ABSTRACT
Background
Muscle protein synthesis (MPS) can be stimulated by ingestion of protein sources, such as whey, casein, or soy. Protein supplementation can enhance muscle protein synthesis after exercise and may preserve skeletal muscle mass and function in aging adults. Therefore, identifying protein sources with higher anabolic potency is of high significance.
Objective
The aim of this study was to determine the anabolic potency and efficacy of a novel whey protein hydrolysate mixture (WPH) on mechanistic target of rapamycin complex 1 (mTORC1) signaling and skeletal MPS in healthy young subjects.
Methods
Ten young men (aged 28.7 ± 3.6 y, 25.2 ± 2.9 kg/m2 body mass index [BMI]) were recruited into a double-blind two-way crossover trial. Subjects were randomized to receive either 0.08 g/kg of body weight (BW) of WPH or an intact whey protein (WHEY) mixture during stable isotope infusion experiments. Fractional synthetic rate, leucine and phenylalanine kinetics, and markers of amino acid sensing were assessed as primary outcomes before and 1–3 h after protein ingestion using a repeated measures mixed model.
Results
Blood leucine concentration, delivery of leucine to muscle, transport of leucine from blood into muscle and intracellular muscle leucine concentration significantly increased to a similar extent 1 h after ingestion of both mixtures (P < 0.05). Phosphorylation of S6K1 (i.e. a marker of mTORC1 activation) increased equally by ∼20% 1-h postingestion (P < 0.05). Ingestion of WPH and WHEY increased mixed MPS similarly in both groups by ∼43% (P < 0.05); however, phenylalanine utilization for synthesis increased in both treatments 1-h postingestion but remained elevated 3-h postingestion only in the WPH group (P < 0.05).
Conclusions
We conclude that a small dose of WPH effectively increases leucine transport into muscle, activating mTORC1 and stimulating MPS in young men. WPH anabolic potency and efficacy for promoting overall muscle protein anabolism is similar to WHEY, an intact protein source. This trial was registered at clinicaltrials.gov as NCT03313830.
Keywords: amino acid transport, human muscle protein turnover, hyperaminoacidemia, muscle protein anabolism, whey protein, anabolic signaling, leucine
Introduction
Muscle mass is controlled by the dynamic and concurrent cellular processes of protein synthesis (MPS) and breakdown (MPB). The balance between synthesis and breakdown rates determine whether a muscle undergoes growth or atrophy. The intake of dietary protein, and in particular essential amino acids (EAA), has been shown to stimulate protein synthesis via nutrient-sensitive cell signaling pathways regulated by activation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway (1–5). Of the EAAs, the branched-chain amino acids (BCAAs) appear to be the amino acids responsible for activating protein synthesis, with leucine being the most potent (1, 6–12).
Over the past few years, the effects of protein supplementation on muscle adaptation have been extensively described. Studying protein ingestion following an acute bout of resistance exercise training, we found that a protein blend and intact whey protein have a similar effect on hyperaminoacidemia, mTORC1 pathway activation, MPS and MPB, both in young adults (13). Protein blend ingestion following resistance exercise also promotes human muscle protein synthesis in older adults (14). However, the protein blend was able to prolong hyperaminoacidemia, muscle protein fractional synthetic rate (FSR), and muscle protein anabolism via mTORC1 signaling (15). The rapid digestion and absorption of whey protein increases blood amino acid concentrations shortly following ingestion, whilst ingestion of casein produces a slower but more prolonged (6 h) hyperaminoacidemia that can prolong postexercise muscle protein FSR (13, 15). Those findings confirm that the ability of a protein to stimulate muscle protein anabolism is associated with its overall protein quality/source (i.e. amino acid composition) (17, 18) and its digestion rate (i.e. fast, intermediate, or slow) (19, 20).
Based on the above assumption, recent studies have focused on whey protein hydrolysate (WPH). WPH contains mostly peptides, which have physiological effects and can be absorbed more rapidly. Bioactive peptides, such as Ile-Val, Leu-Val, Val-Leu, Ile-Ile, Ile-Leu, and Leu-Leu, can increase muscle glycogen repletion by promoting glucose uptake (21, 22). It is not known whether these bioactive peptides can enhance MPS in skeletal muscle to a greater extent than leucine, which is believed to be the major nutritional anabolic stimulus. Preliminary data from our preclinical study has also demonstrated that WPH can stimulate MPS at lower doses compared with intact whey protein (WHEY). Tang et al. demonstrated, for example, that WPH stimulates MPS to a greater extent than casein and soy (23) in the rested state and after exercise. Other studies conducted in animal models by Kanda et al. showed that dietary WPH can increase muscle glycogen content after exercise, by regulating glycogen synthase (21), and promote postexercise recovery and MPS (24). The most valid hypothesis to explain these results is based on the pattern of appearance of amino acids in the systemic circulation. When hydrolyzed whey is consumed, the rise in EAA, BCAA, and leucine are greater than with other sources, such as soy or casein (23).
Given its dynamic role in glucose metabolism and its role in the activation of mTORC1 signaling, WPH may be an important nutritional supplement for promoting skeletal MPS in individuals with sarcopenia or other muscle-wasting conditions. Although studies have examined the effect of WPH and other different sources of protein (e.g. soy, casein, or whey) in combination with exercise (13–15), no study to our knowledge has compared the effect of WPH and WHEY on muscle protein net balance and amino acid transport into muscle. Thus, the purpose of this study was to compare the response of amino acid uptake, mTORC1 signaling, and MPS after the ingestion of WHEY or WPH in healthy young men. The rationale for comparing WHEY with WPH (rather than comparison to a variety of other protein sources) is 2-fold: 1) the higher prevalence of bioactive peptides in the composition of WPH is significantly different from WHEY and may have a different effect on muscle protein metabolism; 2) WHEY stimulates MPS to a greater extent than plant sources containing the same amount of protein (25). Thus, by comparing WPH directly to WHEY we can examine the role of WPH composition by using an identical low dose of protein for each group. Unfortunately, our current understanding of peptide regulation of muscle anabolism in humans is limited. Therefore, we hypothesized that WPH would stimulate MPS to a greater extent than WHEY when both are given at an equivalent low dose.
Methods
Subjects
Ten healthy young men (aged 28.7 ± 3.6 y; BMI 25.2 ± 2.9 kg/m2) were recruited from the Galveston, TX community via advertisements in local newspapers and websites. After we carefully explained procedures and risks, informed consent was obtained from all subjects. Eligibility was determined using clinical history, physical exam, and laboratory tests. Subjects with morbid obesity, diabetes, cancer, active infections, or significant cardiovascular disease, or renal, liver or pulmonary disease were excluded. Subjects were all recreationally active but not involved in regular and/or strenuous physical activity (>2 times/wk) and they were not regularly consuming protein supplements. Subject characteristics are described in Table 1.
TABLE 1.
Characteristics of the 10 young men that participated in the double-blind crossover trial1
| Subjects (N = 10) | |
|---|---|
| Age, y | 28.7 ± 3.6 |
| Weight, kg | 79.3 ± 12.2 |
| BMI, kg/m2 | 25.5 ± 2.9 |
| Lean mass, % | 73.0 ± 6.0 |
| Fat mass, % | 23.0 ± 6.3 |
1All values are means ± SDs, subjects n = 10. Sample group included 9 Caucasians and 1 Asian.
Subjects came in fasting for their screening visit for blood measurements. A dual-energy X-ray absorptiometry (Lunar iDEXA) scan analysis was performed to determine body composition. Interventional protein intake was then adjusted according to the body weight (BW) of each subject. The CONSORT (Consolidated Standards of Reporting Trials) diagram is shown in Figure 1.
FIGURE 1.
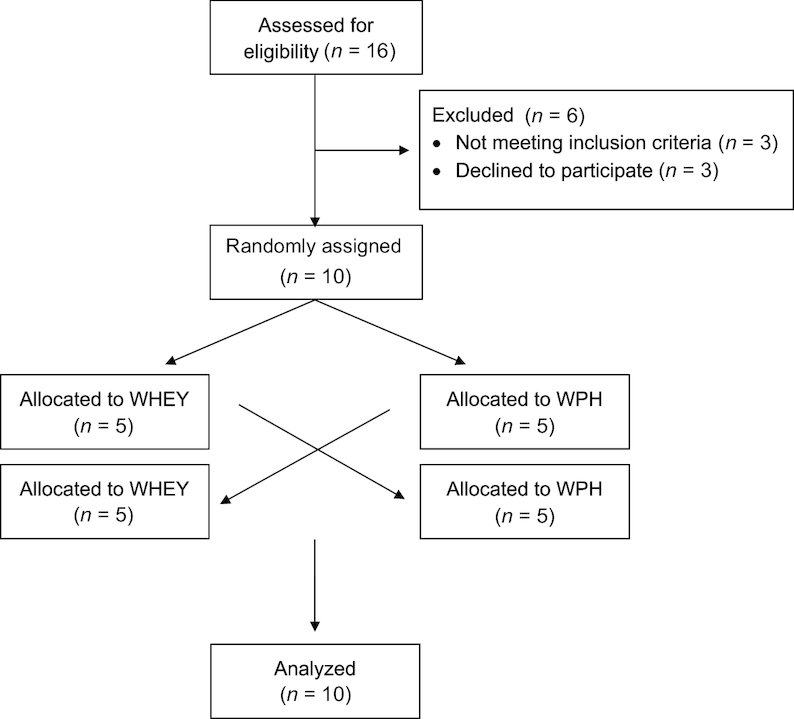
Consolidated Standards of Reporting Trials (CONSORT) diagram of study recruitment, enrollment, randomization, follow-up, and analysis. Abbreviations: WHEY, intact whey protein beverage; WPH, whey protein hydrolyzed beverage.
Study design
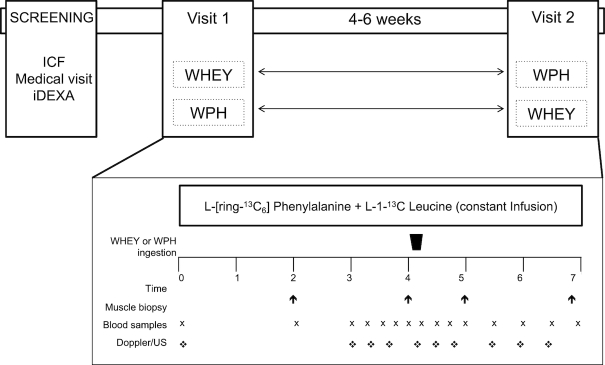
The study was designed as a double-blind, randomized crossover trial. After the screening procedure, eligible subjects were randomly assigned to receive either WPH or WHEY protein on the first study day. After a period of 4–6 wk, subjects were asked to return to our laboratory, where they consumed the other intervention drink and were studied. The investigators and all subjects were blinded to the treatments. The general design is depicted in Figure 2.
FIGURE 2.
Schematic showing infusion study design. Fractional synthesis rate was determined using muscle biopsies taken from the participant on postabsorptive status (basal period) and 1 h and 3 h from protein ingestion (treatment period). Abbreviations: ICF, informed consent procedure; iDEXA, dual-energy X-ray absorptiometry; WHEY, intact whey protein beverage; WPH, whey protein hydrolyzed beverage.
Acute metabolic study
Subjects were studied in the postabsorptive state, after an overnight fast (only water was allowed ad libitum). Subjects reported to the Institute for Translational Sciences Clinical Research Center (ITS-CRC) on the morning of the infusion study day. Following admission, a catheter was placed into a forearm vein for tracer infusion and into the contralateral hand vein, which was heated for arterialized blood sampling. After collecting a background blood sample, a primed-continuous infusion of L-[ring-13C6]phenylalanine and L-[1-13C]leucine (priming dose: 2 µmol/kg; infusion rate: 0.05 µmol/kg/min) was started to measure MPS. The basal period and the treatment period (postprotein ingestion) lasted, respectively, 2 and 3 h in order to measure the time course of muscle protein turnover and the associated signaling proteins in response to the different treatments (WPH or WHEY). At 2 h after tracer infusion initiation, a first muscle biopsy was obtained from the vastus lateralis. The biopsy was performed using a 5-mm Bergström biopsy needle, under a sterile procedure and local anesthesia. Immediately after the first biopsy, a femoral line was placed in the same leg from which the muscle biopsies were obtained to collect blood samples. At 2 h after the first biopsy, a second muscle sample was obtained from the same incision and was immediately followed by the ingestion of 1 of the 2 intervention drinks (WPH or WHEY). A third and fourth muscle biopsy was obtained from a second incision at 1 and 3 h, respectively, after consumption of the drink. Frequent blood samples were also taken simultaneously from the femoral vein and from the arterialized hand vein for measurement of amino acid enrichment. Blood flow was determined at each blood sample using Doppler ultrasound as described by Harris et al. (26).
Protein beverages
The intervention beverages (WHEY or WPH) were dissolved in ∼300 mL of water. In order to maintain the isotopic steady state, each solution was enriched (7.5%) with L-[ring-13C6]phenylalanine and L-[1-13C]leucine. The composition of the beverages is reported in Supplemental Table 1. Subjects ingested 0.08 g of total protein per kg of BW.
Analytical methods
Enrichments of blood leucine and phenylalanine were determined using GC-MS (6890 Plus GC, 5973 N MSD/DS, 7683 autosampler, Agilent Technologies) as previously described (17, 27, 28). Muscle tissue samples were ground, and intracellular free amino acids and muscle proteins extracted. Intracellular free enrichment and the phenylalanine concentration was determined by GC-MS using L-[13C9–15N]phenylalanine as an internal standard. Skewed isotopomer abundance distribution and overlapping spectra of tracer and internal standards were accounted for, and a 15% correction factor was used to account for dilution by interstitial amino acids.
Phenylalanine and leucine kinetics were calculated using the 3-pool model of leg muscle amino acid kinetics, as follows:
delivery to the leg: {
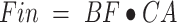 };
};transport into the muscle:
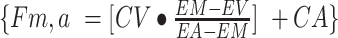
intracellular availability:
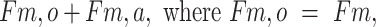
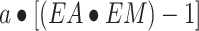
For phenylalanine, kinetics were also calculated:
net balance:
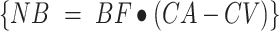
release from proteolysis:
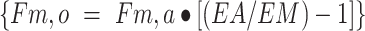
utilization for protein synthesis:
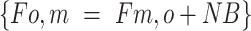
CA and CV are plasma concentrations in the arterialized wrist vein and femoral vein, respectively. EA, EV, and EM are free tracer enrichments in the arterialized wrist vein and femoral vein and in muscle, respectively. BF is leg blood flow. The FSR of mixed muscle proteins was calculated from phenylalanine as 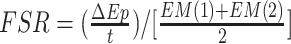
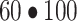 , where Δ
, where Δ is the incremental change in enrichment of protein-bound phenylalanine between the 2 biopsies, t is the time between biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the given 2 biopsies.
is the incremental change in enrichment of protein-bound phenylalanine between the 2 biopsies, t is the time between biopsies, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the given 2 biopsies.
Plasma glucose and lactate concentration were measured for each time point during the infusion study using an automated glucose and lactate analyzer (YSI). Basal and postingestion insulin concentrations were determined in serum with an enzyme-linked immunosorbent assay (Millipore), according to the manufacturer's instructions.
Western blot analysis
Specific details of the immunoblotting analysis can be found elsewhere (15). Muscle tissue samples were used to measure phosphorylation status of protein kinase B (AktSer473; Cell Signaling 9271), ribosomal protein S6 kinase (S6K1Thr389; Cell Signaling 9505), ribosomal protein S6 (rpS6Ser235/236; Cell Signaling 2211), eukaryotic elongation factor 2 (eEF2Thr56; Cell Signaling 2331), and 4E-binding protein 1 (4E-BP1Thr37/46; Cell Signaling 2459). Membranes were stripped and reprobed for total protein. A dilution of 1:500 and 1:1,000 were used respectively for phosphorylated and total expression of each protein. Data were normalized to a rat control and presented as phosphorylation status relative to their total protein expression. Representative blots for each protein are presented in Supplemental Figure 1.
Statistical analysis
Statistical analyses were performed using the statistical software GraphPad Prism version 7.00 for Mac OS X (GraphPad Software). Sample size and power were calculated for all primary outcomes. Primary outcome variables for all aims were measures of MPS and mTORC1 signaling. We first computed the basal-to-acute EAA ingestion change for each variable in each subject. Experiments were designed using a 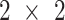 factorial approach with repeated measures across time. We then used a standard repeated measure
factorial approach with repeated measures across time. We then used a standard repeated measure 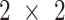 ANOVA model to test the main effects, treatment by group interactions, and contrasts of interest, particularly the interaction with WPH and WHEY. Post hoc testing was carried out using the Tukey–Kramer test.
ANOVA model to test the main effects, treatment by group interactions, and contrasts of interest, particularly the interaction with WPH and WHEY. Post hoc testing was carried out using the Tukey–Kramer test.
Since the results of protein phosphorylation and other associated anabolic and catabolic signaling proteins are usually expressed in arbitrary units, we calculated the between-subject variability and expressed it as a CV to simplify the power calculation and the presentation of the results for these variables. Previous investigations performed in our laboratory indicate that the SD for phosphorylation of mTORC1 signaling proteins is typically 0.02 AU. With 8 subjects per group, we were able to detect mean differences of 0.0337 in protein phosphorylation at 80% power. Differences were considered significant at 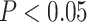 . All results are presented as mean ± SEs.
. All results are presented as mean ± SEs.
Results
Blood amino acid concentrations
Plasma BCAA and phenylalanine concentrations are represented in Figure 3. Ingestion of WPH and WHEY increased plasma valine for 60 min, leucine for 90 min, isoleucine for 60 min, and phenylalanine for 45-min postingestion (P < 0.05). Plasma amino acid concentrations did not differ between the 2 beverages at any time point (valine, P = 0.82; leucine, P = 0.55; isoleucine, P = 0.84; and phenylalanine, P = 0.85). Only valine tended to be slightly higher at 15 min after WPH compared with after WHEY ingestion (P = 0.06).
FIGURE 3.
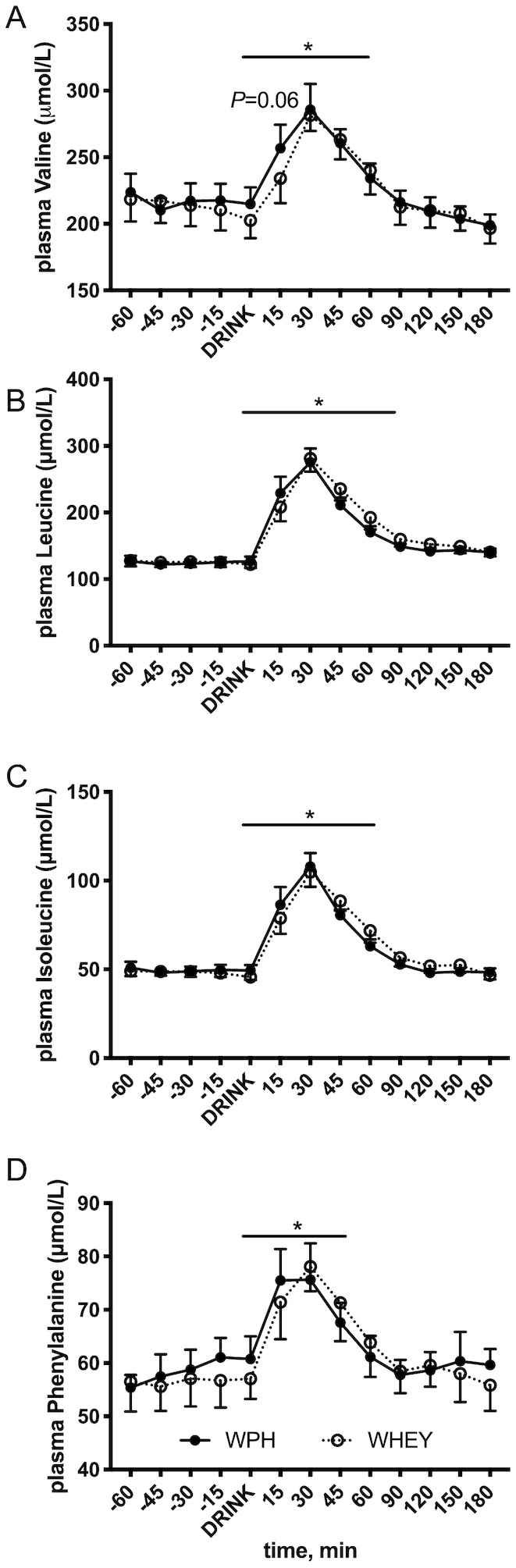
Branched-chain and phenylalanine amino acid blood concentrations before and after whey protein hydrolyzed beverage (WPH) and intact whey protein beverage (WHEY) ingestion in young men. A) valine, B) leucine, C) isoleucine, and D) phenylalanine. Values are means ± SEs, n = 10. Line with * denotes difference from basal for all time points with no difference between groups, P < 0.05.
Blood flow, glucose, lactate, and insulin
Blood flow was unchanged (P = 0.85) following ingestion of WPH and WHEY and did not differ between groups or across time (Figure4A). Arterial plasma glucose gradually decreased from prenutrient ingestion without a significant difference between groups; however, the concentration at 180-min postnutrient ingestion was significantly lower compared with baseline in both groups (WPH: −3.5 ± 2.2%, WHEY −4.7 ± 2.2%; Figure4B, P < 0.05) with no group difference. Plasma lactate concentrations gradually decreased in both groups after 60 min from nutrient ingestion; however, only the WPH group presented a significant decrease at 120 and 150 min (−21.6 ± 4.8% and −17.1 ± 6.8%, respectively, Figure4C). Plasma insulin concentrations increased immediately after nutrient ingestion and remained elevated for 30 min (Figure4D, P < 0.05) with no between-group differences.
FIGURE 4.
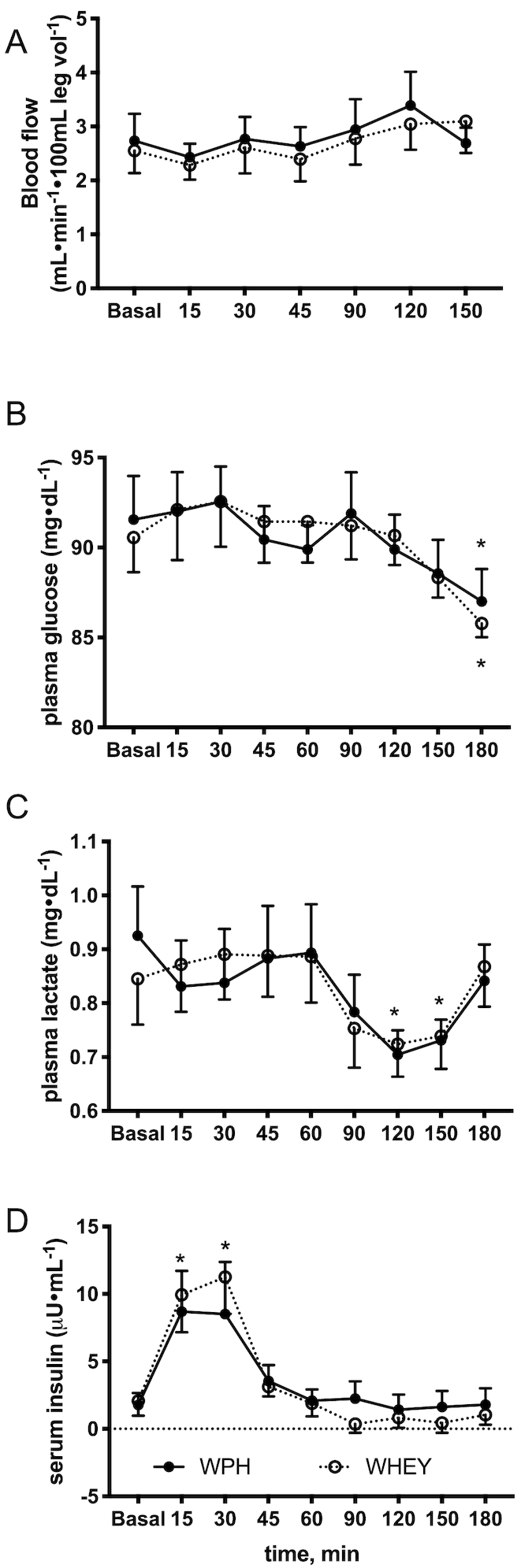
Blood flow A), glucose B), lactate C), and insulin D) concentration before and after whey protein hydrolyzed beverage (WPH) and intact whey protein beverage (WHEY) ingestion in young men. Values are means ± SEs, n = 10. *Denotes difference from basal with no difference between groups, P < 0.05.
Leucine kinetics
Arterial leucine concentrations increased (P < 0.05) in both groups at 1-h postnutrient ingestion but remained slightly elevated at 3 h only in the WHEY group (Figure 5A). Leucine delivery to the leg (i.e. blood flow x leucine concentration) increased equally at 1-h postingestion (P < 0.05) in both groups and returned to baseline values after 3 h (Figure 5B). Leucine transport into the muscle increased at 1-h postingestion in both groups but was significantly greater in the WPH group at 1 h (P < 0.05 compared with WHEY; Figure 5C). The rate of leucine transport into muscle remained elevated in WPH at 3 h compared with WHEY (P < 0.05). Intracellular availability of leucine increased (P < 0.05) in both groups after 1 h but remain elevated at 3 h only in the WPH group (Figure 5D).
FIGURE 5.
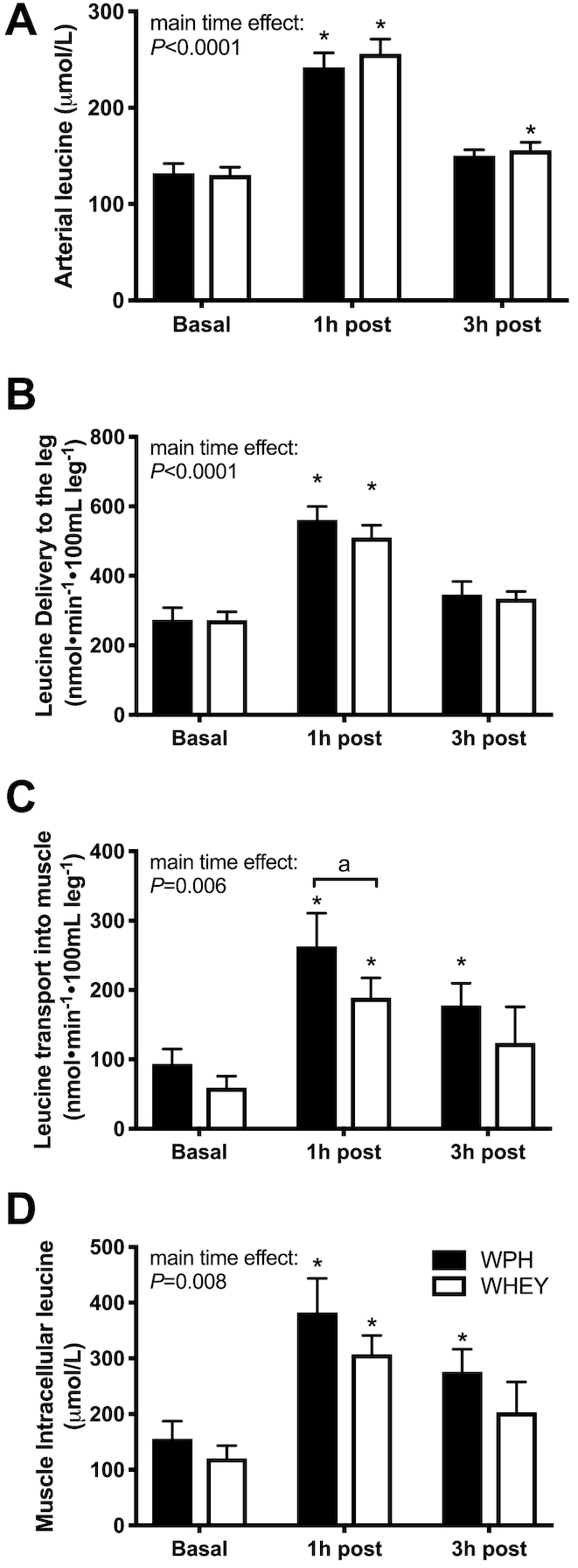
Leucine kinetics after whey protein hydrolyzed beverage (WPH) and intact whey protein beverage (WHEY) ingestion in young men. A) arterial concentration, B) delivery and C) transport into the muscle, and D) intracellular concentrations in the basal state (basal) and 1 and 3 h following mixture ingestion. Each time point represents hourly averages. Values are means ± SEs, n = 10. *Significantly different from basal; P < 0.05; a significant difference between groups.
mTORC1 signaling
Phosphorylation of AktS473 increased significantly at 1-h postingestion (P < 0.05, Table 2) and was similar in both groups. S6K1Thr389 increased significantly (P < 0.05) at 1 h, and was elevated at 3-h postingestion for both treatments, whilst rpS6Ser235/236 increased significantly only after WPH ingestion (P < 0.05, Table 2). 4E-BP1Thr37/46 was significantly lower 3-h postingestion in the WHEY group. Phosphorylation status of eEF2Thr56 was unchanged postingestion and was similar in both groups (P > 0.05).
TABLE 2.
Western-blot analysis of proteins associated with muscle protein synthesis following ingestion of whey protein hydrolyzed beverage (WPH) or intact whey protein beverage (WHEY) in young men1
| WPH | WHEY | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 h | 3 h | Baseline | 1 h | 3 h | |
| Akt (Ser473) | 0.85 ± 0.15 | 1.96 ± 0.32* | 0.91 ± 0.12 | 1.43 ± 0.28 | 1.81 ± 0.40* | 0.78 ± 0.14 |
| S6K1 (Thr389) | 0.32 ± 0.07 | 0.38 ± 0.06* | 0.36 ± 0.09 | 0.34 ± 0.11 | 0.41 ± 0.12* | 0.41 ± 0.11 |
| 4E-BP1 (Thr37/46) | 1.00 ± 0.18 | 1.44 ± 0.25 | 0.97 ± 0.16 | 1.08 ± 0.22 | 1.64 ± 0.35 | 0.97 ± 0.16* |
| rpS6 (Ser240/244) | 0.26 ± 0.05 | 0.56 ± 0.08* | 0.63 ± 0.38 | 0.30 ± 0.06 | 0.58 ± 0.15 | 0.73 ± 0.22 |
| eEF2 (Thr56) | 1.02 ± 0.16 | 1.13 ± 0.18 | 1.23 ± 0.38 | 0.89 ± 0.08 | 0.93 ± 0.10 | 0.86 ± 0.10 |
1Data represent values for phosphorylated form normalized by total protein, WPH and WHEY. All values are means ± SEs, n = 10. *Different from baseline, P < 0.05.
Representative blots for each protein can be found in Supplemental Figure 1.
Muscle-protein FSR and phenylalanine kinetics
Mixed muscle FSR during the 3-h postingestion period was increased in both groups (P < 0.05, Figure 6A). FSR at 60-min postingestion did not differ significantly between groups (Figure 6B). Phenylalanine arterial and venous concentrations increased in both WPH and WHEY at 1 h after ingestion compared with baseline (P < 0.05, effect of time: P < 0.05). There was no main treatment difference for arterial, venous, or muscle intracellular phenylalanine concentrations (Table 3). Arterial, venous, and intracellular muscle phenylalanine enrichments are shown in Table 3.
FIGURE 6.
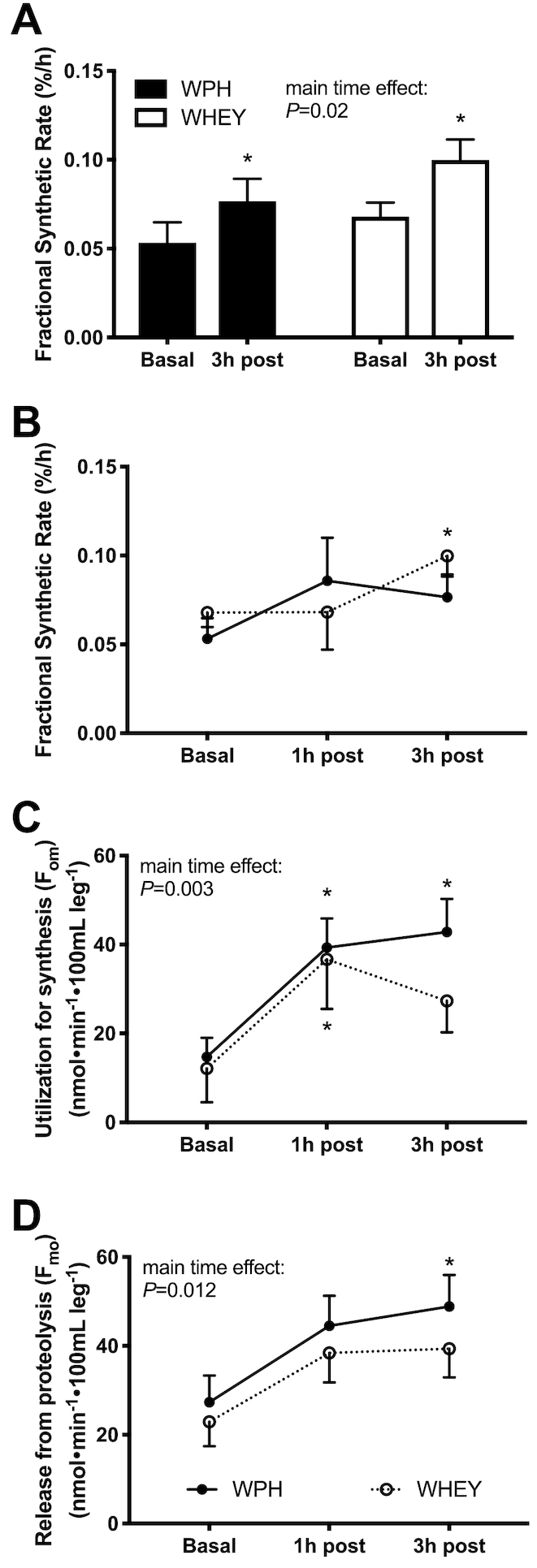
Phenylalanine kinetics after whey protein hydrolyzed beverage (WPH) and intact whey protein beverage (WHEY) ingestion in young men. A) Fractional synthesis rate (FSR) in the basal state (basal) and 1 and 3 h following mixture ingestion, B) FSR in the basal state and 3 h following mixture ingestion, C) intracellular amino acids utilization (om) indicating protein synthesis, and D) release from proteolysis (mo), reflecting protein breakdown following ingestion of WPH and WHEY in young men. Values are means ± SEs, n = 10. *Significantly different from basal; P < 0.05.
TABLE 3.
Phenylalanine enrichments and concentrations following ingestion of whey protein hydrolyzed beverage (WPH) or intact whey protein beverage (WHEY) in young men1
| WPH | WHEY | |||||
|---|---|---|---|---|---|---|
| Baseline | 1h | 3h | Baseline | 1h | 3h | |
| Enrichment, % | ||||||
| Artery† | 7.1 ± 0.4 | 7.4 ± 0.3* | 8.0 ± 0.5 | 7.1 ± 0.3 | 7.9 ± 0.4* | 8.0 ± 0.4* |
| Vein† | 6.4 ± 0.4 | 6.3 ± 0.2 | 6.4 ± 0.3 | 6.4 ± 0.3 | 6.8 ± 0.3* | 6.7 ± 0.3* |
| Muscle IC† | 3.7 ± 0.3 | 4.9 ± 0.4* | 4.3 ± 0.3 | 3.7 ± 0.4 | 4.2 ± 0.3 | 3.9 ± 0.4 |
| Concentration (nmol/mL) | ||||||
| Artery† | 61.0 ± 2.4 | 73.3 ± 4.3* | 61.6 ± 3.0 | 55.7 ± 3.4 | 71.3 ± 4.1* | 57.8 ± 3.3 |
| Vein† | 65.2 ± 4.1 | 73.3 ± 3.7* | 62.2 ± 3.5 | 61.9 ± 3.8 | 72.7 ± 4.8* | 63.1 ± 4.1 |
| Muscle IC† | 50.0 ± 5.3 | 53.3 ± 4.9 | 53.7 ± 3.9 | 50.4 ± 4.1 | 58.0 ± 5.9* | 58.7 ± 7.4 |
1Data represents value for arterialized and venous blood and intracellular muscle phenylalanine enrichment (expressed as tracer-to-tracee ratio, %) and concentration (expressed as nmol/mL) at rest, 1-h and 3-h postingestion of WPH and WHEY. All values are means ± SEs, n = 10. *Different from baseline, P < 0.05; † main effect of time, P < 0.05.
Abbreviation: IC, intracellular.
The 3-pool model for assessing phenylalanine kinetics was used to provide a measurement of protein utilization for synthesis (Fo,m). WPH and WHEY showed a similar increase in amino acid utilization for protein synthesis at 1 h, but only remained elevated at 3-h postingestion in the WPH treatment (Figure6C). The index of muscle protein breakdown (Fm,o) as determined by the 3-pool model showed an increase over time in both groups and remained higher at 3-h postingestion only in the WPH group (P < 0.05 from baseline, Figure6D). The net phenylalanine balance increased at 15 min and 30 min (P < 0.05 from baseline) and decreased at 60 min from nutrient ingestion, with no difference between groups (effect of group: P = 0.08; Figure 7).
FIGURE 7.
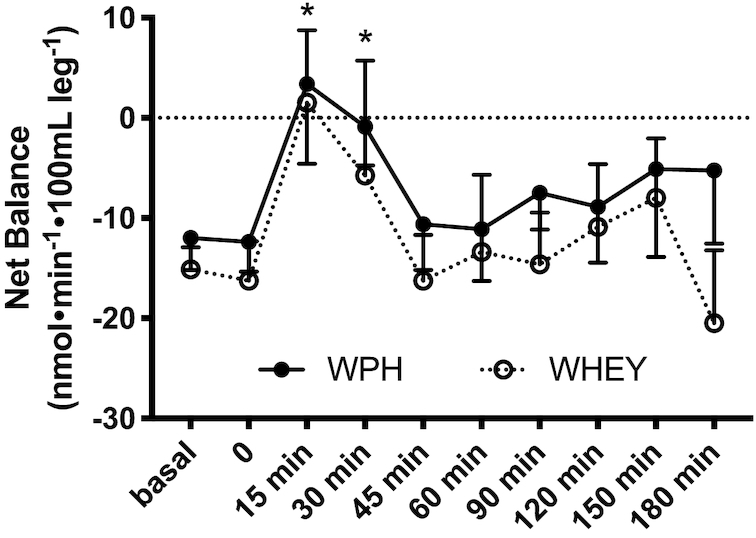
Phenylalanine net balance in the basal state and at 3-h postingestion for subjects given intact whey protein beverage (WHEY) and whey protein hydrolyzed beverage (WPH) mixture in young men. Values are means ± SEs, n = 10. No difference was observed between the 2 groups.
Discussion
The aim of this study was to compare the effect of WHEY and its hydrolysate form (WPH) on amino acid delivery and transport into muscle, mTORC1 signaling, and MPS. The primary finding from our study is that WPH and WHEY ingestion in young men produces a similar muscle protein anabolic response. Ingestion of protein or EAA has a potent effect on stimulating MPS in human subjects (1, 4, 29) due to a transient increase in circulating amino acid concentrations (30). In some conditions, such as bed rest, aging, or frailty, the link between circulating amino acids and the stimulation of MPS can be disrupted (31). In other situations acute exercise combined with ingestion of protein or amino acids can enhance the muscle protein anabolic response by activating the mTORC1 pathway (32–37). It is generally assumed that the acute anabolic response to protein ingestion is beneficial for promoting recovery following exercise and may improve muscle mass and quality over the long term (33, 38, 39). However, many studies providing protein supplements to healthy young or older adults are unable to show beneficial effects of protein supplementation on muscle size, quality, or function which is likely due to the relatively high protein content of the Western diet. On the other hand, designing highly anabolic protein supplements should have clinical relevance as these may be beneficial to vulnerable populations such as frail older adults with sarcopenia or muscle cachexia, often seen in cancer patients. For example, WPH may be beneficial for specific patients who have a difficult time consuming sufficient amounts of protein as it is considered to be a protein source with high anabolic potency (i.e. ability to maximize MPS at low doses) and may influence other aspects of muscle quality (40). In some clinical patients the condition of anabolic resistance (i.e. the inability or reduced ability of protein to stimulate MPS) can be a significant problem (41). Therefore, the development of dietary supplements with a high anabolic potency may be an important nutritional intervention to prevent muscle loss (37, 41, 42).
Recent evidence suggests that hydrolyzed whey protein (WPH) contains peptides that can be easily digested and absorbed compared with a whole protein source and may have a greater effect on MPS (23, 42, 43). Our hypothesis was that a dose of 0.08 g/kg BW of WPH would induce a greater increase in the muscle protein anabolic response than the same amount of WHEY, which could translate to a potential greater gain or maintenance of muscle mass. As mentioned above, we were not able to confirm our hypothesis as a small dose of WPH did not further increase MPS above what was found with a similar dose of WHEY (WPH: +67%; WHEY: +57%). The increase in MPS is associated with the activation of mTORC1 signaling (e.g. rpS6, S6K1, and 4E-BP1 phosphorylation) observed at 1-h postingestion of both mixtures. However, to our knowledge, this is the first time WPH has been studied for its amino acid kinetics and we found some interesting results. For example, mixed MPS was similar between treatments following nutrient ingestion, however, when we examined phenylalanine kinetics using the 3-pool model we discovered that protein synthesis appears to be prolonged (3-h postingestion) for the WPH, whilst muscle protein breakdown was similar between treatments. Overall, net phenylalanine balance across the leg increased in the 30-min postingestion in both groups. However, the general trend (2-way ANOVA group effect: P = 0.08) was for WPH to be slightly higher (i.e. more anabolic) than WHEY over the 3-h time course postingestion. These results may appear to be contradictory, however a brief comment on the differences in methods used in this study is worth mentioning. The main difference between the 3-pool model and FSR measures of MPS is the time range at which each is taken. The 3-pool model is measured instantaneously at discrete time points whereas the FSR is measured over a period of hours. Thus, they provide quite different measures of protein turnover. FSR provides an overall average of how the muscle responded across the experimental period, the 3-pool model provides an instantaneous picture of what happens at specific time points. Therefore, it is possible that certain events may be missed with the 3-pool model due to the sampling timing.
The postprandial muscle protein synthetic response to protein ingestion is related to plasma hyperaminoacidemia (27, 44, 45), which can be influenced by the dose (46, 47), the source (14, 15, 43), or the digestibility of protein consumed (23, 48). Due to its short chain structure, WPH has a faster absorption rate and digestion (49, 50), and thus, can rapidly increase blood amino acid concentrations. However, in our study, we observed no statistically significant difference in the plasma concentration of BCAAs (valine, leucine, isoleucine). All the amino acids studied reached their peak 30 min after beverage ingestion and returned to baseline values within 90 min; the WPH group consistently presented a higher (but not significantly so) concentration at 15 min from consumption compared with WHEY. However, to examine leucine kinetics to a greater extent we also infused a stable isotope tracer of leucine and discovered a novel finding. Specifically, we found that the rate of leucine transport into muscle was significantly higher in the WPH group compared with WHEY (see discussion below). This resulted in a slightly higher muscle intracellular concentration of leucine and may help to explain the faster MPS response in the WPH group as discussed above. On the other hand, our total BCAA blood data contrast with previous findings of Morifuji et al. (43), who found a higher plasma concentration of BCAA with protein hydrolysates compared with the intact form. A possible explanation for this discrepancy could be the total amount of protein (and thus BCAA) ingested in the 2 studies. In our study, subjects received ∼6 g of protein (1.4 g of BCAA), whilst Morifuji's protocol called for 12 g of protein (∼2.8 g of BCAA). This larger amount of ingested intact protein could have increased the time needed to digest, slowing and lowering the rate of appearance in the blood. A recent article by Nakayama et al. (51) showed that, at lower doses of WPH (i.e. 2.5 or 5 g), plasma EAA concentrations reached their peak at 20 min. In the present study we didn't measure EAA concentrations at this time point, which may partially explain why we didn't find a difference in hyperaminoacidemia between the 2 mixtures. Our data confirm, however, that WPH can rapidly induce hyperaminoacidemia.
Moreover, insulin concentrations did not differ between groups. Insulin is a potent anabolic stimulus (52) which increases following protein ingestion (53, 54), contributing to the promotion of muscle protein anabolism. The hyperinsulinemic response to WPH is dose dependent (51), and it has been suggested that ingestion of >3.3 g of WPH is necessary to promote the proper anabolic response of muscle metabolism. In the present study, 6 g of WPH had the same effect on insulin concentration as the same amount of WHEY protein, with a transient increase in the first 30 min following protein ingestion, and a return to basal concentrations within 45-min postingestion.
Of all the amino acids, leucine appears to be the most potent stimulus for activating protein synthesis via mTORC1 signaling (17, 29, 55). For example, in 2014, Churchward-Venne et al. demonstrated that when added to a small protein beverage containing 6 g of whey protein, 5 g of leucine enhances protein synthesis as much as 25 g of whey protein in rested conditions as well as following exercise in young men (39). On the other hand, a study on whey protein hydrolysate demonstrated that WPH stimulated MPS more than casein and soy (23), in both the rested state and postexercise. The most valid hypothesis to explain these results is based on the pattern of appearance of amino acids in the systemic circulation. When hydrolyzed whey is consumed, the rise in EAA, BCAA, and leucine are greater than for other sources, such as soy or casein (23). Although we observed no difference in the concentrations of blood leucine between the 2 sources, after consuming WPH, leucine transport in the muscle was greater and remained elevated even 3 h after ingestion (Figure 4). With a similar trend, the intracellular leucine concentration was also higher 1 h after ingestion and remained significantly higher compared with baseline only after WPH ingestion. As previously shown (17), leucine transport into the muscle and its intracellular availability are important contributors to MPS. It is possible that the hydrolyzed form of whey protein, with its higher proportion of peptides, can induce a prolonged transport of leucine into the muscle, and thus, induce a greater intracellular leucine accumulation during the postprandial period. For example, one hypothesis is that WPH promoted greater incretin release from the gut than WHEY which enhanced the uptake of leucine by muscle. Incretin hormones (e.g. glucagon-like-pepetide-1, GLP-1, and glucose-dependent insulintropic polypeptide, GIP) are involved in protein-stimulated release of insulin (16) and GLP-1 can acutely enhance microvascular recruitment in muscle (56). Furthermore, amino acid transport may be enhanced by the proton-dependent oligopeptide transporter (hPHT1) which transports di- and tri-peptides (57). Therefore, incretin-induced microvascular recruitment and specific peptide transporters may be involved in WPH-enhanced leucine uptake. Future work specifically designed to test this hypothesis is needed.
In summary, we found that WPH ingestion leads to amino acid transport into muscle, activation of mTORC1 signaling, and an increase in the rate of MPS. This stimulation of muscle protein anabolism was similar to that observed following ingestion of intact WHEY protein. Of interest is that this response occurred at relatively small dosages in comparison to currently used doses of protein or EAA supplements. Although the overall muscle protein anabolic response was similar between WPH and WHEY, the utilization of the 3-pool model allowed us to discover some important differences between the 2 beverages. For example, WPH induced a greater transport and accumulation of leucine into muscle which increased amino acid utilization for protein synthesis compared with WHEY. We conclude that the bioactive peptides contained in the hydrolyzed form of whey can be easily digested and absorbed compared with a whole protein source, and may be an alternative nutritional strategy to improve protein synthesis in clinical conditions associated with muscle wasting.
Supplementary Material
Acknowledgments
We thank Paula Skinkis for assistance in subject recruitment and Cayla Perez N.P. and Adetutu Odejimi, N.P., for assisting in screening participants, performing muscle biopsies, and placing femoral catheters.
The authors’ responsibilities were as follows—TM, BBR, KN, and CS: designed the research; TM, CRB, EV, and BBR: conducted the research; TM, CRB, BV, CSF, EV, and BBR: analyzed the data; TM and BBR: wrote the manuscript; TM, CRB, EV, BV, KN, CS, and BBR: edited the manuscript; TM and BBR: have primary responsibility for the final content. All authors were responsible for the study design, data collection and analysis, decision to publish, preparation of the manuscript, and have read and approved the final manuscript. KN and CS are employees of the company that supported the study. They were responsible for the study design, decision to publish, preparation of the manuscript, and were not involved in data collection and analysis as all studies were carried out independently at University of Texas Medical Branch.
Notes
Supported by Meiji Co., Ltd.
Author disclosures: TM, CRB, BV, CSF, EV, and BBR, no conflicts of interest. KN and CS are employees of the company that supported the study. They were responsible for the study design, decision to publish, preparation of the manuscript, and have read and approved the final manuscript. KN and CS were not involved in data collection and analysis as all studies were carried out independently at University of Texas Medical Branch.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Akt, protein kinase B; BCAA, branched-chain amino acid; BW, body weight; EAA, essential amino acid; FSR, fractional synthetic rate; MPS, muscle protein synthesis; mTOR, mammalian/mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; rpS6, ribosomal protein S6; S6K1, 70-kDa S6 protein kinase; 4E-BP1, 4E binding protein 1.
References
- 1. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB. Exercise, amino acids and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc. 2011;43(12):2249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimball SR, Jefferson LS.. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–7. [DOI] [PubMed] [Google Scholar]
- 7. Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136:264S–8S. [DOI] [PubMed] [Google Scholar]
- 8. Kimball SR, Jefferson LS.. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–31S. [DOI] [PubMed] [Google Scholar]
- 9. Nair KS, Short KR.. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135:1547S–52S. [DOI] [PubMed] [Google Scholar]
- 10. Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem Biophys Res Commun. 2004;313:417–22. [DOI] [PubMed] [Google Scholar]
- 11. Kimball SR, Jefferson LS.. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2001;4:39–43. [DOI] [PubMed] [Google Scholar]
- 12. Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–34. [DOI] [PubMed] [Google Scholar]
- 13. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Volpi E et al.. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr. 2013;143:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borack MS, Reidy PT, Husaini SH, Markofski MM, Deer RR, Richison AB, Lambert BS, Cope MB, Mukherjea R, Volpi E et al.. Soy-dairy protein blend or whey protein isolate ingestion induces similar postexercise muscle mechanistic target of rapamycin complex 1 signaling and protein synthesis responses in older men. J Nutr. 2016;146:2468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E et al.. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol (1985). 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mignone LE, Wu T, Horowitz M, Rayner CK. Whey protein: the “whey” forward for treatment of type 2 diabetes?. World J Diabetes. 2015;6:1274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorissen SH, Rémond D, van Loon LJ. The muscle protein synthetic response to food ingestion. Meat Sci. 2015;109:96–100. [DOI] [PubMed] [Google Scholar]
- 19. Sarwar Gilani G, Wu Xiao C, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108:(Suppl 2):S315–32. [DOI] [PubMed] [Google Scholar]
- 20. Kanda A, Nakayama K, Sanbongi C, Nagata M, Ikegami S, Itoh H. Effects of whey, caseinate, or milk protein ingestion on muscle protein synthesis after exercise. Nutrients. 2016;8(6):E339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanda A, Morifuji M, Fukasawa T, Koga J, Kanegae M, Kawanaka K, Higuchi M. Dietary whey protein hydrolysates increase skeletal muscle glycogen levels via activation of glycogen synthase in mice. J Agric Food Chem. 2012;60:11403–8. [DOI] [PubMed] [Google Scholar]
- 22. Morifuji M, Kanda A, Koga J, Kawanaka K, Higuchi M. Preexercise ingestion of carbohydrate plus whey protein hydrolysates attenuates skeletal muscle glycogen depletion during exercise in rats. Nutrition. 2011;27:833–7. [DOI] [PubMed] [Google Scholar]
- 23. Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985). 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 24. Kanda A, Nakayama K, Fukasawa T, Koga J, Kanegae M, Kawanaka K, Higuchi M. Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br J Nutr. 2013;110:981–7. [DOI] [PubMed] [Google Scholar]
- 25. van Vliet S, Burd NA, van Loon LJ. The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr. 2015;145:1981–91. [DOI] [PubMed] [Google Scholar]
- 26. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wolfe RR, Chinkes DL.. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken (NJ): Wiley-Liss; 2005. [Google Scholar]
- 29. Dickinson JM, Gundermann DM, Walker DK, Reidy PT, Borack MS, Drummond MJ, Arora M, Volpi E, Rasmussen BB. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr. 2014;144:1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bohé J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paddon-Jones D, Leidy H.. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014;17:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab. 2004;14:255–71. [DOI] [PubMed] [Google Scholar]
- 33. Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol (1985). 2009;106:1692–701. [DOI] [PubMed] [Google Scholar]
- 34. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93:322–31. [DOI] [PubMed] [Google Scholar]
- 35. Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–9. [DOI] [PubMed] [Google Scholar]
- 36. Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino acid sensing in skeletal muscle. Trends Endocrinol Metab. 2016;27:796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moore DR. Nutrition to support recovery from endurance exercise: optimal carbohydrate and protein replacement. Curr Sports Med Rep. 2015;14:294–300. [DOI] [PubMed] [Google Scholar]
- 39. Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK et al.. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr. 2014;99:276–86. [DOI] [PubMed] [Google Scholar]
- 40. Lollo PCB, Amaya-Farfan J, Faria IC, Salgado JVV, Chacon-Mikahil MPT, Cruz AG, Oliveira CAF, Montagner PC, Arruda M. Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. International Dairy Journal. 2014;34:19–24. [Google Scholar]
- 41. Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. [DOI] [PubMed] [Google Scholar]
- 42. Koopman R, Walrand S, Beelen M, Gijsen AP, Kies AK, Boirie Y, Saris WH, van Loon LJ. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J Nutr. 2009;139:1707–13. [DOI] [PubMed] [Google Scholar]
- 43. Morifuji M, Ishizaka M, Baba S, Fukuda K, Matsumoto H, Koga J, Kanegae M, Higuchi M. Comparison of different sources and degrees of hydrolysis of dietary protein: effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J Agric Food Chem. 2010;58:8788–97. [DOI] [PubMed] [Google Scholar]
- 44. Wolfe RR. Effects of amino acid intake on anabolic processes. Can J Appl Physiol. 2001;26:(Suppl):S220–S7. [DOI] [PubMed] [Google Scholar]
- 45. Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:3219S–24S. [DOI] [PubMed] [Google Scholar]
- 46. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. [DOI] [PubMed] [Google Scholar]
- 47. Calbet JA, MacLean DA.. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J Nutr. 2002;132:2174–82. [DOI] [PubMed] [Google Scholar]
- 48. Morifuji M, Koga J, Kawanaka K, Higuchi M. Branched-chain amino acid-containing dipeptides, identified from whey protein hydrolysates, stimulate glucose uptake rate in L6 myotubes and isolated skeletal muscles. J Nutr Sci Vitaminol (Tokyo). 2009;55:81–6. [DOI] [PubMed] [Google Scholar]
- 49. Adibi SA. Intestinal transport of dipeptides in man: relative importance of hydrolysis and intact absorption. J Clin Invest. 1971;50:2266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adibi SA, Morse EL, Masilamani SS, Amin PM. Evidence for two different modes of tripeptide disappearance in human intestine. Uptake by peptide carrier systems and hydrolysis by peptide hydrolases. J Clin Invest. 1975;56:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakayama K, Sanbongi C, Ikegami S. Effects of whey protein hydrolysate ingestion on postprandial aminoacidemia compared with a free amino acid mixture in young men. Nutrients. 2018;10(4):E507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95:3848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59:44–55. [DOI] [PubMed] [Google Scholar]
- 54. Walker DK, Drummond MJ, Dickinson JM, Borack MS, Jennings K, Volpi E, Rasmussen BB. Insulin increases mRNA abundance of the amino acid transporter SLC7A5/LAT1 via an mTORC1-dependent mechanism in skeletal muscle cells. Physiol Rep. 2014;2:e00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–20. [DOI] [PubMed] [Google Scholar]
- 56. Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.