ABSTRACT
Background
The menopause has adverse effects on cardiometabolic profiles that are linked to an increased risk of atherosclerosis in women. A healthy diet during the menopausal transition may counteract the menopause-induced atherosclerotic risk.
Objective
This prospective cohort study aimed to examine the associations between empirically derived dietary patterns and subclinical carotid atherosclerosis in midlife women.
Methods
A total of 1246 midlife women (average age at baseline: 46.3 y) from the Study of Women's Health Across the Nation who completed dietary assessments and had a carotid ultrasound scan were included. Dietary data were collected at 3 time points, during 1996–1997, 2001–2003, and 2005–2007. Measures of carotid atherosclerosis included common carotid artery intima-media thickness (CCA-IMT), adventitial diameter (AD), and carotid plaque index collected during 2009–2013. Three statistical methods, including principal component analysis (PCA), reduced rank regression (RRR), and partial least squares regression (PLS), were used to identify dietary patterns.
Results
A Western dietary pattern was identified from each method and a Prudent dietary pattern from PCA. High adherence to the Western pattern was associated with higher CCA-IMT. Women in the fourth quartile of the Western pattern identified by PCA, RRR, and PLS had 0.042 mm (95% CI: 0.011, 0.073), 0.033 mm (95% CI: 0.0086, 0.057), and 0.049 mm (95% CI: 0.025, 0.074), respectively, larger CCA-IMT than women in the first quartile; these differences correspond to 30%, 24%, and 35% of the sample SD, respectively. The Prudent pattern was not significantly associated with CCA-IMT. No significant associations were found between the identified dietary patterns and AD or carotid plaque.
Conclusions
The positive association between the Western diet and CCA-IMT was robust under different dietary pattern derivation methods. The adoption of a diet low in red meat, processed meat, deep-fried products, and sugar-sweetened beverages among midlife women is associated with a lower future risk of atherosclerosis.
Keywords: cardiovascular disease, atherosclerosis, intima-media thickness, diet, dietary patterns, menopausal transition, midlife women, principal component analysis, reduced rank regression, partial least squares regression
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity in the USA (1). Over and above the effects of chronological aging, the menopause in women has been linked with unfavorable changes in cardiometabolic profiles, including increases in visceral fat (2) and elevations in total cholesterol, LDL cholesterol, and apolipoprotein B (3–6). Due to these adverse changes in CVD risk factors, the menopause is associated with an elevated risk of clinical CVD (7) and an accelerated progression of atherosclerosis (8, 9) in women.
Diet is a major modifiable risk factor for CVD. The traditional approach of nutritional epidemiology focuses on the potential impacts of individual foods or nutrients (10, 11). Dietary pattern analysis is a complementary approach that allows examination of the overall diet and is less subject to several methodological limitations common in the traditional “single food/nutrient” approach (e.g., small effects of individual foods, complicated interaction among nutrients, and mutual confounding between dietary exposures). Results from dietary pattern analyses can also be more directly translated into dietary guidelines (10, 11).
The atherosclerotic risk induced by the menopause may be counteracted by the adoption of a healthy diet during the menopausal transition (8, 12, 13). However, the association between dietary patterns during midlife and atherosclerosis later in life among women is inconclusive (14–22), which is partially due to certain methodological issues. Most previous studies have used dietary data collected at a single time point to derive dietary patterns. As an individual's dietary intake may change over time, only 1 dietary measurement may not accurately capture the long-term dietary habits (23, 24). Furthermore, multiple variable reduction techniques are available for dietary pattern identification (25, 26), which can potentially lead to inconsistent results. The principal component analysis (PCA) is the most commonly used method for empirical dietary pattern analysis (26). Reduced rank regression (RRR) and partial least squares regression (PLS) are alternative techniques and allow the incorporation of a priori hypotheses of potential pathophysiological pathways in dietary pattern identification (25–27). However, few studies have compared the performances of different variable reduction techniques (27–29).
Midlife is a crucial period for CVD risk prevention in women, and midlife women may benefit considerably from targeted dietary interventions. Therefore, the associations of dietary patterns with subclinical atherosclerosis in this understudied group warrant further investigations. We aimed to: 1) use repeatedly collected dietary data from the Study of Women's Health Across the Nation (SWAN) to evaluate the prospective associations between dietary patterns during midlife and measures of subclinical carotid atherosclerosis later in life, and 2) compare the performances of 3 variable reduction techniques in the estimation of diet–disease associations.
Methods
Study design and study population
The SWAN is a multi-center, multi-ethnic, prospective cohort study initiated in 1996 to study the natural history of menopause (30). The participants were recruited from 7 sites, including Boston, Chicago, Southeastern Michigan, Los Angeles, Newark (NJ), Pittsburgh, and Oakland (CA). Participants were women who self-identified as African American (Pittsburgh, Chicago, Detroit, and Boston), Chinese (Oakland), Japanese (Los Angeles), Hispanic (Newark), or non-Hispanic white (all sites). Baseline eligibility criteria included age 42–52 y, having an intact uterus and ≥1 ovary, not pregnant or lactating, not using oral contraceptives or hormone therapy in the past 3 mo, and having ≥1 menstrual cycle in the past 3 mo. At baseline, 3302 women were enrolled. Clinic assessments began in 1996, and participants have been followed up for 16 examinations conducted approximately annually, most recently in 2016–2018. The SWAN protocols were approved by the Institutional Review Board at each site. All participants provided written informed consent at each study visit.
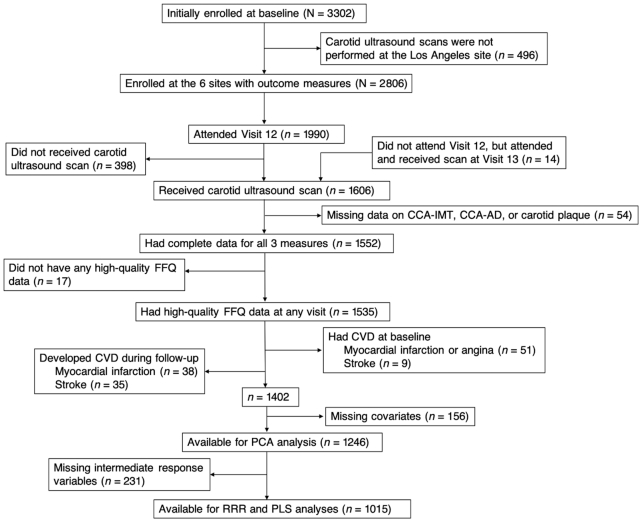
Carotid ultrasound scans were performed at all sites, except the Los Angeles site, at follow-up Visit 12 (2009–2011) or Visit 13 (2011–2013), with the vast majority of scans conducted at Visit 12. Among the 2806 women initially enrolled at the 6 sites, 1990 (70.9%) participants attended Visit 12, 1592 (80.0%) of whom had a carotid scan at Visit 12 or Visit 13. An additional 14 women did not attend Visit 12, but attended and received the carotid scan at Visit 13. Thus, a total of 1606 women had a carotid scan. For this analysis, we excluded women who had incomplete data on the 3 specific measures of carotid atherosclerosis (n = 54); who did not have high-quality dietary data at any visit [defined as not reporting too few (<4/d) or too many (>16/d) solid foods, not skipping >10 food items on the questionnaire, and not reporting total energy intake that was too low (<500 kcal/d) or too high (>5000 kcal/d)] (n = 17); who self-reported having myocardial infarction or angina (n = 51) or stroke (n = 9) at baseline or experienced myocardial infarction (n = 38) or stroke (n = 35) during the follow-up before their carotid scans; and who had missing data for the major covariates (n = 156). After the aforementioned exclusions, the final analytical sample for PCA consisted of 1246 women. Additionally, 231 women had missing data on the intermediate response variables [C-reactive protein (CRP) and plasminogen activator inhibitor 1 (PAI-1)], so the final analytical sample for RRR and PLS included 1015 women (Figure 1). Compared with the excluded participants, the women retained in the analysis were less likely to be African American, to report economic strain, to have depressive symptoms, and to have abdominal obesity, elevated blood pressure, elevated glucose, and reduced serum HDL cholesterol. The retained participants were more likely to have a college degree and to self-report having excellent or very good overall health.
FIGURE 1.
Exclusion flow of participants for the association between dietary patterns and subclinical carotid atherosclerosis in the Study of Women's Health Across the Nation. High-quality FFQ data were defined as not reporting too few (<4/d) or too many (>16/d) solid foods, not skipping >10 food items on the questionnaire, and not reporting total energy intake that was too low (<500 kcal/d) or too high (>5000 kcal/d). AD, adventitial diameter; CCA, common carotid artery; CVD; cardiovascular disease; IMT, intima-media thickness.
Assessment of exposures
Dietary intake was measured at baseline (1996–1997), Visit 5 (2001–2003), and Visit 9 (2005–2007). We used a modified 1995 version of the Block FFQ, which has previously been validated against dietary records (31) and 24-h recalls (32). The questionnaire included 103 food items. Trained personnel asked the participants how often they consumed each food item during the past year, as well as the usual portion size for each item. Up to 9 predefined frequencies (ranging from never to 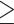 5 times/d) and 3 predefined portion sizes (ranging from small to large) were available for each item. Total energy intake and nutrient intake were calculated by multiplying the reported frequency, the reported portion size, and the corresponding nutrient content (33). We aggregated the 103 foods into 46 prespecified groups based on nutrient profile or culinary use (34, 35) (Supplemental Table 1). We calculated the intake (in grams) for each food group and adjusted it for total energy intake using the residual method (36). We then computed the energy-adjusted food group values averaged across ≤3 available dietary measurements (baseline, Visit 5, and Visit 9) to capture the long-term intake; 63.6%, 25.4%, and 10.9% of the participants had 3, 2, and 1 available dietary measurements, respectively.
5 times/d) and 3 predefined portion sizes (ranging from small to large) were available for each item. Total energy intake and nutrient intake were calculated by multiplying the reported frequency, the reported portion size, and the corresponding nutrient content (33). We aggregated the 103 foods into 46 prespecified groups based on nutrient profile or culinary use (34, 35) (Supplemental Table 1). We calculated the intake (in grams) for each food group and adjusted it for total energy intake using the residual method (36). We then computed the energy-adjusted food group values averaged across ≤3 available dietary measurements (baseline, Visit 5, and Visit 9) to capture the long-term intake; 63.6%, 25.4%, and 10.9% of the participants had 3, 2, and 1 available dietary measurements, respectively.
Assessment of outcomes
Centrally trained and certified sonographers obtained carotid ultrasound images at Visit 12 or Visit 13 using a Terason t3000 Ultrasound System (Teratech Corp.) with a variable frequency linear array transducer (37). Two digitized images were obtained for each of the left and right distal common carotid artery (CCA). From each of these 4 images, near and far wall intima-media thickness (IMT) measures of the CCA were obtained. The mean of the maximal readings of all 4 images was used in the analyses. Adventitial diameter (AD) was measured as the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole. The presence and extent of plaque in each of 5 segments of the left and right carotid artery (distal and proximal CCA, carotid bulb, and proximal internal and external carotid arteries) was evaluated. The degree of the plaque for each segment was graded between 0 (no observable plaque) and 3 (plaque obstructing ≥50% of the luminal diameter). The grades from all segments were summed to create the plaque index (38). All carotid scan images were read centrally at the SWAN Ultrasound Reading Center at the University of Pittsburgh. The outcomes of this study included the intima-media thickness of the common carotid artery (CCA-IMT), the adventitial diameter of the common carotid artery (CCA-AD), and the carotid plaque index. We treated CCA-IMT and CCA-AD as continuous variables and the carotid plaque index as a binary variable (≥2 versus <2). Graphical examinations of CCA-IMT and CCA-AD showed normal distributions, so no transformations were performed.
Assessment of covariates
Self-reported baseline covariates included age, race/ethnicity, education level ( high school, some college, or college degree/postcollege), economic strain (dichotomized as somewhat/very hard compared with not hard to pay for basic necessities) (39), self-rated overall health (excellent/very good, good, or fair/poor), depressive symptoms (dichotomized by the Center for Epidemiologic Studies Depression Scale:
high school, some college, or college degree/postcollege), economic strain (dichotomized as somewhat/very hard compared with not hard to pay for basic necessities) (39), self-rated overall health (excellent/very good, good, or fair/poor), depressive symptoms (dichotomized by the Center for Epidemiologic Studies Depression Scale:  16 or <16) (40), smoking status (never, past, or current), nonoccupational physical activity (assessed on 5-point Likert-scale questions with total scores ranging from 3 to 15) (41), and menopausal status based on self-reported menstrual bleeding patterns (dichotomized as premenopausal or early perimenopausal). Self-reported use of hormone therapy from baseline through to the carotid scan visit was dichotomized as ever use or never use, with ever use defined as the use of hormone therapy at any visit from baseline to the carotid scan visit. Waist circumference (centimeters) was measured with a measuring tape placed horizontally at the level of the natural waist or the narrowest part of the torso from the anterior aspect. Blood pressure was measured by a trained technician; the average of 2 seated measurements using a standard mercury sphygmomanometer was used in the analysis.
16 or <16) (40), smoking status (never, past, or current), nonoccupational physical activity (assessed on 5-point Likert-scale questions with total scores ranging from 3 to 15) (41), and menopausal status based on self-reported menstrual bleeding patterns (dichotomized as premenopausal or early perimenopausal). Self-reported use of hormone therapy from baseline through to the carotid scan visit was dichotomized as ever use or never use, with ever use defined as the use of hormone therapy at any visit from baseline to the carotid scan visit. Waist circumference (centimeters) was measured with a measuring tape placed horizontally at the level of the natural waist or the narrowest part of the torso from the anterior aspect. Blood pressure was measured by a trained technician; the average of 2 seated measurements using a standard mercury sphygmomanometer was used in the analysis.
Blood samples were taken to conduct blood assays, as described previously (42, 43). Briefly, serum triglyceride concentrations were measured using enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics). Serum HDL cholesterol was measured using heparin-2 M manganese chloride. The serum glucose concentration was measured from fasting samples using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics). CRP was measured in plasma using an ultrasensitive rate immunonephelometric method (Dade-Behring). PAI-1 was measured in plasma with a sandwich procedure using a solid phased monoclonal antibody and enzyme-labeled goat second antiserum for detection (IMUBIND plasma PAI-1 ELISA, American Diagnostica). Cardiometabolic conditions were defined using established harmonized guidelines (44). Specifically, abdominal obesity was defined as waist circumference 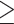 80 cm for Chinese women and
80 cm for Chinese women and 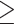 88 cm for others. Elevated blood pressure was defined as systolic blood pressure
88 cm for others. Elevated blood pressure was defined as systolic blood pressure 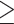 130 mm Hg, or diastolic blood pressure
130 mm Hg, or diastolic blood pressure 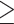 85 mm Hg, or antihypertensive drug treatment. Elevated serum glucose was defined as fasting serum glucose
85 mm Hg, or antihypertensive drug treatment. Elevated serum glucose was defined as fasting serum glucose 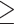 100 mg/dL or drug treatment of elevated glucose. Elevated serum triglycerides was defined as fasting serum triglycerides
100 mg/dL or drug treatment of elevated glucose. Elevated serum triglycerides was defined as fasting serum triglycerides 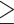 150 mg/dL. Reduced serum HDL cholesterol was defined as fasting serum HDL cholesterol <50 mg/dL.
150 mg/dL. Reduced serum HDL cholesterol was defined as fasting serum HDL cholesterol <50 mg/dL.
Statistical analysis
We used 3 complementary statistical techniques, including PCA, RRR, and PLS, to identify dietary patterns. PCA combines correlated food items into weighted linear combinations (i.e., dietary patterns) that account for the maximum variation of the original food variables (26). RRR aims to identify food combinations that explain the maximum variation in a set of prespecified intermediate response variables, and PLS aims to derive food combinations that explain variation in both the intermediate response variables and the food variables (25). The intermediate response variables are the potential mediators for the diet–disease association based on a priori hypotheses. RRR and PLS can be used to explore potential biological mechanisms for the diet–disease associations due to their ability to incorporate the intermediate response variables.
We used the 46 energy-adjusted food groups (averaged across ≤3 visits) as the inputs of PCA, RRR, and PLS. Inflammatory biomarker CRP and thrombotic biomarker PAI-1 were used as the intermediate response variables for RRR and PLS. We chose plasma CRP and plasma PAI-1 because the effect of diet on subclinical atherosclerosis may be partly mediated by proinflammatory and prothrombotic pathways (16, 19). We used the values at Visit 6 (2002–2004) for CRP and PAI-1 and natural log-transformed both variables to improve normality. The number of dietary patterns initially derived by PCA and PLS is restricted by the number of food variables used (i.e., 46 in this study), whereas the number of patterns derived by RRR is constrained by the number of intermediate response variables used (i.e., 2 in this study). The number of patterns to retain from PCA for further analysis was based on the scree plot (45), the eigenvalue >1 criterion, and the overall interpretability of the principal components (46). For RRR and PLS, the first patterns were retained for further analysis as they explained more variation in the intermediate response variables than the succeeding patterns. We calculated the factor scores for each retained pattern, where a higher score represented a higher adherence to the corresponding pattern.
We estimated the associations of quartiles of dietary pattern factor scores with CCA-IMT/CCA-AD using multivariate linear regression models, and with a high carotid plaque index (≥2) using modified Poisson models (47). The selection of confounders was based on prior knowledge of CVD risk factors. We adjusted for age, race/ethnicity, education level, economic strain, self-rated overall health, depressive symptoms, smoking status, nonoccupational physical activity level, total energy intake, menopausal status, use of hormone therapy, abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, reduced HDL cholesterol, and the number of missing visits for dietary measurements. To test for linear trends, we assigned the median factor score of each quartile to participants in the corresponding quartile as a continuous variable in the models.
We conducted several sensitivity analyses to examine the robustness of the results. First, to assess potential selection bias due to missing data, we used inverse probability weighting to incorporate a nonresponse weight for each participant based on her baseline predictors of attrition including race, education level, economic strain, self-rated overall health, depressive symptoms, smoking status, physical activity level, menopausal status, abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, and reduced HDL cholesterol. Second, we used the average food groups of baseline and Visit 5 to derive dietary patterns (i.e., ignoring dietary data at Visit 9). The motivation of this sensitivity analysis was to assess whether the inclusion of the Visit 9 dietary data appreciably influenced the estimates as the intermediate response variables were the Visit 6 values due to data availability (PAI-1 data were not available after Visit 9). Third, we derived dietary patterns separately for baseline and Visit 5 using the food groups at each visit and then used the average factor scores (in quartiles) in the analyses. Fourth, to examine potential time-varying confounding by variables that are confounders for future exposures but mediators for past exposures, we used the inverse-probability-of-treatment weighting and marginal structural models (48) to additionally adjust for cardiometabolic outcomes measured at Visit 3 (1999–2001), including abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, and reduced HDL cholesterol. Fifth, we adjusted for waist circumference, systolic blood pressure, serum glucose, serum triglycerides, and serum HDL cholesterol as continuous variables rather than dichotomous variables. Sixth, to account for multiple comparisons due to the simultaneous examination of numerous dietary patterns and outcomes, we used Bonferroni correction and the false discovery rate adjustment (49) for the 12 exposure–outcome combinations. Seventh, we used traditional cardiometabolic risk factors, including waist-to-hip ratio, systolic blood pressure, serum triglycerides, serum HDL cholesterol, and serum glucose, as the intermediate response variables for RRR and PLS. All analyses were conducted using SAS 9.4 (SAS Institute Inc.) at a 2-sided α level of 0.05.
Results
The general characteristics of the analytical sample are presented in Table 1. The mean age of the participants at baseline was 46.3 y with an SD of 2.64 y. Approximately half of the participants (52.8%) were non-Hispanic white, 28.6% were African American, 12.7% were Chinese, and 5.94% were Hispanic. At baseline, 62.2% of the participants were never smokers, 25.7% were past smokers, and 12.1% were current smokers. The mean CCA-IMT and CCA-AD at the carotid scan visit were 0.923 mm (SD: 0.136) and 7.19 mm (SD: 0.649), respectively, and 25.3% of the participants had a high carotid plaque index. Plasma CRP was correlated with CCA-IMT, CCA-AD, and plaque index with a Spearman correlation coefficient of 0.22, 0.18, and 0.11, respectively. Plasma PAI-1 was correlated with CCA-IMT, CCA-AD, and plaque index with a Spearman correlation coefficient of 0.17, 0.15, and 0.13, respectively.
TABLE 1.
General characteristics of the 1246 participants included for the association between dietary patterns and subclinical carotid atherosclerosis in the Study of Women's Health Across the Nation (USA), 1996–20131
| PCA-Western | PCA-Prudent | RRR-Western | PLS-Western | ||||||
|---|---|---|---|---|---|---|---|---|---|
| General characteristics | Total | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 | Quartile 1 | Quartile 4 |
| n | 1246 | 311 | 311 | 311 | 311 | 253 | 254 | 253 | 254 |
| Age at baseline, y | 46.3 ± 2.64 | 46.3 ± 2.73 | 46.4 ± 2.61 | 46.1 ± 2.49 | 46.7 ± 2.69 | 46.7 ± 2.68 | 46.4 ± 2.69 | 46.8 ± 2.77 | 46.3 ± 2.63 |
| Age at the carotid scan, y | 60.2 ± 2.69 | 60.2 ± 2.77 | 60.2 ± 2.69 | 59.9 ± 2.60 | 60.5 ± 2.75 | 60.6 ± 2.73 | 60.1 ± 2.75 | 60.7 ± 2.82 | 60.1 ± 2.68 |
| Race and ethnicity | |||||||||
| African American, % | 28.6 | 20.3 | 41.2 | 42.8 | 23.8 | 11.1 | 50.8 | 11.5 | 56.7 |
| Hispanic, % | 5.94 | 6.43 | 6.43 | 11.9 | 0.965 | 0.00 | 1.97 | 0.00 | 1.97 |
| Chinese, % | 12.7 | 27.0 | 6.75 | 5.14 | 15.8 | 27.3 | 1.57 | 30.0 | 1.97 |
| Non-Hispanic white, % | 52.8 | 46.3 | 45.7 | 40.2 | 59.5 | 61.7 | 45.7 | 58.5 | 39.4 |
| Education level | |||||||||
| High school or less, % | 21.0 | 19.9 | 21.9 | 33.4 | 9.32 | 13.4 | 26.4 | 13.4 | 28.7 |
| Some college, % | 30.3 | 22.5 | 36.3 | 35.1 | 26.7 | 24.9 | 42.1 | 20.6 | 43.3 |
| College degree/postcollege, % | 48.7 | 57.6 | 41.8 | 31.5 | 64.0 | 61.7 | 31.5 | 66.0 | 28.0 |
| Somewhat/very hard to pay for basics, % | 32.3 | 31.2 | 30.6 | 42.8 | 26.1 | 24.5 | 34.3 | 26.1 | 33.9 |
| Self-rated overall health | |||||||||
| Excellent/very good, % | 63.5 | 64.0 | 61.4 | 50.5 | 71.1 | 75.9 | 49.6 | 74.3 | 54.3 |
| Good, % | 27.4 | 25.4 | 28.6 | 37.6 | 19.9 | 17.4 | 38.2 | 15.8 | 35.0 |
| Fair/poor, % | 9.15 | 10.6 | 10.0 | 11.9 | 9.00 | 6.72 | 12.2 | 9.88 | 10.6 |
| Depressive symptoms,2 % | 22.6 | 19.3 | 21.5 | 33.4 | 18.0 | 17.8 | 27.6 | 16.6 | 28.0 |
| Total energy intake,3 Mcal/d | 1.75 ± 0.560 | 1.21 ± 0.216 | 2.35 ± 0.591 | 1.95 ± 0.665 | 1.60 ± 0.459 | 1.53 ± 0.415 | 1.77 ± 0.615 | 1.53 ± 0.535 | 1.72 ± 0.473 |
| Number of missing dietary measurements | |||||||||
| 0, % | 63.6 | 66.2 | 55.3 | 50.5 | 72.7 | 79.1 | 63.8 | 80.6 | 61.4 |
| 1, % | 25.4 | 23.8 | 31.8 | 30.2 | 20.9 | 18.6 | 28.0 | 16.6 | 27.6 |
| 2, % | 10.9 | 10.0 | 12.9 | 19.3 | 6.43 | 2.37 | 8.27 | 2.77 | 11.0 |
| Smoking status | |||||||||
| Never, % | 62.2 | 67.5 | 63.3 | 56.3 | 64.0 | 63.6 | 53.5 | 68.4 | 57.1 |
| Past, % | 25.7 | 23.5 | 22.5 | 21.5 | 29.3 | 28.9 | 24.8 | 28.5 | 20.9 |
| Current, % | 12.1 | 9.00 | 14.2 | 22.2 | 6.75 | 7.51 | 21.7 | 3.16 | 22.1 |
| Nonoccupational physical activity4 | 7.7 ± 1.8 | 8.0 ± 1.9 | 7.6 ± 1.7 | 7.1 ± 1.7 | 8.4 ± 1.8 | 8.2 ± 1.8 | 7.3 ± 1.6 | 8.3 ± 1.8 | 7.3 ± 1.6 |
| Menopausal status | |||||||||
| Early perimenopausal, % | 44.4 | 42.4 | 45.7 | 49.2 | 43.7 | 39.9 | 50.0 | 40.3 | 46.9 |
| Premenopausal, % | 55.6 | 57.6 | 54.3 | 50.8 | 56.3 | 60.1 | 50.0 | 59.7 | 53.2 |
| Hormone therapy use,5 % | 43.2 | 39.6 | 40.2 | 37.9 | 49.2 | 48.2 | 40.9 | 47.8 | 40.6 |
| Abdominal obesity,6 % | 39.0 | 42.8 | 39.6 | 44.1 | 34.1 | 23.7 | 51.6 | 29.3 | 48.0 |
| Elevated blood pressure,7 % | 26.5 | 22.5 | 34.1 | 29.6 | 26.7 | 20.2 | 37.4 | 19.0 | 38.2 |
| Elevated serum glucose,8 % | 21.4 | 22.5 | 23.5 | 22.8 | 17.0 | 12.7 | 29.9 | 13.8 | 26.0 |
| Elevated serum triglycerides,9 % | 17.4 | 18.7 | 18.3 | 19.0 | 13.5 | 13.0 | 21.7 | 13.4 | 20.1 |
| Reduced serum HDL-C,10 % | 33.3 | 32.8 | 33.1 | 36.7 | 29.9 | 24.1 | 37.8 | 22.9 | 34.3 |
| CCA-IMT,11 mm | 0.923 ± 0.136 | 0.919 ± 0.132 | 0.934 ± 0.135 | 0.941 ± 0.140 | 0.913 ± 0.136 | 0.895 ± 0.123 | 0.960 ± 0.145 | 0.890 ± 0.121 | 0.964 ± 0.145 |
| CCA-AD,11 mm | 7.19 ± 0.649 | 7.17 ± 0.656 | 7.20 ± 0.679 | 7.25 ± 0.640 | 7.13 ± 0.646 | 7.08 ± 0.613 | 7.30 ± 0.687 | 7.08 ± 0.633 | 7.34 ± 0.686 |
| High carotid plaque index,11, 12 % | 25.3 | 28.9 | 22.2 | 26.1 | 23.5 | 26.1 | 32.7 | 22.1 | 28.4 |
Values are means (SDs) for continuous variables and percentages for categorical variables. Values of polytomous variables may not sum to 100% due to rounding. The sample size for reduced rank regression and partial least squares regression was 1015 due to missing data on the intermediate response variables. The variables are the baseline (1996–1997) measures unless specified otherwise.
Defined as the Center for Epidemiologic Studies Depression Scale 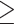 16 (40).
16 (40).
Average value across available visits of baseline (1996–1997), Visit 5 (2001–2003), and Visit 9 (2005–2007). Mcal, 1000 kcal.
Assessed on 5-point Likert and ordinal quantitative scales with total scores ranging from 3 to 15, with higher values indicating more frequent engagement in nonoccupational physical activity (41).
Defined as reported use of hormone therapy at any time from baseline to the carotid scan visit.
Defined as waist circumference 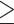 80 cm for Chinese women and
80 cm for Chinese women and 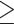 88 cm for others (44).
88 cm for others (44).
Defined as systolic blood pressure 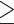 130 mm Hg, or diastolic blood pressure
130 mm Hg, or diastolic blood pressure 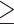 85 mm Hg, or antihypertensive drug treatment (44).
85 mm Hg, or antihypertensive drug treatment (44).
Defined as fasting serum glucose 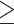 100 mg/dL or drug treatment of elevated glucose (44).
100 mg/dL or drug treatment of elevated glucose (44).
Defined as fasting serum triglycerides 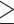 150 mg/dL (44).
150 mg/dL (44).
Defined as fasting serum HDL cholesterol <50 mg/dL (44).
Measured at Visit 12 (2009–2011) or Visit 13 (2011–2013).
Defined as carotid plaque index 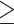 2.
2.
AD, adventitial diameter; CCA, common carotid artery; IMT, intima-media thickness; PCA, principal component analysis; PLS, partial least squares regression; RRR, reduced rank regression.
Two dietary patterns were retained from PCA, which jointly explained 17.2% of the variation in food intake but only 2.35% of the variation in the intermediate response variables. The first pattern was named the PCA-Western pattern, and the second pattern was named the PCA-Prudent pattern. The PCA-Western pattern explained 10.7%, 1.43%, and 0.00% of the variation in food intake, CRP, and PAI-1, respectively. The PCA-Prudent pattern explained 6.49%, 2.07%, and 1.21% of the variation in food intake, CRP, and PAI-1, respectively (Table 2). Only the first patterns from RRR and PLS were retained for further analyses as they explained more variation in the intermediate response variables. These 2 patterns were consequently named the RRR-Western pattern and the PLS-Western pattern, respectively. The RRR-Western pattern explained 3.65%, 12.5%, and 4.20% of the variation in food intake, CRP, and PAI-1, respectively. The PLS-Western pattern explained 8.06%, 8.41%, and 1.89% of the variation in food intake, CRP, and PAI-1, respectively (Table 2). Thus, the PCA-derived patterns explain a high percentage of variation in food intake but a low amount of variation in both the intermediate response variables and the atherosclerosis outcomes. The RRR-derived pattern accounts for more variation in the intermediate response variables and the outcomes but explains low variation in food intake. The PLS-derived pattern accounts for a similar amount of variation in the intermediate response variables and the outcomes as the RRR-derived pattern but also explains a much higher amount of variation in food intake than the RRR-derived pattern.
TABLE 2.
Percentage of variation in the intermediate response variables and food variables explained by the dietary patterns derived by multiple statistical methods among 1246 participants of the Study of Women's Health Across the Nation (USA), 1996–20131
| Explained variation in intermediate response variables,2 % | |||||||
|---|---|---|---|---|---|---|---|
| Plasma CRP | Plasma PAI-1 | Both CRP and PAI-1 | Explained variation in food groups, % | Explained variation in CCA-IMT, % | Explained variation in CCA-AD, % | Explained variation in the carotid plaque index, % | |
| PCA | |||||||
| PCA-Western | 1.43 | 0.00 | 0.72 | 10.7 | 0.273 | 0.101 | 0.0391 |
| PCA-Prudent | 2.07 | 1.21 | 1.63 | 6.49 | 0.550 | 0.667 | 0.194 |
| Both PCA patterns | 3.50 | 1.21 | 2.35 | 17.2 | 0.823 | 0.769 | 0.233 |
| RRR-Western | 12.5 | 4.20 | 8.32 | 3.65 | 3.97 | 2.00 | 0.227 |
| PLS-Western | 8.41 | 1.89 | 5.15 | 8.06 | 3.46 | 1.77 | 0.209 |
The dietary patterns were derived using the energy-adjusted food groups averaged across available visits of baseline (1996–1997), Visit 5 (2001–2003), and Visit 9 (2005–2007). The sample size for reduced rank regression and partial least squares regression was 1015 due to missing data on the intermediate response variables.
The intermediate response variables were the log-transformed values at Visit 6 (2002–2004).
AD, adventitial diameter; CCA, common carotid artery; CRP, C-reactive protein; IMT, intima-media thickness; PAI-1, plasminogen activator inhibitor 1; PCA, principal component analysis; PLS, partial least squares regression; RRR, reduced rank regression.
The PCA-Western pattern was characterized by high intakes of dairy products, pizza, unprocessed red meat, processed red meat, and salad dressings; the PCA-Prudent pattern included high intakes of vegetables, legumes, and fruits (Table 3 and Supplemental Table 2). The PCA-Western pattern was weakly correlated with a higher concentration of CRP (Spearman correlation coefficient: 0.10); the PCA-Prudent pattern was weakly correlated with lower concentrations of CRP (Spearman correlation coefficient: −0.18) and PAI-1 (Spearman correlation coefficient: −0.09) (Table 3). The RRR-Western pattern was characterized by high intakes of margarine, fried fish, artificially sweetened beverages, processed red meat, and sugar-sweetened beverages, and low intakes of soup, fiber cereals, fruits, cruciferous vegetables, and wine; the PLS-Western pattern included high intakes of French fries, processed red meat, fried fish, margarine, and sugar-sweetened beverages, and low intakes of fruits, legumes, dark-yellow vegetables, cruciferous vegetables, and soup (Table 3 and Supplemental Table 2). Both the RRR-Western pattern and the PLS-Western pattern were positively correlated with CRP (Spearman correlation coefficient: 0.39 and 0.32, respectively) and PAI-1 (Spearman correlation coefficient: 0.22 and 0.15, respectively) (Table 3). The Spearman correlation coefficient was 0.14 between PCA-Western and RRR-Western patterns, 0.51 between PCA-Western and PLS-Western patterns, and 0.77 between RRR-Western and PLS-Western patterns.
TABLE 3.
Correlations among empirically derived dietary patterns, food groups, and intermediate response variables in 1246 participants of the Study of Women's Health Across the Nation (USA), 1996–20131
| Spearman correlation coefficients with intermediate response variables2 | |||
|---|---|---|---|
| Factor loading | CRP | PAI-1 | |
| PCA-Western | 0.10 | — | |
| High | |||
| Dairy products | 0.28 | 0.09 | — |
| Pizza | 0.27 | — | — |
| Unprocessed red meat | 0.26 | 0.13 | — |
| Processed red meat | 0.25 | 0.25 | 0.08 |
| Salad dressings | 0.24 | — | — |
| Low | |||
| Fruits | −0.11 | −0.12 | — |
| Milk with lower fat content | −0.09 | −0.10 | — |
| Legumes | −0.07 | −0.13 | — |
| Cruciferous vegetables | −0.05 | −0.12 | −0.09 |
| Tomatoes | −0.04 | −0.09 | — |
| PCA-Prudent | −0.18 | −0.09 | |
| High | |||
| Dark-yellow vegetables | 0.39 | −0.07 | −0.07 |
| Green leafy vegetables | 0.38 | — | — |
| Cruciferous vegetables | 0.37 | −0.12 | −0.09 |
| Legumes | 0.31 | −0.13 | — |
| Fruits | 0.31 | −0.12 | — |
| Low | |||
| Whole milk | −0.13 | — | — |
| Margarine | −0.07 | 0.21 | 0.11 |
| Sweets and desserts | −0.07 | — | — |
| Organ meat | −0.05 | 0.06 | — |
| Beer | −0.04 | −0.07 | — |
| RRR-Western | 0.39 | 0.22 | |
| High | |||
| Margarine | 0.32 | 0.20 | 0.10 |
| Fried fish | 0.22 | 0.15 | — |
| Artificially sweetened beverages | 0.22 | 0.19 | — |
| Processed red meat | 0.22 | 0.25 | 0.08 |
| Sugar-sweetened beverages | 0.17 | 0.17 | 0.09 |
| Low | |||
| Soup | −0.30 | −0.14 | — |
| Fiber cereals | −0.26 | −0.06 | −0.13 |
| Fruits | −0.23 | −0.13 | — |
| Cruciferous vegetables | −0.23 | −0.12 | −0.09 |
| Wine | −0.22 | −0.12 | −0.11 |
| PLS-Western | 0.32 | 0.15 | |
| High | |||
| French fries | 0.27 | 0.13 | — |
| Processed red meat | 0.25 | 0.25 | 0.08 |
| Fried fish | 0.22 | 0.15 | — |
| Margarine | 0.22 | 0.20 | 0.10 |
| Sugar-sweetened beverages | 0.20 | 0.17 | 0.09 |
| Low | |||
| Fruits | −0.27 | −0.13 | — |
| Legumes | −0.25 | −0.12 | — |
| Dark-yellow vegetables | −0.22 | −0.07 | −0.07 |
| Cruciferous vegetables | −0.22 | −0.12 | −0.09 |
| Soup | −0.21 | −0.14 | — |
The food groups were the energy-adjusted values averaged across available visits of baseline (1996–1997), Visit 5 (2001–2003), and Visit 9 (2005–2007). For simplicity, only the 5 positively associated and the 5 inversely associated food groups with the largest factor loadings (absolute value) are shown. The sample size for reduced rank regression and partial least squares regression was 1015 due to missing data on the intermediate response variables.
The intermediate response variables were the log-transformed values at Visit 6 (2002–2004). For simplicity, only statistically significant (P <0.05) correlation coefficients are shown.
CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor 1; PCA, principal component analysis; PLS, partial least squares regression; RRR, reduced rank regression.
Higher adherences to PCA-Western, RRR-Western, and PLS-Western patterns were all associated with higher CCA-IMT after adjusting for covariates (P-trend: 0.013, 0.0058, and <0.001, respectively) (Table 4). Women in the fourth quartile of the PCA-Western, the RRR-Western, and the PLS-Western patterns had 0.042 mm (95% CI: 0.011, 0.073), 0.033 mm (95% CI: 0.0086, 0.057), and 0.049 mm (95% CI: 0.025, 0.074), respectively, larger CCA-IMT than women in the first quartile; these differences correspond to 30%, 24%, and 35% of the sample SD of CCA-IMT, respectively. Adherence to the PCA-Prudent pattern was not significantly associated with CCA-IMT (P-trend = 0.38). We found no associations between any identified dietary patterns and CCA-AD or carotid plaque.
TABLE 4.
Quartiles of dietary pattern factor scores from multiple statistical methods and subclinical carotid atherosclerosis among 1246 participants of the Study of Women's Health Across the Nation (USA), 1996–20131
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean difference | RR | n | Mean difference | RR | n | Mean difference | RR | n | Mean difference | RR | P-trend2 | |
| CCA-IMT, mm | |||||||||||||
| PCA-Western | 311 | 0 (Ref) | — | 312 | 0.014 (−0.0083, 0.037) | — | 312 | 0.035 (0.0086, 0.061)* | — | 311 | 0.042 (0.011, 0.073)* | — | 0.013 |
| PCA-Prudent | 311 | 0 (Ref) | — | 312 | 0.0042 (−0.017, 0.025) | — | 312 | −0.018 (−0.039, 0.0038) | — | 311 | −0.0060 (−0.028, 0.016) | — | 0.38 |
| RRR-Western | 253 | 0 (Ref) | — | 254 | 0.0035 (−0.019, 0.026) | — | 254 | 0.016 (−0.0074, 0.040) | — | 254 | 0.033 (0.0086, 0.057)* | — | 0.0058 |
| PLS-Western | 253 | 0 (Ref) | — | 254 | 0.019 (−0.0039, 0.041) | — | 254 | 0.019 (−0.0042, 0.042) | — | 254 | 0.049 (0.025, 0.074)* | — | < 0.001 |
| CCA-AD, mm | |||||||||||||
| PCA-Western | 311 | 0 (Ref) | — | 312 | 0.036 (−0.072, 0.14) | — | 312 | 0.091 (−0.034, 0.21) | — | 311 | 0.057 (−0.091, 0.20) | — | 0.65 |
| PCA-Prudent | 311 | 0 (Ref) | — | 312 | 0.040 (−0.059, 0.14) | — | 312 | −0.014 (−0.12, 0.088) | — | 311 | −0.017 (−0.12, 0.090) | — | 0.51 |
| RRR-Western | 253 | 0 (Ref) | — | 254 | 0.052 (−0.056, 0.16) | — | 254 | 0.027 (−0.086, 0.14) | — | 254 | 0.040 (−0.075, 0.15) | — | 0.56 |
| PLS-Western | 253 | 0 (Ref) | — | 254 | 0.034 (−0.074, 0.14) | — | 254 | 0.026 (−0.085, 0.14) | — | 254 | 0.11 (−0.0021, 0.23) | — | 0.071 |
| Carotid plaque | |||||||||||||
| PCA-Western | 311 | — | 1.00 (Ref) | 312 | — | 0.87 (0.65, 1.15) | 312 | — | 0.85 (0.61, 1.17) | 311 | — | 0.79 (0.53, 1.17) | 0.35 |
| PCA-Prudent | 311 | — | 1.00 (Ref) | 312 | — | 1.14 (0.88, 1.47) | 312 | — | 1.02 (0.77, 1.35) | 311 | — | 1.09 (0.82, 1.50) | 0.74 |
| RRR-Western | 253 | — | 1.00 (Ref) | 254 | — | 0.77 (0.56, 1.05) | 254 | — | 0.86 (0.63, 1.18) | 254 | — | 1.01 (0.75, 1.35) | 0.79 |
| PLS-Western | 253 | — | 1.00 (Ref) | 254 | — | 1.17 (0.86, 1.58) | 254 | — | 1.06 (0.78, 1.46) | 254 | — | 1.10 (0.80, 1.51) | 0.66 |
Values are mean differences (95% CIs) for CCA-IMT/CCA-AD, and risk ratios (95% CIs) for carotid plaque. The dietary patterns were derived using the energy-adjusted food groups averaged across available visits of baseline (1996–1997), Visit 5 (2001–2003), and Visit 9 (2005–2007). The outcomes were measured at Visit 12 (2009–2011) or Visit 13 (2011–2013). The sample size for reduced rank regression and partial least squares regression was 1015 due to missing data on the intermediate response variables. All models were adjusted for age at the carotid scan (continuous), race/ethnicity (non-Hispanic white or not), education level (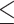 high school, some college, or college degree/postcollege), economic strain (somewhat/very hard paying for basics, or not hard paying for basics), self-rated overall health (excellent/very good, good, or fair/poor), Center for Epidemiologic Studies Depression scale (
high school, some college, or college degree/postcollege), economic strain (somewhat/very hard paying for basics, or not hard paying for basics), self-rated overall health (excellent/very good, good, or fair/poor), Center for Epidemiologic Studies Depression scale (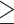 16 or <16), smoking status (ever or never), nonoccupational physical activity level (continuous), total energy intake (continuous average across available visits of baseline, Visit 5, and Visit 9), menopausal status (premenopausal or early perimenopausal), use of hormone therapy from baseline to the visit of the carotid scan (ever or never), abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, reduced HDL cholesterol, and the number of missing visits for dietary measurements (0, 1, or 2). The baseline covariates were used except age, total energy intake, hormone therapy use, and the number of missing visits for dietary measurements.
16 or <16), smoking status (ever or never), nonoccupational physical activity level (continuous), total energy intake (continuous average across available visits of baseline, Visit 5, and Visit 9), menopausal status (premenopausal or early perimenopausal), use of hormone therapy from baseline to the visit of the carotid scan (ever or never), abdominal obesity, elevated blood pressure, elevated glucose, elevated triglycerides, reduced HDL cholesterol, and the number of missing visits for dietary measurements (0, 1, or 2). The baseline covariates were used except age, total energy intake, hormone therapy use, and the number of missing visits for dietary measurements.
Computed by assigning the median factor score of each quartile to participants in the corresponding quartile as a continuous variable.
Different from Quartile 1, P <0.01.
AD, adventitial diameter; CCA, common carotid artery; IMT, intima-media thickness; PCA, principal component analysis; PLS, partial least squares regression; Ref, reference group; RR, risk ratio; RRR, reduced rank regression.
The results did not change appreciably after accounting for missing data using nonresponse weights, using the average food groups of baseline and Visit 5 to derive dietary patterns, deriving dietary patterns separately for baseline and Visit 5, adjusting for time-varying confounders using marginal structural models, or adjusting for certain covariates as continuous variables. The association between the PCA-Western pattern and CCA-IMT became marginally significant (P-trend = 0.052) when using the separately derived patterns and became nonsignificant (P-trend = 0.12) when adjusting for time-varying confounders, possibly due to the reduced sample sizes in these sensitivity analyses. Results from these sensitivity analyses are shown in Supplemental Tables 3–5. After false discovery rate adjustment, the associations between the 3 Western patterns and CCA-IMT remained significant or marginally significant (adjusted P-trend: 0.052, 0.035, and 0.0012 for PCA-Western, RRR-Western, and PLS-Western patterns, respectively); the association between the PLS-Western pattern and CCA-IMT remained significant also under the Bonferroni correction (adjusted P-trend = 0.0012). RRR- and PLS-derived patterns using traditional cardiometabolic risk factors were not significantly associated with subclinical carotid atherosclerosis (results available upon request).
Discussion
This study evaluated the prospective associations between empirically derived dietary patterns during midlife and subclinical carotid atherosclerosis later in life among women. After extensively adjusting for covariates, higher adherences to Western dietary patterns derived by PCA, RRR, and PLS were all associated with higher CCA-IMT. The Prudent pattern was not significantly associated with CCA-IMT, and no significant associations were found between the identified dietary patterns and CCA-AD or carotid plaque.
The literature on dietary patterns and subclinical atherosclerosis is inconclusive with some studies suggesting the presence of an association (14, 16, 19, 21, 22) and others finding no such evidence (15, 17, 18, 20). Few studies have specifically examined the associations between empirical dietary patterns during midlife and atherosclerosis later in life among women. In the Framingham Study, women (mean age ∼47 y at baseline) with a cluster-analysis-derived dietary pattern high in fat and sugar and low in fruit and vegetables had significantly higher odds of carotid artery stenosis after 12 y compared with women with a “Heart-Healthy” diet at baseline (14). In a cross-sectional study in Spain using cluster analysis (participants aged 40–54 y; 37% women), compared with participants following the Mediterranean diet, those with a “social-business” diet high in red meat, premade foods, snacks, and sugar-sweetened beverages had significantly higher odds of coronary artery calcification (22). As a counterexample, in a Finnish cohort of participants aged 24–39 y at baseline (54% women), a PCA-derived pattern with high intakes of rye, potatoes, butter, sausages, milk, and coffee was not associated with CCA-IMT in women 21 y later (17). In a French cohort of women aged 35–60 y at baseline, none of the 4 PCA-derived patterns, including a Western pattern high in processed meat and a Prudent pattern high in fruits, vegetables, and fish, were significantly associated with CCA-IMT or carotid plaque measured 7.5 y later (18). All but 1 (17) of the studies mentioned above used a single dietary assessment to measure diet and thus were unlikely to have accurately captured the long-term diet. Furthermore, all 4 studies included almost exclusively white participants, so the generalizability of the results to other racial and ethnic groups may be limited.
The use of different dietary pattern derivation techniques and intermediate response variables may partially explain the inconsistency in the literature. PCA uses the correlations among food variables, and the derived patterns are heavily influenced by cultural backgrounds. RRR and PLS allow the incorporation of intermediate variables and thus may assess potential etiological mechanisms and inform prevention strategies targeted toward specific pathways. RRR has gained popularity in nutritional epidemiology, but few studies have compared the performances of RRR (and PLS) to the more traditionally used PCA (27–29). Two prior studies have used RRR (but not PLS) to evaluate the association between dietary patterns and subclinical atherosclerosis. In a cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis, an RRR-derived pattern high in total and saturated fat and low in fiber and micronutrients was associated with higher CCA-IMT and coronary artery calcification (16). Similarly, in the Insulin Resistance Atherosclerosis Study, a baseline RRR-derived pattern with high intakes of refined grains, red meat, cheese, and sugar-sweetened beverages was positively associated with CCA-IMT after 5 y (19).
The association we observed between the RRR-derived pattern and CCA-IMT was consistent with prior studies, although we used somewhat different intermediate response variables (CRP and PAI-1) than those used in the Multi-Ethnic Study of Atherosclerosis (CRP, IL-6, homocysteine, and fibrinogen) and the Insulin Resistance Atherosclerosis Study (PAI-1 and fibrinogen). CRP as an inflammatory biomarker and PAI-1 as a thrombotic biomarker are both potential predictors for CVD risk (50, 51). Further, the effect of diet on subclinical atherosclerosis may be partly mediated by proinflammatory and prothrombotic processes (16, 19). Contrary to the results from the Multi-Ethnic Study of Atherosclerosis, which reported that a PCA-derived Western pattern was not associated with CCA-IMT (16), the PCA-Western pattern in the present study was associated with CCA-IMT with a magnitude comparable to that for the RRR-Western pattern despite the fact that the PCA-Western pattern explained little variation in the intermediate response variables. Therefore, the PCA-Western pattern likely promotes atherosclerosis independent of the inflammation or hemostasis processes, or through alternative inflammatory and hemostatic mechanisms that are not well accounted for by CRP or PAI-1. Other potential inflammatory and hemostatic intermediate response variables we considered included IL-6, homocysteine, and fibrinogen, which were unavailable in SWAN. The null finding when traditional cardiometabolic risk factors were used as the intermediate response variables is consistent with Liese et al. (19), who also initially used traditional CVD factors as the intermediate response variables of RRR and found that only the selection of PAI-1 and fibrinogen yielded dietary patterns that were significantly associated with CCA-IMT. Therefore, our study provides evidence that the association between diet and subclinical carotid atherosclerosis may be mediated by inflammatory and hemostatic mechanisms rather than through traditional cardiometabolic risk factors.
Among the 3 Western patterns in this study, the PLS-Western pattern has the strongest association with CCA-IMT. PLS is a compromise between PCA and RRR, with the capability of identifying food combinations that are not only commonly consumed in the population but also associated with intermediate risk factors (25). The PLS-derived pattern accounts for a similar amount of variation in the intermediate response variables and the outcomes as the RRR-derived pattern and explains much more variation in food intake than the RRR-derived pattern. PLS offers more flexibility over RRR, especially in exploratory analyses in the presence of uncertainty regarding the mechanistic pathways (27). PLS has been underutilized in the nutritional epidemiologic literature. Future studies can take advantage of this relatively novel technique to uncover the interrelations among dietary exposures, intermediate risk factors, and disease outcomes.
In the present study, in comparison with being in the lowest quartile, being in the highest quartile of the Western patterns was associated with differences in CCA-IMT equivalent to 24% to 35% of the sample SD. Previous studies report that every 1-SD increment in CCA-IMT is associated with a 26% higher risk of myocardial infarction and a 32% higher risk of stroke (52). Although it is unclear to what extent the small differences across quartiles from the present study are clinically meaningful for an individual woman, the results are of public-health relevance at the population level due to the high prevalence of Western diet in the USA. Also, the estimates from the present study may be conservative as the women retained in the analysis were generally healthier compared with the baseline sample of women who were initially enrolled in SWAN. Still, the average CCA-IMT in the SWAN population is 0.09 mm higher than that among women of comparable age in the French population (18, 53), indicating a high burden of subclinical atherosclerosis among midlife women in the USA. Although it was not possible to pinpoint the nutrients that were most responsible for the observed associations, all 3 Western patterns were characterized by high intakes of foods rich in saturated fat (processed red meat, French fries, margarine, and dairy products), trans fat (French fries and margarine), cholesterol (processed red meat and dairy products), and added sugar (sugar-sweetened beverages), all of which have been associated with larger CCA-IMT (54, 55). Interestingly, the Prudent dietary pattern per se, with relatively high loadings on fruits, vegetables, and legumes, is not associated with subclinical atherosclerosis in this study. This lack of association may be explained by the overall low intake of healthy foods and the relatively narrower distribution of the Prudent pattern factor scores compared with the 3 Western patterns. The lowest quartiles of the Western dietary patterns in our study all have >2 servings of fruit per day, meeting the recommended daily intake of 2 servings in the Healthy US-Style Eating Pattern of the 2015–2020 Dietary Guidelines (56). However, the lowest quartiles of the Western dietary patterns all have <2 servings of vegetables per day, failing to reach the recommended daily intake of 2.5 servings (56).
The primary strengths of this study include the focus on multi-ethnic midlife women, the comparison of multiple data reduction methods, the extensive adjustment of covariates, and the use of multiple prospective measures of dietary intake to reduce random within-person variation in diet and to more accurately capture the long-term diet (11). This study also has some limitations. First and foremost, carotid atherosclerosis was measured only once. Without baseline outcome measures, it was not possible to assess the change in atherosclerosis over time, or when atherosclerosis began to accumulate. As a result, it is unclear whether midlife is the most causally pertinent period for the effect of diet (12). Future studies should collect data on both diet and atherosclerosis repeatedly to better understand the longitudinal associations. Second, the self-reported dietary intake inevitably had a certain degree of measurement error, which was minimized by the use of repeated dietary measures from a validated FFQ and by the restriction to women with high-quality dietary data. Also, the validity of a dietary pattern analysis does not depend on the accurate quantification of the absolute intake, but the relative ranking of the individuals; the FFQ is relatively robust in ranking the levels of dietary intake (11). Third, CRP and PAI-1 may not represent the inflammatory and hemostatic processes that are most pertinent to the development of atherosclerosis, in which case the patterns derived by RRR and PLS may underestimate the true associations. Fourth, we had to exclude 231 participants with missing data on CRP or PAI-1. Fifth, aggregating the original 103 food items into 46 food groups is arbitrary, and differences in food groupings may affect the patterns derived (57), although we used the same food grouping that has been used in previous studies (34, 35). Sixth, this study was subject to multiple testing due to the inclusion of numerous dietary patterns and outcomes. However, the associations remained robust after controlling for the false discovery rate, and the key findings did not change considerably under various sensitivity analyses. Evaluation of potential effect modification by race/ethnicity was not among the aims of this work but is of interest for future studies as the effect of dietary intake may vary by racial and ethnic backgrounds (58).
In conclusion, this prospective study identifies 3 Western dietary patterns that are associated with a higher level of subclinical carotid atherosclerosis in midlife women. The menopausal transition in women represents a vulnerable window of increased CVD risk and a crucial period for CVD prevention. The results from this study indicate that the positive association between the Western diet and CCA-IMT is robust under various statistical methods of dietary pattern identification. The adoption of a diet low in red meat, processed meat, deep-fried products, and sugar-sweetened beverages among midlife women is associated with lower future atherosclerosis.
Supplementary Material
ACKNOWLEDGEMENTS
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, principal investigator (PI) 2011–present; MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011–present; Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair. We thank the study staff at each site and all the women who participated in SWAN. We thank Siobán D Harlow for her feedback on the manuscript.
The authors’ responsibilities were as follows—DW and AB: designed the research; DW: analyzed the data and wrote the manuscript; CAK-G, EAJ, MRE, BMA, EB-M, LFB, M-HH, and AB: contributed to the interpretation of the results and the editing of the manuscript; DW and AB: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
The Study of Women's Health Across the Nation (SWAN) has grant support from the NIH, Department of Health and Human Services (DHHS), through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) , and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495).
Author disclosures: The authors report no conflicts of interest.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or NIH.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AD, adventitial diameter; CCA, common carotid artery; CRP, C-reactive protein; CVD, cardiovascular disease; IMT, intima-media thickness; PAI-1, plasminogen activator inhibitor 1; PCA, principal component analysis; PLS, partial least squares regression; RRR, reduced rank regression; SWAN, Study of Women's Health Across the Nation.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Abdulnour J, Doucet E, Brochu M, Lavoie J-M, Strychar I, Rabasa-Lhoret R, Prud'homme D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause. 2012;19:760–7. [DOI] [PubMed] [Google Scholar]
- 3. Li Z, McNamara JR, Fruchart J-C, Luc G, Bard JM, Ordovas J, Wilson P, Schaefer E. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37:1886–96. [PubMed] [Google Scholar]
- 4. Carr MC, Kim KH, Zambon A, Mitchell ES, Woods NF, Casazza CP, Purnell JQ, Hokanson JE, Brunzell JD, Schwartz RS. Changes in LDL density across the menopausal transition. J Investig Med. 2000;48:245–50. [PubMed] [Google Scholar]
- 5. Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition?. J Am Coll Cardiol. 2009;54:2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thurston RC, Karvonen-Gutierrez CA, Derby CA, El Khoudary SR, Kravitz HM, Manson JE. Menopause versus chronologic aging: their roles in women's health. Menopause. 2018;25:849–54. [DOI] [PubMed] [Google Scholar]
- 8. Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579–85. [DOI] [PubMed] [Google Scholar]
- 9. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 11. Willett W. Nutritional Epidemiology. 3rd ed New York, NY: Oxford University Press; 2012. [Google Scholar]
- 12. Wang D, Jackson EA, Karvonen-Gutierrez CA, Elliott MR, Harlow SD, Hood MM, Derby CA, Sternfeld B, Janssen I, Crawford SL et al.. Healthy lifestyle during the midlife is prospectively associated with less subclinical carotid atherosclerosis: The Study of Women's Health Across the Nation. J Am Heart Assoc. 2018;7:e010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Karvonen-Gutierrez CA, Jackson EA, Elliott MR, Appelhans BM, Barinas-Mitchell E, Bielak LF, Baylin A. Prospective associations between beverage intake during the midlife and subclinical carotid atherosclerosis: The Study of Women's Health Across the Nation. PLoS One. 2019;14:e0219301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Millen BE, Quatromoni PA, Nam B-H, O'Horo CE, Polak JF, D'Agostino RB. Dietary patterns and the odds of carotid atherosclerosis in women: the Framingham Nutrition Studies. Prev Med. 2002;35:540–7. [DOI] [PubMed] [Google Scholar]
- 15. Millen BE, Quatromoni PA, Nam B-H, O'Horo CE, Polak JF, Wolf PA, D'Agostino RB. Dietary patterns, smoking, and subclinical heart disease in women: opportunities for primary prevention from the Framingham Nutrition Studies. J Am Diet Assoc. 2004;104:208–14. [DOI] [PubMed] [Google Scholar]
- 16. Nettleton JA, Steffen LM, Schulze MB, Jenny NS, Barr RG, Bertoni AG, Jacobs DR. Associations between markers of subclinical atherosclerosis and dietary patterns derived by principal components analysis and reduced rank regression in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2007;85:1615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mikkilä V, Räsänen L, Laaksonen MM, Juonala M, Viikari J, Pietinen P, Raitakari OT. Long-term dietary patterns and carotid artery intima media thickness: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2009;102:1507–12. [DOI] [PubMed] [Google Scholar]
- 18. Kesse-Guyot E, Vergnaud A-C, Fezeu L, Zureik M, Blacher J, Péneau S, Hercberg S, Galan P, Czernichow S. Associations between dietary patterns and arterial stiffness, carotid artery intima-media thickness and atherosclerosis. Eur J Cardiovasc Prev Rehabil. 2010;17:718–24. [DOI] [PubMed] [Google Scholar]
- 19. Liese AD, Nichols M, Hodo D, Mellen PB, Schulz M, Goff DC, D'Agostino RB. Food intake patterns associated with carotid artery atherosclerosis in the Insulin Resistance Atherosclerosis Study. Br J Nutr. 2010;103:1471–9. [DOI] [PubMed] [Google Scholar]
- 20. Hoebeeck L, Rietzschel E, Langlois M, De Buyzere M, De Bacquer D, De Backer G, Maes L, Gillebert T, Huybrechts I. The relationship between diet and subclinical atherosclerosis: results from the Asklepios Study. Eur J Clin Nutr. 2011;65:606. [DOI] [PubMed] [Google Scholar]
- 21. Gardener H, Wright CB, Cabral D, Scarmeas N, Gu Y, Cheung K, Elkind MS, Sacco RL, Rundek T. Mediterranean diet and carotid atherosclerosis in the Northern Manhattan Study. Atherosclerosis. 2014;234:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peñalvo JL, Fernández-Friera L, López-Melgar B, Uzhova I, Oliva B, Fernández-Alvira JM, Laclaustra M, Pocock S, Mocoroa A, Mendiguren JM. Association between a social-business eating pattern and early asymptomatic atherosclerosis. J Am Coll Cardiol. 2016;68:805–14. [DOI] [PubMed] [Google Scholar]
- 23. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 24. Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol. 2011;26:877–86. [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann K, Schulze MB, Schienkiewitz A, Nöthlings U, Boeing H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol. 2004;159:935–44. [DOI] [PubMed] [Google Scholar]
- 26. Schulze MB, Hoffmann K. Methodological approaches to study dietary patterns in relation to risk of coronary heart disease and stroke. Br J Nutr. 2006;95:860–9. [DOI] [PubMed] [Google Scholar]
- 27. DiBello JR, Kraft P, McGarvey ST, Goldberg R, Campos H, Baylin A. Comparison of 3 methods for identifying dietary patterns associated with risk of disease. Am J Epidemiol. 2008;168:1433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer J, Döring A, Herder C, Roden M, Koenig W, Thorand B. Dietary patterns, subclinical inflammation, incident coronary heart disease and mortality in middle-aged men from the MONICA/KORA Augsburg cohort study. Eur J Clin Nutr. 2011;65:800. [DOI] [PubMed] [Google Scholar]
- 29. Melaku YA, Gill TK, Taylor AW, Adams R, Shi Z. A comparison of principal component analysis, partial least-squares and reduced-rank regressions in the identification of dietary patterns associated with bone mass in ageing Australians. Eur J Nutr. 2018;57:1969–83. [DOI] [PubMed] [Google Scholar]
- 30. Sowers MFR, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans DA, Neer R, Matthews KA, Sherman S. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R,eds. Menopause: Biology and Pathology. San Diego, CA: Academic Press; 2000. p. 175–88. [Google Scholar]
- 31. Block G, Thompson F, Hartman A, Larkin F, Guire K. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–93. [PubMed] [Google Scholar]
- 32. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 33. Huang M-H, Schocken M, Block G, Sowers M, Gold E, Sternfeld B, Seeman T, Greendale GA. Variation in nutrient intakes by ethnicity: results from the Study of Women's Health Across the Nation (SWAN). Menopause. 2002;9:309–19. [DOI] [PubMed] [Google Scholar]
- 34. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–21. [DOI] [PubMed] [Google Scholar]
- 35. Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–62. [DOI] [PubMed] [Google Scholar]
- 36. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 37. Thurston RC, Chang Y, Derby CA, Bromberger JT, Harlow SD, Janssen I, Matthews KA. Abuse and subclinical cardiovascular disease among midlife women. Stroke. 2014;45:2246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sutton-Tyrrell K, Kuller LH, Matthews KA, Holubkov R, Patel A, Edmundowicz D, Newman A. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–16. [DOI] [PubMed] [Google Scholar]
- 39. Hall MH, Matthews KA, Kravitz HM, Gold EB, Buysse DJ, Bromberger JT, Owens JF, Sowers M. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32:73–82. [PMC free article] [PubMed] [Google Scholar]
- 40. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 41. Sternfeld B, Ainsworth BE, Quesenberry Jr. C. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. [DOI] [PubMed] [Google Scholar]
- 42. Thurston RC, El Khoudary SR, Sutton-Tyrrell K, Crandall CJ, Gold E, Sternfeld B, Selzer F, Matthews KA. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women's Health Across the Nation. Menopause (New York, NY). 2011;18:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Appelhans BM, Baylin A, Huang M-H, Li H, Janssen I, Kazlauskaite R, Avery EF, Kravitz HM. Beverage intake and metabolic syndrome risk over 14 years: the Study of Women's Health Across the Nation. J Acad Nutr Diet. 2017;117:554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 45. Cattell RB. The scree test for the number of factors. Multivariate Behav Res. 1966;1:245–76. [DOI] [PubMed] [Google Scholar]
- 46. O'Rourke N, Hatcher L. A Step-by-step Approach to using SAS for Factor Analysis and Structural Equation Modeling. 2nd ed Cary, NC: SAS Institute; 2013. [Google Scholar]
- 47. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 48. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 49. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodol). 1995;57:289–300. [Google Scholar]
- 50. Blake G, Ridker P. Inflammatory bio‐markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. [DOI] [PubMed] [Google Scholar]
- 51. Vaughan DE. PAI‐1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–83. [DOI] [PubMed] [Google Scholar]
- 52. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. [DOI] [PubMed] [Google Scholar]
- 53. Thurston RC, El Khoudary SR, Derby CA, Barinas-Mitchell E, Lewis TT, McClure CK, Matthews KA. Low socioeconomic status over 12 years and subclinical cardiovascular disease: The Study of Women's Health Across the Nation. Stroke. 2014;45:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Markus RA, Mack WJ, Azen SP, Hodis HN. Influence of lifestyle modification on atherosclerotic progression determined by ultrasonographic change in the common carotid intima-media thickness. Am J Clin Nutr. 1997;65:1000–4. [DOI] [PubMed] [Google Scholar]
- 55. Merchant AT, Kelemen LE, de Koning L, Lonn E, Vuksan V, Jacobs R, Davis B, Teo KK, Yusuf S, Anand SS. Interrelation of saturated fat, trans fat, alcohol intake, and subclinical atherosclerosis. Am J Clin Nutr. 2008;87:168–74. [DOI] [PubMed] [Google Scholar]
- 56. 2015–2020 Dietary guidelines for Americans. US Department of Health and Human Services. Washington (DC): USDA; 2015. [Google Scholar]
- 57. Newby P, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 58. De Goede J, Soedamah‐Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta‐analysis of prospective cohort studies. J Am Heart Assoc. 2016;5:e002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.