Abstract
Background
The voriconazole and echinocandin combination has been found to be synergistic in vitro and in vivo against most Aspergillus fumigatus isolates, both with a WT azole phenotype and an azole-resistant phenotype. The interaction between isavuconazole and echinocandins is less well studied. This is especially true for azole-resistant isolates.
Objectives
We investigated the in vitro interaction between isavuconazole and anidulafungin for 30 A. fumigatus isolates including 18 azole-resistant isolates with various isavuconazole resistance phenotypes.
Methods
The isavuconazole/anidulafungin interaction was studied by using an adapted EUCAST-based 2D (12 × 8) chequerboard broth microdilution colorimetric assay using XTT. The interaction was analysed by FIC index (FICi) analysis and Bliss independence (BI) interaction analysis.
Results
Both the FICi analysis and the BI analysis showed synergistic interaction between isavuconazole and anidulafungin for the majority of WT and azole-resistant isolates. As we did not see significant beneficial effects of combination therapy in TR46/Y121F/T289A isolates at clinically achievable drug concentrations, it is unlikely that TR46/Y121F/T289A infections would benefit from isavuconazole and anidulafungin combination therapy.
Conclusions
In regions with high azole resistance rates this combination may benefit patients with WT disease, azole-resistant invasive aspergillosis and those with mixed azole-susceptible and azole-resistant infection, but may not be beneficial for aspergillosis due to isolates with high isavuconazole resistance, such as TR46/Y121F/T289A isolates.
Introduction
It is estimated that worldwide approximately 300 000 people suffer from invasive aspergillosis annually.1 Voriconazole and isavuconazole are currently recommended as first-line agents for the treatment of patients with invasive aspergillosis.2–4 However, the emergence of azole resistance in Aspergillus fumigatus greatly challenges the management of invasive aspergillosis. As resistance selection may take place in the environment, any patient is at risk and may present with azole-resistant disease. Environmental resistance is dominated by isolates that harbour a tandem repeat (TR) in the promoter region of the cyp51A target gene combined with amino acid alterations, i.e. TR34/L98H and TR46/Y121F/T289A. Although these mutations alter the activity of all medical triazoles, TR34 isolates are highly resistant to itraconazole, whereas TR46 isolates are highly resistant to voriconazole.5,6
In regions with endemic resistance levels exceeding 10%, an international expert panel recommended to move away from azole first-line therapy and treat with liposomal amphotericin B, or to use voriconazole combined with an echinocandin.7 The voriconazole and echinocandin combination was found to be synergistic in vitro and in vivo against most A. fumigatus isolates with a WT azole phenotype.8–19 Importantly, similar beneficial effects of this combination were also seen in most azole-resistant A. fumigatus isolates.8–12,18 However, these studies involved isolates with voriconazole EUCAST MICs of 0.5–8 mg/L and it remains unclear whether an azole and echinocandin combination can be beneficially used in isolates with high resistance to voriconazole (MICs >8 mg/L). There is a risk that in patients with infection due to highly voriconazole- or isavuconazole-resistant isolates the benefit of combination therapy is lost and therapy is based on the echinocandin efficacy, which is suboptimal in (neutropenic) patients with invasive aspergillosis.20,21
Isavuconazole was shown to be non-inferior to voriconazole for the treatment of invasive aspergillosis and thus received an A-I recommendation in most guidelines, similar to that of voriconazole.2–4,22 Given the favourable pharmacokinetics and toxicity profile of isavuconazole, the drug is increasingly used in patients with invasive aspergillosis. There is currently limited evidence to support isavuconazole and echinocandin combination therapy. To the best of our knowledge, only two studies to date have investigated the interaction of isavuconazole with an echinocandin and only five azole-resistant A. fumigatus isolates (three with TR34/L98H mutation, one with G54W and one with P216L) were tested.23,24 Therefore, we investigated the in vitro interaction between isavuconazole and anidulafungin for 30 A. fumigatus isolates including 18 azole-resistant isolates with various isavuconazole resistance phenotypes.
Materials and methods
Isolates
A total of 30 A. fumigatus isolates were used in the study, of which 18 were azole-resistant with a known cyp51A mutation [6 isolates with TR34/L98H, 2 with TR46/Y121F/T289A, 3 with G54 (G54R, G54E, G54E), 4 with M220 (M220I, M220K, M220R, M220V), 1 with P216L, 1 with G448S and 1 with G138C] and 12 phenotypically azole WT isolates.
Antifungal agents and XTT/menadione
Isavuconazole (Sigma–Aldrich, St Louis, MO, USA) and anidulafungin (Sigma–Aldrich) were dissolved in DMSO at a concentration of 3200 mg/L. Antifungals were further diluted in RPMI 1640 to obtain the desired concentrations (described below) by serial dilutions according to the ISO scheme for preparing antifungal dilution series.25,26 Final antifungal concentrations ranged from 0.008 to 8 mg/L for isavuconazole and 0.008 to 0.5 mg/L for anidulafungin.
XTT (Sigma–Aldrich) was dissolved in sterile saline to produce a concentration of 500 mg/L. Menadione (Sigma–Aldrich) was first diluted in absolute ethanol at a concentration of 10 000 mg/L and was then added to the XTT solution to produce an XTT/menadione solution of 31.25 μM menadione and 500 mg/L XTT. Fifty microlitres of XTT/menadione solution was added to the wells after 48 h of incubation, resulting in final concentrations of 100 mg/L XTT and 6.25 μM menadione. The latter concentrations of XTT and menadione were chosen as these were previously found to be optimal for caspofungin.27
MIC determination and chequerboard XTT assay
The isavuconazole/anidulafungin interaction was studied by using an adapted EUCAST-based 2D (12 × 8) chequerboard broth microdilution colorimetric assay using XTT, as described previously.28 Four-fold the final concentration of isavuconazole or anidulafungin was diluted in 2-fold concentrated medium. Fifty microlitres of both isavuconazole and anidulafungin concentrations were added to 96-well microtitre plates to obtain 2-fold final concentrated medium and antifungal combinations. Prepared microtitre plates were stored at −70°C for a maximum of 4 weeks. Spore suspensions were prepared spectrophotometrically (Spectronic GENESYS 20) in sterile saline supplemented with 0.1% Tween 20 (Sigma–Aldrich) and further diluted in sterile saline to obtain a dilution of 1 × 106–4.2 × 106 conidia. One hundred microlitres of inoculum was added to the 96-well plates and the plates were incubated at 35–37°C. Microtitre plates were then incubated for 48 h. The activities of isavuconazole and anidulafungin were determined in accordance with the EUCAST reference methods.25 The anidulafungin minimal effective concentration (MEC) was read visually after 24 h and the isavuconazole MIC was read visually after 48 h. After 48 h, 50 μL of prepared XTT/menadione solution (to produce final concentrations of 6.25 μM menadione and 100 mg/L XTT) was added to the wells and incubated for a further 2 h. Conversion of XTT to formazan was measured as OD with a microtitre plate reader (Anthos Labtec Instruments HT3 Microtiter Plate Reader) at 450 nm with 630 nm as reference. Background OD from a similar incubated microtitre plate with added XTT/menadione solution but without inoculum was subtracted from the measurements.11,28 Measurements were performed in triplicate. Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality controls.
Interaction models
The interaction was analysed using two interaction models. The first was the FIC index (FICi) with inhibition endpoints based on XTT metabolism, where an FICi of ≤0.5 indicates synergism and an FICi of >4.0 indicates antagonism. An FICi between 0.5 and 4 indicates indifferent interaction. Two different endpoints were used. FIC1 was defined as 90% inhibition of formazan production compared with growth control. This endpoint, based on inhibition of formazan production, corresponded well to the isavuconazole visual MIC endpoint. FIC2 was defined as 75% inhibition compared with the growth control. Offscale MICs were adjusted to the next 2-fold higher or 2-fold lower concentration. FIC1 and FIC2 were calculated for each replicate individually. When only 2/3 samples were in accordance, the interaction conclusion of the majority was used for the isolate.
The second model used was the 3D surface interaction model based on the Bliss independence (BI) theory as previously described.11,29 The expected growth was calculated based on the BI model using Excel (2010) and GraphPad Prism (5.03). The expected growth was subtracted from observed growth for all three replicates and the mean was used as the BI result. BI results were tested for significance using a t-test. The sum of all significant BI results was used to calculate Bliss interaction (ΔE), where a positive ΔE indicates synergism and a negative ΔE indicates antagonism. The zero surface indicates no interaction.
Results
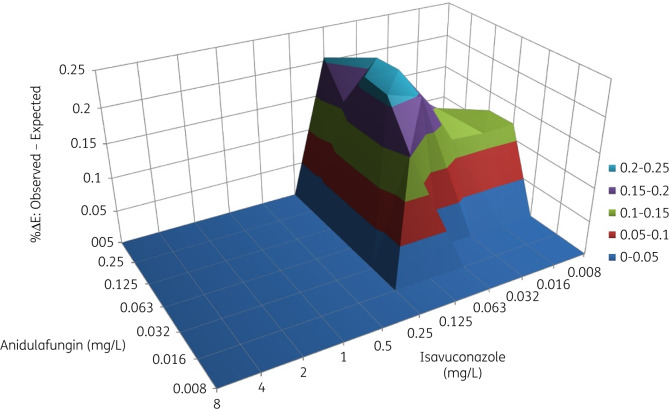
The interaction between isavuconazole and anidulafungin was synergistic for the majority of isolates (Table 1). As the MEC endpoint for anidulafungin without isavuconazole, which corresponded to 50% inhibition, was out of range for most isolates, this endpoint was excluded from the analysis. The FICi could not be determined for TR46/Y121F/T289A isolates as all defined FIC endpoints were out of the tested range. For all other isolates combined, the FIC1 was between 0.27 and 1.02. The geometric mean (GM) of FIC1 was between 0.5 and 4 when isolates were analysed in subgroups (WT, isolates with TR34/L98H and isolates with cyp51A point mutations, respectively). The interaction was synergistic for 2 isolates and indifferent for 26 isolates using the FIC1 endpoints. No antagonism was observed. The FIC2 endpoint ranged between 0.002 and 1.02. The FIC2 GMs of subgroups (WT, isolates with TR34/L98H and isolates with cyp51A point mutations) were <0.5. The interaction was synergistic for 7/12 WT isolates, 6/6 isolates with TR34/L98H and 7/10 isolates with cyp51A point mutations. No antagonism was observed (Table 1). TR46/Y121F/T289A isolates were excluded as all FIC endpoints were offscale and interaction based on FIC analyses could not be calculated. The results of the FIC analysis were confirmed with BI analysis, with a mean ΔE of 138%, indicating synergism (Table 1). Five isolates had an antagonistic interaction according to the BI analysis, while 24 had a synergistic interaction (Table S1, available as Supplementary data at JAC Online). The 3D surface diagram is displayed for the WT isolate AZN8196 with an isavuconazole MIC of 0.5 mg/L (Figure 1). The mean ΔE was higher for WT isolates compared with TR34/L98H isolates and isolates with cyp51A point mutations, but not significantly different. Only minor interaction was seen for TR46/Y121F/T289A isolates. At the range of concentrations used in the experiment, no favourable results were observed. The ΔE was 0% for one TR46 isolate while only minor antagonistic interaction was observed for the other isolate (ΔE of −19%).
Table 1.
FIC and BI analysis of 30 azole-susceptible or -resistant A. fumigatus isolates
| No. of isolates | Resistance genotype | ISA MIC (mg/L)a | FIC1 |
FIC2 |
BI, sum ΔE, mean (range) | ||||
|---|---|---|---|---|---|---|---|---|---|
| synergism (≤0.5) | indifference (0.5–4) | antagonism (>4) | synergism (≤0.5) | indifference (0.5–4) | antagonism (>4) | ||||
| 6 | TR34/L98H | 7.12 (4–>8) | 6 | 6 | 89.33 (−24 to 408) | ||||
| 2 | TR46/Y121F/T289A | >8 (>8) | OOR | OOR | −9.5 (−19 to 0) | ||||
| 10 | cyp51A point mutations | 0.63 (0.25–8) | 2 | 8 | 7 | 3 | 89 (−109 to 362) | ||
| 12 | WT isolates | 0.59 (0.25–4) | 12 | 7 | 5 | 201.83 (−112 to 522) | |||
ISA, isavuconazole; OOR, out of range.
Values shown are GM (range).
Figure 1.
BI surface diagram for the combination of isavuconazole and anidulafungin for A. fumigatus isolate AZN8196. The 0 surface indicates no interaction whereas the surface above 0 indicates synergistic interaction. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
We found predominantly synergistic in vitro interaction between isavuconazole and anidulafungin for most azole-susceptible and azole-resistant A. fumigatus isolates. Both statistical methods, FIC analyses and BI analyses were in concordance. However, the FIC results varied between the FIC endpoints that were defined.
In vitro and in vivo data already support a beneficial interaction between voriconazole and anidulafungin for invasive aspergillosis.8–17,19 Similar results were found for posaconazole or itraconazole with an echinocandin.29–33 This study indicates a favourable effect of combination therapy with isavuconazole and anidulafungin for A. fumigatus for both azole-susceptible and -resistant isolates with isavuconazole MICs between 1 and 8 mg/L.
Importantly, the favourable effects of combination therapy were not observed in our interaction model for isolates with TR46/Y121F/T289A mutations having isavuconazole MICs >8 mg/L. To investigate drug interactions, the range of drug combinations used in the interaction model should be around the MIC of the drug. However, the precise isavuconazole MICs for TR46/Y121F/T289A isolates are not known as the standard susceptibility testing range is truncated at 8 or 16 mg/L for isavuconazole and the exposure needed to treat isolates with such high MICs is not achievable in humans. Therefore, we did not extend the range of isavuconazole drug concentrations tested in our in vitro model. Thus, we cannot exclude a synergistic interaction between isavuconazole and anidulafungin in isavuconazole highly resistant A. fumigatus isolates. As we did not see significant beneficial effects of combination therapy at clinically achievable drug concentrations, it is unlikely that TR46/Y121F/T289A infections would benefit from isavuconazole and anidulafungin combination therapy. This implies that when using isavuconazole plus anidulafungin for infections with TR46/Y121F/T289A, only the efficacy of the echinocandin remains, which is suboptimal.20,21 Thus, in geographic regions with a high incidence of TR46/Y121F/T289A or in cases with documented TR46/Y121F/T289A infection, liposomal amphotericin B alone, or in combination with an azole, should be considered.
The in vitro results of FIC2 and the BI analysis of this study are comparable to the in vitro results of the combination of voriconazole and anidulafungin that was tested with the same methodology11 and confirmed in a mouse model of invasive aspergillosis.10 However, when we used the FIC1 endpoint the interactions were predominantly indifferent whereas most interactions of voriconazole and anidulafungin remained synergistic at the FIC1 endpoint. However, the difference in mean FICi was only small (0.6 for isavuconazole/anidulafungin versus 0.4 for voriconazole/anidulafungin), which challenges the clinical significance of the observed difference.11 Therefore, we anticipate similar beneficial effects of isavuconazole/anidulafungin when used in vivo compared with voriconazole/anidulafungin.
Similar interactions of isavuconazole/anidulafungin were found in an in vitro study that evaluated the combination of isavuconazole and anidulafungin for five azole-resistant A. fumigatus isolates.24 That study also found that the interaction was predominantly indifferent when complete inhibition was used as the endpoint (e.g. FIC1 in the current study), while 2/5 isolates showed synergistic interaction when incomplete inhibition (e.g. FIC2) was used as the FIC endpoint. This indicates that addition of anidulafungin does not reduce the MIC of isavuconazole, but does add to the inhibitory effect at subMIC levels.
Although the combination of voriconazole with anidulafungin did not result in significantly better efficacy in a randomized control trial, post hoc analysis indicated that the combination may be beneficial for a subgroup population with Aspergillus galactomannan indices between 0.5 and 1.5.34 Thus, in regions with high azole resistance rates the combination may benefit patients with WT disease, azole-resistant invasive aspergillosis and those with mixed azole-susceptible and azole-resistant infection, but may not be beneficial for aspergillosis due to isolates with high isavuconazole resistance such as isolates with TR46/Y121F/T289A. As similar in vitro interactions were found for isavuconazole and anidulafungin compared with the voriconazole and anidulafungin combination, the results of this study indicate that voriconazole can be replaced by isavuconazole when used in combination with an echinocandin.
Supplementary Material
Acknowledgements
Part of the results have previously been presented at the 8th Trends in Medical Mycology meeting, 6–9 October 2017, Belgrade, Serbia. Abstract number: P030.
Funding
This study was supported by internal funding.
Transparency declarations
R.J.M.B. has served as a consultant to Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp. and Pfizer, Inc., and has received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp. and Pfizer, Inc. outside the submitted work. All contracts were through Radboudumc, and all payments were invoiced by Radboudumc. P.E.V. reports grants from Gilead Sciences, Merck Sharp & Dohme Corp., Pfizer, Inc. and F2G, and non-financial support from OLM diagnostics and IMMY, outside the submitted work. All other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Bongomin F, Gago S, Oladele RO. et al. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ullmann AJ, Aguado JM, Arikan-Akdagli S. et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24 Suppl 1: e1–38. [DOI] [PubMed] [Google Scholar]
- 3. Patterson TF, Thompson GR 3rd, Denning DW. et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tissot F, Agrawal S, Pagano L. et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017; 102: 433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buil JB, Hagen F, Chowdhary A. et al. Itraconazole, voriconazole, and posaconazole CLSI MIC distributions for wild-type and azole-resistant Aspergillus fumigatus isolates. J Fungi (Basel) 2018; 4: E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Ingen J, van der Lee HA, Rijs TA. et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother 2015; 70: 178–81. [DOI] [PubMed] [Google Scholar]
- 7. Verweij PE, Ananda-Rajah M, Andes D. et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 2015; 21-22: 30–40. [DOI] [PubMed] [Google Scholar]
- 8. Cuenca-Estrella M, Gomez-Lopez A, Garcia-Effron G. et al. Combined activity in vitro of caspofungin, amphotericin B, and azole agents against itraconazole-resistant clinical isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 2005; 49: 1232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krishnan-Natesan S, Wu W, Chandrasekar PH.. In vitro efficacy of the combination of voriconazole and anidulafungin against voriconazole-resistant cyp51A mutants of Aspergillus fumigatus. Diagn Microbiol Infect Dis 2012; 73: 135–7. [DOI] [PubMed] [Google Scholar]
- 10. Seyedmousavi S, Bruggemann RJ, Melchers WJ. et al. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother 2013; 68: 385–93. [DOI] [PubMed] [Google Scholar]
- 11. Seyedmousavi S, Meletiadis J, Melchers WJ. et al. In vitro interaction of voriconazole and anidulafungin against triazole-resistant Aspergillus fumigatus. Antimicrob Agents Chemother 2013; 57: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siopi M, Siafakas N, Vourli S. et al. Dose optimization of voriconazole/anidulafungin combination against Aspergillus fumigatus using an in vitro pharmacokinetic/pharmacodynamic model and response surface analysis: clinical implications for azole-resistant aspergillosis. J Antimicrob Chemother 2016; 71: 3135–47. [DOI] [PubMed] [Google Scholar]
- 13. Chandrasekar PH, Cutright JL, Manavathu EK.. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin Microbiol Infect 2004; 10: 925–8. [DOI] [PubMed] [Google Scholar]
- 14. Kirkpatrick WR, Perea S, Coco BJ. et al. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 2002; 46: 2564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang M, Su X, Sun WK. et al. Efficacy of the combination of voriconazole and caspofungin in experimental pulmonary aspergillosis by different Aspergillus species. Mycopathologia 2014; 177: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clemons KV, Espiritu M, Parmar R. et al. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 2005; 49: 4867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Planche V, Ducroz S, Alanio A. et al. In vitro combination of anidulafungin and voriconazole against intrinsically azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother 2012; 56: 4500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fakhim H, Vaezi A, Dannaoui E. et al. In vitro combination of voriconazole with micafungin against azole-resistant clinical isolates of Aspergillus fumigatus from different geographical regions. Diagn Microbiol Infect Dis 2018; 91: 266–8. [DOI] [PubMed] [Google Scholar]
- 19. Petraitis V, Petraitiene R, Hope WW. et al. Combination therapy in treatment of experimental pulmonary aspergillosis: in vitro and in vivo correlations of the concentration- and dose-dependent interactions between anidulafungin and voriconazole by Bliss independence drug interaction analysis. Antimicrob Agents Chemother 2009; 53: 2382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viscoli C, Herbrecht R, Akan H. et al. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J Antimicrob Chemother 2009; 64: 1274–81. [DOI] [PubMed] [Google Scholar]
- 21. Herbrecht R, Maertens J, Baila L. et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: a European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant 2010; 45: 1227–33. [DOI] [PubMed] [Google Scholar]
- 22. Maertens JA, Raad II, Marr KA. et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387: 760–9. [DOI] [PubMed] [Google Scholar]
- 23. Katragkou A, McCarthy M, Meletiadis J. et al. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob Agents Chemother 2014; 58: 6934–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raffetin A, Courbin V, Jullien V. et al. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant Aspergillus spp. Antimicrob Agents Chemother 2017; 62: e01382–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez-Tudela JL, Arendrup MC, Arikan S. et al. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 2008; 14: 982–4. [DOI] [PubMed] [Google Scholar]
- 26.International Organization for Standardization. Clinical laboratory testing and in vitro diagnostic test systems – Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices – Part 1: Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases (ISO 20776-1:2006). 2006.
- 27. Antachopoulos C, Meletiadis J, Sein T. et al. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob Agents Chemother 2007; 51: 881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meletiadis J, Mouton JW, Meis JF. et al. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 2001; 39: 3402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mavridou E, Meletiadis J, Rijs A. et al. The strength of synergistic interaction between posaconazole and caspofungin depends on the underlying azole resistance mechanism of Aspergillus fumigatus. Antimicrob Agents Chemother 2015; 59: 1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin-Vicente A, Capilla J, Guarro J.. Synergistic effect of anidulafungin combined with posaconazole in experimental aspergillosis. Med Mycol 2017; 55: 457–60. [DOI] [PubMed] [Google Scholar]
- 31. Bedin Denardi L, Pantella Kunz de Jesus F, Keller JT. et al. Evaluation of the efficacy of a posaconazole and anidulafungin combination in a murine model of pulmonary aspergillosis due to infection with Aspergillus fumigatus. Diagn Microbiol Infect Dis 2018; 90: 40–3. [DOI] [PubMed] [Google Scholar]
- 32. Lepak AJ, Marchillo K, VanHecker J. et al. Impact of in vivo triazole and echinocandin combination therapy for invasive pulmonary aspergillosis: enhanced efficacy against Cyp51 mutant isolates. Antimicrob Agents Chemother 2013; 57: 5438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shalit I, Shadkchan Y, Samra Z. et al. In vitro synergy of caspofungin and itraconazole against Aspergillus spp.: MIC versus minimal effective concentration end points. Antimicrob Agents Chemother 2003; 47: 1416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marr KA, Schlamm HT, Herbrecht R. et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med 2015; 162: 81–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.