Abstract
Previous findings concerning gastric atrophy as a potential risk factor for esophageal squamous cell carcinoma (ESCC) have been inconsistent. We aimed to test whether gastric atrophy and, further, its interaction with poor oral health elevated the risk of ESCC in a high-risk region of China. Our population-based case-control study in Taixing, China (2010–2014), recruited cases from local hospitals and the local cancer registry. Controls were selected randomly from the local population registry. Ultimately, 1,210 cases and 1,978 controls answered questionnaires and provided blood samples for assay of pepsinogens. Unconditional logistic regression models were used to estimate odds ratios and 95% confidence intervals. Gastric atrophy (defined as a serum level of pepsinogen I of <55 μg/L) was associated with an increased risk for ESCC (odds ratio = 1.61; 95% confidence interval: 1.33, 1.96), even after full adjustment for potential confounding factors. In addition, suggestion of an additive interaction between gastric atrophy and poor oral health was observed (relative excess risk due to interaction = 1.28, 95% confidence interval: 0.39, 2.18). We conclude that gastric atrophy appears to be a risk factor for ESCC in a high-risk region of China, and there is a suggested additive interaction with poor oral health that increases this risk even further.
Keywords: esophageal squamous cell carcinoma, gastric atrophy, pepsinogens, poor oral health
Abbreviations
- CI
confidence interval
- ESCC
esophageal squamous cell carcinoma
- PGI
pepsinogen I
- PGII
pepsinogen II
Esophageal cancer, the sixth most common cause of cancer death, is associated with very poor survival (mortality-to-incidence ratio of 0.88) and was responsible for 500,000 deaths worldwide in 2018 (1). In that year, the highest mortality (10.7 deaths per 100,000 person-years) and incidence rates were both reported in eastern Asia. In 2012, 87% of the esophageal cancer cases were esophageal squamous cell carcinoma (ESCC), 79% of which occurred in China, India, and Southeast and Central Asia (2).
Gastric atrophy caused by chronic Helicobacter pylori infection is already known to be involved in the development of gastric cancer (3). Moreover, Ye et al. (4) first reported an unexpected association between gastric atrophy and ESCC risk, a result subsequently confirmed by 2 case-control studies based on endoscopy findings in Japan (5, 6). One of these also reported a rising risk with increasing severity of the gastric atrophy (histological fundic atrophy and fundic intestinal metaplasia) (5). Another retrospective, register-based study of endoscopy findings in Japan reported a higher proportion of gastric atrophy in ESCC patients compared with patients with esophageal adenocarcinoma (7).
However, although a cohort study in the Netherlands also observed a positive association between gastric atrophy and the risk for ESCC, they could not confirm that the risk increased with the severity of gastric atrophy (8). Therefore, they concluded that a causal relationship seems unlikely and that the association can be explained by confounding factors such as smoking (8). Furthermore, a prospective case-cohort study did not find an association between the level of serum pepsinogen, a marker for gastric atrophy, and ESCC in Linxian, China, a high-risk area for this carcinoma (9).
Poor oral health has been reported to be an independent risk factor for ESCC in high-risk areas (10, 11). In a case-control study on a high-risk population in Iran by Nasrollahzadeh et al. (12), they not only once again confirmed the association between gastric atrophy (assessed on the basis of serum pepsinogens) and ESCC but also observed a potential interaction with poor oral health that led to a further increase in the risk. However, that study did not have sufficient statistical power (293 cases and 524 controls), particularly for interaction analysis, and it also had certain limitations in study design (e.g., the utilization of neighborhood controls and individual matching, which might have introduced overmatching bias and hampered appropriate analysis of interaction, respectively). The mechanism underlying the relationship between gastric atrophy and ESCC is still unknown (13); it could involve reductions in the number/activity of gastric glands and less acid secretion in the atrophic stomach, which would enable bacterial proliferation (14). Carcinogens produced by bacteria, such as nitrosamines and acetaldehyde, might then enter the esophagus through regurgitation and cause esophageal cancer (15); nonacidic reflux has also been proposed to be the missing link between gastric atrophy and ESCC (16). Moreover, poor oral health, with altered bacterial flora in the oral cavity and additional carcinogens entering the esophagus, might further elevate the risk for ESCC (10).
In the light of such inconsistent findings regarding the association between gastric atrophy and ESCC, as well as the earlier lack of sufficient statistical power to examine the possible interaction between gastric atrophy and poor oral health, we designed the present large population-based, case-control study in a high-risk region in China.
METHODS
Subject recruitment
The research design and flow of subject recruitment have been described in detail previously (17–19). In brief, we performed a population-based, case-control study from 2010–2014 in Taixing, Jiangsu province, China, where the incidence of ESCC is high (11). More than 90% of the esophageal cancer patients in this area are referred to the 4 largest hospitals (the People’s Hospital of Taixing, the Second People’s Hospital of Taixing, the Third People’s Hospital of Taixing, and the Hospital of Traditional Chinese Medicine of Taixing).
Individuals diagnosed by the endoscopy units in these hospitals between October 2010 and September 2013 were invited to participate. This approach was designed to reduce the risk of nondifferential recall bias, given that these patients were unaware of their cancer diagnosis at the time of recruitment and data collection. We complemented this case recruitment with linkage to the local cancer registry during the same period. Altogether, 1,681 suspected cases of esophageal cancer were identified by the hospitals and through linkage to the local cancer registry. On the basis of available sections from formalin-fixed and paraffin-embedded tissue blocks stained with hematoxylin-eosin, a pathologist histopathologically verified 1,499 cases of cancer (78.3% of the total number indicated in the local cancer registry), including 1,418 cases of ESCC and 81 tumors of other types. Here only the cases of ESCC are analyzed (Figure 1).
Figure 1.
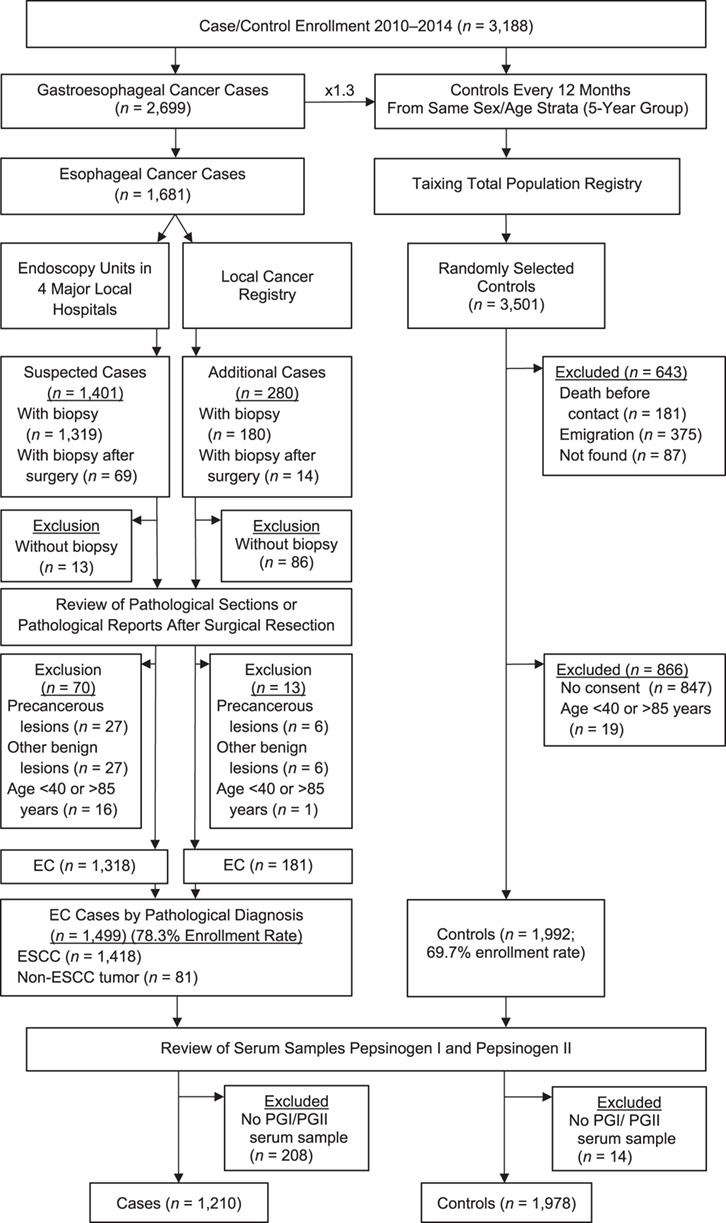
Flowchart illustrating the recruitment of cases of esophageal squamous cell carcinoma (ESCC) and control individuals in connection with a population-based, case-control study conducted in Taixing, China, 2010–2014. EC, esophageal cancer; PGI, pepsinogen I; PGII, pepsinogen II.
We utilized the Taixing Total Population Registry to randomly select control individuals who frequency-matched the patients in sex and age (within the same 5-year group) every 12 months. Simultaneously, cases of gastric cancer were enrolled. Because the age distributions for development of these 2 types of cancer are similar, a common set of controls was employed.
In total, 2,699 potential cases of gastroesophageal cancer were identified. Anticipating a moderately high nonresponse rate (20%–30%) among controls, we randomly selected 1.3 controls per case, for a total of 3,501. Of these, 643 were excluded due to death, emigration out of the study area, or inability to contact, leaving 2,858 eligible controls, of whom 2,011 provided informed consent and 1,992 were aged 40–85 years, giving a participation rate of 70.4% (2,011/2,858) (Figure 1).
Quality control
The inclusion criteria were an age of 40–85 years and residency in Taixing for at least the last 5 years. We attempted to reduce the risk of information bias by tape recording the interviews and assigning each interviewer the same number of cases and controls to balance information misclassification.
To examine the influence of potential selection bias, we compared the age and sex distribution of the enrolled (78.3%) and nonenrolled (21.7%) cases and enrolled (69.7%) and nonenrolled (30.3%) controls and found no statistically significant differences (Web Table 1, available at https://academic.oup.com/aje). We could not assess any potential differences in other demographic, clinical, or social characteristics, because we did not have such information concerning the nonrespondents.
Data collection and assays for biomarkers
The staff involved were specially trained to perform face-to-face interviews using a structured electronic questionnaire, covering factors proposed to be potential confounders in connection with previous studies in this area (demographic characteristics, lifestyle, family structure, and history of cancer).
Our aim was to collect 10-mL blood samples (2 tubes) from all participants, but 208 cases and 14 controls did not have available samples upon analysis. Serum prepared from 1,210 cases and 1,978 controls was stored at −80°C for later determination of pepsinogen I and II (PGI and PGII) concentrations by enzyme-linked immunosorbent assay (Pepsinogen I and II Kits; Biohit Healthcare, Helsinki, Finland) at Qilu Hospital of Shandong University, China. The interassay coefficients of variation were 11.3% and 14.9%, respectively. Immunoglobulin G antibodies directed against H. pylori were quantified by immunoblotting (H. pylori IgG Antibody Detection Kit; Syno Gene Digital Technology, Taizhou, China).
Statistical analysis
Although a low level of serum PGI correlates with gastric fundic atrophy (20), there are no generally accepted cutoff values for PGI, PGII, or the PGI:PGII ratio in this connection. On the basis of our previous study of an Asian population (12), we chose a serum level of PGI below 55 μg/L (sensitivity 61.9%, specificity 94.8%, positive predictive value 48.1%, negative predictive value 97.2%, and area under the curve 0.78) as the serological definition of this condition. A similar criterion was proposed on the basis of an investigation on a Chinese population (21). In addition, we examined potential associations employing other definitions of gastric atrophy (22).
The distributions of demographic and pepsinogen values for cases and controls were compared using the Mann-Whitney unpaired test for continuous variables and χ2 test or Fisher’s exact (observed number < 5) test for categorical variables (after excluding missing values). We used unconditional logistic regression models to estimate the odds ratios with 95% confidence intervals for association of ESCC with gastric atrophy as indicated by the serum concentration of PGI or PGII or PGI:PGII ratio.
Model fitting.
Our multivariate analysis involved fitting 3 models. In the first model, we adjusted only for the frequency-matched variables age (continuous) and sex. For the “almost fully adjusting” model, we also adjusted for education (illiteracy, primary school, middle school, high school and above), marital status (unmarried, married, divorced or widowed), occupation (farmer, worker, service/clerk/professional/administrator), family wealth score (quartiles 1–5), body mass index 10 years before (<18.5, 18.5–23.9, 24.0–27.9, ≥28.0), tea drinking (never/ever), history of esophageal cancer among first-degree relatives (no/yes), cigarette smoking (in pack years: never-smoker, former-smoker, current light smoker (≤18), current medium smoker (19–40), current heavy smoker (>41)), alcohol consumption in g/day (never drinker, former drinker, current low consumption (≤40), current medium consumption (41–135), current high consumption (>135)), and H. pylori serostatus (negative/positive). The full model also took into account the sum of missing and filled teeth (quartiles: 0, 1–3, 3–11, ≥12) and number of daily toothbrushings (≤1, ≥2). Potential confounding variables were identified through careful evaluation of risk factors for ESCC previously proposed to be important in the scientific literature (15, 23–25), together with our own background knowledge of this field. Missing data were disregarded when constructing the multivariate logistic regression models. In a separate analysis of ESCC in association with gastric atrophy, we also assessed the influence of missingness by using dummy variables indicating missing.
Assessment of interactions.
We examined both potential multiplicative and additive interactions between gastric atrophy and poor oral health, alcohol consumption, cigarette smoking, and tea drinking. In the case of multiplicative interactions, a cross-product term was introduced into the regression model and the P value was derived from Wald test. To test additive interactions, we used the relative excess risk due to interaction (RERI), synergy index (S), and attributable proportion (AP) due to interaction, and calculations were performed using the spreadsheet developed by Andersson et al. (26). Because alcohol consumption, cigarette smoking, and tea drinking were not common among our female subjects, we examined the interaction between these factors and gastric atrophy only among the men.
A 2-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were carried out with SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Sensitivity analysis
Sensitivity analysis was attempted by restricting the analysis of combined effects of gastric atrophy and oral health to patients with a serum level of PGII of <11.8 μg/L, as proposed in our earlier investigation in an area with a high incidence of ESCC (12). To assess the potential influence of nondifferential misclassification, we also performed such an analysis only on cases identified by the endoscopy units.
Ethical considerations
This study was approved by the Institutional Review Board of the School of Life Sciences, Fudan University; the Institutional Review Board of Qilu Hospital, Shandong University; and the Regional Vetting Board of Stockholm (Dnr 2018/357–31). All participants provided written informed consent.
RESULTS
The ratio of men to women (2:1) among our cases is typical for areas with a high incidence of ESCC (Table 1). Our frequency matching ensured that there were no differences in sex or age between the cases and controls. Although male cases and controls were similar in age, marital status, occupation, missing and filled teeth, and H. pylori serostatus, they did differ with regard to the other variables (Table 1). Female cases and controls were similar in marital status, tea drinking, smoking, alcohol consumption, and H. pylori serostatus, but they differed in all other respects (Table 1). The median PGI and PGII levels and PGI:PGII ratio differed statistically between male, but not female, cases and controls (Table 2).
Table 1.
Demographic Information for Study Subjects Enrolled in a Case-Control Study of Esophageal Squamous Cell Carcinoma (n = 3,188), Taixing, China, 2010–2014
| Men (n = 2,180) | Women (n = 1,008) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Controls
(n = 1,363) |
Cases
(n = 817) |
Controls
(n = 615) |
Cases
(n = 393) |
|||||||
| Variable | No. | % | No. | % | P Value | No. | % | No. | % | P Value |
| Age at interview, yearsa | 65.6 (8.4) | 65.5 (8.5) | 0.547b | 67.6 (9.4) | 69.5 (7.6) | 0.016b | ||||
| Age group at interview, years | 0.132c | <0.001c | ||||||||
| 40–49 | 53 | 3.89 | 25 | 3.06 | 29 | 4.72 | 5 | 1.27 | ||
| 50–59 | 274 | 20.10 | 180 | 22.03 | 89 | 14.47 | 28 | 7.12 | ||
| 60–69 | 584 | 42.85 | 352 | 43.08 | 208 | 33.82 | 162 | 41.22 | ||
| 70–79 | 402 | 29.49 | 216 | 26.44 | 235 | 38.21 | 160 | 40.71 | ||
| 80–85 | 50 | 3.67 | 44 | 5.39 | 54 | 8.78 | 38 | 9.67 | ||
| Educational level | 0.003c | <0.001d | ||||||||
| Illiterate | 171 | 12.55 | 145 | 17.75 | 366 | 59.51 | 285 | 72.52 | ||
| Primary school | 585 | 42.92 | 357 | 43.70 | 171 | 27.80 | 91 | 23.16 | ||
| Middle school | 459 | 33.68 | 235 | 28.76 | 66 | 10.73 | 15 | 3.82 | ||
| High school and above | 148 | 10.86 | 80 | 9.79 | 12 | 1.95 | 2 | 0.51 | ||
| Marital status | 0.574c | 0.677d | ||||||||
| Unmarried | 63 | 4.62 | 41 | 5.02 | 5 | 0.81 | 2 | 0.51 | ||
| Married | 1,151 | 84.45 | 676 | 82.74 | 426 | 69.27 | 264 | 67.18 | ||
| Divorced/widowed | 149 | 10.93 | 100 | 12.24 | 184 | 29.92 | 127 | 32.32 | ||
| Occupation | 0.494c | 0.006c | ||||||||
| Farmer | 709 | 52.02 | 446 | 54.59 | 545 | 88.62 | 371 | 94.40 | ||
| Worker | 354 | 25.97 | 198 | 24.24 | 43 | 6.99 | 16 | 4.07 | ||
| Service/clerk/professional/administrator | 300 | 22.01 | 173 | 21.18 | 27 | 4.39 | 6 | 1.53 | ||
| Family wealth score | <0.001c | 0.011c | ||||||||
| Quintile 1 (lowest) | 288 | 21.13 | 238 | 29.13 | 119 | 19.35 | 110 | 27.99 | ||
| Quintile 2 | 231 | 16.95 | 135 | 16.52 | 119 | 19.35 | 74 | 18.83 | ||
| Quintile 3 | 285 | 20.91 | 191 | 23.38 | 144 | 23.41 | 91 | 23.16 | ||
| Quintile 4 | 295 | 21.64 | 150 | 18.36 | 130 | 21.14 | 72 | 18.32 | ||
| Quintile 5 (highest) | 264 | 19.37 | 103 | 12.61 | 103 | 16.75 | 46 | 11.70 | ||
| Body mass indexe 10 years prior | <0.001c | 0.001c | ||||||||
| <18.5 | 59 | 4.33 | 63 | 7.71 | 56 | 9.11 | 52 | 13.23 | ||
| 18.5–23.9 | 857 | 62.88 | 555 | 67.93 | 340 | 55.28 | 219 | 55.73 | ||
| 24.0–27.9 | 363 | 26.63 | 166 | 20.32 | 172 | 27.97 | 112 | 28.50 | ||
| ≥28.0 | 82 | 6.02 | 30 | 3.67 | 46 | 7.48 | 9 | 2.29 | ||
| Missing | 2 | 0.15 | 3 | 0.37 | 1 | 0.16 | 1 | 0.25 | ||
| Sum of missing and filled teeth | 0.121c | 0.009c | ||||||||
| 0 | 366 | 26.85 | 193 | 23.62 | 129 | 20.98 | 55 | 13.99 | ||
| 1–3 | 349 | 25.61 | 187 | 22.89 | 137 | 22.28 | 72 | 18.32 | ||
| 4–11 | 345 | 25.31 | 211 | 25.83 | 164 | 26.67 | 107 | 27.23 | ||
| ≥12 | 285 | 20.91 | 197 | 24.11 | 177 | 28.78 | 140 | 35.62 | ||
| Missing | 18 | 1.32 | 29 | 3.55 | 8 | 1.30 | 19 | 4.83 | ||
| Frequency of toothbrushing per day | <0.001c | <0.001c | ||||||||
| Once or less | 896 | 65.74 | 649 | 79.44 | 385 | 62.60 | 312 | 79.39 | ||
| Twice or more | 449 | 32.94 | 141 | 17.26 | 223 | 36.26 | 69 | 17.56 | ||
| Missing | 18 | 1.32 | 27 | 3.30 | 7 | 1.14 | 12 | 3.05 | ||
| Tea drinking | <0.001c | 0.220c | ||||||||
| Never | 852 | 62.51 | 421 | 51.53 | 574 | 93.33 | 363 | 92.37 | ||
| Ever | 491 | 36.02 | 358 | 43.82 | 31 | 5.04 | 13 | 3.31 | ||
| Missing | 20 | 1.47 | 38 | 4.65 | 10 | 1.63 | 17 | 4.33 | ||
| History of esophageal cancer among first-degree relatives | <0.001c | <0.001c | ||||||||
| No | 1,098 | 80.56 | 532 | 65.12 | 496 | 80.65 | 257 | 65.39 | ||
| Yes | 250 | 18.34 | 256 | 31.33 | 111 | 18.05 | 122 | 31.04 | ||
| Missing | 15 | 1.10 | 29 | 3.55 | 8 | 1.30 | 14 | 3.56 | ||
| Smoking statusf | <0.001c | 0.841d | ||||||||
| Never-smoker | 315 | 23.11 | 171 | 20.93 | 591 | 96.10 | 374 | 95.17 | ||
| Former-smoker | 152 | 11.15 | 61 | 7.47 | 4 | 0.65 | 2 | 0.51 | ||
| Current light smoker | 231 | 16.95 | 104 | 12.73 | 13 | 2.11 | 10 | 2.54 | ||
| Current medium smoker | 411 | 30.15 | 262 | 32.07 | 6 | 0.98 | 5 | 1.27 | ||
| Current heavy smoker | 253 | 18.56 | 215 | 26.32 | 1 | 0.16 | 2 | 0.51 | ||
| Missing | 1 | 0.07 | 4 | 0.49 | 0 | 0.00 | 0 | 0.00 | ||
| Alcohol drinking statusg | <0.001c | 0.425d | ||||||||
| Never drinker | 580 | 42.55 | 191 | 23.38 | 570 | 92.68 | 348 | 88.55 | ||
| Former drinker | 72 | 5.28 | 34 | 4.16 | 5 | 0.81 | 2 | 0.51 | ||
| Current low consumption | 159 | 11.60 | 106 | 12.97 | 18 | 2.93 | 19 | 4.83 | ||
| Current medium consumption | 361 | 26.49 | 298 | 36.47 | 9 | 1.46 | 5 | 1.27 | ||
| Current high consumption | 170 | 12.47 | 150 | 18.36 | 2 | 0.33 | 0 | 0.00 | ||
| Missing | 21 | 1.54 | 38 | 4.65 | 11 | 1.79 | 19 | 4.83 | ||
| Helicobacter pylori serostatus | 0.119c | 0.848c | ||||||||
| Negative | 448 | 32.87 | 242 | 29.62 | 187 | 30.41 | 122 | 31.04 | ||
| Positive | 902 | 66.18 | 566 | 69.28 | 422 | 68.62 | 268 | 68.19 | ||
| Missing | 13 | 0.95 | 9 | 1.10 | 6 | 0.98 | 3 | 0.76 | ||
a Values are expressed as mean (standard deviation).
b Mann-Whitney unpaired test for continuous variables was used to derive P values after excluding the corresponding missing value.
c χ 2 test was used to derive P values after excluding the corresponding missing value.
d Fisher exact test for categorical variables was used to derive P values after excluding the corresponding missing value.
e Weight (kg)/height (m)2
f Current smokers: light, ≤18 pack-years; medium, 19–40 pack-years; and heavy, ≥41 pack-years.
g Current drinkers: low consumption, ≤40 g/day; medium consumption, 41–135 g/day; and high consumption, >135 g/day.
Table 2.
Serum Pepsinogen Values for Enrollees in a Case-Control Study of Esophageal Squamous Cell Carcinoma (n = 3,188), Taixing, China, 2010–2014
| Sex and Group | No. | Missing |
Mean
(SD) |
Median
(IQR) |
Range | 25th–75th Percentile | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| PGI, μg/L | |||||||
| Women | |||||||
| Cases | 391 | 2 | 0.51 | 97.52 (75.31) | 79.32 (65.23) | 0.00–469.40 | 51.77–117.00 |
| Controls | 611 | 4 | 0.65 | 92.94 (50.05) | 84.48 (53.37) | 0.00–350.40 | 60.43–113.80 |
| Men | |||||||
| Cases | 813 | 4 | 0.49 | 109.95 (84.29) | 89.55 (77.84) | 0.00–521.00 | 56.56–134.40 |
| Controls | 1,350 | 13 | 0.95 | 104.08 (55.82) | 95.74 (59.96) | 0.00–487.80 | 69.34–129.30 |
| PGII, μg/L | |||||||
| Women | |||||||
| Cases | 392 | 1 | 0.25 | 12.90 (13.81) | 9.88 (12.06) | 0.03–184.10 | 4.86–16.92 |
| Controls | 611 | 4 | 0.65 | 13.78 (11.64) | 10.04 (14.67) | 0.05–105.70 | 5.16–19.85 |
| Men | |||||||
| Cases | 814 | 3 | 0.37 | 13.96 (15.09) | 10.75 (11.87) | 0.05–251.20 | 5.79–17.66 |
| Controls | 1,352 | 11 | 0.81 | 14.76 (12.42) | 12.12 (12.89) | 0.05–244.00 | 6.94–19.83 |
| PGI:PGII Ratio | |||||||
| Women | |||||||
| Cases | 391 | 2 | 0.51 | 32.42 (178.40) | 8.54 (8.85) | 0.00–2996.00 | 5.17–14.03 |
| Controls | 611 | 4 | 0.65 | 33.56 (163.27) | 8.10 (7.99) | 0.00–2244.00 | 5.03–13.02 |
| Men | |||||||
| Cases | 813 | 4 | 0.49 | 39.55 (306.54) | 8.87 (7.72) | 0.00–6614.00 | 5.94–13.66 |
| Controls | 1,349 | 14 | 1.03 | 26.08 (155.90) | 7.93 (6.75) | 0.00–2732.00 | 5.32–12.08 |
Abbreviations: IQR, interquartile range; PGI, pepsinogen I; PGII, pepsinogen II; SD, standard deviation.
The odds ratio for ESCC in association with gastric atrophy (defined as a PGI level of <55 μg/L), when adjusted for age and sex, was 1.60 (95% confidence interval (CI): 1.34, 1.90) (Table 3). The estimate was quite similar when fully adjusted for age, sex, education, marital status, occupation, family wealth score, body mass index 10 years before, tea drinking, history of esophageal cancer among first-degree relatives, smoking status, alcohol consumption, and H. pylori serostatus (odds ratio = 1.63, 95% CI: 1.35, 1.97), and even when adjusted further for the sum of missing and filled teeth and frequency of daily toothbrushing (odds ratio = 1.61, 95% CI: 1.33, 1.96) (Table 3). Analyses stratified by sex showed statistically significant odds ratios for ESCC in association with gastric atrophy in both men and women (data not shown). In a subset of the cases for whom we had information about tumor location, the odds ratio for ESCC in the upper part of the esophagus was 1.35 (95% CI: 0.87, 2.09) while the odds ratio for ESCC in the mid and lower part of the esophagus was 1.87 (95% CI 1.50, 2.34). When other cutoff values were employed to define gastric atrophy, the resulting odds ratios were always greater than 1 but not always statistically significant (Web Table 2). H. pylori serostatus did not differ significantly between the cases and controls (Table 3).
Table 3.
Odds Ratios for Esophageal Squamous Cell Carcinoma in Association With Gastric Atrophy, Defined by Pepsinogen I Values of <55 μg/L, and Helicobacter pylori Seropositivity in a Case-Control Study, Taixing, China, 2010–2014
| Variable | Controls | Cases | Age/Sex-Adjusted b | Fully Adjusted (Except MFT and Toothbrushing) c | Fully Adjusted d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. a | % | No. a | % | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| PGI, μg/L | <0.001 | <0.001 | <0.001 | ||||||||||
| ≥55 | 1,592 | 83.3 | 841 | 75.1 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| <55 | 319 | 16.7 | 279 | 24.9 | 1.60 | 1.34, 1.90 | 1.63 | 1.35, 1.97 | 1.61 | 1.33, 1.96 | |||
| H. pylori serostatus | 0.215 | 0.211 | 0.248 | ||||||||||
| Negative | 622 | 32.5 | 340 | 30.3 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Positive | 1,291 | 67.5 | 782 | 69.7 | 1.10 | 0.95, 1.29 | 1.11 | 0.94, 1.32 | 1.11 | 0.93, 1.31 | |||
Abbreviations: CI, confidence interval; MFT, missing and filled teeth; OR, odds ratio; PGI, pepsinogen I.
a Complete observations in the full model.
b Adjusted for age (continuous) and sex.
c Adjusted for age (continuous), sex, education, marital status, occupation, family wealth score, body mass index 10 years prior, tea drinking, history of esophageal cancer among first-degree relatives, smoking status, alcohol drinking status, and H. pylori serostatus (except age, all other variables are categorized as in Table 1).
d Additionally adjusted for sum of missing and filled teeth and frequency of toothbrushing per day.
When gastric atrophy was defined as a serum level of PGI of <55 μg/L, none of the multiplicative interactions between gastric atrophy and missing and filled teeth or number of daily toothbrushings were statistically significant (Table 4). However, indicators of additive interaction—the relative excess risk due to interaction, synergy index, and attributable proportion due to interaction—were all significant in the case of toothbrushing. When gastric atrophy was defined as a PGI of <30 μg/L, the relative excess risk due to interaction was 1.17, the synergy index was 2.38, and the attributable proportion due to interaction was 0.39 for the sum of missing and filled teeth, with corresponding values of 2.40, 2.95, and 0.52 for toothbrushing (Web Table 3). Sensitivity analysis limited to those with PGII < 11.8 μg/L produced similar results (Web Table 4). No significant positive interactions between gastric atrophy (defined as PGI < 55 μg/L) and alcohol consumption, cigarette smoking, or tea drinking were observed (Web Table 5). Sensitivity analysis restricted to cases identified by endoscopy units did not change the results materially (data not shown). Including subjects with missing data in the multivariate logistic regression model also did not change the estimates markedly (data not shown).
Table 4.
Estimation of Combined Effects of Gastric Atrophy (Defined by Pepsinogen I Values of < 55 μg/L), Dental Health, and Oral Hygiene on Esophageal Squamous Cell Carcinoma Risk in a Case-Control Study in Taixing, China, 2010–2014
| No Gastric Atrophy | Gastric Atrophy Present | Interaction Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | Controls | Cases | Additive Interaction | Multiplicative Interaction | ||||||||||
| Interaction Variable | No. a | No. a | OR b | 95% CI | No. a | No. a | OR b | 95% CI | RERI OR | 95% CI | S | 95% CI | AP c | 95% CI | P Value |
| Missing and filled teethd | |||||||||||||||
| <4 | 824 | 388 | 1.00 | Referent | 143 | 105 | 1.66 | 1.23, 2.25 | |||||||
| ≥4 | 768 | 453 | 1.55 | 1.18, 2.03 | 176 | 174 | 2.45 | 1.76, 3.93 | 0.23 | −0.53, 0.99 | 1.19 | 0.67, 2.10 | 0.09 | −0.20, 0.39 | 0.786 |
| No. of toothbrushings per daye | |||||||||||||||
| ≥2 | 545 | 158 | 1.00 | Referent | 106 | 44 | 1.27 | 0.84, 1.93 | |||||||
| ≤1 | 1,047 | 683 | 2.14 | 1.72, 2.65 | 213 | 235 | 3.67 | 2.80, 4.85 | 1.28 | 0.39, 2.18 | 1.91 | 1.16, 3.15 | 0.35 | 0.15, 0.55 | 0.203 |
Abbreviations: AP, attributable proportion; CI, confidence interval; OR, odds ratio; RERIOR, relative excess risk due to interaction; S, synergy index.
a Complete observations.
b All ORs were adjusted for age (continuous), sex, education, marital status, occupation, family wealth score, body mass index 10 years prior, tea drinking, history of esophageal cancer among first-degree relatives, smoking status, alcohol drinking status, Helicobacter pylori serostatus (except age, all other variables are categorized as in Table 1).
c Attributable proportion due to interaction.
d Additionally adjusted for frequency of toothbrushing per day.
e Additionally adjusted for sum of missing and filled teeth.
DISCUSSION
Previous reports on the potential association between gastric atrophy and ESCC have been inconsistent, probably due to inappropriate study design and small sample sizes in some studies. Although a prospective cohort study on patients diagnosed histopathologically with gastric atrophy would be ideal, such a study is almost impossible to perform because of ethical considerations and the relatively low incidence of ESCC. A population-based case-control study with a large sample size would be the next best choice, but again, endoscopic examination of all cases and control subjects is not possible. Although gastric atrophy defined serologically is an inherent limitation due to the poor sensitivity of pepsinogen biomarkers, such nondifferential misclassification would bias the true association towards null. Thus, our present population-based, case-control study, with a large sample size, would provide a good possibility of detecting any potential blurred association and any interactions with other risk factors.
Our findings confirm that gastric atrophy (defined as a PGI serum level of <55 μg/L) is a risk factor for ESCC in a high-risk region in eastern China, even after adjusting for a variety of known potential confounders. The cutoff point of <55 μg/L was validated with the same test system (Pepsinogen I and II Kits; Biohit HealthCare) in a prior study in northern Iran with similar prevalence of H. pylori infection and pangastritis as well as ESCC incidence and mortality as in this study.
Our conclusion is contradictory to that arrived at on the basis of a case-cohort study in another high-risk area in China (9). However, with a similar cutoff value of ≤50 μg/L for PGI, the point estimate of 1.64 (hazard ratio) in that earlier investigation (although only borderline statistically significant due to the small sample size) is very close to our estimate here.
However, their nonlinear continuous model, using the median of the 4th quartile of PGI:PGII ratio as a reference, showed no obvious trend. Here, we also found that the median PGI:PGII ratio was higher among cases than controls. Smoking (27) and use of proton pump inhibitors (28) (probably more common among cases than controls), might raise PGI levels and explain this difference. Moreover, because the biological meaning of a high PGI level or PGI:PGII ratio remains unclear, the usefulness of treating these as continuous variables is uncertain.
A key limitation to the present investigation is the risk of misclassification of exposure, because gastric atrophy was measured by serum pepsinogen levels and not endoscopy. Due to the population-based study design, we did not ask healthy control subjects to undergo endoscopy for ethical reasons and logistics constraints, and therefore we did not collect the histopathological reports from gastric biopsies taken for clinical purposes from ESCC patients either, although they could have been used for histopathological confirmation of gastric atrophy in a subset of the ESCC patients. We attempted to minimize this limitation by considering a broad span of cutoff values for serum levels of PGI and/or the PGI:PGII ratio. Many cutoff values proposed previously showed robust indications that gastric atrophy is a risk factor for ESCC.
Even though we made considerable efforts to reduce the risk of bias and confounding here, this risk always remains. Although the age and sex distributions of respondents and nonrespondents were similar (Web Table 1), these groups might have differed with respect to other demographic, clinical, or social characteristics. If such differences were similar for the cases and controls, the results would be biased towards null, but a differential difference could distort the findings in an unknown manner. However, such differences would have to be of a magnitude that we do not consider reasonable. The situation is similar for other forms of information bias, such as misclassification of diagnosis. The 208 cases that were unavailable for analysis were similar to the 1,210 cases who provided a blood sample for analysis in most aspects, except for age, BMI, missing and filled teeth, and frequency of toothbrushing (Web Table 6). If all of them did not have gastric atrophy, our estimate would be deflated to a maximally adjusted odds ratio of 1.28 (95% CI: 1.06, 1.55), which is still statistically significant. If however all of them had gastric atrophy, that would increase our estimate to a maximally adjusted odds ratio of 2.73 (95% CI: 2.30, 3.25).
The generalizability of our study results might be limited due to the fact that our study population has one of the highest incidence rates of ESCC in the world. Another limitation is lack of oral health information regarding medical conditions such as untreated decay, tooth bleeding, leukoplakia, and gingivitis, which are closely related to the risk of ESCC.
The major strengths of this investigation are its truly population-based, case-control study design and our considerable efforts to minimize the influence of selection bias and recall bias. We collected detailed information concerning potential confounding factors. Furthermore, our large sample size guaranteed sufficient statistical power to detect even small effects and examine interactions.
Our overall interpretation of present and previous findings is that gastric atrophy appears to be a risk factor for ESCC, although the evidence is not yet definitive. The 2 possible underlying causal relationships proposed previously require further elucidation. First, oxidative damage caused by nonacidic gastroesophageal reflux (16) might lead to malignant transformation of epithelial cells as it did in an animal model (29). This is partly supported by a higher odds ratio for ESCC in the mid and lower part compared with that in the upper part of the esophagus in our study. Second, alterations in the gastric microbiota might be involved, given that we have detected dysbiosis among patients with ESCC and squamous dysplasia (30). The altered gastric microbiome might harbor species that can carry out N-nitrosation, producing substances known to be carcinogenic towards esophageal mucosa (16).
We also found an additive interaction with poor oral hygiene, which might be due to a biological interaction mechanism. Again, altered microbiota in the oral cavity, due to poor dental health and oral hygiene, might lead to distant effects from inflammation (10) or enhanced production of carcinogenic nitrosamines (11, 31). Furthermore, gastric atrophy might act as a source of such altered microbiota (12). Another explanation for our observed findings could be that the microbiome associated with poor oral health could predispose the development of gastric atrophy and subsequent carcinogenesis in susceptible individuals, and if so, gastric atrophy might lie in the pathway between poor oral health and ESCC. However poor oral health might also be a confounder for the association between gastric corpus atrophy and ESCC. Altogether, we recommend that future research in this area employ histopathological diagnosis of gastric atrophy and explore the role of an altered gastric and oral microbiome in the development of ESCC (30).
In summary, we conclude that gastric atrophy (as defined by the serum level of pepsinogen), appears to be a risk factor for ESCC in a high-risk region of eastern China, and furthermore, there are indications of an additive interaction with poor oral hygiene.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (Isabella Ekheden, Weimin Ye); Clinical Epidemiology Unit, Qilu Hospital of Shandong University, Jinan, China (Xiaorong Yang, Hui Chen, Ming Lu); State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China (Xingdong Chen, Li Jin); Fudan University Taizhou Institute of Health Sciences, Taizhou, China (Xingdong Chen, Ziyu Yuan, Li Jin, Ming Lu, Weimin Ye); Human Phenome Institute, Fudan University, Shanghai, China (Xingdong Chen, Li Jin); Department of Epidemiology, School of Public Health, Shandong University, Jinan, China (Ming Lu); Department of Epidemiology and Health Statistics, Fujian Medical University, Fuzhou, China (Weimin Ye); and Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China (Weimin Ye).
I.E. and X.Y. contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (grants 81573229 and 81273151 to M.L.), the International S&T Cooperation Program of China (grant 2015DFE32790 to X.C., M.L., and W.Y.), the Swedish Cancer Society (grant 2016/510 to W.Y.), and the Swedish Research Council (grant 2015-02625 to W.Y.). I.E. is supported in part by a scholarship from the Karolinska Institutet MD/PhD program.
We thank the interviewers and technicians at the Taizhou Institute of Health of Fudan University for data collection and sample preparation, the staff at the Taixing Center for Disease Control and Prevention for organizing the field work, and the staff at the study hospitals for their help with sample collection.
Conflict of interest: none declared.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. [DOI] [PubMed] [Google Scholar]
- 3. Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48(13):3554–3560. [PubMed] [Google Scholar]
- 4. Ye W, Held M, Lagergren J, et al. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. J Natl Cancer Inst. 2004;96(5):388–396. [DOI] [PubMed] [Google Scholar]
- 5. Iijima K, Koike T, Abe Y, et al. Extensive gastric atrophy: an increased risk factor for superficial esophageal squamous cell carcinoma in Japan. Am J Gastroenterol. 2007;102(8):1603–1609. [DOI] [PubMed] [Google Scholar]
- 6. Akiyama T, Inamori M, Iida H, et al. Macroscopic extent of gastric mucosal atrophy: increased risk factor for esophageal squamous cell carcinoma in Japan. BMC Gastroenterol. 2009;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okada M, Ishimura N, Mikami H, et al. Circumferential distribution and clinical characteristics of esophageal cancer in lower esophagus: differences related to histological subtype. Esophagus. 2019;16(1):98–106. [DOI] [PubMed] [Google Scholar]
- 8. de Vries AC, Capelle LG, Looman CW, et al. Increased risk of esophageal squamous cell carcinoma in patients with gastric atrophy: independent of the severity of atrophic changes. Int J Cancer. 2009;124(9):2135–2138. [DOI] [PubMed] [Google Scholar]
- 9. Ren JS, Kamangar F, Qiao YL, et al. Serum pepsinogens and risk of gastric and oesophageal cancers in the General Population Nutrition Intervention Trial cohort. Gut. 2009;58(5):636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Yuan Z, Lu M, et al. Poor oral health is associated with an increased risk of esophageal squamous cell carcinoma—a population-based case-control study in China. Int J Cancer. 2017;140(3):626–635. [DOI] [PubMed] [Google Scholar]
- 12. Nasrollahzadeh D, Malekzadeh R, Aghcheli K, et al. Gastric atrophy and oesophageal squamous cell carcinoma: possible interaction with dental health and oral hygiene habit. Br J Cancer. 2012;107(5):888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McColl KE. Helicobacter pylori and oesophageal cancer—not always protective. Gut. 2007;56(4):457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 15. Kamangar F, Chow WH, Abnet CC, et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38(1):27–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cook MB. Non-acid reflux: the missing link between gastric atrophy and esophageal squamous cell carcinoma? Am J Gastroenterol. 2011;106(11):1930–1932. [DOI] [PubMed] [Google Scholar]
- 17. Suo C, Yang Y, Yuan Z, et al. Alcohol intake interacts with functional genetic polymorphisms of aldehyde dehydrogenase (ALDH2) and alcohol dehydrogenase (ADH) to increase esophageal squamous cell cancer risk. J Thorac Oncol. 2019;14(4):712–725. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Ni Y, Yuan Z, et al. Very hot tea drinking increases esophageal squamous cell carcinoma risk in a high-risk area of China: a population-based case-control study. Clin Epidemiol. 2018;10:1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Chen X, Zhuang M, et al. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: a population-based case-control study in China. Sci Rep. 2017;7(1):17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samloff IM, Varis K, Ihamaki T, et al. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83(1):204–209. [PubMed] [Google Scholar]
- 21. Tong Y, Wu Y, Song Z, et al. The potential value of serum pepsinogen for the diagnosis of atrophic gastritis among the health check-up populations in China: a diagnostic clinical research. BMC Gastroenterol. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang YK, Yu JC, Kang WM, et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic review and meta-analysis. PLoS One. 2015;10(11):e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24(5):729–735. [DOI] [PubMed] [Google Scholar]
- 24. Tran GD, Sun XD, Abnet CC, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113(3):456–463. [DOI] [PubMed] [Google Scholar]
- 25. Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17(1):2–9. [DOI] [PubMed] [Google Scholar]
- 26. Andersson T, Alfredsson L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579. [DOI] [PubMed] [Google Scholar]
- 27. Tatemichi M, Kabuto M, Tsugane S. Effect of smoking on serum pepsinogen I level depends on serological status of Helicobacter pylori. Jpn J Cancer Res. 2001;92(3):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee CT, Kuo BI, Chen CY, et al. Helicobacter pylori clearance and serum gastrin and pepsinogen I concentrations in omeprazole treatment of duodenal ulcer patients. Eur J Clin Pharmacol. 1999;54(11):817–820. [DOI] [PubMed] [Google Scholar]
- 29. Inayama M, Hashimoto N, Tokoro T, et al. Involvement of oxidative stress in experimentally induced reflux esophagitis and esophageal cancer. Hepatogastroenterology. 2007;54(75):761–765. [PubMed] [Google Scholar]
- 30. Nasrollahzadeh D, Malekzadeh R, Ploner A, et al. Variations of gastric corpus microbiota are associated with early esophageal squamous cell carcinoma and squamous dysplasia. Sci Rep. 2015;6:8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen X, Winckler B, Lu M, et al. Oral microbiota and risk for esophageal squamous cell carcinoma in a high-risk area of China. PLoS One. 2015;10(12):e0143603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


