Abstract
Pulmonary arterial hypertension is associated with tyrosine kinase inhibitors used in the treatment of chronic myeloid leukemia. Dasatinib is a known cause of drug-induced pulmonary arterial hypertension. There have been case reports linking Bosutinib with deterioration of pre-existing pulmonary arterial hypertension. Here, we present a case of a 37-year-old woman with chronic myeloid leukemia treated with Bosutinib who was diagnosed with pulmonary arterial hypertension. Prior to Bosutinib, she had received Dasatinib without documented cardiopulmonary toxicity. Withdrawal of Bosutinib led to partial reversal of pulmonary arterial hypertension, and with the addition of pulmonary arterial hypertension-targeted treatment, there was near normalization of hemodynamics.
Keywords: drug-induced pulmonary hypertension, tyrosine kinase inhibitors, chronic myeloid leukemia, Macitentan
Introduction
Chronic myeloid leukemia (CML) is characterized by an acquired translocation between chromosomes 9 and 22 resulting in the BCR-ABL fusion gene which encodes a constitutively active tyrosine kinase. The prognosis of CML has improved since the introduction of BCR-ABL tyrosine kinase inhibitors (TKIs).1
There is accumulating data on the cardiopulmonary toxicities of TKIs.2 Dasatinib, a second-generation TKI, has a definite association with pulmonary arterial hypertension (PAH).3
Bosutinib, another second-generation TKI, is considered to have a “possible association” with PAH,3 based on reports of deterioration on Bosutinib in patients with pre-existing Dasatinib-induced PAH.4–6
We present a case of incident PAH associated with Bosutinib. Although the patient had prior exposure to Dasatinib, there was no documented evidence of PAH prior to treatment with Bosutinib.
Written informed consent was obtained from the patient for publication of anonymized health information.
Case description
This 37-year-old woman was diagnosed with CML in 2002. She was treated initially with Imatinib for seven years, which was switched to Dasatinib because of alopecia, peripheral edema, and periorbital edema. An echocardiogram two years after commencing Dasatinib was normal. She remained on Dasatinib for five years, with no symptoms of cardiopulmonary dysfunction. However, she developed proteinuria of 3 g/24 h, a recognized toxicity associated with Dasatinib,7 that necessitated a switch to Bosutinib in August 2014. After that change her proteinuria improved to 0.89 g/24 h and she had normal creatinine and serum albumin (43 g/L). She maintained major molecular response to CML throughout this period.
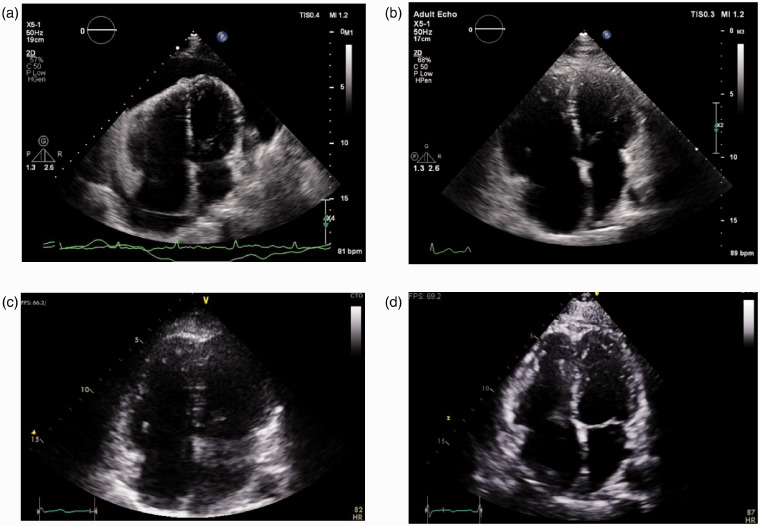
In May 2018, she presented with a six-month history of progressive dyspnea graded as New York Heart Association (NYHA) functional class (FC) III. She was normotensive with no signs of heart failure. Echocardiography revealed a large pericardial effusion with strong evidence for tamponade, that is, inversion of the right atrium (RA) and right ventricle (RV) and a “swinging” appearance. The RV was moderately dilated (Fig. 1a). RV systolic pressure (RVSP) could not be obtained due to incomplete tricuspid regurgitation Doppler spectrum. Electrocardiogram demonstrated electrical alternans. She was admitted to hospital under the general cardiology unit and pericardiocentesis was performed with 1 L of serosanguinous fluid drained over 24 h. At the time, the pericardial effusion was thought to be a direct drug-related toxicity, and Bosutinib was ceased.
Figure 1.
Echocardiogram apical four chamber views. (a) May 2018. Large pericardial effusion. Moderately dilated right ventricle (RV) with diastolic collapse. (b) June 2018, after drainage of pericardial effusion. Severely dilated RV and dilated right atrium (RA). (c) November 2018, 6 months after Bosutinib withdrawal. Moderate RV dilation. Normal RA size. (d) March 2019, 10 months after Bosutinib withdrawal. Mild RV dilation. Normal RA size.
Following pericardiocentesis, her dyspnea improved (NYHA FC II). Repeat echocardiography demonstrated RVSP of 52 mmHg, severe RV enlargement, moderately reduced RV systolic function and moderate RA enlargement. There was flattening of the interventricular septum and no features of left-sided heart disease (Fig. 1b). No residual pericardial effusion. These findings raised, for the first time, concerns about underlying severe pulmonary hypertension (PH).
Ventilation/perfusion lung scan excluded pulmonary embolism. Computed tomography scan of the chest and pulmonary function testing did not reveal chronic lung disease. Connective tissue disease, HIV, and liver disease were excluded. There was no history of anorexigen or recreational drug use. Cardiac magnetic resonance imaging did not demonstrate any evidence of a direct myocardial or pericardial pathology.
Right heart catheterization (RHC) confirmed pre-capillary PH: RA pressure (RAP) 6 mmHg, pulmonary artery pressure (PAP) 52/22/35 mmHg (systolic/diastolic/mean), pulmonary capillary wedge pressure (PCWP) 11 mmHg, cardiac output (CO) 2.75 L/min, cardiac index (CI) 1.6 L/min/m2, pulmonary vascular resistance (PVR), 8.7 Wood units (WU), mixed venous oxygen saturation (MVO2) 61%, and RV stroke work index (RVSWI) 6.6 g·m/m2/beat.
Following the RHC, the patient was referred to our PH clinic. She continued to improve clinically following the withdrawal of Bosutinib. In November 2018, she was NYHA FC II, with a 6-min walk distance (6MWD) of 585 m, BNP <10 ng/L and echocardiogram showed moderate RV dilation, mildly reduced RV systolic function, normal RA size, and no pericardial effusion (Fig. 1c). RVSP could not be obtained.
Given her clinical improvement, RHC was repeated in January 2019. This showed partial hemodynamic improvement: RAP 1 mmHg, PAP 43/7/27 mmHg, PCWP 7 mmHg, CO 3.19 L/min, CI 1.8 L/min/m2, PVR 6.3 WU, MVO2 73%, and RVSWI 7.5 g·m/m2/beat. We elected to not treat the patient with PAH-specific therapy at this stage, in hopes of further improvement over time. In June 2019, after 13 months off Bosutinib, RHC again demonstrated persistent PAH: RAP 2 mmHg, PAP 41/16/27 mmHg, PCWP 8 mmHg, CO 3.36 L/min, CI 1.9 L/min/m2, PVR 5.7 WU, MVO2 72%, and RVSWI 7.6 g·m/m2/beat. She was NYHA FC II and 6MWD was 577 m. Brain natriuretic peptide (BNP) <10 ng/L. Echocardiography showed mild RV dilation, mildly reduced RV systolic function, normal RA size, and no pericardial effusion (Fig. 1d). As per guideline recommendations,8 given her mild PAH and low-risk status, monotherapy was commenced in the form of Macitentan 10 mg PO once daily. At this time she was started on Nilotinib for her CML.
In December 2019, six months after starting Macitentan, she was clinically stable in NYHA FC II and 6MWD was 570 m. She had near-normal hemodynamics: RAP 7 mmHg, PAP 35/16/25 mmHg, PCWP 13 mmHg, CO 4.17 L/min, CI 2.4 L/min/m2, PVR 2.9 WU, MVO2 75%, and RVSWI 8.4 g·m/m2/beat. Our cardiac catheterization laboratory employs the indirect Fick method to determine CO, wherein oxygen uptake is estimated using the LaFarge formula. Unfortunately, this is known to produce inaccurate results and is the likely explanation for the patient’s incongruously low CI. In contrast, her MVO2 and RVSWI results were favorable, in keeping with her clinical status. Acute vasoreactivity testing with nitric oxide showed no change in PAP. We had not performed vasoreactivity testing after the two prior RHC because, based on her clinical status, we had anticipated her PAP would be near-normal and were not contemplating starting treatment.
Discussion
Here, we presented a case of incident PAH associated with Bosutinib. Our patient had prior treatment with Dasatinib without documented Dasatinib-induced cardiopulmonary toxicity. She presented with progressive exercise intolerance and a large pericardial effusion after exposure to Bosutinib for 45 months. Her moderate-to-severe PAH improved partially after withdrawal of Bosutinib. Treatment with Macitentan resulted in near normalization of hemodynamics.
The introduction of BCR-ABL TKIs transformed the prognosis of CML, and patients who achieve complete cytogenetic remission have survival rates comparable to the general population.2 Bosutinib was commercially introduced more recently than Dasatinib, in 2012 and 2006, respectively. Bosutinib was originally introduced as a second-line agent for CML resistant or intolerant to prior therapy.1 More recently, Bosutinib received approval for first-line treatment based on a phase III study demonstrating superior response rates compared to Imatinib.9 However, TKIs are associated with cardiopulmonary toxicity,2 which can cause significant morbidity and even mortality.10 These toxicities have been attributed to off-target effects.2
Dasatinib is a cause of PAH,3 with the lowest estimated incidence of 0.45% based on French PH registry data.10 There have been four reported cases of Bosutinib associated with deterioration of pre-existing Dasatinib-induced PAH.4–6 The sixth World Symposium on PH in 2018 recognized Bosutinib as having a “possible association” with PAH.3 More recently, analysis of pharmacovigilance data found a signal for PAH and Bosutinib, along with several other protein kinase inhibitors. However, there was insufficient granularity in the data to distinguish new onset from aggravation of PAH.11 TKI-induced PAH is usually a late complication; the median duration of Dasatinib exposure at diagnosis of PAH is 42 (range 8–74) months.12 Therefore, it is conceivable that more cases of Bosutinib-associated PAH will be discovered with accumulating long-term clinical experience and pharmacovigilance.
Our patient had been previously exposed to Dasatinib for five years, which was switched to Bosutinib because of severe proteinuria associated with Dasatinib.7 Echocardiogram was normal two years into treatment with Dasatinib. She did not have any cardiopulmonary symptoms on Dasatinib, and only began to develop exercise intolerance 39 months after starting Bosutinib.
As there was no echocardiogram for three years preceding the switch from Dasatinib to Bosutinib, we cannot entirely discount the possibility that PAH was present prior to commencing Bosutinib. However, that is unlikely considering that she only started to develop symptoms of PAH more than three years after switching from Dasatinib to Bosutinib. Data from the French PH registry showed that all 21 patients with Dasatinib-induced PAH were receiving Dasatinib at the time of diagnosis of PAH.12 Additionally, the four previously reported cases of Bosutinib-associated exacerbation of pre-existing PAH occurred within 12 months of exposure to Bosutinib.4–6 Therefore, it is most probable that our patient developed PAH secondary to Bosutinib. Nonetheless, one has to entertain the possibility that previous Dasatinib exposure had a priming effect or additive toxicity.
A postulated mechanism for TKI-induced PAH is off-target inhibition of Src, a family of kinases involved in modulating pulmonary vascular tone and remodelling.11,13 A significant correlation between pharmacovigilance signals and affinity for Src kinases has been found.11 Dasatinib and Bosutinib are potent inhibitors of Src. PAH has also been reported with Ponatinib,14 a third-generation TKI which also inhibits Src.2 Nevertheless, considering that PAH only occurs in a small minority of patients exposed to these agents, it is likely that the pathobiology is multifactorial. More work is evidently needed to elucidate the complex mechanisms involved in the pathogenesis of drug-induced PAH.
Author contributions
All authors contributed equally to the writing and editing of the manuscript.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Saglio G, Jabbour E. First-line therapy for chronic phase CML: selecting the optimal BCR-ABL1-targeted TKI. Leuk Lymphoma 2018; 59: 1523–1538. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015; 33: 4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickey PM, Thompson AAR, Charalampopoulos A, et al. Bosutinib therapy resulting in severe deterioration of pre-existing pulmonary arterial hypertension. Eur Respir J 2016; 48: 1514–1516. [DOI] [PubMed] [Google Scholar]
- 5.Riou M, Seferian A, Savale L, et al. Deterioration of pulmonary hypertension and pleural effusion with bosutinib following dasatinib lung toxicity. Eur Respir J 2016; 48: 1517–1519. [DOI] [PubMed] [Google Scholar]
- 6.Seegobin K, Babbar A, Ferreira J, et al. A case of worsening pulmonary arterial hypertension and pleural effusions by bosutinib after prior treatment with dasatinib. Pulm Circ 2017; 7: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alahmari A, Lipton JH, Kim D. Dasatinib induced reversible nephrotic range proteinuria occurs more frequently compared to other tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Blood 2017; 130: 2880. [Google Scholar]
- 8.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol 2018; 36: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montani D, Bergot E, Günther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 2012; 125: 2128–2137. [DOI] [PubMed] [Google Scholar]
- 11.Cornet L, Khouri C, Roustit M, et al. Pulmonary arterial hypertension associated with protein kinase inhibitors: a pharmacovigilance-pharmacodynamic study. Eur Respir J 2019; 5: 1802472. [DOI] [PubMed] [Google Scholar]
- 12.Weatherald J, Chaumais MC, Savale L, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J 2017; 50: 1700217. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraj C, Tang B, Bálint Z, et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J 2013; 41: 85–95. [DOI] [PubMed] [Google Scholar]
- 14.Quilot FM, Georges M, Favrolt N, et al. Pulmonary hypertension associated with ponatinib therapy. Eur Respir J 2016; 47: 676–679. [DOI] [PubMed] [Google Scholar]