). A random sample of 100 households was targeted from each selected ward, resulting in a possible 1200 sampling units. A total of 727/1200 households (response rate of 60.6%) were inspected by trained entomologists to collect mosquito larvae and pupae. The entomological survey was carried out by IDRC-sponsored project personnel during August and September (monsoon) of 2013.
Figure 1.
Location of sampled wards (n=12) by IUEZs for the 2013 entomological survey (in colour) and sampled communities (four wards) for the 2014 KAAP survey (patterned) in the DCC area.
Sampling of households for the KAAP survey
For the purpose of administering the KAAP survey in the selected 12 wards, one ward from each IUEZ was randomly chosen, resulting in the following: ward 20 in the RVBS, ward 26 in the RAWB, ward 69 in the PASC and ward 60 in the CRIA zone. Using a probability proportional sampling procedure, a total of 360 households were targeted in the four selected wards, of which 330 responded (91.7%). The first author interviewed the corresponding household heads during June–July 2014.
Data analysis
Descriptive and computational statistical data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and the results are presented in sequence: an analysis of Stegomyia indices in the IUEZs, the status and explanatory factors of knowledge and awareness among city dwellers, and the status and explanatory factors of vector control measures being undertaken by them. The association of the Stegomyia indices was evaluated through the χ2 test for equality of proportions among the three IUEZs (RVBS, RAWB and PASC); considering the unmet minimum number in the CRIA zone, data for this zone were merged with the RVBS. The descriptive analysis involved 18 variables related to participant responses regarding KAAP and WTP (see Supplementary Table 2).
The χ2 tests were performed to identify factors associated with knowledge and awareness and IUEZ differentials, determine if there are differences in control measures among the IUEZs, identify factors influencing vector control measures and determine if there are differences in WTP for vector control among the IUEZs. The multinomial logistic regression (MLR) method was applied to identify the factors influencing WTP. To single out important variables related to WTP, univariate analysis of 18 explanatory variables was performed. Based on a cut-off p-value ˂0.1, 11 explanatory variables were included in the MLR model and a stepwise selection method was applied to identify the key explanatory factors of WTP.
Results
Stegomyia indices in the IUEZs
With an overall response rate of 60.6% for the entomological survey and coverage of a total of 727 inspected households that met the minimum required response rate of ≥50%,15 the likelihood of a nonresponse bias is minimal. A total of 674 wet containers were identified, of which 224 were positive in terms containing Aedes larvae and/or pupae (see Supplementary Table 3), including a total of 1120 Aedes pupae (identification methods are detailed elsewhere16).
The 2013 monsoon season survey results reveal that 15% (109/727) of the inspected households were positive for Aedes larvae and/or pupae. The distribution and prevalence of vector mosquitoes can be described by the traditional Stegomyia indices. Overall, 30.8 positive containers were observed per 100 households inspected, with a range between 0.26 in the RAWB and 0.38 in the PASC zones (Table 1). The mean PPI was 0.54: 0.57 in the RVBS, 0.21 in the RAWB and 0.65 in the PASC, the area dominated by paved/built areas, residential buildings and public and commercial service infrastructure.
Table 1.
Stegomyia indices by IUEZ, Dhaka (12 wards), 2013
| IUEZ | House index | 95% CI | Container index | 95% CI | Breteau index | 95% CI | PPI |
|---|---|---|---|---|---|---|---|
| RVBSa | 14.3 | 10.2 to 17.8 | 30.8 | 25.5 to 36.5 | 26.8 | 23.0 to 33.0 | 0.57 |
| RAWB | 10.9 | 5.6 to 16.4 | 25.8 | 18.4 to 33.6 | 25.8 | 18.4 to 33.6 | 0.21 |
| PASC | 17.5 | 13.5 to 22.4 | 39.1 | 33.2 to 44.8 | 37.5 | 32.4 to 43.6 | 0.65 |
| All zones | 14.9 | 12.3 to 17.5 | 33.2 | 29.7 to 36.8 | 30.8 | 27.5 to 34.2 | 0.54 |
The RVBS was merged with the CRIA in consideration of the unmet minimum size of the CRIA for statistical tests.
House index: number of positive households/number of households visited (%); Container index: number of positive containers/number of wet containers (%); Breteau index: number of positive containers/number of households visited (per 100 households); PPI: number of pupae collected/number of people who slept last night.
As shown in Table 1, the Stegomyia indices, except for the house index, vary significantly among the IUEZs (container index 33.2 [95% CI 29.7 to 36.8], p=0.018; Breteau index 30.8 [95% CI 27.5 to 34.2], p=0.007; PPI 0.54, p=0.0001). These results indicate that in Dhaka, IUEZs have profound impacts on mosquito abundance and density, and that urban ecology and functional characteristics have significant impacts on vector abundance and spatial distribution. Differences were detected in mosquito abundance and density as a function of urban ecology (IUEZ).
Respondents’ demographic and socio-economic characteristics
The sociodemographic breakdown of the KAAP survey respondents (i.e. household heads) showed that among the 330 respondents, 56.7% (187/330) were women and most were adults (the 31–50 y age group accounted for 43.7% [135/309]). Almost a quarter (24.5% [78/318]) of the respondents had never attended school and 39.3% (125/318) had an educational background beyond grade 12. The majority of the sampled households had incomes <25 000 Bangladeshi Taka (BDT; approximately US$300) per month.
Status of knowledge and awareness of dengue and its associated factors
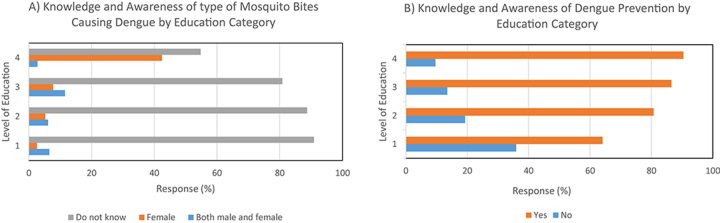
Results of bivariate analyses of respondents’ knowledge and awareness of dengue and its associated factors are presented in Table 2. Of 330 respondents, 309 (93.9%; 95% CI 92.2 to 96.6) had some knowledge about dengue from various sources. However, while 261 of 329 respondents (79.3%; 95% CI 74.9 to 83.7) were unaware of whether male or female mosquito bites can cause dengue infection, 297 respondents (90.3%; 95% CI 86.7 to 93.5) agreed that DF and severe dengue can have ‘serious’ health implications. The majority of respondents were aware of the association between dengue incidence and land use (52.6% [172/327]; 95% CI 47.2 to 58.0) or weather (57.0% [187/328]; 95% CI 51.6 to 62.3), as well as with rainy early summer and monsoon seasonality (68.6% [225/328]; 95% CI 63.6 to 73.6). An overwhelming majority of respondents agreed that dengue diseases can be prevented by various measures (79.3% [261/329]; 95% CI 74.9 to 83.7). Knowledge and awareness variables (GenMosbite, DEpiPrev, LUcozD, WcozD) (see Supplementary Table 2 for description of variables) were found through χ2 tests to be significantly associated with the level of education (p<0.0001, 0.0003, 0.0002 and 0.026, respectively, at the 5% level) (Figure 2).
Table 2.
Distribution and bivariate test results of responses concerning knowledge of dengue by KAAP survey participants (n=330) and by IUEZ, Dhaka (four wards), 2014
| Variable/category | Respondents all zones | IUEZs | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RVBS | RAWB | PASC | CRIA | ||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||
| Prior knowledge of dengue/heard of dengue | |||||||||||
| Yes No |
309 20 |
(93.9) (6.1) |
97 8 |
(92.4) (7.6) |
85 3 |
(96.6) (3.4) |
64 4 |
(94.1) (5.9) |
63 5 |
(92.7) (7.3) |
0.630 |
| Knowledge of magnitude of dengue fever | |||||||||||
| Serious Mild Do not know |
297 14 18 |
(90.3) (4.3) (5.4) |
96 5 4 |
(91.4) (4.8) (3.8) |
86 1 1 |
(97.8) (1.1) (1.1) |
60 4 4 |
(88.2) (5.9) (5.9) |
55 4 9 |
(80.9) (5.89) (13.2) |
0.019* |
| Knowledge of association of land-use with dengue | |||||||||||
| Yes Do not know |
172 155 |
(52.6) (47.4) |
63 41 |
(60.6) (39.4) |
42 46 |
(47.7) (52.3) |
37 30 |
(55.2) (44.8) |
30 38 |
(44.1) (55.9) |
0.131 |
| Knowledge of association of weather with dengue | |||||||||||
| Yes Do not know |
187 141 |
(57.0) (43.0) |
61 44 |
(58.1) (41.9) |
44 44 |
(50.0) (50.0) |
38 29 |
(56.7) (43.3) |
44 24 |
(64.7) (35.3) |
0.326 |
| Knowledge of association of seasonality with dengue | |||||||||||
| Early summer Monsoon Winter |
75 150 103 |
(22.9) (45.) (31.4) |
20 42 44 |
(18.9) (39.6) (41.5) |
20 42 26 |
(22.7) (47.7) (29.6) |
22 34 12 |
(32.3) (50.0) (17.7) |
13 32 21 |
(19.7) (48.5) (31.8) |
0.049* |
| Knowledge of types of mosquito bites causing dengue | |||||||||||
| Both male and female mosquito Female mosquito Do not know |
22 46 261 |
(6.7) (14.0) (79.3) |
4 24 78 |
(3.8) (22.6) (73.6) |
2 11 75 |
(2.3) (12.5) (85.2) |
5 6 57 |
(7.4) (8.8) (83.8) |
11 5 51 |
(16.4) (7.5) (76.1) |
0.0006* |
| Knowledge of dengue prevention | |||||||||||
| Yes No |
261 68 |
(79.3) (20.7) |
90 15 |
(85.7) (14.3) |
69 19 |
(78.4) (21.4) |
51 17 |
(75.0) (25.0) |
51 17 |
(75.0) (25.0) |
0.240 |
| Total | 330 | (100) | 106 | (100) | 88 | (100) | 68 | (100) | 68 | (100) | |
Early summer: April–May; Monsoon: June–September; Winter: December–January.
*Significant at p˂0.05 level.
Figure 2.
Distribution (%) of knowledge and awareness among respondents (n=329) by education category (1=no schooling/primary education, 2=secondary, 3=higher secondary/diploma, 4=graduate and postgraduate). (A) Type of mosquito bites causing dengue. (B) Dengue prevention options.
In terms of IUEZ communities, significant differences were detected among the respondents regarding their knowledge of the severity of dengue infection (p=0.019), its association with seasonality (p=0.049) and the types of mosquito bites that may cause dengue infection (p=0.0006; see Table 2). The extent of knowledge of DF and its severity was noticeably higher among the respondents from the RAWB (ward 26) than other IUEZs.
Attitudes toward vector control measures and WTP
Of the 329 respondents, 261 (79.3% [95% CI 74.9 to 83.7]) demonstrated an affirmative attitude towards prevention and/or control of dengue infection. Bivariate analyses of such attitudes towards the prevention or control of dengue via vector control show (Table 3) that the majority of respondents are inclined towards using coils and/or mosquito repellent spray (72.1% [238/330]; 95% CI 67.3 to 76.9) and bed nets (57.6% [190/330]; 95% CI 52.3 to 62.9). In addition, two-thirds of the respondents (66.7% [220/330]; 95% CI 61.6 to 71.8) reported that government and/or institutional measures were not effective in limiting the propagation of the dengue vector or the spread of dengue infection.
Table 3.
Distribution and bivariate analyses of responses concerning vector control measures, spending per month by household and WTP for vector control (n=330) and by IUEZ, Dhaka (four wards), 2014
| Variable/category | Respondents | IUEZs | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| all zones | RVBS | RAWB | PASC | CRIA | ||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| Attitude and practice of control measures using bed net | ||||||||||||
| (ConBedN) | Yes No |
190 140 |
(57.6) (42.4) |
65 41 |
(61.3) (38.7) |
58 30 |
(65.9) (34.1) |
29 39 |
(42.7) (57.3) |
38 30 |
(55.9) (44.1) |
0.025* |
| Attitude and practice of control measures using coil/spray | ||||||||||||
| (ConCS) | Yes No |
238 92 |
(72.1) (27.9) |
86 20 |
(81.1) (18.9) |
57 31 |
(64.8) (35.2) |
47 21 |
(69.1) (30.9) |
48 20 |
(70.6) (29.4) |
0.071 |
| Attitude and practice of control measures using electric gadgets | ||||||||||||
| (ConElecGaz) | Yes No |
57 273 |
(17.3) (82.7) |
16 90 |
(15.1) (84.9) |
12 76 |
(13.6) (86.4) |
22 46 |
(32.4) (67.6) |
7 61 |
(10.3) (89.7) |
0.003* |
| Attitude and practice of control measures using fan | ||||||||||||
| (ConFan) | Yes No |
162 168 |
(49.1) (50.9) |
51 55 |
(48.1) (51.9) |
44 44 |
(50.0) (50.0) |
38 30 |
(55.9) (44.1) |
29 39 |
(42.6) (57.4) |
0.484 |
| Awareness of government control measures | ||||||||||||
| (GovCon) | Yes No |
110 220 |
(33.3) (66.8) |
38 68 |
(35.8) (64.2) |
20 68 |
(22.7) (77.3) |
27 41 |
(39.7) (60.3) |
25 43 |
(36.8) (63.2) |
0.095 |
| Average spending for mosquito control/moth by households | ||||||||||||
| (AvgCPM) | 0–100 101–400 >400 |
88 144 63 |
(29.8) (48.8) (21.4) |
19 53 22 |
(20.2) (56.4) (23.4) |
20 45 14 |
(25.3) (57.0) (17.7) |
25 27 7 |
(42.4) (45.8) (11.8) |
24 19 20 |
(38.1) (30.1) (31.8) |
0.002* |
| Willingness to pay for mosquito vector control | ||||||||||||
| (WTP) | 0–100 101–400 >400 |
117 96 117 |
(35.5) (29.0) (35.5) |
34 28 44 |
(32.1) (26.4) (41.5) |
30 23 35 |
(34.1) (26.1) (39.8) |
25 24 19 |
(36.8) (35.3) (27.9) |
28 21 19 |
(41.2) (30.9) (27.9) |
0.393 |
| Total | 330 | (100) | 106 | (100) | 88 | (100) | 68 | (100) | 68 | (100) | ||
*Significant at p˂0.05 level.
Given the general failure of public services in vector control measures,17 an overwhelming majority of respondents (89.4% [295/330]; 95% CI 86.1 to 92.7) paid privately for vector control measures. Of them, 48.8% (144/330) spent a modest amount (101–400 BDT/mo) for this purpose. All respondents, including the 35 respondents who had not spent any money on vector control, expressed their WTP for control measures (Table 3). Notably, while only 63 of 295 respondents (21.4% [95% CI 16.7 to 26.1]) had been spending more than 400 BDT at the time of the survey, 117 (35.5%) respondents reported they would pay more than 400 BDT in the future.
The χ2 test results revealed that there were significant differences among the IUEZs in the use of bed nets (p=0.025), electronic gadgets (p=0.003) and average monthly spending for vector control (p=0.002). Among the IUEZs, no significant difference in terms of WTP for vector control was registered.
Explanatory factors associated with attitudes towards WTP
An MLR model with stepwise method, based on 11 variables, was carried out to identify factors related to WTP for vector control purposes. This resulted in five significant variables (AvgCPM, Income, LUcozD, SeasonMos, ConAeElecGaz) (see Supplementary Table 2 for a description of the variables) associated with WTP (p<0.0001, 0.006, 0.018, 0.001 and 0.009, respectively; Table 4). The findings indicate that if monthly household income increases, WTP for vector control would correspondingly increase. For example, the OR (2.56 [95% CI 1.00 to 6.52]) implies that WTP 101–400 BDT vs ≤100 BDT per month would increase by 2.56 times if household monthly income increased from ˂25 000 to ≥25 000 BDT, after adjusting for other factors. The MLR results also revealed that enhancement of knowledge of the effects of land use on dengue, effects of seasonality on dengue incidence and adoption of electronic gadgets would all increase WTP for vector control measures (Table 4).
Table 4.
Results of the MLR model identifying factors influencing WTP to control dengue vector mosquitoes (n=330), Dhaka (four wards), 2014
| Explanatory variables | Explanation of the variables | p-Value | OR | 95% CI |
|---|---|---|---|---|
| Average cost per month (AvgCPM) AvgCPM 2 vs 1 WTP 2 AvgCPM 2 vs 1 WTP 3 AvgCPM 3 vs 1 WTP 2 AvgCPM 3 vs 1 WTP 3 |
When AvgCPM 101–400 vs ≤100, then WTP 101–400 vs ≤100a When AvgCPM 101–400 vs ≤100, then WTP >400 vs ≤100a When AvgCPM >400 vs ≤100, then WTP 101–400 vs ≤100a When AvgCPM >400 vs ≤100, then WTP >400 vs ≤100a |
<0.0001* | 8.12 9.80 10.26 61.23 |
3.74 to 17.62 4.15 to 23.15 2.96 to 35.57 17.55 to 213.62 |
| Income Income 2 vs 1 WTP 2 Income 2 vs 1 WTP 3 Income 3 vs 1 WTP 2 Income 3 vs 1 WTP 3 |
When income 25 000–50 000 vs 0–25 000, then WTP 101–400 vs ≤100 When income 25 000–50 000 vs 0–25 000, then WTP >400 vs ≤100 When income >50 000 vs 0–25 000, then WTP 101–400 vs ≤100 When income >50 000 vs 0–25 000, then WTP >400 vs ≤100 |
0.006* | 2.56 5.22 0.99 2.73 |
1.00 to 6.52 2.03 to 13.39 0.36 to 2.75 1.04 to 7.16 |
| Knowledge of land use causing dengue (LUcozD) Yes vs do not know WTP 2 Yes vs do not know WTP 3 |
When LUcozD yes vs do not know, then WTP 101–400 vs ≤100a When LUcozD yes vs do not know, then WTP >400 vs ≤100 |
0.018* | 0.46 1.16 |
0.22 to 0.98 0.52 to 2.58 |
| Knowledge of season causing dengue (SeasonMos) Summer vs monsoon WTP 2 Summer vs monsoon WTP 3 Winter vs monsoon WTP 2 Winter vs monsoon WTP 3 |
When SeasonMos summer vs monsoon, then WTP 101–400 vs ≤100a When SeasonMos summer vs monsoon, then WTP >400 vs ≤100 When SeasonMos winter vs monsoon, then WTP 101–400 vs ≤100a When SeasonMos winter vs monsoon, then WTP >400 vs ≤100 |
0.001* | 3.03 6.98 1.89 3.96 |
1.20 to 7.64 2.65 to 18.36 0.82 to 4.37 1.66 to 9.42 |
| Using electronic gadget for mosquito control (ConAeElecGaz) Yes vs no WTP 2 Yes vs no WTP 3 |
When ConElecGaz yes vs no, then WTP 101–400 vs ≤100a When ConElecGaz yes vs no, then WTP>400 vs ≤100 |
0.009* | 2.13 4.42 |
0.78 to 5.82 1.67 to 11.68 |
aReference category is 1 for WTP.
*Significant at p˂0.05 level.
Discussion
The results of the present study show that urban ecological and functional characteristics have profound impacts on Aedes abundance and spatial distribution in Dhaka, Bangladesh. From analyses of the traditional Stegomyia indices and PPI, it is evident that Aedes abundance (reflected in modest to high index values) is significantly influenced by city dwellers’ KAAP, particularly in respect to managing water containers on their premises as sources of infestation.10,18 As about one-third of the residents do not have access to piped potable water,16,17 they tend to store water and inadvertently create sites for vector mosquitoes to develop. This problem is augmented by frequent power failures and interruptions in water delivery.11,16 In several Latin and Central American cities, Barrera et al.19 and Stewart Ibarra20 observed a similar influence of environmental and human behavioural factors on Aedes productivity and abundance. Although several studies observed tyres as a major breeding site of Aedes,21–22 due to the limited coverage of our entomological survey, primarily to household premises, we did not find tyres to be a major breeding site in Dhaka. In urban areas of Thailand, Barbazan et al.23 calculated a PPI of 0.8, and in Cambodia, Seng et al.24 observed PPIs between 1.0 and 4.4. In Dhaka, a relatively low PPI – 0.58 by Dhar-Chowdhury et al.16 and 0.54 in the present study (Table 1) – compared with other Southeast Asian countries is likely the result of Dhaka’s extremely high population density.
Our study identified that education level is significantly associated with the KAAP of city dwellers regarding dengue, and that variations among the IUEZs in land use/cover patterns and the educational status of the respondents noticeably influenced differences in their Stegomyia indices. These were reflected in the significant differences in the container index, Breteau index and PPI among the IUEZs. For instance, the PASC zone, characterized by the predominance of paved and built-up areas, including residential, service and commercial buildings and other infrastructure, had the highest mosquito density in terms of the house index, Breteau index and container index. Our overall findings of varying mosquito density by IUEZ are similar to a recent study in Rio de Janeiro, Brazil that observed variable dengue vector distribution among and within neighbourhoods.25 Our findings imply a strong positive correlation of vector productivity and abundance with urban infrastructure, the surrounding environment and supportive habitat within the households. Several studies in this context have asserted that environmental factors induced by urbanization and associated changes in land use/surface area, water bodies, vegetation coverage and surface type are significant factors enhancing Aedes habitats, which in turn may result in a high risk of disease transmission.26,27
In the present study we found that the majority of the respondents (93.9%) had heard about dengue via different sources, including collaborative health education programmes, and were aware of the serious adverse effects of DF and severe dengue (90.3%). An overwhelming majority (79.3%) of the respondents agreed that dengue infection and spread can be prevented, and a majority knew that land use changes, weather and seasonal differences contribute to dengue incidence. In this regard, collaborative education campaigns in Nicaragua28 and the Philippines29 have proven effective in enhancing knowledge and awareness of the disease and its transmission. Consistent with our findings, numerous studies have confirmed that people with better knowledge of the vector and the disease were associated with lower breeding rates.8,9 According to these studies, knowledge and education led to changes in human practices and behaviour, which eventually reduces vector breeding and dengue infection rates.
Similar to our findings, a Peruvian study observed that knowledge about dengue was positively associated with higher levels of education, and improved prevention practice was associated with higher socio-economic status.9 A nationwide study in Malaysia also revealed that income, occupation and knowledge about the dengue vector, dengue disease and its risks were closely associated with rigorous dengue prevention practices.30 However, other studies have cautioned that knowledge about dengue does not always transfer to practice among community members.8,9
According to the findings of our study, most of the community members (79.3%) are unaware of the types of mosquito bites that can cause dengue infection, and a high degree of confusion in understanding dengue transmission is evident. Dhar-Chowdhury et al.’s11 findings revealed that substantial gaps exist between local knowledge and experts’ views regarding dengue risk and severity, causes and symptoms of dengue, dengue vector ecology, and dengue transmission risk and control. Despite these gaps, the results of our study revealed some improvements in local community members’ basic knowledge about dengue and its vector, serious adverse effects of DF and severe dengue, and the association of weather and seasonality with dengue incidence and disease prevention. This change is likely attributable to recent awareness-building campaigns through the media and collaborative health education programmes by the governments and non-governmental organizations (NGOs).
According to the WHO,31 several specific measures can be taken to prevent and control dengue, including eliminating mosquito breeding grounds; effective solid waste disposal; covering, emptying and cleaning water storage containers every week; applying insecticide; and using window screens, coils and vaporizers. In recent years, some newer measures, including mosquito control through genetic manipulation, sterile insect technique and attractive toxic sugar baits, have also produced promising outcomes.32 A recent multicountry study in Asia found spraying with insecticide was the major vector control measure taken by governments.33 However, as Mahmood17 found, two-thirds of the respondents agreed that government control measures were not effective. Also, continued use of pyrethroid-based aerosol products should be guided by the insecticide resistance status of the key vectors in sentinel sites. While inquiring about control measures, we found that the most commonly used measures were coils and spraying repellents and insecticides, because of their convenience and affordability to all income groups. These practices indicate an inclination towards private spending, in part as a response to the failure or limitations of public programmes.
Despite recognition that dengue is a rapidly growing and important disease, there are only a few studies carried out thus far related to the economic aspects (e.g. WTP for vector control and/or vaccine) of the disease.10,34 Since government authorities, including the DCC and the national government, are not very active or effective in controlling the dengue vector and transmission in Bangladesh,16–18 exploring citizens’ WTP privately is crucial. These are all the more important when considering the declining trend in public funding for the health sector at large.
Our study, the first of its kind in Bangladesh examining WTP, revealed that the average household spending per month for mosquito control was modest (101–400 BDT [approximately US$1.2–5]) and the majority of respondents were willing to spend more in the future. A similar study in Florida and Arizona, USA reported that about two-thirds of the study population expressed that they would be willing to pay US$25 more annually, on top of government dengue control measures.34 Similar to our findings in Dhaka, the US study also observed that education, income and perceived knowledge about the disease are important factors influencing people’s WTP. Among the limited studies on WTP thus far, most have revealed that socio-economic status is highly correlated with WTP, especially in the case of a vaccine, if any were available.10,34
Overall, the present study revealed that the majority of local people have some knowledge of dengue, they attempt to control the vector privately considering the local context and they are willing to pay for mosquito control to reduce and prevent dengue. However, the use of control measures taken at the individual level is unlikely to be very effective. For example, usage of personal protective insect spray, mosquito coils, bed nets or electric gadgets, commonly used by the respondents, would only be effective within their household premises. The public spaces where exposure often occurs, such as roads, sewerage and storm drains, public and commercial buildings, vegetated areas, meadows, canals and streams, are not covered by such measures.
It is therefore critically important for the public authorities to take up effective dengue vector control measures such as spraying/fogging neighbourhoods regularly, applying appropriate insecticide to vector breeding sites and providing subsidized window/door screens. Further education and training of community members on how to control vectors (e.g. using lidded containers, proper waste disposal) and avoid mosquito bites (e.g. wearing clothes properly covering the body, use spray/coil) is recommended to eliminate the remaining inequities in knowledge attributable to differences in socio-economic status and education levels. Public health officials and local NGOs can act as a bridge between the government and the community in controlling and preventing dengue. An integrated and collaborative mosquito control programme by the government, NGOs and the community can thus effectively reduce the spread and incidence of dengue.
The present study was limited in scope by several factors. First, the urban ecological classification part of the study covered only 12 wards and the KAAP survey covered only 4 of 90 wards, and therefore any generalization should be made with caution. Second, the entomological data used in this study refer to a cross-sectional survey period (the monsoon season of 2013) and therefore an analysis of any trends could not be performed. Third, a detailed questionnaire on institutional (both governmental and NGO) control measures was not included. Further research on these issues, along with individual and private-sector contributions concerning effective vector and dengue prevention and control, is necessary.
Conclusions
In this study we found that overall dengue vector abundance in the city of Dhaka is modest to high, primarily affected by household-level water storage and vector spatial distribution, and is significantly associated with the land use/cover characteristics of the IUEZs. The water containers possessed and used by households produce large populations of Aedes, especially during the monsoon, enhancing the risk of pathogen transmission.35 Such water container management has likely been influenced by the lack of awareness among a majority of city dwellers about the virus–vector–host relationships and dengue transmission. Effective public and community education campaigns are needed to promote and encourage the implementation of water and waste management strategies by city dwellers as a means of dengue vector control. In addition, the private sector, from the perspective of corporate social responsibility, needs to pay serious attention to dengue prevention and control. This can be achieved in part by implementing and complying with international regulations and standards (e.g. International Health Regulation 2005 to control vectors and reservoirs at the point of entry by airport authorities).
Reaffirming findings of other recent studies,11,16–18,35 our research revealed that government and other institutional interventions to control vector mosquitoes have been sporadic and generally ineffective. Although most households currently spend some of their own funds on vector control within their premises, control in public spaces is largely non-existent or ineffectual. In our study, which to our knowledge is the first in Bangladesh on WTP for vector control, we found that all respondents were willing to pay more for effective vector control, because they recognized the associated high health risk. However, government programmes must continue to support communities through sustainable vector control measures, such as subsidizing the price of window and door screens and mosquito traps, and to provide logistic support for the systematic reduction of mosquito breeding sites. The materialization of such private citizen and private sector roles in citywide vector and dengue control, however, would require good governance, transparency and accountability from the public institutions dealing with health risks.
Supplementary Material
Acknowledgements
We are thankful to Khandakar Hasan Mahmud, Department of Geography and Environment, Jahangirnagar University, Savar, Bangladesh for his assistance in preparing the map. We thank the field entomologists, North South University students and Dhaka ward participants for their contributions to this study. We acknowledge the extensive support provided by the Department of Public Health of North South University, Directorate General of Health Services of the Government of Bangladesh, Dhaka, Bangladesh.
Contributor Information
Sabrina Islam, School of Health and Life Sciences, North South University, Dhaka 1229, Bangladesh.
C Emdad Haque, Natural Resources Institute, University of Manitoba, 70 Dysart Rd, Winnipeg, MB, Canada R3T 2N2.
Shakhawat Hossain, Department of Mathematics and Statistics, University of Winnipeg, Winnipeg, MB, Canada R3B 2E9.
David Walker, Department of Environment and Geography, University of Manitoba, Winnipeg, MB, Canada R3T 2N2.
Authors’ contributions
SI and CEH conceived and designed the study, finalized the study tools and collected data. SI conducted the KAAP survey interviews. SI, CEH and SH contributed to the literature review. SI and SH contributed to the data analysis. SI, CEH, SH and DW contributed to the data interpretation and preparation of the manuscript. All authors read and approved the final version. CEH is the guarantor of the paper.
Funding
This research was funded by the IDRC, Ottawa, Canada (grant 106040-001) and the University of Manitoba, Winnipeg, MB, Canada. Further financial assistance was received from the Social Science and Humanities Research Council, InSight Grant (grant 435-2012-1748), Ottawa, ON, Canada; from the IDRC through the IDRC Doctoral Research Awards (no. 107099-99906075-050) to the first author; and from the Natural Sciences and Engineering Research Council, Ottawa, ON, Canada (grant 419428) to the third author.
Competing interests
None declared.
Ethical approval;
This research was approved by the Bangladesh Medical Research Council, Dhaka, Bangladesh and the University of Manitoba Joint Faculty Research Ethics Board, Winnipeg, MB, Canada. All participants provided informed consent verbally before participation in the study. In Bangladesh, many respondents are illiterate and are unwilling to provide written consent due to fear of forgery or deception. In this regard, both the Bangladeshi and Canadian ethics boards, considering the sociocultural contexts, approved oral consent procurement, with witnesses and their signatures.
References
- 1. World Health Organization Dengue and severe dengue. Geneva: World Health Organization; 2018. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 2. Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization Dengue and severe dengue. Geneva: World Health Organization; 2019. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 5. Saied KG, Al-Taiar A, Altaire A, Alqadsi A, Alariqi EF, Hassan M. Knowledge, attitude and preventive practices regarding dengue fever in rural areas of Yemen. Int Health. 2015;7(6):420–5. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization Dengue guidelines for diagnosis, treatment, prevention, and control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 7. Pickett STA, Cadenasso ML. Advancing urban ecological studies: frameworks, concepts, and results from the Baltimore Ecosystem Study. Austral Ecol. 2006;31(2):114–25. [Google Scholar]
- 8. Elsinga J, Schmidt M, Lizarazo EF, Vincenti-Gonzalez MF et al. Knowledge, attitudes, and preventive practices regarding dengue in Maracay, Venezuela. Am J Trop Med Hyg. 2018;99(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paz-Soldán VA, Morrison AC, Lopez JJC et al. Dengue knowledge and preventive practices in Iquitos, Peru. Am J Trop Med Hyg. 2015;93(6):1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harapana H, Anwar S, Bustamam A, et al. Willingness to pay for a dengue vaccine and its associated determinants in Indonesia: a community-based, cross-sectional survey in Aceh. Acta Trop. 2017;166:249–56. [DOI] [PubMed] [Google Scholar]
- 11. Dhar-Chowdhury P, Haque CE, Driedger SM et al. Community perspectives on dengue transmission in the city of Dhaka, Bangladesh. Int Health. 2014;6(4):306–16. [DOI] [PubMed] [Google Scholar]
- 12. Banu S, Hu W, Hurst C, et al. Space-time clusters of dengue fever in Bangladesh. Trop Med Int Health. 2012;17(9):1086–91. [DOI] [PubMed] [Google Scholar]
- 13. Roy P, Alam H. Dengue risk rising. Daily Star, 6 October 2018.
- 14. Struyf A, Hubert M, Rousseeuw PJ. Clustering in an object-oriented environment. J Stat Softw. 1996;1(4):1–30. [Google Scholar]
- 15. Davern M, McAlpine D, Beebe TJ, et al. Are lower response rates hazardous to your health survey? An analysis of three state telephone health surveys. Health Serv Res. 2010;45(5 Pt 1):1324–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhar-Chowdhury P, Haque CE, Lindsay R, et al. Socioeconomic and ecological factors influencing Aedes aegypti prevalence, abundance, and distribution in Dhaka, Bangladesh. Am J Trop Med Hyg. 2016;94(6):1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahmood SAI. Dengue: an epidemic is largely a failure in public health administration! The role of Dhaka City Corporation. World Health Popul. 2006. January;1–10. [Google Scholar]
- 18. Ferdousie F, Yoshimatsu S, Ma E, et al. Identification of essential containers for Aedes larval breeding to control dengue in Dhaka, Bangladesh. Trop Med Health. 2015;43(4):253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrera R, Amador M, MacKay A. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5(12):e1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stewart Ibarra AM, Ryan SJ, Beltran E, et al. Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS One. 2013;8(11):e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Getachew D, Tekie H, Gebre-Michael T, et al. Breeding sites of Aedes aegypti: potential dengue vectors in Dire Dawa, East Ethiopia. Interdiscip Perspect Infect Dis. 2015;2015:706276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higa Y, Yen NT, Kawada H, et al. Geographic distribution of Aedes aegypti and Aedes albopictus collected from used tires in Vietnam. J Am Mosq Control Assoc. 2010;26(1):1–9. [DOI] [PubMed] [Google Scholar]
- 23. Barbazan P, Tuntaprasart W, Souris M et al. Assessment of a new strategy, based on Aedes aegypti (L.) pupal productivity, for the surveillance and control of dengue transmission in Thailand. Ann Trop Med Parasitol. 2008;102(2):161–71. [DOI] [PubMed] [Google Scholar]
- 24. Seng CM, Setha T, Nealon J, et al. Pupal sampling for Aedes aegypti (L.) surveillance and potential stratification of dengue high-risk areas in Cambodia. Trop Med Int Health. 2009;14(10):1233–40. [DOI] [PubMed] [Google Scholar]
- 25. Xavier DR, Magalhães MA, Gracie R, et al. Spatial–temporal diffusion of dengue in the municipality of Rio de Janeiro, Brazil, 2000–2013. Cad Saúde Pública. 2017;33(2):e00186615. [DOI] [PubMed] [Google Scholar]
- 26. Delmelle E, Hagenlocher M, Kienberger S, et al. A spatial model of socioeconomic and environmental determinants of dengue fever in Cali, Colombia. Acta Trop. 2016;164:169–76. [DOI] [PubMed] [Google Scholar]
- 27. Sarfraz MS, Tripathi NK, Tipdecho T, et al. Analyzing the spatio-temporal relationship between dengue vector larval density and land-use using factor analysis and spatial ring mapping. BMC Public Health. 2012;12:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayers G. Education and community sensitization are the keys to preventing dengue. Geneva: International Federation of Red Cross and Red Crescent Societies; 2013.
- 29. Yboa BC, Labrague LJ. Dengue knowledge and preventive practise among rural residents in Samar Province, Philippines. Am J Public Health Res. 2013;1(2):47–52. [Google Scholar]
- 30. Wong LP, Shakir SMM, Atefi N et al. Factors affecting dengue prevention practices: nationwide survey of the Malaysian public. PLoS One. 2015;10(4):e0122890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization Dengue and severe dengue. Geneva: World Health Organization; 2014. http://apps.who.int/iris/bitstream/handle/10665/204161/Fact_Sheet_WHD_2014_EN_1629.pdf?sequence=1 (accessed 12 March 2019).
- 32. World Health Organization Mosquito (vector) control emergency response and preparedness for Zika virus. Geneva: World Health Organization; 2016. https://www.who.int/neglected_diseases/news/mosquito_vector_control_response/en/.
- 33. Arunachalam N, Tyagi BK, Samuel M, et al. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City, India. Pathog Glob Health. 2012;106(8):488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dickinson KL, Hayden MH, Haenchen S, et al. Willingness to pay for mosquito control in Key West, Florida and Tucson, Arizona. Am J Trop Med Hyg. 2016;94(4):775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paul KK, Dhar-Chowdhury P, Haque CE, et al. Risk factors for the presence of dengue vector mosquitoes, and determinants of their prevalence and larval site selection in Dhaka, Bangladesh. PLoS One. 2018;13(6):e0199457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.