Significance
NFAT5 mediates protection from adverse effects of hypertonicity. Cells, e.g. kidney, in hypertonic conditions take up salts and protective organic osmolytes. By mechanisms not fully understood, NFAT5 is activated and induces genes including those that code enzymes responsible for synthesizing protective osmolytes. We show that in solution the NFAT5 NTD, which contains one of NFAT5′s three transcription activation domains, undergoes hypertonicity- and/or osmolyte-induced disorder-to-order conformational rearrangement. In its more folded conformation, interaction of NFAT5 NTD with specific proteins is enhanced, including that with high mobility group protein (HMGI-C), which has been shown to protect against apoptosis. These findings suggest that in vivo, increased intracellular ionic strength, coupled with osmolytes, may directly activate NFAT5. They encourage further pursuit of this possibility.
Keywords: NFAT5, hypertonicity, osmolytes, intrinsically disordered region, protein–protein interactions
Abstract
Nuclear Factor of Activated T cells 5 (NFAT5) is a transcription factor (TF) that mediates protection from adverse effects of hypertonicity by increasing transcription of genes, including those that lead to cellular accumulation of protective organic osmolytes. NFAT5 has three intrinsically ordered (ID) activation domains (ADs). Using the NFAT5 N-terminal domain (NTD), which contains AD1, as a model, we demonstrate by biophysical methods that the NTD senses osmolytes and hypertonicity, resulting in stabilization of its ID regions. In the presence of sufficient NaCl or osmolytes, trehalose and sorbitol, the NFAT5 NTD undergoes a disorder-to-order shift, adopting higher average secondary and tertiary structure. Thus, NFAT5 is activated by the stress that it protects against. In its salt and/or osmolyte-induced more ordered conformation, the NTD interacts with several proteins, including HMGI-C, which is known to protect against apoptosis. These findings raise the possibility that the increased intracellular ionic strength and elevated osmolytes caused by hypertonicity activate and stabilize NFAT5.
Mammalian cells have adaptive responses that enhance survival during various forms of stress (1). Among these, uncompensated extracellular hyperosmotic stress results in osmotic outflow of water with a concomitant reduction in cell volume and an increase in intracellular ionic strength. This leads to cell cycle delay, DNA breakage, oxidative stress, and apoptotic death (2–4). Cells adapt to hypertonic stress by accumulating organic osmolytes, which are known to compensate for the cell volume reduction induced by the hyperosmotic environment by allowing for the osmotic influx of water into cells. Under isosmotic conditions, a reduction or loss of intracellular osmolytes may also lead to a functional reduction in cell volume and an increase in ionic strength sufficient to compromise normal cellular metabolic and biochemical function (5–8). Another known function of such osmolytes is to protect proteins from denaturation by preserving their native structure in the face of potentially denaturing conditions. The possibility that this function may apply in hyperosmotic stress has, to our knowledge, not been investigated, nor has the possibility that high salt alone can stabilize any intrinsically ordered (ID) region of Nuclear Factor of Activated T cells 5 (NFAT5).
The osmosensitive TF NFAT5/TonEBP/OREBP plays a crucial role protecting cells against deleterious effects of hyperosmotic stress upon urinary concentration, the adaptive immune response, and other physiological systems, particularly in tissues that experience large fluctuations in tonicity, such as the renal medulla (9–12). NFAT5 modulates cellular response to osmotic changes by enhancing the expression of target genes, including those responsible for synthesis and/or transport of multiple organic osmolytes, such that the intracellular concentration of compatible protective osmolytes is increased and compensates for increased extracellular tonicity (13–15). NFAT5 is a multidomain transcription factor (TF) in which the ID N-terminal domain (NTD) includes a tonicity-dependent auxiliary export region responsible for nuclear and cytoplasmic localization and AD1 (amino acids 1–76), one of NFAT5’s three activation domains (16–22). NFAT5 ID C-terminal domain (CTD) contains two tonicity-dependent transactivation domains, AD2 and AD3. Although AD1 alone is the weakest of the three, its inclusion in test constructs doubles the hypertonicity response of AD2 or AD2AD3 (18, 19).
ID regions are disproportionately higher in cell-signaling proteins, giving them an advantage over proteins with ordered conformations, since proteins with ID regions can more efficiently and selectively interact with appropriate target binding partner proteins and enhance allosteric responses (23–33). The ID regions of many TFs are known to undergo disorder-to-order conformational transition upon interacting with organic osmolytes and encounters with specific target binding molecules (26, 27). Due to their lack of stable structure, the detailed mechanism behind the functions of NFAT5’s ID regions are unknown. We chose to investigate first the simpler of NFATs two major ID regions, hypothesizing that in the presence of appropriate concentrations of compatible osmolyte(s) or simple inorganic salts, the ID NTD of NFAT5 would undergo disorder-to-order conformational rearrangements that would enhance protein–protein interactions.
In this study, we show our hypothesis to be correct: When incubated with the natural organic osmolytes trehalose or sorbitol, NFAT5 NTD changes from ID to more ordered conformation, consistent with a two-state transition. In this folded conformation, the interaction of NFAT5 NTD with specific proteins, e.g., high mobility group protein (HMGI-C), is significantly enhanced. We also found that ID NTD can adopt higher secondary/tertiary structure in the presence of NaCl. Sorbitol and NaCl are known to work together to maintain cellular tonicity (6), and our data suggest that their structural effects are greater than the additive. These data show that NFAT5’s ID NTD can become more structured in the presence of osmolytes and inorganic salt, and that this enhances important protein–protein interactions. Such interactions are the natural function of TFs. These results provide proof of principle of a physical basis for salt and osmolyte actions on NFAT5 structure and function.
Materials and Methods
Bacterial Expression, Protein Purification, and Biophysical Analyses.
Plasmid of human NFAT5 NTD (amino acids 2–220) in pET41b was transformed into HMS174 Escherichia coli competent cells. Recombinant protein was expressed and purified to near homogeneity as described (SI Appendix, Supplemental Methods). The far-ultraviolet (UV) and near-UV circular dichroism (CD) spectra of the purified recombinant NFAT5 NTD protein were recorded at 22 °C on a Jasco 815 spectropolarimeter by using a 0.1-cm and 1.0-cm quartz cell, respectively, as described (34–37). The spectra were recorded at a fixed protein concentration. All spectra recorded were corrected for the contribution of solute concentrations. Each spectrum is a result of five spectra accumulated, averaged, and smoothed. Fluorescence emission spectra of protein in solution were monitored at excitation wavelength of 278 or 295 nm as described (36, 37). All measurements were made using 1-cm rectangular cuvettes at 22 °C, and all data were corrected for the contribution of the respective solute concentrations.
Protein–Protein Interactions.
Pulldowns were performed from HEK293 cell lysates comprising a 1:1 mix of cytoplasm and membrane extracts or a 1:1 mix of soluble and chromatin bound nuclear extracts as prepared (SI Appendix, Supplemental Methods). After a detergent removal and buffer exchange as described (20), NFAT5 NTD and trehalose (1.4 M) were added to the proteins (including a control without trehalose) and incubated overnight at 4 °C with rotation. The samples were passed through a 0.22-μm filter and mixed with preequilibrated Co2+ resin (GE Health Care Life Sciences) to purify NFAT5 NTD and any associated proteins. The resin was first washed with 10 column volumes (CVs) of 25 mM NaPi, pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 20 mM imidazole buffer. The His-tag NFAT5 NTD and associated proteins were eluted with 5 CVs of 25 mM NaPi, pH 7.4, 150 mM NaCl, 1 mM EDTA, 250 mM imidazole buffer. All fractions were first analyzed using 4–12% Bis-Tris gels (Life Tech) to determine the pulled down NFAT5 NTD and the associated proteins in the presence and absence of trehalose. Samples were buffer exchanged as described (20). Protease digestion was achieved by incubation at 37 °C overnight. The digested supernatant was removed to a clean protein LoBind tube and adjusted to pH 3, using 1% trifluoracetic acid (TFA), and concentrated via SpeedVac before being resuspended in 50 μL of 1% TFA. The tryptic peptides were then desalted, dried, and resuspended in 0.1% formic acid for liquid chromatography–mass spectrometry (LC-MS/MS) analysis as described (20).
LC-MS/MS Analysis and Protein Identification.
Tryptic peptides were analyzed on an Orbitrap Elite mass spectrometer as described (20). Briefly, samples were purified using an Agilent Zorbax 300SB-C18 trap column in a buffer containing 0.1% formic acid in water at a flow rate of 6 μL/min for 10 min (20). Trapped peptides were separated using reversed-phase C18 column followed by MS analysis as described (20). The Mascot search algorithm and relative protein abundance were used to identify proteins from the raw MS data, and peptides were searched against a target-decoy protein (Swiss-Prot human) as described (20).
Results
NFAT5 NTD Shows ID Characteristics.
Secondary structural analysis of the NFAT5 NTD (amino acids 2–220) predicted a large amount of sequence in random coil (ID) configuration (SI Appendix, Supplementary Methods), with more than 75% of the NTD sequence random coil and only a small proportion as helix or sheet (SI Appendix, Fig. S1). We used several independent predictors to analyze the ID nature of NFAT5 NTD. Analysis by IUPred showed ID regions in NFAT5 (SI Appendix, Fig. S2). We also applied ANCHOR, which predicts potential binding regions within ID proteins. Such regions function by undergoing a disorder-to-order transition upon binding to a protein partner. ANCHOR identifies segments in a generally disordered region that cannot form enough favorable intrachain interactions to fold spontaneously and have the energetic capability to gain structure by interacting with a globular partner protein (SI Appendix, Fig. S2). Analysis by three different versions of PONDR for ID prediction further strengthened the case for the ID nature of NFAT5 NTD, evident from PONDR scores over 0.5 (SI Appendix, Fig. S3). Further, computational analysis of ID predisposition of the NFAT5 NTD was extended to include the CH-CDF plot method, which is based on the combined use of the Charge/Hydropathy and CDF (Cumulative Distribution Function) binary disorder predictors (SI Appendix, Figs. S4 and S5). In addition to providing plots showing ID predisposition of NTD, we utilized IUPRED and PONDR analyses to show the per-residue disorder predisposition of the full-length NFAT5 protein (SI Appendix, Figs. S6 and S7). These plots provide the overall disorder status of NFAT5 protein. Together, these data support the belief that NFAT5 NTD possesses characteristics of an ID protein. Solution biophysical data confirm this notion.
Trehalose Induces Secondary/Tertiary Structure in NFAT5 NTD.
We expressed and purified NFAT5 NTD protein to near homogeneity (SI Appendix, Figs. S8–S12). The effects of increasing concentrations of trehalose on purified NFAT5 NTD were analyzed using far-UV CD spectroscopy (Fig. 1A). As predicted (SI Appendix, Figs. S1–S7), the spectrum of NFAT5 NTD in buffer shows little secondary structural content, as evident from a minimum around 200 nm in the CD spectrum (black circle; Fig. 1A). Increasing concentrations of trehalose (0.20–1.40 M) resulted in a concentration-dependent increase in minima around 220 nm, a pattern indicative of increased helical content (Fig. 1A). The trehalose-induced conformational transition in the NFAT5 NTD appears to be cooperative, from the sigmoidal curve of ellipticity at 220 nm (Φ220), suggestive of folding to a natural configuration (Fig. 1B). Limited proteolytic digestions of NFAT5 NTD in the presence and absence of trehalose show that at 0.0 M trehalose, the protein is nearly completely digested (compare lanes 2 and 3; SI Appendix, Fig. S13), whereas it is increasingly protected by 0.2–1.0 M trehalose (lanes 3–7). The effect appears to reach saturation beyond 1.0 M trehalose (lanes 7–9; SI Appendix, Fig. S13). This suggests that NFAT5 NTD has regions that have folded into a tertiary structure that secludes the residues attacked by the protease to positions not easily reached. To acquire further evidence for tertiary structure occurring in NFAT5 NTD, we recorded the near-UV CD spectra of this protein in the presence and absence of 1 M NaCl, sorbitol, or trehalose (SI Appendix, Fig. S14). The spectra show changes typical of those arising from adding differing solutes. Such changes may arise from movement of residues from polar to more hydrophobic environments in the protein. These data suggest that trehalose induces, on average, a compact tertiary structural arrangement in NFAT5 NTD.
Fig. 1.
Presence of trehalose induces secondary structure in ID NFAT5 NTD. (A) Far-UV CD spectra of NFAT5 NTD protein in the absence and presence of increasing concentrations of trehalose [0.0–1.4 M]. Each spectrum represents an average of three spectra recorded, corrected for the contribution of the buffer, and smoothed. (B) Trehalose-induced conformational transition of NFAT5 NTD as assessed by increase in ellipticity at 220-nm wavelength.
Trehalose Causes the NFAT5 NTD Protein to Fold into a Conformation that Interacts with Specific Proteins.
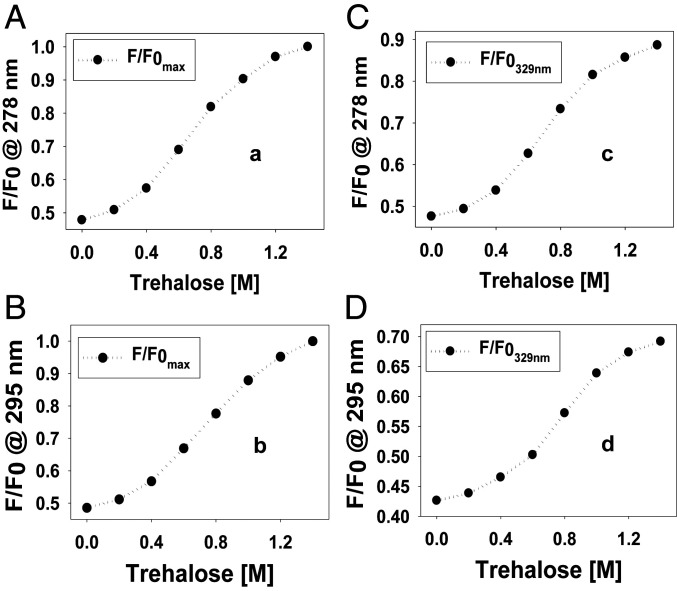
To monitor the environment around the Trp and Tyr residues of the NFAT5 NTD, we employed inherent fluorescence emission, with excitation at 278 nm to monitor both amino acids and at 295 nm to monitor Trp only (SI Appendix, Figs. S15 and S16). In both sets of spectra, the quantum yield of the fluorescence significantly increases with respect to increasing concentrations of trehalose (SI Appendix, Figs. S15 and S16). Trp residues buried in nonpolar regions of the protein can be followed at 329 nm (38, 39). Our results based on emission at 329 nm (Fig. 2 C and D) showed that in the presence of trehalose, Trp may be buried, strongly suggestive of acquired tertiary structure. There was a similar trend for the shift in fluorescence emission maximum after excitation at 278 or 295 nm in the presence of trehalose (Fig. 2 A and B). These fluorescence emission studies, together with the CD data, thus indicate that the trehalose causes, on average in the conformer ensemble, secondary and tertiary structures to form cooperatively in NFAT5 NTD. We estimated the degree of exposure of Trp residues to solvent in native NFAT5 NTD by measuring the efficiency with which their fluorescence was quenched by acrylamide, a dynamic quencher of Trp fluorescence. The fluorescence emission spectra of NFAT5 NTD recorded in the absence and presence of increasing concentrations of acrylamide showed that the fluorescence intensity for the Trp maximum was significantly reduced (≥40%) as sufficient acrylamide was added (SI Appendix, Fig. S17), suggesting that without osmolyte, the Trp residues in NFAT5 NTD are readily accessible.
Fig. 2.
(A and B) Reversible trehalose-induced conformational transition of NFAT5 NTD monitored at maximum wavelength (F/F0 max) at an excitation wavelength of 278 and 295 nm, respectively. (C and D) Reversible trehalose-induced conformational transition of NFAT5 NTD monitored at 329-nm emission wavelength (F/F0 329 nm) after exciting at a wavelength of 278 and 295 nm, respectively.
The NFAT5 NTD is known to interact with several cellular proteins important for NFAT5-mediated transcriptional activity. Since disorder-order transition in ID regions often facilitates their interactions with specific proteins, we tested for such interactions of NFAT5 NTD in nuclear and cytoplasmic extracts of HEK293 cells by use of peptide affinity chromatography followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Fig. 3 A and B) and mass spectrometry (Fig. 3C). In osmo-stressed cell nuclear extracts, the MS data showed that trehalose facilitates interaction of NFAT5 NTD with high mobility group protein (HMGI-C) and other proteins (Fig. 3). Of note, high mobility group protein HMGI-C/HMGA-2 was present in bioreplicates of nuclear proteins plus NFAT5 NTD when trehalose was present (Fig. 3C). HMGI-C has previously been shown to be present in N-terminal NFAT5 peptide affinity pulldown from nuclear extracts (20, 22).
Fig. 3.
HEK293 cells under osmotic stress (500 mOsm NaCl), followed by nuclear (N) and cytoplasmic (C) protein extraction and addition of N-term (amino acids 2–220) NFAT5, incubated both with and without trehalose (T) and His-tag purification, using a Co2+ column. (A) Nuclear extraction. (B) Cytoplasmic extraction. Lane 1: MW marker. Lane 2: Flow through. Lane 3: 20 mM imidazole wash. Lane 4: 250 mM imidazole elution. Lane 5: Flow through + 1.4 M trehalose. Lane 6: 20 mM imidazole wash + 1.4 M trehalose. Lane 7: 250 mM imidazole elution + 1.4 M trehalose. Lane 8: bovine serum albumin std, 0.1 mg/mL. (C) In-solution digestion followed by LC-MS/MS determination of proteins present in each extraction (Nuclear ± 1.4 M trehalose, Cytoplasmic ± 1.4 M trehalose).
NaCl and Sorbitol Produce Independent, Greater than Additive Effects to Fold NFAT5 NTD Protein.
Since many cells adapt to an increase in intracellular ionic strength by accumulating sorbitol, we determined whether exposure to NaCl or sorbitol can cause folding of ID NFAT5 NTD. In concentrations of NaCl up to 2.0 M, NFAT5 NTD shows an increased level of secondary structural elements, as evident from a dip in the CD spectrum around 220 nm (Fig. 4A). Similar results were observed in the presence of sorbitol (Fig. 4B). Thus, at higher concentrations of either NaCl or sorbitol, ID NFAT5 NTD adopts, on average, a more ordered conformation. Further, in 1 M NaCl or sorbitol alone, the degree of NFAT5 NTD structure is similar (Fig. 4C). However, when both solutes are present, the level of secondary structural elements appears to be higher (Fig. 4C). A comparison of the theoretical sum of the change due to the two treatments singly to experimental CD spectra collected from a mixture of both solutes present together indicates that the effects of NaCl and sorbitol on the folding of NFAT5 NTD protein appear to be greater than additive (Fig. 4D). Interactive effects of mixed osmolytes have been previously explored (40).
Fig. 4.
Incubation of NFAT5 NTD with NaCl (N) or sorbitol (S) induces secondary structure in ID NFAT5 NTD. Far-UV CD spectra of NFAT5 NTD protein in the absence and presence of increasing concentrations of NaCl (A) and sorbitol (B), [0.0–2.0 M], respectively. Each spectrum represents an average of three spectra recorded, corrected for the contribution of the buffer, and smoothed. (C) Far-UV CD spectra of NFAT5 NTD protein in buffer (black), 1.0 M NaCl (red), 1.0 M sorbitol, and 1.0 M NaCl + 1.0 M sorbitol. (D) Far UV CD spectra (black) showing the average of individually recorded NFAT5 NTD in the presence of 1.0 M NaCl and 1.0 M sorbitol (theoretical sum [theo]), and average when recorded in the presence of 1.0 M NaCl + 1.0 M sorbitol together (experimental [exp]).
Discussion
Hypertonicity increases NFAT5 expression and activates it, resulting in NFAT5-dependent expression of target genes important for cell survival, such as aldose reductase, BGT1, SMIT, HSP70, and TAUT (41–51). In addition to its osmoadaptive responses in the kidney, NFAT5 also regulates the expression of the urea transporter, aquaporins and serum- and glucocorticoid-inducible kinase (Sgk1). NFAT5 is also expressed in many nonrenal tissues or cells in which osmoadaptation is less critical under physiological conditions (52–57). Such protection could become important in extreme conditions, e.g., severe dehydration. Hypertonic activation of NFAT5 also increases the expression of target genes that synthesize or transport multiple organic osmolytes (12). Certain organic osmolytes are known to act as chemical chaperones to fold/stabilize proteins including ID regions of many transcription factors (58–61). NFAT5 contains multiple regions predicted to be ID, including its NTD and CTD, both important for NFAT5 function. The NTD contains an auxiliary export region, involved in nuclear and cytoplasmic localization, a monopartite nuclear localization signal, and transcription activation function AD1 (20, 62). The CTD contains transactivation domains AD2 and AD3 (18, 19, 22). Since several predictors of protein structure agreed that all these domains are ID, we tested the hypothesis that protective organic osmolytes and/or NaCl could promote their folding. To the best of our knowledge, the existence of these ID regions and their capacity to take on secondary and tertiary structure has not been experimentally validated.
We selected the NFAT5 NTD for the present study, as proof of principle. Our biophysical analyses provide physical proof that the NFAT5 NTD is ID. CD, fluorescence emission, and limited proteolytic digestion data clearly demonstrate that in aqueous solution, the NTD possesses little or no secondary/tertiary structure. We further show that protective osmolytes often found in cells, as well as NaCl, can cause the protein to fold.
It has been reported that the ID regions of several TF proteins undergo disorder-order conformational transition in the presence of organic osmolytes, such that an ordered conformation with expected function is acquired in the protein (23, 24). Our results demonstrate that the ID NTD of NFAT5 undergoes such a transition in the presence of natural osmolytes, trehalose and sorbitol, or even simply high NaCl. The presence of AD1 doubles the hyperosmotic response of AD2 or AD2AD3-containing constructs. This effect is significant because physiologic activation of NFAT5 regulates the expression of genes that can synthesize cellular osmolytes in response to tonicity. These findings therefore suggest a mechanism for controlling the synthesis of organic osmolytes by the allosteric response of NFAT5 to the cellular osmotic state.
Since NFAT5-mediated regulation of NaCl (ionic strength) and sorbitol (osmolyte) are involved in counterbalancing cellular hypertonicity in the renal medulla and possibly in other cells/tissues, we tested whether these two solutes could work in conjunction to maintain the disorder-order transition in NTD conformation. Our biophysical data showing the folding of the ID NTD in the presence of each solute alone strongly suggests that these specific solutes may be involved in providing structural stability to ID NFAT5 protein, specifically its NTD. Further, the combined effects of these solutes suggest that under hypertonic and even physiological conditions, the activity of NFAT5 may be regulated by the fine-tuning response to concentrations of NaCl and sorbitol.
Due to their structural flexibility, the ID regions of many TFs play important roles in their biological functions. In the large ensembles of transient structures that compose ID regions are some that facilitate multiple, specific protein–protein interactions with coregulatory proteins. These cofactors are critical for initiation and maintenance of transcriptional activity (27, 63–66). To investigate whether increased natural structure in its NTD affects relevant cellular protein–protein interactions, we looked for a correlation between change in NFAT5 NTD structure and association with other proteins. MS identified several NFAT5 NTD protein binding partners. Among these, HMGI-C was present only when NFAT5 NTD was incubated with nuclear extracts in the presence of osmolyte. We thus have demonstrated that the increase in NFAT5 structure caused by osmolyte enhances physiologically relevant protein–protein interactions. Uncompensated osmotic stress damages DNA, interrupts cellular proliferation, and kills cells (3, 12). This is prevented by accumulation of compatible organic osmolytes, whose function is shared by trehalose. Our finding that trehalose induces association of NFAT5 with HMGI-C contributes to explaining the association since HMGI-C suppresses apoptosis (67, 68) and is involved with proliferation of cancer cells (68–70).
It has been demonstrated thermodynamically that in multidomain proteins, the presence of ID domains enhances the allosteric response of binding (e.g., a protein or DNA). The quantitative nature of interdomain coupling in holoNFAT5 will determine the extent to which this occurs (29, 63). The allosteric model, which explains why ID enhances allosteric responses, shows that interdomain coupling can enhance or repress folding of other domains, thus altering their function. This has been verified experimentally for a two-domain version of the GR/NR3C1 (37). Even a brief consideration of the implications of the ensemble allosteric model leads to the conclusion that in NFAT5, with its three separate, interactive, ID activation domains the coupling responses will be complex (71, 72). However, based on observations with many other proteins (73, 74), it is likely that the NFAT NTD and CTD exist in an ID conformation in the full-length protein. Extensive future studies will be required to determine the degree to which the NTD is unstructured in the full-length NFAT5 protein and how its ID/folded domains interact in vivo.
In sum, we have demonstrated that, in solution: the NFAT5 NTD is ID; protective natural osmolytes or NaCl increase its secondary and tertiary structure and salt plus osmolyte effects are additive (or greater than additive); the dose–response curves of NFAT5 to these agents suggests a two-state transition (without excluding the possibility of intermediate transient states), a hallmark of a switch from disorder to ordered structure; the more structured ensemble of NFAT5 isomers enhanced binding of HMGI-C, a physiologically relevant protein. The degree of dynamic behavior in proteins ranges widely, from slightly varying interactions between well-ordered subunits to complete ID. This gives the opportunity for varying regional and quantitative changes in structure to have important discriminatory effects on protein function (71, 72, 75, 76). A few other stress–response transcription factors are believed to respond directly to the stress itself or its immediate products, e.g., the responders to heat shock, unfolded proteins in the ER, and oxidative stress (77–80). Our results suggest a physical mechanism by which NFAT5 responds to hypertonic stress by direct structural reactions to salt and osmolytes.
Supplementary Material
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911680117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Hoffmann E. K., Lambert I. H., Pedersen S. F., Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Lang F. et al., Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247–306 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Dmitrieva N. I., Cai Q., Burg M. B., Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc. Natl. Acad. Sci. U.S.A. 101, 2317–2322 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dmitrieva N. I., Michea L. F., Rocha G. M., Burg M. B., Cell cycle delay and apoptosis in response to osmotic stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 130, 411–420 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N., Living with water stress: Evolution of osmolyte systems. Science 217, 1214–1222 (1982). [DOI] [PubMed] [Google Scholar]
- 6.Gallazzini M., Ferraris J. D., Kunin M., Morris R. G., Burg M. B., Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl. Proc. Natl. Acad. Sci. U.S.A. 103, 15260–15265 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Perez A., Burg M. B., Renal medullary organic osmolytes. Physiol. Rev. 71, 1081–1115 (1991). [DOI] [PubMed] [Google Scholar]
- 8.Burg M. B., Kwon E. D., Kültz D., Regulation of gene expression by hypertonicity. Annu. Rev. Physiol. 59, 437–455 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Rodríguez C., Aramburu J., Rakeman A. S., Rao A., NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. U.S.A. 96, 7214–7219 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyakawa H., Woo S. K., Dahl S. C., Handler J. S., Kwon H. M., Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. U.S.A. 96, 2538–2542 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko B. C., Turck C. W., Lee K. W., Yang Y., Chung S. S., Purification, identification, and characterization of an osmotic response element binding protein. Biochem. Biophys. Res. Commun. 270, 52–61 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Burg M. B., Ferraris J. D., Dmitrieva N. I., Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441–1474 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Burg M. B., Ferraris J. D., Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 283, 7309–7313 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraris J. D. et al., ORE, a eukaryotic minimal essential osmotic response element. The aldose reductase gene in hyperosmotic stress. J. Biol. Chem. 271, 18318–18321 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Ko B. C., Ruepp B., Bohren K. M., Gabbay K. H., Chung S. S., Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 272, 16431–16437 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Cheung C. Y., Ko B. C., NFAT5 in cellular adaptation to hypertonic stress–Regulations and functional significance. J. Mol. Signal. 8, 5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi Y. et al., Mutations that reduce its specific DNA binding inhibit high NaCl-induced nuclear localization of the osmoprotective transcription factor NFAT5. Am. J. Physiol. Cell Physiol. 303, C1061–C1069 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S. D., Colla E., Sheen M. R., Na K. Y., Kwon H. M., Multiple domains of TonEBP cooperate to stimulate transcription in response to hypertonicity. J. Biol. Chem. 278, 47571–47577 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Ferraris J. D. et al., Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc. Natl. Acad. Sci. U.S.A. 99, 739–744 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DuMond J. F. et al., Peptide affinity analysis of proteins that bind to an unstructured NH2-terminal region of the osmoprotective transcription factor NFAT5. Physiol. Genomics 48, 290–305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuMond J. F., He Y., Burg M. B., Ferraris J. D., Expression, fermentation and purification of a predicted intrinsically disordered region of the transcription factor, NFAT5. Protein Expr. Purif. 115, 141–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumond J. F. et al., Peptide affinity analysis of proteins that bind to an unstructured region containing the transactivating domain of the osmoprotective transcription factor NFAT5. Physiol. Genomics 48, 835–849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyson H. J., Wright P. E., Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Wright P. E., Dyson H. J., Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 16, 18–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar R., McEwan I. J., Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation. Endocr. Rev. 33, 271–299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan S. H., Kumar R., An overview of the importance of conformational flexibility in gene regulation by the transcription factors. J. Biophys. 2009, 210485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garza A. S., Ahmad N., Kumar R., Role of intrinsically disordered protein regions/domains in transcriptional regulation. Life Sci. 84, 189–193 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Garza A. M., Khan S. H., Kumar R., Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Mol. Cell. Biol. 30, 220–230 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilser V. J., Thompson E. B., Intrinsic disorder as a mechanism to optimize allosteric coupling in proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 8311–8315 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan S. H. et al., Binding of the N-terminal region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations. J. Biol. Chem. 287, 44546–44560 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R. et al., Regulation of the structurally dynamic N-terminal domain of progesterone receptor by protein-induced folding. J. Biol. Chem. 288, 30285–30299 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan S. H., Arnott J. A., Kumar R., Naturally occurring osmolyte, trehalose induces functional conformation in an intrinsically disordered activation domain of glucocorticoid receptor. PLoS One 6, e19689 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R., Litwack G., Structural and functional relationships of the steroid hormone receptors’ N-terminal transactivation domain. Steroids 74, 877–883 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baskakov I. V. et al., Trimethylamine N-oxide-induced cooperative folding of an intrinsically unfolded transcription-activating fragment of human glucocorticoid receptor. J. Biol. Chem. 274, 10693–10696 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Garza A. S., Khan S. H., Moure C. M., Edwards D. P., Kumar R., Binding-folding induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain. PLoS One 6, e25875 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R. et al., Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J. Biol. Chem. 274, 24737–24741 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Kumar R., Lee J. C., Bolen D. W., Thompson E. B., The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J. Biol. Chem. 276, 18146–18152 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Bushueva T. L., Tonevitsky A. G., The effect of pH on the conformation and stability of the structure of plant toxin-ricin. FEBS Lett. 215, 155–159 (1987). [DOI] [PubMed] [Google Scholar]
- 39.Watanabe K., Honjo E., Tsukamoto T., Funatsu G., Fluorescence studies on the interaction of adenine with ricin A-chain. FEBS Lett. 304, 249–251 (1992). [DOI] [PubMed] [Google Scholar]
- 40.Holthauzen L. M., Bolen D. W., Mixed osmolytes: The degree to which one osmolyte affects the protein stabilizing ability of another. Protein Sci. 16, 293–298 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Rodríguez C. et al., Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. U.S.A. 101, 2392–2397 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyakawa H. et al., Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am. J. Physiol. 274, F753–F761 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Na K. Y., Woo S. K., Lee S. D., Kwon H. M., Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J. Am. Soc. Nephrol. 14, 283–288 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Ito T. et al., Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem. J. 382, 177–182 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo S. K., Lee S. D., Na K. Y., Park W. K., Kwon H. M., TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol. Cell. Biol. 22, 5753–5760 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakshmipathi J. et al., Identification of NFAT5 as a transcriptional regulator of the EDN1 gene in collecting duct. Am. J. Physiol. Renal Physiol. 316, F481–F487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Go W. Y., Liu X., Roti M. A., Liu F., Ho S. N., NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. U.S.A. 101, 10673–10678 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves R., Nuclear functions of the HMG proteins. Biochim. Biophys. Acta 1799, 3–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Perez A. et al., Molecular cloning of cDNA coding for kidney aldose reductase. Regulation of specific mRNA accumulation by NaCl-mediated osmotic stress. J. Biol. Chem. 264, 16815–16821 (1989). [PubMed] [Google Scholar]
- 50.Sheikh-Hamad D., García-Pérez A., Ferraris J. D., Peters E. M., Burg M. B., Induction of gene expression by heat shock versus osmotic stress. Am. J. Physiol. 267, F28–F34 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Ferraris J. D., Burg M. B., Williams C. K., Peters E. M., García-Pérez A., Betaine transporter cDNA cloning and effect of osmolytes on its mRNA induction. Am. J. Physiol. 270, C650–C654 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Nakayama Y., Peng T., Sands J. M., Bagnasco S. M., The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 275, 38275–38280 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Lanaspa M. A. et al., The expression of aquaporin-1 in the medulla of the kidney is dependent on the transcription factor associated with hypertonicity, TonEBP. J. Biol. Chem. 285, 31694–31703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam A. K. M. et al., Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J. Biol. Chem. 279, 48048–48054 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Hasler U. et al., Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J. Am. Soc. Nephrol. 17, 1521–1531 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Chen S. et al., Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J. Clin. Invest. 119, 1647–1658 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trama J., Lu Q., Hawley R. G., Ho S. N., The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J. Immunol. 165, 4884–4894 (2000). [DOI] [PubMed] [Google Scholar]
- 58.Khan S. H., Ahmad N., Ahmad F., Kumar R., Naturally occurring organic osmolytes: From cell physiology to disease prevention. IUBMB Life 62, 891–895 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Kumar R., Role of naturally occurring osmolytes in protein folding and stability. Arch. Biochem. Biophys. 491, 1–6 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Rumjanek F. D., Osmolyte induced tumorigenesis and metastasis: Interactions with intrinsically disordered proteins. Front. Oncol. 8, 353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansouri A. L. et al., Folding propensity of intrinsically disordered proteins by osmotic stress. Mol. Biosyst. 12, 3695–3701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tong E. H. et al., Regulation of nucleocytoplasmic trafficking of transcription factor OREBP/TonEBP/NFAT5. J. Biol. Chem. 281, 23870–23879 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Li J., Hilser V. J., Assessing allostery in intrinsically disordered proteins with ensemble allostery model. Methods Enzymol. 611, 531–557 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Liu J. et al., Intrinsic disorder in transcription factors. Biochemistry 45, 6873–6888 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayan V. et al., A multiprotein binding interface in an intrinsically disordered region of the tumor suppressor protein interferon regulatory factor-1. J. Biol. Chem. 286, 14291–14303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugase K., Dyson H. J., Wright P. E., Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447, 1021–1025 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Fujikane R., Komori K., Sekiguchi M., Hidaka M., Function of high-mobility group A proteins in the DNA damage signaling for the induction of apoptosis. Sci. Rep. 6, 31714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun J., Qiao Y., Song T., Wang H., MiR-495 suppresses cell proliferation by directly targeting HMGA2 in lung cancer. Mol. Med. Rep. 19, 1463–1470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Ayachi I. et al., The WNT10B network is associated with survival and metastases in chemoresistant triple-negative breast cancer. Cancer Res. 79, 982–993 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou Q. et al., RKIP suppresses the proliferation and metastasis of breast cancer cell lines through up-regulation of miR-185 targeting HMGA2. Arch. Biochem. Biophys. 610, 25–32 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Motlagh H. N., Wrabl J. O., Li J., Hilser V. J., The ensemble nature of allostery. Nature 508, 331–339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Motlagh H. N., Li J., Thompson E. B., Hilser V. J., Interplay between allostery and intrinsic disorder in an ensemble. Biochem. Soc. Trans. 40, 975–980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue B., Brown C. J., Dunker A. K., Uversky V. N., Intrinsically disordered regions of p53 family are highly diversified in evolution. Biochim. Biophys. Acta 1834, 725–738 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuznetsova I. M., Povarova O. I., Uversky V. N., Turoverov K. K., Native globular actin has a thermodynamically unstable quasi‐stationary structure with elements of intrinsic disorder. FEBS J. 283, 438–445 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Uversky V. N., p53 proteoforms and intrinsic disorder: An illustration of the protein structure-function continuum concept. Int. J. Mol. Sci. 17, 1874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kulkarni P. et al., Phosphorylation-induced conformational dynamics in an intrinsically disordered protein and potential role in phenotypic heterogeneity. Proc. Natl. Acad. Sci. U.S.A. 114, E2644–E2653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen S., Wang X., Nisar M. F., Lin M., Zhong J. L., Heme oxygenases: Cellular multifunctional and protective molecules against UV-induced oxidative stress. Oxid. Med. Cell. Longev. 2019, 5416728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valera-Alberni M., Canto C., Mitochondrial stress management: A dynamic journey. Cell Stress 2, 253–274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dokladny K., Myers O. B., Moseley P. L., Heat shock response and autophagy–Cooperation and control. Autophagy 11, 200–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rashid H. O., Yadav R. K., Kim H. R., Chae H. J., ER stress: Autophagy induction, inhibition and selection. Autophagy 11, 1956–1977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.