Abstract
Colon cancer continues to be one of the leading causes of mortality and morbidity throughout the world despite the availability of reliable screening tools and effective therapies. The majority of patients with colon cancer are diagnosed at an early stage (stages I to III), which provides an opportunity for cure. The current treatment paradigm of early stage colon cancer consists of surgery followed by adjuvant chemotherapy in a select group of patients, which is directed at the eradication of minimal residual disease to achieve a cure. Surgery alone is curative for the vast majority of colon cancer patients. Currently, surgery and adjuvant chemotherapy can achieve long term survival in about two-thirds of colon cancer patients with nodal involvement. Adjuvant chemotherapy is recommended for all patients with stage III colon cancer, while the benefit in stage II patients is not unequivocally established despite several large clinical trials. Contemporary research in early stage colon cancer is focused on minimally invasive surgical techniques, strategies to limit treatment-related toxicities, precise patient selection for adjuvant therapy, utilization of molecular and clinicopathologic information to personalize therapy and exploration of new therapies exploiting the evolving knowledge of tumor biology. In this review, we will discuss the current standard treatment, evolving treatment paradigms, and the emerging biomarkers, that will likely help improve patient selection and personalization of therapy leading to superior outcomes.
Keywords: Adjuvant, Circulating tumor DNA, Immunoscore, Minimally invasive, Neoadjuvant, FOxTROT, Minimal residual disease, International duration evaluation of adjuvant chemotherapy
Core tip: Although the majority of patients with colon cancer are diagnosed in an early stage, cancer recurrence after initial curative therapy is frequent, underscoring the need for novel approaches. The challenges in the current treatment paradigm include the lack of precise patient selection tools for adjuvant therapy, disabling toxicities, and modest efficacy of the adjuvant therapies. Herein we provide a contemporaneous appraisal of the early stage colon cancer treatment and discuss how evolving technologies, including circulating tumor DNA, can potentially transform the standard of care.
INTRODUCTION
Colorectal cancer (CRC) continues to be a major global health problem, with approximately 1.09 million new cases diagnosed and 551000 deaths from it each year[1]. Globally the burden of CRC is expected to increase by 60% resulting in more than 2.2 million new cases and 1.1 million deaths annually by the year 2030[2]. Recent data from the western countries suggest that the incidence of CRC is increasing in population under age 50[3]. Approximately 75% of newly diagnosed CRC patients present with non-metastatic early stage disease[4], which presents an opportunity of curative-intent treatment. Despite surgery and adjuvant therapy, 5% to 30% patients with colon cancer (CC) endure recurrence[5,6].
Colorectal carcinogenesis is a protracted multistage process which evolves over several decades. Most CRCs arise from adenomatous polyps that gradually progress to dysplasia and eventually to carcinoma over a period of 5-15 years[7], which opens up an opportunity for early detection and cure. Screening can identify early stage CRC that is easier to treat and has a lower mortality rate than advanced CRC. In addition, screening can prevent CRC by detecting and removing premalignant polyps before they progress to carcinoma. CRC incidence and mortality rates have been declining in the United States, likely due to widespread adoption of screening[8]. However, conventional colonoscopy has about 25% of false-negative results due to flat or depressed precancerous lesions[9]. A systematic review of colonoscopy studies reported a pooled miss rate of 22% for all polyps and 26% for polyps smaller than 5 mm in size[10].
While current treatment modalities with proven efficacy save thousands of lives, short- and long-term toxicities of the treatment often significantly compromise the quality of life. To improve efficacy and reduce toxicity, contemporary research is focusing on the following areas: (1) Minimizing the invasiveness of surgical resection and improving surgical recovery; (2) Refining patient selection for adjuvant therapy based on novel biomarkers; (3) Precise risk stratification to calibrate treatment type, intensity and duration; and (4) Exploration of new systemic therapies incorporating targeted agents. In this article, we present a review of the current standard treatment strategies and evolving treatment paradigms that might improve outcomes in the near future.
EARLY STAGE COLON CANCER: OVERVIEW OF TREATMENT STRATEGIES
Current standard treatment of early stage CC consists of upfront resection of the primary tumor along with regional lymph nodes and, in selected patients, administration of adjuvant chemotherapy (AC) 4 to 8 wk after the surgery[11-13]. For stage I CC, the current standard of care is surgery alone, which results in a 5-year disease-free survival (DFS) rate of 95%[6], and AC is not recommended. In stage II CC, the reported 5-year DFS rate with surgery alone ranges from 82% to 88%[6,14], and the benefit of AC remains controversial. Current major guidelines recommend AC guided by risk stratification based on clinicopathologic features for patients with stage II CC[11-13]. AC, preferably with a combination of fluoropyrimidine and oxaliplatin, is recommended for all resected stage III patients[11]. Of note, surgery alone can achieve a 5-year DFS rate of 45%-50% in stage III patients[14,15], and the administration of oxaliplatin-based AC after surgery results in a 5-year DFS rate of 67%-70%[5,16,17]. These data highlight that only 17%-20% of stage III patients survive long term because of AC. The gain in survival with oxaliplatin-based AC needs to be considered in the light of treatment-related toxicities, especially 12.5% incidence of grade 3 neuropathy after 6 months of treatment[5]. Table 1 summarizes the role of surgery and chemotherapy in early stage CC.
Table 1.
Role of surgery and adjuvant chemotherapy in early stage colon cancer (American Joint Committee on cancer stages I to III)
| Stage I | Stage II | Stage III | |
| Definition | The tumor has grown through the colonic mucosa and has invaded the muscular layer of the colon | The tumor has grown through the wall of the colon or invaded adjacent organ, but has not involved the regional lymph nodes | The tumor has spread to the regional lymph nodes, but not to the distant organs |
| Contribution of surgery | 5-yr DFS rate of 95% with surgery alone[6] | 5-yr DFS rate of 82% to 88% with surgery alone[6,14] | 5-yr DFS rate of 45%-50% with surgery alone[14,15] |
| Contribution of adjuvant chemotherapy | Adjuvant chemotherapy not recommended | Only offered to “high-risk” group-magnitude of benefit is uncertain | Recommended for all patients. Absolute improvement of 5-yr DFS rate is about 20% because of adjuvant chemotherapy[5,16,17] |
DFS: Disease free survival.
SURGERY FOR EARLY STAGE COLON CANCER
The techniques for surgical resection have changed dramatically in the last three decades with the invention of minimally invasive techniques such as laparoscopy and robotic surgery. Endoscopic techniques that can be employed for select stage I tumors are currently an active area of research and offer the potential to significantly reduce the risk of complications, which averages about 20% in patients undergoing traditional surgical resection[18]. The expansion of laparoscopy for colectomy, along with the rapid growth of robotics has allowed surgeons to perform colectomy with significant reductions in complications and faster recovery for patients. In general, the goal of surgical resection is three-fold: To resect visible malignant disease, to remove the tumor in the wall of the colon and to remove the lymph nodes in the drainage basin for the tumor. By accomplishing this, accurate pathologic staging can be determined, and patients can be stratified into risk categories based on histologic and pathologic features. Such risk stratification is nearly impossible to perform without detailed histopathologic information.
Endoscopic resection
In select cases of large non-invasive premalignant polyps or early invasive tumors with favorable features, endoscopic resection can be employed. Clearly, for advanced adenomas such as tubulovillous adenomas or intramucosal adenocarcinoma, lymph node resection is not indicated, and only complete removal of the mucosal based dysplastic tissue is needed. Additionally, certain malignant polyps with favorable features, such as well or moderate differentiation, pedunculated morphology, at least 2 mm from the cauterized edge, without lymphovascular invasion and no evidence of distant or nodal metastases, are amenable to endoscopic resection with very low risk of lymph node metastasis and excellent long-term overall survival[19]. In a study of patients with malignant polyps who were lacking only one listed favorable feature, the risk of lymph node metastasis was 8% and residual carcinoma was 3% following surgery; with the risk of surgical complications at 13%, the balance remained even suggesting that only patients with multiple poor prognostic features would benefit from surgery (Table 2)[20,21]. Furthermore, some features are high-risk enough on their own to warrant resection even if others are lacking, specifically poorly differentiated tumors or mucinous or signet ring histology or those with deeper submucosal invasion, as these morphologies are associated with rates of lymph node metastases as high as 17%-46%[21,22]. Thus, for malignant polyps and very small stage I disease, the recommendation for full segmental colonic resection should be an individualized decision based upon the patients’ tumor risk factors and surgical risk factors. In some guidelines, specific recommendations are laid out for when such treatments should be employed to help guide surgeons on risk management in such complicated settings[23]. There are three general advanced endoscopic techniques: Endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and combined endoscopic-laparoscopic surgery (CELS). EMR, which involves the injection of fluid to “lift the polyp” from the submucosa followed by polypectomy using snare technique, differs from ESD, where endoscopic knives are used to create an incision in the bowel wall after fluid injection, and the lesion is removed circumferentially[24]. Both EMR and ESD allow higher rates of en bloc resection of the colon lesion and minimize piecemeal resection, which can make margin identification difficult and can lead to higher polyp recurrence rates[25]. EMR is technically less challenging, has lower complication risks, and can be repeated multiple times if necessary in the case of recurrent non-invasive dysplasia[25]. ESD is employed with larger lesions and for those where invasion into the submucosa is suspected as this technique allows resection into the deeper (submucosal) layers of the bowel wall[25]. If deeper invasion is a concern, endoscopic ultrasound or chromoendoscopy during the procedure can be performed with good accuracy in predicting the depth of submucosal invasion, and this can help guide the choice of endoscopic resection[22]. Once the lesions are removed, if poor prognostic features are present (as defined in Table 2) then surgery is recommended due to elevated risk of nodal metastases[26]. Overall, the results of ESD have been very good, with local recurrence rates of 2% in one single-institution high volume center, all of which were high-grade dysplasia without invasion and piecemeal resection was shown to be the significant predictor of recurrence[27].
Table 2.
Prognostic features of malignant polyps
| Features consistent with low risk of lymph node metastases (Low risk/favorable features)[26] | Features consistent with high risk of lymph node metastases (Poor prognostic features)[26] |
| Margins with no dysplasia or malignancy | Poorly differentiated |
| Well or moderately differentiated | Mucin/mucinous |
| No angiolymphatic invasion | Signet ring or cribriform histology |
| Superficial invasion into submucosa (≤ 2 mm) | Tumor budding |
| Lymphovascular invasion | |
| Deeper invasion into submucosa (> 2 mm) |
In cases where endoscopic resection is difficult, or the risk of complications is high, a combined surgical and endoscopic approach, known as CELS, can be utilized with great effect. Because the occult rate of invasive cancer for patients with benign appearing endoscopically unresectable polyps is low, 8.4% in one study, surgical resection may be avoided in select patients[28]. In CELS, the surgeon laparoscopically mobilizes the colon, offers assistance with positioning the colon in redundant patients, and repairs any perforation or controls bleeding when needed while the endoscopist performs the mucosal resection to remove the lesion. This technique has been shown to have acceptable risks with complication rates of 11% and failure rates of 6% in one study[29]. Furthermore, the cost of such procedures are lower than formal colectomy, most due to reductions in hospital length of stay for the CELS procedure[30].
Principles of surgical resection
The goal of surgical resection is three-fold: To resect visible malignant disease, to remove the affected segment of intestine, and to remove the correlating draining lymph nodes with vascular ligation and mesocolon integrity[31]. In the absence of synchronous lesions, the surgeon should inspect the abdominal cavity for evidence of other disease, and plan operative resection based upon the location of the tumor in the colon and its lymphovascular drainage such that a margin of colon 5-7 cm proximal and distal to the tumor is removed en bloc with the associated mesentery extending to the origin of the named primary blood vessel feeding the segment of bowel[26]. A minimum of 12 lymph nodes should be resected to allow accurate pathologic staging and improved survival[32]. When feasible, anastomosis of the proximal and distal resection margins should be considered to allow bowel continuity.
In the last few years, the idea of a complete mesocolic excision (CME) has gained popularity. This idea is similar to the complete mesorectal excision for rectal cancer–a sharp dissection along anatomic embryologic planes to dissect the colon mesentery from the retroperitoneum and isolate the angiolymphatic drainage to its most central location[33,34]. Studies indicate that the rate of central nodal metastasis is approximately 2%-3%, even when other nodes closer to the tumor location do not harbor metastases (i.e. skip metastases), thus if surgical resection reduced even just this recurrence, CME would be as effective as AC for low-risk stage II patients, in part by identifying micro-metastatic disease and optimizing lymph node harvest[35]. This dissection is not without cost, as overly aggressive clearance of lymphatic tissues around origin vessels on the aorta can not only damage the vessels, but also the nerve plexus resulting in diarrhea, delayed gastric emptying, as well as urologic and sexual dysfunction[35,36]. While several retrospective cohort studies have shown favorable oncologic outcomes, there remains no randomized controlled study to support the benefit of CME at this time, and a recent meta-analysis did not find any significant difference in complications or oncologic outcomes[37-39]. A corollary to CME, sentinel lymph node biopsy (removing the first draining node for a given tumor to determine if additional nodal resection is needed) has been commonly used in many other malignancies, including breast cancer and melanoma; however, in CC in vivo sentinel node biopsy has not been routinely utilized due to technical considerations with the procedure. Sentinel lymph node mapping (identifying the location of the first draining node within the resected lymphatic tissue) may have more utility. While it is not routinely recommended as part of the pathologic assessment, there is the potential to identify nodal micro-metastases and thereby more accurately stage patients, yet even in doing so long-term outcomes may not be appreciably affected[40,41]. Future studies will need to be performed to understand the full benefits of costs of CME and nodal mapping techniques.
Minimally invasive surgical resection
Laparoscopic surgical techniques were first described in the late 1980s and has spread widely throughout the surgical community with its principles impacting every facet of surgical care; CRC treatments are no exception. In the early 2000s, several randomized controlled trials validated the safety and oncologic utility of laparoscopic surgery for CC[42-44]. Laparoscopic resections have been shown to have less operative blood loss, faster return of bowel function, fewer complications, shorter hospital stays, with no differences in oncologic outcomes such as positive margins, lymph node harvest, or survival[45-47]. This interest in reducing the impact of surgery with ever smaller incisions and ever less invasive approaches has led to a number of novel surgical techniques including hand-assisted laparoscopy (using a smaller approximately 4 cm port to allow a single hand into the abdominal cavity), single-incision surgery (all ports through one incision about 2-3 cm long), and robotic surgery (using a “robotic” platform with fine and flexible instruments). These various techniques, which use the same oncologic principles discussed previously, are appropriate options with comparable oncologic outcomes, and the choice of technique ultimately lies with the surgeon[26]. Despite enthusiasm and recommendations from multiple societies, the rates of minimally invasive surgery utilization in many countries only reaches 50% with considerable geographic variability; it is not entirely clear why this is the case, but the long training needed for mastery of complex laparoscopic procedures and higher equipment costs are certainly contributory[26,31].
ADJUVANT CHEMOTHERAPY: GOAL, ENDPOINTS AND TIMING
The primary goal of adjuvant therapy is eradication of micro-metastatic residual disease after surgical removal of the primary tumor to achieve a cure. Since micro-metastatic disease cannot be reliably identified or monitored, historically improvement in 5-year overall survival (OS) had been the gold standard to confirm the benefit of AC. Overall, the 5-year OS correlates well with the long term disease control, as demonstrated in 2 large retrospective analyses[48,49] including an ACCENT (Adjuvant Colon Cancer End Points ) database analysis of 20898 patients enrolled in 18 randomized trials. The ACCENT database analysis reported recurrence rates of less than 1.5% per year after 5 years and less than 0.5% per year after 8 years from the study enrollment[49]. These data support the view that 5-year OS is a reliable surrogate marker of long-term survival and provides the “evidence for cure”. However, a long follow up period is needed to demonstrate an improvement in 5-year OS with the newer therapies in clinical trials, which underscored a need for an alternative strategy. A separate ACCENT database analysis of patients treated with 5-FU-based AC suggested that the 3-year DFS rate is an excellent predictor of 5-year OS, especially for stage III patients[50], and the 3-year DFS rate could be a surrogate endpoint for adjuvant CC trials. Subsequent retrospective analyses, which included patients receiving oxaliplatin-based AC, supported this view[51,52] although extended survival after recurrence as a result of improved therapy of metastatic disease weakened the strength of association between 3-year DFS and 5-year OS. Overall, 3-year DFS rate is considered a reliable endpoint to assess the efficacy of adjuvant therapy. The Drugs Advisory Committee of the United States Food and Drug Administration (FDA) has accepted a 3-year DFS rate as a regulatory endpoint for adjuvant therapy trials in CC. The adjuvant therapy with FOLFOX (5-FU, LV and oxaliplatin) was approved in the United States for stage III CC based on the improvement of the 3-year DFS rate reported in the MOSAIC trial[53]. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) pooled analysis[54], which evaluated the non-inferiority of AC administered for 3 mo vs 6 mo in stage III CC patients, also chose 3-year DFS rate as the primary endpoint.
The ideal time interval between surgery and initiation of AC is unknown, and a randomized clinical trial has not been conducted to date to address this question. Although the major guidelines do not specifically recommend a time window after the surgery, initiation of AC within 6 to 8 wk of surgery is required in most adjuvant clinical trials and has been accepted as a preferred practice. However, AC often does not begin within 8 wk of surgery in routine clinical practice due to a variety of reasons, including delay in recovery from the surgery. In this regard, laparoscopic surgery has an advantage over open resection, as recovery from the surgery is faster[55]. The impact of delaying initiation of AC on survival has been investigated in several retrospective studies and meta-analyses, which reached conflicting conclusions. A recent SEER-Medicare database analysis of 18491 patients reported significantly worse OS with initiation of AC after 8 wk of surgery, although benefit still persisted with a delay of up to 5 mo[56]. Two meta-analyses of fluoropyrimidine-based AC trials reported a higher risk of mortality with delayed initiation of AC beyond 8 wk[57] and 12 wk[58]. Conversely, a population-based analysis by the British Columbia Cancer Agency reported no adverse impact on outcome with a delay beyond 8 wk in patients with stage III CC who received oxaliplatin-based AC, implying that analyses based on fluoropyrimidine-based AC may not apply to patients who receive oxaliplatin-based AC[59]. The results of retrospective studies should be viewed in the light of possible biases, most important of which is the possibility that adverse tumor biology may have been responsible for both delays in initiation of AC as well as adverse survival outcome. For example, surgery of T4 CC is associated with higher post-operative morbidity[60] which can potentially delay the initiation of AC, and at the same time, the T4 disease is an independent predictor of poor survival[61]. In absence of conclusive data, we recommend initiation of AC within 8 wk of surgery. However, it is important to recognize that delayed initiation of AC, even up to 24 wk from the surgery, is associated with some degree of benefit[62].
DURATION OF ADJUVANT CHEMOTHERAPY
Duration of AC has evolved over the last 3 decades through a series of clinical trials[63-66] from 18 mo in the 1980s to 3 mo currently for a select group of patients. The MOSAIC trial, which established FOLFOX as the preferred adjuvant therapy for stage III CC, used chemotherapy for 6 mo[53]. However, oxaliplatin-based regimens for 6 mo are associated with several disabling toxicities, especially the oxaliplatin-induced peripheral sensory neuropathy. Some degree of neuropathy occurs in nearly all patients[53], and approximately two-thirds will have symptoms one-year post-treatment or beyond[67,68]. Moreover, the neuropathy often peaks several months after the last dose of oxaliplatin, which makes the preemptive dose adjustment to prevent neuropathy difficult[69]. In consideration of the potential curability and long survival of patients undergoing AC, the efficacy of a shorter duration of adjuvant therapy was explored in a pooled analysis of six large randomized trials with stage III CC patients (IDEA study) which evaluated the primary hypothesis that 3 mo of adjuvant oxaliplatin-based therapy would be non-inferior to standard 6 mo with a primary endpoint of 3-year DFS rate[54]. This pooled analysis had a non-inferiority design in which non-inferiority of 3 mo vs 6 mo would be established if the upper limit of the two-sided 95% confidence interval (CI) of the hazard ratio (HR) did not exceed 1.12. The rationale behind choosing this non-inferiority margin was it corresponded to a worsening of the 3-year DFS rate by 2.7% compared to the standard therapy (from 72% to 69.3%), an outcome that was considered acceptable. Overall, about 40% of the patients received CAPOX (capecitabine and oxaliplatin), and 60% received FOLFOX. After a median follow-up of 41.8 months, although there was only 0.9 % difference in the 3-year DFS rate (74.6% vs 75.5%), the non-inferiority of 3 mo vs 6 mo was not confirmed in the overall study population (HR 1.07; 95%CI: 1.00-1.15). In a preplanned subgroup analysis by chemotherapy regimen, the non-inferiority of 3 mo was observed for CAPOX but not for FOLFOX. Of the patients who received CAPOX, 3 mo was found to be non-inferior to 6 mo (DFS rates of 75.9% vs 74.8%, respectively; HR 0.95; 95%CI: 0.85-1.06). Conversely, for patients receiving FOLFOX, 6 mo was found to be superior to 3 mo (DFS rate of 73.6% for 3 mo vs 76% for 6 mo; HR 1.16; 95%CI: 1.06-1.26; P = 0.001). Furthermore, an exploratory analysis revealed that in the ‘low risk ‘ patient group (T1–3 and N1; 58.7% of patients), 3 mo of therapy was non-inferior to 6 mo for both CAPOX and FOLFOX regimens, with the 3-year DFS rates of 83.1% and 83.3%, respectively (HR 1.12; 95%CI: 0.90-1.12). Conversely, in patients with “high risk” tumors (T4/N1-2 or any T/N2 ; 41.3% of patients), the 6-month therapy was superior to the 3-month (3-year DFS rate of 64.4% vs 62.7% for the treatments combined; HR 1.12; 95%CI: 1.03-1.23; P = 0.01 for superiority). As expected, there was a substantial reduction in neurotoxicity with the 3-mo treatment. The incidence of neurotoxicity of grade 2 or higher with the 3-month regimens was 16.6% with FOLFOX and 14.2% with CAPOX compared to the 6-mo regimens, 47.7% with FOLFOX and 44.9% with CAPOX. Thus, the IDEA analysis provided a basis for treating low-risk stage III CC patients with 3 mo of therapy, especially if CAPOX is utilized. Based on this data, the most recent National Comprehensive Cancer Network (NCCN) guidelines recommend CAPOX for 3 mo as the preferred regimen for patients with low-risk stage III CC. For patients with high-risk stage III CC, CAPOX for 3 to 6 mo (with category 1 evidence for 6 mo) or FOLFOX for 6 mo (category 1) are recommended. Although CAPOX appears to have superior efficacy than FOLFOX in IDEA analysis, the evidence is not conclusive. The choice of using CAPOX vs. FOLFOX was not randomized, which increased the potential for selection bias. This is an important consideration in view of the fact that capecitabine is often poorly tolerated in the US population[70].
Four trials in IDEA collaboration (SCOT, TOSCA, ACHIEVE-2, and HORG) enrolled patients with high-risk stage II CC, with a total of 3273 patients randomly assigned to 3 mo vs 6 mo of adjuvant therapy, of whom 2019 received CAPOX and 1254 received FOLFOX[71]. The overall analysis failed to establish the non-inferiority of 3 mo vs 6 mo of treatment in terms of efficacy. In the entire population, five-year DFS rate was 80.7% vs 84% for 3 mo vs 6 mo of therapy, respectively (HR 1.18; 95%CI: 1.05-1.31; absolute difference of 3.3%). A subset analysis by regimen showed that 3 mo of CAPOX was non-inferior, with a 5-year DFS rate of 81.7% for 3 mo vs 82.0% for 6 mo (HR 1.02; 95%CI: 0.88-1.17). By contrast, the 5-year DFS rate for FOLFOX was 79.2% for 3 mo of treatment vs 86.5% for 6 mo, an absolute 7.3% difference in favor of longer treatment duration (HR 1.42; 95%CI: 1.19-1.70). It was concluded that 3 mo of CAPOX is a reasonable choice for high-risk stage II CC patients.
ADJUVANT CHEMOTHERAPY: CURRENT STANDARD
AC following the surgery is routinely recommended for all patients of resected stage III CC based on the unequivocal survival benefit demonstrated in numerous clinical trials, both with the 5-FU monotherapy[49] and oxaliplatin-based regimens[72]. The benefit with AC for the stage II group as a whole is debatable. Table 3 summarizes landmark adjuvant chemotherapy trials conducted in stage II and III CC patients.
Table 3.
Landmark adjuvant trials in early stage colon cancer
| Study (Reference) | Study population | Patients (n) | Experimental arm | Control arm | Study result/Conclusion |
| Intergroup (INT) 0035[64] | Stage II and III | 1296 | 5-FU bolus + Levamisole for 1 yr. | Observation. | Stage III: 5-FU/Levamisole reduced recurrence rate by 41% (P < 0.0001) and the death rate by 33% (P = 0.006). Stage II- No survival benefit with 5-FU/Levamisole. One year of 5-FU based adjuvant chemotherapy became the standard for stage III patients. |
| NSABP C-03[66] | Duke stage B and C | 1081 | Bolus 5-FU plus LV for 1 yr. | MOF for 1 year. | 5-yr DFS rates- 54% vs 66% in favor of 5-FU/LV, P = 0.0004. 5-yr OS rates - 66% vs 76% in favor of 5-FU/LV, P = 0.003. |
| IMPACT B2[77] | Stage II | 1016 | Bolus 5-FU/LV for 6 mo. | Observation. | Pooled analysis of B2 CC in 5 randomized trials. No significant improvement in survival with the adjuvant chemotherapy. The 5-yr EFS: 73% for controls and 76% for 5-FU + LV (HR, 0.83; 90%CI: 0.72-1.07). The 5-yr OS: 80% for controls and 82% for 5-FU + LV (HR, 0.86; 90%CI: 0.68-1.07). |
| Intergroup (INT) 0089[63] | High-risk stage II and stage III | 3794 | (1) Low-dose LV plus 5-FU (Mayo Clinic regimen); (2) High-dose LV plus 5-FU (Roswell Park regimen); and (3) Low-dose LV plus Levamisole plus 5-FU. Each for 30-32 wk. | Bolus 5-FU plus levamisole for 1 year. | None among the 4 arms was statistically superior in terms of DFS or OS. Roswell park regimen was better tolerated than Mayo Clinic regimen in terms of diarrhea. 6 mo of 5-FU/LV replaced 12 mo of 5-FU/Levamisole as standard of care. |
| GERCOR C96.1[85,86] | Stage II and stage III | 905 | Semimonthly infusional 5-FU/LV (de Gramont regimen). Duration- 24 vs 36 wk. | Monthly bolus 5-FU /LV (Mayo Clinic regimen). Duration- 24 vs 36 wk. | DFS and OS were not statistically different between treatment groups and treatment durations. Semimonthly infusional 5-FU/LV regimen had better toxicity profile and was adopted as the standard arm for the MOSAIC trial. |
| QUASAR[75] | Stage I-III | 3239 (Colon stage II = 2291) | 5-FU/LV monthly bolus (Mayo clinic regimen) for 6 mo. | Observation. | 3.6% (95%CI: 1.0–6.0) absolute improvement in 5-year OS with adjuvant chemotherapy in stage II CC patients. |
| X-ACT trial[92] | Stage III | 1987 | Capecitabine- 6 mo. | 5-FU/LV (Mayo Clinic regimen)- 6 mo. | 5-yr OS rates 71.4% with capecitabine vs 68.4% with 5-FU/LV (P = 0.06). Capecitabine was at least equivalent to 5-FU/LV in terms of OS and DFS. |
| MOSAIC[53,76] | High-risk Stage II and stage III | 2246 | FOLFOX4 for 6 mo. | de Gramont regimen (infusional 5-FU/LV) for 6 mo. | 10-year OS rates for stage III - 67.1% vs 59.0 % (HR, 0.80; P = 0.016) in favor of FOLFOX. 10-year OS rates for stage II - 78.4% vs 79.5% (HR, 1.00; P = 0.980). FOLFOX replaced 5-FU/LV as the standard adjuvant therapy in resected stage III CC. |
| NSABP C-07[17,90] | Stage II and stage III | 2407 | FLOX for 6 mo. | Bolus 5-FU/LV (Roswell Park) for 6 mo. | 5-yr DFS 69.4 vs 64.2% favoring FLOX (HR, 0.82; 95%CI, 0.72–0.93; P = 0.002) corresponding to an 18% relative reduction in the risk of a DFS event. 5-yr OS was similar between treatment groups. |
| NO169968/ XELOXA[16] | Stage III | 1886 | CAPOX- 6 mo. | bolus 5-FU/LV (Mayo Clinic or Roswell Park regimen) for 6 mo. | 7-yr DFS rates 63% versus 56% in favor of CAPOX (HR, 0.80; 95%CI, 0.69–0.93; P = 0.004). 7-year OS rates 73% vs 67% in favor of CAPOX (HR, 0.83; 95%CI, 0.70–0.99; P = 0.04). |
| IDEA meta-analysis[54] | Stage III | 12834 | FOLFOX or CAPOX for 3 mo. | FOLFOX or CAPOX for 6 mo. | Noninferiority of 3 mo versus 6 mo treatment was not confirmed in the overall study population. Among the patients with low-risk tumors (T1-T3, N1), 3 mo of therapy with CAPOX was noninferior to 6 mo, with a 3-year rate of disease-free survival of 85.0% versus 83.1% (hazard ratio, 0.85; 95%CI, 0.71-1.01). |
CC: Colon cancer; 5-FU: 5 Fluorouracil; LV: Leucovorin; MOF: Lomustine + vincristine + 5-FU; NS: Not significant; DFS: Disease free survival; EFS: Event free survival; OS: Overall survival; HR: Hazard ratio; CAPOX: Capecitabine and oxaliplatin.
Stage II colon cancer
Despite several randomized trials and meta-analyses, an unequivocal robust survival benefit from AC has not been demonstrated in stage II CC patients. The challenges to show a clear benefit with AC in stage II patients include marked prognostic heterogeneity within this patient group (5-year survival rate of 66.5% in T3N0 tumors vs 37.3% in T4bN0 tumors[73]), stage migration as a result of improved lymph node sampling over the years[74], excellent prognosis with the surgery alone[6] and a smaller number of stage II patients enrolled in randomized studies. The important studies which evaluated AC in stage II patients include QUASAR[75], MOSAIC[76], NSABP C-07[17], IMPACT B2 analysis[77] and the Cancer Care Ontario group analysis[78]. The QUASAR trial randomized 3239 patients with CRC (1073 patients of stage II CC in each arm) to observation vs monthly bolus 5-FU/LV for 6 mo. Among the patients with stage II CC, there was only a trend towards better OS in favor of the group who received AC with a five-year OS of 83.9% vs 81.5 % (HR 0.86; 95%CI: 0.54-1.19). The major criticism of the QUASAR trial was the small number of lymph nodes harvested (median number 6). The IMPACT B2 and the Cancer Care Ontario group analysis, both designed to evaluate the benefit of 5-FU-based AC, also failed to show a clear survival benefit. Conversely, in an ACCENT database analysis of nearly 6900 patients, 5-FU-based AC was associated with a 5% absolute improvement in survival at eight years (72% vs 66.8%, P = 0.026)[49]. A National Cancer Database (NCDB) analysis, which included 153110 patients of stage II CC diagnosed between 1998 and 2011, also showed a benefit with AC[79]. The 5- and 10-year OS rates were 73% and 51% with chemotherapy, as opposed to 62 % and 35% without chemotherapy.
The impact of adding oxaliplatin to 5-FU/LV backbone in stage II patients was explored in two prospective randomized trials, the MOSAIC[76] and the NSABP- C07[17] trials. The final report of the MOSAIC trial[76] reported identical 10-year OS rates with 5-FU/LV vs FOLFOX4: 79.5% vs 78.4% (HR 1.00; P = 0.98), respectively. NSABP-C07 trial also did not show any benefit of oxaliplatin containing regimen FLOX over 5FU/LV (5-year DFS rate 82.1% vs 80.1%, respectively; P = 0.67). Of note, no prospective randomized trial has been conducted to date comparing oxaliplatin-based AC with observation alone in resected stage II CC patients. In summary, evidences are lacking to support the routine use of AC in stage II CC patients.
Several studies have suggested that certain clinicopathologic high-risk features might be predictive of benefit from AC in stage II CC patients[80,81]. The current NCCN guideline recommends consideration of AC in stage II CC patients with following high-risk features[11]- T4 primary tumor, poorly differentiated histology (exclusive of tumors with deficient mismatch repair), lymphovascular invasion (LVI), perineural invasion (PNI), bowel obstruction, localized perforation, inadequately sampled lymph nodes (< 12 nodes) and close, indeterminate, or positive margin. The MOSAIC[5] trial included 569 patients with high-risk stage II CC- 282 patients randomized to the FOLFOX4 arm and 287 patients to the 5-FU/LV arm. The 5-year DFS rate was numerically higher with FOLFOX4, 82.3% (95%CI: 77.2%-86.28%) vs 74.6% (95%CI: 69.1%-79.34%), a difference that was not statistically significant. The NCDB analysis[79] mentioned above demonstrated a benefit with AC with a 5-year OS improvement from 57% to 76% (P < 0.001) in the high-risk group.
An important limitation of the studies described above is that these studies analyzed the high-risk stage II patients collectively as a group, despite the possibility that biologic heterogeneity among the various high-risk features may exist. A retrospective study, which analyzed the patients based on a single predominant high-risk feature[82], showed that AC was associated with improved OS only among the patients with T4 tumor as the single high-risk feature (HR 0.51; 95%CI: 0.34–0.78) or combinations involving T4 tumors as T4/< 12 sampled lymph nodes (HR 0.31; 95%CI: 0.11–0.90), T4/high grade histology (HR 0.26; 95%CI: 0.11-0.61), and T4/LVI (HR 0.16; 95%CI: 0.04–0.61). A prospective randomized trial to evaluate the benefit of AC exclusively in the high-risk stage II CC patients has not been conducted to date.
Stage III colon cancer
Once the NSABP C-01 trial[65] demonstrated a survival benefit with 5-FU-based AC in patients with resected Duke B and C colon cancer and the enhancement of the antitumor activity of 5-FU by leucovorin (LV) was reported[83], clinical trials over the next decades were conducted with three major schedules of 5-FU and LV combinations: (1) Monthly bolus 5-FU and LV (Mayo clinic regimen); (2) Weekly bolus FU and LV, 6 wk out of 8 wk (Roswell Park Memorial Institute regimen, RPMI); and (3) Semimonthly infusional 5-FU/LV regimen (de Gramont schedule)[63,66,84-87]. These clinical trials led to two important conclusions : (1) Stage III CC patients derived unequivocal survival benefit from the AC whereas stage II patients did not; and (2) All three 5-FU/LV schedules had comparable efficacy, but the semimonthly regimen had better tolerability[85,86,88,89]. These trials established 5-FU/LV based regimens as the standard adjuvant therapy for stage III CC in the pre-oxaliplatin era. The GERCOR C96.1 trial[85,86] compared semimonthly regimen with monthly bolus 5-FU/LV in stage II and III patients, each given over 24 wk and 36 wk. There were no significant differences in DFS with either treatment arm (HR, 1.04) or between 24 wk or 36 wk of therapy (HR, 0.94) at a median follow up of 6-year. The semimonthly regimen was less toxic, particularly with regards to hematologic and gastrointestinal adverse events (P < 0.001). As a result, the semimonthly regimen was adopted as the standard arm in the subsequent MOSAIC trial[53].
In the next phase, several randomized adjuvant trials were conducted in which oxaliplatin was added to the 5-FU/LV backbone[16,17,76]. The MOSAIC trial, which randomized resected stage II and III patients to semimonthly 5-FU/LV vs oxaliplatin-based FOLFOX4 for 6 mo, demonstrated a superior 3-year DFS in stage III patients treated with FOLFOX4[53] and the benefit sustained long term. Most recent publication of MOSAIC data, after a median follow up of 9.5 years, reported a 10-year OS of 67.1% with FOLFOX4 vs 59% with 5-FU/LV (HR 0.80; 95%CI: 0.66-0.96; P = 0.016)[76]. In the XELOXA trial[16], resected stage III CC patients were assigned to CAPOX vs bolus 5-FU/LV (as Mayo Clinic regimen or RPMI) for 6 mo. After a median follow up of about 7 years, the 7-year DFS rates (the primary endpoint of the study) were 63% and 56% with CAPOX and 5-FU/LV, respectively (HR 0.80; 95%CI: 0.69-0.93; P = 0.004). In the NSABP C-07 trial[17,90], oxaliplatin was added to the weekly bolus 5-FU/LV (FLOX) and was compared to the RPMI regimen for 6 mo in stage II and III patients. This trial reported outcome after 8 years of median follow up which showed a favorable 5-year DFS with FLOX in the combined stage II and III population- 69% vs 64% (HR 0.82; 95%CI: 0.72-0.93; P = 0.002), but no OS benefit (5- year OS of 80% vs 78 % with an HR of 0.88; 95%CI: 0.75-1.02; P = 0.08). Based on these trial results, FOLFOX and CAPOX emerged as the preferred adjuvant regimens for resected stage III CC. FLOX regimen is rarely used in the current clinical practice because of toxicities, particularly diarrhea and neutropenia. However, the FLOX regimen could be a logical alternative for patients who experience chest pain with capecitabine or infusional 5-FU[91].
Capecitabine as adjuvant therapy was evaluated in stage III CC patients in the Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) study[92] which randomly assigned 1987 patients to six months of capecitabine alone (1250 mg/m2 twice daily for 14 of every 21 d) or monthly bolus 5-FU/LV . With a median follow-up of 6.9 years, capecitabine was at least equivalent to 5-FU/LV in terms of DFS (HR 0.88; 95%CI: 0.77-1.01) and OS (HR 0.86; 95%CI: 0.74-1.01). This pattern was maintained in all subgroups, including patients aged 70 years or older.
AC in the elderly population (aged ≥ 70 years) poses a number of unique challenges, which include limited bone marrow reserve, impaired functional capacity, co-morbidities, and increased risk of toxicities from chemotherapy. Analysis of pooled clinical trial data[93] as well as population-based studies[94-96] have provided evidence that 5-FU/LV based AC confers as much OS benefit in elderly population as in younger population and the rate of toxicities are not higher in the older population. However, the benefit from the addition of oxaliplatin to 5-FU/LV in the elderly population is controversial. Post-hoc analyses of MOSAIC[97] and NSABP C-07[17] trials, as well as an ACCENT database analysis[98] have failed to demonstrate a significant survival benefit with oxaliplatin-based regimens in patients aged ≥ 70 years. On the other hand, a benefit was suggested in a pooled analysis of four randomized trials comparing an oxaliplatin-containing vs a non-oxaliplatin containing regimen[99]. In this analysis, OS was significantly improved in all age groups, although the benefits of oxaliplatin were attenuated in those aged ≥ 70 years (HR 0.78; 95%CI: 0.61-0.99, vs HR 0.62; 95%CI: 0.54-0.72). Furthermore, patients aged ≥ 70 years are more likely to discontinue oxaliplatin earlier than younger patients[17]. As a result, oxaliplatin-based regimens are not routinely recommended for patients aged ≥ 70 years, although not contraindicated for those in good general health. For elderly patients considered to have low-risk disease and/or considered unsuitable for oxaliplatin-based chemotherapy, capecitabine or 5-FU based regimens are reasonable alternatives. A subgroup analysis of the X-ACT trial confirmed the efficacy of capecitabine in stage III patients aged ≥ 70 years[92]. If tolerance to capecitabine is poor, which is prevalent in the United States[70], intravenous 5-FU/LV based regimens are reasonable alternatives, especially the semimonthly regimen, because of its favorable toxicity profile[86].
Oxaliplatin based AC is the current standard of care for stage III patients with dMMR/MSI-H tumors, which is supported by a retrospective study[100]. 5-FU monotherapy is contraindicated in this group, as discussed in the following section. The role of immunotherapy in this setting is currently being investigated in clinical trials[101].
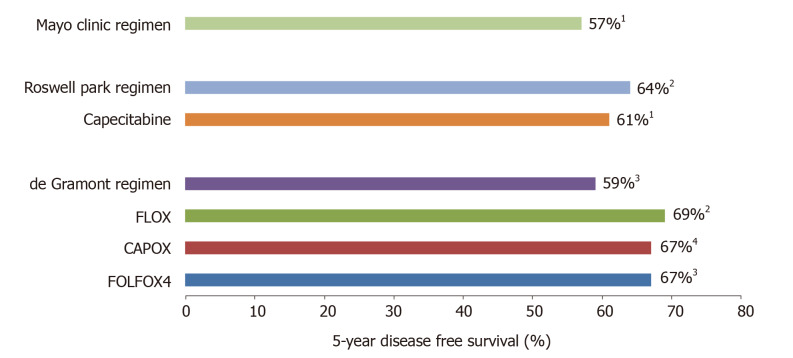
Several drugs active in metastatic setting have failed to show any benefit in the adjuvant setting, including the addition of irinotecan to 5-FU/LV[102-104], the addition of bevacizumab to oxaliplatin-based regimens[105,106], the addition of bevacizumab to capecitabine[107] and finally the addition of cetuximab to FOLFOX in the N0147[108] and PETACC8[109] trials. Figure 1 illustrates 5-year DFS rates with standard adjuvant regimens in stage III CC.
Figure 1.
5-year disease free survival rate in stage III colon cancer patients treated with standard adjuvant chemotherapy regimens. 1X-ACT trial[92]; 2NSABP-C07[17,90]; 3MOSAIC trial[53,76]; 4NO16968/XELOXA[16].
REFINING PATIENT SELECTION AND PERSONALIZATION OF ADJUVANT THERAPY
The most important challenge in the current treatment paradigm of early stage CC is the inability to detect micro-metastatic residual disease after the surgery. Clinicopathologic characteristics currently utilized to optimize adjuvant therapy imperfectly prognosticate the risk of cancer recurrence. As a result, AC is recommended in all resected stage III CC patients, although only about 20% of these patients are the actual beneficiary of the adjuvant therapy, as discussed earlier. Conversely, AC is withheld in all average risk stage II patients, and 12%-18% of these patients endure cancer recurrence[6,14]. Recent research has unveiled a variety of promising tools and biomarkers which might enable precise patient selection and therapy personalization. These biomarkers/tools broadly belong to the following categories: (1) Circulating tumor DNA (ctDNA) based assays; (2) Tools based on immune contexture of the primary tumor (“immunoscore”); and (3) Molecular markers and genomic profiling. Table 4 summarizes the leading prognostic and/or predictive biomarkers.
Table 4.
Evolving tools and biomarkers which may help precise patient selection for adjuvant therapy and therapy personalization in early stage colon cancer
| Biomarker/tool | Clinical significance | Potential use and relevance | Ref. |
| ctDNA | Prognostic | ctDNA detection in the bloodstream after surgical resection and adjuvant chemotherapy provides direct evidence of residual micro-metastatic disease and correlates with a very high risk of cancer recurrence in resected stage II and III patients. Sensitivity, specificity, positive and negative predictive values are 48%, 100%, 100% and 91%, respectively. Reported studies suggest that ctDNA can potentially serve as a real time marker of adjuvant therapy efficacy in stage II and III patients. | [110-115] |
| Immunoscore | Prognostic | High immunoscore is associated with favorable prognosis in both stage II and III patients independent of patient T stage, N stage and microsatellite instability. High-risk stage II patients with high Immunoscore had similar time to recurrence compared with average risk stage II patients in a recent report. | [118-122] |
| dMMR | Prognostic and predictive | Associated with favorable prognosis in stage II and possibly low-risk (IDEA defined) stage III patients. Predicts lack of benefit and possibly harm with 5-FU based adjuvant chemotherapy in both stage II and III patients. | [124-137] |
| KRAS and BRAFV600E mutation | Prognostic | KRAS and BRAFV600E mutations have been reported to be associated with a worse prognosis in several large retrospective studies, in both stage II and III patients. dMMR status attenuates adverse prognostic impact of BRAFV600E mutation, possibly except in IDEA defined high-risk stage III CC. | [133,137 -141] |
| Genomic profiling (Oncotype Dx Colon Cancer®) | Prognostic | Prognostic discrimination capacity is insufficient to guide therapy in routine clinical practice. | [142-147] |
| PIK3CA mutations | Predictive | Retrospective analysis suggests an association between the use of aspirin and improved survival among the patients with mutated-PIK3CA colorectal cancer including stage I-III patients. | [152] |
| CDX2 expression | Prognostic and predictive | Retrospective analysis suggested lack of CDX2 expression was associated with worse outcome in stage II and III CC. Lack of CDX2 expression appears to be predictive of benefit from adjuvant chemotherapy in stage II patients. | [153] |
| CMS | Prognostic | CMS1 tumors have a good prognosis, the CMS4 tumors have a poor prognosis, and the CMS2 and CMS3 types have an intermediate prognosis. Not validated to guide therapy in routine clinical practice. | [148-151] |
ctDNA: Circulating tumor DNA; dMMR: Deficient mismatch repair status; CC: Colon cancer; CMS: Consensus molecular subtypes.
Circulating tumor DNA
The ctDNA is the fraction of cell-free DNA in the circulation that originates from the apoptotic or necrotic tumor cells and carries tumor-specific genetic or epigenetic alterations. A rapidly increasing body of research indicates that the presence of tumor-specific ctDNA in the bloodstream after completion of the curative surgery can identify patients with residual, radiographically occult cancer who are at a substantially higher risk of cancer recurrence[110-115]. Two recently reported cohort studies, designed to determine the prognostic value of ctDNA in newly diagnosed resected stage II and III CC patients who had at least one tumor-specific DNA mutation commonly found in CC, are of particular importance[113,114]. The first study analyzed 230 patients with stage II CC using a next-generation sequencing panel on blood collected 4-10 wk after surgical resection[114]. The study showed that, among the patients who did not receive AC, 79% (11 out of 14) with detectable ctDNA post-surgery had a cancer relapse at a median follow-up of 27 mo. On the other hand, recurrence occurred in only 16 (9.8 %) of 164 patients with negative postoperative ctDNA (HR 18; 95%CI: 7.9-40; P < 0.001). Kaplan-Meier estimates of relapse-free survival at 3 years were 0% for the ctDNA-positive and 90% for the ctDNA-negative groups. Detectable ctDNA following resection had a positive predictive value of 100% and a negative predictive value of 92%. Among the stage II patients who received AC, the presence of ctDNA after completion of chemotherapy was also associated with an inferior recurrence-free survival (HR 11; 95%CI: 1.8-68; P = 0.001). On multivariate analysis, the detection of ctDNA was associated with the highest risk for recurrence (HR 28; P < 0.001), and the other well-known high-risk clinicopathologic features (i.e., < 12 lymph nodes examined, presence of lymphovascular invasion, microsatellite status) did not meet statistical significance. In the other study with stage III patients[113], 47% of patients with detectable ctDNA post-surgery were disease-free at 3 years compared with 76% of those with undetectable ctDNA (HR 3.8; 95%CI: 2.4-21.0; P < 0.001). On multivariate analysis, ctDNA status after surgery had the strongest independent association with cancer recurrence among the clinicopathological variables studied, including T and N stage. Disease recurrence at 3 years was also higher in the patients with detectable ctDNA after AC than in those without ctDNA after AC (77% vs 30%; HR 6.8; 95%CI: 11.0-157.0; P < 0.001). Furthermore, conversion from positive to negative ctDNA status after AC resulted in a lower recurrence rate compared to the patients with persistent ctDNA (HR 3.7; P = 0.04). In both studies, the risk of cancer recurrence was substantially higher in those who had detectable ctDNA post-surgery, which did not turn undetectable after standard AC, suggesting the possibility that ctDNA can potentially serve as a real-time marker of efficacy of the adjuvant therapy. A recently reported analysis of the IDEA-France data (presented at the ESMO 2019 Congress) also confirmed that the presence of ctDNA post-operatively is an independent adverse prognostic marker (adjusted HR 1.85; P < 0.001) in stage III patients[116]. These data, taken together, suggest that ctDNA can serve as a tool to detect minimal residual disease following resection and AC in early stage CC patients, independent of known clinicopathologic risk factors.
The ctDNA, although looks promising, has several important limitations, which include modest sensitivity in the adjuvant setting (50%-60%)[117], a lack of standardization across the platforms, and a lack of validation cohorts in the reported studies. Moreover, among the stage III patients[113] who completed at least 12 wk of prescribed adjuvant therapy (78 out of 96), 9 patients had detectable ctDNA post-surgery which turned undetectable after AC, and 3 of these 9 patients had disease recurrence with a time to recurrence between 15.7 to 20 mo. This observation highlights a potential drawback of ctDNA as a marker of efficacy of adjuvant therapy.
Immunoscore
Immunoscore, derived from the density of CD3+ and CD8+ T-cells within the tumor and its invasive margin, is an emerging tool that may play an important role in the near future to risk-stratify early stage CC patients into distinct prognostic groups with significant therapeutic implications[118-121]. Immunoscore has recently been validated prospectively in a large trial population of stage I-III CC patients and has been demonstrated to have a stronger association with survival characteristics than a variety of other risk parameters, including the AJCC/UICC TNM classification system[120]. A separate study reported that high-risk stage II patients with high Immunoscore had a time to recurrence similar to the low-risk stage II patients implying that Immunoscore can potentially risk-stratify high-risk stage II CC patients and help precise patient selection for adjuvant therapy[119]. A meta-analysis to evaluate the prognostic value of immunoscore in CC, which included 8 studies, confirmed that low immunoscore was significantly correlated with poor OS (HR 1.74; 95%CI: 1.43-2.13) and DFS (HR 1.82; 95%CI: 1.64-2.03)[122]. Clinical trials are needed to assess the value of Immunoscore in guiding therapeutic decision making. Immunoscore, once prospectively validated, has the potential to help select patients for observation who would otherwise be candidates for AC based on current guidelines. Perhaps of even greater importance is the potential for the immunoscore to be used to identify the subset of patients who might be responsive to immunotherapy-based adjuvant therapy.
Molecular markers and genomic profiling
A variety of molecular markers are reported to have prognostic and predictive value with important therapeutic implications in early stage CC. Microsatellite instability (MSI), a characteristic genetic signature of deficient mismatch repair mechanism (dMMR), is an important prognostic and predictive biomarker which currently influences treatment decision. High levels of MSI (MSI-H), defined as instability in ≥ 30% of microsatellite loci, occurs approximately in 15% to 20% of early stage CRC patients[123] with higher prevalence in stage II as compared with stage III CC (21 vs 14% in one study)[124]. Patients with dMMR stage II CC have an excellent prognosis with surgery alone, and AC does not improve survival[125-128]. Current NCCN guideline does not recommend AC in MSI-H/dMMR stage II patients, even in patients with high-risk features such as T4 tumors[11].
For the patient group with stage III disease, the MSI-H/dMMR status has also been shown to be associated with favorable prognosis in some[129,130] but not in all studies[131]. Furthermore, it has been suggested in a retrospective analysis that the favorable effect of dMMR status is limited to patients with right-sided stage III tumors treated with FOLFOX -based AC[132]. A recently reported pooled analysis of stage III CC patients (n = 5337) enrolled in 2 adjuvant trials with FOLFOX ± cetuximab [N0147 (Alliance) and PETACC-8] reported that the prognostic advantage of MSI-H status is limited to IDEA study defined low-risk stage III patients[133].
A number of retrospective analyses support the view that MSI-H phenotype predicts the lack of efficacy or even potential harm with 5-FU based AC[126,127,134,135]. Furthermore, in vitro studies suggest that dMMR CC cell lines are less susceptible to 5-FU induced cytotoxicity[136]. Based on these data, AC with 5-FU/LV alone is not recommended for stage II or III CC patients. Conversely, both DNA mismatch repair-proficient and –deficient CC cell lines are sensitive to oxaliplatin[137], and AC with oxaliplatin-based regimens retains its efficacy in MSI-H stage III CC patients[100].
Poor survival associated with the presence of KRAS[138-140] and BRAFV600E mutations[140,141] in early stage CC patients have been reported in several large retrospective studies. In a recently reported retrospective analysis, KRAS mutation was found to be a strong predictor of shorter time to relapse in both IDEA analysis defined low- and high-risk stage III patients who received FOLFOX-based AC for 6 mo[133]. However, sufficient data do not exist at this time to use KRAS or BRAF mutation status to guide adjuvant therapy.
Several multigene assays have been explored as prognostic and predictive tools to identify higher-risk patients in a given TNM stage group. Oncotype dx colon is the most extensively studied gene panel[142-146]. The validation studies with stage II and III patients in QUASAR and NSABP C-07 trials showed that the Oncotype dx recurrence scores are prognostic for recurrence, DFS, and OS but not predictive of benefit from AC[142,146]. ColoPrint, a gene expression classifier similar to Oncotype dx, has been shown to significantly improve prognostic accuracy in stage II patients independent of other clinical factors[147]. However, sufficient data do not exist to recommend these tools for routine clinical use at this time.
Consensus Molecular Subtypes (CMS), proposed by the CRC Subtyping Consortium based on unsupervised gene expression profile to refine the classification of CRC and facilitate prognostication and development of expression signature-based targeted therapies[148], is another area of development. Among the four subtypes, CMS4 or the mesenchymal subtype has the worst survival rate. Although CMS system has been demonstrated to have prognostic significance[149-151], this system has not been extensively validated for clinical use at this time.
A few other molecular markers deserve a mention, which include PIK3CA mutation and CDX2 expression. A retrospective analysis of 964 rectal or CC patients in Nurses' Health Study and the Health Professionals Follow-up Study revealed that PIK3CA mutation status could predict a survival advantage from adjuvant therapy with aspirin[152]. The loss of CDX2 expression was identified as a negative prognostic marker in a retrospective cohort of patients with stage II and stage III CC[153]. Furthermore, a lack of CDX2 expression identified a subgroup of patients with stage II CC who appeared to benefit from AC. However, these hypothesis-generating results need prospective validation before being deployed into routine clinical practice.
PERIOPERATIVE CHEMOTHERAPY
Accumulating preclinical and clinical data suggest that the surgical trauma can influence several pathophysiological processes potentially leading to tumor metastasis and recurrence[154], which provides a biologic basis for exploration of an alternative strategy in which a part of the systemic chemotherapy is delivered for “chemical debulking” prior to the surgery. The rest is delivered after the surgery, referred as “perioperative” chemotherapy. Potential benefits of administration of chemotherapy before surgery are several, which include earlier treatment of occult micro-metastatic disease, improved tolerability, and dose intensity, opportunity to assess response to preoperative chemotherapy to inform adjuvant therapy, reduction of tumor cell shedding during surgery and improved R0 resection rates. A retrospective NCDB analysis reported a 23% lower risk of death at 3 years in T4b non-metastatic CC patients treated with preoperative chemotherapy followed by surgery compared to patients who had upfront resection followed by AC (HR 0.77; 95%CI: 0.60-0.98; P = 0.04)[155]. Several single-arm studies, including the pilot phase of the randomized FOxTROT trial, have explored the feasibility of perioperative chemotherapy in operable, locally advanced CC and reported significant tumor downstaging with acceptable toxicity[156,157]. Recently two studies that explored the efficacy of perioperative chemotherapy have reported their results- the phase II PRODIGE 22 trial[158] and the phase III FOxTROT trial[159].
The preliminary result of the ongoing FOxTROT trial (NCT00647530) has been presented at the American Society of Clinical Oncology annual meeting (2019). In this trial, 1052 patients (median age of 65 years) with operable, non-obstructed early stage CC ( T3 to T4, N0 to N2 and M0 based on CT scan) who were fit for modified FOLFOX (mFOLFOX) and surgery, were randomized in a 2: 1 ratio to the novel neoadjuvant treatment arm consisting of 6 wk of mFOLFOX followed by surgery and 18 wk of mFOLFOX post-operatively (n = 698) or control arm (n = 354). Patients in the control arm underwent upfront surgery, followed by 24 wk of adjuvant mFOLFOX. The trial allowed physicians to replace mFOLFOX with CAPOX as the chemotherapy backbone and to shorten the duration of chemotherapy from 24 wk to 12 wk in older, low-risk patients. Attempted curative surgery was successful in 98% of patients in both treatment groups. In this trial, the perioperative therapy arm was associated with a trend towards an improved 2-year rate of failure, the primary endpoint of the study, defined as relapse or persistent disease at 2 years (13.6% in the perioperative arm vs 17.2% in the control arm). This difference, however, did not reach the target statistical significance (HR 0.75; 95%CI: 0.55–1.04; P = 0.08). The absence of statistically significant benefit in this trial was attributed to the lower than expected failure rate in control arm-18% vs expected 25% to 32% used for the power calculation. The perioperative treatment protocol was well-tolerated and safe, with no increase in perioperative morbidity, and a trend toward fewer serious postoperative complications ( 4.7% vs 7.4% rate of anastomotic leak or intra-abdominal abscess, 4.3% vs 7.1% rate of complications requiring further surgery, and 12% vs 14% rate of complications prolonging postoperative stay). Furthermore, perioperative arm had marked reduction in the rate of incomplete resections, 5% vs 11% (P = 0.001).
An exploratory subgroup analysis of the FOxTROT trial provided important information regarding the patients with dMMR tumors. In this analysis, exclusion of the patients with dMMR tumors (n = 173) resulted in a drop of the HR for a 2-year treatment failure rate, suggesting that the neoadjuvant therapy was less effective in patients with dMMR tumors. On pathological examination of the resected tumors from patients who received pre-operative chemotherapy, tumor regression induced by chemotherapy was absent in nearly 74% of the dMMR tumors as compared to 26.6% in the pMMR (proficient mismatch repair) tumors. This result suggests that upfront surgery probably would be the preferred option for the early stage CC patients with dMMR tumors. The role of pre-operative immunotherapy is unknown at this time for this patient group.
The phase II randomized study, PRODIGE 22[158], had the similar design in which the patients with resectable localized stage III or high-risk stage II CC determined by CT scans were randomized to receive either 6 months of adjuvant FOLFOX after colectomy (control) or 4 cycles of FOLFOX before surgery and 8 cycles after surgery (perioperative arm). The primary endpoint of the study was the histological tumor regression grade (TRG). In this trial, TRG was not significantly improved in the perioperative arm, but overall mortality and morbidity rates were similar in both arms. It is important to note that the CT scan criteria were associated with a 33% rate of over staging in the control group. Based on these results, it can be inferred that perioperative chemotherapy should not be adopted as a standard treatment option at this time. However, these trial results provide a rationale for using perioperative chemotherapy in selected patient groups, such as T4b patients who are at risk of incomplete resection.
CONCLUSION
Although the treatment of early stage CC has evolved at a slower pace in last decades, research involving novel and biomarker-guided therapies is likely to advance this field in the near future. The likely areas of focus are: (1) Personalization of therapy, based on clinicopathologic and molecular characteristics, in terms of type, duration, and intensity; and (2) Discovery of novel treatment with improved efficacy.
Although oxaliplatin-based chemotherapy is the current standard adjuvant therapy for resected stage III dMMR patients, the efficacy of chemotherapy in this tumor type is limited[160,161]. Based on the data confirming the efficacy of checkpoint inhibitors in patients with metastatic dMMR CC[162], investigators have moved on to evaluating immunotherapy agents in the adjuvant setting. The ATOMIC trial (NCT02912559) is currently ongoing whichcompares mFOLFOX for 6 mo plus 12 mo of atezolizumab vs 6 mo of mFOLFOX in patients with resected stage III dMMR CC. In the POLEM trial (NCT03827044), patients who have undergone surgical resection for stage III dMMR or POLE exonuclease domain–mutant CC will be assigned to chemotherapy (CAPOX for 12 wk or capecitabine for 24 wk) or chemotherapy followed by avelumab for 24 wk.
The PIK3CA mutated CC patients are another molecular subgroup of patients currently under study. A retrospective study, which primarily included stage I-III CRC patients, reported a potential benefit of aspirin on CC specific mortality in PIK3CA mutated patients. Several trials are underway to assess the impact of aspirin as an adjuvant treatment in stage III or high-risk stage II patients with PIK3CA mutation [PRODIGE 50-ASPIK trial (NCT02467582), Add-Aspirin (ISRCTN74358648)].
As discussed above, it is uncertain if the addition of oxaliplatin to 5-FU benefits elderly patients with stage III CC. The PRODIGE 34-ADAGE trial (NCT02355379) is currently underway to assess the benefit of adjuvant chemotherapy with or without oxaliplatin in patients over 70 years who have stage III CC.
Recently published encouraging data with HER2[163,164] and BRAF[165] directed therapy in metastatic CC may translate into new trials in the adjuvant setting. A trial is currently assessing dual HER2 inhibition (with pertuzumab plus trastuzumab) in unresectable CC, including non-metastatic locally advanced patients (NCT03365882). A recently reported pooled analysis of stage III CC patients treated with FOLFOX-based AC showed that the IDEA meta-analysis defined high-risk stage III patients with BRAFV600E mutant tumors had a much worse prognosis compared to the rest of stage III patients[133]. This patient group should possibly be the target of adjuvant trial with BRAFV600E directed therapy.
The high risk stage III patients have a 3-year DFS rate of around 65%, even with 6 months of adjuvant oxaliplatin-based chemotherapy[54]. This group represents a population in need of more effective treatments. A trial is exploring the intensification of adjuvant treatment for this group (IROCAS, NCT02967289) with the addition of irinotecan to the FOLFOX backbone. The evolving perioperative chemotherapy approach utilized in the FOxTROT trial described above, which led to an increase in R0 resection rate with no increase in postsurgical complications, may also potentially improve the outcome of high-risk stage III patients.
The ability of traditional clinicopathologic characteristics to define the risk of cancer recurrence and optimize the adjuvant therapy for patients with resected early stage CC is limited. In this regard, ctDNA is a promising tool that has shown a very high prognostic value in both stage II and III CC patients. One of the major obstacles to utilizing this platform is the need to have a marker mutation unique for a given patient in order to determine that ctDNA is actually pathologic. Each patient will need to have unique mutation profile, limiting the applicability of this tool. Furthermore, mutations may not be present in all clones of a malignancy. Thus, each marker must be patient-specific and highly conserved across all clones of a patient’s tumor. At this time, the use of ctDNA technology is limited by the absence of prospective data confirming its value as a predictive biomarker for adjuvant therapy. Nonetheless, the early results are promising, and several randomized clinical trials are underway to further evaluate the prognostic value of ctDNA (NCT02842203, NCT03312374, NCT03637686), and to explore the value of ctDNA-directed adjuvant therapy in resected stage II and III CC [DYNAMIC-II (ACTRN12615000381583), DYNAMIC-III (ACTRN12617001566325), NRG-GI005 (COBRA) for stage IIA CC, CIRCULATE-IDEA].
In conclusion, despite a lack of newer agents with improved efficacy, a number of advances have altered the treatment landscape of early stage CC. Existing treatment regimens have been modified and refined to decrease the impact on patients, improve tolerability and optimize patient outcomes. As we move to an era dominated by the utilization of advanced surgical technologies, targeted therapies, and immunotherapy, it is likely that outcome will continue to improve with a reduction in treatment-related complications. The use of biomarkers and genomic signatures to risk stratify individual patients presents an enormous opportunity to personalize treatment. We anticipate that the use of ctDNA-based tools will improve patient selection for adjuvant therapy and help the detection of early, curable recurrences.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript. All the authors have no conflict of interest related to the manuscript.
Manuscript source: Invited manuscript
Peer-review started: January 21, 2020
First decision: March 5, 2020
Article in press: August 1, 2020
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perse M, Fahrner R S-Editor: Zhang H L-Editor: A P-Editor: Li JH
Contributor Information
Sakti Chakrabarti, Division of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI 53226, United States. schakrabarti@mcw.edu.
Carrie Y Peterson, Department of Surgery, Medical College of Wisconsin, Milwaukee, WI 53226, United States.
Deepika Sriram, Division of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI 53226, United States.
Amit Mahipal, Division of Medical Oncology, Mayo Clinic, Rochester, MN 55905, United States.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, Ladabaum U. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975-2015. JAMA. 2019;321:1933–1934. doi: 10.1001/jama.2019.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 6.Osterman E, Glimelius B. Recurrence Risk After Up-to-Date Colon Cancer Staging, Surgery, and Pathology: Analysis of the Entire Swedish Population. Dis Colon Rectum. 2018;61:1016–1025. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 7.Kelloff GJ, Schilsky RL, Alberts DS, Day RW, Guyton KZ, Pearce HL, Peck JC, Phillips R, Sigman CC. Colorectal adenomas: a prototype for the use of surrogate end points in the development of cancer prevention drugs. Clin Cancer Res. 2004;10:3908–3918. doi: 10.1158/1078-0432.CCR-03-0789. [DOI] [PubMed] [Google Scholar]
- 8.Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120:2893–2901. doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]
- 9.Orlando FA, Tan D, Baltodano JD, Khoury T, Gibbs JF, Hassid VJ, Ahmed BH, Alrawi SJ. Aberrant crypt foci as precursors in colorectal cancer progression. J Surg Oncol. 2008;98:207–213. doi: 10.1002/jso.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 11.Benson AB, Venook AP, Al-Hawary MM. NCCN Guidelines version 1. 2020 Colon Cancer. Available from: https://wwwnccnorg/professionals/physician_gls/pdf/colonpdf 2020. [Google Scholar]
- 12.Costas-Chavarri A, Nandakumar G, Temin S, Lopes G, Cervantes A, Cruz Correa M, Engineer R, Hamashima C, Ho GF, Huitzil FD, Malekzadeh Moghani M, Sharara AI, Stern MC, Teh C, Vázquez Manjarrez SE, Verjee A, Yantiss R, Shah MA. Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J. Glob Oncol. 2019;5:1–19. doi: 10.1200/JGO.18.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 14.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson NW, Yothers G, Lopa S, Costantino JP, Petrelli NJ, Wolmark N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17:959–966. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 17.Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Bentrem DJ, Merkow RP, Nelson H, Wang E, Ko CY, Soper NJ. Laparoscopic-assisted vs. open colectomy for cancer: comparison of short-term outcomes from 121 hospitals. J Gastrointest Surg. 2008;12:2001–2009. doi: 10.1007/s11605-008-0568-x. [DOI] [PubMed] [Google Scholar]
- 19.Freeman HJ. Long-term follow-up of patients with malignant pedunculated colon polyps after colonoscopic polypectomy. Can J Gastroenterol. 2013;27:20–24. doi: 10.1155/2013/380389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benizri EI, Bereder JM, Rahili A, Bernard JL, Vanbiervliet G, Filippi J, Hébuterne X, Benchimol D. Additional colectomy after colonoscopic polypectomy for T1 colon cancer: a fine balance between oncologic benefit and operative risk. Int J Colorectal Dis. 2012;27:1473–1478. doi: 10.1007/s00384-012-1464-0. [DOI] [PubMed] [Google Scholar]
- 21.Song BR, Xiao CC, Wu ZK. Predictors of Lymph Node Metastasis and Prognosis in pT1 Colorectal Cancer Patients with Signet-Ring Cell and Mucinous Adenocarcinomas. Cell Physiol Biochem. 2017;41:1753–1765. doi: 10.1159/000471868. [DOI] [PubMed] [Google Scholar]
- 22.Shimura T, Ebi M, Yamada T, Hirata Y, Nishiwaki H, Mizushima T, Asukai K, Togawa S, Takahashi S, Joh T. Magnifying chromoendoscopy and endoscopic ultrasonography measure invasion depth of early stage colorectal cancer with equal accuracy on the basis of a prospective trial. Clin Gastroenterol Hepatol. 2014;12:662–8.e1-2. doi: 10.1016/j.cgh.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Bianco F, Arezzo A, Agresta F, Coco C, Faletti R, Krivocapic Z, Rotondano G, Santoro GA, Vettoretto N, De Franciscis S, Belli A, Romano GM Italian Society of Colorectal Surgery. Practice parameters for early colon cancer management: Italian Society of Colorectal Surgery (Società Italiana di Chirurgia Colo-Rettale; SICCR) guidelines. Tech Coloproctol. 2015;19:577–585. doi: 10.1007/s10151-015-1361-y. [DOI] [PubMed] [Google Scholar]
- 24.Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol. 2017;33:315–319. doi: 10.1097/MOG.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 25.Fukami N. Surgery Versus Endoscopic Mucosal Resection Versus Endoscopic Submucosal Dissection for Large Polyps: Making Sense of When to Use Which Approach. Gastrointest Endosc Clin N Am. 2019;29:675–685. doi: 10.1016/j.giec.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis Colon Rectum. 2017;60:999–1017. doi: 10.1097/DCR.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Qin WZ, Yao LQ, Zhong YS, Zhang YQ, Chen WF, Hu JW, Ooi M, Chen LL, Hou YY, Xu MD, Zhou PH. Long-term outcomes of endoscopic submucosal dissection for high-grade dysplasia and early-stage carcinoma in the colorectum. Cancer Commun (Lond) 2018;38:3. doi: 10.1186/s40880-018-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorgun E, Benlice C, Church JM. Does Cancer Risk in Colonic Polyps Unsuitable for Polypectomy Support the Need for Advanced Endoscopic Resections? J Am Coll Surg. 2016;223:478–484. doi: 10.1016/j.jamcollsurg.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Gorgun E, Benlice C, Abbas MA, Steele S. Experience in colon sparing surgery in North America: advanced endoscopic approaches for complex colorectal lesions. Surg Endosc. 2018;32:3114–3121. doi: 10.1007/s00464-018-6026-2. [DOI] [PubMed] [Google Scholar]
- 30.Jayaram A, Barr N, Plummer R, Yao M, Chen L, Yoo J. Combined endo-laparoscopic surgery (CELS) for benign colon polyps: a single institution cost analysis. Surg Endosc. 2019;33:3238–3242. doi: 10.1007/s00464-018-06610-z. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzon L, Biondi A, Carus T, Dziki A, Espin E, Figueiredo N, Ruiz MG, Mersich T, Montroni I, Tanis PJ MISiCOL Task Force, Benz SR, Bianchi PP, Biebl M, Broeders I, De Luca R, Delrio P, D'Hondt M, Fürst A, Grosek J, Guimaraes Videira JF, Herbst F, Jayne D, Lázár G, Miskovic D, Muratore A, Helmer Sjo O, Scheinin T, Tomazic A, Türler A, Van de Velde C, Wexner SD, Wullstein C, Zegarski W, D'Ugo D. Achieving high quality standards in laparoscopic colon resection for cancer: A Delphi consensus-based position paper. Eur J Surg Oncol. 2018;44:469–483. doi: 10.1016/j.ejso.2018.01.091. [DOI] [PubMed] [Google Scholar]
- 32.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 33.Wells KO, Senagore A. Minimally Invasive Colon Cancer Surgery. Surg Oncol Clin N Am. 2019;28:285–296. doi: 10.1016/j.soc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Hameed I, Aggarwal P, Weiser MR. Robotic Extended Right Hemicolectomy with Complete Mesocolic Excision and D3 Lymph Node Dissection. Ann Surg Oncol. 2019;26:3990–3991. doi: 10.1245/s10434-019-07692-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Søndenaa K, Quirke P, Hohenberger W, Sugihara K, Kobayashi H, Kessler H, Brown G, Tudyka V, D'Hoore A, Kennedy RH, West NP, Kim SH, Heald R, Storli KE, Nesbakken A, Moran B. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery : proceedings of a consensus conference. Int J Colorectal Dis. 2014;29:419–428. doi: 10.1007/s00384-013-1818-2. [DOI] [PubMed] [Google Scholar]
- 36.Prevost GA, Odermatt M, Furrer M, Villiger P. Postoperative morbidity of complete mesocolic excision and central vascular ligation in right colectomy: a retrospective comparative cohort study. World J Surg Oncol. 2018;16:214. doi: 10.1186/s12957-018-1514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo A, Iversen ER, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Born PW, Kristensen B, Kleif J. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol. 2019;20:1556–1565. doi: 10.1016/S1470-2045(19)30485-1. [DOI] [PubMed] [Google Scholar]
- 38.Sammour T, Malakorn S, Thampy R, Kaur H, Bednarski BK, Messick CA, Taggart M, Chang GJ, You YN. Selective central vascular ligation (D3 lymphadenectomy) in patients undergoing minimally invasive complete mesocolic excision for colon cancer: optimizing the risk-benefit equation. Colorectal Dis. 2020;22:53–61. doi: 10.1111/codi.14794. [DOI] [PubMed] [Google Scholar]
- 39.Alhassan N, Yang M, Wong-Chong N, Liberman AS, Charlebois P, Stein B, Fried GM, Lee L. Comparison between conventional colectomy and complete mesocolic excision for colon cancer: a systematic review and pooled analysis : A review of CME versus conventional colectomies. Surg Endosc. 2019;33:8–18. doi: 10.1007/s00464-018-6419-2. [DOI] [PubMed] [Google Scholar]
- 40.Weixler B, Rickenbacher A, Raptis DA, Viehl CT, Guller U, Rueff J, Zettl A, Zuber M. Sentinel Lymph Node Mapping with Isosulfan Blue or Indocyanine Green in Colon Cancer Shows Comparable Results and Identifies Patients with Decreased Survival: A Prospective Single-Center Trial. World J Surg. 2017;41:2378–2386. doi: 10.1007/s00268-017-4051-2. [DOI] [PubMed] [Google Scholar]
- 41.Estrada O, Pulido L, Admella C, Hidalgo LA, Clavé P, Suñol X. Sentinel lymph node biopsy as a prognostic factor in non-metastatic colon cancer: a prospective study. Clin Transl Oncol. 2017;19:432–439. doi: 10.1007/s12094-016-1543-8. [DOI] [PubMed] [Google Scholar]
- 42.Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 43.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 44.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM COlon cancer Laparoscopic or Open Resection Study Group (COLOR) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 45.Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49–57. doi: 10.7150/jca.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34:498–504. doi: 10.1016/j.ctrv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Peterson CY, Palazzi K, Parsons JK, Chang DC, Ramamoorthy SL. The prevalence of laparoscopy and patient safety outcomes: an analysis of colorectal resections. Surg Endosc. 2014;28:608–616. doi: 10.1007/s00464-013-3216-9. [DOI] [PubMed] [Google Scholar]
- 48.Bouvier AM, Launoy G, Bouvier V, Rollot F, Manfredi S, Faivre J, Cottet V, Jooste V. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer. 2015;137:2133–2138. doi: 10.1002/ijc.29578. [DOI] [PubMed] [Google Scholar]
- 49.Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O'Callaghan C, Seitz JF, Francini G, Haller D, Yothers G, Goldberg R, de Gramont A. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, Grothey A, O'Connell M, Catalano PJ, Kerr D, Green E, Wieand HS, Goldberg RM, de Gramont A ACCENT Group. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 51.de Gramont A, Hubbard J, Shi Q, O'Connell MJ, Buyse M, Benedetti J, Bot B, O'Callaghan C, Yothers G, Goldberg RM, Blanke CD, Benson A, Deng Q, Alberts SR, Andre T, Wolmark N, Grothey A, Sargent D. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol. 2010;28:460–465. doi: 10.1200/JCO.2009.23.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sargent D, Shi Q, Yothers G, Van Cutsem E, Cassidy J, Saltz L, Wolmark N, Bot B, Grothey A, Buyse M, de Gramont A Adjuvant Colon Cancer End-points (ACCENT) Group. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;47:990–996. doi: 10.1016/j.ejca.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 54.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malietzis G, Mughal A, Currie AC, Anyamene N, Kennedy RH, Athanasiou T, Jenkins JT. Factors Implicated for Delay of Adjuvant Chemotherapy in Colorectal Cancer: A Meta-analysis of Observational Studies. Ann Surg Oncol. 2015;22:3793–3802. doi: 10.1245/s10434-015-4479-2. [DOI] [PubMed] [Google Scholar]
- 56.Gao P, Huang XZ, Song YX, Sun JX, Chen XW, Sun Y, Jiang YM, Wang ZN. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer. 2018;18:234. doi: 10.1186/s12885-018-4138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 59.Peixoto RD, Kumar A, Speers C, Renouf D, Kennecke HF, Lim HJ, Cheung WY, Melosky B, Gill S. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin Colorectal Cancer. 2015;14:25–30. doi: 10.1016/j.clcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Mohan HM, Evans MD, Larkin JO, Beynon J, Winter DC. Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol. 2013;20:2929–2936. doi: 10.1245/s10434-013-2967-9. [DOI] [PubMed] [Google Scholar]
- 61.Chu QD, Zhou M, Medeiros KL, Peddi P, Kavanaugh M, Wu XC. Poor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapy. BMC Cancer. 2016;16:460. doi: 10.1186/s12885-016-2446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turner MC, Farrow NE, Rhodin KE, Sun Z, Adam MA, Mantyh CR, Migaly J. Delay in Adjuvant Chemotherapy and Survival Advantage in Stage III Colon Cancer. J Am Coll Surg. 2018;226:670–678. doi: 10.1016/j.jamcollsurg.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 63.Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 64.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 65.Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, Jones J, Glass A, Lerner H, Lawrence W. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C-01. J Natl Cancer Inst. 1988;80:30–36. doi: 10.1093/jnci/80.1.30. [DOI] [PubMed] [Google Scholar]
- 66.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 67.Beijers AJ, Mols F, Vreugdenhil G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer. 2014;22:1999–2007. doi: 10.1007/s00520-014-2242-z. [DOI] [PubMed] [Google Scholar]
- 68.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 69.Grothey A. Oxaliplatin-safety profile: neurotoxicity. Semin Oncol. 2003;30:5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 70.Haller DG, Cassidy J, Clarke SJ, Cunningham D, Van Cutsem E, Hoff PM, Rothenberg ML, Saltz LB, Schmoll HJ, Allegra C, Bertino JR, Douillard JY, Gustavsson BG, Milano G, O'Connell M, Rustum Y, Tabernero J, Gilberg F, Sirzén F, Twelves C. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26:2118–2123. doi: 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 71.Iveson T, Sobrero AF, Yoshino T, Sougklakos I, Ou F-S, Meyers JP, Shi Q, Saunders MP, Labianca R, Yamanaka T, Boukovinas I, Hollander NH, Torri V, Yamazaki K, Georgoulias V, Lonardi S, Harkin A, Rosati G, Paul J, Collaboration obotI. Prospective pooled analysis of four randomized trials investigating duration of adjuvant (adj) oxaliplatin-based therapy (3 vs 6 months {m}) for patients (pts) with high-risk stage II colorectal cancer (CC) J Clin Oncol. 2019;37:3501. [Google Scholar]
- 72.Shah MA, Renfro LA, Allegra CJ, André T, de Gramont A, Schmoll HJ, Haller DG, Alberts SR, Yothers G, Sargent DJ. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J Clin Oncol. 2016;34:843–853. doi: 10.1200/JCO.2015.63.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th ed. 2010. Available from: https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%207th%20Ed%20Cancer%20Staging%20Manual.pdf. [Google Scholar]
- 74.Shi Q, Andre T, Grothey A, Yothers G, Hamilton SR, Bot BM, Haller DG, Van Cutsem E, Twelves C, Benedetti JK, O'Connell MJ, Sargent DJ. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database. J Clin Oncol. 2013;31:3656–3663. doi: 10.1200/JCO.2013.49.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 76.André T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T, Tabernero J, Van Laethem JL, Banzi M, Maartense E, Shmueli E, Carlsson GU, Scheithauer W, Papamichael D, Möehler M, Landolfi S, Demetter P, Colote S, Tournigand C, Louvet C, Duval A, Fléjou JF, de Gramont A. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol. 2015;33:4176–4187. doi: 10.1200/JCO.2015.63.4238. [DOI] [PubMed] [Google Scholar]
- 77.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 78.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care's gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 79.Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AV. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer. 2016;122:3277–3287. doi: 10.1002/cncr.30181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, Labianca R, Chen W, Cha SS, Heldebrant MP, Goldberg RM. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 81.O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, Wieand HS. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 82.Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA, Lum SS, Senthil M. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann Surg Oncol. 2018;25:1980–1985. doi: 10.1245/s10434-018-6484-8. [DOI] [PubMed] [Google Scholar]
- 83.Moran RG, Keyomarsi K. Biochemical rationale for the synergism of 5-fluorouracil and folinic acid. NCI Monogr. 1987:159–163. [PubMed] [Google Scholar]
- 84.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 85.Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, Colbert N, Boaziz C, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Buyse M, de Gramont A. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003;21:2896–2903. doi: 10.1200/JCO.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 86.André T, Quinaux E, Louvet C, Colin P, Gamelin E, Bouche O, Achille E, Piedbois P, Tubiana-Mathieu N, Boutan-Laroze A, Flesch M, Lledo G, Raoul Y, Debrix I, Buyse M, de Gramont A. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: final results of GERCOR C96.1. J Clin Oncol. 2007;25:3732–3738. doi: 10.1200/JCO.2007.12.2234. [DOI] [PubMed] [Google Scholar]
- 87.Wolmark N, Rockette H, Mamounas E, Jones J, Wieand S, Wickerham DL, Bear HD, Atkins JN, Dimitrov NV, Glass AG, Fisher ER, Fisher B. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 88.Köhne CH, Bedenne L, Carrato A, Bouché O, Popov I, Gaspà L, Valladares M, Rougier P, Gog C, Reichardt P, Wils J, Pignatti F, Biertz F. A randomised phase III intergroup trial comparing high-dose infusional 5-fluorouracil with or without folinic acid with standard bolus 5-fluorouracil/folinic acid in the adjuvant treatment of stage III colon cancer: the Pan-European Trial in Adjuvant Colon Cancer 2 study. Eur J Cancer. 2013;49:1868–1875. doi: 10.1016/j.ejca.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 89.Poplin EA, Benedetti JK, Estes NC, Haller DG, Mayer RJ, Goldberg RM, Weiss GR, Rivkin SE, Macdonald JS. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23:1819–1825. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]
- 90.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN, Zapas JL, Goodwin JW, Fehrenbacher L, Ramanathan RK, Conley BA, Flynn PJ, Soori G, Colman LK, Levine EA, Lanier KS, Wolmark N. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 91.Chakrabarti S, Sara J, Lobo R, Eiring R, Finnes H, Mitchell J, Hartgers M, Okano A, Halfdanarson T, Grothey A. Bolus 5-fluorouracil (5-FU) In Combination With Oxaliplatin Is Safe and Well Tolerated in Patients Who Experienced Coronary Vasospasm With Infusional 5-FU or Capecitabine. Clin Colorectal Cancer. 2019;18:52–57. doi: 10.1016/j.clcc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Twelves C, Scheithauer W, McKendrick J, Seitz JF, Van Hazel G, Wong A, Díaz-Rubio E, Gilberg F, Cassidy J. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012;23:1190–1197. doi: 10.1093/annonc/mdr366. [DOI] [PubMed] [Google Scholar]
- 93.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 94.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 95.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 96.Zuckerman IH, Rapp T, Onukwugha E, Davidoff A, Choti MA, Gardner J, Seal B, Mullins CD. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with Stage III colon cancer. J Am Geriatr Soc. 2009;57:1403–1410. doi: 10.1111/j.1532-5415.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 97.Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L, de Gramont A. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 98.McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, O'Connell M, Twelves CJ, Saltz LB, Haller DG, Sargent DJ. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600–2606. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haller DG, O'Connell MJ, Cartwright TH, Twelves CJ, McKenna EF, Sun W, Saif MW, Lee S, Yothers G, Schmoll HJ. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol. 2015;26:715–724. doi: 10.1093/annonc/mdv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tougeron D, Mouillet G, Trouilloud I, Lecomte T, Coriat R, Aparicio T, Des Guetz G, Lécaille C, Artru P, Sickersen G, Cauchin E, Sefrioui D, Boussaha T, Ferru A, Matysiak-Budnik T, Silvain C, Karayan-Tapon L, Pagès JC, Vernerey D, Bonnetain F, Michel P, Taïeb J, Zaanan A. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv438. [DOI] [PubMed] [Google Scholar]
- 101.Sinicrope FA, Ou F-S, Shi Q, Nixon AB, Mody K, Levasseur A, Dueck AC, Dhanarajan AR, Lieu CH, Cohen DJ, Innocenti F, Behrens RJ, Peters W, Sargent DJ, Sommer N, O'Reilly EM, Meyerhardt J. Randomized trial of FOLFOX alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient DNA mismatch repair or microsatellite instability (ATOMIC, Alliance A021502) J Clin Oncol. 2017;35:TPS3630. [Google Scholar]
- 102.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, Fields AL, Mayer RJ. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 103.Van Cutsem E, Labianca R, Bodoky G, Barone C, Aranda E, Nordlinger B, Topham C, Tabernero J, André T, Sobrero AF, Mini E, Greil R, Di Costanzo F, Collette L, Cisar L, Zhang X, Khayat D, Bokemeyer C, Roth AD, Cunningham D. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 104.Ychou M, Raoul JL, Douillard JY, Gourgou-Bourgade S, Bugat R, Mineur L, Viret F, Becouarn Y, Bouché O, Gamelin E, Ducreux M, Conroy T, Seitz JF, Bedenne L, Kramar A. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol. 2009;20:674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 105.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Lopa SH, Wolmark N. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31:359–364. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault-Goebel F, Shacham-Shmueli E, Bajetta E, Makrutzki M, Shang A, André T, Hoff PM. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 107.Kerr RS, Love S, Segelov E, Johnstone E, Falcon B, Hewett P, Weaver A, Church D, Scudder C, Pearson S, Julier P, Pezzella F, Tomlinson I, Domingo E, Kerr DJ. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): an open-label, randomised phase 3 trial. Lancet Oncol. 2016;17:1543–1557. doi: 10.1016/S1470-2045(16)30172-3. [DOI] [PubMed] [Google Scholar]
- 108.Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, Kahlenberg MS, Shields AF, Quesenberry JT, Webb TA, Farr GH, Jr, Pockaj BA, Grothey A, Goldberg RM. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN, Blons H, Collette L, Van Cutsem E, Rougier P, Salazar R, Bedenne L, Emile JF, Laurent-Puig P, Lepage C PETACC-8 Study Investigators. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:862–873. doi: 10.1016/S1470-2045(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 110.Diehn M, Alizadeh AA, Adams H-P, Lee JJ, Klassen S, Palma JF. Early prediction of clinical outcomes in resected stage II and III colorectal cancer (CRC) through deep sequencing of circulating tumor DNA (ctDNA) J Clin Oncol. 2017;35:3591. [Google Scholar]
- 111.Parikh AR, Seventer EEV, Boland GM, Hartwig A, Jaimovich A, Raymond VM, Talasaz A, Corcoran RB. A plasma-only integrated genomic and epigenomic circulating tumor DNA (ctDNA) assay to inform recurrence risk in colorectal cancer (CRC) J Clin Oncol. 2019;37:3602–3602. [Google Scholar]
- 112.Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, Heilskov Rasmussen M, Vang S, Shchegrova S, Frydendahl Boll Johansen A, Srinivasan R, Assaf Z, Balcioglu M, Olson A, Dashner S, Hafez D, Navarro S, Goel S, Rabinowitz M, Billings P, Sigurjonsson S, Dyrskjøt L, Swenerton R, Aleshin A, Laurberg S, Husted Madsen A, Kannerup AS, Stribolt K, Palmelund Krag S, Iversen LH, Gotschalck Sunesen K, Lin CJ, Zimmermann BG, Lindbjerg Andersen C. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019;5:1124–1131. doi: 10.1001/jamaoncol.2019.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, Wong R, Kosmider S, Ananda S, McKendrick J, Lee B, Cho JH, Faragher I, Jones IT, Ptak J, Schaeffer MJ, Silliman N, Dobbyn L, Li L, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol. 2019;5:1710–1717. doi: 10.1001/jamaoncol.2019.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, Kosmider S, Skinner I, Wong R, Steel M, Tran B, Desai J, Jones I, Haydon A, Hayes T, Price TJ, Strausberg RL, Diaz LA, Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Li L, Cohen JD, Kinde I, Ptak J, Popoli M, Schaefer J, Silliman N, Dobbyn L, Tie J, Gibbs P, Tomasetti C, Kinzler KW, Papadopoulos N, Vogelstein B, Olsson L. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019;5:1118–1123. doi: 10.1001/jamaoncol.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taieb J, Taly V, Vernerey D, Bourreau C. Analysis of circulating tumor DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: prognostic and predictive value for adjuvant treatment duration. Annals of Oncology. 2019;30:V867. [Google Scholar]
- 117.Dasari A, Grothey A, Kopetz S. Circulating Tumor DNA-Defined Minimal Residual Disease in Solid Tumors: Opportunities to Accelerate the Development of Adjuvant Therapies. J Clin Oncol. 2018;36:JCO2018789032. doi: 10.1200/JCO.2018.78.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D'Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pagès F. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galon J, Hermitte F, Mlecnik B, Marliot F. Immunoscore clinical utility to identify good prognostic colon cancer stage II patients with high-risk clinico-pathological features for whom adjuvant treatment may be avoided. J Clin Oncol. 2019;37:487. [Google Scholar]
- 120.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 121.Yomoda T, Sudo T, Kawahara A, Shigaki T, Shimomura S, Tajiri K, Nagasu S, Fujita F, Kinugasa T, Akagi Y. The Immunoscore is a Superior Prognostic Tool in Stages II and III Colorectal Cancer and is Significantly Correlated with Programmed Death-Ligand 1 (PD-L1) Expression on Tumor-Infiltrating Mononuclear Cells. Ann Surg Oncol. 2019;26:415–424. doi: 10.1245/s10434-018-07110-z. [DOI] [PubMed] [Google Scholar]
- 122.Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of immunoscore in patients with colorectal cancer: A systematic review and meta-analysis. Cancer Med. 2019;8:182–189. doi: 10.1002/cam4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, Colacchio TA, Saltz LB, Warren RS. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer--a study of CALGB 9581 and 89803. J Clin Oncol. 2011;29:3153–3162. doi: 10.1200/JCO.2010.33.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koenig JL, Toesca DAS, Harris JP, Tsai CJ, Haraldsdottir S, Lin AY, Pollom EL, Chang DT. Microsatellite Instability and Adjuvant Chemotherapy in Stage II Colon Cancer. Am J Clin Oncol. 2019;42:573–580. doi: 10.1097/COC.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 126.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 127.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sargent DJ, Shi Q, Yothers G, Tejpar S. Prognostic impact of deficient mismatch repair (dMMR) in 7,803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol. 2014;32:3507. [Google Scholar]
- 129.Zaanan A, Fléjou JF, Emile JF, Des GG, Cuilliere-Dartigues P, Malka D, Lecaille C, Validire P, Louvet C, Rougier P, de Gramont A, Bonnetain F, Praz F, Taïeb J. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res. 2011;17:7470–7478. doi: 10.1158/1078-0432.CCR-11-1048. [DOI] [PubMed] [Google Scholar]
- 130.Zaanan A, Shi Q, Taieb J, Alberts SR, Meyers JP, Smyrk TC, Julie C, Zawadi A, Tabernero J, Mini E, Goldberg RM, Folprecht G, Van Laethem JL, Le Malicot K, Sargent DJ, Laurent-Puig P, Sinicrope FA. Role of Deficient DNA Mismatch Repair Status in Patients With Stage III Colon Cancer Treated With FOLFOX Adjuvant Chemotherapy: A Pooled Analysis From 2 Randomized Clinical Trials. JAMA Oncol. 2018;4:379–383. doi: 10.1001/jamaoncol.2017.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim JE, Hong YS, Kim HJ, Kim KP, Kim SY, Lim SB, Park IJ, Kim CW, Yoon YS, Yu CS, Kim JC, Kim JH, Kim TW. Microsatellite Instability was not Associated with Survival in Stage III Colon Cancer Treated with Adjuvant Chemotherapy of Oxaliplatin and Infusional 5-Fluorouracil and Leucovorin (FOLFOX) Ann Surg Oncol. 2017;24:1289–1294. doi: 10.1245/s10434-016-5682-5. [DOI] [PubMed] [Google Scholar]
- 132.Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, Nelson GD, Goldberg RM, Sargent DJ, Alberts SR. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sinicrope FA, Huebner LJ, Laurent-Puig P, Smyrk TC, Tabernero J, Mini E, Goldberg RM, Folprecht G, Zaanan A, Malicot KL, Shi Q, Alberts SR, Taieb J. Relative contribution of clinical and molecular features to outcome within low and high risk T and N groups in stage III colon cancer (CC) J Clin Oncol. 2019;37:3520. [Google Scholar]
- 134.Jover R, Zapater P, Castells A, Llor X, Andreu M, Cubiella J, Piñol V, Xicola RM, Bujanda L, Reñé JM, Clofent J, Bessa X, Morillas JD, Nicolás-Pérez D, Payá A, Alenda C Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55:848–855. doi: 10.1136/gut.2005.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, Zaniboni A, Seitz JF, Sinicrope F, Gallinger S. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, Boland CR. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehmé A, Christen RD, Howell SB. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–4886. [PubMed] [Google Scholar]
- 138.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst. 1998;90:675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 139.Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouché O, Tabernero J, Mini E, Goldberg RM, Folprecht G, Luc Van Laethem J, Sargent DJ, Alberts SR, Emile JF, Laurent Puig P, Sinicrope FA. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst. 2017;109:djw272. doi: 10.1093/jnci/djw272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taieb J, Zaanan A, Le Malicot K, Julié C, Blons H, Mineur L, Bennouna J, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Lepage C, Emile JF, Laurent-Puig P. Prognostic Effect of BRAF and KRAS Mutations in Patients With Stage III Colon Cancer Treated With Leucovorin, Fluorouracil, and Oxaliplatin With or Without Cetuximab: A Post Hoc Analysis of the PETACC-8 Trial. JAMA Oncol. 2016;2:643–653. doi: 10.1001/jamaoncol.2015.5225. [DOI] [PubMed] [Google Scholar]
- 141.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 142.Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN, Lee M, Watson D, Shak S, Kerr DJ. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29:4611–4619. doi: 10.1200/JCO.2010.32.8732. [DOI] [PubMed] [Google Scholar]
- 143.O'Connell MJ, Lavery I, Yothers G, Paik S, Clark-Langone KM, Lopatin M, Watson D, Baehner FL, Shak S, Baker J, Cowens JW, Wolmark N. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol. 2010;28:3937–3944. doi: 10.1200/JCO.2010.28.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Venook AP, Niedzwiecki D, Lopatin M, Ye X, Lee M, Friedman PN, Frankel W, Clark-Langone K, Millward C, Shak S, Goldberg RM, Mahmoud NN, Warren RS, Schilsky RL, Bertagnolli MM. Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol. 2013;31:1775–1781. doi: 10.1200/JCO.2012.45.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamanaka T, Oki E, Yamazaki K, Yamaguchi K, Muro K, Uetake H, Sato T, Nishina T, Ikeda M, Kato T, Kanazawa A, Kusumoto T, Chao C, Lopatin M, Krishnakumar J, Bailey H, Akagi K, Ochiai A, Ohtsu A, Ohashi Y, Yoshino T. 12-Gene Recurrence Score Assay Stratifies the Recurrence Risk in Stage II/III Colon Cancer With Surgery Alone: The SUNRISE Study. J Clin Oncol. 2016;34:2906–2913. doi: 10.1200/JCO.2016.67.0414. [DOI] [PubMed] [Google Scholar]
- 146.Yothers G, O'Connell MJ, Lee M, Lopatin M, Clark-Langone KM, Millward C, Paik S, Sharif S, Shak S, Wolmark N. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol. 2013;31:4512–4519. doi: 10.1200/JCO.2012.47.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kopetz S, Tabernero J, Rosenberg R, Jiang ZQ, Moreno V, Bachleitner-Hofmann T, Lanza G, Stork-Sloots L, Maru D, Simon I, Capellà G, Salazar R. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. Oncologist. 2015;20:127–133. doi: 10.1634/theoncologist.2014-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Sousa E Melo F, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, de Jong JH, de Boer OJ, van Leersum R, Bijlsma MF, Rodermond H, van der Heijden M, van Noesel CJ, Tuynman JB, Dekker E, Markowetz F, Medema JP, Vermeulen L. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. 2013;19:614–618. doi: 10.1038/nm.3174. [DOI] [PubMed] [Google Scholar]
- 150.Roepman P, Schlicker A, Tabernero J, Majewski I, Tian S, Moreno V, Snel MH, Chresta CM, Rosenberg R, Nitsche U, Macarulla T, Capella G, Salazar R, Orphanides G, Wessels LF, Bernards R, Simon IM. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int J Cancer. 2014;134:552–562. doi: 10.1002/ijc.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song N, Pogue-Geile KL, Gavin PG, Yothers G, Kim SR, Johnson NL, Lipchik C, Allegra CJ, Petrelli NJ, O'Connell MJ, Wolmark N, Paik S. Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016;2:1162–1169. doi: 10.1001/jamaoncol.2016.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, Sun R, Nosho K, Meyerhardt JA, Giovannucci E, Fuchs CS, Chan AT, Ogino S. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dalerba P, Sahoo D, Paik S, Guo X, Yothers G, Song N, Wilcox-Fogel N, Forgó E, Rajendran PS, Miranda SP, Hisamori S, Hutchison J, Kalisky T, Qian D, Wolmark N, Fisher GA, van de Rijn M, Clarke MF. CDX2 as a Prognostic Biomarker in Stage II and Stage III Colon Cancer. N Engl J Med. 2016;374:211–222. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tohme S, Simmons RL, Tsung A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017;77:1548–1552. doi: 10.1158/0008-5472.CAN-16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dehal A, Graff-Baker AN, Vuong B, Fischer T, Klempner SJ, Chang SC, Grunkemeier GL, Bilchik AJ, Goldfarb M. Neoadjuvant Chemotherapy Improves Survival in Patients with Clinical T4b Colon Cancer. J Gastrointest Surg. 2018;22:242–249. doi: 10.1007/s11605-017-3566-z. [DOI] [PubMed] [Google Scholar]
- 156.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jakobsen A, Andersen F, Fischer A, Jensen LH, Jørgensen JC, Larsen O, Lindebjerg J, Pløen J, Rafaelsen SR, Vilandt J. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol. 2015;54:1747–1753. doi: 10.3109/0284186X.2015.1037007. [DOI] [PubMed] [Google Scholar]
- 158.Karoui M, Rullier A, Piessen G, Legoux JL, Barbier E, De Chaisemartin C, Lecaille C, Bouche O, Ammarguellat H, Brunetti F, Prudhomme M, Regimbeau JM, Glehen O, Lievre A, Portier G, Hartwig J, Goujon G, Romain B, Lepage C, Taieb J for PRODIGE 22 investigators/collaborators. Perioperative FOLFOX 4 Versus FOLFOX 4 Plus Cetuximab Versus Immediate Surgery for High-Risk Stage II and III Colon Cancers: A Phase II Multicenter Randomized Controlled Trial (PRODIGE 22) Ann Surg. 2020;271:637–645. doi: 10.1097/SLA.0000000000003454. [DOI] [PubMed] [Google Scholar]
- 159.Seymour MT, Morton D, Investigators obotIFT. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin Oncol. 2019;37:3504. [Google Scholar]
- 160.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman MJ. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA., Jr Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 165.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]