Abstract
Background
Initial appropriate anti-infective therapy is associated with improved outcomes in patients with severe infections. In critically ill patients, altered pharmacokinetic (PK) behaviour is common and known to influence the achievement of PK/pharmacodynamic targets.
Objectives
To describe population PK and optimized dosing regimens for flucloxacillin in critically ill patients.
Methods
First, we developed a population PK model, estimated between-patient variability (BPV) and identified covariates that could explain BPV through non-linear mixed-effects analysis, using total and unbound concentrations obtained from 35 adult critically ill patients treated with intermittent flucloxacillin. Second, we validated the model using external datasets from two different countries. Finally, frequently prescribed dosing regimens were evaluated using Monte Carlo simulations.
Results
A two-compartment model with non-linear protein binding was developed and validated. BPV of the maximum binding capacity decreased from 42.2% to 30.4% and BPV of unbound clearance decreased from 88.1% to 71.6% upon inclusion of serum albumin concentrations and estimated glomerular filtration rate (eGFR; by CKD-EPI equation), respectively. PTA (target of 100%fT>MIC) was 91% for patients with eGFR of 33 mL/min and 1 g q6h, 87% for patients with eGFR of 96 mL/min and 2 g q4h and 71% for patients with eGFR of 153 mL/min and 2 g q4h.
Conclusions
For patients with high creatinine clearance who are infected with moderately susceptible pathogens, therapeutic drug monitoring is advised since there is a risk of underexposure to flucloxacillin. Due to the non-linear protein binding of flucloxacillin and the high prevalence of hypoalbuminaemia in critically ill patients, dose adjustments should be based on unbound concentrations.
Introduction
Severe infection is recognized as an important determinant of outcome for patients in the ICU.1 Initial appropriate anti-infective therapy, implying both a timely commencement of treatment with a spectrum appropriate for the targeted pathogen and adequate exposure to this antimicrobial agent, is associated with significantly improved clinical outcomes in patients with severe infections.2–4 However, antibiotic selection and dosing is often challenging in critically ill patients because of disease complexity, reduced antibiotic susceptibility of causative pathogens and pathophysiological changes associated with critical illness. These pathophysiological changes, for example altered renal function or hypoalbuminaemia, can influence antibiotic pharmacokinetics (PK) and consequently the achievement of PK/pharmacodynamic (PD) targets.5,6
Flucloxacillin is a β-lactam antibiotic frequently used in the treatment of different infections caused by Gram-positive bacteria, such as penicillinase-producing staphylococci, including MSSA. It is metabolized to a limited extent and the unchanged drug and metabolites are excreted in the urine by glomerular filtration and tubular secretion. Flucloxacillin is approximately 95% bound to serum proteins and has a half-life of about 1 h.7 The clinical outcome of β-lactam antibiotics is related to the time the unbound (or free) drug concentration remains above the MIC of the targeted pathogen, fT>MIC.8 In order to devise rational and individualized dosing regimens of flucloxacillin that ensure sufficient fT>MIC, it is of importance to understand its PK behaviour and to identify relevant covariates influencing this PK. Because of its high protein binding and the relationship between unbound drug and outcome, the unbound PK of flucloxacillin are of special interest. There is, however, limited knowledge on the PK of flucloxacillin, especially in critically ill patients.
In view of the above, we performed a population PK study of total and unbound flucloxacillin in an adult critically ill patient population. Our objectives were to describe population PK and optimized dosing regimens for flucloxacillin in critically ill patients. Furthermore, we sought to externally validate the model as well as quantify the value of various patient-specific covariates explaining altered flucloxacillin PK behaviour.
Patients and methods
Patients and samples
PK data were obtained from 35 patients treated with intermittent flucloxacillin for a (suspected) infection in the 30 bed tertiary referral ICU at the Royal Brisbane and Women’s Hospital in Brisbane, Australia. This study comprised data from two different sources. The first dataset was from a previously published prospective clinical study, performed between May and December 2009.5 This dataset consisted of 10 patients with hypoalbuminaemia (serum albumin concentration ≤32 g/L). After at least 24 h of treatment, timed samples were collected at 30, 45, 60, 90, 120, 150 and 180 min (q4h dosing regimens) or 300 min (q6h dosing regimens) after the 30 min infusion. In all samples, both total and unbound flucloxacillin concentrations were measured. Data for the other 25 patients originated from a prospective observational study that was conducted as part of the β-lactam therapeutic drug monitoring (TDM) programme, between May 2012 and July 2014.9 For 19 of these patients, only unbound flucloxacillin concentrations were available; for the other 6 patients, both total and unbound concentrations were available. For the majority of these patients, both mid and trough levels were obtained in one dosing interval. Samples were obtained after at least four doses, between Days 1 and 5.
Bioanalytical method
The samples obtained for the prospective study were frozen at −80°C and analysed within 8 months after the sample was drawn, in accordance with the results of the long-term stability investigation.10 Samples collected during the TDM programme were centrifuged within 1 h of collection and analysed directly. All samples were analysed using a validated HPLC-UV method.
Detailed information on the bioanalytical methods is available as Supplementary data (Appendices 1–3) at JAC Online.
PK analysis
We developed an integrated PK model for total and unbound flucloxacillin PK using the non-linear mixed-effects modelling package NONMEM. Detailed information on methodological model building and validation is available in the Supplementary data.
External validation
Two external datasets were used for the external validation of the predictive performance of the PK model. The first external dataset, the Brisbane dataset, consisted of 28 unbound flucloxacillin concentrations from 20 critically ill patients admitted to the ICU of the Royal Brisbane and Women’s Hospital. The second external dataset, the Nijmegen dataset, consisted of 34 total and unbound flucloxacillin concentrations from 14 critically ill patients admitted to the ICU of the Radboud University Medical Center in Nijmegen, The Netherlands. Detailed information on the external patient cohorts is available in the Supplementary data.
Total and unbound flucloxacillin plasma concentrations were predicted by fixing the population PK parameters to the final estimates of the previously developed model and setting maximum evaluations (MAXEVAL) to 0. The impact of the identified covariates was validated by means of differences in predictive performance of the population PK model without covariates (the structural model) and with covariates (the final model). To this end, the differences in bias (percentage error) and precision (absolute percentage error), calculated using Equations 1 and 2, between the structural and final model were assessed. A P value of 0.05 was used as a cut-off value for statistical significance. Since the data were not normally distributed, as tested with the Shapiro–Wilk test, the median errors were compared.11
| (1) |
| (2) |
where and represent the ith predicted (PRED, population prediction) and observed concentration, respectively.
Also, visual predictive checks (VPCs) were compared visually and it was evaluated whether there was bias present in the model itself. Models where 0 was included in the IQR of the median percentage error were considered unbiased.
Monte Carlo dosing simulations
Using the final population PK model, total and unbound flucloxacillin concentration–time profiles were predicted based on Monte Carlo simulations following two frequently prescribed dosing regimens: 1 g q6h and 2 g q4h. These dosing regimens were simulated for the first 24 h of treatment of a typical patient with all median characteristics of the population but with an estimated glomerular filtration rate (eGFR) of the 10th (33 mL/min) and 90th percentile (153 mL/min) and with a serum albumin concentration of the 10th (15 g/L) and 90th percentile (30 g/L).
Second, the PTA, being the percentage of patients with an unbound flucloxacillin concentration remaining above a specified MIC during the whole dosing interval (100%fT>MIC),8 was calculated for different dosing regimens. Simulations of four frequently prescribed dosing regimens (1 g q6h, 1 g q4h, 2 g q6h and 2 g q4h) for patients with all median characteristics of the population but with three different eGFR values (33, 96 and 153 mL/min) were performed. Each dosing regimen was simulated 1000 times per eGFR value. Since the MIC distribution of flucloxacillin for Staphylococcus aureus is lacking but suggested to be similar to that of cloxacillin,12 the target MICs were based on the MIC distribution of cloxacillin for S. aureus according to EUCAST. The epidemiological cut-off value (ECOFF) of this pathogen for cloxacillin is 0.5 mg/L, although the WT distribution of this pathogen shows that the majority (55%) of the isolated S. aureus have an MIC of 0.25 mg/L.13 Additionally, the PTA for the ECOFF of oxacillin as a surrogate for flucloxacillin12 for MSSA, 2.0 mg/L,13 was also evaluated.
Statistical analysis
Spearman correlation tests were used to test the correlation between the protein binding (%) of flucloxacillin, calculated as (1 − unbound fraction) × 100, and (i) the total flucloxacillin concentration and (ii) the serum albumin concentration. The Shapiro–Wilk test was used to assess whether the data were normally distributed. Wilcoxon matched-pairs tests were used to assess the differences between the predictive performance of the structural model and the predictive performance of the final model in the external datasets. All abovementioned tests were performed using GraphPad Prism version 8.0.2 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com.
Ethics
Approval for the prospective study, from which 10 patients for the method development dataset originated, was obtained by the ethics committee of the Royal Brisbane and Women’s hospital (protocol HREC/09/QRBW/85) and written informed consent was obtained for each patient prior to entering the study. For the other 25 patients included in the model-building dataset and the 20 patients in the Brisbane external dataset, a waiver for informed consent was granted by the ethics committee of the Royal Brisbane and Women’s Hospital, since blood sampling was performed as part of the local TDM programme. For the Nijmegen external dataset, the study protocol was evaluated by the local ethics committee and the need for written informed consent was waived due to its observational nature.
Results
Patients and samples
Patient characteristics are shown in Table 1. The majority of the patients were treated with flucloxacillin for bloodstream or respiratory infections, at doses ranging from 1 g q6h to 2 g q2h. For all patients, flucloxacillin dose was at the discretion of the treating intensivist. In total, 79 total and 104 unbound flucloxacillin plasma concentrations were collected, obtained at a median of 30 h after the start of flucloxacillin treatment (range: 0.5–168 h) and a median of 2 h (range: 0.5–6 h) after the most recent administration. Measured total concentrations ranged from 1.0 to 202 mg/L and measured unbound concentrations ranged from 0.1 to 30 mg/L.
Table 1.
Patient characteristics
| Characteristic | Model-building patient cohort (n = 35) | External patient cohorts |
|
|---|---|---|---|
| Brisbane (n = 20) | Nijmegen (n = 14) | ||
| Female, n (%) | 12 (34) | 7 (35) | 3 (21) |
| Age (years) | 52 (43–67) | 55 (41–62) | 61 (51–71) |
| Total body weight (kg) | 95 (73–120) | 80 (62–115) | 83 (74–96) |
| BMI (kg/m2) | 31 (25–35) | 26 (20–34) | 27 (24–29) |
| SOFA score | 8 (5–13) | 6 (3–10) | 9 (5–11) |
| eGFR (mL/min) | 96 (26–166) | 52 (11–159) | 51 (22–177) |
| RRT, n (%) | 4 (11) | 3 (15) | 3 (21) |
| Albumin (g/L) | 21 (15–34) | 21 (15–33) | 15 (10–26) |
Values are expressed as median (IQR), unless stated otherwise. RRT, renal replacement therapy.
Protein binding
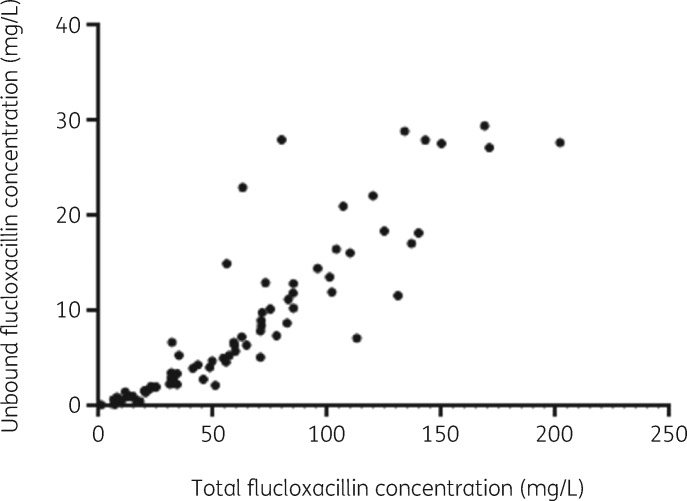
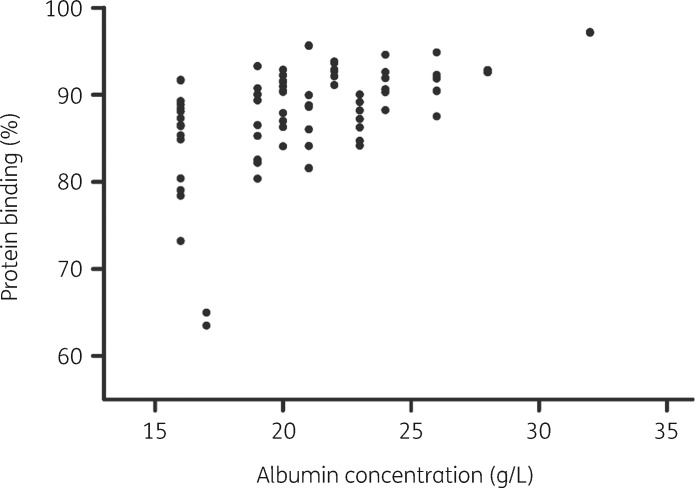
The observed protein binding of flucloxacillin (%), calculated per analysed patient sample as (1 − unbound fraction) × 100, ranged from 63.4% to 97.2%, with a median of 89.4%. The relationship between unbound and total flucloxacillin concentrations is depicted in Figure 1; it shows non-linear, saturable protein binding that is concentration dependent. This was confirmed by the association between the protein binding of flucloxacillin and total flucloxacillin concentrations, which was not constant (Spearman correlation r = −0.63, P = <0.0001). In line with this finding, the protein binding of flucloxacillin was positively associated with serum albumin concentrations (Figure 2, Spearman correlation r = 0.52, P = <0.0001). Analysed serum albumin concentrations ranged from <15 mg/L to 34 g/L, with a median of 21 g/L.
Figure 1.
Unbound versus total flucloxacillin concentrations, measured in 79 samples from 16 patients.
Figure 2.
Protein binding of flucloxacillin (%), calculated as (1 − unbound fraction) × 100, versus serum albumin concentrations, based on 79 patient samples.
PK analysis
A two-compartmental model with first-order elimination provided the best fit for logarithmically transformed data. In line with the observations in Figure 1, non-linear protein binding resulted in a better fit than linear protein binding and was described by the following equation:14
| (3) |
In Equation 3, Ctotal is the total flucloxacillin concentration, Cunbound is the unbound flucloxacillin concentration and (Bmax × Cunbound)/(Kd + Cunbound) represents the bound flucloxacillin concentration, where Bmax is the maximum binding capacity and Kd is the equilibrium dissociation constant.
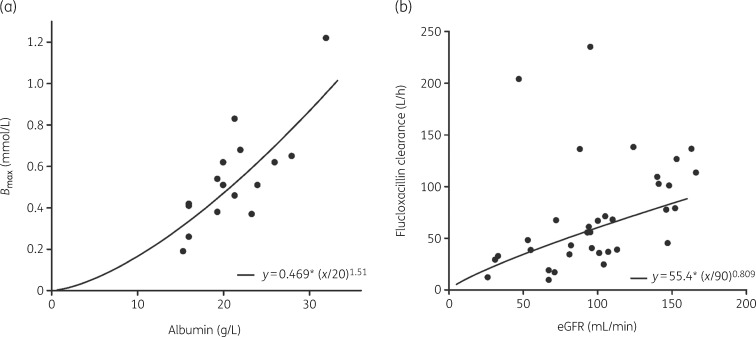
The multivariate covariate analysis revealed that there was a statistically significant association between serum albumin concentration and Bmax and between eGFR and CL, as described by Equations 4 and 5.
| (4) |
| (5) |
The associations between the covariates and the PK parameters are depicted in Figure 3. With these associations in the model, the between-patient variability (BPV) of Bmax decreased from 42.2% to 30.4% and the BPV of CL decreased from 88.1% to 71.6%.
Figure 3.
Covariate relationship between (a) serum albumin concentrations and Bmax and (b) eGFR and CL of unbound flucloxacillin for the final model, for all patients for whom both total and unbound concentrations were measured (n = 16). The dots represent the individual estimates of (a) Bmax and (b) unbound flucloxacillin CL. The line represents the model-predicted association between the parameter estimate and the covariate of interest.
Parameter estimates of the different model-building steps are shown in Table 2. Detailed information on the results of methodological model building and validation, including the VPC (Figure S1), is available in the Supplementary data (Appendix 2).
Table 2.
Parameter estimates of the different model-building steps
| Parameters | Structural model |
Final model |
Bootstrap (n = 1000) of model with covariates |
|||
|---|---|---|---|---|---|---|
| estimate | RSE (%) | estimate | RSE (%) | estimate | 95% CI | |
| B max (mmol/L) | 0.46 | 14.5 | 0.469 | 14.1 | 0.478 | 0.316–0.622 |
| Kd (mmol/L) | 0.0397 | 15.3 | 0.0441 | 16.6 | 0.0450 | 0.0260–0.0621 |
| CL (L/h) | 54.6 | 13.6 | 55.4 | 11.4 | 55.2 | 42.8–68.1 |
| V 1 (L) | 51.5 | 11.5 | 52.7 | 12.0 | 53.3 | 36.9–68.6 |
| V 2 (L) | 55.9 | 11.9 | 56.8 | 11.8 | 57.3 | 41.6–72.0 |
| Q (L/h) | 66.4 | 25.8 | 67.2 | 26.0 | 65.6 | 32.6–102 |
| BPV | ||||||
| Bmax (%CV) | 42.2 | 23.3 | 30.4 | 19.2 | 28.5 | 14.4–41.1 |
| CL (%CV) | 88.1 | 11.7 | 71.6 | 15.8 | 68.9 | 43.7–96.1 |
| Residual variability | ||||||
| proportional error, unbound flucloxacillin | 0.222 | 11.6 | 0.222 | 11.2 | 0.212 | 0.169–0.275 |
| proportional error, total flucloxacillin | 0.161 | 11.5 | 0.160 | 11.6 | 0.150 | 0.112–0.203 |
| Covariates | ||||||
| albumin | — | — | 1.51 | 28.6 | 1.52 | 0.521–2.50 |
| eGFR | — | — | 0.809 | 24.2 | 0.809 | 0.365–1.02 |
CV, coefficient of variation; RSE, relative standard error.
External validation
Table 1 shows the characteristics of the patients in the different datasets. Table 3 shows that, for both external datasets, the predictive performance of the final model was statistically significantly better than the predictive performance of the structural model, with lower median percentage errors and lower median absolute percentage errors. For both external datasets, the structural as well as the final model proved to be unbiased for predicting the unbound and total flucloxacillin concentrations. The VPCs (Figures S2 and S3) show that the final model, with covariates, better predicted the observed concentration–time data of both external datasets than the model without covariates.
Table 3.
Predictive performance of the structural and final model in two external datasets
| Brisbane external dataset (n = 20) |
Nijmegen external dataset (n = 14) |
|||||
|---|---|---|---|---|---|---|
| Characteristics | structural model | final model | P | structural model | final model | P |
| Total flucloxacillin | ||||||
| error (%) | NA | NA | 18.1 (−54.8 to 66.4) | −11.0 (−57.1 to 28.3) | 0.0005 | |
| absolute error (%) | NA | NA | 55.3 (28.5–77.6) | 39.6 (19.2–59.6) | 0.004 | |
| Unbound flucloxacillin | ||||||
| error (%) | −59.4 (−83.4 to 14.3) | −5.80 (−36.9 to 29.7) | 0.01 | −49.1 (−86.1 to 9.80) | −27.8 (−64.5 to 21.2) | 0.04 |
| absolute error (%) | 70.3 (40.4–92.0) | 33.4 (10.9–58.4) | 0.0005 | 59.2 (32.3–88.9) | 51.7 (25.2–74.9) | 0.01 |
Values are expressed as median (IQR). A one-sided Wilcoxon matched-pairs test was used to test differences between the performance of the structural model and the final model. NA, not available.
Monte Carlo dosing simulations
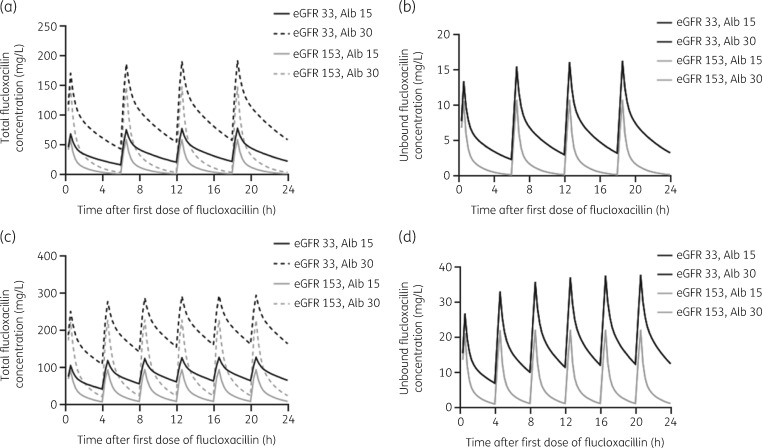
Patients with higher eGFRs and lower serum albumin concentrations had lower total flucloxacillin concentrations (Figure 4a and c). For unbound flucloxacillin, simulations indicated that higher eGFRs resulted in lower unbound concentrations. Serum albumin concentrations did not affect unbound concentrations (Figure 4b and d).
Figure 4.
Illustration of the effect of the covariates eGFR (mL/min) and serum albumin concentration (g/L) on the concentration–time curve of flucloxacillin as assessed by Monte Carlo simulations of the first 24 h of treatment of a virtual critically ill patient, with all median characteristics of the population, but with two different eGFR values and two different serum albumin concentrations. Both total and unbound flucloxacillin concentrations were simulated for two different IV dosing regimens: (a) total flucloxacillin concentrations after 1 g q6h; (b) unbound flucloxacillin concentrations after 1 g q6h; (c) total flucloxacillin concentrations after 2 g q4h; and (d) unbound flucloxacillin concentrations after 2 g q4h. Alb, serum albumin concentration.
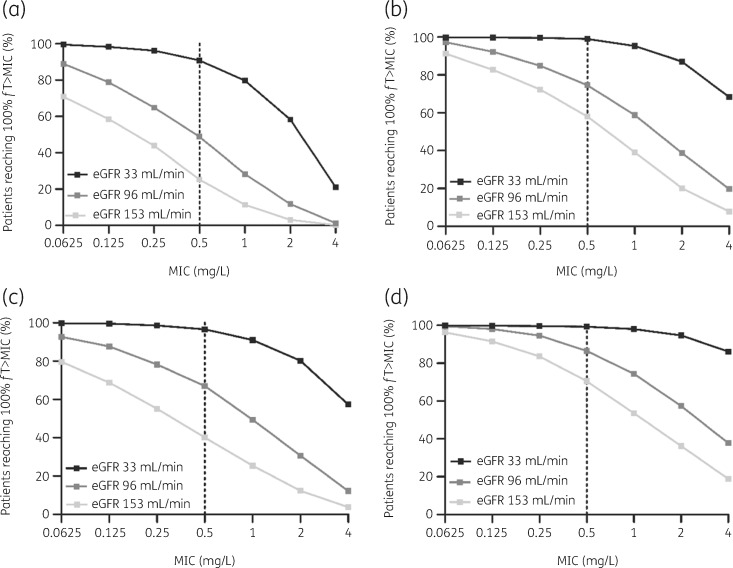
Figure 5 shows the difference in PTA for the frequently prescribed dosing regimens 1 g q6h, 1 g q4h, 2 g q6h and 2 g q4h, for different simulated eGFRs and different MICs of the targeted pathogen. When targeting the ECOFF of cloxacillin for S. aureus, 0.5 mg/L, a dosing regimen of 1 g q6h resulted in a PTA of 91% for patients with an eGFR of 33 mL/min. A dosing regimen of 2 g q4h resulted in a PTA of 87% for patients with an eGFR of 96 mL/min and a PTA of 71% for patients with an eGFR of 153 mL/min.
Figure 5.
Monte Carlo simulations (n = 1000) and PTA for achieving 100%fT>MIC at t = 24 h for various eGFRs calculated by the CKD-EPI equation, for four different IV flucloxacillin dosing regimens administered to critically ill patients: (a) 1 g q6h; (b) 1 g q4h; (c) 2 g q6h; and (d) 2 g q4h. The ECOFF of cloxacillin (as a surrogate for flucloxacillin) for S. aureus is 0.5 mg/L, according to EUCAST.14
The PTA for an MIC of 0.25 mg/L, the MIC of the majority of targeted pathogens,13 was >90% for patients with an eGFR of ≤96 mL/min treated with a dosing regimen of 2 g q4h. For patients with a higher eGFR, a PTA of >90% was only reached for pathogens with an MIC of ≤0.125 mg/L and a dosing regimen of 2 g q4h. The PTA for an MIC of 2.0 mg/L, the ECOFF of oxacillin for MSSA, and a dosing regimen of 2 g q4h was 95% for patients with an eGFR of 33 mL/min, 57% for patients with an eGFR of 96 mL/min and 36% for patients with an eGFR of 153 mL/min.
Discussion
This is, to the best of our knowledge, the first study where both the PK and the covariates affecting the PK of total and unbound flucloxacillin in critically ill patients are described with the aim of optimizing flucloxacillin dosing regimens. In a previous study performed at the Royal Brisbane and Women’s Hospital,5 in which 10 of the patients from this current study were included, patients with hypoalbuminaemia (serum albumin concentration ≤32 g/L) and without severe renal dysfunction (serum creatinine <170 μmol/L) were recruited to develop a PK model. Only unbound flucloxacillin concentrations were used for that PK model and thus no protein-binding model was applied. As expected, our PK parameter estimates for central volume of distribution (V1), peripheral volume of distribution (V2) and intercompartmental clearance (Q) were similar to those reported in the previously performed study. The PK parameter estimate for CL was lower in the current study, which can be explained by the fact that patients with severe renal dysfunction were excluded from the previously published study, but not from the current study. In a recently published report describing the total and unbound PK of flucloxacillin in non-critically ill patients, a two-compartment model with non-linear protein binding was also found to best describe the data, with a parameter estimate for Bmax in the same range.15
We showed that a lower eGFR was related to a lower flucloxacillin CL; BPV of flucloxacillin CL decreased from 88.1% to 71.6% upon inclusion of eGFR. The large remaining BPV can be explained by: (i) flucloxacillin is not only eliminated by glomerular filtration, but also via tubular secretion and non-renal mechanisms, where non-renal mechanisms account for approximately 30% of total CL;7,16,17 and (ii) eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is not validated in critically ill patients and it is known that serum creatinine has demerits in this population, considering the rapid (patho)physiological changes in these patients.18 However, our results show that eGFR was related to unbound flucloxacillin CL, and thus flucloxacillin exposure and PTA, and this is an easily accessible parameter. Furthermore, augmented CLCR (>130 mL/min/1.73 m2) is present in approximately 50% of patients admitted to the ICU.19 Therefore, we believe this parameter is of value when optimizing dosing regimens for the individual critically ill patient.
We demonstrated that, due to non-linear protein binding across the observed concentration range, total flucloxacillin concentrations are not representative of unbound concentrations. This is in line with the finding of a previous study, where significant differences between predicted (from total concentrations) and measured unbound concentrations for flucloxacillin were reported.20 Most assays used in TDM programmes and PK studies of critically ill patients measure total β-lactam antibiotic concentrations.21,22 In order to individualize dosing regimens, published plasma protein-binding percentages derived from studies performed in non-critically ill patients are used to calculate unbound concentrations from the measured total concentrations.20,23 Our results show that these assays may not be suitable in clinical practice, where the target is defined as the unbound concentration, i.e. fT>MIC. An explanation for the non-linear association between unbound and total concentrations is that when the concentration of flucloxacillin in plasma increases, binding sites on proteins are increasingly saturated, resulting in a higher fraction of unbound drug in plasma, i.e. saturable protein binding.24 In our data, differences in the unbound fraction of flucloxacillin could be partly explained by differences in serum albumin concentrations; serum albumin concentrations were associated with Bmax. A lower serum albumin concentration was related to lower protein binding (and a higher unbound fraction) and resulted in a lower exposure of total flucloxacillin, but not unbound flucloxacillin. This finding is similar to what was observed in non-critically ill patients.15 Consequently, patients with low serum albumin concentrations are most likely to have lower flucloxacillin protein binding than the values observed in the literature. This is of special importance in the ICU, where 40%–50% of the patients have low serum albumin concentrations (<25 g/L).25 In clinical practice, this means that when only total flucloxacillin concentrations are measured and converted into unbound concentrations using protein-binding values observed in the literature, i.e. 95%,7 in order to evaluate the attainment of the PK/PD target of 100%fT>MIC, there is a risk of underestimating unbound concentrations. As a consequence, unnecessarily high flucloxacillin doses may be selected when dosing regimens are based on measured total concentrations.
We validated the impact of eGFR and serum albumin concentrations on the flucloxacillin PK in two external datasets from two different countries. Since there is no defined therapeutic window for flucloxacillin and TDM is not routinely applied, we chose to compare the population-predicted concentrations (PRED) from the model with and without the covariates. There are no standardized requirements for bias and precision when applying a PK model to an external dataset, therefore we used a statistically significant endpoint to evaluate the predictive performance. For both datasets, we found a significant improvement in predictive performance when the covariates were added to the model. This confirms that the identified covariates, serum albumin concentration and eGFR, are of relevance for flucloxacillin exposure in critically ill patients.
Our results show that large differences in PTA are encountered for different eGFRs and target MICs, indicating that it is of importance to combine information on the targeted pathogen (MIC) with information on the patient (e.g. renal function) to devise a rational dosing regimen. In general, when aiming for an effectiveness target of 100%fT>MIC,8 a dosing regimen of 2 g q4h is adequate for patients with an eGFR of <96 mL/min and where no MIC of the targeted pathogen is available. For patients with an eGFR of ≤33 mL/min, a dosing regimen of 1 g q6h should be adequate. For patients with an eGFR of ≥96 mL/min and who are infected with moderately susceptible pathogens, TDM using unbound flucloxacillin concentrations is advised to devise an optimal dosing regimen. For patients infected with MSSA, currently used intermittent dosing regimens are unlikely to result in an acceptable PTA, particularly in patients without renal impairment. For these patients, measurement of the pathogen MIC and TDM using unbound concentrations is advised.
Our study has several limitations. First, the limited number of patients and total and unbound flucloxacillin concentrations that were used for model building has likely resulted in a limited power to identify covariates that have a significant impact. This might, for example, explain why no statistically significant relationship was found between renal replacement therapy (RRT) (n = 4 patients) and flucloxacillin. However, we believe that with this dataset we were able to identify the most clinically relevant covariates, which was also confirmed in two external patient cohorts. Also, although our dataset consisted of a limited number of patients, it is the largest ICU patient dataset used for population PK modelling of flucloxacillin to date. Second, our PK model is solely based on patients receiving intermittent, and not continuous, infusion of flucloxacillin. This is due to the nature of this retrospective cohort in a centre where intermittent infusion is current standard practice in the ICU. Third, all patients in the model development and both external datasets had a serum albumin concentration below 35 g/L. Therefore, extrapolation of this model to patients with higher serum albumin concentrations should be performed with caution.
Nevertheless, our data represent an important step forward, as this study is the first to develop and validate a PK model incorporating both total and unbound flucloxacillin. This resulted in several relevant findings that are easily applicable to the optimization of flucloxacillin dosing regimens in daily clinical practice.
Conclusions
We showed that both eGFR and serum albumin concentration have a significant impact on flucloxacillin exposure and PK/PD target attainment and should be taken into account when devising a rational dosing regimen for critically ill patients. For patients with high CLCR and infected with moderately susceptible pathogens, TDM is advised, as a risk of underexposure exists. Dose adjustments should be based on unbound concentrations, due to the non-linear protein binding of flucloxacillin and the high prevalence of hypoalbuminaemia in this patient population.
Funding
This study was supported by internal funding.
Transparency declarations
R.M.vH. has provided consultancies for Nordic Pharma. R.J.M.B. has served as a consultants to Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp., and Pfizer, Inc., and has received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp., and Pfizer, Inc. All contracts were through Radboudumc, and all payments were invoiced by Radboudumc. J.L. has received honoraria from Pfizer and MSD. J.A.R. wishes to acknowledge funding from the Australian National Health and Medical Research Council for Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065). J.A.R. has provided lectures/consultancies or had grant funding provided to his institution from MSD, Astellas, BioMérieux, Accelerate Diagnostics and Cardeas Pharma. All other authors: none to declare.
Supplementary data
Figures S1 to S3 and Appendices 1 to 3 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1. Vincent J, Rello J, Marshall J. et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–9. [DOI] [PubMed] [Google Scholar]
- 2. Kollef MH, Sherman G, Ward S. et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999; 115: 462–74. [DOI] [PubMed] [Google Scholar]
- 3. MacArthur RD, Miller M, Albertson T. et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 2004; 38: 284–8. [DOI] [PubMed] [Google Scholar]
- 4. Kumar A, Roberts D, Wood K. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–96. [DOI] [PubMed] [Google Scholar]
- 5. Ulldemolins M, Roberts JA, Wallis SC. et al. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J Antimicrob Chemother 2010; 65: 1771–8. [DOI] [PubMed] [Google Scholar]
- 6. Roberts JA, Abdul-Aziz MH, Lipman J. et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sweetman SC, Flucloxacillin In: Martindale: The Complete Drug Reference. 36th edn Pharmaceutical Press, 2009; 277. [Google Scholar]
- 8. Jager NGL, van Hest RM, Lipman J. et al. Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 2016; 9: 961–79. [DOI] [PubMed] [Google Scholar]
- 9. Wong G, Briscoe S, McWhinney B. et al. Therapeutic drug monitoring of β-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother 2018; 73: 3087–94. [DOI] [PubMed] [Google Scholar]
- 10. McWhinney BC, Wallis SC, Hillister T. et al. Analysis of 12 β-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2039–43. [DOI] [PubMed] [Google Scholar]
- 11. Sheiner LB, Beal SL.. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 1981; 9: 503–12. [DOI] [PubMed] [Google Scholar]
- 12. Sutherland R, Croydon EA. et al. Flucloxacillin, a new isoxazolyl penicillin, compared with oxacillin, cloxacillin, and dicloxacillin. Br Med J 1970; 4: 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EUCAST. Antimicrobial wild type distributions of microorganisms, cloxacillin/Staphylococcus aureus 2019. https://mic.eucast.org/Eucast2/SearchController/search.jsp?action=performSearch&BeginIndex=0&Micdif=mic&NumberIndex=50&Antib=-1&Specium=14
- 14. Toutain PL, Bousquet-Melou A.. Free drug fraction vs. free drug concentration: a matter of frequent confusion. J Vet Pharmacol Ther 2002; 25: 460–3. [DOI] [PubMed] [Google Scholar]
- 15. Wilkes S, van Berlo I, Ten Oever J. et al. Population pharmacokinetic modelling of total and unbound flucloxacillin in non-critically ill patients to devise a rational continuous dosing regimen. Int J Antimicrob Agents 2019; 53: 310–7. [DOI] [PubMed] [Google Scholar]
- 16. Landersdorfer CB, Kirkpatrick CMJ, Kinzig-Schippers M. et al. Population pharmacokinetics at two dose levels and pharmacodynamic profiling of flucloxacillin. Antimicrob Agents Chemother 2007; 51: 3290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thijssen HH, Wolters J.. The metabolic disposition of flucloxacillin in patients with impaired kidney function. Eur J Clin Pharmacol 1982; 22: 429–34. [DOI] [PubMed] [Google Scholar]
- 18. Sunder S, Jayaraman R, Mahapatra HS. et al. Estimation of renal function in the intensive care unit: the covert concepts brought to light. J Intensive Care 2014; 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Claus BOM, Hoste EA, Colpaert K. et al. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care 2013; 28: 695–700. [DOI] [PubMed] [Google Scholar]
- 20. Wong G, Briscoe S, Adnan S. et al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 2013; 57: 6165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pinder N, Brenner T, Swoboda S. et al. Therapeutic drug monitoring of β-lactam antibiotics – influence of sample stability on the analysis of piperacillin, meropenem, ceftazidime and flucloxacillin by HPLC-UV. J Pharm Biomed Anal 2017; 143: 86–93. [DOI] [PubMed] [Google Scholar]
- 22. El-Najjar N, Hösl J, Holzmann T. et al. UPLC–MS/MS method for therapeutic drug monitoring of 10 antibiotics used in intensive care units. Drug Test Anal 2018; 10: 584–91. [DOI] [PubMed] [Google Scholar]
- 23. Carlier M, Stove V, Wallis SC. et al. Assays for therapeutic drug monitoring of β-lactam antibiotics: a structured review. Int J Antimicrob Agents 2015; 46: 367–75. [DOI] [PubMed] [Google Scholar]
- 24. Zeitlinger MA, Derendorf H, Mouton JW. et al. Protein binding: do we ever learn? Antimicrob Agents Chemother 2011; 55: 3067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finfer S, Bellomo R, McEvoy S. et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ 2006; 333: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.