Significance
In humans and other social animals, early life adversity and weak social relationships can both alter adult stress responses. The causal connections between these variables are debated; resolving this debate requires data on all three variables in the same individuals. Here, we unite these components in wild female baboons. We find that early adversity elevates adult females’ glucocorticoid levels, one measure of the stress response. This effect was largely explained by the direct effect of early adversity on glucocorticoids and was not mediated by poor social relationships in adulthood. Hence, early adversity and adult social relationships appear to have independent effects on adult stress responses.
Keywords: stress response, developmental origins of health and disease, causal inference, social relationships, HPA axis
Abstract
In humans and other animals, harsh conditions in early life can have profound effects on adult physiology, including the stress response. This relationship may be mediated by a lack of supportive relationships in adulthood. That is, early life adversity may inhibit the formation of supportive social ties, and weak social support is itself often linked to dysregulated stress responses. Here, we use prospective, longitudinal data from wild baboons in Kenya to test the links between early adversity, adult social bonds, and adult fecal glucocorticoid hormone concentrations (a measure of hypothalamic–pituitary–adrenal [HPA] axis activation and the stress response). Using a causal inference framework, we found that experiencing one or more sources of early adversity led to a 9 to 14% increase in females’ glucocorticoid concentrations across adulthood. However, these effects were not mediated by weak social bonds: The direct effects of early adversity on adult glucocorticoid concentrations were 11 times stronger than the effects mediated by social bonds. This pattern occurred, in part, because the effect of social bonds on glucocorticoids was weak compared to the powerful effects of early adversity on glucocorticoid levels in adulthood. Hence, in female baboons, weak social bonds in adulthood are not enough to explain the effects of early adversity on glucocorticoid concentrations. Together, our results support the well-established notions that early adversity and weak social bonds both predict poor adult health. However, the magnitudes of these two effects differ considerably, and they may act independently of one another.
Both early life experiences and social environments during adulthood can have profound effects on individual health and survival. Children who experience socioenvironmental traumas—from famine to parental loss to abuse—fare worse on a wide range of health outcomes in adulthood (1–9). They are also disproportionately likely to find themselves in negative social environments as adults, including having dysfunctional or unsupportive relationships, or being socially isolated (10–12). Likewise, characteristics of adult social environments, including social support and social status, have repeatedly been linked to adult health and longevity (13).
These observations have led to multiple hypotheses, all of which propose that adult social environments could contribute to the negative health effects of early adversity. For instance, the social causation hypothesis posits a causal effect of adult social environments on health (14–17); the social buffering hypothesis proposes that supportive social relationships modulate the impact of stressful events (18, 19); and, relevant to early life effects, aspects of the biological embedding hypothesis posit that social dysregulation can exacerbate health problems in adulthood (9, 12, 20). Under this model, harsh conditions in early life program a highly “reactive” phenotype, one symptom of which is hypothalamic–pituitary–adrenal (HPA) axis dysfunction (12, 21, 22). While this phenotype may help children cope with immediate challenges, it could have costly long-term consequences for responsiveness to social and environmental stressors, and for disease burden in adulthood.
While some predictions of these hypotheses have been supported (22–25), studies that measure early life adversity, adult social environments, and adult health outcomes in the same subjects are rare (but see ref. 26). Measuring them together in the same population, and in the same individuals over their life spans, is important for two reasons. First, in isolation, relationships between any two of the three variables may be consistent with multiple models that explicitly link them. For instance, the social causation hypothesis, the social buffering hypothesis, and the biological embedding hypothesis all predict that poor social conditions lead to poor health outcomes. Second, testing the pieces independently fails to capture critical mechanistic information: namely, the mediating effect that social relationships may have on the connection between early experiences and adult health (Fig. 1). Filling this gap for human subjects is challenging because it is difficult to measure early adversity in real time, and to connect these experiences to relationships and to health measures across adulthood.
Fig. 1.
Early adversity has negative effects on adult health outcomes in many species, including humans (solid arrow). However, early adversity is also frequently associated with social dysfunction (e.g., greater isolation or less supportive relationships) across the life course, which may itself contribute to negative health outcomes. The dashed arrows represent the proposed mediating effect of weak social bonds on the relationship between early adversity and adult health.
Animal models can help to fill this gap. While the connections between early adversity, social bonds, and health have been most thoroughly explored in humans, similar effects are widely documented in other mammals, birds, reptiles, amphibians, and invertebrates (reviewed in ref. 27). This ubiquity suggests that the mechanisms that link these variables have deep biological roots. Nonhuman primates are particularly well suited to testing predictions derived from theoretical models of human health and behavior. Like humans, they are highly social and have slow life histories; they can face a variety of social or ecological forms of early life adversity; extensive evidence indicates that such adversity produces negative health and behavioral outcomes (28–31); and primatology’s traditional focus on long-term, individual-based data can provide the prerequisite longitudinal data that span the life course (32). Primates in the wild, rather than in captivity, are especially valuable for testing evolutionary hypotheses because they are exposed to selective pressures under which their (and to some degree our own) species evolved.
Here, we test whether the links between early life adversity, the strength of adult social bonds, and adult HPA axis activity are consistent with the idea that social bonds in adulthood mediate a relationship between early experiences and adult stress responses. To do this, we use data from 192 female baboons who were studied from birth by the Amboseli Baboon Research Project in Kenya (32, 33). Founded in 1971, the Amboseli Baboon Research Project has prospective longitudinal data on early life experiences, and fine-grained longitudinal data on adult social bonds and glucocorticoid (GC) hormone levels (a measure of HPA axis activation and the “stress response”).
Importantly, prior research in Amboseli found that female baboons who experienced three or more sources of early adversity have lifespans which are >50% shorter, on average, than their peers who experienced no known sources of adversity (29). The six sources of early adversity analyzed in (29) are as follows: 1) drought in the first year of life; 2) the presence of a competing younger sibling that may divert maternal attention; 3) high population density; 4) maternal death; 5) being born to a low-ranking mother; and 6) being born to a mother who is socially isolated (Table 1). This same paper also showed that females who experienced more sources of early adversity were more socially isolated from other females in adulthood than those who experienced fewer adversities, although they were not more isolated from males. Additionally, an earlier, separate analysis found that social relationships in adulthood predicted female longevity (34): Greater “social connectedness” to both adult females and males was associated with a 34 to 45% reduced risk of death in any given year, compared to females with lower social connectedness. Together, these observations suggest that early adversity may influence these animals’ ability to form or maintain close social relationships, and that low social connectedness to either males or females may be detrimental for adult females’ health.
Table 1.
Sources of early life adversity for Amboseli baboons and the number of subjects in our study who experienced each source of adversity
| Type of adversity | Definition | Females that did not experience the adversity | Females that experienced the adversity | Total females |
| Drought | <200 mm of rain during the first year of life | 164 | 28 | 192 |
| Competing sibling | Birth of a sibling <1.5 y after the subject’s birth (lowest quartile of interbirth intervals) | 153 | 39 | 192 |
| High group density | Group size in the top quartile of the population (>33 individuals) on the day the subject was born | 161 | 31 | 192 |
| Maternal loss | Death of the subject’s mother before the subject reached age 4 y (the approximate age of reproductive maturity for females) | 157 | 35 | 192 |
| Low maternal rank | Mother was in the lowest quartile of proportional dominance ranks on the day the subject was born | 152 | 40 | 192 |
| Maternal social isolation | Average maternal social bond strength was in the lowest quartile over the first 2 y of the subject’s life | 140 | 52 | 192 |
Early life circumstances and adult social bonds are also linked to HPA axis activity in baboons and other social species (35, 36). Since the HPA axis plays an important regulatory role in many aspects of physiology, from immune function to metabolism to circadian rhythm (37–39), and is highly conserved across vertebrate taxa (40), it is a plausible common pathway through which the myriad outcomes associated with early adversity might emerge. Here, we focus on GCs, a class of steroid hormones that are key indicators of HPA axis activity. They have been connected to a variety of types of early adversity in many different species (9, 41–45) (but see refs. 46 and 47 for alternative perspectives). They are an appropriate biomarker to use in this context for two reasons: First, some hypotheses propose HPA axis regulation as a mechanism by which early adversity “embeds” itself (9, 12); and second, because GCs are themselves a biologically relevant indicator of adult health status in this baboon population. Specifically, controlling for a range of environmental, social, and individual challenges experienced by an adult female, having higher fecal GC (fGC) concentrations is linked to significantly increased risk of mortality (48).
One advance in the present study is that both fGCs and females’ social bonds are measured throughout all available adult years of the subjects’ lives. We evaluate females’ social bonds both with other adult females and with adult males; we treat these bonds separately because prior work indicates they respond differently to early adversity, and have somewhat different relationships with female survival (29, 34). Social bond strength with females and males are measured annually, and fGCs are also measured repeatedly [n = 9,863 total samples, with a mean of 51.4 samples from our 192 subjects (33)]. While these longitudinal data are ideal for evaluating our predictions, they also present modeling challenges for established methods of mediation analysis. Here, we use a statistical approach (49) that leverages functional principal components analysis (FPCA) to reduce the dimensionality of these longitudinal trajectories—for both social bond strength and fGC concentrations across adulthood—and uses the resulting principal component scores in a well-established mediation analysis framework (50). We use this modeling strategy to test four predictions: 1) females who experienced early adversity will have higher fGC concentrations in adulthood than those who did not; 2) females who experienced early adversity will have weaker social bonds in adulthood than females who did not; 3) part of the relationship between early adversity and elevated fGC concentrations will be mediated through weak social bonds; and 4) independent of early adversity, females who have weaker social bonds in adulthood will have higher fGC concentrations in adulthood.
Testing these predictions together provides insight into the pathways by which early adversity and fGCs may be connected. If weak social bonds in adulthood are linked with elevated fGCs, and if animals who experienced early adversity are less likely to make and maintain bonds, then the connection between early adversity and this measure of the stress response may primarily run through social bonds. Alternatively, early adversity may not have strong effects on fGCs, or any negative effect of social bonds on health may be too small to compensate for early adversity. Either way, our analysis represents a comprehensive test of the relationships among early experience, adult social bonds, and a measure of adult stress responses in a single system, and introduces a useful statistical approach for using longitudinal data in the context of mediation modeling.
Mediation Model Structure and Assumptions
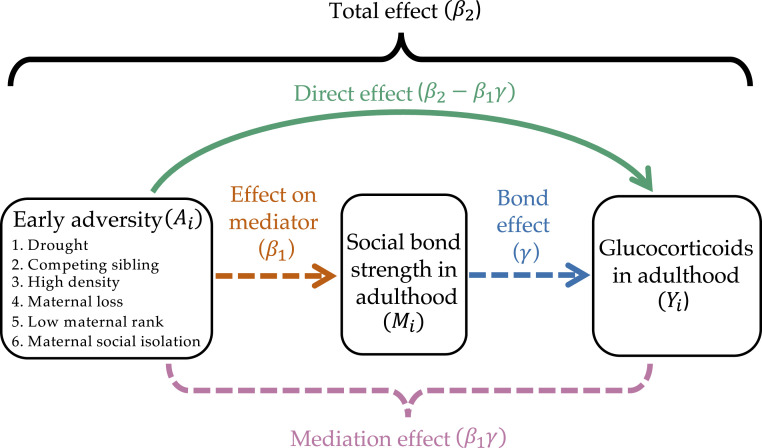
We begin by briefly summarizing the mediation modeling framework we used, because familiarity with the associated structure and terminology are necessary to interpret our results. Mediation modeling provides information about different pathways by which a third variable might influence a relationship between a predictor and an outcome. The underlying assumption of the mediation framework is that the “total effect” of early life adversity on fGCs (outcome ) may be broken down into two separate pathways: one that runs through a female’s social bond strength (mediator ; the “mediation effect” in Fig. 2), and one that goes directly between the two variables (the “direct effect” in Fig. 2) (50, 51). We tested the mediating effects of two types of social bonds: those females have with other females, and those they have with adult males. These social bond measures capture the strength of each female’s top three social bonds—with adult females and adult males separately—relative to all other females alive in the population at the same time (see Materials and Methods for details).
Fig. 2.
Visual representation of the modeling framework used to test the relationships among early adversity, social bond strength, and glucocorticoid (GC) concentrations. Mediation models provide estimates of the total effect, or “global” relationship between early adversity and GCs , which are subdivided into estimates of the mediation effect and direct effect . , the relationship between early adversity and social bond strength is the effect (of early adversity) on the mediator (the dashed orange arrow), while is the bond effect (i.e., the effect of weak social bonds on adult GC concentrations, independent of early adversity; this is the dashed blue arrow). Collectively, the effect on the mediator and the bond effect make up the mediation effect, encompassed by the pink bracket. The colors of the arrows in the diagram correspond to the colors of the column headings in the results tables; for example, the green direct effect arrow is the pathway represented in the green direct effect column in Tables 2 and 3. The dashed arrows represent the causal pathways captured by the dashed mediation effect bracket. The colors in this figure also correspond to the visual representation of these effects in Figs. 3 and 4. Information on our sources of early adversity can be found in Table 1, and information on measuring social bond strength and GC concentrations can be found in Materials and Methods.
To estimate the effect of on and the mediation effect of , we fit three equations, simplified below for clarity (full versions are in SI Appendix). The first equation characterizes the relationship between early adversity and the mediator , conditional on covariates (variables known to influence social bond strength, but that are independent of early adversity; see Materials and Methods, Control variables). Individual baboons (i) have multiple observations , indexed on their age at the time of observation. The “effect on the mediator” from this equation (the dashed orange arrow in Fig. 2) answers the question: What is the relationship between early adversity and social bond strength?
| [1] |
The second equation captures the total effect (i.e., the relationship between and ) conditional on covariates (covariates known to influence fGCs, but that are independent of early adversity; see Materials and Methods, Control variables), but does not differentiate between the part of the relationship that runs through mediator (social bond strength) vs. the part that is due to a direct relationship. The total effect from this equation (the black bracket in Fig. 2) answers the question: What is the relationship between early adversity and fGCs?
| [2] |
The third equation is identical to the second, except that it also conditions on the values of the mediator :
| [3] |
(from Eq. 1) multiplied by (from Eq. 3) answers the question: How much of the relationship between early adversity and fGCs is imposed through weak social bonds? That is, it estimates the size of the mediation effect. In contrast, estimates the relationship between social bond strength and fGCs, independent of early adversity (the “bond effect”; dashed blue arrow in Fig. 2).
Results
Early Adversity Is Linked to Elevated Glucocorticoids Across Adulthood.
We began by testing the total effect ( black bracket in Fig. 2) of early adversity on females’ fGC concentrations across adulthood [n = 9,863 fecal samples from 192 females (33)], focusing on six different adversity sources and their cumulative effects (Table 1 and Fig. 2). In support of our first prediction, we found a strong total effect of early adversity on females’ fGC concentrations across adulthood (Tables 2 and 3 and Fig. 3). Females who experienced one or more sources of adversity had fGC concentrations that were ∼9% higher than their peers who did not experience any adversity (cumulative adversity (0 vs. 1+) models in Tables 2 and 3). Similarly, females who experienced two or more sources had fGC concentrations that were roughly 14% higher than females who experienced only one source (cumulative adversity [1 vs. 2] models in Tables 2 and 3), and 21% higher than females who experienced no adversity (model in SI Appendix, Table S1).
Table 2.
Effect sizes and 95% CIs (in brackets) for models that test the mediation effect of social bonds with females on fGC concentrations
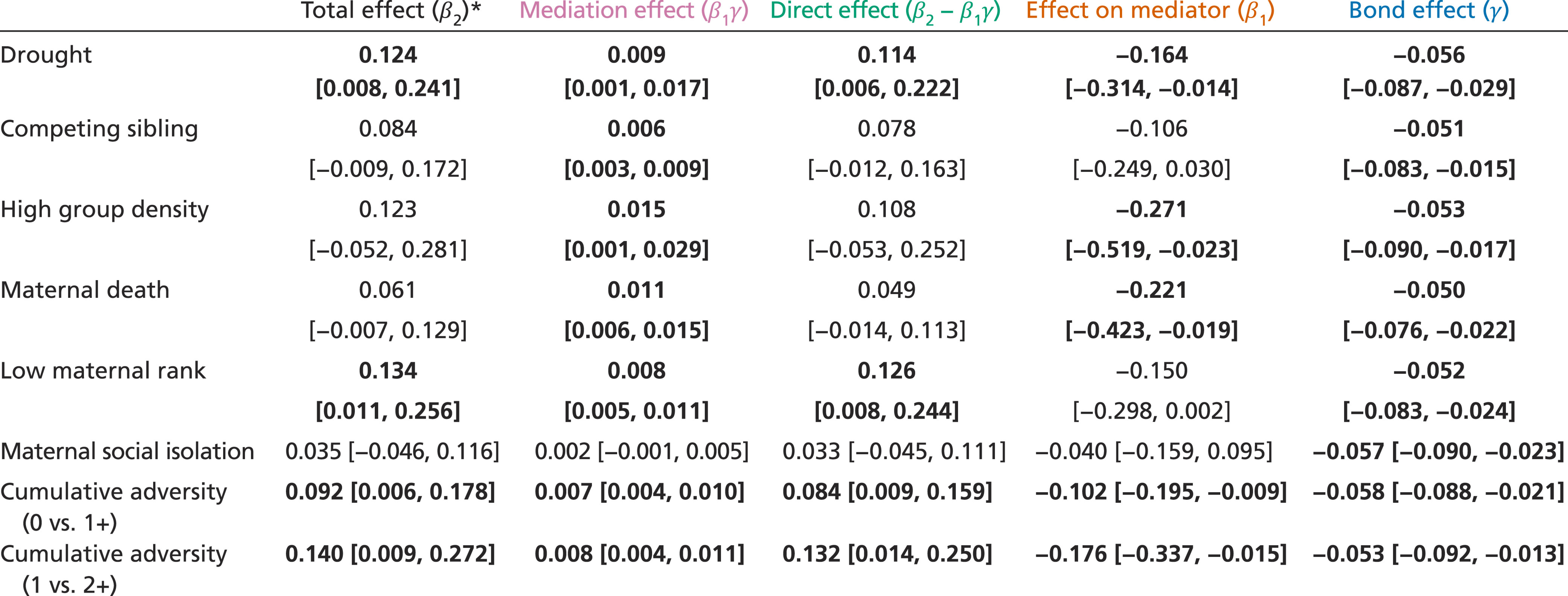 |
Table 3.
Effect sizes and 95% CIs (in brackets) for models that test the mediation effect of bonds with males on fGC concentrations
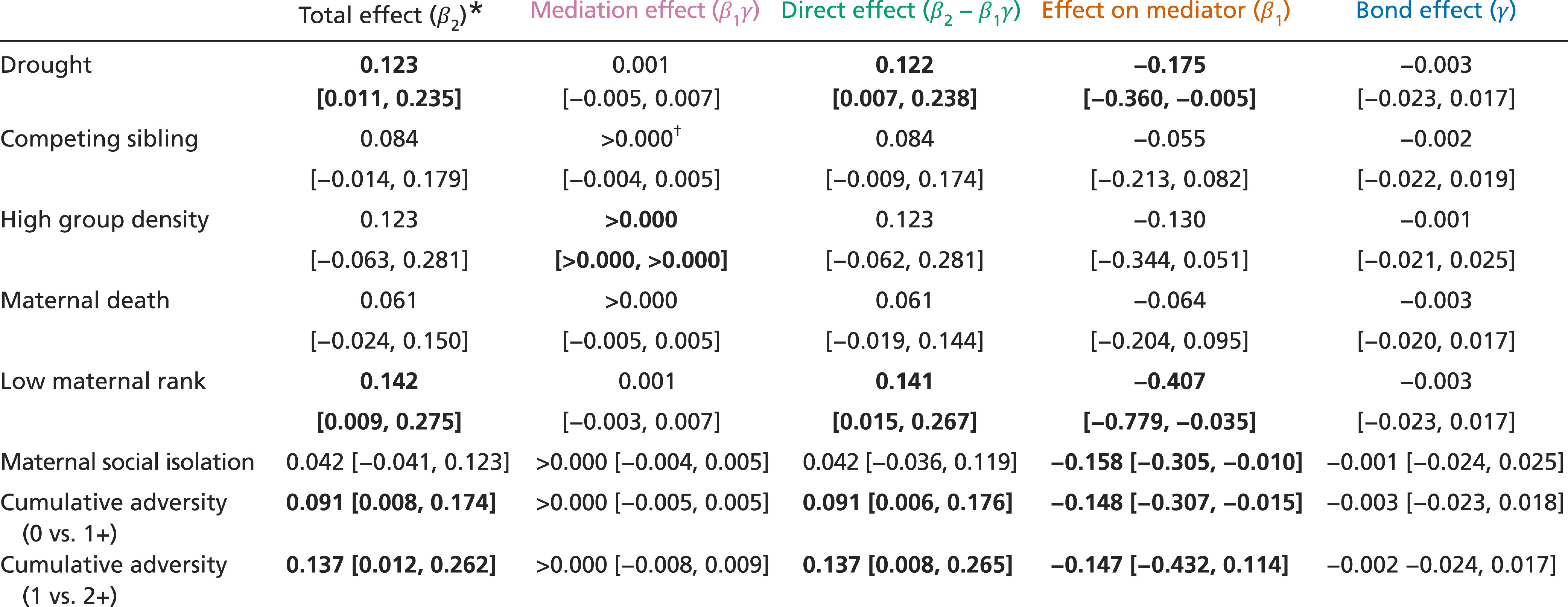 |
Entries in bold have 95% CIs that exclude zero.
The column headings in Table 3 match the color of the relationship arrow they correspond to in Fig. 2, and the visual representation of the effect on mediator result in Fig. 4.
Estimates of zero that include > or < indicate whether the direction of the effect was positive or negative. In these cases, the effect size was small enough that rounding to the nearest thousandth means there are no visible nonzero digits.
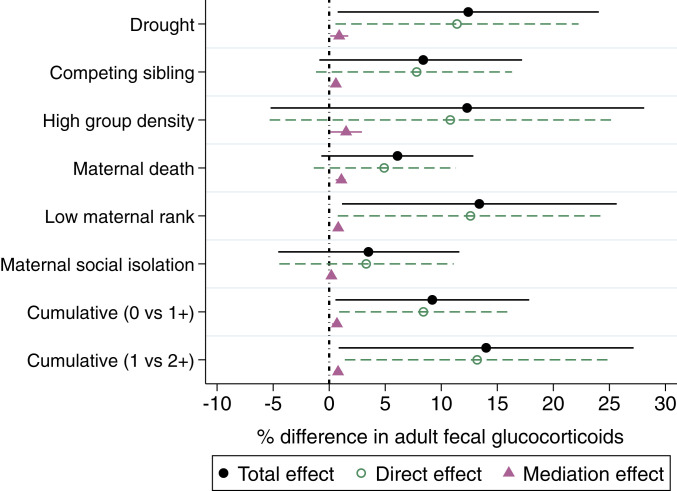
Fig. 3.
Effect sizes and CIs for total, direct, and mediation effects of early adversity on adult fGC concentrations, from models testing social bond strength with adult females as the mediator variable. The colors associated with the different types of effects correspond to the colors of the pathway arrows in Fig. 2, and the column headings in Table 2. Black (filled circles) indicates the total effect of early adversity on adult fGCs; green (open circles) indicates the direct effect that does not pass through the mediator, and pink (triangles) represents the mediation effect, where social bond strength with other females is the mediator.
Although the range of total effect sizes across all 16 models in Tables 2 and 3 varied from 4 to 14%, the results were always directionally consistent toward higher fGCs, even in the models in which the CI included zero (Fig. 3). Among our individual sources of adversity, females who were born during a drought, into a high-density group, or to a low-ranking mother had especially elevated fGC concentrations (12 to 14%) in adulthood, although the CI in the model of high group density included zero (Fig. 3).
Weak Social Bonds Play a Minor Role in the Link between Early Adversity and Elevated fGCs.
While females who experienced early adversity exhibited elevated fGCs across adulthood, we found no evidence that these effects were strongly mediated by the absence of strong social bonds, either with adult females or with adult males (prediction 2). The mediation effect (; dashed pink bracket in Fig. 2) was consistently small. The strength of females’ social bonds with other females accounted for a difference in fGCs of only 0.85% when averaged across the six individual adversity models in Table 2 (pink column heading), even though the CIs did not include zero for five of the six individual sources of adversity, as well as for the two models of cumulative adversity (Table 2 and Fig. 3).
When averaged across the six models measuring individual sources of adversity in Table 2, the direct effect of early adversity on adult GCs was 11.6 times stronger than the mediation effect running through social bonds with adult females (Fig. 3; mediation [pink] vs. direct [green] effect in Table 2). For example, for females who experienced one or more sources of early adversity, the direct effect explained an increase in fGCs of 8.4%, while the mediation effect only accounted for an increase in fGCs of 0.7%, compared to females that did not experience any sources of early adversity. The mediation effect running through social bonds with adult males was negligible. Across all models, we found no evidence that weaker social bonds with males contributed to the negative effects of early adversity on female fGC concentrations (mediation effect in Table 3; averaged across the six models that evaluated individual sources of adversity, effect size = 0.04% difference in fGCs).
Early Adversity Has Modest Effects on Social Bond Strength, and Social Bond Strength Has Modest Effects on fGC Concentrations.
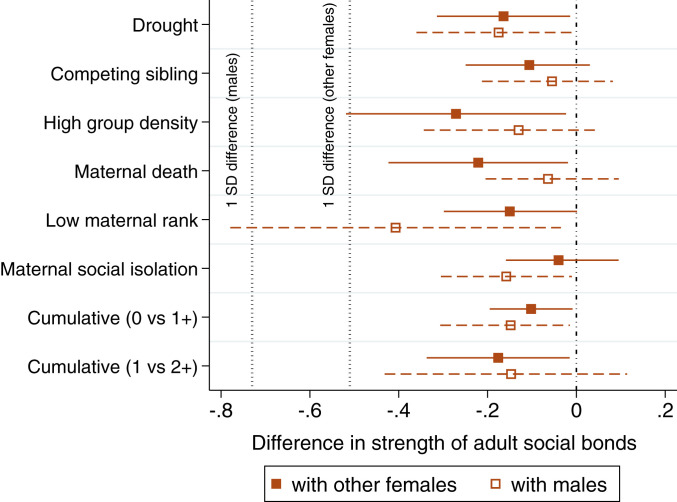
Two patterns in our models point to why the mediation effect, which runs through social bonds with females or males, is weak compared to the direct effect of early adversity on adult fGC concentrations. First, in support of our second prediction, the experience of early adversity was linked to weaker social bonds across adulthood, but the effect sizes were modest (effect on the mediator; dashed orange arrow in Fig. 2 and orange column heading in Tables 2 and 3). For instance, when averaging the effects across the six models that tested each individual source of adversity, females who experienced a given adversity were, on average, 0.16 units less socially connected with other females than peers who did not experience that adversity (Table 2 and Fig. 4). A 0.16-unit decrease in social bond strength represents just 31% of 1 SD of female–female social bond strength (±1 SD difference in social bond strength to other females = 0.51 units; 0.16/0.51 = 31%; see SI Appendix, Fig. S1 for an illustration of the effect size in the context of female social bonds). Hence, the effects of early adversity on female bond strength with other females are small compared to the overall variation in female social bonds. The weakest female–female bonds were observed in females who were born into high-density conditions, or lost their mothers before age 4 (Fig. 4).
Fig. 4.
Effect sizes and CIs for the relationship between early adversity and social bond strength with adult females (filled squares and solid lines), and adult males (open squares and dashed lines). These effects correspond to the pathway of the same color, labeled “effect on mediator” in Fig. 2, and to the corresponding columns in Tables 2 and 3. The dashed black vertical lines represent a 1 SD difference in adult social bond strength to other females (0.51 units), and to males (0.73 units).
Females who experienced early adversity also exhibited weaker bonds with adult males in adulthood, but the effect sizes were smaller than for bonds with adult females (effect on the mediator; Table 3). Although females who experienced any source of early adversity were also, on average, 0.16 units less strongly socially bonded with males, this represented just 22% of 1 SD of female bond strength with males (1 SD difference in social bond strength with males = 0.73; 0.16/0.73 = 22%). The source of adversity linked to the weakest female–male bonds was being born to a mother who held a low dominance rank (56% of 1 SD; Table 3 and Fig. 4).
A second reason why the mediation effect was weak compared to the direct effect is that we observed only a modest bond effect (blue arrow in Fig. 2, blue column heading in Tables 2 and 3). Specifically, in support of prediction four, females with weak social bonds to adult females had higher fGC concentrations across adulthood (bond effect in Table 2), but these effects were small compared to the direct effect of early adversity on fGCs. Given these effect sizes, in order to accomplish an 8.4% rise in fGCs, female social bond strength would need to drop in response to early adversity by 2.8 SDs (i.e., a one-unit decline in social bond strength predicts only a 5.8% increase in fGC concentrations; a one-unit change in female social bond strength corresponds to 2 SDs of this variable). This modest bond effect may also mean that the potential for social buffering (i.e., for social bonds to alleviate the effects of early adversity on social ties) are limited, but a detailed analysis of the potential for this kind of buffering is beyond the scope of this analysis.
Additionally, the bond effect with males was negligible (Table 3): We found no evidence that weaker social bonds with adult males led to elevated fGCs (bond effect, Table 3). This lack of a bond effect explains why female–male social bonds do not mediate the relationship between early adversity and fGCs (mediation effect, Table 3). If social bond strength with males does not predict lower fGC concentrations, then these relationships cannot be a pathway through which early adversity and fGC concentrations are linked.
Discussion
The well-established connections between early adversity and poor health, and negative adult social environments and poor health, suggest that aspects of adult sociality could play a substantial mediating role in the connection between early adversity and poor adult health (e.g., refs. 8, 9, 13 and 26). This may include HPA axis dysregulation in adulthood and its concomitant health risks (12). Here, we measured the mediating role of social bonds, comparing the direct effects of early adversity to the mediating effects of weak social bonds on HPA axis activation across adulthood. We find that in wild female baboons, the effects of early adversity on adult GC concentrations are powerful, and that the vast majority of this relationship can be explained by the direct effect of early adversity itself, rather than by the mediating effect of weak social bonds in adulthood. Baboons are highly social primates in which prior tests have found that strong social bonds are linked to longevity (29, 34) and regulate the physiological response to stressors (35, 36, 52). Our findings indicate that in baboons, and perhaps other social animals, the social environment is not a powerful mediator of the negative effects of early adversity on GC concentrations. They also highlight the potential dangers of assuming that independent tests of individual predictions will yield the full story of the relationships among more than two variables. Our data are consistent with the well-established notions that early adversity is bad for health, and that weak social bonds can mediate these effects. However, our findings show that the magnitude of the effects can differ considerably, and they may even largely be independent of one another.
Our results are also consistent with many studies on human subjects that find connections between early adversity and various measures of altered HPA axis activity, including differences in GC reactivity, GC secretion patterns, and circadian rhythm dynamics (9, 53, 54). They also point toward the possibility that HPA axis activity plays a role in the relationship between early adversity and life span in baboons. Across models, females who experienced early adversity had 9 to 14% higher fGC concentrations, across their adult lives, than females who did not experience early adversity. This result suggests that these animals contend with chronically elevated HPA axis activity (i.e., chronic stress), a physiological response associated with a variety of potentially fitness-reducing costs such as immune suppression, poor inflammatory control, and less efficient energy expenditure (55). Health effects like these may help explain why a separate analysis in our study population found that relatively high fGC concentrations were associated with shorter life spans in adult female baboons, after adjusting for other factors that influence fGCs (48). In the aggregate, our data indicate that HPA axis function is a plausible contributor to the relationship between early adversity and shorter life spans in these baboons.
The observation that, for female baboons, the direct effect of early adversity on HPA axis activation is 11 times stronger than the mediating effect of weak social bonds is interesting in light of prior studies in humans, baboons, and many other social mammals that find that greater isolation (including weak social bonds) is linked to elevated GCs (56–59). Consistent with these studies, we found that female baboons with weaker social bonds had elevated fGCs, but these effects were too small to contribute substantially to the link between early adversity and HPA axis activation across adulthood. Intriguingly, social bonds with adult males did not predict fGC concentrations, even though bonds with adult females did. While female–female social bonds form the backbone of baboon societies (60–63), females’ relationships with males also predict survival (34), and male–female bonds provide mothers and their infants protection against harassment by other baboons (64–66). In wild olive baboons, lactating females without close male social partners have higher fGCs than those who do (67). It may be that the lack of a relationship between male–female bond strength and female fGC concentrations in our data reflect the more short-lived nature of male–female bonds, compared to female–female bonds. Male–female bonds are strongest when females are nursing young infants and may otherwise be transient (65, 67). Because our data focus on lifetime measures, rather than specific cross-sectional time windows, any temporary impact of male–female social bonds may be harder to detect.
The weak effect of social bonds on adult fGC concentrations is only part of the reason that bonds did not meaningfully mediate the early adversity–fGC relationship in our data. We also found that while early adversity did lead to weaker social bonds across adulthood, the size of this effect was modest. Females that experienced early adversity had social bonds with other females that were less than a third of a SD weaker than those who did not. For their bonds with males, the effect size was just over a fifth of a SD. These effects would need to be much larger for bond strength to mediate the relationship between early adversity and fGCs.
It was also clear that the link between early adversity and adult bond strength varied among sources of early adversity, confirming and extending two interesting observations about baboon social relationships. First, while females whose mothers died when they were young had weaker social bonds with other females, this was not true of their bonds with males. Since mothers are key social partners for female baboons (68, 69), females who experience maternal loss may have weaker bonds with other females, because they cannot replace this important female–female bond. Furthermore, their mothers cannot facilitate bonds with other females, including bonds with maternal sisters (62, 68, 69). Previous work indicates that social bonds between adult maternal sisters get stronger after their mother dies (68), but if the mother dies when one or more of her daughters are especially young, the loss might inhibit the young female’s ability to form bonds with her sisters in the first place. In addition, while mothers may facilitate their daughters’ relationships with other females, they may not play this same role for their daughters' connections to males. In baboons, males regularly move between social groups (70), so mothers might be limited in their ability to serve as “bridges” between their daughters and a regularly rotating cast of males. Moreover, mothers’ own relationships to males may be unstable or short term, limiting their ability to act as facilitators. In olive baboons, close female relatives rarely share a common most-preferred male social partner (71), also suggesting that female–female social network benefits do not necessarily extend to male–female networks.
Second, while maternal death was not an important predictor of females’ social bonds with males, maternal dominance rank (and by extension subject rank, since rank is “inherited” in this species) was. Low maternal dominance rank was by far the strongest predictor of weak social bonds with males. Females whose mothers were in the bottom dominance rank quartile had social bond strength values that were more than one-half of a SD lower than their counterparts whose mothers were higher ranking. This result supports previous findings that male social partners represent a resource over which females compete, and that low-ranking females are less effective competitors (34, 64, 72). It also raises the interesting possibility that females may not work to facilitate their daughters' relationships with males even if they can, because once their daughters mature, they could theoretically compete for the attention of their mother’s preferred male social partner(s) (72).
Our findings on the relationships among early adversity, social bonds, and fGC concentrations in wild baboons are consistent with observations in many social species that early adversity and weak relationships both predict poor health, and that early adversity predicts various forms of social dysfunction, including weaker relationships. However, they call into question the notion that social bonds play a major role in mediating the effect of early adversity on poor health. In wild female baboons, any such effect appears to be functionally biologically irrelevant, and what little exists is limited strictly to their relationships with other females. More work will be needed to determine what alternative pathways (for example, resource competition) might connect early adversity to health and longevity in this species, as well to as establish whether the mediating effects of social bonds are similarly small in systems with different socioecologies.
Materials and Methods
Study Subjects and Population.
Our subjects were wild female savannah baboons (primarily Papio cynocephalus with some naturally occurring admixture from neighboring Papio anubis populations) monitored by the Amboseli Baboon Research Project in the Amboseli ecosystem, Kenya (32). We used longitudinal demographic, ecological, life history, and behavioral data collected from known females on a near-daily basis since 1994, as well as hormone metabolite data collected since 2000 (33). Each female subject (n = 192) was at least 4 y old. We had complete information on her experience of six well-characterized sources of early adversity (Table 1) (29, 73), as well as information on her adult social bonds and fGC concentrations. Methods for measuring early adversity and other variables are described below. Over the 19-y time span of the data used in this study (1998 to 2017), these subjects lived in 12 different social groups, all of which were fission products of two study groups observed since 1971 and 1980, respectively. Males are not included as subjects in our analyses because they disperse around sexual maturity (70); hence data are often missing for male social bonds and hormone concentrations.
Measuring Early Adversity.
Following ref. 29, we used six sources of early adversity (Table 1). For each source, we tested its relationship to outcome variables both independently and cumulatively. For the cumulative analyses, we summed the number of distinct sources of early adversity experienced by each female subject (mean, 1.17 sources of adversity; SD, 0.94; range, 0 to 5 adversities). Four of our sources were derived from continuously distributed variables (high group density, low maternal social rank, competing younger sibling, and maternal social isolation). For these, the presence of adversity was defined as the subject falling in the worst quartile in the population (i.e., the top quartile of group densities, and the lowest quartiles of maternal dominance rank, interbirth intervals, and our social bond strength measure, defined below). The quartile cutoffs were based on all females that attained 4 y of age over the history of the project (n = 627); hence the proportion of subjects in this dataset that experienced each adverse circumstance may differ from 0.25.
Longitudinal Measurement of fGCs.
We measured GC concentrations in 9,863 fecal samples collected opportunistically from our 192 subjects. On average, we had 51.36 fGC measures from each female (SD, 48.43; range, 3 to 288 samples per subject), which were collected on average across 5.49 y of their lives (SD, 3.95; range, 0.01 to 13.75 y). The mean age at sample collection was 9.49 y (SD, 3.56; range, 4.03 to 17.99). Compared to GC measurements from plasma, blood, or saliva, fGCs provide an integrated measure of GC concentrations over a period of hours to days (74). As such, fGCs do not capture circadian changes in GCs or short-term responses to stress. Instead, considered together as longitudinal trajectories across adulthood, fGCs synthesize several patterns of HPA axis dysregulation, including chronically elevated GCs, flattened circadian GC patterns (i.e., lack of decline after the morning rise in GCs), and slow recovery after exposure to stressors (74).
After collection in the field, samples were freeze dried, sifted to remove undigested vegetation, and shipped to the United States. In the United States, the samples were extracted, purified, and analyzed via radioimmunoassay, using well-established, previously described protocols and commercially available corticosterone kits that have been validated for use in this baboon population (75–77). As expected, fGC concentrations were not normally distributed, so we log-transformed the values for use in our models.
Longitudinal Measurement of Social Bond Strength.
We measured the strength of females’ social bonds with both adult females and adult males across adulthood. Our measure captures the bond strength of each female’s top three social bonds—with adult females and adult males separately—relative to all other females alive in the population at the same time. Following previous studies, our measure of bond strength is based on observations of dyadic grooming interactions, which are the most common form of affiliative behavior in baboons and many other primates (60, 68, 78, 79).
Grooming interactions were collected via representative interaction sampling, during which data collectors move through a baboon group recording all visible observations of grooming, while conducting 10-min focal animal follows on a randomized, preselected list of females. We used these grooming observations to first calculate a “dyadic sociality index” (DSI) (68) that measures bond strength between each pair of adult animals in each group in a given year. We first calculated the dyadic grooming rate as follows:
| [4] |
where is the number of observed grooming interactions in a given period between the dyad, is the number of days in the year that the dyad partners were coresident in the same social group, and identifies a given dyad. To account for variation in observer effort, which arises as a result of different sampling intensity in different-sized groups (groups with fewer females receive more per-female observer effort), we operationalize observer effort as follows:
| [5] |
where is the number of focal animal samples collected during the coresidence period, and is the average number of females in the group on those days. Groups with fewer females or more behavioral samples will have higher observer effort values. Finally, we fit the regression:
| [6] |
where is the slope associated with observer effort for all dyads of a given type (i.e., female–female dyads, or male–female dyads) in a given year . Using this “universal” slope for all dyads of a given type, rather than relying on the slope generated by any specific individual, helps correct for biases in observer effort that are a by-product of either especially small groups, or groups who were observed few times in a given period.
The residuals of this regression are the DSI values for all dyads, which are then z-scored within years to account for year-to-year variability in overall sociality. A DSI of zero for a given dyad means that the dyad in question exhibited the expected amount of grooming (neither unusually high nor unusually low), given the population’s overall dyadic grooming rate during that time window and the observer effort for that particular group (SI Appendix, Fig. S1). We calculated two measures of social bond strength for each female: Her bond strength with other adult females, and her bond strength with adult males, by averaging the values of her three highest DSI scores with females and males, respectively. Lower numbers mean that a female has weaker social bonds, while higher numbers mean that she has stronger social bonds.
For analysis purposes, we calculated each female’s measure of social bond strength over the year prior to each of her fGC samples. Thus, each fGC sample had a specific social bond strength value associated with it. For example, an fGC sample collected on December 31, 2016, is associated with a social bond strength measure calculated from all of the female’s observed grooming interactions between January 1 and December 31 of 2016. Rarely, a female had fewer than three grooming partners of a given sex during a given year. In these instances, we divided her summed DSI scores by the number of grooming partners she actually had (one or two), in order to avoid conflating bond quality with bond quantity. In aggregate, our DSI calculations capture 1,214 total female life-years (mean per individual, 6.32; SD, 3.81; range, 1 to 15).
Analysis Implementation.
The structure of our mediation models is summarized above in Mediation Model Structure and Assumptions. Here, we describe the details of how we implemented these analyses. First, we describe the process of dimension reduction of the mediator and outcome variables via functional principal components analysis (FPCA). Second, we describe the control variables used to account for environmental and demographic variables known to predict variation in social bond strength or fGC concentrations in our subjects. Third, we describe the model implementation procedures, including how FPCA was incorporated into the models, the set of models that we ran, and specifics of the interpretation of the model results.
Dimension reduction via FPCA.
Mediation models require lower-dimensional data than our social bond strength and fGC measures provide, which both comprised repeated measurements at many time points across the course of the females’ lives (49, 80). To remedy this, we used FPCA to reduce the dimensionality of these trajectories of females’ fGC concentrations and social bond strengths. We then treated the resulting dominant principal components as the outcome and mediator variables, respectively, in mediation models (49).
FPCA is analogous to standard PCA, except that instead of reducing multiple, correlated variables into a smaller number of uncorrelated axes of variation, FPCA captures differences in “functional data” (i.e., trajectories, curves, or anything that varies over time) (81, 82). As such, it is useful for summarizing irregularly spaced longitudinal data using a few principal components (49, 80). We performed FPCA separately on the mediator and outcome trajectories to generate the respective components used in the models.
In this approach, each female baboon has a separate trajectory for social bond strength (i.e., mediator) values and fGC (i.e., outcome) values observed at time grids , where is the identity of an individual female, is the total number of observations for an individual female, and the and values constitute the observed trajectories. We assume these trajectories, after controlling for relevant covariates and random effects, are represented by linear combinations of a few underlying smooth curves. Specifically, the model for the mediator trajectory is as follows:
| [7] |
where is an index variable for a given observation (in our case, female age is used as the index), are the covariates on social bond strength and are random effects for social group and hydrological year (see Control variables below), respectively; are a set of functional principal components (i.e., orthogonal eigenfunctions), which are ordered by the proportion of the variation of the trajectories that a component explains; is the principal score corresponding to principal component for unit ; and is an error term. The number is chosen by the fraction of the variance the first components collectively explain. In our models, we required that the K components must explain at least 90% of the variance, and K never exceeded 4.
Similarly, the model for the outcome trajectory is as follows:
| [8] |
where are the set of functional principal components that depict the first dominant modes of variation in the outcome trajectories (after controlling for relevant covariates and random effects ; see Control variables below), are the corresponding principal scores, and is an error term. The component scores for both the mediator and the outcome variable are then backtranslated to individual trajectories for model interpretation purposes (see Model implementation and interpretation below and SI Appendix). More technical details of the FCPA are provided in SI Appendix.
Control variables.
In this baboon population, several environmental or demographic variables are known to predict variation in social bond strength, fGC concentrations, or both (83–85). To account for these variables, we adjusted each female’s fGC and social bond strength measures using the covariates outlined in Tables 4 and 5. Details on how we measured these covariates are in SI Appendix.
Table 4.
fGC concentrations were adjusted for the following covariates
| fGC covariate | Definition |
| Reproductive state | The female’s reproductive state on the day the sample was collected (cycling, pregnant, or lactating) |
| Group density | The number of adult members in the female’s social group on the day the sample was collected |
| Group density squared* | The square of group density |
| Mean maximum temperature | The mean maximum temperature in the 30 d prior to sample collection |
| Season | Whether the sample was collected in the wet season or dry season |
| Delta rainfall | A measure of how much rainfall deviated from expectation during the 3-mo period preceding sample collection |
| Proportional (i.e., relative) dominance rank | Proportion of adult females in the social group who are lower ranking than the focal female |
Included to account for known nonlinear relationships between group density and fGCs (85).
Table 5.
Social bond strength was adjusted for the following covariates
| Covariate | Definition | Rationale |
| Group density | Mean number of adult members in the subject’s social group during the year in which bond strength was calculated | Females who live with different numbers of potential social partners may exhibit different bond strengths. |
| Mean number of coresident adult maternal relatives (R > 0.25)* | Mean number of adult maternal sisters, adult daughters, and mother living in the same social group as the subject during the year in which bond strength was calculated | For female baboons, maternal kin may offer additional support, beyond the scope of social bonds, which could be relevant to fGC concentrations (e.g., physical protection) (69, 70). Controlling for number of relatives is a conservative approach that helps isolate the effects of bonds per se, because animals with smaller matrilines may receive less kin support. |
| Percent of prior year with young infant* | Percent of days in the year that a female had an infant less than 3 mo old | Adult females are attracted to other females' young infants (86, 87), so females with young infants may have stronger social bonds with other females. |
| Percent of prior year cycling† | Percent of days in the year that a female was cycling | Males socialize more with females who are cycling (88, 89), so females who cycled for a larger fraction of the year may have stronger social bonds to males. |
Used only in models where the mediator was social bonds with females.
Used only in models where the mediator was social bonds with males.
Age is used to index within-individual observations for FPCA on both social bond strength and fGCs; because this implicitly captures age-related information, it is not treated as a separate covariate. However, data on both fGCs and social bond strength for old females (>18 y of age) were sparse [only ∼20% of female baboons who live to 5 y of age survive until age 18 (90)]. As such, we truncated all trajectories at age 18, which trimmed the data for 32 of our 192 subjects (16.7%).
In addition to the covariates outlined in Tables 4 and 5, the models also cluster on two random effects that our exploratory analyses indicated correlate with variation in measures of social bond strength, and with fGC concentrations: hydrological year (in Amboseli, November through the following October, which encompasses the full dry/wet season cycle), and social group identity. Individual female identity is not treated as a random effect because individual effects are implicitly captured in the trajectory modeling and resulting FPCA scores.
Model implementation and interpretation.
Model set.
Analyses were conducted in R, version 3.3.2, using the MASS package and custom code to implement the models. We ran 16 models. For each of the two mediators (social bond strength with females or males), we tested each of the six sources of adversity independently, plus two cumulative adversity models that compared: 1) females who did not experience any sources of early adversity (n = 48) vs. those who experienced one or more (n = 144), and 2) females who experienced one source of adversity (n = 82) vs. those who experienced two or more sources of adversity (n = 62). All models contained three components: the predictor of interest (a source of adversity), the mediator variable (the FPCA-generated components of social bond strength, to either females or males), and the outcome (the FPCA-generated components of fGCs). All sources of adversity were treated as binary variables, where 0 indicated that the female did not experience the source of adversity and 1 indicated that she did. We did not treat number of early adversities as a continuous variable, because it would require the strong and unproven assumption that each additional source of adversity has a constant effect on the outcome.
Interpreting coefficients.
The effect sizes for any given pathway (e.g., the direct effect or effect on mediator) reflect effects across females’ lifetime trajectories. Hence the model coefficients can be interpreted as indicating that a female who experienced a given adversity has fGC concentrations, or social bond strength values, that are y units different from counterparts who did not, over the course of her life span. The magnitude of the changes in log fGCs are all <20%, and are small enough to be functionally interpretable as percent change in untransformed fGCs. We present differences in social bond strength in SDs, since the units associated with this measure are difficult to interpret. For an illustration of the effect size in the overall context of female social bonds, see SI Appendix, Fig. S1.
Causal assumptions.
In order to interpret the above effects as causal, two additional structural assumptions are required (46). The first is unconfoundedness: i.e., that there are no unmeasured confounding variable(s), besides the observed covariates , between early adversity and the social bond and fGC processes. In other words, early adversity is randomized among the baboons with the same covariate values. The second is sequential unconfoundedness: that there are no unmeasured confounding variable(s), besides the observed covariates and the history of social bond strength , between the social bond process and the fGC process. Exploratory checks of robustness ruled out the possibility of “feedback” between the social bond and fGC processes (see SI Appendix for further details, including SI Appendix, Tables S2 and S3); this is one scenario in which the sequential unconfoundedness assumption would be violated. We are not aware of, but also cannot rule out, the existence of additional sequential confounders. This is a limitation of our study.
Data Availability Statement.
Data used for this study are available on Dryad at https://doi.org/10.5061/dryad.2280gb5pm. The code used to implement the analyses is available at GitHub, https://github.com/zengshx777/ABRP_Mediation.
Supplementary Material
Acknowledgments
We thank two reviewers for their exceptionally helpful reviews, as well as the members of the J. Tung, S.C.A., and E.A.A. laboratories, whose insights greatly improved the manuscript. We gratefully acknowledge the support of the NIH, especially the National Institute on Aging, and the NSF for the majority of the data represented here. Our work is currently supported through NIH Grants R01AG053330, R01AG053308, R01HD088558, and P01AG031719, and NSF Award IOS 1456832. In the past decade, we also acknowledge support from Awards/Grants IOS 1053461, IBN 9985910, IBN 0322613, IBN 0322781, BCS 0323553, BCS 0323596, DEB 0846286, DEB 0846532, IOS 0919200, R01AG034513-01, R21AG049936, and P01AG031719. We thank the Kenya Wildlife Service, Institute of Primate Research, National Museums of Kenya, National Council for Science and Technology, the Kajiado County Council, the members of the Amboseli–Longido pastoralist communities in Kenya, and the Enduimet Wildlife Management Area. We also thank Tortilis Camp and Ker and Downey Safaris for their help and cooperation in Kenya. Many people have contributed to the long-term data over the years, but we are particularly grateful for the work of the Amboseli Baboon Project long-term field team (R. S. Mututua, S. Sayialel, and J. K. Warutere), and V. Somen and T. Wango for their assistance in Nairobi. We thank Karl Pinc for his database design and management expertise, as well as the Amboseli Baboon Research Project’s database technicians, D. Onderdonk, C. Markham, T. Fenn, N. Learn, L. Maryott, P. Onyango, and J. Gordon. We also thank Duke University, Princeton University, the Chicago Zoological Society, the Max Planck Institute for Demographic Research, the L. S. B. Leakey Foundation, and the National Geographic Society for support at various times over the years.
Footnotes
The authors declare no competing interest.
Data deposition: The data (CSV file) reported in this paper have been deposited in the Dryad repository at https://datadryad.org/stash/dataset/doi:10.5061/dryad.2280gb5pm. The code used to implement the analyses is available at GitHub, https://github.com/zengshx777/ABRP_Mediation.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004524117/-/DCSupplemental.
References
- 1.Fryers T., Brugha T., Childhood determinants of adult psychiatric disorder. Clin. Pract. Epidemiol. Ment. Health 9, 1–50 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter L. L. et al., Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 35, 2617–2623 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slopen N. et al., Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom. Med. 72, 694–701 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hostinar C. E., Ross K. M., Chen E., Miller G. E., Early-life socioeconomic disadvantage and metabolic health disparities. Psychosom. Med. 79, 514–523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto Pereira S. M., Stein Merkin S., Seeman T., Power C., Understanding associations of early-life adversities with mid-life inflammatory profiles: Evidence from the UK and USA. Brain Behav. Immun. 78, 143–152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickrama K. A. S., Bae D., O’Neal C. W., Explaining the association between early adversity and young adults’ diabetes outcomes: Physiological, psychological, and behavioral mechanisms. J. Youth Adolesc. 46, 2407–2420 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Friedman E. M., Karlamangla A. S., Gruenewald T. L., Koretz B., Seeman T. E., Early life adversity and adult biological risk profiles. Psychosom. Med. 77, 176–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker D. J. P., Fetal origins of coronary heart disease. Br. Heart J. 69, 195–196 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller G. E. et al., Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 14716–14721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umberson D., Williams K., Thomas P. A., Liu H., Thomeer M. B., Race, gender, and chains of disadvantage: Childhood adversity, social relationships, and health. J. Health Soc. Behav. 55, 20–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebbert A. M., Infurna F. J., Luthar S. S., Lemery-Chalfant K., Corbin W. R., Examining the link between emotional childhood abuse and social relationships in midlife: The moderating role of the oxytocin receptor gene. Child Abuse Negl. 98, 104151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller G. E., Chen E., Parker K. J., Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 137, 959–997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt-Lunstad J., Smith T. B., Layton J. B., Social relationships and mortality risk: A meta-analytic review. PLoS Med. 7, e1000316 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elovainio M. et al., Socioeconomic differences in cardiometabolic factors: Social causation or health-related selection? Evidence from the Whitehall II Cohort Study, 1991–2004. Am. J. Epidemiol. 174, 779–789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foverskov E., Holm A., Socioeconomic inequality in health in the British household panel: Tests of the social causation, health selection and the indirect selection hypothesis using dynamic fixed effects panel models. Soc. Sci. Med. 150, 172–183 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Warren J. R., Socioeconomic status and health across the life course: A test of the social causation and health selection hypotheses. Soc. Forces 87, 2125–2153 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kröger H., Pakpahan E., Hoffmann R., What causes health inequality? A systematic review on the relative importance of social causation and health selection. Eur. J. Public Health 25, 951–960 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Cohen S., Wills T. A., Stress, social support, and the buffering hypothesis. Psychol. Bull. 98, 310–357 (1985). [PubMed] [Google Scholar]
- 19.Praharso N. F., Tear M. J., Cruwys T., Stressful life transitions and wellbeing: A comparison of the stress buffering hypothesis and the social identity model of identity change. Psychiatry Res. 247, 265–275 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Hertzman C., The biological embedding of early experience and its effects on health in adulthood. Ann. N. Y. Acad. Sci. 896, 85–95 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Berens A. E., Jensen S. K. G., Nelson C. A. 3rd, Biological embedding of childhood adversity: From physiological mechanisms to clinical implications. BMC Med. 15, 135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrlich K. B., Ross K. M., Chen E., Miller G. E., Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype? Dev. Psychopathol. 28, 1273–1283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danese A. et al., Biological embedding of stress through inflammation processes in childhood. Mol. Psychiatry 16, 244–246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen L. J. H. et al., Cumulative childhood risk is associated with a new measure of chronic inflammation in adulthood. J. Child Psychol. Psychiatry 60, 199–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraro K. F., Schafer M. H., Wilkinson L. R., Childhood disadvantage and health problems in middle and later life: Early imprints on physical health? Am. Sociol. Rev. 81, 107–133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Case A., Paxson C., The long reach of childhood health and circumstance: Evidence from the Whitehall II study. Econ. J. (Lond.) 121, F183–F204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyck H. J. F., Buchanan K. L., Crino O. L., Jessop T. S., Effects of developmental stress on animal phenotype and performance: A quantitative review. Biol. Rev. Camb. Philos. Soc. 94, 1143–1160 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Conti G. et al., Primate evidence on the late health effects of early-life adversity. Proc. Natl. Acad. Sci. U.S.A. 109, 8866–8871 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tung J., Archie E. A., Altmann J., Alberts S. C., Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dettmer A. M., Novak M. A., Suomi S. J., Meyer J. S., Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology 37, 191–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinelli S. et al., Early-life stress induces long-term morphologic changes in primate brain. Arch. Gen. Psychiatry 66, 658–665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberts S. C., Altmann J., “The Amboseli Baboon Research Project: 40 years of continuity and change” in Long-Term Field Studies of Primates, Kappeler P. M., Watts D. P., Eds. (Springer, Heidelberg, Germany, 2012), chap. 12, pp. 261–288. [Google Scholar]
- 33.Rosenbaum S., et al. , Data from: Social bonds do not mediate the relationship between early adversity and adult glucocorticoids in wild baboons. Dryad. 10.5061/dryad.2280gb5pm. Deposited 2 July 2020. [DOI] [PMC free article] [PubMed]
- 34.Archie E. A., Tung J., Clark M., Altmann J., Alberts S. C., Social affiliation matters: Both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc. Biol. Sci. 281, 20141261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engh A. L. et al., Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. Biol. Sci. 273, 707–712 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittig R. M. et al., Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 54, 170–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chrousos G. P., The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332, 1351–1362 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Buckley T. M., Schatzberg A. F., On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 90, 3106–3114 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Björntorp P., Rosmond R., The metabolic syndrome—a neuroendocrine disorder? Br. J. Nutr. 83 (suppl. 1), S49–S57 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Wingfield J. C., The concept of allostasis: Coping with a capricious environment. J. Mammal. 86, 248–254 (2005). [Google Scholar]
- 41.Nusslock R., Miller G. E., Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol. Psychiatry 80, 23–32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlamangla A. S. et al., Early-life adversity and dysregulation of adult diurnal cortisol rhythm. J. Gerontol. B Psychol. Sci. Soc. Sci. 74, 160–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birnie A. K., Taylor J. H., Cavanaugh J., French J. A., Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi). Psychoneuroendocrinology 38, 3003–3014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez M. M., The impact of early adverse care on HPA axis development: Nonhuman primate models. Horm. Behav. 50, 623–631 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Anisman H., Zaharia M. D., Meaney M. J., Merali Z., Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16, 149–164 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Fogelman N., Canli T., Early life stress and cortisol: A meta-analysis. Horm. Behav. 98, 63–76 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Dowd J. B., Goldman N., Do biomarkers of stress mediate the relation between socioeconomic status and health? J. Epidemiol. Community Health 60, 633–639 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alberts S. C., Campos F. A., Gesquiere L., Archie E. A., Glucocorticoid levels predict lifespan in wild female baboons. Am. J. Phys. Anthropol. 168, 4 (2019).30408154 [Google Scholar]
- 49.Zeng S., Rosenbaum S., Archie E., Alberts S., Li F., Causal mediation analysis for sparse and irregular longitudinal data. arXiv:2007.01796 (03 July 2020).
- 50.Baron R. M., Kenny D. A., The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182 (1986). [DOI] [PubMed] [Google Scholar]
- 51.Lange T., Hansen K. W., Sørensen R., Galatius S., Applied mediation analyses: A review and tutorial. Epidemiol. Health 39, e2017035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engh A. L. et al., Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons. Anim. Behav. 71, 1227–1237 (2006). [Google Scholar]
- 53.Hunter A. L., Minnis H., Wilson P., Altered stress responses in children exposed to early adversity: A systematic review of salivary cortisol studies. Stress 14, 614–626 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Engert V., Efanov S. I., Dedovic K., Dagher A., Pruessner J. C., Increased cortisol awakening response and afternoon/evening cortisol output in healthy young adults with low early life parental care. Psychopharmacology (Berl.) 214, 261–268 (2011). [DOI] [PubMed] [Google Scholar]
- 55.McEwen B. S., Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cacioppo J. T., Hawkley L. C., Norman G. J., Berntson G. G., Social isolation. Ann. N. Y. Acad. Sci. 1231, 17–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cacioppo J. T., Cacioppo S., Capitanio J. P., Cole S. W., The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sapolsky R. M., Alberts S. C., Altmann J., Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch. Gen. Psychiatry 54, 1137–1143 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Crockford C., Wittig R. M., Whitten P. L., Seyfarth R. M., Cheney D. L., Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm. Behav. 53, 254–265 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Silk J. B. et al., Female chacma baboons form strong, equitable, and enduring social bonds. Behav. Ecol. Sociobiol. (Print) 64, 1733–1747 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silk J. B., Alberts S. C., Altmann J., Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Silk J. B., Alberts S. C., Altmann J., Cheney D. L., Seyfarth R. M., Stability of partner choice among female baboons. Anim. Behav. 83, 1511–1518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silk J. B. et al., Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Palombit R. A., Cheney D. L., Seyfarth R. M., Female–female competition for male “friends” in wild chacma baboons (Papio cynocephalus ursinus). Anim. Behav. 61, 1159–1171 (2001). [Google Scholar]
- 65.Nguyen N., Van Horn R. C., Alberts S. C., Altmann J., “Friendships” between new mothers and adult males: Adaptive benefits and determinants in wild baboons (Papio cynocephalus). Behav. Ecol. Sociobiol. (Print) 63, 1331–1344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moscovice L. R. et al., Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Anim. Behav. 79, 1007–1015 (2010). [Google Scholar]
- 67.Shur M. D., Hormones Associated with Friendship between Adult Male and Lactating Female Olive Baboons, Papio hamadryas anubis, (Rutgers The State University of New Jersey, New Brunswick, NJ, 2008). [Google Scholar]
- 68.Silk J. B., Altmann J., Alberts S. C., Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 61, 183–195 (2006). [Google Scholar]
- 69.Silk J. B., Alberts S. C., Altmann J., Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav. Ecol. Sociobiol. 61, 197–204 (2006). [Google Scholar]
- 70.Alberts S. C., Altmann J., Balancing costs and opportunities: Dispersal in male baboons. Am. Nat. 145, 279–306 (1995). [Google Scholar]
- 71.Silk J. B., Roberts E. R., Barrett B. J., Patterson S. K., Strum S. C., Female–male relationships influence the form of female–female relationships in olive baboons, Papio anubis. Anim. Behav. 131, 89–98 (2017). [Google Scholar]
- 72.Cheney D. L., Silk J. B., Seyfarth R. M., Evidence for intra-sexual selection in wild female baboons. Anim. Behav. 84, 21–27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zipple M. N., Archie E. A., Tung J., Altmann J., Alberts S. C., Intergenerational effects of early adversity on survival in wild baboons. eLife 8, e47433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Touma C., Palme R., Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Ann. N. Y. Acad. Sci. 1046, 54–74 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Khan M. Z., Altmann J., Isani S. S., Yu J., A matter of time: Evaluating the storage of fecal samples for steroid analysis. Gen. Comp. Endocrinol. 128, 57–64 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Beehner J. C., Bergman T. J., Cheney D. L., Seyfarth R. M., Whitten P. L., Testosterone predicts future dominance rank and mating activity among male chacma baboons. Behav. Ecol. Sociobiol. 59, 469–479 (2006). [Google Scholar]
- 77.Gesquiere L. R. et al., Coming of age: Steroid hormones of wild immature baboons (Papio cynocephalus). Am. J. Primatol. 67, 83–100 (2005). [DOI] [PubMed] [Google Scholar]
- 78.King A. J., Clark F. E., Cowlishaw G., The dining etiquette of desert baboons: The roles of social bonds, kinship, and dominance in co-feeding networks. Am. J. Primatol. 73, 768–774 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Mitani J. C., Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640 (2009). [Google Scholar]
- 80.Yao F., Müller H.-G., Wang J.-L., Functional data analysis for sparse longitudinal data. J. Am. Stat. Assoc. 100, 577–590 (2005). [Google Scholar]
- 81.Lever J., Krzywinski M., Altman N., Principal component analysis. Nat. Methods 14, 641 (2017). [Google Scholar]
- 82.Greven S., Crainiceanu C., Caffo B., Reich D., Longitudinal functional principal component analysis. Electron. J. Stat. 4, 1022–1054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gesquiere L. R. et al., Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus). Horm. Behav. 54, 410–416 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gesquiere L. R., Onyango P. O., Alberts S. C., Altmann J., Endocrinology of year-round reproduction in a highly seasonal habitat: Environmental variability in testosterone and glucocorticoids in baboon males. Am. J. Phys. Anthropol. 144, 169–176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markham A. C., Gesquiere L. R., Alberts S. C., Altmann J., Optimal group size in a highly social mammal. Proc. Natl. Acad. Sci. U.S.A. 112, 14882–14887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Altmann J., Baboon Mothers and Infants, (Harvard University Press, Cambridge, MA, 1980). [Google Scholar]
- 87.Altmann J., . “Infant independence in yellow baboons” in The Development of Behavior: Comparative and Evolutionary Aspects, Burghardt G. M., Bekoff M., Eds. (Garland STPM Press, Oxford, UK, 1978), pp. 253–277. [Google Scholar]
- 88.Alberts S. C., Altmann J., Wilson M. L., Mate guarding constrains foraging activity of male baboons. Anim. Behav. 51, 1269–1277 (1996). [Google Scholar]
- 89.Gesquiere L. R., Wango E. O., Alberts S. C., Altmann J., Mechanisms of sexual selection: Sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm. Behav. 51, 114–125 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Bronikowski A. M. et al., Female and male life tables for seven wild primate species. Sci. Data 3, 160006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this study are available on Dryad at https://doi.org/10.5061/dryad.2280gb5pm. The code used to implement the analyses is available at GitHub, https://github.com/zengshx777/ABRP_Mediation.