ABSTRACT
Background
25-Hydroxycholecalciferol [25(OH)D] is the predominant circulating metabolite of vitamin D and serves as the precursor for 1α,25-dihydroxycholecalciferol [1,25(OH)2D], the hormonally active form. The presence of 1α-hydroxylase (1α-OHase) in the intestine suggests that 1,25(OH)2D can be produced from 25(OH)D, but the effects of oral 25(OH)D on the intestine have not been determined.
Objectives
We investigated the acute intestinal response to orally consumed 25(OH)D in mice by assessing mRNA induction of cytochrome p450 family 24 subfamily A member 1 (Cyp24), a vitamin D–dependent gene. The mechanism of action then was determined through in vitro analyses with Caco2 and HT-29 cells.
Methods
Adult male C57BL6 mice were given a single oral dose of 40, 80, 200, or 400 ng 25(OH)D (n = 4 per dose) or vehicle (n = 3), and then killed 4 h later to evaluate the duodenal expression of Cyp24 mRNA by qPCR and RNA in situ hybridization. The 25(OH)D-mediated response was also evaluated with Caco2 and HT-29 cells by inhibition assay and dose-response analysis. A cytochrome p450 family 27 subfamily B member 1 (CYP27B1) knockdown of HT-29 was created to compare the dose-response parameters with wild-type HT-29 cells.
Results
Oral 25(OH)D induced expression of Cyp24 mRNA in the duodenum of mice with 80 ng 25(OH)D by 3.3 ± 0.8 ΔΔCt compared with controls (P < 0.05). In vitro, both Caco2 and HT-29 cells responded to 25(OH)D treatment with 200-fold and 175-fold greater effective concentration at 50% maximal response than 1,25(OH)2D, yet inhibition of 1α-OHase and knockdown of CYP27B1 had no effect on the responses.
Conclusions
In mice, orally consumed 25(OH)D elicits a vitamin D–mediated response in the duodenum. In vitro assessments suggest that the response from 25(OH)D does not require activation by 1α-OHase and that 25(OH)D within the intestinal lumen acts as a vitamin D receptor agonist.
Keywords: 25-hydroxyvitamin D, CYP27B1, vitamin D, VDR, intestine
Introduction
25-Hydroxycholecalciferol [25(OH)D] is a metabolic product of vitamin D and the precursor to the calcium-regulating hormone, 1α,25-dihydroxycholecalciferol [1,25(OH)2D]. Because of its intrinsic role, 25(OH)D has a natural presence in animal-based food products. Egg yolks, for example, contain 25(OH)D ranging from 9.8 to 25 ng/g yolk (1, 2). In meats, 25(OH)D content varies from undetectable to 2.1 ng/g for chicken breast (2–4), 0.9 ng/g for beef (3–8), and 0.6 ng/g for pork (5, 9, 10). Bovine milk also contains 25(OH)D that is in concentrations typically between 0.1 and 0.2 ng/mL (5, 11). Comparisons made between 25(OH)D and vitamin D3 have found that both have similar concentrations (4, 9, 12, 13). Unlike vitamin D3, 25(OH)D content in foods is not reported in nutrition labels and, to our knowledge, no reference data on 25(OH)D consumption have been compiled. Taylor et al. (14) used the USDA Retail Food Commodity Intakes database to establish an approximation that adults consume 1.7–2.9 μg 25(OH)D/d. This estimated intake is much less than the current 10 μg/d recommendation for vitamin D; thus, 25(OH)D from the diet is thought to have minimal impact on vitamin D status, even when accounting for a greater potency (15, 16), and efforts to understand the effects of dietary 25(OH)D on the intestine have received limited attention.
The enzyme responsible for the conversion of 25(OH)D to 1,25(OH)2D is 1α-hydroxylase (1α-OHase). Although 1α-OHase is primarily located in the kidneys for providing endocrinal regulation of calcium homeostasis, small amounts have been observed in the intestines (17, 18). The presence of 1α-OHase in the intestine would imply that dietary 25(OH)D could be activated to 1,25(OH)2D. The locally produced 1,25(OH)2D then would bind to and stimulate the vitamin D receptor (VDR) to promote gene expression for increased calcium absorption within the intestinal cells. We considered that dietary 25(OH)D may be a substrate for 1α-OHase in the duodenum. Therefore, we hypothesized that orally consumed 25(OH)D would elicit a vitamin D hormonal response in the intestine via enteric 1α-hydroxylation and subsequent activation of the VDR. To test the hypothesis, an evaluation of the duodenal response to 25(OH)D was performed in mice, then the mechanism of action was investigated through in vitro inhibition and knockdown analyses with human Caco2 and HT-29 cells.
Methods
Experimental reagents
Vitamin D, 25(OH)D, and 1,25(OH)2D (>98% pure, Sigma Aldrich) were prepared as previously described (19, 20). Ketoconazole (Tocris Bioscience), a cytochrome p450 inhibitor, was dissolved in DMSO and stored at 4°C for use with in vitro inhibition assays.
Animal experiments
Male C57BL6 mice (Jackson Laboratories), 5–7 wk old, were housed in groups of 3 or 4 in a 12-h light/dark cycle. Mice were fed ad libitum the Teklad 2014 rodent maintenance diet (Envigo) containing 14.3% crude protein, 4.0% fat (ether extract), 4.1% crude fiber, 4.7% ash, 0.7% calcium, and 0.6 IU vitamin D3/g. Care and handling of mice were approved by the Iowa State University Institutional Animal Care and Use Committee. Mice weighing between 20 and 22 g were divided randomly into treatment groups to receive ethanol, 25(OH)D, or 1,25(OH)2D. Ethanol was used as a placebo treatment for control mice (n = 3). Sixteen mice were given a dose of 40, 80, 200, or 400 ng 25(OH)D (n = 4 per dose), and the remaining 12 mice received 2, 4, or 8 ng 1,25(OH)2D (n = 4 per dose). Each mouse was given the designated treatment in a single oral dose carried in 50 μL peanut oil. Four hours after treatments, the mice were killed and two 1-cm intestinal segments of the duodenum that were ∼1 cm distal from the pylorus were collected. The first segment was homogenized in TRIzol Reagent (Invitrogen) for RNA extraction, and the second was fixed for 24 h in 10% neutral formalin solution and embedded in paraffin for RNA in situ hybridization (RISH).
Cell culture experiments
Human colon adenocarcinoma Caco2 and HT-29 cell lines (American Type Culture Collection) were cultured under standard conditions (5% CO2 in room air, 37°C) in DMEM (Gibco) and Eagle's Minimum Essential Media (EMEM) (Corning Cellgro), respectively, and supplemented with 10% FBS and 0.2% penicillin-streptomycin. Caco2 cells were maintained up to passage 16 and HT-29 cells up to passage 20. All in vitro experiments were conducted in 6-well plates seeded with 2 × 106 cells per well of Caco2 or HT-29 cells, and growth media were replaced 24 h later with 3 mL vitamin D treatment media. Vitamin D treatment media contained 0.1% FBS unless otherwise indicated. Experiments were repeated independently 3 times (n = 3) with 1 control in each experiment. At the end of the indicated time, treatment media were removed and then cells were lysed in 500 μL TRIzol for RNA extraction.
Cell transformation
HT-29 cells were transformed by clustered regularly interspaced short palindromic repeats endonuclease-mediated mutation with Cas9 2NLS nuclease (Synthego) and a synthetic guide RNA (Synthego) for human cytochrome p450 family 27 subfamily B member 1 (CYP27B1) (5′-GUGGUACUCUCGGUAGCCUA-3′). Transformations were performed by using Lipofectamine 3000 (Invitrogen) with Cas9 2NLS nuclease and synthetic guide RNA in Opti-Mem medium (Gibco). Mutagenesis was verified by Western blot analysis of the expressed CYP27B1 protein, 1α-OHase.
RNA quantification
Total RNA was extracted from tissue homogenates and from cell lysates for cDNA synthesis followed by qPCR analysis to determine relative expression of cytochrome p450 family 24 subfamily A member 1 (Cyp24). RNA was separated as previously described (21) by using an RNeasy Mini Prep isolation kit (Qiagen). Prep columns were eluted with 50 μL nuclease-free water for tissue homogenates and 30 μL nuclease-free water for cell lysates. cDNA was synthesized from 1 μg RNA by using random hexamer primers and SuperScript III First Strand Synthesis (Invitrogen). cDNA samples were diluted 1:5 for tissue homogenates and 1:3 for cell lysates with Tris-EDTA buffer (10 mM Tris and 1 mM EDTA) and stored at −20°C. Tissue homogenates were diluted again 1:6 with Millipore water for qPCR. Tissue and cell cDNA samples were amplified with Cyp24 or Gapdh primers (Supplemental Table 1) as previously described (21).
RISH
RISH was performed to qualitatively assess expression of Cyp24 in the mouse duodenum. Formalin-fixed and paraffin-embedded tissues were sectioned (5 μm) and then hybridized with Cyp24 oligo probes by using the RNAScope 2.0 HD Red Manual Detection Kit (Advanced Cell Diagnostics). Oligo probes for RNAScope are proprietary to Advanced Cell Diagnostics. Positive (Mus peptidylprolyl isomerase B, expressed in all mouse cells) and negative (Escherichia coli dihydrodipicolinate reductase, not found in mammalian cells) probes were used as procedural controls. After hybridization, sections were counterstained with hematoxylin and 0.02% ammonia for bluing. Tissues were examined qualitatively by light microscopy at 40× magnification.
Protein and Western blot
Effectiveness of the endonuclease-mediated mutagenesis of CYP27B1 in the HT-29 and Caco2 cell lines was determined by Western blotting. Trypsinized cells were resuspended in radioimmunoprecipitation assay buffer with protease inhibitor and quantified by using the Quick Start Bradford protein assay (Bio-Rad). Proteins were separated by gel electrophoresis, then transferred to a polyvinylidene difluoride membrane for Western blotting. Primary antibodies used included mouse anti-CYP27B1 (1α-OHase; mol wt. 56 kD) at 1:500 dilution and mouse monoclonal anti-GAPDH (mol wt. 37 kD) at 1:2000 dilution (Santa Cruz Biotechnologies). The secondary used for both primaries was goat anti-mouse secondary antibody in a dilution of 1:10,000 (Santa Cruz Biotechnologies). Blots were developed by using enhanced chemiluminescence (Pierce) and imaged in a ChemiDoc imaging system (Bio-Rad).
Dose-response mathematical modeling
Dose-response analyses were modeled after the Michaelis–Menten kinetics equation where reaction rate (ν) is represented by relative responses from qPCR (ΔΔCt), maximal velocity (Vmax) is represented by maximal effect (Emax), the Michaelis constant (KM) is represented by the effective concentration at 50% of the maximal response (EC50), and the substrate concentration remains notated as [S].
Michaelis–Menten kinetics equation:
 |
(1) |
Modified Michaelis–Menten dose-response equation:
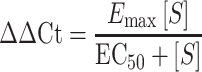 |
(2) |
Statistical analyses
Statistical analyses were performed on GraphPad Prism 5 (GraphPad Software) and SAS version 9.4 (SAS Institute Inc.). Bar graph data depict analyses by t test or ANOVA comparisons with Tukey–Kramer adjustments for multiple comparisons. Analysis of residual plots and the Shapiro–Wilk test were used to determine unequal variance for dose-response data. Unequal data were log transformed to achieve normal distribution and then verified by the same tests. Modified Michaelis–Menten dose-response line graphs were analyzed by nonlinear least-squares regression with subset comparison for best fit lines by the extra sums of squares F test.
Results
25(OH)D elicits a transcriptional response in the duodenum of mice
Four hours after ingestion, duodenal Cyp24 mRNA expression was upregulated compared with controls with 80 ng 25(OH)D (P = 0.016; Figure 1A). All doses of 1,25(OH)2D induced Cyp24 mRNA compared with controls. Qualitatively with RISH, we observed that Cyp24 mRNA induced by 25(OH)D appeared only in the epithelial cells lining the duodenal villi and was similar to the observations with 1,25(OH)2D (Figure 1B). This pattern of response was identical to previously published RISH data by our group (21).
FIGURE 1.
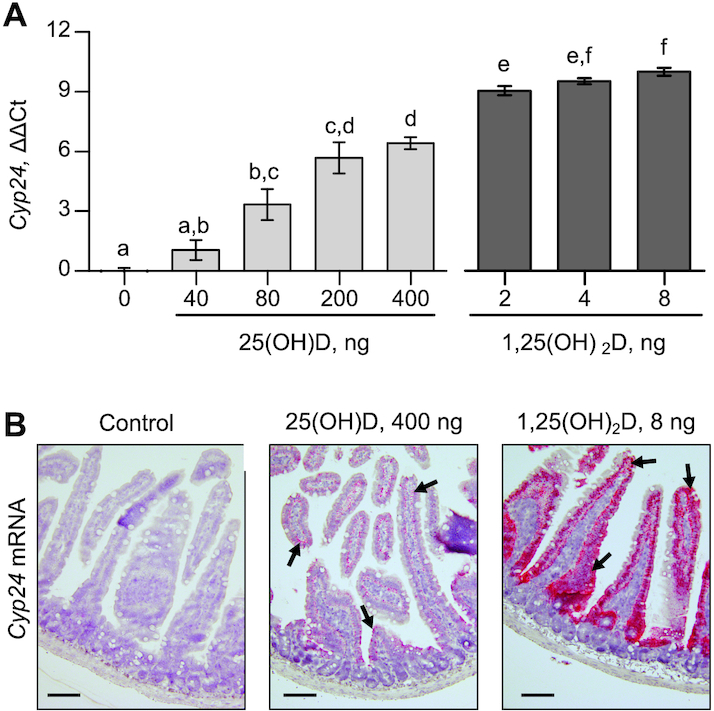
Transcriptional response of Cyp24 to oral 25(OH)D and 1,25(OH)2D in the duodenum of mice. (A) qPCR of Cyp24 mRNA expressed in duodenal tissue. ΔΔCt values are determined as relative to GAPDH and normalized to the mean of controls. Data are mean ± SEM, n = 3 for 0 pmol control and n = 4 for all others. Means without a common letter differ, P < 0.05. (B) RNA in situ hybridization of Cyp24 in control mice, mice given 400 ng 25(OH)D, and mice given 8 ng 1,25(OH)2D at 40× magnification. Hybridized mRNA is stained red as indicated by black arrows. Cyp24, cytochrome p450 family 24 subfamily A member 1; 1,25(OH)2D, 1,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxycholecalciferol.
Effect of 25(OH)D with cultured cells
Under cell culture conditions supplemented with 10% FBS, both Caco2 and HT-29 human colon adenocarcinoma cell lines exhibited the ability to upregulate CYP24 from 100 nM 1,25(OH)2D, but not from equimolar amounts of 25(OH)D or vitamin D (Figure 2). We concluded that these cell conditions were not adequate for evaluating the mechanism of the response that we had observed in mice.
FIGURE 2.
In vitro transcriptional responses of Cyp24 to 100 nM vitamin D (A, D), 25(OH)D (B, E), and 1,25(OH)2D (C, F) with varying enrichments of FBS in treatment media by Caco2 (A–C) and HT-29 cells (D–F). Analysis of CYP24 mRNA is by qPCR. ΔΔCt values are relative to GAPDH and normalized to the control included in each experiment. Data are mean ± SEM, n = 3. Means without a common letter differ, P < 0.05. CYP24, cytochrome p450 family 24 subfamily A member 1; 1,25(OH)2D, 1,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxycholecalciferol.
Removal of FBS from treatment media enables the response to 25(OH)D
We found that using less FBS (1% or 0.1%) in culture media enabled a response by Caco2 and HT-29 to 100 nM 25(OH)D (Figure 2). These data correspond with those from others who observed that 25(OH)D treatment stimulated vitamin D–mediated responses when FBS was removed from media (22–24). No effect of FBS-enrichment was found with the responses to 1,25(OH)2D (Caco2, P = 0.22; HT-29, P = 0.11). Equimolar concentrations of vitamin D did not induce a response in any of the conditions tested (Caco2, P = 0.15; HT-29, P = 0.21). These data suggest that serum proteins impair the response from 25(OH)D in cultured intestinal cells.
Inhibition of 1α-OHase using ketoconazole has no negative effect on the 25(OH)D-mediated response
Inboth Caco2 and HT-29 cell lines, ketoconazole had no effect on the CYP24 expression induced by 1,25(OH)2D (Caco2, P = 0.82; HT-29, P = 0.93; Figure 3). Caco2 cells cotreated with ketoconazole responded to 25(OH)D, as did DMSO cotreated cells (P = 0.4). HT-29 cells cotreated with ketoconazole exhibited a greater response to 25(OH)D than did DMSO controls (P = 0.001). Our explanation for the increased response is that HT-29 cells demonstrated greater expression of CYP24 than Caco2 cells, based on ΔCt values from HT-29 cells that were 2.5 ± 0.4 ΔCt less than those for CYP24 in Caco2 cells (relative to GAPDH, P = 0.0002; data not shown). As a cytochrome p450 enzyme, CYP24 is also a target of ketoconazole; therefore, we suspect that our use of ketoconazole unintentionally inhibited CYP24 from catabolizing the 25(OH)D needed for full potential of the response. No such effect was observed with 1,25(OH)2D, which may be because the 100-nM treatment provides an excess of ligand for VDR activation. These data demonstrate that inhibition of 1α-OHase does not affect the responses and suggest that the protein may not be involved in the response from 25(OH)D.
FIGURE 3.

Inhibition of 1α-hydroxylase by 10 μM ketoconazole or DMSO in Caco2 (A, B) and HT-29 (C, D) cells treated with 100 nM 25(OH)D or 1,25(OH)2D. Experiments were performed in 0.1% FBS media conditions. ΔΔCt values are determined from qPCR of CYP24 mRNA relative to GAPDH and normalized to the control included in each experiment. Data are mean ± SEM, n = 3. Means without a common letter differ, P < 0.05. CYP24, cytochrome p450 family 24 subfamily A member 1; 1,25(OH)2D, 1,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxycholecalciferol.
Knockdown of 1α-OHase and dose-response analyses reveal agonist activation
The lower expression of 1α-OHase in HT-29 CYP27B1em1Jgoff was verified by Western blot analysis (Figure 4). Expression of 1α-OHase was not detectable in Caco2 cells; therefore, a knockdown of these cells was not created. We then performed dose-response analyses for HT-29 CYP27B1+, HT-29 CYP27B1em1Jgoff, and Caco2 cells by measuring the CYP24 expression response to various doses of 25(OH)D and 1,25(OH)2D (Figure 5). In compiling our data, we did not use the relative 100% scale for response effect as is traditionally done for dose-response curves. Instead, we used a modified Michaelis–Menten analysis (described in the Methods) that enables assessment of the response as a measure of VDR activation. This analysis allows interpretation of the Emax and EC50 as kinetic dimensions similar to Vmax and KM, respectively. The calculated Emax and EC50 values are shown in Table 1. Emax values describe the VDR ligand-activated transcriptional response. The Emax values between 1,25(OH)2D and 25(OH)D ligands were consistent for each cell line and confirm that the activity downstream of VDR activation is identical between ligands. No difference in Emax values was observed between wild-type and CYP27B1-knockdown HT-29 cells. Analysis of the EC50 data describes differences in ligand-receptor affinity and reveals characteristics of the activation process. In all cell lines tested, the EC50 was greater for 25(OH)D than for 1,25(OH)2D. We found that knockdown of 1α-OHase did not affect the EC50 for 1,25(OH)2D or for 25(OH)D. Affinity of 25(OH)D compared with 1,25(OH)2D was estimated by relative EC50 data presented in Table 1. These kinetic data demonstrate an independence of the 25(OH)D-mediated response from the expression of 1α-OHase, suggesting that 25(OH)D acts directly as a VDR agonist with lower affinity than 1,25(OH)2D.
FIGURE 4.

Expressed 1α-OHase in wild-type Caco2, wild-type HT-29 CYP27B1+, and knockdown HT-29 CYP27B1em1Jgoff cells. Gel loading is demonstrated by the abundance of GAPDH. CYP27B1, cytochrome p450 family 27 subfamily B member 1; 1α-OHase, 1α-hydroxylase.
FIGURE 5.

Dose-response curves for 25(OH)D (A, C) and 1,25(OH)2D (B, D) from Caco2 cells (A, B) and HT-29 CYP27B1+ and CYP27B1em1Jgoff cells (C, D). Responses are measured by qPCR analysis of CYP24 mRNA (ΔΔCt, relative to GAPDH and normalized to the control per experiment) and plotted by log-scale substrate concentration. Experiments were performed in 0.1% FBS conditions. Symbols represent mean ± SEM, n = 3. Curves depict best-fit line from modified Michaelis–Menten analysis. The x axis vertical lines represent calculated EC50 values and the y axis tabs represent calculated Emax values. CYP24, cytochrome p450 family 24 subfamily A member 1; CYP27B1, cytochrome p450 family 27 subfamily B member 1; EC50, effective concentration at 50% response; Emax, maximal effect; 1,25(OH)2D, 1,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxycholecalciferol.
TABLE 1.
Parameters calculated from modified Michaelis–Menten analysis of dose-responses to 1,25(OH)2D and 25(OH)D in Caco2 CYP27B1+, HT-29 CYP27B1+, and HT-29 CYP27B1em1Jgoff cells1
| Caco2 | HT-29 | ||
|---|---|---|---|
| CYP27B1+ | CYP27B1+ | CYP27B1 em1Jgoff | |
| 1,25(OH)2D | |||
| Emax,2 ΔΔCt | 10.6 ± 0.4 | 9.6 ± 0.2 | 9.0 ± 0.3 |
| EC50, nM | 0.07 ± 0.01 | 0.18 ± 0.01 | 0.23 ± 0.04 |
| 25(OH)D | |||
| Emax,2 ΔΔCt | 10.6 ± 0.5 | 9.8 ± 0.8 | 9.8 ± 0.6 |
| EC50, nM | 16 ± 4* | 28 ± 9* | 40 ± 8* |
| Relative EC50 | |||
| [25(OH)D, nM]/[1,25(OH)2D, nM] | 200 | 175 | 174 |
Experiments were performed in 0.1% FBS conditions. Values are mean ± SEM, n = 3. *Different from 1,25(OH)2D parameter, P < 0.0001, as determined by comparison of best-fit lines. CYP27B1, cytochrome p450 family 27 subfamily B member 1; Emax, maximal effect; EC50, effective concentration at 50% of response; 1,25(OH)2D, 1,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxycholecalciferol.
ΔΔCt values are determined from qPCR relative to GAPDH and normalized to the mean of controls.
Discussion
In the present study, we show that orally consumed 25(OH)D results in an upregulation of the vitamin D hormonal response in the duodenum of mice. This response may hold biological relevance if we consider an extreme, yet plausible example. In our mouse model, we showed that 80 ng 25(OH)D was needed to elicit a significant effect on the VDR-mediated response. Assuming that the mouse ingests 3 g/d, then it would need to eat a food item with 26.7 ng 25(OH)D/g. As mentioned, egg yolks have been shown to contain ≤25 ng 25(OH)D/g (2). If the mouse were to feast on this egg yolk, then the 25(OH)D in egg could feasibly elicit a similar magnitude of hormonal response. However, the mouse would likely consume the egg yolk over a longer period of time and not all at once. Such dietary limitations may offer protective effects from the potential toxicity of 25(OH)D as a hormonal agonist.
Use of a low-FBS in vitro model has important relevance for examining the vitamin D hormonal response in the intestine. We considered that the FBS used in cell culture contains proteins that bind to 25(OH)D such as vitamin D binding protein and albumin. Because orally consumed proteins within the intestinal lumen are denatured by digestion, and because the intestinal epithelium receives nourishment from the lumen rather than from sera, then one can assume that the 25(OH)D in digesta would be free and not bound to protein. Thus, use of FBS in media would not reflect the environment of the intestinal lumen. Because we are focused only on the epithelium of the intestines, we feel that this model appropriately represents actions by vitamin D metabolites on the intestinal tissue. However, it is important to recognize that our experiments used proliferating cells instead of differentiated cells, which may affect the response observed by the cells.
Our hypothesis suggested that 1α-OHase is required for 25(OH)D to induce CYP24 expression in intestinal cells, but inhibition of the 1α-OHase had no effect on the responses. To ensure that the lack of effect by ketoconazole was not a result of failure to inhibit the 1α-OHase, we then knocked-down the expression of 1α-OHase in HT-29 cells. The resulting change in expression had no negative effect on the response to 25(OH)D either. Furthermore, Caco2 cells had less abundance of CYP27B1 than did the HT-29 cells and elicited a remarkably similar response with a slightly lower EC50 concentration. These findings suggest that 25(OH)D is not dependent on CYP27B1 for VDR activation and may act as a VDR agonist. Previous mechanistic studies have also demonstrated the agonistic activity of 25(OH)D. Lou et al. (25) found that primary cells from Cyp27b1 knockout mice are able to respond to 25(OH)D, despite the inability to produce 1,25(OH)2D. Furthermore, they showed that responses in human MCF-7 cells were not impaired by inhibiting the 1α-OHase, and knocking out VDR in the cells did prevent the response from occurring (25). Verone-Boyle et al. (26) used small interfering RNA (siRNA) against CYP27B1 in combination with their CYP27B1 knockout in a human lung cancer cell line, but they could not impair the 25(OH)D-mediated stimulation of VDR and found that the response was independent of 1,25(OH)2D production in the cells. Susa et al. (27) used siRNA against both CYP27B1 and VDR in human prostate cancer cells and also came to the conclusion that 25(OH)D can act as a hormonal agonist. The idea that 25(OH)D will stimulate the vitamin D–mediated response is not new; however, we demonstrate a physiological application of 25(OH)D functioning as an agonistic ligand specifically within the intestine.
Dose-response analyses revealed detailed characterization of the mechanism for the 25(OH)D-mediated response. If we consider our initial hypothesis that 25(OH)D undergoes 1α-hydroxylation before VDR activation, then we would have expected a 2-step reaction for 25(OH)D to elicit the same response as 1,25(OH)2D. This 2-step model would include hydroxylation by 1α-OHase to form 1,25(OH)2D (step 1), followed by substrate binding to VDR (step 2), then yielding activation of the hormonal response. The KM of 1α-OHase (step 1) has been previously determined to be 2.7 μM (28), whereas the Kd of 1,25(OH)2D binding to VDR (step 2) ranges from 0.13 to 1.2 nM (29–31), with 1 exception of 32 nM by Falsone et al. (32). Given these estimates, we note that the KM for 1α-OHase is much greater than the Kd for VDR activation; therefore, we expect the hydroxylation of step 1 to be rate-limiting with values on the order of 1–10 μM. Incredibly, our calculations of EC50 correspond with the previous kinetic experiments. The EC50 values for 1,25(OH)2D were similar to previous estimates of the Kd for VDR. The EC50 values for 25(OH)D, on the other hand, were much less than expected for the action of the 1α-OHase and are within the range of estimates for VDR binding. Binding affinity for 25(OH)D to VDR has not been experimentally determined but has been estimated to be ∼50- to 150-fold less than the affinity for 1,25(OH)2D (25, 32–34). These estimates closely correspond to our calculations of 175- and 200-fold differences in relative affinity for HT-29 and Caco2 cells, respectively. On the basis of data from the present study, our hypothesis for enteric 1,25(OH)2D synthesis from 1-hydroxylation was not substantiated. Instead, we conclude that these data substantiate the claim that 25(OH)D in the intestinal lumen acts as a VDR agonist and activates the transcriptional response.
Estimations of 25(OH)D content in animal products correlate with the animal's serum [25(OH)D] and can be increased by supplementation in the animal's diet (2, 6, 10). We argue that these small amounts of 25(OH)D may have substantial value when considered as a VDR agonist with hormonal activity. If we consider a vitamin D–deficient mammal with increased VDR expression, then its nutritional source of 25(OH)D may have a physiological importance in maintaining health for the human or animal in times of insufficient sunlight when vitamin D3 is not endogenously made. The question that remains is whether the hormonal activity of 25(OH)D will translate to increased calcium absorption. Some investigations have demonstrated such calcemic effects of 25(OH)D in the intestines (6, 35, 36), but the analyses assumed that 25(OH)D is converted to 1,25(OH)2D by the kidneys before stimulating the intestine. In humans, for example, Vaes et al. (37) gave healthy subjects 10 μg 25(OH)D/d for 24 wk and observed decreases in serum parathyroid hormone that may reflect improvements in calcium homeostasis, but the research group did not account for the potential activity of 25(OH)D itself. Interestingly, we also have noted controversies that suggest diets low in animal protein have negative effects on bone calcification (38, 39). Perhaps the effects of diet on bone can be attributed to the 25(OH)D present in the animal-based food source more so than its composition of amino acids. More studies are needed to understand the full impact of 25(OH)D consumed in the diet.
Although we used vitamin D–normal mice, it is possible that a vitamin D–deficient animal may exhibit increased sensitivity to 25(OH)D. This consideration is based on a previous study where the authors observed an inverse relation between the abundance of VDR in the prostate gland and serum 25(OH)D in rats (40). If the abundance of VDR in the intestines also increases during vitamin D deficiency, then we would expect a lower dose of 25(OH)D to initiate transcriptional events. Conversely, it is also possible that VDR can decrease during vitamin D deficiency. Given the high prevalence of vitamin D deficiency and insufficiency among industrialized nations (41), it may be necessary to investigate the response to 25(OH)D during altered vitamin D states to further understand the importance of 25(OH)D in intestinal signaling.
Limitations of our in vitro investigations are important to consider. Although we demonstrated an effective model, our use of human colon adenocarcinoma cells may not precisely reflect the expression and responsiveness of in vivo duodenal cells for human and mouse. In addition, mice may elicit a response to 25(OH)D that differs from humans. As a result, direct translation of our in vitro and in vivo data to human application may vary.
In conclusion, we found that oral consumption of 25(OH)D induces a vitamin D response in the epithelial layer of the duodenum in mice. In addition, our in vitro analyses confirm that 25(OH)D present in the lumen of the intestine acts as a VDR agonist to the intestinal epithelium. Our new perspective that orally administered 25(OH)D acts as a hormone itself adds important nutritive value to the small amounts of 25(OH)D present in animal-based foods that had not previously been considered.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Catherine Martens for invaluable technical assistance throughout this study. The authors’ responsibilities were as follows—CJR and NJK: designed the research; CJR: conducted the research, analyzed the data, performed the statistical analyses, and wrote the manuscript; NJK, RLH, and JPG: provided essential materials (vitamin D compounds); DCB: provided critical scientific guidance and input; CJR and JPG: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
An abstract containing portions of this work was presented on June 9–12, 2018, at the annual meeting of the American Society for Nutrition, Boston, MA.
Supported by NIH grant CA173628-02 (to JPG).
Author disclosures: JPG and RLH are joint owners of GlycoMyr, Inc., a company that synthesizes modified vitamin D compounds. All other authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Cyp24, cytochrome p450 family 24 subfamily A member 1; CYP27B1, cytochrome p450 family 27 subfamily B member 1; EC50, effective concentration at 50% of the maximal response; Emax, maximal effect; RISH, RNA in situ hybridization; siRNA, small interfering RNA; VDR, vitamin D receptor; 1,25(OH)2D, 1α,25-dihydroxycholecalciferol; 1α-OHase, 1α-hydroxylase; 25(OH)D, 25-hydroxycholecalciferol.
References
- 1. Mattila P, Piironen V, Uusi-Rauva E, Koivistoinen P. Determination of 25-hydroxycholecalciferol content in egg yolk by HPLC. J Food Compos Anal. 1993;6(3):250–5. [Google Scholar]
- 2. Mattila PH, Valkonen E, Valaja J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs and chicken meat. J Agric Food Chem. 2011;59(15):8298–303. [DOI] [PubMed] [Google Scholar]
- 3. Bilodeau L, Dufresne G, Deeks J, Clément G, Bertrand J, Turcotte S, Robichaud A, Beraldin F, Fouquet A. Determination of vitamin D3 and 25-hydroxyvitamin D3 in foodstuffs by HPLC UV-DAD and LC–MS/MS. J Food Compos Anal. 2011;24(3):441–8. [Google Scholar]
- 4. Strobel N, Buddhadasa S, Adorno P, Stockham K, Greenfield H. Vitamin D and 25-hydroxyvitamin D determination in meats by LC–IT-MS. Food Chem. 2013;138(2–3):1042–7. [DOI] [PubMed] [Google Scholar]
- 5. Mattila PH, Piironen VI, Koivistoinen PE, Uusi-Rauva EJ. Contents of cholecalciferol, ergocalciferol, and their 25-hydroxylated metabolites in milk products and raw meat and liver as determined by HPLC. J Agric Food Chem. 1995;43(9):2394–9. [Google Scholar]
- 6. Foote MR, Horst RL, Huff-Lonergan EJ, Trenkle AH, Parrish FC, Beitz DC. The use of vitamin D3 and its metabolites to improve beef tenderness. J Anim Sci. 2004;82(1):242–9. [DOI] [PubMed] [Google Scholar]
- 7. Montgomery JL, King MB, Gentry JG, Barham AR, Barham BL, Hilton GG, Blanton JR, Horst RL, Galyean ML, Morrow KJ et al.. Supplemental vitamin D3 concentration and biological type of steers. II. Tenderness, quality, and residues of beef. J Anim Sci. 2004;82(7):2092–104. [DOI] [PubMed] [Google Scholar]
- 8. Wertz AE, Knight TJ, Trenkle A, Sonon R, Horst RL, Huff-Lonergan EJ, Beitz DC. Feeding 25-hydroxyvitamin D to improve beef tenderness. J Anim Sci. 2004;82(5):1410–8. [DOI] [PubMed] [Google Scholar]
- 9. Clausen I, Jakobsen J, Leth T, Ovesen L. Vitamin D3 and 25-hydroxyvitamin D3 in raw and cooked pork cuts. J Food Compos Anal. 2003;16(5):575–85. [Google Scholar]
- 10. Burild A, Lauridsen C, Faqir N, Sommer HM, Jakobsen J. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J Nutr Sci. 2016;5:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reeve LE, Jorgensen NA, Deluca HF. Vitamin D compounds in cows’ milk. J Nutr. 1982;112(4):667–72. [DOI] [PubMed] [Google Scholar]
- 12. Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with?. Ann Nutr Metab. 2003;47(3–4):107–13. [DOI] [PubMed] [Google Scholar]
- 13. Purchas R, Zou M, Pearce P, Jackson F. Concentrations of vitamin D3 and 25-hydroxyvitamin D3 in raw and cooked New Zealand beef and lamb. J Food Compos Anal. 2007;20:90–8. [Google Scholar]
- 14. Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, Yetley EA. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr. 2014;144:654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cashman KD, Seamans KM, Lucey AJ, Sto E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95(3):1350–6. [DOI] [PubMed] [Google Scholar]
- 16. Guo J, Lovegrove JA, Givens DI. 25(OH)D3-enriched or fortified foods are more efficient at tackling inadequate vitamin D status than vitamin D3. Proc Nutr Soc. 2018;77(3):282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–94. [DOI] [PubMed] [Google Scholar]
- 18. Balesaria S, Sangha S, Walters JRF. Human duodenum responses to vitamin D metabolites of TRPV6 and other genes involved in calcium absorption. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goff JP, Koszewski NJ, Haynes JS, Horst RL. Targeted delivery of vitamin D to the colon using β-glucuronides of vitamin D: therapeutic effects in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2012;302:G460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koszewski NJ, Horst RL, Goff JP. Importance of apical membrane delivery of 1,25-dihydroxyvitamin D3 to vitamin D-responsive gene expression in the colon. Am J Physiol Gastrointest Liver Physiol. 2012;303:G870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reynolds CJ, Koszewski NJ, Horst RL, Beitz DC, Goff JP. Localization of the 1,25-dihydroxyvitamin D-mediated response in the intestines of mice. J Steroid Biochem Mol Biol. 2019;186:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136(11):2754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taparia S, Fleet JC, Peng JB, Xiang DW, Wood RJ. 1,25-Dihydroxyvitamin D and 25-hydroxyvitamin D-mediated regulation of TRPV6 (a putative epithelial calcium channel) mRNA expression in Caco-2 cells. Eur J Nutr. 2006;45(4):196–204. [DOI] [PubMed] [Google Scholar]
- 24. Munetsuna E, Kawanami R, Nishikawa M, Ikeda S, Nakabayashi S, Yasuda K, Ohta M, Kamakura M, Ikushiro S, Sakaki T. Anti-proliferative activity of 25-hydroxyvitamin D3 in human prostate cells. Mol Cell Endocrinol. 2014;382(2):960–70. [DOI] [PubMed] [Google Scholar]
- 25. Lou YR, Molnár F, Peräkylä M, Qiao S, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D3 is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol. 2010;118(3):162–70. [DOI] [PubMed] [Google Scholar]
- 26. Verone-Boyle AR, Shoemaker S, Attwood K, Morrison CD, Makowski AJ, Battaglia S, Hershberger PA. Diet-derived 25-hydroxyvitamin D3 activates vitamin D receptor target gene expression and suppresses EGFR mutant non-small cell lung cancer growth in vitro and in vivo. Oncotarget. 2016;7(1):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Susa T, Iizuka M, Okinaga H, Tamamori-Adachi M, Okazaki T. Without 1α-hydroxylation, the gene expression profile of 25(OH)D3 treatment overlaps deeply with that of 1,25(OH)2D3 in prostate cancer cells. Sci Rep. 2018;8(1):9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inouye K, Sakaki T. Enzymatic studies on the key enzymes of vitamin D metabolism; 1α-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24). Biotechnol Annu Rev. 2001;7:179–94. [DOI] [PubMed] [Google Scholar]
- 29. MacDonald PN, Haussler CA, Terpening CM, Galligan MA, Reeder MC, Whitfield GK, Haussler MR. Baculovirus-mediated expression of the human vitamin D receptor. J Biol Chem. 1991;266(28):18808–13. [PubMed] [Google Scholar]
- 30. Nakajima S, Hsieh JC, Macdonald PN, Haussler CA, Galligan MA, Jurutka PW, Haussler MR. Purified human vitamin D receptor overexpressed in Escherichia coli and baculovirus systems does not bind 1,25-dihydroxyvitamin D3 hormone efficiently unless supplemented with a rat liver nuclear extract. Biochem Biophys Res Commun. 1993;197(2):478–85. [DOI] [PubMed] [Google Scholar]
- 31. Juntunen K, Rochel N, Moras D, Vihko P. Large-scale expression and purification of the human vitamin D receptor and its ligand-binding domain for structural studies. Biochem J. 1999;344(2):297–303. [PMC free article] [PubMed] [Google Scholar]
- 32. Falsone SF, Kurkela R, Chiarandini G, Vihko P, Kungl AJ. Ligand affinity, homodimerization, and ligand-induced secondary structural change of the human vitamin D receptor. Biochem Biophys Res Commun. 2001;285(5):1180–5. [DOI] [PubMed] [Google Scholar]
- 33. Link RP, DeLuca HF. On the specificity of vitamin D compounds binding to chick pig intestinal 1,25-dihydroxyvitamin D3 receptor. Steroids. 1988;51(5–6):583–98. [DOI] [PubMed] [Google Scholar]
- 34. Bouillon R, Okamura WA, Norman AW. Structure-function relationship in the vitamin D endocrine system. Endocr Rev. 1995;16(2):200–57. [DOI] [PubMed] [Google Scholar]
- 35. Blunt JW, Tanaka Y, DeLuca HF. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1968;61(4):1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winter M, Morava E, Simon G, Gyüre A. The effect of vitamin D3 and 25-hydroxycholecalciferol on intestinal transport of calcium in vivo and in vitro. Experientia. 1972;28(6):659–60. [DOI] [PubMed] [Google Scholar]
- 37. Vaes AMM, Tieland M, de Regt MF, Wittwer J, van Loon LJC, de Groot LCPGM. Dose–response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37(3):808–14. [DOI] [PubMed] [Google Scholar]
- 38. Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68(4):859–65. [DOI] [PubMed] [Google Scholar]
- 39. Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78(3):584S–92S. [DOI] [PubMed] [Google Scholar]
- 40. Campolina-Silva GH, Maria BT, Mahecha GAB, Oliveira CA. Reduced vitamin D receptor (VDR) expression and plasma vitamin D levels are associated with aging-related prostate lesions. Prostate. 2018;78(7):532–46. [DOI] [PubMed] [Google Scholar]
- 41. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem?. J Steroid Biochem Mol Biol. 2014;144:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



