Abstract
Introduction
Liver fibrosis increases progressively with aging and has been associated with poorer cognitive performance in middle‐aged and older adults. We investigated the relationships between a non‐invasive score for advanced liver fibrosis (non‐alcoholic fatty liver disease [NAFLD] fibrosis score [NFS]) and dementia risk. We also assessed physical frailty, a common geriatric condition which is associated to dementia. We tested the joint effects of physical frailty and fibrosis on dementia incidence.
Methods
A total of 1061 older adults (65 to 84 years), from the Italian Longitudinal Study on Aging, were prospectively evaluated for the risk of dementia in a period between 1992 and 2001. Liver fibrosis was defined according to the NFS. Physical frailty was assessed according to the Fried's criteria. Cox proportional hazards models were used to estimate the short‐ and long‐term risk of overall dementia, associated to the NFS, testing the effect modifier of physical frailty status.
Results
Older adults with only high NFS (F3‐F4) did not exhibit a significant increased risk of overall dementia. Over 8 years of follow‐up, frail older adults with high NFS had an increased risk of overall dementia (hazard ratio [HR]: 4.23; 95% confidence interval [CI]: 1.22 to 14.70, P = .023). Finally, physically frail older adults with low albumin serum levels (albumin < 4.3 g/dL) and with advanced liver fibrosis (F3‐F4 NFS) compared to those with lower liver fibrosis score (F0‐F2 NFS) were more likely to have a higher risk of overall dementia in a long term‐period (HR: 16.42; 95% CI: 1.44 to 187.67, P = .024).
Discussion
Advanced liver fibrosis (F3‐F4 NFS) could be a long‐term predictor for overall dementia in people with physical frailty. These findings should encourage a typical geriatric, multidisciplinary assessment which accounts also for the possible co‐presence of frail condition in older adults with chronic liver disease and liver fibrosis.
Keywords: dementia, frailty, NAFLD fibrosis score, older adults, population‐based study
1. INTRODUCTION
Chronic liver diseases (CLDs) are a major cause of multimorbidity and mortality worldwide. Data from the Third National Health and Nutrition Examination Survey (NHANES‐III), conducted in the U.S. population between 1988 and 2008, showed that the prevalence of CLDs increased over time from 12% to 15%. 1 In Western countries, this tendency is mainly driven by rising impact of non‐alcoholic fatty liver disease (NAFLD). The number of patients affected by this condition is expected to further increase due to population aging. In fact, NAFLD is highly recognized in older adults and its prevalence increases with age reaching 30% in the seventh decade. 2 This can be explained by the accumulation, during aging, of many risk factors which on turn predispose to NAFLD (ie, obesity, dyslipidemia, type 2 diabetes, metabolic syndrome). 3 Although natural history of CLDs encompasses progression to cirrhosis and hepatocellular carcinoma, not all the affected subjects experience such complications. It seems that liver fibrosis is the main determinant factor of disease progression; indeed, people with a higher degree of liver fibrosis are more prone to poorer long‐term outcomes. 4 A recent population‐based study demonstrated that liver fibrosis is largely unrecognized in subjects with previously unknown liver disease. 5 Moreover, the rate of liver fibrosis increased with age. 5
It is becoming clear that the relevance of this condition extends far beyond the liver and cardiovascular multimorbidity and mortality. It has been shown that subjects with cirrhosis or CLDs are more likely to be pre‐frail or frail compared to those without CLDs. 6 Frailty is a common geriatric condition which may predispose to future dementia. 7 Recent evidence from the Framingham study showed that higher levels of liver fibrosis are related to poor cognitive performance in middle‐aged and older adults. 8 However, it is conceivable that this relationship could be moderated in part by the presence of physical frailty rather than traditional vascular and metabolic risk factors. Therefore, we hypothesized that liver fibrosis and frailty in older adults might be interacting aspects determining an increased dementia risk.
Today, the gold standard to detect liver fibrosis remains liver biopsy; however, biopsy presents some glaring limitations (eg, high cost, procedure‐related risk, sampling error) that do not allow the use as screening tool in general population, and, in particular, among older adults. Non‐invasive tests of liver fibrosis might represent a feasible tool for earlier diagnosis in community‐based settings. 9 Among these tools, the NAFLD fibrosis score (NFS) is a simple and well‐validated instrument which accurately stratifies subjects with NAFLD based on risk of liver fibrosis. 10 , 11 When applied in community‐dwelling older adults, NFS also exhibits good diagnostic accuracy, 12 , 13 , 14 and is able to predict adverse health outcomes such as disability and mortality. 15 In the present study, we examined the relationship between NFS and the incidence of dementia, also investigating the role of physical frailty as a possible effect modifier in a large Italian population‐based sample.
2. METHODS
2.1. Participants
Participants of this study were enrolled from a large population‐based study, the Italian Longitudinal Study on Aging (ILSA), promoted by the Italian National Research Council‐CNR‐Targeted Project on Aging. More specific information on ILSA data collection have been reported elsewhere. 16 A sample of 5632 subjects aged 65 to 84 years, independent or institutionalized, was randomly selected from the electoral rolls of eight Italian municipalities, after stratification for age and sex. The data of the present study have been obtained during the first prevalence survey study between March 1992 and June 1993, the second prevalence survey study between September 1995 and October 1996, and the third prevalence survey study between March 2000 and September 2001. Participants with known history of viral hepatitis were excluded from this analysis. From the original study population (N = 5632), we selected 4521 potentially eligible participants. Thereafter, we excluded patients who did not undergo frailty screening (N = 1830). From the remaining 2691 subjects, we removed those with any missing data in variables necessary for the computation of the NFS (N = 1630), resulting in a final sample size of 1061.
2.2. Standard protocol approvals, registrations, and patient consents
Approval was received by the Institutional Review Board of the eight municipalities of the ILSA. Informed consent was obtained from all subjects and/or their relatives before enrollment.
2.3. Clinical examination and laboratory analyses
Comorbidities including coronary artery disease (myocardial infarction or angina pectoris), congestive heart failure, type 2 diabetes mellitus, hypertension, and stroke were identified with a two‐phase procedure, using clinical criteria described in detail elsewhere. 16 Metabolic syndrome was diagnosed according to the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP‐ATP III) criteria. 17 Based on self‐reports, smoking habits were categorized as “ever” or “never” smoker, and the variable “pack‐years cigarettes” (number of years smoked × [usual number of cigarettes smoked per day/20 cigarettes per pack]) was generated to represent the total smoking exposure. We collected information on alcohol consumption by food frequency questionnaires asking participants how many drinks they had consumed per day in the previous year, according to three categories (1) one glass (equal to 0.125 L), (2) two glasses (equal to 0.25 L), (3) four glasses (equal to 0.50 L). These data were transformed in drink as reported elsewhere. 18 Body mass index (BMI) was calculated as weight/height2 (kg/m2). Fasting blood glucose (FBG), and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured, in blood samples, early in the morning after a 13‐hour overnight fast. Serum albumin was measured by electrophoresis. Serum concentrations of apolipoproteins B and A‐I were determined by the nephelometric method with a Behring Nephelometer 100 Analyzer (Behring, Marburg, Germany). Functional status was assessed with the Instrumental Activities of Daily Living (IADL) scale. 19 Depressive symptoms were investigated using the Italian version of the 30‐item Geriatric Depression Scale (GDS‐30). 20 Physical frailty has been retrospectively assessed according to modified Fried's criteria and using the operationalized criteria. 21 The Charlson comorbidity index, a weighted index that takes into account the number and the seriousness of comorbid disease, was calculated. 22
RESEARCH IN CONTEXT
Systematic review: We searched electronic databases (MEDLINE, Embase, Scopus, and Web of Science) for manuscripts published in English, from database inception to January 31, 2020 using the following search terms: “liver fibrosis” and “non‐alcoholic fatty liver disease” and (“cognitive function” or “dementia”). Previous study reported limited association between chronic liver diseases, including non‐alcoholic fatty liver disease (NAFLD), and incident dementia, but reduced cognitive performance has been observed in older adults with advanced liver fibrosis. On the other hand, degree of liver fibrosis is strongly related with physical frailty and both conditions are associated with worse clinical outcomes. Therefore, liver fibrosis and frailty in older adults might be interacting aspects determining an increased dementia risk. To the best of our knowledge, no one study investigated the effect of liver fibrosis on dementia risk in older adults, bearing in mind also the role of co‐occurring frail condition.
Interpretation: In a large 8‐year population‐based longitudinal study, enrolling 1061 older adults (65 to 84 years old), we retrospectively assessed the degree of liver fibrosis using the Angulo's NAFLD fibrosis score (NFS). We observed that liver fibrosis per se was not related to incident dementia. Only frail older adults with advanced liver fibrosis had an increased risk of dementia.
Future directions: Further prospective studies should verify whether frailty could have an independent role in the progression of liver disease and in the long‐term prognosis of older adults with chronic liver diseases, also using a more accurate assessment of liver fibrosis (eg, transient elastography). If our findings are confirmed, randomized controlled trials are warranted to test if interventions acting on frailty conditions or potential underlying common mechanisms with liver fibrosis (eg, albumin deficit, inflammation, thrombosis) could reduce the risk of dementia in older adults.
2.4. Neuropsychological evaluation and classification of dementia
Mini‐Mental State Examination (MMSE) was used to evaluate global cognitive function (orientation, attention, immediate and delayed verbal memory, constructional praxis, and language). 23 A complete neuropsychological battery was administered for evaluating episodic memory and selective attention, comprehensive of the diagnosis of mild cognitive impairment, as detailed elsewhere. 16 Episodic memory was tested with the Babcock Story Recall Test (BSRT; score ranging from 0 to 16). 24 , 25 This test measures immediate and delayed recall, using a 21‐unit story. For the purpose of scoring, an event‐weighted, hierarchical system was used, based upon the degree of organization of the recollection provided by the subject. Selective attention was assessed by the Digit Cancellation Test (DCT; score ranging from 0 to 60). 24 This test has a basic format with three different matrices made up of 13 strings of 10 digits (zero to nine in random sequence), each line including up to five targets. Digits are required to be crossed out within a time limit (45 seconds/matrix). The finding strategy of dementia cases was described in detail elsewhere. 16 Briefly, the diagnosis was based on the Diagnostic and Statistical Manual of Mental Disorders, Third Edition Revised criteria for dementia syndrome; 26 the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association criteria for possible and probable Alzheimer's disease (AD); 27 and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision criteria for vascular dementia (VaD). 28
2.5. Liver fibrosis assessment
Severity of liver fibrosis was estimated by NFS, which combines clinical and serum markers, obtained at first survey, using the following algorithm: −1.675 + 0.037 × age [years] + 0.094 × BMI [kg/m2] + 1.13 × impaired fasting glucose (IFG) or type 2 diabetes [yes = 1, no = 0] + 0.99 × AST to ALT ratio—0.013 × platelet [×109/L]—0.66 × albumin [g/dl]. 10 Participants were characterized into three categories based on previously published cut‐offs: high probability of advanced fibrosis (F3‐F4) with NFS >0.676, indeterminate probability of advanced fibrosis (−1.455 < NFS ≤ 0.676), and low probability of advanced fibrosis (F0‐F2) with NFS <−1.455. 10
2.6. Statistical analysis
All analyses were performed using STATA 16 statistical software (StataCorp, 2019, Stata Statistical Software: Release 16, College Station, TX: StataCorp LLC). Sociodemographics, and clinical and laboratory parameters were evaluated by the three NFS categories (F0‐F2, Indeterminate, and F3‐F4) using the Kruskall‐Wallis method due to the high heteroskedasticity among the compared groups. Pairwise comparisons have been analyzed by the Mann‐Whitney method and the P value has been set at .017 (0.05/3 = 1.7%). Time to new events of overall dementia, in the period between 1992 and 1995 (first survey to second survey) and in the period between 1992 and 2001 (first survey to third survey) were modelled separately using Cox proportional hazards. The proportional hazard assumption over time for the covariates of interest was checked including in the Cox model each covariate by time as a predictor variable. Collinearity between NFS and parameters involved in the statistical models (demographic and clinical characteristics) was investigated by Spearman's ρ coefficients setting its values ≥0.80. Partially adjusted models (adjusted for age, sex [coded 0 for women as reference group and 1 for men], metabolic syndrome [coded 0 if not affected, coded 1 if affected]), and intake of alcohol in drink as a categorical variable (no alcohol intake coded = 1, ≤1 drink/day coded = 2, one or more drinks but ≤2 drinks/day coded = 3, >2 drinks/day coded = 4), identified as models 1, were estimated for each outcome. As models 2 were identified the fully adjusted models which considered the variables in model 1 plus education, pack‐years (coded 0 for pack‐years cigarettes = 0 [never smoking], and 1 for pack‐years cigarettes ≥0.5), MMSE score at baseline, Charlson Comorbidity Index, albumin serum levels, and apolipoprotein B:apolipoprotein A‐I ratio. In considering which variables should be retained in the models we considered the hierarchy principle according to modelling strategy with three stages: variable specification, interaction assessment, and confounding assessment. If a product variable is retained in the model, then all lower‐order components of that variable must be retained in the model. 29 In deciding to retain NFS scores x physical frailty product in the models, we compared the log likelihood statistics of the models with and without those with the interaction term by a likelihood ratio test. These evaluations were calculated also by low serum levels of albumin (<4.3 g/dL coded 1 median value). To check the proportional hazard assumption over time for the covariates for each type of event, we included in the Cox model each covariate by time as a predictor variable. Interaction between NAFLD fibrosis score and physical frailty was estimated. To examine the interaction between frailty and CLD on dementia risk the comparisons of the hazards between groups were estimated by LINCOM procedure in Stata. The statistical significance threshold was set at 5%.
2.7. Data availability
Any anonymized data not published within the article will be shared by request from the corresponding author.
3. RESULTS
The sociodemographic and clinical characteristics (mean ± standard deviation [SD] or %) of subjects across the three NFS categories (“F0‐F2,” “Indeterminate,” and “F3‐F4”) are shown in Table 1. NFS is a composite score but any collinearity was identified with the parameters included in the statistical models (Spearman's ρ coefficients in Table 1). Figure 1 shows the sample size and attrition of the study population. The total analysis time at risk and under observation were in the short‐term period 4272 persons/year, while in the long‐term period were 8769 persons/year. The incident cases of overall dementia in the first period were 51 (31 AD, 29 VaD, and 12 other dementias), while in the second period were 90 (41 AD, 31 VaD, and 18 other dementias). Differences in age (72.39 ± 5.34 vs 74.69 ± 5.74, P < .01 evaluated by separate variance t test) and sex (Pearson χ2 = 16.7, P < .01) were observed between participants and non‐participants (1061 participants: 588 [20.88%] men and 473 [16.80%] women; 4571 non‐participants: 2228 [79.12%] men and 2343 [83.20%] women). The mean (SD) age of the participants at baseline was 72.39 (5.34) years, 44.58% were women, and 6.6% were frail (out of them, 13.5 were in the F3‐F4 NFS category). Compared to participants with F0‐F2 NFS, those with F3‐F4 NFS were more likely to be men (63.83%), with higher BMI and more frail (Table 1). In this study, the prevalence of the highest NFS category was 15.66% (95% confidence interval [CI], 13.39 to 18.23) and it increased with age (65 to 69 years: 7.63%, 95% CI 5.34% to 10.79%; 69 to 74 years: 11.04%, 95% CI 8.05 to 14.96; 75 to 79 years: 17.54%, 95% CI 13.13% to 23.04%; and 80 to 84 years: 25.65%, 95% CI 19.50% to 33.00%; test for trend odd ratio [OR]: 1.65, 95% CI: 1.39 to 1.95) and it was higher in men than in women (men: 18.46%, 95% CI: 15.25% to 22.16% and women: 12.02%, 95% CI: 9.22% to 15.52%; OR: 1.65, 95% CI: 1.04 to 2.17). Moreover, F3‐F4 NFS category was associated to physical frailty independently by age (OR: 1.96, 95% CI: 1.07 to 3.58) and sex (OR: 2.99, 95% CI: 1.66 to 5.38).
TABLE 1.
Demographic and clinical characteristics (mean [standard deviation] or %) of individuals by non‐alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) categories in the Italian Longitudinal Study on Aging (first prevalence survey, 1992 to 1993)
| Entire sample (n = 1061) | NFS F0‐F2 (n = 303) | NFS indeterminate (n = 617) | NFS F3‐F4 (n = 141) | P value | Spearman's ρ coefficients | |
|---|---|---|---|---|---|---|
| Women (%) | 473 (44.58) | 146 (48.18) | 276 (44.81) | 51 (36.17) | .060 | 0.07 # |
| Age (y) | 72.39 (5.34) | 70.64 (4.85) | 72.70 (5.28) ‡ | 74.76 (5.48) * , † | .0001 | 0.23 # |
| Education (y) | 6.81 (5.00) | 8.13 (5.62) | 6.30 (4.55) ‡ | 6.15 (4.95) * | .0001 | −0.14 * |
| Pack‐years | 17.24 (26.16) | 16.41 (24.42) | 17.52 (26.96) | 17.83 (26.36) | .8734 | −0.03 |
| Alcohol consumption (%) | 192 (18.20) | 63 (20.86) | 109 (17.78) | 20 (14.29) | .6719 | 0.01 |
| BMI (kg/m2) | 26.94 (4.35) | 25.54 (3.60) | 27.17 (4.20) ‡ | 28.97 (5.36) * , † | .0001 | 0.23 * |
| Mini‐Mental State Examination | 26.10 (3.93) | 26.65 (3.91) | 26.06 (3.74) | 25.06 (4.53) * , † | .0001 | −0.13 * |
| Instrumental activities of daily living | 8.04 (3.25) | 7.68 (2.80) | 8.00 (3.06) | 8.96 (4.52) * , † | .0515 | 0.08 # |
| Geriatric Depression Scale‐30 | 8.96 (5.69) | 8.41 (5.65) | 9.35 (5.63) | 8.47 (5.94) | .005 | 0.03 |
| Charlson Comorbidity Index | 0.94 (0.80) | 0.87 (0.81) | 0.95 (0.78) | 1.04 (0.87) | .5041 | −0.01 |
| Hypertension (%) | 588 (55.42) | 147 (48.51) § | 369 (59.81) ¶ | 72 (51.06) | .003 | 0.05 |
| Type 2 diabetes (%) | 126 (12.33) | 48 (16.49) § | 61 (10.30) | 17 (12.23) | .031 | −0.06 |
| Coronary artery disease (%) | 104 (9.80) | 32 (10.56) | 60 (9.72) | 12 (8.51) | .792 | −0.03 |
| Stroke (%) | 12 (1.17) | 4 (1.37) | 5 (0.84) | 3 (2.16) | .404 | 0.01 |
| Congestive heart failure (%) | 92 (8.67) | 22 (7.26) | 49 (7.94) | 21 (14.89) § | .018 | 0.06 |
| Metabolic syndrome (%) | 450 (42.41) | 115 (37.95) | 271 (43.92) | 64 (45.39) | .169 | 0.07 # |
| Albumin (mg/dL) | 4.31 (0.41) | 4.49 (0.40) | 4.27 (0.39) ‡ | 4.10 (0.39) * , † | .0001 | −0.30 * |
| B/A1 | 0.88 (0.43) | 0.92 (0.53) | 0.88 (0.40) | 0.84 (0.25) | .2688 | −0.05 |
| Physical Frailty (%) | 65 (6.6) | 14 (4.86) | 33 (5.78) | 18 (13.53) | .002 | 0.11 * |
Abbreviations: B/A1, apolipoprotein B to apolipoprotein A1 ratio; BMI, body mass index.
Bonferroni adjusted between‐groups comparison, NFS F3‐F4 versus NFS F0‐F2: P < .01.
Bonferroni adjusted between‐groups comparison, NFS F3‐F4 versus NFS indeterminate: P < .01.
Bonferroni adjusted between‐groups comparison, NFS indeterminate versus NFS F0‐F2: P < .01.
P < .01666, with Bonferroni adjusted comparison.
P < .0033, with Bonferroni adjusted comparison.
P < .05, ** P < .01 values for Spearman's ρ coefficients between NFS categories and demographic and clinical characteristics.
FIGURE 1.
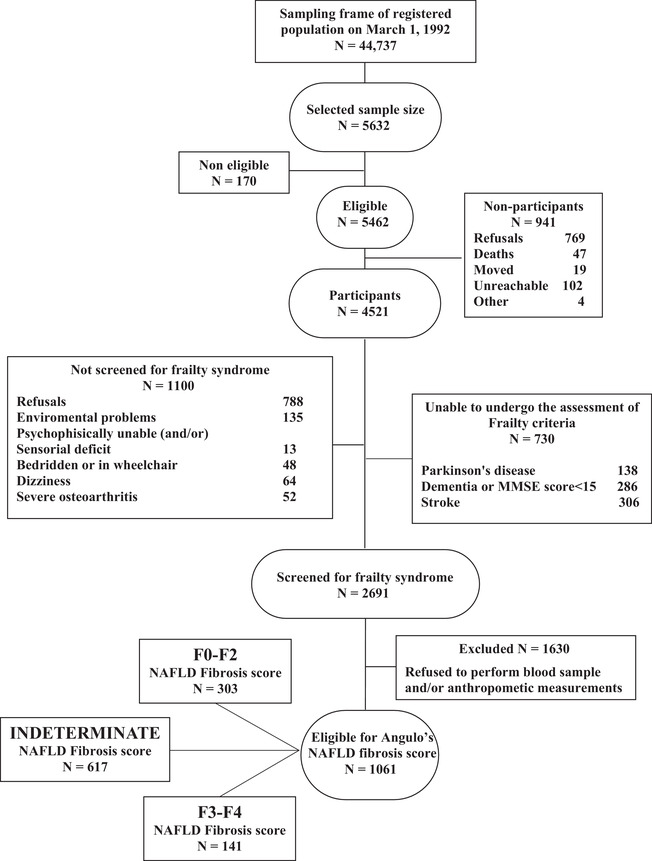
Attrition of the study population at the different phases of the survey in assessing the role of non‐alcoholic fatty liver disease fibrosis score on incident dementia. The Italian Longitudinal Study on Aging, first prevalence survey, 1992 to 1993
In physically frail older adults, the incidence rates (the number of events per 1000 person‐years at risk) of overall dementia for the highest NFS category (F3‐F4 NFS) was roughly five times that in those with the lowest NFS category (Table 2). The physically frail older individuals with higher liver fibrosis score compared to those with lower liver fibrosis score were more likely to have a higher risk of overall dementia in a long‐term period (hazard ratio [HR]: 4.23; 95% CI: 1.22 to 14.70), but not in the short‐term period in a multivariate adjusted model (Table 3). Finally, physically frail older adults with low albumin serum levels (albumin <4.3 g/dL) and with advanced liver fibrosis (F3‐F4 NFS) compared to those with lower liver fibrosis score (F0‐F2 NFS) were more likely to have a higher risk of overall dementia in a long‐term period (HR: 16.42: 95% CI: 1.44 to 187.67, P = .024; Table 3).
TABLE 2.
Incidence rate of overall dementia (per 1000 persons/year) in older adults with (non‐alcoholic fatty liver disease [NAFLD] fibrosis score [NFS] F3‐F4) and without advanced liver fibrosis (NFS F0‐F2) by physical frailty and by low albumin serum levels. The Italian Longitudinal Study on Aging (first and second surveys, 1992 to 1996 and first and third survey, 1992 to 2001)
| New events (1992 to 1995) | Rate | 95% CI | New events (1992 to 2001) | Rate | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Physical frailty | NFS no | 6 | 16.81 | 5.42–52.11 | 7 | 11.24 | 4.22–29.94 |
| NFS yes | 7 | 45.23 | 16.98–120.51 | 11 | 49.87 | 24.94–99.71 | |
| Without physical frailty | NFS no | 29 | 7.58 | 5.12–11.22 | 60 | 8.10 | 6.22–10.56 |
| NFS yes | 9 | 13.49 | 6.06–30.02 | 12 | 9.97 | 5.19–19.16 | |
| Physical frailty by albumin serum level <4.3 g/dL | NFS no | 1 | 9.79 | 1.38–69.48 | 3 | 4.61 | 0.65–32.73 |
| NFS yes | 4 | 56.35 | 21.15–150.13 | 6 | 45.17 | 20.29–100 |
Abbreviation: CI, confidence interval.
TABLE 3.
Hazard ratios (HRs) of incident overall dementia associated to non‐alcoholic fatty liver disease (NAFLD) fibrosis score (NFS) by physical frailty and by low albumin serum levels. The Italian Longitudinal Study on Aging (first and second surveys, 1992 to 1996 and first and third survey, 1992 to 2001)
| New events (N°) | Model 1 HRs partially adjusted (95% CI) a | P value | Model 2 HRs fully adjusted (95% CI) b | P value | ||
|---|---|---|---|---|---|---|
| Overall dementia (1992 to 1995) | 51 | Fibrosis | 1.19 (0.48–2.97) | .71 | 1.09 (0.43–2.78) | .86 |
| Physical frailty | 1.31 (0.39–4.40) | .67 | 0.84 (0.23–2.99) | .78 | ||
| Fibrosis × physical frailty | 1.80 (0.31–10.46) | .51 | 1.91 (0.31–11.85) | .49 | ||
| NFS yes/physical frailty yes | 2.14 (0.47–9.75) | .32 | 2.08 (0.42–10.33) | .37 | ||
| Dementias by albumin serum levels <4.3 g/dL (1992 to 1995) | 28 | Fibrosis | 2.03 (0.71–5.81) | .19 | 2.05 (0.71–5.97) | .19 |
| Physical frailty | 0.78 (0.1–6.18) | .81 | 0.46 (0.05–4.34) | .49 | ||
| Fibrosis × frailty | 1.95 (0.17–22.30) | .59 | 2.54 (0.17–37.13) | .50 | ||
| NFS yes/physical frailty yes | 3.94 (0.42–36.88) | .23 | 5.21 (0.46–58.61) | .18 | ||
| Dementias (1992 to 2001) | 90 | Fibrosis | 0.87 (0.42–1.78) | .70 | 0.91 (0.44–1.88) | .80 |
| Physical frailty | 0.82 (0.29–2.33) | .71 | 0.73 (0.25–2.09) | .56 | ||
| Fibrosis × physical frailty | 4.79 (1.16–19.80) | .030 | 4.64 (1.11–19.43) | .036 | ||
| NFS yes/physical frailty yes | 4.15 (1.21–14.31) | .024 | 4.23 (1.22–14.70) | .023 | ||
| Dementias by Albumin serum level <4.3 g/dL (1992 to 2001) | 47 | Fibrosis | 0.90 (0.39–2.11) | .82 | 1.02 (0.43–2.41) | .97 |
| Physical frailty | 0.15 (0.02–1.50) | .11 | 0.13 (0.01–1.28) | .08 | ||
| Fibrosis × physical frailty | 19.09 (1.51–241.68) | .023 | 16.14 (1.27–205.17) | .032 | ||
| NFS yes/physical frailty yes | 17.24 (1.51–197.0) | .022 | 16.42 (1.44–187.67) | .024 |
Model 1: HRs were adjusted for: age, sex (coded 0 for women and 1 for men), metabolic syndrome (coded 0 if not affected, coded 1 if affected), and alcohol intake (no alcohol intake coded = 1, ≤1 drink/day coded = 2, 1 or more drinks but ≤2 drinks/day coded = 3, >2 drinks/day coded = 4).
Model 2: HRs were adjusted for: Model 1 plus education, pack‐years cigarettes (coded 0 for pack‐years cigarettes = 0 (never smoking), and 1 for pack‐years cigarettes ≥0.5), Mini‐Mental State Examination score at baseline, Charlson Comorbidity Index score, apolipoprotein B:apolipoprotein A‐I ratio, albumin serum level (this last confounder has been excluded in the stratification by albumin levels).
Abbreviation: CI, confidence interval.
4. DISCUSSION
In the present study, during 8‐year follow‐up, we observed that among physically frail older individuals the presence of advanced liver fibrosis (F3‐F4 NFS) was associated to about four times increased risk of overall dementia in comparison to a lower liver fibrosis score. The HR of overall dementia increased by about 16 times in physically frail older adults with low albumin serum levels (albumin <4.3 g/dL).
Epidemiological data showed that the presence of liver fibrosis, assessed using non‐invasive tests as the NFS, may predict multimorbidity and mortality in people with known CLD, 30 , 31 as well as in general population. 15 The estimated prevalence among older adults of NFS F3‐F4 ranged from 7.8% to 18.9%. 13 , 14 , 15 Consistently, in the present large population‐based sample of Italian older adults, we found an overall prevalence of advanced fibrosis of 15.66%. To the best of our knowledge, this is the first study investigating the relationship between liver fibrosis and the risk of dementia and their relationship with physical frailty. Previous data from a large Korean population‐based study highlighted that presence of CLDs in older adults predisposed individuals to slightly higher dementia risk over 10‐year follow‐up. 32 Also in the general population, abnormal liver function tests (higher AST‐to‐ALT ratio and reduced ALT) have been associated with cognitive dysfunction and AD‐related biomarkers. 33 Intriguingly, in a cross‐sectional analysis from the Framingham study, Weinstein et al. showed that NAFLD per se did not influence cognitive performance in middle‐aged and older adults. 8 However, they found that subjects with NAFLD and higher degree of liver fibrosis, assessed with NFS, showed worse executive function and abstract reasoning. 8 Despite this evidence, less is known about potential underlying mechanisms acting in the relationship between liver fibrosis and occurrence of cognitive disorders.
We found that the risk of overall dementia is further increased in physically frail older people with advanced liver fibrosis who reported lower serum albumin levels (<4.3 g/dL). Albumin is a well‐known biomarker related with both physical frailty, as consequence of malnutrition, 34 and with impaired liver biosynthetic capacities as observed in advanced liver fibrosis. 35 It has also been demonstrated that lower albumin levels predict higher risk of incident dementia in community‐dwelling older adults. 36 Albumin is an important carrier protein which is involved in amyloid beta (Aβ) brain deposition through stabilization of Aβ monomers and inhibition of their polymerization. 37 Recent findings showed that albumin binding capacity is significantly and progressively reduced in NAFLD patients. 38 Hence, lower albumin levels and function might contribute to Aβ burden and the development of AD. Also, other mechanisms could explain the link between liver fibrosis and physical frailty and the development of dementia. For example, a dysregulation of immune and coagulation systems occurs in liver fibrosis 39 , 40 as well as during aging and is particularly prominent in frail individuals. 41 Experimental studies suggest that liver fibrosis is characterized by pro‐inflammatory and pro‐thrombotic state. It has been speculated that hepatocyte senescence may induce an infiltration of liver parenchyma with inflammatory cells, mainly a cluster of differentiation 4+ (CD4+) lymphocytes and macrophages, 42 which could increase the susceptibility to fibrosis during aging. 40 , 43 Although it is well known that CLDs are associated with increased risk of vascular diseases, hypercoagulability is often unrecognized. 39 Pro‐thrombotic state might predispose individuals to further liver damage with fibrosis progression, a process also known as parenchymal extinction. 44 It is conceivable that, as happens in the liver, pro‐thrombotic state may induce micro‐infarction also in the brain causing white matter injury. Indeed, Petta et al. found that white matter lesions were not associated with NAFLD per se but rather with advanced liver fibrosis, observing a three‐times risk of vascular lesions in subjects with F2‐F4 fibrosis stage at transient elastography. 45
In older adults, the inappropriate activation of pro‐inflammatory and pro‐thrombotic pathways leads to a catabolic cascade and a progressive worsening of physical frailty. 41 Accumulating evidence shows that physical frailty might represent a peculiar tract of advanced liver disease among older adults. 6 , 15 In the present population, there was a stepwise increase of the physical frailty prevalence from 4.86% to 13.3% as the liver fibrosis progressed. A similar trend was reported in a population of 962 older subjects from the InCHIANTI study, with a physical frailty prevalence of 18.7% in the F3‐F4 NFS category. 15 Recently, Wang et al. proposed a liver frailty index (LFI), which assesses physical frailty based on grip strength, chair stands, and balance. 6 The authors showed that people with cirrhosis, or just with CLD but without cirrhosis, had significantly higher LFI scores compared to subjects without liver disease. Older adults with CLD also had significantly lower grip strength compared to those without liver disease. 6 Sarcopenia, a condition which shares some components with physical frailty characterized by low muscle strength and the presence of low muscle quantity or quality, has been shown to be related with liver cirrhosis and reduced quality of life. 46 Previous studies have reported that people with higher degrees of liver fibrosis also had lower levels of physical activity. 8 , 15 Indeed, international consensus highlights that physical frailty assessment could be a useful step in the evaluation of liver transplantation candidates and to guide therapeutic decisions in these subjects. 47
A major strength of our preliminary exploratory study was the assessment of liver fibrosis in a large population‐based cohort prospectively evaluated that allowed the determination of a temporal sequence between fibrosis score and dementias, hence cause‐effect inferences could be made. Moreover, our study included comprehensive geriatric assessment concerning validated cognitive and functional as well as laboratory tests. There are also several limitations. NAFLD fibrosis was based neither on ultrasound, nor HCV testing, nor on a sum of the biopsy's individual scores but on a score derived from the Angulo's algorithm. Upscaling in subjective under‐reporting of alcohol consumption, though it could be important, was not investigated in our older population because we consider it more relevant in young people and adults and only in a small part in older age. The sensitivity of the NFS is low (51% for the highest cut‐off point >0.676); 10 thus, a proportion of participants identified as having advanced fibrosis was misclassified. We expect this to result in diminution of the association. Finally, the number of participants with the highest risk for advanced fibrosis and, consequently, the new events of overall dementia, were small; thus, statistical power was limited.
5. CONCLUSIONS
Our findings suggest that liver fibrosis could be a potentially modifiable risk factor determining incident dementia in patients with physical frailty. It is conceivable that presence of a common geriatric condition such as frailty could have more weight rather than traditional risk factors in the progression of liver disease and in the long‐term prognosis of older adults with CLDs. These findings should encourage a routine assessment of physical frailty in older adults with known or suspected CLDs. Randomized controlled trials are warranted to verify if strategies acting on physical frailty or potential underlying common mechanisms with liver fibrosis (eg, albumin deficiency, inflammation, thrombosis) could have a beneficial effect on liver fibrosis itself as well as reduce the risk of dementia.
AUTHOR CONTRIBUTIONS
VS conceived and designed the study; VS and CC interpreted the data and wrote the manuscript; VS performed the statistical analysis and takes responsibility for the integrity of the data, the accuracy of data analysis, and had full access to all of the data of the study; NN, GP, FL, AC, BPI, DS, ML, and FP assisted in literature search, interpretation of data, and manuscript preparation; ES, LG, CG, MB, ADC, and DI assisted in study design and data interpretation; AP and CS critically revised the manuscript. All authors approved the final draft submitted. The present article was endorsed by Fondazione Dieta Mediterranea, Ostuni, Italy.
CONFLICTS OF INTEREST
The authors report no competing interests.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
APPENDIX 1.
The ILSA Working Group
Istituto Superiore di Sanità, Rome: E. Scafato, MD (Scientific Coordinator); G. Farchi, MSc; L. Galluzzo, MA; C. Gandin, MD. University of Bari: A. Capurso, MD; F. Panza, MD, PhD; V. Solfrizzi, MD, PhD; V. Lepore, MD; P. Livrea, MD. University of Catania: L. Motta, MD; G. Carnazzo, MD; M. Motta, MD; P. Bentivegna, MD. Italian National Research Centre on Aging, Fermo: S. Bonaiuto, MD; G. Cruciani, MD; D. Postacchini, MD. University of Firenze: D. Inzitari, MD; L. Amaducci, MD. Italian National Research Council, Firenze: A. Di Carlo, MD; M. Baldereschi, MD. University of Genova: C. Gandolfo, MD; M. Conti, MD. San Raffaele Institute, Milan: N. Canal, MD; M. Franceschi, MD. University of Milano: G. Scarlato, MD; L. Candelise, MD; E. Scapini, MD. University of Napoli: F. Rengo, MD; P. Abete, MD; F. Cacciatore, MD. University of Padova: G. Enzi, MD; L. Battistin, MD; G. Sergi, MD; G. Crepaldi, MD. Italian National Research Council, Aging Section, Padova: S. Maggi, MD; N. Minicucci, MD; M. Noale, MD. Institute of Hygiene, University of Padova: F. Grigoletto, ScD; E. Perissinotto, ScD. Università Cattolica del Sacro Cuore, Rome: P. Carbonin, MD.
Solfrizzi V, Scafato E, Custodero C, et al. Liver fibrosis score, physical frailty, and the risk of dementia in older adults: the Italian Longitudinal Study on Aging. Alzheimer's Dement. 2020;6:e12065 10.1002/trc2.12065
See the Appendix for members of the ILSA Working Group.
REFERENCES
- 1. Younossi ZM, Stepanova M, Afendy M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524‐530.e1. quiz e60. [DOI] [PubMed] [Google Scholar]
- 2. Bertolotti M, Lonardo A, Mussi C, et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185‐14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frith J, Day CP, Henderson E, Burt AD, Newton JL. Non‐alcoholic fatty liver disease in older people. Gerontology. 2009;55:607‐613. [DOI] [PubMed] [Google Scholar]
- 4. Angulo P, Kleiner DE, Dam‐Larsen S. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389‐397 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caballeria L, Pera G, Arteaga I, et al. High prevalence of liver fibrosis among european adults with unknown liver disease: a population‐based study. Clin Gastroenterol Hepatol. 2018;16:1138‐1145.e5. [DOI] [PubMed] [Google Scholar]
- 6. Wang CW, Lebsack A, Chau S, Lai JC. The range and reproducibility of the liver frailty index. Liver Transpl. 2019;25:841‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community‐dwelling older people: a systematic review and meta‐analysis. J Am Med Dir Assoc. 2016;17:881‐888. [DOI] [PubMed] [Google Scholar]
- 8. Weinstein G, Davis‐Plourde K, Himali JJ, Zelber‐Sagi S, Beiser AS, Seshadri S. Non‐alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle‐aged adults: the Framingham study. Liver Int. 2019;39(9):1713‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris R, Harman DJ, Card TR, Aithal GP, Guha IN. Prevalence of clinically significant liver disease within the general population, as defined by non‐invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol Hepatol. 2017;2:288‐297. [DOI] [PubMed] [Google Scholar]
- 10. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 11. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med. 2011;43:617‐649. [DOI] [PubMed] [Google Scholar]
- 12. Patel YA, Gifford EJ, Glass LM, et al. Identifying nonalcoholic fatty liver disease advanced fibrosis in the veterans health administration. Dig Dis Sci. 2018;63:2259‐2266. [DOI] [PubMed] [Google Scholar]
- 13. Hartleb M, Baranski K, Zejda J, Chudek J, Wiecek A. Non‐alcoholic fatty liver and advanced fibrosis in the elderly: results from a community‐based Polish survey. Liver Int. 2017;37:1706‐1714. [DOI] [PubMed] [Google Scholar]
- 14. Veysey M, Siow W, Niblett S, King K, Yates Z, Lucock M. Hepatic fibrosis in an elderly population. J Gastroenterol Hepatol. 2014;29:87. [Google Scholar]
- 15. De Vincentis A, Costanzo L, Vespasiani‐Gentilucci U, et al. Association between non‐invasive liver fibrosis scores and occurrence of health adverse outcomes in older people. Dig Liver Dis. 2019;51(9):1330‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882‐1891. [DOI] [PubMed] [Google Scholar]
- 17. Expert Panel on Detection E, Treatment of High Blood Cholesterol in A . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486‐2497. PMID:11368702 [DOI] [PubMed] [Google Scholar]
- 18. Solfrizzi V, D'Introno A, Colacicco AM, et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790‐1799. [DOI] [PubMed] [Google Scholar]
- 19. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179‐186. [PubMed] [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37‐49. [DOI] [PubMed] [Google Scholar]
- 21. Solfrizzi V, Scafato E, Frisardi V, et al. Frailty syndrome and all‐cause mortality in demented patients: the Italian Longitudinal Study on Aging. Age. 2012;34:507‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 24. Spinnler H, Tognoni G. Standardizzazione e Taratura Italiana di Test Neuropsicologici. Ital J Neurol Sci. 1987;6:12‐120. [PubMed] [Google Scholar]
- 25. Barigazzi R, Della Sala S, Laiacona M, Spinnler H, Valenti V. Esplorazione testistica della memoria di prosa. Ric Psicol. 1987;1:50‐80. [Google Scholar]
- 26. American Psychiatric Association., American Psychiatric Association. Work Group to Revise DSM‐III . Diagnostic and Statistical Manual of Mental Disorders: DSM‐III‐R. 3rd ed Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 27. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939‐944. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization . The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research, (29–35). Geneva: World Health Organization; 1993. [Google Scholar]
- 29. Bishop YMM, Fienberg SE, Holland PW. Discrete Multivariate Analysis: Theory and Practice, (1–557). (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA): Springer; 2007. [Google Scholar]
- 30. Treeprasertsuk S, Bjornsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. 2013;19:1219‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782‐789 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim HM, Lee YH, Han K, et al. Impact of diabetes mellitus and chronic liver disease on the incidence of dementia and all‐cause mortality among patients with dementia. Medicine. 2017;96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nho K, Kueider‐Paisley A, Ahmad S, et al. Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open. 2019;2:e197978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheong CY, Nyunt MSZ, Gao Q, et al. Risk factors of progression to frailty: findings from the Singapore Longitudinal Ageing Study. J Nutr Health Aging. 2020;24:98‐106. [DOI] [PubMed] [Google Scholar]
- 35. Ampuero J, Aller R, Gallego‐Duran R, et al. The effects of metabolic status on non‐alcoholic fatty liver disease‐related outcomes, beyond the presence of obesity. Aliment Pharmacol Ther. 2018;48:1260‐1270. [DOI] [PubMed] [Google Scholar]
- 36. Taniguchi Y, Kitamura A, Kaito S, et al. Albumin and hemoglobin trajectories and incident disabling dementia in community‐dwelling older Japanese. Dement Geriatr Cogn Disord. 2019;47:233‐242. [DOI] [PubMed] [Google Scholar]
- 37. Milojevic J, Raditsis A, Melacini G. Human serum albumin inhibits Abeta fibrillization through a “monomer‐competitor” mechanism. Biophys J. 2009;97:2585‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun L, Wang Q, Liu M, etal. Albumin binding function is a novel biomarker for early liver damage and disease progression in non‐alcoholic fatty liver disease. Endocrine. 2020). 69(2), 294–302. [DOI] [PubMed] [Google Scholar]
- 39. Northup PG, Sundaram V, Fallon MB, et al. Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost. 2008;6:2‐9. [DOI] [PubMed] [Google Scholar]
- 40. Mahrouf‐Yorgov M, Collin de l'Hortet A, Cosson C, et al. Increased susceptibility to liver fibrosis with age is correlated with an altered inflammatory response. Rejuv Res. 2011;14:353‐363. [DOI] [PubMed] [Google Scholar]
- 41. Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aravinthan A, Scarpini C, Tachtatzis P, et al. Hepatocyte senescence predicts progression in non‐alcohol‐related fatty liver disease. J Hepatol. 2013;58:549‐556. [DOI] [PubMed] [Google Scholar]
- 43. Collins BH, Holzknecht ZE, Lynn KA, et al. Association of age‐dependent liver injury and fibrosis with immune cell populations. Liver International. 2013;33:1175‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal‐vein thrombosis in cirrhosis ‐ possible role in development of parenchymal extinction and portal‐hypertension. Hepatology. 1995;21:1238‐1247. [PubMed] [Google Scholar]
- 45. Petta S, Tuttolomondo A, Gagliardo C, et al. The presence of white matter lesions is associated with the fibrosis severity of nonalcoholic fatty liver disease. Medicine. 2016;95:e3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jindal A, Jagdish RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19:1896‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any anonymized data not published within the article will be shared by request from the corresponding author.


