Abstract
Introduction
Aboriginal Australians have among the highest rates of dementia worldwide, yet no study has investigated the subtypes, risk factors, or longer term outcomes of mild cognitive impairment (MCI) in this population.
Methods
A total of 336 community‐dwelling Aboriginal Australians aged ≥60 years participated in a longitudinal study, completing a structured interview at baseline. MCI (amnestic subtype, aMCI; non‐amnestic subtype, naMCI) and dementia were diagnosed via cognitive screening, medical assessment, and clinical consensus. Associations between life‐course factors and baseline MCI subtypes were examined using logistic regression. Conversion to dementia was assessed at 6‐year follow‐up.
Results
Prevalent aMCI (n = 24) was associated with older age (odds ratio [OR] = 1.68, 95% confidence interval [CI]: 1.12 to 2.53), head injury (OR = 3.19, 95% CI: 1.35 to 7.56), symptoms of depression (OR = 1.52, 95% CI: 1.04 to 2.24), and lower blood pressure (OR = 0.53, 95% CI: 0.33 to 0.86). Prevalent naMCI (n = 29) was associated with low education (OR = 4.46, 95% CI: 1.53 to 13.05), unskilled work history (OR = 5.62, 95% CI: 2.07 to 13.90), higher body mass index (OR = 1.99, 95% CI: 1.30 to 3.04), and moderate to severe hearing loss (OR = 2.82, 95% CI: 1.06 to 7.55). A small proportion of MCI cases reverted to intact at follow‐up (15%), but most remained stable (44%), developed dementia and/or died (41%).
Discussion
Sociodemographic and clinical factors both contributed to baseline MCI and were distinct for MCI subtypes, with similar patterns of conversion to dementia for amnestic and non‐amnestic MCI.
Keywords: aboriginal Australians, Alzheimer's disease, diagnostic stability, Indigenous, mild cognitive impairment subtypes, mild cognitive impairment, predictive validity, risk factors
1. INTRODUCTION
Global dementia prevalence is rapidly increasing, and with no effective therapeutic treatment options available, earlier points in the disease progression must be targeted to prevent or delay functional decline and dementia onset. 1 Mild cognitive impairment (MCI) is described as an intermediate stage between normal aging and dementia, at which point preventive intervention might still be effective. 2 MCI may be diagnosed as amnestic (aMCI) or non‐amnestic (naMCI), as well as single or multi‐domain subtypes. 3 Focusing on a decline in memory function, aMCI was founded on the earliest definition of MCI, which described a prodromal stage of Alzheimer's disease (AD). In contrast, naMCI refers to cognitive decline without memory impairment. These MCI subtypes are hypothesized to represent the prodromal stage of distinct dementia syndromes. Specifically, aMCI is likely to be associated with AD, while naMCI is more likely to progress to non‐AD dementias (eg, vascular or Lewy body dementias). 3 , 4 However, these distinctions are increasingly called into question, particularly in older people, who account for the majority of MCI and dementia cases.
Many longitudinal studies have confirmed that MCI is a high‐risk dementia state, but the predictive validity of MCI subtypes has been challenged. 5 Although aMCI has been shown to have high rates of conversion to AD, a high proportion (23%‐27%) of naMCI cases also convert to AD and conversion from naMCI to other dementia subtypes is not consistent. 6 , 7 , 8 , 9 Overall, a combination of amnestic and non‐amnestic cognitive deficits (ie, multi‐domain aMCI) appears to confer the highest risk for dementia. In addition, at various follow‐up intervals, 16% to 28% of those diagnosed with MCI at baseline revert to normal levels of cognitive function at follow‐up. 5 , 10 , 11 , 12 In light of this heterogeneity, characterizing the risk factor profiles of these subtypes may provide valuable early intervention targets and help ascertain the clinical utilty and prognostic validity of these subtypes.
RESEARCH IN CONTEXT
Systematic review: We searched Pubmed and OVID databases using the terms “mild cognitive impairment” and “Indigenous.” No previous study had examined mild cognitive impairment (MCI), nor its associated risk factors or long‐term outcomes, in the Aboriginal Australian population.
Interpretation: Our findings provide insight into the life‐course factors that contribute toward a diagnosis of MCI in older Aboriginal Australians, particularly with respect to the role of socioeconomic factors (ie, education and occupation). The distinct risk factor (RF) profiles associated with amnestic and non‐amnestic MCI subtypes indicated that despite little difference in long‐term dementia outcomes, preventative measures may be targeted individually for these subtypes.
Future directions: Future studies may look to investigate (a) the relationships among hypotension, antihypertensive medication, and MCI; (b) the predictive validity of neuropsychological criteria compared to the traditional subtyping method in this population; or (c) the association of biomarkers with specific MCI subtypes in this population.
Australian Aboriginal and Torres Strait Islander peoples (hereafter respectfully referred to as Aboriginal Australians) have among the highest rates of dementia in the world, with a prevalence three to five times higher than the wider Australian population. 13 , 14 , 15 Previous investigations into the life‐course factors contributing to the high dementia prevalence have highlighted significant associations with both biomedical and sociocultural factors. In a cross‐sectional study of Aboriginal Australians from the remote Kimberley region of Western Australia (aged ≥45 years), older age, being male, and having no formal education were associated with dementia; adjusting for these demographic factors, current smoking, stroke, epilepsy, head injury, poor mobility, incontinence, and falls were also associated with dementia. 15 In a follow‐up of this cohort, only older age and head injury predicted cognitive decline. 16
More recently, a study of Aboriginal Australians from urban and regional areas of New South Wales (NSW; aged ≥60 years) 17 highlighted similar dementia risk factors: older age, childhood adversity and trauma, unskilled work, stroke, and head injury. People with dementia also presented with higher levels of comorbidity, such as low body mass index (BMI), depression, high‐risk alcohol use, social isolation, and physical inactivity. In 2015, Radford et al. 14 published an estimated MCI prevalence rate in this population of 17.7% (95% confidence interval [CI], 13.4 to 21.9); however, the risk factors and clinical trajectories of MCI subtypes have not been studied in this population. This is critical as identifying relevant modifiable life‐course factors associated with MCI in this population could provide practical targets for primary and secondary prevention, thereby delaying dementia onset and reducing the burden of dementia on communities. Furthermore, given that Aboriginal Australians often face greater exposure to various life‐course risk factors (eg, social disadvantage and chronic conditions), 18 it is important to investigate the etiology of MCI. These findings could be relevant to other populations, especially those with similarly high exposure to dementia risk factors.
This study aimed to: (1) investigate the prevalence and risk factor profiles of aMCI and naMCI in an Aboriginal Australian population and (2) investigate the 6‐year outcomes of baseline MCI diagnoses.
2. METHODS
2.1. Participants
Inclusion criteria were: identifying as an Aboriginal and/or Torres Strait Islander person, aged ≥60 years at enrolment, and residing in the study catchment area for 6 months minimum. Participants were excluded if they were unwilling, incarcerated, had a recent stroke (<3 months), or could not give informed consent (and had no appropriate person to provide proxy consent). Participants were recruited from five partner Aboriginal communities with support from local Aboriginal research assistants. Study sites were NSW Local Government Areas in metropolitan Sydney (Randwick/Botany Bay, Campbelltown) and the mid‐north coast (Kempsey, Nambucca, Coffs Harbour). Baseline recruitment and data collection occurred between March 2010 and September 2012. Follow‐up was conducted between July 2016 and April 2018.
2.2. Diagnostic process
2.2.1. Baseline
All participants were screened for cognitive impairment using the Mini‐Mental State Examination (MMSE), 19 the Rowland Universal Dementia Assessment Scale (RUDAS), 20 and the modified Kimberley Indigenous Cognitive Assessment (mKICA), 21 , 22 which have been validated in this population. 23 Participants scoring below predetermined cut‐offs on any one of these tests (MMSE ≤26, max = 30; RUDAS ≤25, max = 30; mKICA ≤35, max = 39 23 ) were referred for comprehensive medical assessment and further cognitive testing, as has been reported in detail previously. 14 , 24 The medical assessment also involved a proxy interview with a nominated close family member or friend to obtain collateral functional and medical history. A panel of at least three clinicians then made a final clinical diagnosis based on review of these data. All‐cause dementia was diagnosed based on National Institute on Aging‐Alzheimer's Association (NIA‐AA) criteria. 25 MCI was diagnosed according to international consensus criteria, 3 including: subjective cognitive decline identified by self and/or collateral report, objective impairment on cognitive tasks, maintenance of activities of daily living (ADLs, self and proxy ratings; 26 in addition to open‐ended enquiry regarding changes in function and usual activities), and not meeting criteria for dementia. Subjective cognitive decline was assessed in the medical assessment. Participants were asked if they had noticed any memory problems, what the first sign of change was, and when these problems began. The proxy interview included similar questions about changes to memory, thinking, or behavior, as well as the Cambridge Behavioral Inventory Revised. 27
MCI was categorized as aMCI if cognitive performance in the memory domain (assessed using the memory subscale from the Addenbrooke's Cognitive Examination Revised 28 and a logical memory test 29 ) was impaired (relative to education and language background) and naMCI if memory was intact but impairment was apparent in another cognitive domain (eg, executive functioning or visuospatial abilities). A diagnosis of multiple domain aMCI or naMCI was given if impairment in more than one cognitive domain was present, with consideration of the following domains: memory, executive/frontal lobe function, visuospatial function, language, and praxis.
2.2.2. Follow‐up
All MCI cases underwent cognitive screening again at 6‐year follow‐up, using the MMSE and mKICA. 19 The medical assessment, consensus review, and diagnostic procedure remained consistent with baseline.
Twelve participants who scored below screening cut‐offs did not complete medical assessment (refused [n = 2] or could not be scheduled within study timeframe [n = 10]). For these cases, diagnosis was based on MMSE scores and ADL assessment. Stable MCI was diagnosed if ADLs remained intact and MMSE score had changed by <3 points (<2 standard deviations [SD]) from their baseline score, 3 based on normative data from the baseline study. 23 Participants with follow‐up MMSE score < 22 and ADL impairment were diagnosed with dementia, consistent with baseline procedure. 30 Participants who improved MMSE score by at least 3 points, with scores above dementia cut‐offs, 23 were classified as reverting to cognitively intact.
2.3. Associated factors
Baseline demographic and risk factor data were collected during structured interview. For participants selected to proceed to medical assessment, a contact person was also interviewed to provide complementary information on life‐course risk factors.
2.3.1. Early‐life factors
Participants were asked to recall where they grew up (coded major city = 0; regional/remote areas = 1), and if they were exposed to adverse experiences during childhood (ie, death of one/both parents or removal from their family). Participants completed the Childhood Trauma Questionnaire (CTQ), which screens for abuse and neglect during childhood 31 (range 25‐125, higher scores correspond with higher trauma exposure). Formal education level was dichotomized to reflect conventions in dementia research (0 = secondary school or above; 1 = primary school or below) and informal education was assessed using the Retrospective Indigenous Childhood Enrichment (RICE), comprised of Community, Intellectual and Traditional subscales 32 (range 0‐40, higher scores reflect enriched environment).
2.3.2. Midlife factors
Participants were asked about history of head injury causing loss of consciousness, epilepsy, stroke/transient ischemic attack, hypertension, hypercholesterolemia, diabetes, or heart disease, as well as previous diagnoses of depression, anxiety, or post‐traumatic stress disorder (PTSD; all coded: 0 = absent; 1 = present). Responses to hypertension, hypercholesterolemia, diabetes, and heart disease were computed as a cardiovascular index (summed to create a score ranging 0‐4, where 0 indicates absence of all risk factors). “Highest level” of past alcohol consumption was reported using the Alcohol Use Disorders Identification Test–shortened version (AUDIT‐C). 33 Based on a previous study of Aboriginal Australians 34 scores were categorized as 0 = abstinent; 1‐5 = low risk; ≥6 = high risk/dependent drinking. Lifetime smoking was measured using the pack‐year history method (1 pack‐year = 1 year of pack‐a‐day smoking). Lifetime occupation was coded 1 = unskilled (ie, “laborers”) or 0 = partially skilled/skilled (all other categories), according to standard classifications. 35
2.3.3. Late‐life factors
Current depressive symptoms were measured using the modified Patient Health Questionnaire‐9 (mPHQ‐9; range: 0‐30, higher scores indicate greater depressive symptoms). 36 Weight (kg), height (m), and blood pressure (mmHg) were measured. BMI was calculated as weight (kg)/height (m) 2 . Blood pressure was analyzed as mean arterial pressure (MAP), indicative of whether there is sufficient blood flow for perfusion of major organs (eg, the brain). This value was calculated using the formula: (systolic + 2*diastolic)/3. 37 Self‐reported hearing loss (none‐mild = 0; moderate‐severe = 1), sleeping difficulties (0 = none; 1 = infrequent; 2 = frequent) and current smoking status (no = 0; yes = 1) were recorded. Current alcohol consumption was assessed using the AUDIT‐C and coded using the same method as past alcohol consumption. For physical activity levels, individuals reported if they had participated in mild (eg, walking/gardening), moderate (eg, swimming/dancing), or vigorous (eg, running/football/digging) activity in the last 3 months (coded 0 = nil‐mild; 1 = moderate‐vigorous). Social connections were assessed using marital status (0 = de facto/married; 1 = widowed/divorced/separated/never married), living arrangements (1 = live alone; 0 = with others), and frequency of subjective loneliness (0 = never; 1 = sometimes/quite often). Exposure to racism was measured using the Measure of Indigenous Racism Experiences (MIRE) systematic racism subscale. 38 Total scores, based on average responses across 10 items (5‐point Likert scale) were coded as “none” (all responses “never”), “low” (average response “hardly ever”), or “high” (average response “sometimes”/”often”/”very often”).
2.4. Statistical analysis
Data were analyzed using SPSS version 25 (IBM Corp., Armonk, NY). Bivariate logistic regression was used to examine univariable cross‐sectional associations between life course factors and a diagnosis of aMCI or naMCI at baseline. Baseline diagnosis was the criterion variable and all other variables were predictors. Predictors with P < .1 were subsequently entered into backward stepwise multivariable logistic regression models predicting diagnostic outcome, with significance level set at P < .05. The backward stepwise logistic regression method was chosen due to the large number of variables and to avoid suppressor effects. To maximize inclusion of participants in multivariable analyses, the expectation‐maximization (EM) procedure was used to compute missing values for continuous variables (ie, MAP and BMI). Values were missing completely at random (Little's MCAR > 0.05). Variables likely to be associated with MAP and BMI, (ie, cardiovascular risk factors: smoking, hypercholesterolemia, heart disease, hypertension, diabetes, sex, and age) were also entered into the EM model. All continuous variables (age, CTQ, RICE Community subscale, cardiovascular index, smoking pack‐years, mPHQ‐9, MAP, and BMI) met the linearity assumption 39 and there was no multicollinearity (no variance inflation factor [VIF] values >10 40 or tolerance values >0.1 41 ). Odds ratios (OR) with 95% CIs are reported. Standardized scores (z‐scores calculated within the current sample) for continuous predictor variables were used throughout regression analyses, whereby ORs correspond to an increased risk in the criterion associated with a 1‐SD increase in the predictor.
3. RESULTS
Participants with baseline diagnoses of dementia (n = 45) or significant cognitive impairment other than MCI (n = 2 chronic schizophrenia, n = 1 developmental disorder, n = 1 delirium due to general medical condition) were excluded from further analysis. The remaining cohort (n = 287) had a mean age of 65.9 years (SD = 5.6, range = 60–85, 61.7% female). As illustrated in Figure 1, the majority of participants were cognitively intact (n = 234); 29 had aMCI and 24 had naMCI. All participants spoke English; 58.2% were from regional sites (41.8% urban).
FIGURE 1.
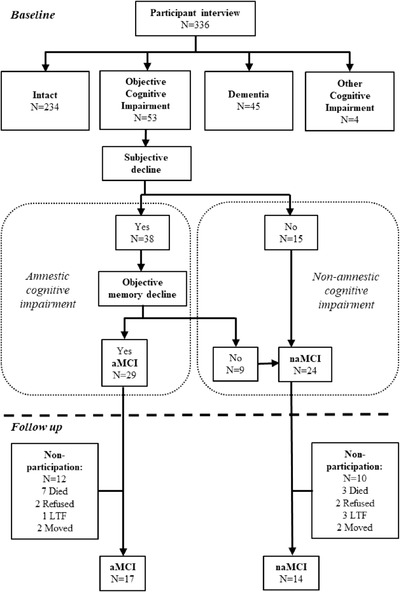
Flowchart of participants included in the baseline and the follow‐up stages of the study. aMCI, amnestic mild cognitive impairment; naMCI, non‐amnestic mild cognitive impairment; LTF, lost to follow‐up; moved, moved out of study area and unable to follow up
The clinical consensus process identified 15 individuals who had cognitive impairment consistent with MCI but did not strictly meet MCI criteria due to a lack of self‐reported decline and/or lack of appropriate contact person to provide a collateral report confirming decline (see Figure 1). A comparison of cognitive profiles between these cases and those diagnosed with MCI subtypes indicated relatively intact memory performance compared to the aMCI group. Comparison to naMCI indicated no significant difference in any cognitive domain (see Table S1 in supporting information). Therefore, for the purpose of this study these individuals were classified as naMCI. Impairment in multiple cognitive domains was detected for the majority (74%) of MCI cases, with no significant difference in the proportion diagnosed with multi‐domain MCI for aMCI versus naMCI groups.
3.1. Risk factors
Univariate risk factor analyses are presented in Tables 1 and 2. Factors that were associated with aMCI at P < .1 were older age; low education,; greater depressive symptoms; lower blood pressure; loneliness; and history of head injury, epilepsy, and stroke. In the final model, older age, history of head injury, symptoms of depression, and lower blood pressure remained significant factors associated with aMCI (P < .05, see Table 3). For naMCI, factors that were associated at P < .1 were low education, greater childhood enrichment (RICE Community subscale), unskilled work history, lower blood pressure, higher BMI, moderate to severe hearing loss, and history of stroke. Low education, unskilled work history, higher BMI, and moderate‐severe hearing loss remained significant (P < .05) in the final model (see Table 3).
TABLE 1.
Univariable associations between early and mid‐life factors (at baseline) and MCI subtypes
| Total sample | Intact (n = 234) | aMCI (n = 29) | naMCI (n = 24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Missing | Observed range | n (%) | Observed range | n (%) | OR (95% CI) | P | Observed range | n (%) | OR (95% CI) | P |
| Early‐life factors | |||||||||||
| Age (y) a | 0 | 60‐85 | 65.62(5.49) | 60‐80 | 67.97(6.37) | 1.45(1.02, 2.05) | .037 | 60‐81 | 66.63(5.74) | 1.19(0.79‐1.78) | .403 |
| Male | 0 | ‐ | 88(38) | ‐ | 13(45) | 1.32(0.61‐2.88) | .480 | ‐ | 8(33) | 0.82(0.34‐1.98) | .651 |
| Grew up | 2 | ||||||||||
| Major city | ‐ | ‐ | 76(33) | ‐ | 8(28) | ‐ | REF | ‐ | 6(25) | ‐ | REF |
| Regional/remote | ‐ | ‐ | 155(67) | ‐ | 21(72) | 1.28(0.54‐3.02) | .575 | ‐ | 18(75) | 1.46(0.56‐3.83) | .440 |
| Parent(s) died | 2 | ‐ | 50(22) | ‐ | 8(28) | 1.46(0.61‐3.52) | .395 | ‐ | 5(21) | 0.96(0.34‐2.71) | .943 |
| Removed from family | 6 | ‐ | 24(11) | ‐ | 4(14) | 1.36(0.44‐4.24) | .596 | ‐ | 1(4) | 0.37(0.05‐2.86) | .340 |
| CTQ score a | 22 | 25‐104 | 36.52(15.92) | 25‐54 | 32.07(8.23) | 0.64(0.34‐1.23) | .181 | 25‐80 | 33.57(13.71) | 0.80(0.47‐1.36) | .406 |
| Education | 1 | ||||||||||
| Primary or less | ‐ | ‐ | 38(16) | ‐ | 18(65) | ‐ | REF | ‐ | 16(67) | ‐ | REF |
| Secondary or higher | ‐ | ‐ | 196(84) | ‐ | 10(35) | 2.87(1.23,6.69) | .015 | ‐ | 8(33) | 2.58(1.03, 6.45) | .043 |
| RICE community a | 1 | 9‐40 | 24.23(6.46) | 15‐35 | 23.72(5.17) | 0.92(0.61‐1.38) | .683 | 9‐36 | 21.50(5.95) | 0.64(0.41, 1.00) | .050 |
| RICE traditional a | 2 | 5‐25 | 7.82(3.38) | 4‐19 | 8.31(3.32) | 1.15(0.79‐1.69) | .463 | 4‐24 | 7.88(5.25) | 1.02(0.67‐1.53) | .941 |
| RICE intellectual a | 15 | 5‐25 | 13.94(5.24) | 5‐25 | 12.32(5.21) | 0.72(0.48‐1.10) | .126 | 5‐23 | 12.27(4.48) | 0.72(0.45‐1.14) | .160 |
| Mid‐life factors | |||||||||||
| History of head injury | 9 | ‐ | 54(24) | ‐ | 13(45) | 2.57(1.16,5.69) | .020 | ‐ | 6(25) | 1.06(0.40‐2.79) | .913 |
| History of epilepsy | 12 | ‐ | 11(5) | ‐ | 4(14) | 3.08(0.91‐10.42) | .070 | ‐ | 2(8) | 1.84(0.38‐8.84) | .449 |
| History of stroke | 10 | ‐ | 37(17) | ‐ | 9(31) | 2.27(0.96‐5.39) | .062 | ‐ | 8(33) | 2.53(1.01, 6.34) | .048 |
| Cardiovascular index a | 19 | 0‐4 | 1.95(1.19) | 0‐4 | 1.96(1.37) | 1.01(0.68‐1.50) | .971 | 0‐4 | 2.04(1.22) | 1.08(0.69‐1.68) | .733 |
| History of anxiety/PTSD | 12 | ‐ | 70(32) | ‐ | 11(38) | 1.33(0.60‐2.96) | .489 | ‐ | 8(33) | 1.09(0.44‐2.66) | .857 |
| History of depression | 11 | ‐ | 74(33) | ‐ | 11(38) | 1.21(0.54‐2.68) | .646 | ‐ | 8(33) | 0.99(0.40‐2.41) | .976 |
| Past alcohol use | 14 | ||||||||||
| Abstinent | ‐ | ‐ | 60(27) | ‐ | 10(35) | ‐ | REF | ‐ | 10(42) | ‐ | REF |
| Low risk | ‐ | ‐ | 63(29) | ‐ | 9(31) | 0.86(0.33‐2.26) | .755 | ‐ | 6(25) | 0.57(0.20‐1.67) | .306 |
| High risk | ‐ | ‐ | 97(44) | ‐ | 10(35) | 0.62(0.24‐1.57) | .313 | ‐ | 8(33) | 0.50(0.19‐1.32) | .161 |
| Smoking pack years a | 14 | 0‐255 | 24.95(33.63) | 0‐125 | 25.18(30.99) | 1.01(0.67‐1.51) | .973 | 0‐176 | 25.54(42.81) | 1.02(0.67‐1.54) | .936 |
| Unskilled work | 12 | ‐ | 70(31) | ‐ | 11(41) | 1.51(0.67‐3.43) | .321 | ‐ | 16(67) | 4.40(1.80‐ 10.76) | .001 |
Abbreviations: aMCI, amnestic mild cognitive impairment; CI, confidence interval; CTQ, Childhood Trauma Questionnaire; naMCI, non‐amnestic mild cognitive impairment; OR, odds ratio; PTSD, post‐traumatic stress disorder; RICE, Retrospective Indigenous Childhood Enrichment scale; SD, standard deviation.
Notes: Values in bold correspond to risk factors (RFs) that were significant at a level <0.1 and entered into the final stepwise models. Standardized scores for continuous predictor variables were used, whereby odds ratios correspond to an increased risk in the criterion associated with a 1‐SD increase in the predictor.
Continuous variables, mean, standard deviation, and observed range reported.
TABLE 2.
Univariable associations between baseline late‐life factors and MCI subtypes
| Total Sample | Intact (n = 234) | aMCI (n = 29) | naMCI (n = 24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Missing | Observed range | n (%) | Observed range | n (%) | OR (95% CI) | P | Observed range | n (%) | OR (95% CI) | P |
| Depression (mPHQ9) a | 13 | 0‐22 | 4.50(4.82) | 0‐17 | 6.54(4.73) | 1.77(1.45, 2.73) | .040 | 0‐19 | 5.26(4.43) | 1.16(0.77‐1.74) | .470 |
| Blood pressure (MAP a ) | 54 | 73.67‐150.00 | 102.67(14.29) | 70.00‐109.67 | 95.49(9.73) | 0.53(0.33, 0.85) | .011 | 75.33‐114.67 | 95.75(11.13) | 0.95(0.92, 0.99) | .025 |
| Obesity (BMI a ) | 49 | 19.2‐56.8 | 31.66(6.94) | 18.1‐41.9 | 30.86(5.62) | 0.87(0.54‐1.41) | .581 | 25.4‐61.3 | 37.68(9.60) | 1.69(1.19, 2.40) | .003 |
| Hearing Loss | 8 | ||||||||||
| None/mild | ‐ | 188(83) | ‐ | 21(72) | ‐ | REF | ‐ | 14(57) | ‐ | REF | |
| Moderate‐severe | ‐ | 38(17) | ‐ | 8(28) | 1.89(0.78‐4.57) | .161 | ‐ | 10(43) | 3.53(1.46, 8.55) | .005 | |
| Sleep disturbed | 13 | ||||||||||
| None | ‐ | ‐ | 100(45) | ‐ | 11(39) | ‐ | REF | ‐ | 9(39) | ‐ | REF |
| Infrequent | ‐ | ‐ | 61(27) | ‐ | 7(25) | 1.04(0.38‐2.84) | .934 | ‐ | 7(30) | 1.28(0.45‐3.60) | .646 |
| Frequent | ‐ | ‐ | 62(28) | ‐ | 10(36) | 1.47(0.59‐3.65) | .411 | ‐ | 7(30) | 1.25(0.44‐3.54) | .668 |
| Smoking | 11 | ‐ | 75(34) | ‐ | 9(31) | 0.45(0.15‐1.33) | .148 | ‐ | 5(21) | 1.15(0.45‐2.90) | .776 |
| Alcohol | 11 | ||||||||||
| Abstinent | ‐ | ‐ | 129(58) | ‐ | 19(66) | ‐ | REF | ‐ | 15(62) | ‐ | REF |
| Low risk | ‐ | ‐ | 75(34) | ‐ | 9(31) | 0.82(0.35‐1.89) | .634 | ‐ | 5(21) | 0.57(0.20‐1.64) | .300 |
| High risk | ‐ | ‐ | 19(9) | ‐ | 1(3) | 0.36(0.05‐2.83) | .329 | ‐ | 4(17) | 1.81(0.54‐6.03) | .334 |
| Physical activity | 11 | ||||||||||
| Nil mild | ‐ | ‐ | 112(50) | ‐ | 15(52) | ‐ | REF | ‐ | 13(54) | ‐ | REF |
| Moderate‐high | ‐ | ‐ | 111(50) | ‐ | 14(48) | 0.94(0.43‐2.04) | .879 | ‐ | 11(46) | 0.85(0.37‐1.99) | .714 |
| Married/de‐facto | 7 | ‐ | 83(37) | ‐ | 15(52) | 1.82(0.84‐3.97) | .129 | ‐ | 9(38) | 1.02(0.43‐2.44) | .962 |
| Live alone | 10 | ‐ | 48(22) | ‐ | 7(24) | 1.17(0.47‐2.89) | .739 | ‐ | 7(29) | 1.51(0.59‐3.85) | .388 |
| Loneliness | 12 | ||||||||||
| Never | ‐ | ‐ | 118(53) | ‐ | 8(29) | ‐ | REF | ‐ | 14(58) | ‐ | REF |
| Sometimes‐often | ‐ | ‐ | 105(47) | ‐ | 20(71) | 2.81(1.19, 6.65) | .019 | ‐ | 10(42) | 0.80(0.34‐1.88) | .614 |
| Racism | 27 | ||||||||||
| None | ‐ | ‐ | 108(51) | ‐ | 13(45) | ‐ | REF | ‐ | 13(62) | ‐ | REF |
| Low | ‐ | ‐ | 68(33) | ‐ | 9(31) | 1.10(0.45‐2.71) | .837 | ‐ | 6(29) | 0.73(0.27‐2.02) | .548 |
| High | ‐ | ‐ | 34(16) | ‐ | 7(24) | 1.71(0.63‐4.63) | .291 | ‐ | 2(10) | 0.49(0.11‐2.27) | .361 |
Abbreviations: aMCI, amnestic mild cognitive impairment; BMI, body mass index; MAP, mean arterial pressure; mPHQ9, modified Patient Health Questionnaire‐9; naMCI, non‐amnestic mild cognitive impairment; OR, odds ratio; SD, standard deviation.
Notes: Values in bold correspond to RFs that were significant at a level <0.1 and entered into the final stepwise models. Standardized scores for continuous predictor variables were used, whereby odds ratios correspond to an increased risk in the criterion associated with a 1‐SD increase in the predictor.
Continuous variables, mean, standard deviation and observed range reported.
TABLE 3.
Multivariable logistic regression models predicting amnestic (n = 29) and non‐amnestic (n = 24) mild cognitive impairment compared to cognitively intact participants (n = 254) at baseline
| Outcome | Factor | OR (95% CI) | Beta (SE) | Wald | P |
|---|---|---|---|---|---|
| aMCI | Constant | ‐ | ‐2.69 (0.32) | 72.22 | ‐ |
| Age | 1.70 (1.09, 2.66) | 0.53 (0.23) | 5.46 | 0.019 | |
| Head injury | 3.31 (1.39, 7.88) | 1.20 (0.44) | 7.31 | 0.007 | |
| Depression (mPHQ9) | 1.78 (1.11, 2.84) | 0.58 (0.24) | 5.83 | 0.016 | |
| Blood pressure (MAP) | 0.57 (0.35, 0.92) | −0.57 (0.25) | 5.32 | 0.021 | |
| naMCI | Constant | ‐ | −3.77 (0.49) | 56.14 | ‐ |
| Low education | 3.91 (1.38, 11.08) | 1.36 (0.53) | 6.60 | 0.010 | |
| Unskilled work | 4.74 (1.78, 12.62) | 1.56 (0.50) | 9.70 | 0.002 | |
| Obesity (BMI) | 1.93 (1.29, 2.91) | 0.66 (0.21) | 10.10 | 0.001 | |
| Hearing loss | 2.62 (1.00, 6.87) | 0.96 (0.49) | 3.83 | 0.050 |
Abbreviations: aMCI, amnestic mild cognitive impairment; BMI, body mass index; CI, confidence interval; MAP, mean arterial pressure; mPHQ9, modified Patient Health Questionnaire 9; naMCI, non‐amnestic mild cognitive impairment; OR, odds ratio; SE, standard error.
3.2. Follow‐up diagnosis
Of the 53 cases classified with MCI at baseline, 58% (n = 31) participated in follow‐up, with a mortality rate of 19% (n = 10) and 23% (n = 12) lost to follow‐up. Overall, 41% (n = 17) of baseline MCI cases progressed to dementia and/or died over 6 years, with a similar number (n = 18; 44%) retaining a stable diagnosis of MCI at follow‐up. Only six participants (15%) diagnosed with MCI at baseline were cognitively intact at follow‐up. Of the dementia cases diagnosed at follow‐up, there were five cases of AD, and two cases of mixed AD/Lewy body dementia. No clear difference was observed in outcomes between aMCI and naMCI, although the numbers were too small to draw reliable conclusions.
4. DISCUSSION
This study found that in a community sample of older Aboriginal Australians living in urban and regional settings, risk factors associated with aMCI and naMCI were distinct. However, there was no clear difference in the proportion of cases that converted to dementia at follow‐up.
Defining MCI subtypes initially arose as a means of identifying prodromal cases of different dementia types, with aMCI predicting conversion to AD while naMCI converted to non‐AD syndromes. In the current study, aMCI was associated with older age, history of head injury with loss of consciousness, greater depressive symptoms, and lower blood pressure. The associations with age and head injury are consistent with possible underlying neurodegenerative processes associated with AD. 42 , 43 , 44 The association between lower late‐life blood pressure and MCI has previously been reported, with two suggested mechanisms: (1) hypotension occurs secondary to AD pathology, 45 or (2) low systemic blood pressure may lead to cerebral hypoperfusion, causing brain injury and poorer cognitive functioning. 46 In this cohort, 65% of participants were taking antihypertensive medication, consistent with a remote cohort. 16 However, there was no association between antihypertensive medication use and MAP in this sample, suggesting the link between MAP and aMCI cannot be readily explained as a by‐product of treatment for mid‐ to late‐life hypertension, which might have accounted for the true risk of cognitive decline. Finally, the link between lifetime depression and the neurodegenerative processes contributing to dementia could relate to glucocorticoid levels, hippocampal atrophy, increased deposition of amyloid beta (Aβ) plaques, inflammatory changes, and deficits of nerve growth factors or neurotrophin. 47 Here, current symptoms of depression, but not lifetime depression, were associated with aMCI, suggesting that late‐life depression might be a comorbid symptom of cognitive decline or an early indicator of underlying neurodegenerative disease rather than a risk factor. 48 , 49 Depression is a significant concern in this population and its impact on cognitive decline and dementia risk requires further investigation.
In contrast to aMCI, we found that naMCI was associated with low educational attainment, unskilled work, higher late‐life BMI, and moderate to severe hearing loss. This profile is indicative of social determinants of health—including cognitive health—and the social disparities faced by Aboriginal Australians, 50 and other Indigenous populations. The association of MCI with low education and unskilled employment has previously been explained by the “reserve” hypothesis, suggesting that engaging in cognitively stimulating activities throughout life increases cognitive reserve, which may protect against cognitive decline and dementia. 51 Obesity (higher BMI) and hearing loss could relate to cognitive decline via multiple mechanisms including inflammatory processes and/or microvascular damage. 52 These factors have been identified as major modifiable risk factors for dementia globally and, regardless of the underlying pathology, are promising early to midlife targets for reducing the risk of dementia with Aboriginal populations, with public health responses to reduce social inequalities through both structural/societal changes 50 and health promotion or prevention initiatives. 52
With respect to the 6‐year clinical outcomes for those diagnosed with MCI at baseline (including participants who died before follow‐up), 44% had a stable diagnosis of MCI at follow up, and 15% returned to a normal level of cognitive function. In contrast to our hypothesis, the clinical dementia syndromes which emerged over 6 years were not clearly different between MCI subtypes: AD was the primary dementia outcome for both aMCI and naMCI. However, most cases of MCI were classified as “multi‐domain” in this study. Irrespective of MCI classification as amnestic or non‐amnestic, a meta‐analysis found that multi‐domain types were the most likely to convert to dementia. 53 Similarly, recent studies have reported that single domain MCI cases are more likely to revert to normal cognition. 54 , 55 In this context, future investigations with larger samples that compare the trajectories and risk factors of single versus multi‐domain subtypes may provide greater insight into the predictive utility of subtyping MCI in this population.
This study has multiple strengths, including the representativeness of the population‐based cohort, which enhances generalizability to Aboriginal people across urban and regional settings; the extensive examination of social and biomedical risk factors; and culturally appropriate methods, including cognitive assessments. 23 , 24 The use of a comprehensive clinical diagnostic procedure compared to algorithmic methods helped identify recent onset of cognitive decline, rather than lower cognitive performance related to other factors (eg, poor literacy).
A study limitation was the use of self‐report measures; however, supplementary information from contact persons and the comprehensive medical assessment improved the reliability of self‐report and retrospective data. Also, the inclusion of cases without evidence of subjective decline to the naMCI group does not strictly adhere to MCI diagnostic criteria. However, a recent review of these criteria indicated that subjective cognitive decline did not improve how an MCI diagnosis predicted dementia; 56 objective decline is a stronger predictor of subsequent dementia and preferable for informing MCI diagnosis. Another limitation was the relatively small cohort size and proportion of MCI cases who were lost to follow‐up. This limits the strength of the conclusions we can draw, but is a challenge of conducting research in partnership with hard‐to‐reach communities. Risk factors (including additive or cluster effects) for incident MCI also need to be examined in the future. Despite this, these results are critical for beginning to understand MCI in the Aboriginal Australian population, particularly for the large majority residing in urban and regional communities; and can be used to inform future studies, including risk reduction trials. Finally, recent studies have reported significant improvement in the prognostic utility of MCI with the inclusion of biomarkers, 57 , 58 which were not available in this study, and future investigations would benefit from such data.
In conclusion, this study provides important insights into key life‐course factors associated with MCI, and correspondingly dementia risk, in Aboriginal Australians. Our findings highlight the significant impact that social and biomedical factors across the life‐course have on the risk of late‐life cognitive decline. These factors (low education and unskilled work, depression, hearing loss and obesity) may be critical targets for early intervention and prevention of dementia, primarily in the early to midlife period. Although memory impairment was a feature of just over half of MCI cases, there were many that presented with relatively preserved memory function. Together, these findings indicate that the assessment of multiple cognitive domains is important for the identification of MCI in this population, for understanding dementia pathogenesis and for implementing effective secondary dementia prevention.
CONFLICTS OF INTEREST
The authors confirm they have no conflicts of interest to declare.
ETHICS STATEMENT
This study was approved by the Aboriginal Health and Medical Research Council (AHMRC 615/07), University of NSW Human Research Ethics Committee (HREC 08003), and NSW Population & Health Services Research Ethics Committee (AU RED Ref: HREC/09/CIPHS/65; Cancer Institute NSW Ref: 2009/10/187). All participants gave written informed consent, and if unable, verbal assent was obtained from the participant and proxy consent from a relevant person responsible.
Supporting information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
This study was supported by an Australian National Health and Medical Research Council (NHMRC) project grant (510347) and Dementia Collaborative Research Centres grant (S1.16.08). KR is supported by a NHMRC‐ARC Dementia Research Development Fellowship (1103312). LL is supported by a Serpentine Foundation Fellowship. KD is supported by a NHMRC Career Development Fellowship (1105106). This work relied on the support of many individuals and organizations. We are extremely grateful to our participants, local Elder guidance groups, KGOWS Aboriginal Reference Group, the Aboriginal Health and Medical Research Council, and our Aboriginal community partners (Durri Abo‐riginal Corporation Medical Service and Booroongen Djugun in Kempsey, Darrimba Maarra Aboriginal Health Clinic in Nambucca, Galambila Aboriginal Health Service in Coffs Harbour, La Perouse Local Aboriginal Land Council, La Perouse Aboriginal Community Health Centre, and Tharawal Aboriginal Corporation in Campbelltown). We recognize the work of our research team including Aboriginal research assistants, investigators, project officers and interviewers, medical doctors, and knowledge translation and support team (Koori Dementia Care Project).
Derrig H, Lavrencic LM, Broe GA, et al. Mild cognitive impairment in Aboriginal Australians. Alzheimer's Dement. 2020;6:e12054 10.1002/trc2.12054
REFERENCES
- 1. Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report 2015—the Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International; 2015. [Google Scholar]
- 2. Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303‐308. [DOI] [PubMed] [Google Scholar]
- 3. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240‐246. [DOI] [PubMed] [Google Scholar]
- 4. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet North Am Ed. 2006;367:1262‐1270. [DOI] [PubMed] [Google Scholar]
- 5. Han J, Kim T, Lee S, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dement. 2012;8:553‐559. [DOI] [PubMed] [Google Scholar]
- 6. Jungwirth S, Zehetmayer S, Hinterberger M, Tragl K, Fischer P. The validity of amnestic MCI and non‐amnestic MCI at age 75 in the prediction of Alzheimer's dementia and vascular dementia. Int Psychogeriatr. 2012;24:959‐966. [DOI] [PubMed] [Google Scholar]
- 7. Rasquin SMC, Lodder J, Visser PJ, Lousberg R, Verhey FRJ. Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: a 2‐year follow‐up study. Dement Geriatr Cogn Disord. 2005;19:113‐119. [DOI] [PubMed] [Google Scholar]
- 8. Ravaglia G, Forti P, Maioli F, et al. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement Geriatr Cogn Disord. 2006;21:51‐58. [DOI] [PubMed] [Google Scholar]
- 9. Fischer PS, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288‐291. [DOI] [PubMed] [Google Scholar]
- 10. Koepsell T, Monsell S. Reversion from mild cognitive impairment to normal or near‐normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malek‐Ahmadi M. Reversion from mild cognitive impairment to normal cognition. Alzheimer Dis Assoc Disord. 2016;30:324‐330. [DOI] [PubMed] [Google Scholar]
- 12. Brodaty H, Heffernan M, Kochan N, et al. Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement. 2013;9:310‐317. [DOI] [PubMed] [Google Scholar]
- 13. Li S, Guthridge S, Aratchige P, et al. Dementia prevalence and incidence among the indigenous and non‐indigenous populations of the Northern territory. Med J Aust. 2014:465‐469. [DOI] [PubMed] [Google Scholar]
- 14. Radford K, Mack HA, Draper B, et al. Prevalence of dementia in urban and regional Aboriginal Australians. Alzheimers Dement. 2015;11:271‐279. [DOI] [PubMed] [Google Scholar]
- 15. Smith K, Flicker L, Dwyer A, et al. Factors associated with dementia in Aboriginal Australians. Aust N Z J Psychiatry. 2010;44:888‐893. [DOI] [PubMed] [Google Scholar]
- 16. Lo Giudice D, Smith K, Fenner S, et al. Incidence and predictors of cognitive impairment and dementia in Aboriginal Australians: a follow‐up study of 5 years. Alzheimers Dement. 2016;12:252‐261. [DOI] [PubMed] [Google Scholar]
- 17. Radford K, Lavrencic LM, Delbaere K, et al. Factors associated with the high prevalence of dementia in older Aboriginal Australians. J Alzheimers Dis. 2019;70:S75‐S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couzos S, Murray R. Aboriginal Primary Health Care: An Evidence‐Based Approach, Melbourne, Australia: Oxford University Press; 2008. [Google Scholar]
- 19. Folstein M, Folstein S, McHugh P. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 20. Storey J, Rowland J, Conforti D, Dickson H. The Rowland universal dementia assessment scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr. 2004;16:13‐31. [DOI] [PubMed] [Google Scholar]
- 21. Lo Giudice D, Smith K, Thomas J, et al. Kimberley Indigenous Cognitive Assessment tool (KICA): development of a cognitive assessment tool for older indigenous Australians. Int Psychogeriatr. 2006;18:269‐280. [DOI] [PubMed] [Google Scholar]
- 22. Pulver L, Broe G, Grayson D, Dementia screening for urban Aboriginal Australians: The modified Kimberly Indigenous Cognitive Assessment (mKICA). Dementia Collaborative Research Centres, Pilot Study Report, 2012. Available at: https://researchdirect.westernsydney.edu.au/islandora/object/uws:35515/ (2012, accessed 10 August 2020).
- 23. Radford K, Mack H, Draper B, et al. Comparison of three cognitive screening tools in older urban and Regional Aboriginal Australians. Dement Geriatr Cogn Disord. 2015;40:22‐32. [DOI] [PubMed] [Google Scholar]
- 24. Radford K, Mack HA, Robertson H, et al. The Koori growing old well study: investigating aging and dementia in urban Aboriginal Australians. Int Psychogeriatr. 2014;26:1033‐1043. [DOI] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akhtar A, Broe G, Crombie A, McLean W, Andrews G, Caird F. Disability and dependence in the elderly at home. Age Ageing. 1973;2:102‐111. [DOI] [PubMed] [Google Scholar]
- 27. Wear HJ, Catherine J, Wedderburn M, et al. The Cambridge behavioural inventory revised. Dement Neuropsychol. 2008;2:102‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges J. The Addenbrooke's Cognitive Examination Revised (ACE‐R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078‐1085. [DOI] [PubMed] [Google Scholar]
- 29. Wechsler D. Wechsler Memory Scale 3rd Edition (WMS‐III). San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 30. Radford K, Mack H, Draper B, et al. Prevalence of dementia in urban and regional Aboriginal Australians. Alzheimers Dement. 2015;11:271‐279. [DOI] [PubMed] [Google Scholar]
- 31. Bernstein D, Stein J, Newcomb M, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169‐190. [DOI] [PubMed] [Google Scholar]
- 32. Minogue C, Delbaere K, Radford K, Broe T, Forder W, Lah S. Development and initial validation of the Retrospective Indigenous Childhood Enrichment scale (RICE). Int Psychogeriatr. 2018;30:519‐526. [DOI] [PubMed] [Google Scholar]
- 33. Bush K, Kivlahan D, McDonell M, Fihn S, Bradley K. The AUDIT alcohol consumption questions (AUDIT‐C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789‐1795. [DOI] [PubMed] [Google Scholar]
- 34. Calabria B, Clifford A, Shakeshaft A, et al. Identifying Aboriginal‐specific AUDIT‐C and AUDIT‐3 cutoff scores for at‐risk, high‐risk, and likely dependent drinkers using measures of agreement with the 10‐item Alcohol Use Disorders Identification Test. Addict Sci Clin Pract. 2014;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Australian Bureau of Statistics . ANZSCO, Australian and New Zealand Standard Classification of Occupations, Version 1.2. 2013. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/DirClassManualsbyTopic/4AF138F6DB4FFD4BCA2571E200096BAD?OpenDocument
- 36. Esler D, Johnston F, Thomas D, Davis B. The validity of a depression screening tool modified for use with Aboriginal and Torres Strait Islander people. Aust N Z J Public Health. 2008;32:317‐321. [DOI] [PubMed] [Google Scholar]
- 37. Sesso HD, Stampfer MJ, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801‐807. [DOI] [PubMed] [Google Scholar]
- 38. Paradies Y, Cunningham J. Experiences of racism among urban Indigenous Australians: findings from the DRUID study. Ethn Racial Stud. 2009;32:548‐573. [Google Scholar]
- 39. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 40. Myers R. Classical and Modern Regression With Applications. 2nd ed. Boston: MA: Duxbury; 1990. [Google Scholar]
- 41. Menard S. Applied Logistic Regression Analysis. Thousand Oaks: CA: Sage; 1995. [Google Scholar]
- 42. LoBue C, Woon F, Rossetti H, Hynan L, Hart J, Jr, Cullum CM. Traumatic brain injury history and progression from mild cognitive impairment to Alzheimer disease. Neuropsychology. 2018;32:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75:S24‐S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson VE, Stewart W, Smith DH. Widespread and amyloid‐pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Aβ generation by altering β‐and γ‐cleavage of APP. Neurobiol Aging. 2009;30:1091‐1098. [DOI] [PubMed] [Google Scholar]
- 46. Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789‐794. [DOI] [PubMed] [Google Scholar]
- 47. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Richard E, Reitz C, Honig LH, et al. Late‐life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70:383‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mirza SS, Ikram MA, Bos D, Mihaescu R, Hofman A, Tiemeier H. Mild cognitive impairment and risk of depression and anxiety: a population‐based study. Alzheimers Dement. 2017;13:130‐139. [DOI] [PubMed] [Google Scholar]
- 50. Marmot MG. Dignity, social investment and the Indigenous health gap. Med J Aust. 2017;207:20‐21. [DOI] [PubMed] [Google Scholar]
- 51. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet North Am Ed. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 53. Mitchell AJ, Shiri‐Feshki M. Rate of progression of mild cognitive impairment to dementia–meta‐analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252‐265. [DOI] [PubMed] [Google Scholar]
- 54. Forlenza OV, Diniz BS, Nunes PV, Memória CM, Yassuda MS, Gattaz WF. Diagnostic transitions in mild cognitive impairment subtypes. Int Psychogeriatr. 2009;21:1088‐1095. [DOI] [PubMed] [Google Scholar]
- 55. Ritchie LJ, Tuokko H. Patterns of cognitive decline, conversion rates, and predictive validity for 3 models of MCI. Am J Alzheimers Dis Other Demen. 2010;25:592‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brodaty H, Aerts L, Crawford JD, et al. Operationalizing the diagnostic criteria for mild cognitive impairment: the salience of objective measures in predicting incident dementia. Am J Geriatr Psychiatry. 2017;25:485‐497. [DOI] [PubMed] [Google Scholar]
- 57. Edmonds EC, McDonald CR, Marshall A, et al. Early versus late MCI: improved MCI staging using a neuropsychological approach. Alzheimers Dement. 2019;15(5):699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eppig JS, Edmonds EC, Campbell L, et al. Statistically derived subtypes and associations with cerebrospinal fluid and genetic biomarkers in mild cognitive impairment: a latent profile analysis. J Int Neuropsychol Soc. 2017;23:564‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information


