Abstract
At the cellular level, cells infected with human immunodeficiency virus type 1 (HIV-1) exhibit immunity to a second infection by the virus that initiated the first infection or by related viruses [superinfection resistance (SIR)]. In the case of HIV infection, SIR was basically attributed to downregulation of the CD4 receptors. We have recently reported on an interaction between HIV-1 Rev and integrase (IN) proteins, which results in inhibition of IN activity in vitro and integration of cDNA in HIV-1-infected cells. A novel function for the viral Rev protein in controlling integration of HIV cDNAs was thus proposed. The results of the present work suggest involvement of the inhibitory Rev in sustaining SIR. A single exposure to wild-type HIV-1 resulted in one to two integrations per cell. The number of integrated proviral cDNA copies remained at this low level even after double infection or superinfection. SIR was dependent on Rev expression by the strain used for the first infection and was eliminated by peptides that disrupt intracellular complex formation between IN and Rev. The same lack of resistance was observed in the absence of Rev, namely following first infection with a ΔRev HIV strain. The involvement of Rev, expressed from either unintegrated or integrated viral cDNA, in promoting SIR was clearly demonstrated. We conclude that SIR involves Rev-dependent control of HIV cDNA integration.
INTRODUCTION
Various observations have indicated that human immunodeficiency virus (HIV) infection can indeed confer resistance, at the cellular level, to a second infection. Evidently, such resistance is mediated by mechanisms other than classical adaptive immune responses (Nethe et al., 2005; Piantadosi et al., 2007; Yeh et al., 2009). The capacity to prevent a second infection, at the cellular level, by a virus that is closely related to that which established the first infection has been termed superinfection resistance (SIR) (Nethe et al., 2005; van der Kuyl & Cornelissen, 2007). Although the molecular details of this mechanism have not yet been completely elucidated, it appears that in most cases it requires the expression of viral proteins (Nethe et al., 2005; Potash & Volsky, 1998).
Downregulation of CD4 from membranes of HIV-1-infected cells has been suggested to play a major role in conferring resistance to a second infection, but this mechanism is still under dispute (Potash & Volsky, 1998; Saha et al., 1999). To date, the viral proteins Nef, as well as Vpu and Env, have been suggested to mediate downregulation of CD4 and thus to play an important role in conferring SIR (Wildum et al., 2006). However, kinetic studies of CD4 downregulation in infected cells have raised doubt as to the relevance of this process to SIR (Potash & Volsky, 1998; Volsky et al., 1996). Furthermore, it appears that CD4 downregulation increases virus replication and promotes release of new virions, indicating stimulation of virus production rather than resistance (Glushakova et al., 2001; Levesque et al., 2003). Several studies have clearly demonstrated that no direct relations exist between SIR and CD4 downregulation (Potash & Volsky, 1998; Saha et al., 1999; Volsky et al., 1996). Even assuming that downregulation of CD4 contributes to the development of SIR, the possibility of additional, CD4-independent mechanisms cannot be excluded and have, in fact, been suggested (Potash & Volsky, 1998; Wildum et al., 2006).
Similar to Nef, the Rev protein is present in the early phase of infection, expressed from unintegrated viral cDNA (Iyer et al., 2009; Kelly et al., 2008; Wu, 2004, 2008; Wu & Marsh, 2003). The late viral Rev encoded by the integrated HIV genome has so far been implicated mainly in promoting nuclear export of unspliced and single-spliced transcripts (Pollard & Malim, 1998). We have recently demonstrated that interaction between the viral Rev and integrase (IN) proteins results in inhibition of IN activity in vitro and integration of cDNA in HIV-1-infected cells (Levin et al., 2009a, b, 2010b; Rosenbluh et al., 2007). Here, we provide the first demonstration of a novel function of Rev, namely a major and important role in conferring SIR, probably via its interaction with viral IN. HIV infection of cultured cells was quantitatively monitored by estimating integration levels of viral cDNA. About the same integration levels, 1.5–2.0 integrations per cell, were obtained following single or multiple infections with wild-type (wt) HIV-1, indicating the establishment of SIR (see also Levin et al., 2009a). However, in the absence of Rev a significant increase in integration levels, i.e. eradication of resistance, was observed, proving the involvement of Rev in conferring SIR.
RESULTS
SIR can be promoted by Rev expressed from unintegrated cDNA
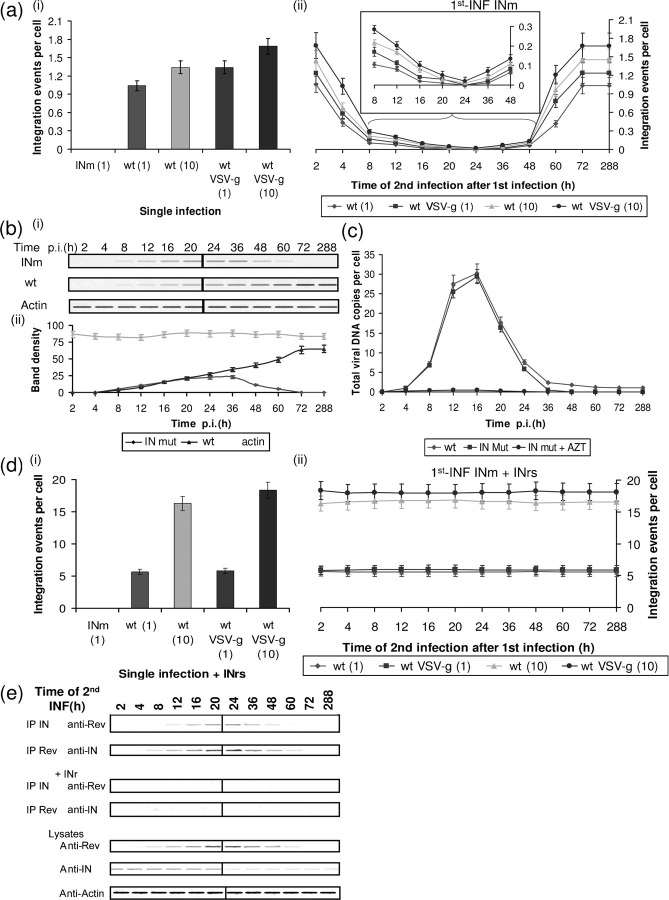
Infection with an HIV mutant (IN D64,116N HIV; INm), being unable to promote genome integration (Nakajima et al., 2001), did not result in any viral DNA integration [Fig. 1a(i)]. However, infection with the wt HIV resulted in a relatively low degree of integration: 1.0–1.8 integrations per cell [Fig. 1a(i) and see Butler et al., 2001; Levin et al., 2009a]. Infection with both viruses leads to the appearance of unintegrated viral cDNA which promotes the expression of several viral proteins, among them Rev (Iyer et al., 2009; Kelly et al., 2008; Wu, 2004, 2008; Wu & Marsh, 2003 and Fig. 1b). Indeed, Rev could clearly be observed at 8 h post-infection (p.i.) with both viruses, well before integration occurs (Fig. 1b and Iyer et al., 2009; Levin et al., 2009a). Rev expressed by the INm cDNA was gradually eradicated and completely disappeared by 72 h p.i. (Fig. 1b) due to the dilapidation of unintegrated cDNA (Fig. 1c). On the other hand, due to integration of the wt viral DNA, the amount of Rev expressed by the integrated cDNA gradually increased, reaching a maximum level by 72 h p.i. (Fig. 1b). Rev expressed by the unintegrated viral DNA was designated Rev-early (Rev-ear).
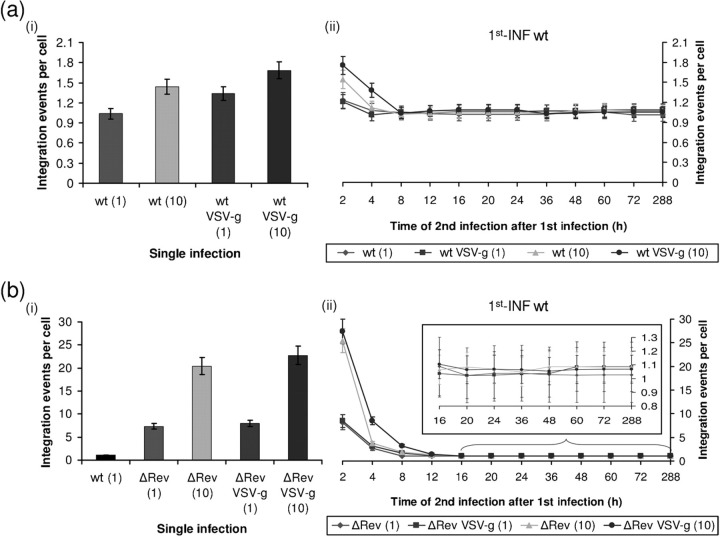
Fig. 1.
Rev expressed from unintegrated DNA can promote SIR. (a) Integration events per cell were estimated from cells which were (i) single infected or (ii) 1st-INF with INm HIV-1 at an m.o.i.=1 and then 2nd-INF with the indicated wt viruses at the specified m.o.i. (1 or 10) and at the indicated times post-1st-INF. (a, ii) Inset, magnification of the marked section. (b, i) Western blot analysis of lysate obtained from cells infected with INm and wt HIV-1 (at different times p.i.) was used to detect Rev or actin (as control). (ii) Bands were quantitatively estimated by Image Gauge V3.0 software from Fujifilm. (c) Quantitative analysis of viral cDNA at different times p.i. by wt and INm HIV-1 (m.o.i.=1). (d) Same as (a) but with the addition of 150 μM INr peptides 2 h before the (i) single infection or (ii) 2nd-INF. (e) Co-immunoprecipitation and Western blot analyses were performed on samples withdrawn from the systems described in (a, ii) and (d, ii). All experimental details are as described in Methods.
In the experiment described in Fig. 1a(ii), infection at zero time (1st-INF) was conducted with the INm HIV-1 (m.o.i.=1) and then at the indicated times, cells were secondly infected (2nd-INF) with wt or VSV-g-coated virus (at an m.o.i.=1 or 10) [Fig. 1a(ii)]. About 1.0–1.8 and 0.4–1.0 integrations per cell were obtained when the 2nd-INF was conducted 2 and 4 h after the 1st-INF, respectively. This must be due to the integration performed by the active viral IN, which was applied by the 2nd-INF. In practice, similar levels of integration were also obtained when the 2nd-INF with the wt or VSV-g-coated viruses was conducted from 60 h post-1st-INF on. At this time, most of the 1st-INF INm viral cDNA had already been abolished (Fig. 1c) and therefore no Rev expressed from the unintegrated cDNA of the INm (Rev-ear-INm) is present (Fig. 1b). Due to the absence of the 1st-INF viral cDNA at this time, infection at 60 h and above can be considered identical to a single infection [Fig. 1a(i) and see Levin et al., 2009a, b]. It should be noted that also infection by wt virus is expected to lead to the appearance of Rev-ear molecules expressed from unintegrated cDNA.
No integration, i.e. complete resistance to the 2nd-INF, was observed when the 2nd-INF was conducted between 8 and 48 h after the 1st-INF [Fig. 1a(ii)]. This was observed when the 2nd-INF was performed at an m.o.i.=10 with either wt or VSV-g-coated viruses [Fig. 1 a(ii)]. These results confirm previous observations regarding complete resistance to 2nd-INF 24 h post-1st-INF with HIV-1 at an m.o.i.=1 (Volsky et al., 1996). The involvement of Rev-ear in promoting complete resistance to infection by wt or VSV-g-coated HIV can be inferred from the results depicted in Fig. 1(d, e). As can be seen [Fig. 1d(ii)], stimulation of integration, namely eradication of resistance to the 2nd-INF, could be achieved by the addition of the IN-derived (INr) peptides, which promote disruption of the Rev-IN interaction (Fig. 1e and see also Levin et al., 2009b). This indicates that the observed resistance, namely inhibition of integration, was promoted by the presence of Rev molecules. Stimulation of integration promoted by the INr peptides (INrs) was also observed following a single infection [Fig. 1d(i)], or even when the 2nd-INF was performed between 4 and 60 h after the 1st-INF when SIR was observed [Fig. 1d(ii)]. This supports the view that the low level of integration obtained during these periods is also due to inhibition by Rev (Levin et al., 2009a, 2010b).
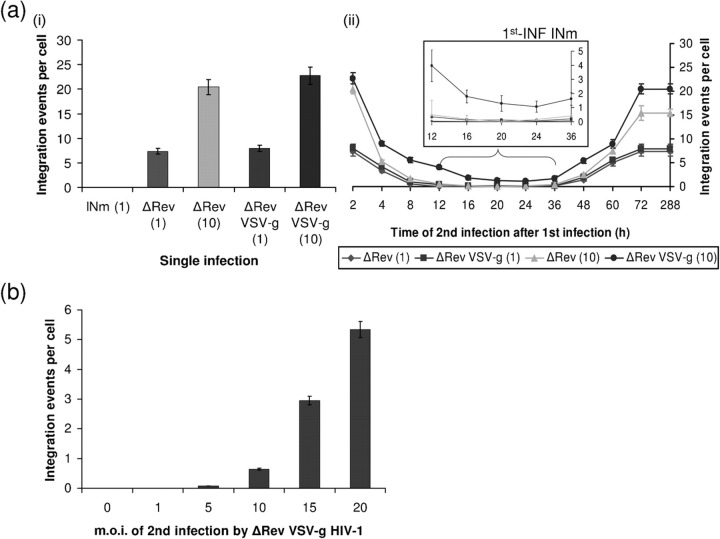
The question of whether a correlation exists between promotion of resistance and expression of Rev-ear (expressed by the unintegrated cDNA) could also be studied following the use of ΔRev HIV for the 2nd-INF. The results in Fig. 2(a) show that even under these conditions – namely, no expression of Rev following the 2nd-INF – complete resistance was obtained when infection was conducted at an m.o.i.=1 or 10 of ΔRev HIV, but only between 12 and 36 h after the 1st-INF [Fig. 2a(ii)]. This indicates that at these time periods the Rev expressed following 1st-INF with INm HIV at an m.o.i.=1 is sufficient to neutralize the relatively high amounts of IN molecule delivered following the 2nd-INF with as much as an m.o.i.=10. A low level of integration, namely less resistance, was obtained when cells were secondly infected by the ΔRev VSV-g-coated virus at an m.o.i.=10 – rather than with wt virus – whose infection is CD4-independent (Fig. 2a and Matlin et al., 1982). This suggests a minor contribution of CD4 downregulation to the resistance observed following 2nd-INF with wt HIV.
Fig. 2.
Dependence of SIR on the quantitative relationship between m.o.i. of 1st-INF and 2nd-INF. (a) Integration events per cell were estimated from cells which were (i) single infection with the indicated HIV-1 or (ii) 1st-INF with INm HIV-1 (m.o.i.=1) and then 2nd-INF with the indicated ΔRev virus (m.o.i.=1 or 10). (a, ii) Inset, magnification of the marked section. (b) m.o.i. titration of ΔRev HIV-1 at 20 h post-1st-INF with INm HIV-1.
The view that the observed resistance is dependent on the quantitative relations between the titre of the first and second infected viruses is evident from the results depicted in Fig. 2(b): no integration, i.e. complete resistance to the 2nd-INF was observed when it was conducted with an m.o.i.=1 of ΔRev VSV-g-coated virus, while about 5.0 integrations per cell was obtained following infection with the same virus at an m.o.i.=20 (Fig. 2b). The non-linear relationship between the titre of the virus added in the 2nd-INF and the level of integration may be explained by assuming that the amount of Rev produced following the 1st-INF is not sufficient to block the IN of the 2nd-INF by the ΔRev HIV-1. However, if the 2nd-INF was carried out with a wt HIV-1, then the Rev expressed by the 2nd-INF limits its own integration to 1–2 events per cell (Levin et al., 2009a). Thus, the Rev of the 1st-INF has less free IN to inhibit, which results in complete SIR [Fig. 1a(ii)]. The high levels of integration observed when the 2nd-INF with ΔRev HIV was performed at 2 h and between 72 and 288 h after the 1st-INF is obviously due to the complete absence of Rev at these times (see also Fig. 3).
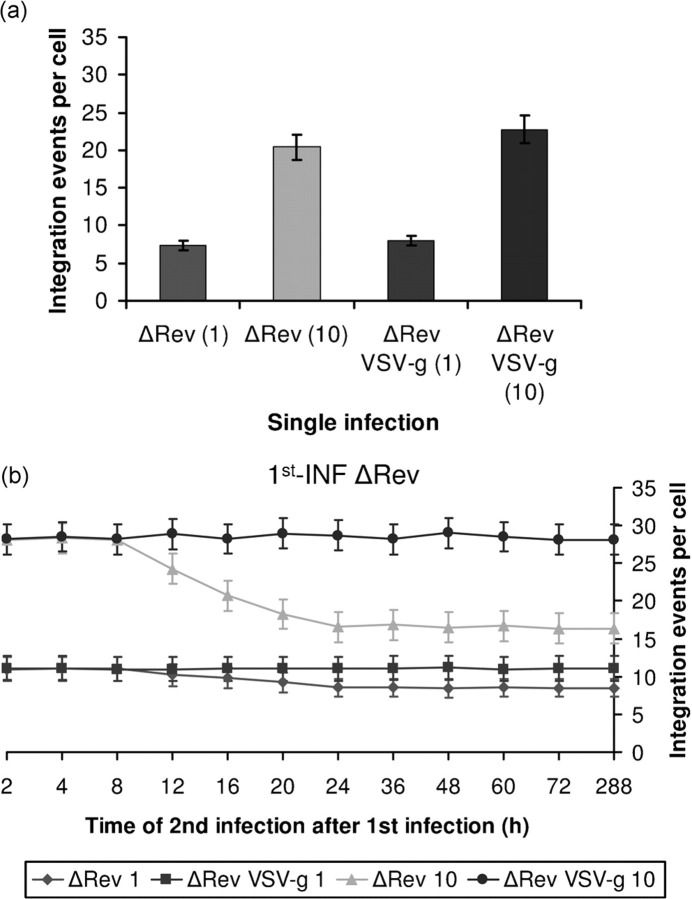
Fig. 3.
SIR is observed in the absence of Rev in cells infected by the wt but not VSV-g-coated viruses. Integration events per cell were estimated following (a) single infection with the indicated HIV-1s or (b) 1st-INF with ΔRev HIV-1 (m.o.i.=1) and 2nd-INF with the indicated ΔRev viruses (m.o.i.=1 or 10) at different time post-1st-INF.
The involvement of Rev expressed by the INm virus in conferring resistance is well confirmed by the results showing eradication of resistance when using the INr peptides (Levin et al., 2009a, b) (Supplementary Fig. S1a, available in JGV Online). These peptides are expected to promote dissociation of the Rev–IN complexes formed between the Rev-ear-INm and the IN of both viruses, as was revealed by co-immunoprecipitation (Supplementary Fig. S1b, available in JGV Online and Levin et al., 2009a, 2010b).
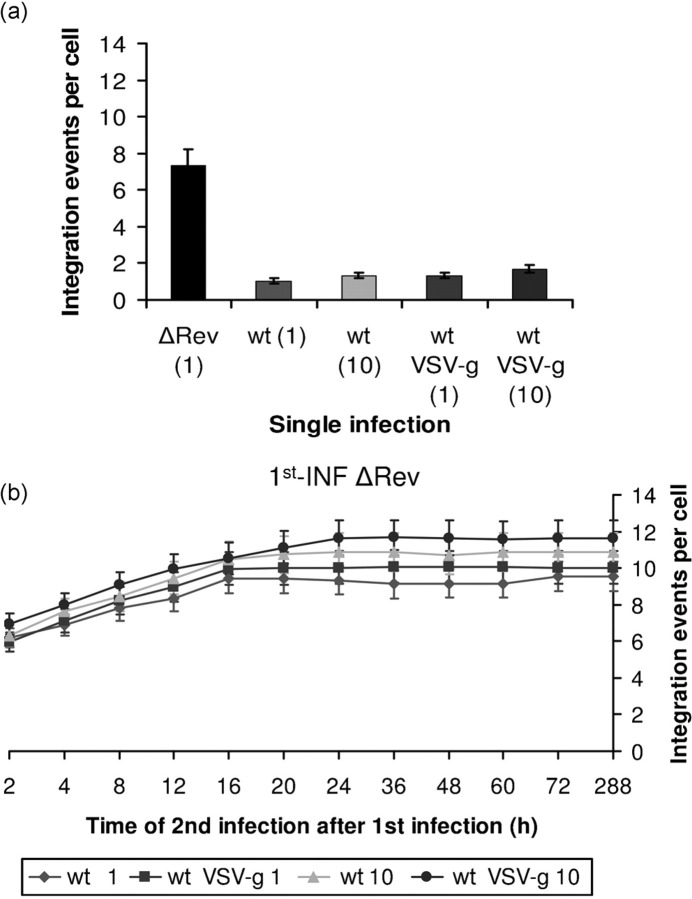
Support for the view that Rev expressed from unintegrated cDNA can confer resistance to 2nd-INF was obtained following the infection of LEDGF/p75-knockdown cells in which integration of wt HIV-1 cDNA hardly occurs (Llano et al., 2006). Indeed our results (Supplementary Fig. S2, available in JGV Online) show promotion of resistance to the 2nd-INF when LEDGF/p75-knockdown cells were infected with the wt virus and secondly infected with either the wt or ΔRev virus. However, no resistance was observed when the 1st-INF was conducted with ΔRev virus and the 2nd-INF with wt HIV (Fig. 4).
Fig. 4.
No SIR is observed in the absence of Rev. Integration events per cell were estimated following (a) single infection with the indicated viruses or (b) 1st-INF with a ΔRev HIV-1 at an m.o.i.=1 and then a 2nd-INF with the indicated wt viruses at an m.o.i.=1 or 10, at different times post-1st-INF. All other experimental details are as described in Methods.
In the complete absence of Rev, only partial resistance is exerted due to CD4 downregulation
It was of interest to determine the level of resistance obtained following double infection with ΔRev viruses, i.e. in the complete absence of Rev. As can be seen (compare results in Fig. 3 to Fig. 2a) relatively high number of integrations per cell was observed when the 1st-INF and the 2nd-INF were conducted with ΔRev viruses. Absolutely no resistance was observed following 2nd-INF with the ΔRev VSV-g-coated virus, again indicating the involvement of Rev in conferring resistance to the 2nd-INF (Fig. 3). However, a 50 % decrease in integration level was obtained from 12 h after the 1st-INF when the 2nd-INF was conducted with the wt-coated (bearing the wt env protein) ΔRev virus. In the absence of Rev, this partial resistance was probably due to CD4 downregulation or occupancy of the CD4 receptors, which mediate entry of the wt but not the VSV-g-coated viruses (Matlin et al., 1982).
Promotion of SIR by double infection with wt HIV-1
Rev plays a major role in conferring SIR, even when the double infection was conducted with wt HIV. When the 2nd-INF was conducted 2 h after the 1st-INF (double infection), both with the wt HIV, about 1.2–1.7 integrations per cell were observed [Fig. 5a(i)]. A slightly lower degree of integration was obtained when the 2nd-INF was performed between 4 and 288 h after the 1st-INF [Fig. 5a(ii)]. These integration levels were practically identical to those observed following a single infection with HIV-1 at an m.o.i. ranging from 1 to 10 [Fig. 5a(i)]. It was well established that integration of wt HIV cDNA should be completed between 16 and 20 h p.i. (Levin et al., 2009b; Pannecouque et al., 2002). Interestingly, the level of integration observed from 16 to 288 h after the 1st-INF was very close or identical to that observed following a single infection [compare Fig. 5a(ii) to 5a(i) and Fig. 1a(ii)]. It can therefore be presumed that the cDNA from the 2nd-INF failed to integrate, indicating complete resistance to the second infection, at least between 16 and 288 h post-1st-INF. Since the levels of integration observed following the 2nd-INF between 4 and 12 h post-1st-INF are very close or identical to those obtained at later times of 2nd-INF (Fig. 5a). At these periods, the observed integration is also merely that of the 1st-INF cDNA. Based on the previously described results (Figs 1–3), it is our assumption that the observed SIR is due to the presence of Rev-ear and Rev expressed by the integrated cDNA (Rev-late).
Fig. 5.
Integration levels following double or superinfection. (a) Integration events per cell were estimated following (i) single infection with the indicated HIV-1s or (ii) 1st-INF with a wt HIV-1 (m.o.i.=1) and then 2nd-INF with the same wt HIV-1 (m.o.i.=1 or 10) at different time post-1st-INF. (b) Same as in (a), but the 2nd-INF was with ΔRev HIV-1. (b, ii) Inset, magnification of the marked section.
Essentially the same results, namely resistance to a 2nd-INF, were obtained when CD4-deficient cells were superinfected with the VSV-g-coated viruses (Supplementary Fig. S3, available in JGV Online). Thus, these results suggest that the CD4 receptors are not involved in conferring resistance to the 2nd-INF. Dissociation of the intracellular Rev–IN complex by the INr peptides, resulted in significant stimulation of integration and clearly indicating involvement of Rev in promoting SIR (Supplementary Fig. S4, available in JGV Online).
Promotion of resistance was also observed when the 1st-INF was conducted with the wt virus and the 2nd-INF with a ΔRev virus (superinfection) (Fig. 5b). It should be noted, however, that much higher levels of integration, reaching up to between 25 and 30 integrations per cell, were obtained when cells were exposed to the ΔRev viruses 2 h after the 1st-INF (Fig. 5b), as compared with the 1.4 integrations per cell obtained under the same conditions following 2nd-INF with the wt HIV-1 strains (Fig. 5a). The involvement of Rev in suppressing the level of integration when the 2nd-INF was performed between 4 and 288 h was further confirmed by the addition of INr peptides (Supplementary Fig. S5, available in JGV Online).
SIR is induced by de novo synthesized Rev
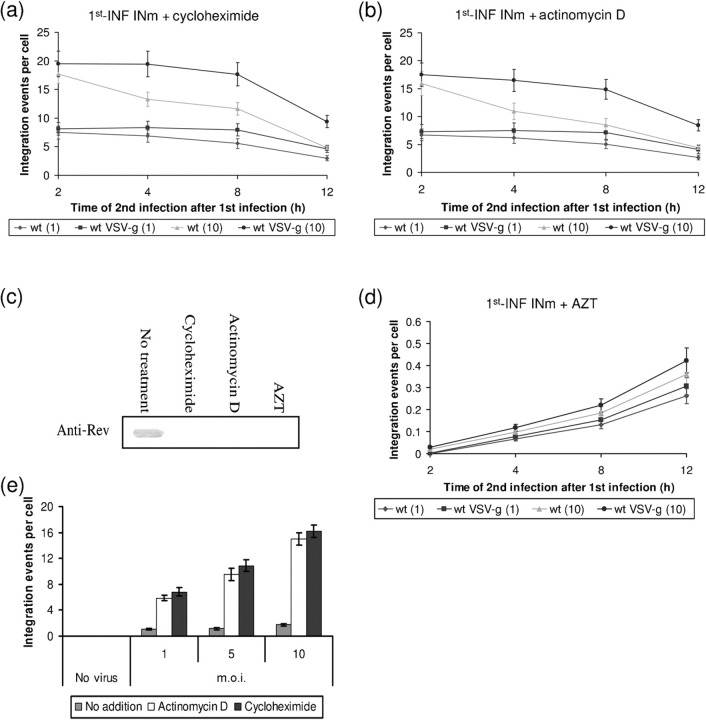
The possibility that SIR can be exerted by Rev molecules, which are delivered by the invading virus particles and not necessarily by newly synthesized molecules should be considered. Therefore, the effect of RNA- and protein-synthesis inhibitors on the appearance of Rev and suppression of integration was studied. As can be seen when the 1st-INF and 2nd-INF were conducted with the INm (to ensure expression of only Rev-ear) and wt HIV, respectively, the addition of cycloheximide and actinomycin D significantly increased the degree of integration (compare results shown in Fig. 6a, b to Fig. 1a) and completely blocked synthesis of Rev (Fig. 6c). These results strongly indicate that the suppression of integration, as an indicator for the promotion of SIR, observed at an early stage of infection results from the appearance of newly synthesized Rev molecules. Furthermore, the possibility that the newly synthesized Rev is encoded by virus-adsorbed cDNA molecules (see Lori et al., 1992) is eliminated by the results showing that AZT caused complete inhibition of the integration observed early on in infection, namely at 2–4 h after the 1st-INF (Fig. 6d). The requirement for an active process of reverse transcription of the viral RNA is thus established. Obviously, the fact that the observed stimulatory effect of cycloheximide and actinomycin D (Fig. 6a, b), as well as the inhibitory effect of AZT (Fig. 6d), are transient is due to their short intracellular half lives (Du & Low, 2001; Jensen et al., 1998; Johnson, 2001). It is worth noting that the integration levels observed following a single infection by a wt virus were also significantly stimulated by cycloheximide and actinomycin (Fig. 6e), probably indicating that also in this case, integration is suppressed by newly synthesized Rev (Iyer et al., 2009; Levin et al., 2009a). It should be mentioned that the appearance of newly assembled viruses as well as newly synthesized viral p24 was completely inhibited in the presence of these inhibitors (data not shown).
Fig. 6.
Inhibition of de novo synthesis of Rev in virus-infected cells abolishes SIR. (a) Integration events per cell were estimated following treatment with cycloheximide as described in Methods and then treated cells were 1st-INF with INm HIV-1 (m.o.i.=1) and 2nd-INF with the indicated wt viruses (m.o.i.=1 or 10). (b) Same as in (a), but cells were treated with actinomycin D. (c) Western blot analysis of INm HIV-1-infected cells incubated with the indicated inhibitors. (d) Same as in (a), but in the presence of AZT. (e) Effects of treatment with cycloheximide or with actinomycin D on integration levels following a single infection with wt HIV-1. All experimental details are as described in Methods.
Involvement of Rev in the regulation of integration events in synchronously infected cells
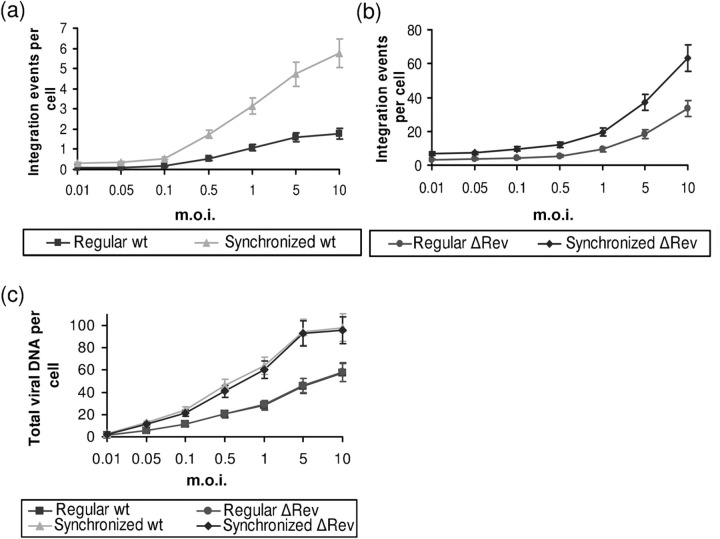
Generally, infection involves variation in the kinetics of virus adsorption, a process which may last for up to 4 h (Levin et al., 2009b). Therefore, a single infection can be considered as a double- or even superinfection process within a relatively short period of time. This pseudo-superinfection process is likely to be avoided by using a synchronized infection system. It was therefore of interest to study whether Rev is also involved in regulation of integration following synchronized infection. A threefold increase was observed in the integration per cell following synchronized infection (see Methods) as compared with the integration obtained following a non-synchronized (regular) single infection (Fig. 7a). Interestingly, as was observed following single (Levin et al., 2009a) or superinfection (Fig. 5a), significantly higher integrations per cell were also obtained when synchronized infection was conducted with the ΔRev virus (Fig. 7b). The observed integration levels were about twofold higher than those observed following a regular single infection (Fig. 7b). As has been observed previously (Levin et al., 2009a, 2010b), infection by the wt and ΔRev viruses resulted in the appearance of the same amount of viral cDNA (Fig. 7c). However, the amount of viral cDNA obtained in synchronized infected cells was about twofold higher than that observed following non-synchronized infection (Fig. 7c).
Fig. 7.
Integration and viral cDNA levels following synchronized and non-synchronized infection. (a) Integration events per cell were estimated following synchronized and normal single infection with wt HIV-1. (b) Same as in (a), but with ΔRev HIV-1. (c) Total viral DNA estimation for the experiments presented in (a) and (b).
DISCUSSION
Resistance of already infected cells to HIV infection has been considered to be mainly due to downregulation of the host cell membrane's CD4 receptors (Potash & Volsky, 1998; Saha et al., 1999). Downregulation of CD4 has been attributed to the expression of the viral Nef, Vpu and Env proteins (Wildum et al., 2006). Although the involvement of the CD4 receptors in conferring resistance is still under debate, there appears to be general agreement regarding the existence of an additional CD4-independent mechanism that promotes SIR (Potash & Volsky, 1998; Wildum et al., 2006). Moreover, the notion that interference with a second infection can be attributed to the presence of viral and not cellular encoded proteins is generally accepted (Nethe et al., 2005; Potash & Volsky, 1998). The mechanism which is responsible for SIR in HIV-1-infected cells is of interest, particularly for the development of novel approaches to anti-HIV therapy. One of the approaches that could be developed based on the mechanism of SIR is to imitate the condition that restricts the 2nd-INF in non-infected cells in order to transform them to resistance for infection.
Here, the involvement of the HIV Rev protein in conferring SIR is suggested. Cells expressing Rev, from either unintegrated or integrated viral cDNA, are resistant to virus infection. These results are supported by our previous studies showing that transfected cells expressing the Rev protein are almost completely immune to infection by HIV-1 (Levin et al., 2009a, 2010b). Our previous results (Levin et al., 2009a, b), as well, show that integration of the viral cDNA into the host chromosomal DNA is regulated by the Rev protein via its interaction with the viral IN. The fact that formation of Rev–IN complexes confers inhibition of IN enzymic activity has been demonstrated under in vitro as well as in vivo conditions (Levin et al., 2009a, b). Disruption of this complex, which can be achieved by peptides derived from IN that interact with Rev (the INrs) (Levin et al., 2009a, b), resulted in reactivation of the viral IN activity allowing multiple integration of the HIV-1 cDNA (Levin et al., 2009a).
Thus, the requirement of Rev for promoting SIR in infected cells can be inferred from two kinds of experiments. First, addition of INr peptides abolished resistance and resulted in stimulation of viral cDNA integration. Second, no resistance – as reflected by integrations per cell – was observed in the absence of Rev, particularly following double infection with ΔRev viruses. The fact that complete resistance can be promoted by merely the presence of Rev should be inferred from the results obtained following second infection with the VSV-g-coated virus, whose penetration into cells is independent of CD4 (Matlin et al., 1982).
Our results may also indicate the contribution of a CD4-dependent mechanism to SIR, especially in the complete absence of Rev. Indeed a 50 % reduction in the integration values was obtained following infection with wt-coated ΔRev virus when compared to infection with VSV-g-coated ΔRev virus. Thus, our observations support previous indications (Potash & Volsky, 1998; Wildum et al., 2006) of the existence of CD4-dependent and -independent mechanisms conferring SIR. Both mechanisms necessitate the presence of actively expressed viral genes. The extent of SIR probably is regulated by two parameters: the degree to which viral encoded proteins (Nef, Vpu, Env and Rev) are expressed following the 1st-INF, and the m.o.i. of the 2nd-INF. Indeed, results in the present work show that the degree of Rev-induced SIR can be decreased by increasing the m.o.i. of the 2nd-INF.
Our results suggest an additional new aspect in the process of Rev-induced resistance. Almost the same degree of integration, on average 1–2 integrations per cell, was observed following double, super and single infection by the wt virus at viral titres between m.o.i.=1 and 10. Similar to the case of double or superinfection, stimulation of virus integration was also observed, in the absence of Rev or in the presence of INrs, following a single infection (see also Levin et al., 2009a, b). This is not necessarily surprising since in a certain respect, the single infection process may present the same features that characterize double infection. It has been well established that the same cell can interact with more than one virus particle and that the course of virus-cell adsorption may extend over a period of about 4 h (Levin et al., 2009b; Pannecouque et al., 2002). Thus, the possibility of the first round of infection conferring, on a cellular level and due to de novo Rev synthesis, immunity to the second round of infection cannot be excluded. This might explain the stimulation of integration observed previously (Levin et al., 2009a, b) and in the present work by the addition of the INrs and in the absence of Rev following a single infection of cultured cells. If indeed this is the case, then no stimulation of integration should be observed in the absence of Rev following synchronized infection. Furthermore, such infection should result in a high level of integration when a wt virus is added. Indeed, the integration levels observed following synchronized infection were higher than those obtained following regular (not synchronized) single infection by the wt virus. However, synchronized infection with a ΔRev virus, i.e. in the absence of Rev, resulted in significantly higher integration levels than synchronized infection by the wt virus. This may indicate that, in addition to the already established inhibition of HIV IN activity by Rev, an additional – as yet non-elucidated – effect may be exerted by Rev, which restricts the level of integration and thus infection in HIV-1-infected cells. It should be noted that the speed with which the infection occurs after absorption can also differ from one entry event to the other. Attempts to reveal if indeed such a mechanism exists are under way in our laboratory. In any event, Rev-induced interference in virus infection as reflected by inhibition of cDNA integration appears to be a more general phenomenon.
METHODS
Cells.
Monolayer adherent HEK293T cells and HEK293T cells overexpressing Rev (Rev10+ cells) were grown in Dulbecco's modified Eagle's medium. The T-lymphocyte cell lines Sup-T1/TL3 and H9 were grown in RPMI 1640 medium. All cells except the Rev10+ and Sup-T1/TL3 cells were provided by the NIH Reagent Program, Division of AIDS, NIAID, NIH, USA. The Rev10+ cells were generated by transfection of HEK293T cells (Cullen, 1987; Levin et al., 2010a). The cells were incubated at 37 °C in a 5 % CO2 atmosphere. All media were supplemented with 10 % (v/v) fetal calf serum, 0.3 g l-glutamine l−1, 100 U penicillin ml−1 and 100 U streptomycin ml−1 (Biological Industries). Sup-T1/TL3 cells, a generous gift from Professor Poeschla (Mayo Foundation), were grown as described previously (Llano et al., 2006).
Viruses.
Wt HIV-1 [HXB2 (Ratner et al., 1985)] as well as the IN mutant D64N D116N (Nakajima et al., 2001) were generated by transfection of HEK293T cells (Cullen, 1987) with the virus-containing plasmid or co-transfection with a plasmid containing VSV-g (Levin et al., 2009b). ΔRev HIV (Verhoef et al., 1997) was generated by transfection of Rev10+ cells with pLAIY47H2 (Cullen, 1987). Viruses were harvested and stored as described previously (Levin et al., 2009b). The pLAIY47H2 (Verhoef et al., 1997) plasmid was a generous gift from Professor Berkhout (Department of Human Retrovirology, University of Amsterdam, The Netherlands). The INm D64N D116N-carrying plasmid was a generous gift from Professor Engelman (Dana-Farber Cancer Institute and Division of AIDS, Harvard Medical School, Boston, MA, USA).
Virus stock titration and normalization.
Quantitative titration of HIV-1 was carried out using the MAGI assay, as described by Kimpton & Emerman (1992). Briefly, TZM-b1 cells were grown in 96-well plates at 1×104 cells per well. The cells were infected with 50 μl serially diluted virus (wt or ΔRev HIV-1) as described previously (Kimpton & Emerman, 1992). Cultured cells were fixed (2 days p.i.) and β-galactosidase was estimated exactly as described previously (Kimpton & Emerman, 1992). Blue cells were counted under a light microscope at ×200 magnification. In the case of IN mutant viruses (D64N D116N) the amount of viral RNA was estimated by real-time reverse transcription PCR as described in Pizzato et al. (2009). This was also performed on the other HIV-1 viruses and was used to normalize the titre of the IN mutant virus. Virus stocks were concentrated by ultracentrifugation (25 000 r.p.m. at 15 °C for 105 min) using a Beckman SW28 rotor (Reiser, 2000) and then virus titre was determined as described above.
Peptide synthesis, labelling and purification.
Peptides were synthesized on an Applied Biosystems (ABI) 433A peptide synthesizer. For cellular-uptake studies, the peptides were labelled with fluorescein at their N terminus (Hayouka et al., 2007). Peptides were also labelled with Trp at their N terminus for UV spectroscopy. Peptide purification was performed on a Gilson HPLC using a reverse-phase C8 semi-preparative column (ACE; Advanced Chromatography Technologies) with a gradient of 5–60 % acetonitrile in water. Peptide concentrations were determined using a UV spectrophotometer (Shimadzu Kyoto) as described previously (Köhler et al., 1989).
Infection of cultured lymphocytes with HIV-1.
Cultured lymphocytes (1×105) were centrifuged for 5 min at 500 g and after removal of the supernatant, the cells were resuspended in 0.2–0.5 ml RPMI 1640 medium containing virus at different m.o.i. Following absorption for 2 h at 37 °C, the cells were washed to remove unbound virus and then incubated at the same temperature for up to 12 days (Rosenbluh et al., 2007). In the case of 2nd-INF, cells were infected with the indicated viruses at different m.o.i. at the indicated times after the first infection. For synchronized infection, virus adsorption was carried out for 2 h at 4 °C then cells were washed as described above and further incubated at 37 °C as described previously (Kopetzki et al., 2008).
Quantitative analysis of the copy numbers of HIV-1 DNA integrated into the host cellular genome.
Cells (samples of 200 μl) were removed 24 h post-2nd-INF, washed three times with PBS, and the integration levels of the viral cDNA were estimated exactly as described previously (Rosenbluh et al., 2007). Briefly, integrated HIV-1 sequences were amplified by two PCR replication steps using the HIV-1 LTR-specific primer (LTR-TAG-F 5′-ATGCCACGTAAGCGAAACTCTGGCTAACTAGGGAACCCACTG-3′) and Alu-targeting primers (first-Alu-F 5′-AGCCTCCCGAGTAGCTGGGA-3′ and first-Alu-R 5′-TTACAGGCATGAGCCACCG-3′) (Yamamoto et al., 2006). During the second-round PCR, the first-round PCR product could be specifically amplified by using the tag-specific primer (tag-F 5′-ATGCCACGTAAGCGAAACTC-3′) and the LTR primer (LTR-R 5′-AGGCAAGCTTTATTGAGGCTTAAG-3′) designed by PrimerExpress (Applied Biosystems) using default settings. The standard linear curve was in the range of 5 ng to 0.25 fg (R=0.99). DNA samples were assayed in quadruplicates of each sample.
Quantitative analysis of the copy numbers of total HIV-1 DNA.
Total viral DNA was estimated using SYBR green real-time quantitative PCR 12 h p.i., exactly as described by Casabianca et al. (2007). Briefly, DNA samples (1 μg DNA) were added to 95 μl containing 1× Hot-Rescue Real-time PCR kit-SG (Diatheva s.r.l.) and 100 nM of each primer-binding site primer: F5 (5′ primer, 5′-TAGCAGTGGCGCCCGA-3′) and R5 (3′ primer, 5′-TCTCTCTCCTTCTAGCCTCCGC-3′). All amplification reactions were carried out using an ABI Prism 7700 Sequence Detection System (Applied Biosystems): one cycle at 95 °C for 10 min, followed by 45 cycles of 15 s at 95 °C and 35 s at 68 °C. In each PCR run, three replicates were performed.
Estimation of inhibitor effects on SIR.
Inhibitors were added 1 h before the 1st-INF. The final concentration of each inhibitor was: 2 μM AZT, 5 μg actinomycin D ml−1 and 50 μg cycloheximide ml−1.
Western blot analysis.
Cells were infected with the indicated viruses at an m.o.i.=10 and harvested at different times p.i., washed three times with PBS and lysed by the addition of PBS containing 1 % (v/v) Triton X-100. The lysate was subjected to SDS-PAGE [using E-PAGE 48.8 % High-Throughput Pre-Cast Gel System (Invitrogen)] and immunoblotted with either monoclonal anti-Rev antibody (α-Rev) (Kramer-Hammerle et al., 2005) or anti-actin (α-Actin) antibody (Santa Cruz) and complement HRP-conjugated antibodies (Jackson) as second antibodies.
Study of in vivo protein–protein interactions using the co-immunoprecipitation methodology.
Co-immunoprecipitation experiments were conducted as described previously (Iordanskiy et al., 2004) with several modifications. Briefly, cells were secondarily infected with an m.o.i.=10 and 12 h post-1st-INF, and lysed as described above. The lysate was incubated for 1 h at 4 °C with α-IN antiserum raised against aa 276–288 (α-IN) (NIH AIDS Research & Reference Reagent Program, catalogue number 758) or α-Actin antibodies. Following 3 h incubation with protein G-agarose beads (Santa Cruz) at 4 °C, the samples were washed three times with PBS containing 1 % (v/v) Nonidet P40. SDS buffer was added and following SDS-PAGE [using E-PAG 48.8 % High-Throughput Pre-Cast Gel System (Invitrogen)], the appropriate membranes were immunoblotted with α-Rev, α-IN or α-Actin antibodies, and complement HRP-conjugated antibodies as second antibodies.
When peptides were used, cells were incubated with 150 μM of the indicated peptide for 2 h prior to infection.
All the experiments described in the present work have been repeated at least three times and the difference between the results never exceeded±10 %.
Supplementary Data
Supplementary Data
Acknowledgments
This work was supported by the Israeli Science Foundation (A. Loyter) and by a starting grant from the European Research Council (ERC) (to A. F.). The authors are imbedded to Professor Z. Debyser (Molecular Medicine, K. U. Leuven, Flanders, Belgium) and Professor Poeschla (Department of Molecular Medicine, Mayo Foundation, Rochester MN, USA) for the LEDGF/p75 knockdown cells. The authors are also indebted to Professor B. Berkhout (Department of Human Retrovirology, Academic Medical Center, University of Amsterdam, The Netherlands) and Professor A. Engelman (Department of Cancer Immunology and AIDS Dana-Farber Cancer Institute and Division of AIDS, Harvard Medical School, Boston, MA, USA) for the mutated viral strands. Authors' contributions: A. Loyter, A. Levin, A. F., D. J. V. and R. B. W. designed research; A. Levin performed the immunoprecipitation experiments and cultured cells infections assays; Z. H. performed peptides synthesis and purification; A. Levin and A. Loyter wrote the paper.
Footnotes
Supplementary material is available with the online version of this paper.
References
- Butler, S. L., Hansen, M. S. & Bushman, F. D. ( 2001. ). A quantitative assay for HIV DNA integration in vivo Nat Med 7, 631–634.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Casabianca, A., Gori, C., Orlandi, C., Forbici, F., Federico Perno, C. & Magnani, M. ( 2007. ). Fast and sensitive quantitative detection of HIV DNA in whole blood leucocytes by SYBR green I real-time PCR assay. Mol Cell Probes 21, 368–378.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Cullen, B. R. ( 1987. ). Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol 152, 684–704. [DOI] [PubMed] [Google Scholar]
- Du, X. & Low, M. G. ( 2001. ). Down-regulation of glycosylphosphatidylinositol-specific phospholipase D induced by lipopolysaccharide and oxidative stress in the murine monocyte-macrophage cell line RAW 264.7. Infect Immun 69, 3214–3223.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakova, S., Munch, J., Carl, S., Greenough, T. C., Sullivan, J. L., Margolis, L. & Kirchhoff, F. ( 2001. ). CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo J Virol 75, 10113–10117.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayouka, Z., Rosenbluh, J., Levin, A., Loya, S., Lebendiker, M., Veprintsev, D., Kotler, M., Hizi, A., Loyter, A. & other authors ( 2007. ). Inhibiting HIV-1 integrase by shifting its oligomerization equilibrium. Proc Natl Acad Sci U S A 104, 8316–8321.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanskiy, S., Zhao, Y., Dubrovsky, L., Iordanskaya, T., Chen, M., Liang, D. & Bukrinsky, M. ( 2004. ). Heat shock protein 70 protects cells from cell cycle arrest and apoptosis induced by human immunodeficiency virus type 1 viral protein R. J Virol 78, 9697–9704.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, S. R., Yu, D., Biancotto, A., Margolis, L. B. & Wu, Y. ( 2009. ). Measurement of human immunodeficiency virus type 1 preintegration transcription by using Rev-dependent Rev-CEM cells reveals a sizable transcribing DNA population comparable to that from proviral templates. J Virol 83, 8662–8673.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, B. L., Lehle, U., Muller, M., Wagner, C. & Kurtz, A. ( 1998. ). Interleukin-1 inhibits renin gene expression in As4.1 cells but not in native juxtaglomerular cells. Pflugers Arch 436, 673–678.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Johnson, S. ( 2001. ). Low-dose, sublingual AZT-monophosphate therapy for HIV+ patients? Med Hypotheses 56, 409–410.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Kelly, J., Beddall, M. H., Yu, D., Iyer, S. R., Marsh, J. W. & Wu, Y. ( 2008. ). Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology 372, 300–312.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpton, J. & Emerman, M. ( 1992. ). Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol 66, 2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler, F., Cardon, G., Pohlman, M., Gill, R. & Schieder, O. ( 1989. ). Enhancement of transformation rates in higher plants by low-dose irradiation: are DNA repair systems involved in incorporation of exogenous DNA into the plant genome? Plant Mol Biol 12, 189–199.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Kopetzki, E., Jekle, A., Ji, C., Rao, E., Zhang, J., Fischer, S., Cammack, N., Sankuratri, S. & Heilek, G. ( 2008. ). Closing two doors of viral entry: intramolecular combination of a coreceptor- and fusion inhibitor of HIV-1. Virol J 5, 56 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle, S., Ceccherini-Silberstein, F., Bickel, C., Wolff, H., Vincendeau, M., Werner, T., Erfle, V. & Brack-Werner, R. ( 2005. ). Identification of a novel Rev-interacting cellular protein. BMC Cell Biol 6, 20 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque, K., Zhao, Y. S. & Cohen, E. A. ( 2003. ). Vpu exerts a positive effect on HIV-1 infectivity by down-modulating CD4 receptor molecules at the surface of HIV-1-producing cells. J Biol Chem 278, 28346–28353.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Levin, A., Hayouka, Z., Brack-Werner, R., Volsky, D. J., Friedler, A. & Loyter, A. ( 2009a. ). Novel regulation of HIV-1 replication and pathogenicity: Rev inhibition of integration. Protein Eng Des Sel 22, 753–763.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, A., Hayouka, Z., Helfer, M., Brack-Werner, R., Friedler, A. & Loyter, A. ( 2009b. ). Peptides derived from HIV-1 integrase that bind Rev stimulate viral genome integration. PLoS One 4, e4155 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, A., Hayouka, Z., Friedler, A. & Loyter, A. ( 2010a. ). Over expression of the HIV-1 Rev promotes death of non-dividing eukaryotic cells. Virus Genes (in press) [DOI] [PubMed]
- Levin, A., Rosenbluh, J., Hayouka, Z., Friedler, A. & Loyter, A. ( 2010b. ). Integration of HIV-1 DNA is regulated by interplay between viral Rev and cellular LEDGF/p75 proteins. Mol Med 16, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano, M., Saenz, D. T., Meehan, A., Wongthida, P., Peretz, M., Walker, W. H., Teo, W. & Poeschla, E. M. ( 2006. ). An essential role for LEDGF/p75 in HIV integration. Science 314, 461–464.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Lori, F., di Marzo Veronese, F., de Vico, A. L., Lusso, P., Reitz, M. S., Jr & Gallo, R. C. ( 1992. ). Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol 66, 5067–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin, K. S., Reggio, H., Helenius, A. & Simons, K. ( 1982. ). Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol 156, 609–631.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Nakajima, N., Lu, R. & Engelman, A. ( 2001. ). Human immunodeficiency virus type 1 replication in the absence of integrase-mediated DNA recombination: definition of permissive and nonpermissive T-cell lines. J Virol 75, 7944–7955.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethe, M., Berkhout, B. & van der Kuyl, A. C. ( 2005. ). Retroviral superinfection resistance. Retrovirology 2, 52 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannecouque, C., Pluymers, W., Van Maele, B., Tetz, V., Cherepanov, P., De Clercq, E., Witvrouw, M. & Debyser, Z. ( 2002. ). New class of HIV integrase inhibitors that block viral replication in cell culture. Curr Biol 12, 1169–1177.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Piantadosi, A., Chohan, B., Chohan, V., McClelland, R. S. & Overbaugh, J. ( 2007. ). Chronic HIV-1 infection frequently fails to protect against superinfection. PLoS Pathog 3, e177 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzato, M., Erlwein, O., Bonsall, D., Kaye, S., Muir, D. & McClure, M. O. ( 2009. ). A one-step SYBR Green I-based product-enhanced reverse transcriptase assay for the quantitation of retroviruses in cell culture supernatants. J Virol Methods 156, 1–7.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Pollard, V. W. & Malim, M. H. ( 1998. ). The HIV-1 Rev protein. Annu Rev Microbiol 52, 491–532.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Potash, M. J. & Volsky, D. J. ( 1998. ). Viral interference in HIV-1 infected cells. Rev Med Virol 8, 203–211.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Ratner, L., Haseltine, W., Patarca, R., Livak, K. J., Starcich, B., Josephs, S. F., Doran, E. R., Rafalski, J. A., Whitehorn, E. A. & other authors ( 1985. ). Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature 313, 277–284.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Reiser, J. ( 2000. ). Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther 7, 910–913.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Rosenbluh, J., Hayouka, Z., Loya, S., Levin, A., Armon-Omer, A., Britan, E., Hizi, A., Kotler, M., Friedler, A. & other authors ( 2007. ). Interaction between HIV-1 Rev and integrase proteins: a basis for the development of anti-HIV peptides. J Biol Chem 282, 15743–15753.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Saha, K., Volsky, D. J. & Matczak, E. ( 1999. ). Resistance against syncytium-inducing human immunodeficiency virus type 1 (HIV-1) in selected CD4+ T cells from an HIV-1-infected nonprogressor: evidence of a novel pathway of resistance mediated by a soluble factor(s) that acts after virus entry. J Virol 73, 7891–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kuyl, A. C. & Cornelissen, M. ( 2007. ). Identifying HIV-1 dual infections. Retrovirology 4, 67 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef, K., Koper, M. & Berkhout, B. ( 1997. ). Determination of the minimal amount of Tat activity required for human immunodeficiency virus type 1 replication. Virology 237, 228–236.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Volsky, D. J., Simm, M., Shahabuddin, M., Li, G., Chao, W. & Potash, M. J. ( 1996. ). Interference to human immunodeficiency virus type 1 infection in the absence of downmodulation of the principal virus receptor, CD4. J Virol 70, 3823–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildum, S., Schindler, M., Munch, J. & Kirchhoff, F. ( 2006. ). Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol 80, 8047–8059.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. ( 2004. ). HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology 1, 13 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. ( 2008. ). The second chance story of HIV-1 DNA: Unintegrated? Not a problem!. Retrovirology 5, 61 [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. & Marsh, J. W. ( 2003. ). Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J Virol 77, 10376–10382.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, N., Tanaka, C., Wu, Y., Chang, M. O., Inagaki, Y., Saito, Y., Naito, T., Ogasawara, H., Sekigawa, I. & other authors ( 2006. ). Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes 32, 105–113.[CrossRef] [DOI] [PubMed] [Google Scholar]
- Yeh, W. W., Jaru-Ampornpan, P., Nevidomskyte, D., Asmal, M., Rao, S. S., Buzby, A. P., Montefiori, D. C., Korber, B. T. & Letvin, N. L. ( 2009. ). Partial protection of Simian immunodeficiency virus (SIV)-infected rhesus monkeys against superinfection with a heterologous SIV isolate. J Virol 83, 2686–2696.[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data