Abstract
Biochemically, interleukin-6 belongs to the class of four-helical cytokines. The cytokine can be synthesised and secreted by many cells. It acts via a cell surface-expressed interleukin-6 receptor, which is not signalling competent. This receptor, when complexed with interleukin-6, associates with the signalling receptor glycoprotein 130 kDa (gp130), which becomes dimerised and initiates intracellular signalling via the Janus kinase/signal transducer and activator of transcription and rat sarcoma proto oncogene/mitogen-activated protein kinase/phosphoinositide-3 kinase pathways. Physiologically, interleukin-6 is involved in the regulation of haematopoiesis and the coordination of the innate and acquired immune systems. Additionally, interleukin-6 plays an important role in the regulation of metabolism, in neural development and survival, and in the development and maintenance of various cancers. Although interleukin-6 is mostly regarded as a pro-inflammatory cytokine, there are numerous examples of protective and regenerative functions of this cytokine. This review will explain the molecular mechanisms of the, in part opposing, activities of the cytokine interleukin-6.
Keywords: gp130, sgp130Fc, IL-6, IL-6R, sIL-6R, trans-signalling, ADAM17
Introduction
Interleukin-6 (IL-6) is considered one of the most prominent pro-inflammatory cytokines 1. Blockade of IL-6 by the neutralising monoclonal antibody tocilizumab has been approved in more than 100 countries for the treatment of patients with autoimmune disorders such as rheumatoid arthritis 2. Additionally, the cytokine storm sometimes encountered when cancer patients are treated with chimeric antigen receptor (CAR) T-cells 3 could be effectively treated with the antibody tocilizumab, leading to US Food and Drug Administration (FDA) approval of the drug for this condition. Even more recently, it has been recognised that many patients experience a similar cytokine storm upon infection with SARS-CoV-2 (COVID-19) virus 4 and that these patients could also be treated with tocilizumab 5. These new data led to a rekindled general interest in the cytokine IL-6.
IL-6 was initially discovered and cloned in the Kishimoto laboratory as a B-cell stimulatory factor 6. Immediately after the molecular cloning, it was evident that IL-6 was identical to hepatocyte stimulating factor 7, hybridoma-plasmacytoma growth factor 8, interferon β2 9, and 26 kDa protein 10. This already indicated the pleiotropic nature of the cytokine. Later on, it was also recognised that IL-6 shows profound activities in the brain 11, 12, in the regulation of metabolism 13, 14, in the response of the body to exercise 15, and in the development and maintenance of various cancers 16.
This review article gives a short overview of the complex biology of IL-6 and explains how one cytokine can have extremely different biologic effects on different cells and in different physiologic states of the human body 17.
The interleukin-6 receptor complex
The four-helical cytokine IL-6 ( Figure 1) on cells binds to a membrane-bound IL-6 receptor (IL-6R), and the complex of IL-6 and IL-6R associates with a second receptor protein, glycoprotein 130 kDa (gp130), which dimerises and initiates intracellular signalling via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and rat sarcoma proto oncogene (ras)/mitogen-activated protein kinase and phosphoinositide-3 kinase pathways ( Figure 2) 18. Importantly, IL-6 exhibits only a measurable affinity to the IL-6R but not to gp130, and the IL-6R does not bind on its own to gp130. It is only the complex of IL-6 and IL-6R that binds to gp130 and induces its dimerisation ( Figure 2). All cells in the body express gp130, but only a few cells such as hepatocytes and some leukocytes express IL-6R. It follows that cells that express only gp130 but not IL-6R cannot be stimulated by IL-6 1.
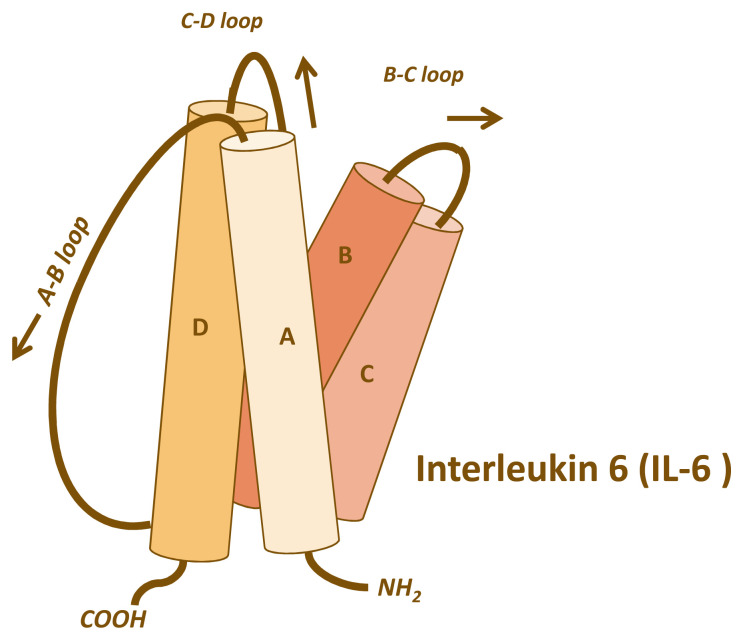
Figure 1. Four-helical topology of the interleukin-6 (IL-6) protein.
IL-6 belongs to the family of four-helical cytokines. The figure shows the four helices with the connecting loops. The A–B and the C–D loops are long enough to reach the length of a helix, whereas the B–C loop is short. Consequently, IL-6 has an up-up-down-down topology, meaning that helices A and B point upwards, whereas helices C and D point downwards. This topology is common to most cytokines such as IL-2, IL-4, IL-7, IL-11, IL-15, leukaemia inhibitory factor, oncostatin M, growth hormone, leptin, and many others.
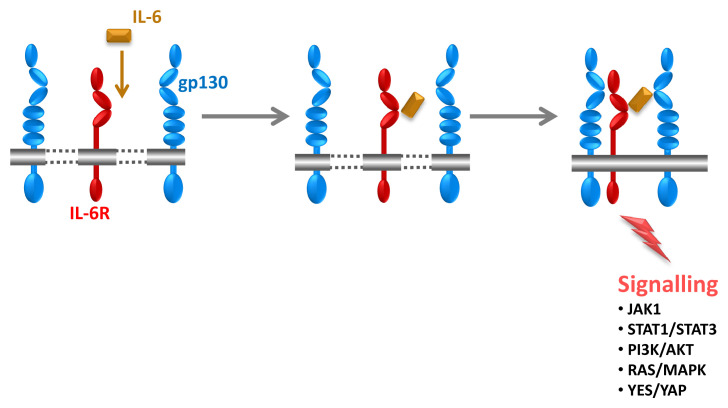
Figure 2. Stimulation of target cells by interleukin-6 (IL-6).
IL-6 (orange) first binds to the IL-6 receptor (IL-6R) (red). The complex of IL-6 and IL-6R associates with glycoprotein 130 kDa (gp130) (blue), which dimerises and leads to intracellular signalling. It is important to note that IL-6 and IL-6R alone exhibit no measurable affinity to gp130. Only the complex of IL-6 and IL-6R binds to and activates gp130. Therefore, IL-6 cannot stimulate cells that do not express IL-6R. Signalling occurs via the signal transducer and activator of transcription (STAT) 1/STAT3, Yamaguchi sarcoma viral oncogene homolog (YES)/YES-associated protein (YAP), phosphoinositide-3 kinase (PI3K)/AKT, and rat sarcoma proto oncogene (RAS)/mitogen-activated protein kinase (MAPK) pathways. JAK, Janus kinase.
Noteworthy, gp130 is a component of the receptor complexes of the so-called gp130 cytokine family, which besides IL-6 comprises IL-11, ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), cardiotrophin-like cytokine (CLC), leukaemia inhibitory factor (LIF), oncostatin M (OSM), and IL-27. For details, please refer to recent reviews 19, 20.
It has, however, been noticed that the membrane-bound IL-6R can be cleaved by the membrane-bound metalloprotease a disintegrin and metalloprotease 17 (ADAM17) to generate a soluble IL-6R (sIL-6R) 21. To a minor extent, the human—but not the murine—sIL-6R can be generated by translation from a differentially spliced mRNA 22. Intriguingly, the sIL-6R can still bind IL-6, and the complex of IL-6 and sIL-6R can associate with gp130 and induce signalling, even on cells that lack the membrane-bound IL-6R 23. This process has been named IL-6 trans-signalling ( Figure 3) 24. Strikingly, following this paradigm, IL-6 can, in the presence of sIL-6R, stimulate any cell in the body since all cells express gp130 17.
Interestingly, most IL-6R-expressing cells including hepatocytes express far more gp130 than IL-6R molecules. Therefore, stimulation of such cells with IL-6 alone will only lead to engagement of few gp130 molecules, whereas stimulation with the complex of IL-6 and sIL-6R will stimulate all cellular gp130 proteins. A threshold for a given response might not be reached with IL-6 stimulation but only with stimulation of all gp130 molecules via IL-6 trans-signalling. This might be an explanation for the observed differences in signalling between trans-signalling and classical signalling that lead to different phenotypes 25.
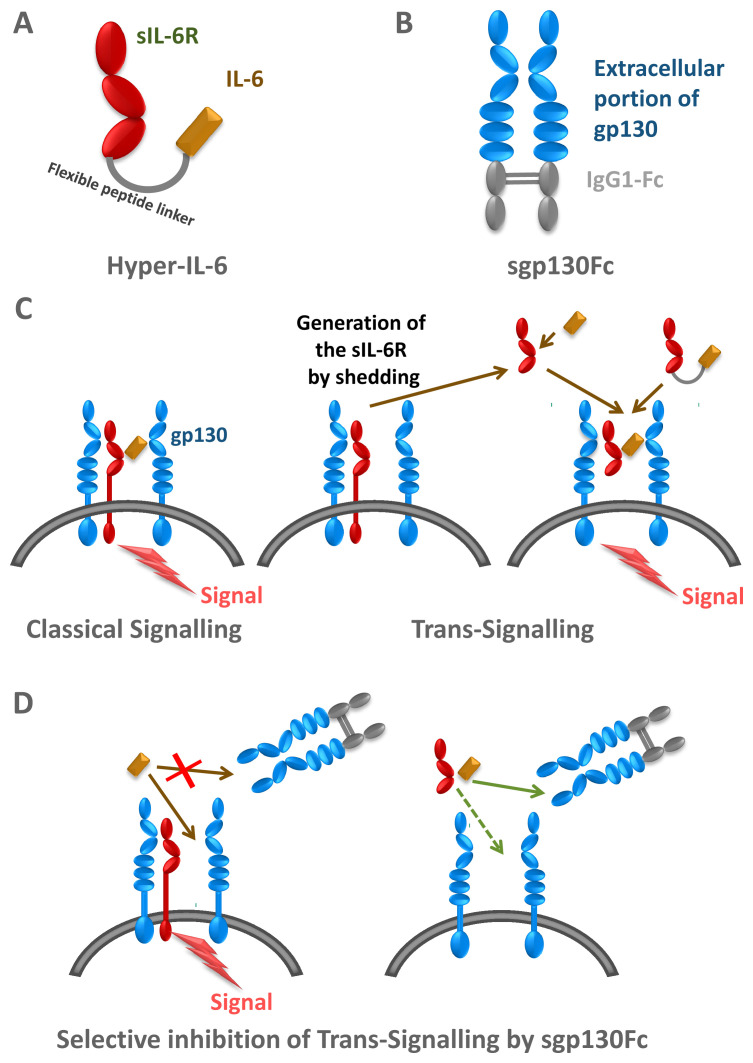
Figure 3. Designer proteins to probe for modes of interleukin-6 (IL-6) signalling.
( A) Hyper-IL-6 is a fusion protein between IL-6 and soluble IL-6 receptor (sIL-6R). ( B) sgp130Fc is a fusion protein of the extracellular portion of glycoprotein 130 kDa (gp130) and the constant part of a human immunoglobulin G1 (IgG1) antibody. ( C) IL-6 can signal via the membrane-bound IL-6R (classical signalling) and via the sIL-6R (trans-signalling). Hyper-IL-6 can be used to mimic IL-6 trans-signalling. ( B) The sg130Fc protein does not interfere with classical IL-6 signalling, but it specifically blocks IL-6 trans-signalling.
Molecular tools to elucidate the functions of interleukin-6
The concept of IL-6 trans-signalling has been corroborated by the use of two designer proteins. The first such protein consists of IL-6 covalently fused to the sIL-6R via a 40 Å flexible peptide linker, which allowed the placement of IL-6 at the correct distance to reach the IL-6 binding site of the sIL-6R. This protein was called Hyper-IL-6 ( Figure 3A) 26. This protein was shown to stimulate gp130-expressing cells in vitro and in vivo, and it was shown that liver regeneration 27, stimulation of neural cells 28, and expansion of hematopoietic cells 29 was far more efficient in the presence of Hyper-IL-6 as compared to IL-6 alone 30.
While Hyper-IL-6 demonstrated only the biologic potential of IL-6 trans-signalling, these experiments did not prove that this process occurred in vivo. A second soluble protein was designed, which consisted of the entire extracellular portion of gp130 covalently fused to the Fc region of human IgG1 ( Figure 3B). The resulting protein, named soluble gp130Fc (sgp130Fc), turned out to exhibit similar properties as membrane-bound gp130: it did not bind IL-6 or IL-6R alone, but it bound with high affinity the complex of IL-6 and sIL-6R 31, 32. Consequently, the sgp130 protein in vitro and in vivo specifically inhibited IL-6 trans-signalling without compromising IL-6 signalling via the membrane-bound IL-6R, i.e. classic signalling 32. The sgp130Fc protein could be used to define IL-6-mediated biologic responses, which were dependent on classic or trans-signalling. This was accomplished by comparing the treatment of animals with sgp130Fc or with neutralising antibodies against IL-6 or IL-6R, which blocked all IL-6 signalling ( Figure 3C, D). Using animal models of human inflammatory diseases or inflammation-associated cancer, it turned out that autoimmune disorders and inflammation-associated cancers were mainly driven by IL-6 trans-signalling whereas regenerative and protective activities of IL-6 were mediated by classic IL-6 signalling via the membrane-bound IL-6R ( Figure 4) 20.
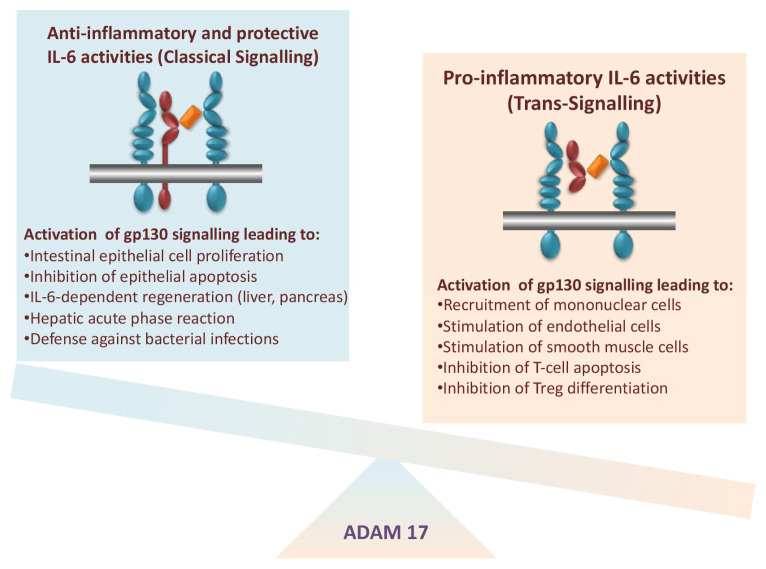
Figure 4. Pro- and anti-inflammatory activities of interleukin-6 (IL-6).
Left, anti-inflammatory and protective activities of the cytokine IL-6 are associated with signalling via the membrane-bound IL-6 receptor (IL-6R). Right, pro-inflammatory activities of the cytokine IL-6 are associated with signalling via the soluble IL-6R (sIL-6R). The membrane-bound metalloprotease a disintegrin and metalloprotease 17 (ADAM17) orchestrates the pro- and anti-inflammatory activities of IL-6. Treg, regulatory T cell.
Physiologic and pathophysiologic functions of interleukin-6
Under homeostatic conditions, IL-6 levels in the circulation are as low as 1–5 pg/ml, but during inflammatory states these levels can rise more than 1,000-fold, and under extreme conditions leading to sepsis IL-6 levels in the µg/ml range have been reported 33. IL-6 is produced by myeloid cells upon Toll-like receptor stimulation together with the cytokines IL-1β and tumor necrosis factor α (TNFα), which, via a feed-forward loop, lead to an immense amplification of IL-6 production during inflammatory conditions 34. There is perhaps no other protein in the human body whose level can go up by six orders of magnitude. This lets us conclude that IL-6 is the major alarm signal in the human body in response to infection, inflammation, and possibly cancer 35.
However, under normal conditions, IL-6 plays an important role in organ/cellular homeostasis. Mice in which the IL-6 gene has been ablated (IL-6 knockout mice) become obese late in life 13, cannot regenerate their liver upon hepatectomy 36, and show no signs of osteoporosis upon ovariectomy 37, indicating roles for IL-6 in body weight regulation, liver physiology, and bone metabolism. In pathophysiologic states, however, there are marked differences between IL-6 knockout mice and wild-type mice. IL-6 knockout mice are completely protected in animal models of rheumatoid arthritis 38 and multiple sclerosis 39, indicating a key role for IL-6 in these autoimmune disorders.
With the help of the sgp130Fc protein and of neutralising monoclonal antibodies, it was possible to selectively block IL-6 trans-signalling or to block all IL-6 signalling, respectively. Using this approach, it was shown that classic IL-6 signalling via the membrane-bound IL-6R was responsible for the defence of the body against bacteria 40, 41, intestinal regeneration upon polymicrobial sepsis 42, prevention of aortic rupture in animal models of abdominal aortic aneurysm 43, and healing of bone fractures 44, 45, indicating that these important processes are severely compromised under blockade of global IL-6 activity 46. It has been hypothesised that the same might apply for the treatment of COVID-19 patients 46 ( Figure 4).
Besides being the major alarm signal in the human body, IL-6 plays a dominant role in various types of cancer. One important reason could be that IL-6, via stimulation of the STAT3 pathway, is a prominent growth factor of many cancer cells. The following scenario has been worked out in pancreatic cancer 47. It was noted that in the Kras G12D model, the massive activation of the STAT3 pathway, which led to tumour progression, was induced by tumour-infiltrating myeloid cells, which stimulated the neoplastic cells via IL-6 trans-signalling 47. Selective blockade of this pathway by the sgp130Fc protein blocked progression of pancreatic intraepithelial neoplasias to pancreatic ductal adenocarcinomas 47, indicating a prominent role for IL-6 trans-signalling in the development of pancreatic cancer. In the murine APC min/+ model of colon cancer, it was established that the genetic deletion of ADAM17, which is responsible for generating not only sIL-6R but also soluble TNFα and soluble ligands of the epidermal growth factor receptor (EGFR), resulted in completely abrogated tumour development 16. Moreover, the formation of neoplasias stimulated ADAM17 on macrophages, leading to EGFR ligand cleavage and subsequent EGFR stimulation. These macrophages now produced IL-6 and sIL-6R, which led to the outgrowth of the tumours. Again, selective blockade of the IL-6 trans-signalling pathway by the sgp130Fc protein blocked tumour development in the APC min/+ model and an additional mouse model of colon cancer 16. This was highly reminiscent of a study in liver cancer, in which it was shown that the EGFR expressed in macrophages but not EGFR in hepatocytes was involved in the development of hepatocellular carcinoma 48. Apparently, macrophage activation may be an important step in the initiation and progression of tumours via the IL-6 trans-signalling pathway 20 ( Figure 4).
Therapeutic targeting of interleukin-6 activity
Therapeutic targeting of the pro-inflammatory cytokine TNFα was introduced as an efficient strategy to treat patients with autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease 49. Subsequently, blockade of the biologic activity of the cytokine IL-6 was shown to be an efficient treatment for patients with rheumatoid arthritis and other autoimmune diseases 2, and it was shown that blocking IL-6 activity was more efficient than blocking TNFα in a monotherapy trial 50. Blockade of IL-6 activity with the IL-6R neutralising monoclonal antibody tocilizumab was also highly effective in the treatment of patients with CAR T cell-induced severe cytokine release syndrome 51. In patients with severe COVID-19 disease, the administration of tocilizumab resulted in a marked improvement of the condition in the majority of patients: the fever subsided, C-reactive protein decreased, and oxygen intake could be lowered. No obvious adverse reactions were observed. These preliminary data indicated that tocilizumab is a candidate for effective treatment of COVID-19 patients 5, 52. Interestingly, treatment of COVID-19 patients with the IL-6R neutralising monoclonal antibody sarilumab resulted in no significant difference in clinical improvement and mortality 53.
Summary
The discovery that the pro-inflammatory activities of IL-6 are mediated by IL-6 trans-signalling whereas the protective and regenerative activities of IL-6 rely on classic signalling via the membrane-bound IL-6R suggested that the sgp130Fc protein might be an ideal candidate for a more specific mode of cytokine blockade as opposed to global cytokine inhibition 20. It was shown in appropriate animal models that blockade of IL-6 trans-signalling was indeed superior to global IL-6 blockade in a bone healing model 44, 45, in a sepsis model 42, in abdominal aortic aneurysm models 43, and in bacterial infection models 40, 41. The sgp130Fc protein was expressed and purified according to GMP regulations. Phase I clinical trials were successfully performed with healthy individuals, and a phase II clinical trial is presently ongoing in patients with inflammatory bowel disease 54. The future will tell whether this elegant therapeutic approach, which was successfully tested in many animal models, leads to a novel paradigm in cytokine-blocking therapies in patients with autoimmune disorders 46. Similarly, blockade of trans-signalling while leaving classical signalling intact may prove to be beneficial for patients experiencing “cytokine storms” from COVID-19 or CAR T-cell therapies. Finally, we suggest that malignancies promoted by high levels of trans-signalling could be contained by this therapeutic modality.
Abbreviations
ADAM17, a disintegrin and metalloprotease 17; EGFR, epidermal growth factor receptor; gp130, glycoprotein 130 kDa; IL-6, interleukin-6; IL-6R, interleukin-6 receptor; ras, rat sarcoma proto oncogene; sgp130Fc, soluble gp130-Fc fusion protein, which under the name of olamkicept is in phase II clinical trials; sIL-6R, soluble IL-6R; STAT, signal transducer and activator of transcription; TNFα, tumor necrosis factor α; YAP, YES-associated protein; YES, Yamaguchi sarcoma viral oncogene homolog.
Acknowledgements
I thank all past and current colleagues of our laboratory for many helpful discussions.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Jacqueline Bromberg, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Hana Algül, Comprehensive Cancer Center Munich, University Hospital Klinikum rechts der isar, Mildred-Scheel-Chair of Tumor Metabolism, Technical University of Munich, Munich, Germany
Elke Roeb, Department of Gastroenterology, Justus Liebig University, Giessen, Germany
Funding Statement
The work of Stefan Rose-John has been supported by grants of the Deutsche Forschungsgemeinschaft Bonn, Germany, under the grant numbers CRC841, project C1, and CRC877, project A1, and by the German Excellence Cluster "Inflammation at Interfaces".
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Kishimoto T: Interleukin-6: From basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. 10.1146/annurev.immunol.23.021704.115806 [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T, Narazaki M, Ogata A, et al. : A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26(1):88–96. 10.1016/j.smim.2014.01.009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 3. Teachey DT, Lacey SF, Shaw PA, et al. : Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–79. 10.1158/2159-8290.CD-16-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore JB, June CH: Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–4. 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Xu X, Han M, Li T, et al. : Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 6. Hirano T, Taga T, Yamasaki K, et al. : Molecular cloning of the cDNAs for interleukin-6/B cell stimulatory factor 2 and its receptor. Ann N Y Acad Sci. 1989;557:167-78, discussion 17880. 10.1111/j.1749-6632.1989.tb24010.x [DOI] [PubMed] [Google Scholar]
- 7. Gauldie J, Richards C, Harnish D, et al. : Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987;84(20):7251–5. 10.1073/pnas.84.20.7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brakenhoff JP, de Groot ER, Evers RF, et al. : Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987;139(12):4116–21. [PubMed] [Google Scholar]
- 9. Zilberstein A, Ruggieri R, Korn JH, et al. : Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986;5(10):2529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haegeman G, Content J, Volckaert G, et al. : Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986;159(3):625–32. 10.1111/j.1432-1033.1986.tb09931.x [DOI] [PubMed] [Google Scholar]
- 11. Rothaug M, Becker-Pauly C, Rose-John S: The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863(6 Pt A):1218–27. 10.1016/j.bbamcr.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 12. Willis EF, MacDonald KPA, Nguyen QH, et al. : Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell. 2020;180(5):833-846.e16. 10.1016/j.cell.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 13. Wallenius V, Wallenius K, Ahrén B, et al. : Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75–9. 10.1038/nm0102-75 [DOI] [PubMed] [Google Scholar]
- 14. Findeisen M, Allen TL, Henstridge DC, et al. : Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature. 2019;574(7776):63–8. 10.1038/s41586-019-1601-9 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 15. Pedersen BK, Febbraio MA: Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 16. Schmidt S, Schumacher N, Schwarz J, et al. : ADAM17 is required for EGF-R-induced intestinal tumors via IL-6 trans-signaling. J Exp Med. 2018;215(4):1205–25. 10.1084/jem.20171696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rose-John S: The biology of interleukin-6 in the 21st century. Semin Immunol. 2014;26(1):1. 10.1016/j.smim.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 18. Schaper F, Rose-John S: Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26(5):475–87. 10.1016/j.cytogfr.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 19. Jones SA, Jenkins BJ: Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–89. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 20. Garbers C, Heink S, Korn T, et al. : Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. 10.1038/nrd.2018.45 [DOI] [PubMed] [Google Scholar]
- 21. Müllberg J, Schooltink H, Stoyan T, et al. : The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993;23(2):473–80. 10.1002/eji.1830230226 [DOI] [PubMed] [Google Scholar]
- 22. Lust JA, Donovan KA, Kline MP, et al. : Isolation of an mRNA encoding a soluble form of the human interleukin-6 receptor. Cytokine. 1992;4(2):96–100. 10.1016/1043-4666(92)90043-q [DOI] [PubMed] [Google Scholar]
- 23. Mackiewicz A, Schooltink H, Heinrich PC, et al. : Complex of soluble human IL-6-receptor/IL-6 up-regulates expression of acute-phase proteins. J Immunol. 1992;149(6):2021–7. [PubMed] [Google Scholar]
- 24. Rose-John S, Heinrich PC: Soluble receptors for cytokines and growth factors: Generation and biological function. Biochem J. 1994;300(Pt 2):281–90. 10.1042/bj3000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rose-John S: The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin Pharmacol Ther. 2017;102(4):591–8. 10.1002/cpt.782 [DOI] [PubMed] [Google Scholar]
- 26. Fischer M, Goldschmitt J, Peschel C, et al. : I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol. 1997;15(2):142–5. 10.1038/nbt0297-142 [DOI] [PubMed] [Google Scholar]
- 27. Galun E, Zeira E, Pappo O, et al. : Liver regeneration induced by a designer human IL-6/sIL-6R fusion protein reverses severe hepatocellular injury. FASEB J. 2000;14(13):1979–87. 10.1096/fj.99-0913com [DOI] [PubMed] [Google Scholar]
- 28. März P, Otten U, Rose-John S: Neural activities of IL-6-type cytokines often depend on soluble cytokine receptors. Eur J Neurosci. 1999;11(9):2995–3004. 10.1046/j.1460-9568.1999.00755.x [DOI] [PubMed] [Google Scholar]
- 29. Audet J, Miller CL, Rose-John S, et al. : Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 2001;98(4):1757–62. 10.1073/pnas.98.4.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rose-John S: Interleukin-6 Family Cytokines. Cold Spring Harb Perspect Biol. 2018;10(2):a028415. 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horsten U, Schmitz-Van de Leur H, Müllberg J, et al. : The membrane distal half of gp130 is responsible for the formation of a ternary complex with IL-6 and the IL-6 receptor. FEBS Lett. 1995;360(1):43–6. 10.1016/0014-5793(95)00053-c [DOI] [PubMed] [Google Scholar]
- 32. Jostock T, Müllberg J, Ozbek S, et al. : Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–7. 10.1046/j.1432-1327.2001.01867.x [DOI] [PubMed] [Google Scholar]
- 33. Waage A, Brandtzaeg P, Halstensen A, et al. : The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169(1):333–8. 10.1084/jem.169.1.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanaka T, Narazaki M, Kishimoto T: IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35. Rose-John S: IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–47. 10.7150/ijbs.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cressman DE, Greenbaum LE, DeAngelis RA, et al. : Liver Failure and Defective Hepatocyte Regeneration in Interleukin-6-Deficient Mice. Science. 1996;274(5291):1379–83. 10.1126/science.274.5291.1379 [DOI] [PubMed] [Google Scholar]
- 37. Poli V, Balena R, Fattori E, et al. : Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13(5):1189–96. 10.1002/j.1460-2075.1994.tb06368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alonzi T, Fattori E, Lazzaro D, et al. : Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187(4):461–8. 10.1084/jem.187.4.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okuda Y, Sakoda S, Bernard CC, et al. : IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int Immunol. 1998;10(5):703–8. 10.1093/intimm/10.5.703 [DOI] [PubMed] [Google Scholar]
- 40. Hoge J, Yan I, Jänner N, et al. : IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J Immunol. 2013;190(2):703–11. 10.4049/jimmunol.1201044 [DOI] [PubMed] [Google Scholar]
- 41. Sodenkamp J, Waetzig GH, Scheller J, et al. : Therapeutic targeting of interleukin-6 trans-signaling does not affect the outcome of experimental tuberculosis. Immunobiology. 2012;217(10):996–1004. 10.1016/j.imbio.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 42. Barkhausen T, Tschernig T, Rosenstiel P, et al. : Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit Care Med. 2011;39(6):1407–13. 10.1097/CCM.0b013e318211ff56 [DOI] [PubMed] [Google Scholar]
- 43. Paige E, Clément M, Lareyre F, et al. : Interleukin-6 Receptor Signaling and Abdominal Aortic Aneurysm Growth Rates. Circ Genom Precis Med. 2019;12(2):e002413. 10.1161/CIRCGEN.118.002413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaiser K, Prystaz K, Vikman A, et al. : Pharmacological inhibition of IL-6 trans-signaling improves compromised fracture healing after severe trauma. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(5):523–36. 10.1007/s00210-018-1483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prystaz K, Kaiser K, Kovtun A, et al. : Distinct Effects of IL-6 Classic and Trans-Signaling in Bone Fracture Healing. Am J Pathol. 2018;188(2):474–90. 10.1016/j.ajpath.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 46. Magro G: SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X. 2020;2(2):100029. 10.1016/j.cytox.2020.100029 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Lesina M, Kurkowski MU, Ludes K, et al. : Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. 10.1016/j.ccr.2011.03.009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48. Lanaya H, Natarajan A, Komposch K, et al. : EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16(10):972–7. 10.1038/ncb3031 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Sacre SM, Andreakos E, Taylor P, et al. : Molecular therapeutic targets in rheumatoid arthritis. Expert Rev Mol Med. 2005;7(16):1–20. 10.1017/S1462399405009488 [DOI] [PubMed] [Google Scholar]
- 50. Gabay C, Emery P, van Vollenhoven R, et al. : Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50. 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Le RQ, Li L, Yuan W, et al. : FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943–7. 10.1634/theoncologist.2018-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Campochiaro C, Della-Torre E, Cavalli G, et al. : Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9. 10.1016/j.ejim.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 53. Della-Torre E, Campochiaro C, Cavalli G, et al. : Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: An open-label cohort study. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-218122 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. Safety and Efficacy of TJ301 IV in Participants With Active Ulcerative Colitis. ClinicalTrials.2020. Reference Source [Google Scholar]