Abstract
The visual phototransduction cascade begins with a cis–trans photoisomerization of a retinylidene chromophore associated with the visual pigments of rod and cone photoreceptors. Visual opsins release their all-trans-retinal chromophore following photoactivation, which necessitates the existence of pathways that produce 11-cis-retinal for continued formation of visual pigments and sustained vision. Proteins in the retinal pigment epithelium (RPE), a cell layer adjacent to the photoreceptor outer segments, form the well-established “dark” regeneration pathway known as the classical visual cycle. This pathway is sufficient to maintain continuous rod function and support cone photoreceptors as well although its throughput has to be augmented by additional mechanism(s) to maintain pigment levels in the face of high rates of photon capture. Recent studies indicate that the classical visual cycle works together with light-dependent processes in both the RPE and neural retina to ensure adequate 11-cis-retinal production under natural illuminances that can span ten orders of magnitude. Further elucidation of the interplay between these complementary systems is fundamental to understanding how cone-mediated vision is sustained in vivo. Here, we describe recent advances in understanding how 11-cis-retinal is synthesized via light-dependent mechanisms.
Keywords: retina, eye, vision, light, chromophore
The physiological response to a visual stimulus requires at the onset the absorption of light by a transducing element capable of converting electromagnetic radiation into a biochemical cascade within a cell (1). From bacteria to mammals, retinylidene proteins accomplish this task (2). They are comprised of a vitamin A-based chromophore conjugated with a Lys residue of the protein moiety, opsin (3). Absorption of light causes isomerization of a double bond within the chromophore, evoking conformational changes in opsin and subsequently the activation of cellular signaling pathways (4, 5). Visual opsins can respond to different wavelengths of light, as well as activate homologous but distinct cellular pathways (6). This versatility is enabled by subtle or, in some cases, profound amino acid sequence diversity among opsin proteins, as well as the isomeric state of their retinal chromophore. Among craniates, there are five families of retinylidene proteins: classical visual pigments expressed in rod and cone photoreceptor cells, ciliary-like opsin-visual pigments (e.g., pinopsin and parapinopsin), melanopsin, neuropsins (e.g., retinal G protein-coupled receptor [RGR] and peropsin), and Opn3/TMT opsins (7). Visual pigments in photoreceptors are comprised of opsin linked via a Schiff base with 11-cis-retinal. Upon absorption of a photon, the chromophore undergoes an ultrafast isomerization on the scale of tens of femtoseconds to an all-trans configuration (8). Slower conformational changes in opsin on the millisecond scale then transiently transform the quiescent receptor into a G protein-coupled signaling molecule triggering the earliest events of vision (1, 4).
Vertebrate photoreceptor cells consist of two types: rods that mediate vision in dim-light settings and cones that sense photons in ambient light and enable color distinction. Rod cells are exquisitely sensitive to light but have a low saturation threshold whereas cones have a relatively low sensitivity but an extremely high saturation threshold. Each rod and cone cell consists of four components: a synaptic terminal, an inner segment (IS), an outer segment (OS), and a connecting cilium attaching the OS to the IS (9). The OS contains the photopigments, opsins, which are G protein-coupled receptors that require a bound chromophore to absorb photons (4). The retinal pigment epithelium (RPE), located in the back of the eye juxtaposed to the photoreceptors, is a postmitotic cell layer that plays a pivotal role in the maintenance and health of the neural retina. In addition to providing nutrients from the bloodstream, it performs the housekeeping function of phagocytosing the oldest portions of the adjacent photoreceptor OS. The RPE is also the major site of vitamin A storage within the vertebrate retina while photoreceptors are beneficiaries of the active chromophore 11-cis-retinal. In photoreceptors, following photobleaching of the visual pigments (rhodopsin and cone opsins), vitamin A generated by the activity of photoreceptor retinol dehydrogenases (RDHs) is rapidly transferred from the retina to the RPE where reisomerization takes place. In the classical visual (retinoid) cycle, the photosensitive active retinoid, 11-cis-retinal, is then produced in the RPE and subsequently delivered to the photoreceptors (Fig. 1) (10–13). Another important cell type involved in retinal function is the retina-specific glial cell type called Müller glia. Each Müller cell spans almost the entire thickness of the retina, including the outer nuclear layer containing rod and cone photoreceptor cell bodies (14).
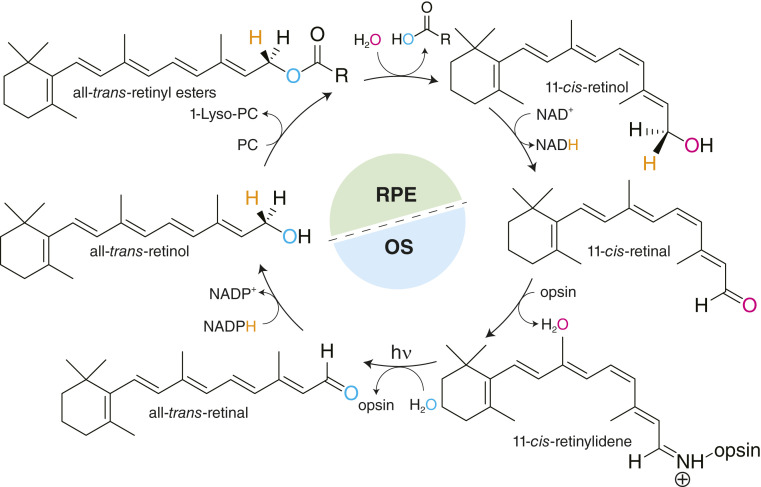
Fig. 1.
The isomerization of retinoids in the classical visual cycle. Vision begins when the 11-cis-retinylidene chromophore of visual opsins undergoes cis-trans photoisomerization, which activates phototransduction. Following this activation process, all-trans-retinal is released from the opsin by hydrolysis and reduced in an NADPH-dependent manner by all-trans-RDHs in the photoreceptor OS. Hydride addition occurs at the re chiral face of the aldehyde to give a proR chiral center at C15. Following movement to the RPE, retinol is esterified by lecithin:retinol acyltransferase in an sn1-regioselective fashion using phosphatidylcholine (PC) as an acyl donor. Retinyl esters serve as substrates for RPE65, which cleaves and isomerizes them in an FeII-dependent manner to form 11-cis-retinol. The C15 prostereo center changes chirality during the isomerization reaction. Finally, the hydride which was added by all-trans-RDHs is removed in an NAD+-dependent manner by 11-cis-RDHs to generate 11-cis-retinal. The 11-cis-retinal is trafficked to the photoreceptor OS to bind with visual opsins to form a ground state visual pigment, thus resetting the system for detection of a subsequent photon. hν, initiated by light.
To sustain vision, however, the photoisomerization (bleaching) of the visual pigments upon exposure to light must be reversible, necessitating de novo 11-cis-retinal production. The 11-cis-retinal is ∼4 kcal/mol higher in free energy compared to all-trans-retinal and thus has a vanishingly low abundance in an equilibrium mixture (15). For this reason, 11-cis-retinal production requires input of energy, either from enzyme-catalyzed processes or through the absorption of light quanta. Moreover, it is known that steady-state cone visual pigment levels are maintained even at absorption rates of >106 photons per second (16). Thus, the retina clearly requires robust and high-efficiency sources of 11-cis-retinal production to remain functional under highly varied illuminances. Studies of the enzymes and binding proteins of the classical visual (retinoid) cycle, driven by a retinoid isomerase (RPE65) and involving photoreceptor cells and the RPE, have been well-described (Fig. 1) (3, 10–13). However, this cycle must be augmented by other mechanisms of chromophore production, especially in bright light, as demonstrated by a variety of animal studies and observations of human vision. In humans, the regeneration of cones in the dark occurs with a time constant (τ) for visual sensitivity recovery of 100 s while that for rods is 400 s, suggesting either that cones have access to alternative sources of visual chromophore or that 11-cis-retinal trafficking to cone OSs is more efficient than to rod OSs. Compelling evidence in support of additional regeneration pathways comes from studies of the null mutation of the RPE65 gene in humans, which blocks the classical visual cycle and affects not only rods but also cone photoreceptors (17). Despite this absolute enzymatic blockade of the visual cycle, some useful vision remains in afflicted individuals for the first two decades of life before photoreceptors fully degenerate, indicating the involvement of an alternative regeneration pathway. Similarly, Rpe65−/− mice remain responsive to light, albeit with greatly reduced sensitivity (18–20).
A few mechanisms have been proposed that could explain residual light sensitivity in Rpe65 knockout mice. It has been shown that 9-cis-retinal formed by either an enzymatic process or by thermal isomerization can generate light-sensitive iso-opsin pigments (21). However, this process requires prolonged dark-rearing of the mice, and thus the extent to which this mechanism is relevant to human vision remains unclear. Other findings indicate that the alternative regeneration pathways supporting vision in these patients and those with normal vision are comprised of photic pathways involving RGR or N-retinylidene-phosphatidylethanolamine (N-ret-PE), which can produce 11-cis-retinal upon exposure to light of a suitable wavelength and even in darkness (22, 23). However, the photic regeneration of visual pigments faces complexities absent in the classical, light-independent visual cycle. Such complexities deserve careful inspection, especially since the regeneration process is not only vital to our normal vision but also has implications for developing new molecular therapies for degenerative diseases of the retina.
Different Opsins and Their Chromophores
Rhodopsin, like all known vertebrate photoreceptor visual pigments, senses light through its Schiff base-linked 11-cis-retinylidene chromophore. The chromophore-binding pocket of rhodopsin has been resolved in atomic detail by determination of its crystal structure (24). The absorption of light by the chromophore allows the fast cis–trans isomerization to proceed. Next, a signaling form of photoactivated rhodopsin called Meta II (λmax = 382 nm) is generated. Upon cis–trans isomerization, retinal forms a fully planar conformation (Fig. 2). Thus, opsin has the capacity to accommodate two different isomers in its active site as the protein relaxes to adopt the new conformation of the chromophore. This is accomplished by specific structural changes within opsin (Fig. 2). For a detailed description of the activation events, recent reviews are available (4, 5). The opsin–all-trans-retinal complex is susceptible to hydrolytic decomposition, releasing free all-trans-retinal for its transformation back to the 11-cis-configuration. It should be noted that solutions of free retinal isomers in n-heptane at equilibrium consist of 13-cis (0.62%), 9-cis (0.23%), 9,13-di-cis (0.04%) and 11-cis (0.001%), and the remainder of the all-trans isomer. Interestingly, in hexane, light illumination leads to almost exclusive formation of the 13-cis isomer whereas solvents of greater polarity allow formation of sizable quantities of the other isomers, including 11-cis-retinal (reviewed in ref. 3). However, such nonenzymatic reactions either do not occur in normal mice, or the resultant isomers are rapidly converted to all-trans- and 11-cis-retinoids.
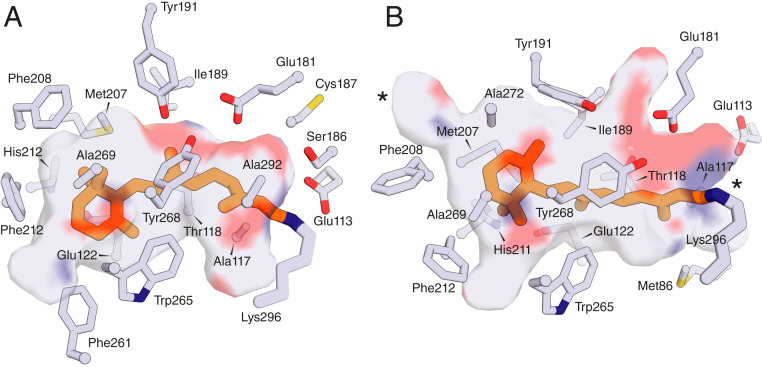
Fig. 2.
Distinct binding pocket geometries for all-trans and 11-cis-retinal as observed in the bovine opsin crystal structure. (A) The crystal structure of ground-state bovine rhodopsin (PDB accession code: 1U19) reveals the 11-cis-retinal chromophore (orange sticks) housed within a bent binding pocket that protects the Schiff base-linked ligand from hydrolysis and spontaneous thermal isomerization. (B) The structure of meta-II rhodopsin (PDB accession code: 3PXD) reveals a more extended binding pocket that accommodates the transform of retinal (orange stick). The pocket rearrangement results in openings to the protein exterior (locations marked by asterisks) that likely enable hydrolysis and release of retinal so that it can be reisomerized to the 11-cis configuration.
Light-activated rhabdomeric and certain ciliary opsins can be restored to their ground state by absorption of a second, lower energy photon—a process referred to as photoreversal (5). For example, in Drosophila, all-trans-3-hydroxy-retinal remains bound to the opsin, and the photopigment remains largely activated. Conversion back to the inactive 11-cis-3-hydroxy-retinal is accomplished exclusively through photoreversal (25). Vertebrate visual pigments, on the other hand, do not undergo photoreversal, instead relying on biochemical regeneration of the visual chromophore. The classical visual cycle has been extensively reviewed (Fig. 3). Briefly, all-trans-retinol from the serum is delivered to the RPE in complex with retinol-binding protein 4 (holo-RBP4), which binds to the retinol transporter Stimulated by Retinoic Acid 6 (STRA6), resulting in delivery of retinol to the interior of the cell (26). This STRA6 receptor is a dimeric protein having one intramembrane and nine transmembrane helices per monomer (27). Once inside these cells, retinol is transported between membranes in association with cellular retinol-binding protein 1 (CRBP1). This protein facilitates the diffusion of all-trans-retinol to the endoplasmic reticulum (ER) where it is further processed. In the first step of this metabolic pathway, lecithin:retinol acyl transferase (LRAT) forms retinyl esters that are sequestered by aggregation into lipid droplet structures termed retinosomes (28, 29), which changes the mass action ratio to favor retinoid uptake from both photoreceptors and the choroidal circulation. LRAT activity depends on a catalytic Cys residue, which serves as a nucleophile attacking the carbonyl carbon of an ester bond in the sn1 position of phosphatidylcholine (lecithin), resulting in transient acylation of the enzyme and formation of a thioester bond (Fig. 3 A and B). Next, this acyl group is transferred onto retinol to generate the retinyl ester product.
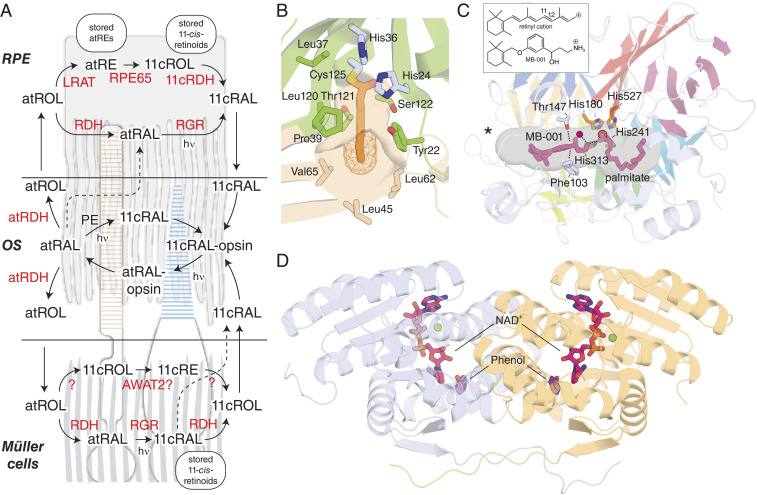
Fig. 3.
Pathways and enzymes for production of 11-cis-retinal in the vertebrate retina. (A) Pathways for 11-cis-retinal production and localization of key classical and nonclassical visual cycle enzymes. The background depicts a rod (red-colored OS) and cone (blue-colored OS) interacting with an RPE cell (Top) and Müller cell apical processes (Bottom). Enzymes are shown in red text, and atRE and 11cROL represent all-trans-retinyl esters and 11-cis-retinol, respectively. The 11-cis-retinal (11cRAL) can be generated in the RPE, Müller glia, and potentially within photoreceptor OSs through light-dependent and light-independent mechanisms. The classical visual cycle occurs mainly in the RPE through an enzymatic pathway involving LRAT, RPE65, and 11-cis-retinol dehydrogenases (11cRDH), most notably RDH5. The 11-cis-retinal is also formed in the RPE through a light-dependent pathway involving the RGR. RGR is also expressed in the Müller glia where it carries out a similar function, but with the generated chromophore being selectivity channeled to cones. Within the photoreceptor OS, all-trans-retinol dehydrogenases (atRDHs) such as RDH8 reduce all-trans-retinal (atRAL) formed during photobleaching to all-trans-retinol (atROL), which is shuttled to adjacent cell layers. Some amount of atRAL may escape this activity and either react with phosphatidylethanolamine (PE) to form an N-ret-PE adduct that can undergo photoisomerization forming 11cRAL, or diffuse directly to the RPE where it can conjugate with RGR for photic 11cRAL production. (B) Structure of an LRAT/phospholipase A2 chimera showing the catalytic Cys-125 residue trapped as an acylated intermediate (PDB accession code: 4Q95). The LRAT-specific loop, which confers retinol acyltransferase activity, is shown in light brown. Note the narrow cavity leading to the catalytic Cys residue and its overall hydrophobicity. (C) Details of the retinoid isomerase catalytic mechanism were revealed by the crystal structure of RPE65 in complex with MB-001 (PDB accession code: 4RSE), a retinyl cation transition state mimetic (Inset), and palmitate (both shown as magenta sticks). The retinoid moiety sits in the active site pocket proximal to the cavity entrance (marked by an asterisk), while the acyl moiety resides in the distal pocket with the carboxylate group situated over the iron. A candidate hydrolytic water (red sphere) is located across from an oxygen of the FeII-bound carboxylate in a position that could account for the chirality inversion that occurs during the reaction. The structure also revealed a close positioning of Phe103 and Thr147 to the carbon atom of MB-001 corresponding to the retinoid C11, explaining the importance of these residues in RPE65 regioselectivity. (D) Structure of an RDH from Drosophila melanogaster in complex with NAD+ and the substrate mimetic phenol (PDB accession code: 5ILG) (90).
Isomerase of the Canonical Visual Cycle
In addition to LRAT, RPE65 (retinoid isomerase) is a key enzyme of the visual cycle (Fig. 3 A and C). It belongs to a family of enzymes, termed carotenoid cleavage oxygenases, that typically catalyze the oxidation of carotenoid substrates to yield apocarotenoid products (30). RPE65 catalyzes the synthesis of 11-cis-retinol from all-trans-retinyl esters (31–33), a process that necessitates temporary lowering of the C11–C12 double bond order. The retinoid-binding site of RPE65 is located within the membrane-proximal region of the cavity (34–36). The retinyl ester (alcohol level of oxidation) cleavage reaction catalyzed by RPE65 involves breaking the O-alkyl bond, generating a retinyl cation with reduced carbon–carbon bond order allowing bond rotation to take place. Because the RPE65 active site cavity is lined primarily by nonpolar side chains, the protein environment is suitable to stabilize the carbocation. During RPE65-catalyzed reaction, a stereochemical inversion of C15 occurs, and the 15-hydroxy oxygen is lost, being replaced by oxygen from bulk solvent. Since 11-cis-retinol is thermodynamically less stable than all-trans-retinol by ∼4 kcal/mol, it has been argued that ester cleavage, which has a ΔG value of about −5 kcal/mol, could be used to drive the isomerization reaction (15). Following rotation of the C11–C12 bond under steric influence by the RPE65 active site, C15 is attacked by water or hydroxide, quenching the retinyl cation with the C11–C12 double bond in a cis configuration (36).
Complexity of RDHs
The rate of this isomerization is accelerated by an intracellular-binding protein, CRALBP, that binds 11- and 9-cis-retinoids but not all-trans- or 13-cis-retinoids (13, 37, 38). In addition to its expression in the RPE, CRALBP is abundantly expressed in Müller cells of the neuronal retina (39). RPE65 activity is enhanced by CRALBP perhaps by limiting the back inhibition by 11-cis-retinol, the product of the reaction. The 11-cis-retinol must be oxidized to the aldehyde form to enable regeneration of the visual pigments. This is accomplished by generic dehydrogenases, such as alcohol dehydrogenase and specialized RDHs that are involved in the redox reaction of retinoids. RDH members of the short-chain dehydrogenase/reductase (SDR) superfamily are the major in vivo contributors to these processes (Fig. 3D). Multiple knockouts of different RDHs in rods, cones, and the RPE point to highly redundant enzymatic systems (40–45). Passive nonionic diffusion is likely sufficient for this process because the chromophore forms a stable covalent complex with opsin, thereby driving the transfer of retinoids to photoreceptors.
After photobleaching, all-trans-retinal released from activated rod and cone visual pigments can diffuse into the outer or inner leaflets of photoreceptor disk membranes. In the former case, all-trans-retinal is directly available to the all-trans-RDH enzymes that quickly convert it to vitamin A. The NADP+/NADPH ratio is about 0.005:1, such that enzymes (RDH8 and RDH12) utilizing this dinucleotide reduce retinal to retinol. To prevent the accumulation of all-trans-retinal in the intradiscal space, photoreceptors express an ∼250-kDa adenosine triphosphate (ATP)-driven transporter called ATP-binding cassette transporter 4 (ABCA4) that is responsible for translocating all-trans-retinal-PE Schiff base conjugates from the intradiscal to the cytosolic side of the disk membrane. There, they dissociate, and the all-trans-retinal is reduced by all-trans-RDHs to enable retinoid cycle entry (46). It was also proposed that ABCA4 functions to recycle 11-cis-retinal released during proteolysis of rhodopsin in RPE endolysosomes following the daily phagocytosis of distal photoreceptor OSs (47).
11-cis-Retinal Production during Dark Adaptation
A major unresolved issue in vision research concerns the precise identity of the different pathways capable of regenerating 11-cis-retinal. In all conditions, it is necessary for both forward and reverse reaction rates to remain balanced; otherwise, the visual pigments would be rapidly depleted. This is particularly important as the individual rate constants differ considerably. The forward reaction of photoisomerization of the chromophore is ultrafast (35 fs) and is followed by release of all-trans-retinal from opsin that occurs within a time frame of seconds (48, 49). The reverse regeneration reaction must match to chromophore depletion. At steady state, the bleaching and regeneration rates of rod and cone pigments are equal at various levels of physiologic illumination. At high intensities, however, visual pigment would be quickly depleted if the classical visual cycle activity were the sole source of 11-cis-retinal. The slow rate of the classical visual cycle (50) suggests that the active visual pigment would be depleted within seconds in normal daylight so no further visual activation could occur. Resolving the timescale discrepancy for rod and cone cells as a function of illumination is fundamental to understanding how our vision is sustained throughout the day. This conundrum has prompted challenges to current dogma and instigated investigations into the possibility of direct all-trans-retinal photoisomerization, as it is released from opsin (aldehyde level isomerization), to produce 11-cis-retinal ready to be recombined with opsins. A combination of modern chemical, biochemical, genetic, and electrophysiological approaches has been applied to advance our understanding of this fundamental process of chromophore regeneration.
Nonphotic RPE65-Independent Isomerization Pathways in Müller Glia
The classical visual cycle is a major source for visual pigment regeneration in rods and contributes to 11-cis-retinal production for cones as well. However, cone photoreceptor dark adaptation, a surrogate for visual pigment regeneration, is much more rapid compared to that of rods, suggesting that cones have access to dedicated pool(s) of visual chromophore. Biochemical and electrophysiological experiments have both supported a cone-specific intraretinal visual cycle involving Müller glia (51–55) although genetic evidence for this pathway has, until recently, been lacking. It should be noted, however, that a cis-retinol oxidation is coupled to the retina-based visual cycle selectively for cone photoreceptor cells. The resulting cis-retinol is oxidized in the cone ISs and then as cis-retinal delivered to OSs to reform photoactive visual pigments, but a similar process does not take place in rod ISs (56, 57).
Candidate enzymes of this alternative visual cycle pathway were identified by screening Müller cell complementary DNA libraries and candidate gene approaches. Using the former approach, a putative isomerase of this pathway was identified as sphingolipid delta4 desaturase 1 (DES1) (58), an enzyme originally known for catalyzing the final step of de novo ceramide biosynthesis. Des1 was suggested to catalyze the isomerization of retinol to form small quantities of 9-cis- and 11-cis-retinol that are trapped by CRALBP or by stereo-selective esterification. Although viral-mediated expression of Des1 in the Müller cells of mice lacking the classical visual cycle isomerase RPE65 boosted cone electroretinography (ERG) responses, the role of DES1 in physiological cone pigment regeneration was left unresolved. This issue was addressed in greater detail through the generation and characterization of Müller cell conditional Des1 knockout mice (59). Des1 knockout specifically in these cells did not interfere with cone visual pigment regeneration in the mouse (59). Genetic knockout of Des1 (degs1) in zebrafish also did not eliminate photopic vision (60). Collectively, those experiments demonstrated a lack of robust retinoid isomerization enzymatic activity, no genetic evidence using the Müller cell-specific knockout, and lack of 9-cis-retinal in a healthy system; thus, the contribution by DES1, if any, appears negligible. In some species, retinyl esters are present in the neuronal retina, and it was suggested that Müller cells could express 11-cis-specific retinyl-ester synthase activity in the form of a multifunctional O-acyltransferase (MFAT) (61).
The existence of an RPE65-independent, cone-specific regeneration pathway remains controversial. To further explore the possibility of an alternative cone-specific regeneration pathway, potent and selective RPE65 inhibitors, such as retinylamine and emixustat-family compounds, were employed (62, 63). These compounds selectively inhibit RPE65 for in vivo and ex vivo ERG measurements in rod-specific G protein knockout (Gnat1−/−) mice that lack rod responses (64). Acute administration of these inhibitors after a bleach suppressed the slow phase of cone dark adaptation without affecting the initial rapid portion, which reflects intraretinal visual cycle function (65). RPE65 inhibitors did not affect the light sensitivity of cone photoreceptors in mice during extended exposure to background light but did slow the subsequent dark recovery. Moreover, cone function was only partially suppressed in cone-dominant ground squirrels and wild-type mice in response to RPE65 pharmacologic inhibition. The detailed mechanism of action for emixustat and similar compounds was reported elsewhere (36, 62, 63, 66). Inhibition of DES1 with fenretinide produced only modest effects on cone recovery. Collectively, these studies demonstrated that RPE65 contributes to mammalian cone pigment regeneration but clearly supported the existence of RPE65-independent regeneration mechanisms that do not depend on DES1.
Photic 11-cis-Retinal Synthesis: N-Ret-PE
In 1952, Ruth Hubbard and George Wald concluded in their seminal studies of vision that the retina obtains new supplies of the active chromophore from one or more sources: 1) eye tissues that contain enzymes which catalyze the isomerization of all-trans-retinal/all-trans-retinol in situ (67); 2) the exchange of all-trans-retinol with “neovitamin Ab” (or 11-cis-retinol in modern parlance) from the blood circulation; or 3) the isomerization of all-trans-retinal in the eye by blue or violet light. Enzymes in the classical visual cycle which are responsible for the consistent replenishment of the visual chromophore reside in the RPE, satisfying the first possibility raised by Hubbard and Wald. No evidence has been found supporting the second notion of an exchange process as 11-cis-retinoids are found in significant amounts only in the eye. Finally, recurrent studies have suggested, but not until recently proven, that photic isomerization can provide an alternative pathway for the generation of 11-cis-retinal in the RPE and Müller cells.
The idea of blue light-mediated isomerization has been revived periodically, but adequate nonenzymatic photoisomerization has yet to be demonstrated convincingly. Following earlier ideas (22, 23), it was proposed that blue light converts all-trans-retinal specifically to 11-cis-retinal through a nonenzymatic process utilizing a phospholipid intermediate in photoreceptor membranes (68). In these extensive studies, it was shown that blue (450 nm) light converts all-trans-ret-PE with high specificity to 11-cis-retinal through a retinyl-phospholipid (N-ret-PE) intermediate in OS photoreceptor membranes. Remarkably, the quantum efficiency of this light-dependent conversion at this wavelength was reported to be similar to the quantum yield of rhodopsin photoisomerization. But there are differences: e.g., the quantum yield of the rhodopsin to bathorhodopsin transformation is 0.67 (69) and is largely wavelength-independent (70). In studies by Kaylor et al. (68), the photoisomerization of N-ret-PE was more effective using white light than light at the maximum absorption of this adduct at 450 nm. The authors then showed that, in mice, exposure to 450-nm light for 10 min following a photobleach accelerated rhodopsin regeneration as compared to mice kept in darkness.
These findings require further investigation, however, to clarify their physiological relevance. For example, the extremely high quantum yield and specificity of N-ret-PE photoisomerization need further chemical analysis. The importance of this pathway to foveal cone pigment regeneration in primates is also unclear given that xanthophyll macular pigments strongly absorb 450-nm light, thus attenuating such a nonenzymatic photochemical reaction (71). Finally, the N-ret-PE mechanism generates significant amounts of 9-cis-retinal, which is not observed during the normal visual cycle. Finally, it is unclear why this mechanism does not appear to operate in older Leber Congenital Amaurosis (LCA) patients suffering from absent or inactivated RPE65 when residual photoreceptors are still present at even significant levels.
Photic 11-cis-Retinal Synthesis: RGR
Decades after Hubbard and Wald (67) first published their findings, Fong and colleagues (72) identified a novel opsin called retinal G protein-coupled receptor (RGR) as a potential source of long wavelength-induced isomerase activity similar to squid retinochrome (73) (reviewed in ref. 74). RGR resembles invertebrate opsins (Fig. 4A), binds all-trans-retinal, and, in visible light, catalyzes the isomerization to active 11-cis-retinal. However, prior biochemical studies on mammalian RGR demonstrated only limited photoisomerase activity (72). The total amount of rhodopsin was lower in Rgr−/− mice than in Rgr+/+ mice under various levels of illuminance (75), suggesting light-dependent production of the chromophore even in the rod cells (Fig. 4B). Using these mice, we demonstrated that 11-cis-retinal production stimulated by white light in mice is attenuated in the absence of RGR (20), suggesting that RGR is a photoisomerase and that chromophore regeneration is receptor-mediated. Others elegantly demonstrated that cones in Rgr−/− retinas lost sensitivity at a faster rate than cones in control retinas (Fig. 4C) (76). Following photoisomerase activity in the RPE, RGR that copurified with RDH5 was identified as a source of chromophore production (77). RGR has robust activity in membranes obtained from RPE cells when light is highly controlled (energy and wavelength). Using expressed and purified RGR, it was demonstrated that RGR accounts for the photoisomerase activity found in the bovine RPE. The presence of CRALBP, which is coexpressed in the RPE with RGR and critical for normal operation of the visual cycle (49), strongly enhances the generation of 11-cis-retinal by RGR (77). CRALBP in Müller cells also was identified as being critical for maintaining normal cone-driven vision and accelerating cone dark adaptation (78). Notably, distant CRALBP homologs are found in mollusks and other invertebrates (79, 80) and play a similar role in supporting RGR photoisomerase activity by acting as retinal shuttles (81). The substitution of a Lys with an Ala residue at position 255 in RGR abolishes the photoisomerization, confirming that the formation of a Schiff base at this Lys residue is critical for substrate binding and isomerization. Like opsin (Fig. 2), RGR can accommodate two retinal isomers; but, in contrast to opsin, all-trans-retinylidene is stable whereas 11-cis-retinylidene is hydrolyzed and released from the active site. The structure of these complexes will provide a clearer mechanistic picture of this “back” isomerization process. In brief, these findings provide compelling biochemical evidence that RGR directly contributes to the regeneration of visual chromophore under sustained light conditions (Fig. 4D).
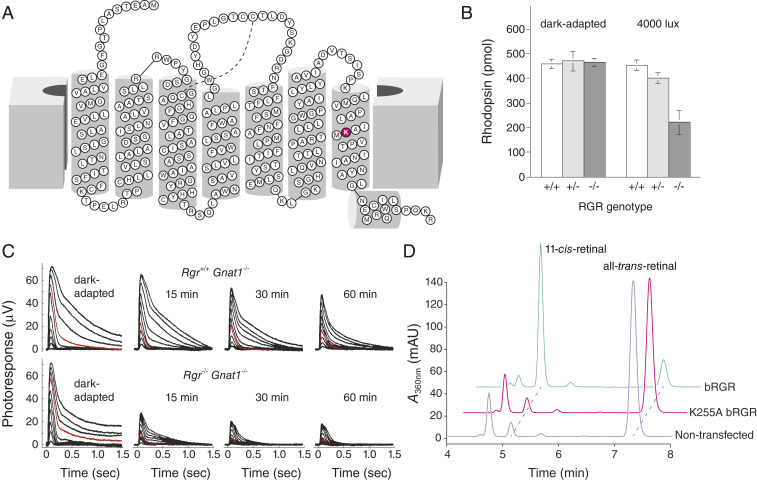
Fig. 4.
Evidence for RGR in supporting light-driven visual chromophore production. (A) A two-dimensional topology diagram showing the heptahelical structure of human RGR. The retinal binding Lys residue is highlighted in magenta. This research was originally published in the Journal of Biological Chemistry, ref. 77. © the American Society for Biochemistry and Molecular Biology. (B) RGR helps maintain visual pigment levels in the setting of intense (4,000 lx) light exposure. Reprinted by permission from ref. 75, Springer Nature: Nature Genetics, copyright (2001). (C) Electrophysiological data documenting a role for RGR in the maintenance of cone photoreceptor responses during exposure to light. Reprinted from ref. 76. Copyright (2019), with permission from Elsevier. (D) Recombinantly expressed and purified RGR directly photoisomerizes all-trans-retinal to 11-cis-retinal upon exposure to 530-nm light. The activity depends on the presence of the Lys255 nucleophile to form a Schiff base conjugate with retinal, where bRGR means bovine RGR, A360nm, absorbance at 360 nm, and mAU, milli-absorbance unit. This research was originally published in the Journal of Biological Chemistry, ref. 77. © the American Society for Biochemistry and Molecular Biology.
Physiological Contribution of Nonphotic and Photic Systems to Rod- and Cone-Mediated Vision
With cessation of light, only enzymatic reactions leading to active chromophore production can take place. This production and the regeneration of visual pigments in humans occur with two exponential phase processes: the faster regeneration of cone pigments in about 8 min and the slower regeneration of rhodopsin that requires roughly 30 min to achieve the fully dark-adapted state (82). The key pathway involved in this chromophore production constitutes the canonical visual cycle with retinoid isomerase RPE65 and other key components, such as LRAT, RDHs, and retinoid-binding proteins. This pathway is potently inhibited by free all-trans-retinal (77).
The excess of the substrate for RPE65 (retinyl esters) guarantees, at least in humans, that the isomerization occurs at the maximum rate (dark adaptation is independent of initial bleaching levels). However, we found that the photic regeneration system can produce 11-cis-retinal at a rate of 1 nmol/min/mg crude protein, which is an order of magnitude greater than the rate of 11-cis-retinol formation by the RPE in the dark (77). These data imply that the photic contribution could be significant, equal to or even exceeding that of RPE65, under certain light conditions. The action spectra for RGR are similar to the absorption maximum of the RGR:all-trans-retinal complex (∼480 nm) (76) but shifted to a long wavelength in isolated RPE or purified RGR (∼530 nm) in another report (77). This discrepancy will need to be addressed experimentally.
At intense bleaches, RGR makes a significant contribution to chromophore production, depending on the availability of all-trans-retinal in the RPE and Müller cells to serve as the substrate for RGR. There are two mechanisms by which this activity can be affected in addition to the wavelength and intensity of light. Studies have shown that all-trans-retinal accumulates in the retina during bright light exposure (83, 84)—conditions under which RGR could act as an efficient photoisomerase. This retinal likely can transfer freely between the photoreceptor OSs and the RPE/Müller cells as demonstrated in model systems (85–87). These results suggest that retinoids may not require active transport or binding protein-mediated transfer between the membranous compartments of rod and cone OSs and the RPE/Müller cells. However, it remains possible that an unidentified active transport mechanism exists in the mammalian retina. The 11-cis-retinal diffuses from the RPE to photoreceptors despite an abundance of CRALBP in the RPE. This occurs because opsin acts as a sink for 11-cis-retinal. Likewise, RGR can act as a sink for all-trans-retinal. Once 11-cis-retinal is generated by RGR, it can enter the visual cycle. RGR also will eliminate 13-cis-retinoids from the healthy eye since it can convert this isomer to all-trans-retinal (88). A second mechanism which can affect RGR activity involves mass action. All-trans-retinol is esterified by LRAT, and the ester can be hydrolyzed (89). The conversion of all-trans-retinol to all-trans-retinal is governed by the reducing power of the cell, and the reaction is reversible and governed by mass action of substrates and products (retinoids and dinucleotides) (Complexity of RDHs). This means that there will always be some amount of all-trans-retinal present in the cell. For the Müller cells, it was proposed that RGR-opsin and RDH10 convert all-trans-retinol to 11-cis-retinol during exposure to visible light in a series of oxidation–isomerization–reduction reactions (76). However, the specific deletion of Rdh10 either in Müller or RPE cells had no impact on light responses (40, 41).
Conclusions/Discussion
Now, it is clearly established that at least two physiologic pathways are involved in visual chromophore production: one that involves the classical visual cycle with a key isomerase, RPE65, that uses retinyl esters to generate 11-cis-retinol; and the second pathway involves an RGR photoisomerase that uses all-trans-retinal as a substrate to directly generate 11-cis-retinal. Together, both pathways meet the need for the chromophore as it is utilized by rod and cone visual pigments. What remains unknown is 1) the relative contributions of both pathways under different light conditions for both rods and cones; 2) the relative contributions of Müller cells and the RPE during photic isomerization for both rod and cone pigment regeneration; and 3) how CRALBP modulates the flow of the chromophore in both regeneration pathways.
Recent gains in our understanding of these different regenerative processes have clarified not only a critical component of the complex biochemistry essential for visual perception but also the etiology and potential treatment options for disparate blinding diseases in humans. A significant number of mutations in visual cycle enzymes and retinoid binding proteins have been identified that cause severe retinal diseases (10, 12). This has led to pharmacological strategies to overcome enzymatic lesions or to modulate visual cycle activity by the inhibition of various visual cycle enzymes, most notably RPE65, to control degenerative damage to photoreceptor cells. In contrast to such strategies, a rapid and efficient production of 11-cis-retinal can be achieved by the RPE and Müller cells by photic stimulation within a narrow band of wavelengths. The resulting 11-cis-retinal can then play a critical role especially in cone opsin regeneration and fulfill the demand for chromophore under daylight conditions. Like rhodopsin and cone opsins, RGR requires a photon to trigger the isomerization of retinal. RGR preferentially binds all-trans-retinal and then produces the 11-cis isomer upon illumination. In RPE65-deficient patients, RGR activity likely plays a key role in their residual vision before retinal degeneration occurs and vision irreversibly declines. The action spectrum of RGR in RPE microsomes and purified protein suggests that a background green light at 510 to 540 nm could be beneficial for generating active chromophore and thereby increasing visual sensitivity in RPE65-deficient patients. Clinical trials will require the control of light intensity not only for safety purposes, but to control the isomerization of the newly formed visual pigments.
In conclusion, recent findings pertaining to the photic generation of 11-cis-retinal in the RPE and Müller cells have added a new physiological element to our understanding of the pathways capable of regenerating the visual chromophore. However, these advances have yet to be fully integrated with what we know about the canonical visual cycle.
Acknowledgments
K.P. is the Leopold Chair of Ophthalmology at the Gavin Herbert Eye Institute, Department of Ophthalmology, University of California, Irvine. This research was supported in part by grants to K.P. from the NIH (EY009339, EY027283, EY025451, and EY019312) and to P.D.K. from the US Department of Veterans Affairs (I01BX004939). We acknowledge support from a Research to Prevent Blindness unrestricted grant to the Department of Ophthalmology, University of California, Irvine. We thank Dr. Vladimir Kefalov for his comments on the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data Availability.
There are no data underlying this work.
References
- 1.Luo D. G., Xue T., Yau K. W., How vision begins: An odyssey. Proc. Natl. Acad. Sci. U.S.A. 105, 9855–9862 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spudich J. L., Yang C. S., Jung K. H., Spudich E. N., Retinylidene proteins: Structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 16, 365–392 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Kiser P. D., Golczak M., Palczewski K., Chemistry of the retinoid (visual) cycle. Chem. Rev. 114, 194–232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palczewski K., G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst O. P., et al. , Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann L., Palczewski K., Advances in understanding the molecular basis of the first steps in color vision. Prog. Retin. Eye Res. 49, 46–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung N. Y., Montell C., Unconventional roles of opsins. Annu. Rev. Cell Dev. Biol. 33, 241–264 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson P. J., et al. , Local vibrational coherences drive the primary photochemistry of vision. Nat. Chem. 7, 980–986 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Mustafi D., Engel A. H., Palczewski K., Structure of cone photoreceptors. Prog. Retin. Eye Res. 28, 289–302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiser P. D., Palczewski K., Retinoids and retinal diseases. Annu. Rev. Vis. Sci. 2, 197–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J. S., Kefalov V. J., The cone-specific visual cycle. Prog. Retin. Eye Res. 30, 115–128 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis G. H., Golczak M., Moise A. R., Palczewski K., Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 47, 469–512 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saari J. C., Vitamin A metabolism in rod and cone visual cycles. Annu. Rev. Nutr. 32, 125–145 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Ramón y Cajal S., (1972) The Structure of the Retina (C. C. Thomas, Springfield, IL: ). [Google Scholar]
- 15.Rando R. R., Membrane phospholipids as an energy source in the operation of the visual cycle. Biochemistry 30, 595–602 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Rodieck R. W., The First Steps in Seeing (Sinauer Associates, Sunderland, MA, 1998). [Google Scholar]
- 17.Jacobson S. G., et al. , Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. U.S.A. 104, 15123–15128 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redmond T. M., et al. , Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 20, 344–351 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Van Hooser J. P., et al. , Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc. Natl. Acad. Sci. U.S.A. 97, 8623–8628 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Hooser J. P., et al. , Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 277, 19173–19182 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan J., Rohrer B., Moiseyev G., Ma J. X., Crouch R. K., Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc. Natl. Acad. Sci. U.S.A. 100, 13662–13667 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenendijk G. W., Jacobs C. W., Bonting S. L., Daemen F. J., Dark isomerization of retinals in the presence of phosphatidylethanolamine. Eur. J. Biochem. 106, 119–128 (1980). [DOI] [PubMed] [Google Scholar]
- 23.Shichi H., Somers R. L., Possible involvement of retinylidene phospholipid in photoisomerization of all-trans-retinal to 11-cis-retinal. J. Biol. Chem. 249, 6570–6577 (1974). [PubMed] [Google Scholar]
- 24.Palczewski K., et al. , Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 (2000). [DOI] [PubMed] [Google Scholar]
- 25.von Lintig J., Kiser P. D., Golczak M., Palczewski K., The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi R., et al. , A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., et al. , Structure of the STRA6 receptor for retinol uptake. Science 353, aad8266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orban T., Palczewska G., Palczewski K., Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J. Biol. Chem. 286, 17248–17258 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imanishi Y., Batten M. L., Piston D. W., Baehr W., Palczewski K., Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 164, 373–383 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poliakov E., Uppal S., Rogozin I. B., Gentleman S., Redmond T. M., Evolutionary aspects and enzymology of metazoan carotenoid cleavage oxygenases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 158665 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redmond T. M., et al. , Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 13658–13663 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X., RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 12413–12418 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H., Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122, 449–459 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiser P. D., et al. , Structure of RPE65 isomerase in a lipidic matrix reveals roles for phospholipids and iron in catalysis. Proc. Natl. Acad. Sci. U.S.A. 109, E2747–E2756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiser P. D., Golczak M., Lodowski D. T., Chance M. R., Palczewski K., Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 106, 17325–17330 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiser P. D., et al. , Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nat. Chem. Biol. 11, 409–415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolze C. S., et al. , Human cellular retinaldehyde-binding protein has secondary thermal 9-cis-retinal isomerase activity. J. Am. Chem. Soc. 136, 137–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X., Lobsiger J., Stocker A., Bothnia dystrophy is caused by domino-like rearrangements in cellular retinaldehyde-binding protein mutant R234W. Proc. Natl. Acad. Sci. U.S.A. 106, 18545–18550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bunt-Milam A. H., Saari J. C., Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J. Cell Biol. 97, 703–712 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Y., et al. , The role of retinol dehydrogenase 10 in the cone visual cycle. Sci. Rep. 7, 2390 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahu B., et al. , Conditional ablation of retinol dehydrogenase 10 in the retinal pigmented epithelium causes delayed dark adaption in mice. J. Biol. Chem. 290, 27239–27247 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda A., et al. , Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl. Acad. Sci. U.S.A. 104, 19565–19570 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haeseleer F., et al. , Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J. Biol. Chem. 277, 45537–45546 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda A., et al. , Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem. 281, 37697–37704 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Driessen C. A., et al. , Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol. Cell. Biol. 20, 4275–4287 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allikmets R., et al. , A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15, 236–246 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Lenis T. L., et al. , Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 115, E11120–E11127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frederiksen R., et al. , Rhodopsin kinase and arrestin binding control the decay of photoactivated rhodopsin and dark adaptation of mouse rods. J. Gen. Physiol. 148, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saari J. C., et al. , Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29, 739–748 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Lamb T. D., Pugh E. N. Jr, Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 23, 307–380 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Mata N. L., Radu R. A., Clemmons R. C., Travis G. H., Isomerization and oxidation of vitamin a in cone-dominant retinas: A novel pathway for visual-pigment regeneration in daylight. Neuron 36, 69–80 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J. S., Kefalov V. J., An alternative pathway mediates the mouse and human cone visual cycle. Curr. Biol. 19, 1665–1669 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J. S., Estevez M. E., Cornwall M. C., Kefalov V. J., Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat. Neurosci. 12, 295–302 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das S. R., Bhardwaj N., Kjeldbye H., Gouras P., Muller cells of chicken retina synthesize 11-cis-retinol. Biochem. J. 285, 907–913 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J., Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 86, 9606–9610 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazono S., Shimauchi-Matsukawa Y., Tachibanaki S., Kawamura S., Highly efficient retinal metabolism in cones. Proc. Natl. Acad. Sci. U.S.A. 105, 16051–16056 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato S., Frederiksen R., Cornwall M. C., Kefalov V. J., The retina visual cycle is driven by cis retinol oxidation in the outer segments of cones. Vis. Neurosci. 34, E004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaylor J. J., et al. , Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat. Chem. Biol. 9, 30–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiser P. D., et al. , Conditional deletion of Des1 in the mouse retina does not impair the visual cycle in cones. FASEB J. 33, 5782–5792 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward R., et al. , Non-photopic and photopic visual cycles differentially regulate immediate, early, and late phases of cone photoreceptor-mediated vision. J. Biol. Chem. 295, 6482–6497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaylor J. J., et al. , Identification of the 11-cis-specific retinyl-ester synthase in retinal Müller cells as multifunctional O-acyltransferase (MFAT). Proc. Natl. Acad. Sci. U.S.A. 111, 7302–7307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golczak M., Kuksa V., Maeda T., Moise A. R., Palczewski K., Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. U.S.A. 102, 8162–8167 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J., et al. , Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J. Clin. Invest. 125, 2781–2794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calvert P. D., et al. , Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl. Acad. Sci. U.S.A. 97, 13913–13918 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiser P. D., et al. , Retinoid isomerase inhibitors impair but do not block mammalian cone photoreceptor function. J. Gen. Physiol. 150, 571–590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golczak M., et al. , Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J. Biol. Chem. 280, 42263–42273 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Hubbard R., Wald G., Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J. Gen. Physiol. 36, 269–315 (1952). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaylor J. J., et al. , Blue light regenerates functional visual pigments in mammals through a retinyl-phospholipid intermediate. Nat. Commun. 8, 16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M., Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature 270, 540–542 (1977). [DOI] [PubMed] [Google Scholar]
- 70.Suzuki T., Callender R. H., Primary photochemistry and photoisomerization of retinal at 77 degrees K in cattle and squid rhodopsins. Biophys. J. 34, 261–270 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Widjaja-Adhi M. A. K., Ramkumar S., von Lintig J., Protective role of carotenoids in the visual cycle. FASEB J. 32, 6305–6315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hao W., Chen P., Fong H. K., Analysis of chromophore of RGR: Retinal G-protein-coupled receptor from pigment epithelium. Methods Enzymol. 316, 413–422 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Hara T., Hara R., Rhodopsin and retinochrome in the squid retina. Nature 214, 573–575 (1967). [DOI] [PubMed] [Google Scholar]
- 74.Choi E. H., Daruwalla A., Suh S., Leinonen H., Palczewski K., Retinoids in the visual cycle: Role of the retinal G protein-coupled receptor. J. Lipid Res., jlr.TR120000850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen P., et al. , A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat. Genet. 28, 256–260 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Morshedian A., et al. , Light-driven regeneration of cone visual pigments through a mechanism involving RGR opsin in Müller glial cells. Neuron 102, 1172–1183.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., et al. , Photic generation of 11-cis-retinal in bovine retinal pigment epithelium. J. Biol. Chem. 294, 19137–19154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xue Y., et al. , CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Invest. 125, 727–738 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albalat R., Evolution of the genetic machinery of the visual cycle: A novelty of the vertebrate eye? Mol. Biol. Evol. 29, 1461–1469 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Tsuda M., et al. , Origin of the vertebrate visual cycle: II. Visual cycle proteins are localized in whole brain including photoreceptor cells of a primitive chordate. Vision Res. 43, 3045–3053 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Terakita A., Hara R., Hara T., Retinal-binding protein as a shuttle for retinal in the rhodopsin-retinochrome system of the squid visual cells. Vision Res. 29, 639–652 (1989). [DOI] [PubMed] [Google Scholar]
- 82.Mahroo O. A., Lamb T. D., Recovery of the human photopic electroretinogram after bleaching exposures: Estimation of pigment regeneration kinetics. J. Physiol. 554, 417–437 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saari J. C., Garwin G. G., Van Hooser J. P., Palczewski K., Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 38, 1325–1333 (1998). [DOI] [PubMed] [Google Scholar]
- 84.Lee K. A., Nawrot M., Garwin G. G., Saari J. C., Hurley J. B., Relationships among visual cycle retinoids, rhodopsin phosphorylation, and phototransduction in mouse eyes during light and dark adaptation. Biochemistry 49, 2454–2463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noy N., Xu Z. J., Kinetic parameters of the interactions of retinol with lipid bilayers. Biochemistry 29, 3883–3888 (1990). [DOI] [PubMed] [Google Scholar]
- 86.Rando R. R., Bangerter F. W., The rapid intermembraneous transfer of retinoids. Biochem. Biophys. Res. Commun. 104, 430–436 (1982). [DOI] [PubMed] [Google Scholar]
- 87.Fex G., Johannesson G., Retinol transfer across and between phospholipid bilayer membranes. Biochim. Biophys. Acta 944, 249–255 (1988). [DOI] [PubMed] [Google Scholar]
- 88.Maeda T., et al. , Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J. Neurochem. 85, 944–956 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isken A., et al. , RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hofmann L., et al. Structural insights into the Drosophila melanogaster retinol dehydrogenase, a member of the short-chain dehydrogenase/reductase family. Biochemistry 55, 6545–6557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.