Abstract
Background:
Microfracture (MFx) is one of the most common techniques used for the treatment of articular cartilage defects, although recently there has been a trend toward the use of drilling rather than MFx for the treatment of these defects.
Purpose:
To perform a systematic review of basic science studies to determine the effect of microfracture versus drilling for articular cartilage repair.
Study Design:
Systematic review.
Methods:
A systematic review was performed by searching PubMed, the Cochrane Library, and EMBASE to identify basic science studies comparing outcomes of MFx versus drilling. The search phrase used was microfracture AND (drilling OR microdrilling). Inclusion criteria were basic science studies that directly compared the effect of MFx versus drilling on subchondral bone, bone marrow stimulation, and cartilage regeneration.
Results:
A total of 7 studies met the inclusion criteria and were included in this systematic review. Of these, 4 studies were performed in rabbits, 1 study in sheep, and 2 studies in humans. All of the included studies investigated cartilage repair in the knee. In the animal studies, microfracture produced fractured and compacted bone and led to increased osteocyte necrosis compared with drilling. Deep drilling (6 mm) was superior to both shallow drilling (2 mm) and MFx in terms of increased subchondral hematoma with greater access to marrow stroma, improved cartilage repair, and increased mineralized bone. However, the overall quality of cartilage repair tissue was poor regardless of marrow stimulation technique. In 2 studies that investigated repair tissue after MFx and/or drilling in human patients with osteoarthritis and cartilage defects, the investigators found that cartilage repair tissue did not achieve the quality of normal hyaline articular cartilage.
Conclusion:
In the limited basic science studies that are available, deep drilling of cartilage defects in the knee resulted in improved biological features compared with MFx, including less damage to the subchondral bone and greater access to marrow stroma. Regardless of marrow stimulation technique, the overall quality of cartilage regeneration was poor and did not achieve the characteristics of native hyaline cartilage. Overall, there is a general lack of basic science literature comparing microfracture versus drilling for focal chondral defects.
Keywords: articular cartilage, bone marrow stimulation, drilling, focal chondral defects, microfracture
Numerous surgical techniques exist for the treatment of isolated articular cartilage defects, including debridement, microfracture (MFx), drilling, osteochondral autograft and allograft transplantation, autologous chondrocyte implantation, and autologous matrix-induced chondrogenesis.22,23,31,32,34 Although subchondral drilling was popular in the 1980s, concerns about osteocyte thermal necrosis led to the development of MFx, in which an awl rather than a motorized drill is used to create subchondral perforations, leading to the release of mesenchymal stem cells and growth factors.14 MFx is often considered a first-line treatment option for these defects, given the ease and low cost of the procedure as well as the good short-term outcomes demonstrated with this technique.1,24,27,31,40 For this reason, new techniques for articular cartilage repair are often compared with the results of MFx.3,13,36 However, recent evidence has suggested that the outcomes of knee MFx may worsen after 5 years postoperatively, particularly for larger lesions and chondral defects in athletes.15,16,33,37,39 Specific concerns regarding the durability of the MFx technique include the effect of microfractures in the subchondral bone, possibly making the bone brittle2 and leading to subchondral cyst formation and subchondral plate disruption.20 Furthermore, MFx leads to the growth of fibrocartilage, which differs in biomechanical properties from the native hyaline cartilage.26 Based on this evidence, some sports medicine surgeons have recently abandoned the traditional MFx technique in exchange for a return to drilling due to a belief that drilling is less detrimental to the subchondral bone and may result in deeper penetration and stimulation of bone marrow cells with higher regenerative potential. The purpose of this study was to perform a systematic review of basic science studies to determine the effect of MFx versus drilling on articular cartilage repair.
Methods
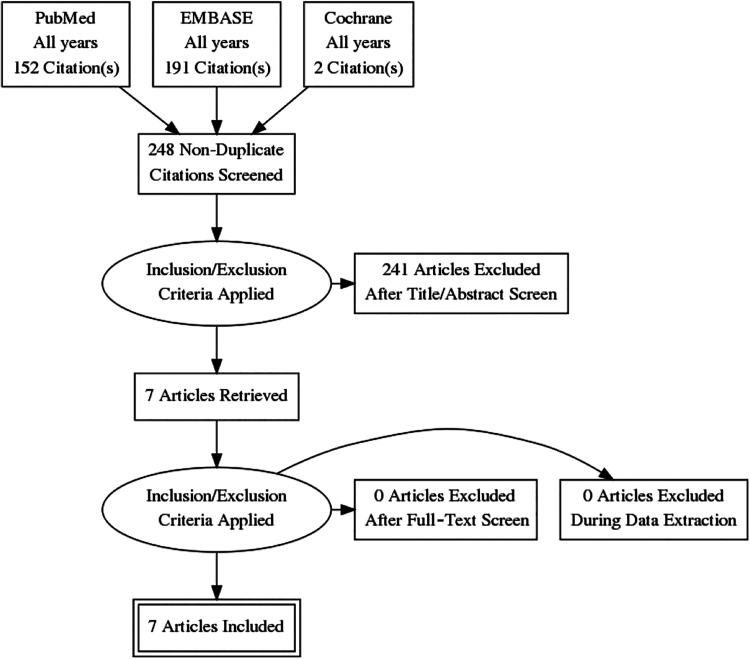
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using a PRISMA checklist. Two independent reviewers (M.J.K., G.M.A.) searched PubMed, EMBASE, and the Cochrane Library up to May 5, 2019. The electronic search strategy used was microfracture AND (drilling OR microdrilling). A total of 248 studies were reviewed by title and/or abstract to determine study eligibility based on inclusion criteria. In cases of disagreement, a third reviewer (M.K.M.) made the final decision. The inclusion criteria were full-text basic science studies that directly compared the effect of MFx versus drilling on the subchondral bone, bone marrow stimulation, and cartilage regeneration. Exclusion criteria included (1) studies without direct comparison between the 2 techniques, (2) studies that reported exclusively clinical outcomes, (3) studies that used biologic agents (eg, platelet-rich plasma or bone marrow aspirate) without reporting the effects of MFx or drilling alone, and (4) studies that either did not clearly define the bone marrow stimulation technique or did not distinguish results between MFx and drilling. Human and animal studies were included. Inclusion criteria were not restricted to 1 particular joint. In total, 7 studies were determined to meet inclusion and exclusion criteria (Figure 1). Data extraction from each study was performed independently and then reviewed by a second author (M.J.K.). There was no need for funding or a third party to obtain any of the collected data.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram.
Reporting Outcomes
Outcomes assessed included cartilage histological features (O’Driscoll score,28 International Cartilage Repair Society [ICRS] score,4,25 Sellers score35), stereology, and micro-computed tomography (micro-CT) for analysis of the subchondral bone. The ICRS histological score includes 6 categories, each scored from 0 to 3; higher total scores indicate complete cartilage regeneration. The Sellers score ranges from 0 to 31; a score of 0 correlates with normal articular cartilage and complete regeneration, and a score of 31 correlates with an empty defect with no repair tissue.
Results
Overall, 7 studies met the inclusion criteria and were therefore included in this systematic review (Figure 1). Of these, 4 studies6–9 were performed in rabbits, 1 study41 in sheep, and 2 studies21,29 in humans. Of note, the 2 human studies21,29 focused on patients who experienced failed marrow stimulation procedures and subsequently underwent total knee arthroplasty (TKA). All included studies investigated cartilage repair in the knee joint (Table 1).
Table 1.
Study Characteristicsa
| Lead Author (Year) | Participants (n) | Outcomes | Summary |
|---|---|---|---|
| Chen9 (2009) | Rabbits (4) | Micro-CT | MFx in the trochlear groove produced fractured and compacted
bone, potentially impeding repair, and led to increased
osteocyte death. Drilling cleanly removed bone from the holes. Deep drilling (6 mm) led to increased subchondral hematoma, allowing for greater access to marrow stroma. |
| Chen7 (2011) | Rabbits (24) | Scoring system for a qualitative assessment of subchondral bone repairs, 3D micro-CT | Both MFx and drilling in the trochlear groove showed repaired
bone with atypical morphologic features, suggesting inability to
reconstitute native structure of subchondral bone. Deep drilling (6 mm) induced increased regions of repair and remodeling bone that positively correlated with improved cartilage repair. |
| Chen8 (2011) | Rabbits (16) | O’Driscoll histological scoring, quantitative histomorphometry | Deep drilling (6 mm) in the trochlear groove showed significant improvement in repair, whereas both shallow drilling (2 mm) and MFx produced a similar quantity and quality of cartilage repair. |
| Chen6 (2013) | Rabbits (48) | Histology, stereology, histomorphometry, micro-CT | The authors compared early repair response of cartilage defects
in the trochlea and MFC after MFx or drilling in mature rabbit
knee joints. Stromal cell density recruitment was similar in defects regardless of location or bone marrow stimulation technique. Trochlear defects showed significantly higher volume fraction of chondrocytes compared with MFC (increased chondrocytes and larger chondrogenic foci). Deep drilling (6 mm) elicited significantly more mineralized bone compared with shallow drilling and MFx. |
| Kaul21 (2012) | Humans (5) | ICRS score, Sellers score | The authors examined repair tissue and adjacent articular
cartilage after failed MFx or Pridie drilling in patients with
early knee OA in MFC. Macroscopic cartilage repair assessment showed ICRS grades II and III, with cartilage defects filled with fibrocartilaginous tissue. Cartilage-specific stains of repair tissue were reduced compared with normal articular cartilage, subchondral bone was incompletely restored, and repair tissue always showed positive immunoreactivity for types II and X collagen. Compared with surrounding native cartilage, repair tissue showed more intense cartilage-specific stains and higher proteoglycan content. Overall, articular cartilage repair did not achieve the quality of normal hyaline articular cartilage. |
| Sakata29 (2013) | Humans (4) | ICRS score | The authors retrospectively investigated cartilage repair after
MFx or drilling for treatment of large cartilage defects in
osteoarthritic human knees in MFC. Marrow stimulation resulted in insufficient cartilage repair on MFCs. Safranin O–stained proteoglycans and type II collagen were seen in only the deep zones of marrow-stimulated holes. Marrow stimulation did not achieve hyaline-like cartilage regeneration in cartilage repair and histological assessment, suggesting low potential for long-term clinical improvement. |
| Zedde41 (2016) | Sheep (4) | Micro-CT | MFx in the MFC showed limited perforation depth and cone-shaped
channels with a high degree of regularity and trabecular bone
compaction leading to a sealing effect. Drilling showed channels with greater depth and smaller diameters with more natural irregularities, absence of trabecular compaction, and improved communication with trabecular canals. Overall, drilling allowed deeper perforation into subchondral bone with less fragmentation and compaction, resulting in improved restoration of the normal subchondral bone architecture. |
a3D, 3-dimensional; CT, computed tomography; ICRS, International Cartilage Repair Society; MFC, medial femoral condyle; MFx, microfracture; OA, osteoarthritis.
Scientific Methods
MFx was performed with an awl to a depth of 2 mm with a 1-mm base in 4 studies,6–9 and drilling was performed at 2 different depths (deep at 6 mm, shallow at 2 mm) with a 0.9-mm base.6–9 Defects were 4 × 4 mm2 in the 4 rabbit studies,6–9 0.50 cm2 in the sheep study,41 2.6 to 4 cm2 in 1 human study,21 and 6 to 8 cm2 in 1 human study.29 In the sheep study, an 8 mm–diameter full-thickness chondral lesion was created by use of an arthroscopic bur; each cartilage lesion was treated with 3 or 5 channels separated by 3 mm.41 MFx was performed with a curved Steadman awl with perforation depth user-controlled with visual feedback from the awl tip, whereas drilling (“nanofracture”) was performed with a cannulated awl and a 1 mm–thick Nitinol needle to a depth of 9 mm.41 One human study21 did not detail the MFx/drilling technique. In the other human study,29 MFx was performed with a 1.5 mm–diameter awl to a depth of 5 mm, at distances of 3 to 4 mm, and drilling was performed with a 1.2-mm Kirschner wire to the same depth and distance. Rabbits were sacrificed 1 day postoperatively in 1 study,9 3 months postoperatively in 2 studies,7,8 and 14 and 21 days postoperatively in 1 study.6 Sheep were sacrificed 6 months after surgery.41 Human knees were analyzed on average 8.8 months after marrow stimulation in 1 study21 and 20.5 months in the other.29
Effect on the Subchondral Bone
In 4 studies,6–9 deep drilling (6 mm) was superior to both shallow drilling (2 mm) and MFx, eliciting significantly more mineralized bone and thereby increased percentage fill of the defects. This was due to an increased volume of repaired and remodeling subchondral bone in the defects created during deep drilling. Deep drilling cleanly removed bone, thereby allowing for free access channels to the marrow space, whereas MFx produced fractured and compacted bone around the MFx holes, potentially impeding repair, and led to increased osteocyte necrosis.9 However, both MFx and drilling showed repaired bone with atypical morphologic features compared with the native structure of subchondral bone, which was less organized and more isotropic, distinct from the surrounding bone.7 Deep (rather than shallow) drilling was shown to produce increased percentage of tissue repair volume within the projected defect, increased Safranin O, increased collagen type II, and decreased collagen type I; when analyzed together via quantitative histomorphometry, these showed significant (P = .021) improvement in tissue repair versus shallow drilling.8 Shallow drilling, however, did not show any statistically significant improvements over MFx.8 In another study, Chen et al6 found that 95% of drill holes, regardless of depth, compared with only half of MFx holes contained chondrogenic foci at 21 days postoperatively. Deep drilling showed a significantly higher percentage of bone fill compared with both shallow drilling and MFx.6 Kaul et al21 reported mostly incomplete restoration of subchondral bone.
Zedde et al41 demonstrated that specimens undergoing MFx showed a limited perforation depth of the awl, with large-diameter channels at the joint surface displaying a high degree of regularity and trabecular bone compaction, leading to a sealing effect and the development of newly formed trabeculae inside the channels. In that study, drilling showed greater depth of the perforations with smaller diameter, more natural irregularities of the walls, absence of trabecular compaction, and improved communication between the canals and the perforation, resulting in a trabecular structure similar to native subchondral bone. Drilling samples did not show any subchondral cyst formation, whereas 3 of 4 MFx samples showed subchondral cysts ranging from 7 to 12 mm in diameter.
Bone Marrow Stimulation
Deep drilling (6 mm) was superior to both shallow drilling (2 mm) and MFx in terms of increased subchondral hematoma with greater access to marrow stroma.9 In 1 study, stromal cell density recruitment was similar in defects regardless of location (trochlea vs medial femoral condyle [MFC]) or bone marrow stimulation technique.6 Trochlear defects, however, showed a significantly higher volume fraction of chondrocytes with increased number of mature foci compared with MFC defects. Based on micro-CT at day 14, MFC defects demonstrated faster woven bone formation through intramembranous ossification compared with trochlear defects, whereas both locations were comparable at day 21.
Cartilage Regeneration
In 2 studies,7,8 deep drilling (6 mm) was superior to both shallow drilling (2 mm) and MFx in terms of significantly improved cartilage repair. Overall, articular cartilage repair did not achieve the quality of normal hyaline cartilage, regardless of marrow stimulation technique.21,29 Kaul et al,21 in a study of 5 patients undergoing TKA at a mean 8.8 months after failed marrow stimulation procedures, reported that cartilage-specific stains of the repair tissue were reduced compared with normal articular cartilage, suggesting poor cartilage quality compared with normal, and the repair tissue always showed positive immunoreactivity for type II collagen (most common type of collagen in hyaline cartilage17) and type X collagen (mainly expressed in hypertrophic chondrocytes18) while only sometimes showing positive immunoreactivity for type I collagen (most abundant collagen, expressed in almost all connective tissues19).
Moreover, Kaul et al21 showed that marrow stimulation resulted in repair tissue with histological and biochemical properties of fibrocartilage. Macroscopic cartilage repair assessment showed ICRS grades II (nearly normal) and III (abnormal), with cartilage defects mostly filled with fibrocartilaginous tissue. Light and polarized light microscopy analysis always showed fibrocartilaginous repair, with weaker birefringence. Microscopic analysis showed cartilage defects completely filled with cell-rich reparative fibrocartilaginous tissue. ICRS analysis showed irregular surface, fibrocartilaginous matrix, cell clusters, and abnormal mineralization in the majority of repairs (≥3).
Compared with adjacent articular cartilage, the repair tissue was more cellular and showed more intense cartilage-specific stains and higher proteoglycan content. The mean Sellers score of the repair tissue was 17.6, and ICRS histological grades ranged from 7 to 9.21,25 In another human study focusing on 4 patients undergoing TKA after failed marrow stimulation of large cartilage defects, Sakata et al29 demonstrated that marrow stimulation resulted in insufficient cartilage repair, and Safranin O–stained proteoglycans and type II collagen were seen mainly in the deep zones of marrow-stimulated holes for both MFx and drilling. Marrow stimulation did not achieve hyaline-like cartilage regeneration and histological assessment via ICRS scoring, and the grade of cartilage repair was not improved compared with the preoperative assessment. In a study by Chen et al,8 Safranin O–stained proteoglycan repair tissue was similarly detected in mostly the deep-mid region of repair tissue at 3 months postoperatively. All treatment groups showed more widespread immunoreactive collagen type II in the repair matrix compared with Safranin O stain. All treatment groups demonstrated repair tissue with poor bonding to adjacent cartilage (O’Driscoll bonding scores <1).
Discussion
Based on the findings of this systematic review, drilling of articular cartilage defects in the knee joint results in several improved biological features compared with MFx. In particular, drilling was found to cause less damage to the subchondral bone, result in greater access to marrow stroma, and lead to a higher volume of cartilage repair tissue. Furthermore, multiple studies demonstrated improved outcomes of deep drilling (6-mm depth) of chondral defects compared with shallow drilling (2-mm depth). However, regardless of marrow stimulation technique, the overall quality of cartilage regeneration was poor and did not achieve the characteristics of the native hyaline cartilage of the articular surfaces.
Due to its limited vascular supply, articular cartilage has limited to no ability to spontaneously repair after injury,5 with full-thickness lesions leading to pain and joint swelling if left untreated.11 Focal chondral defects (FCDs) are common, with a reported incidence of 48% in the medial compartment of the knee and 25% in the lateral compartment among patients undergoing knee arthroscopy.12 MFx was first described by Steadman et al38 as an alternative technique to treat full-thickness cartilage defects in the knee joint by using an awl to make multiple perforations in the subchondral bone plate, thereby releasing bone marrow elements in an effort to enhance tissue regeneration. One benefit suggested during this development was a lower degree of thermal necrosis with the use of a bone awl rather than a motorized drill.14
Given the ease and low cost of the procedure, MFx is often considered a first-line treatment for FCDs.1,24,27,31,40 However, this procedure was first described as one that preserves the integrity of the subchondral bone plate,38 and since that time, MFx has been shown to result in subchondral bone abnormalities including intralesional osteophytes.30 Furthermore, it is known that MFx leads to the growth of fibrocartilage that differs in biomechanical properties from the native hyaline cartilage.26 Although MFx may result in good outcomes in the short term, a systematic review of level 1 and 2 studies demonstrated that MFx often leads to treatment failure after 5 years regardless of lesion size.16 For these reasons, some surgeons have called for the abandonment of MFx and many others have adopted the technique of drilling for lesions that traditionally were indicated for MFx.2 Possible advantages of drilling over MFx include deeper subchondral bone penetration, which may result in the stimulation of higher quality bone marrow products, as well as the creation of small drill holes rather than fracture of the subchondral bone. However, these are only theoretical advantages and must be further studied in a clinical setting.
Despite the recent trend toward drilling rather than MFx, to date, no clinical studies have directly compared outcomes of MFx versus drilling for FCDs of the knee joint. One study10 compared these techniques for the treatment of osteochondral lesions of the talus. In a retrospective cohort study, Choi and Lee10 compared outcomes of drilling (n = 40 ankles) versus MFx (n = 50). Groups were matched for age and sex. At a mean follow-up of 43 months, both groups demonstrated significant improvement in the American Orthopaedic Foot and Ankle Society ankle-hindfoot score, visual analog scale for pain, and ankle activity score, with no significant differences in follow-up scores between groups.
Although the results of this systematic review suggest overall improved biological features of drilling in comparison with MFx, these results are limited to basic science studies. High-quality clinical studies are necessary to determine the clinical effects of MFx and drilling in FCDs of various joints in human patients. Ideally, these studies should randomize patients to treatment with MFx or drilling and should report on a number of outcomes including revision rate, patient-reported outcomes, and magnetic resonance imaging outcomes at different postoperative time points.
The strengths of this study include a comprehensive systematic review performed by 2 independent reviewers. The limitations of this study should also be noted. In particular, this systematic review was limited to basic science studies because no clinical studies that we know of have directly compared outcomes between MFx versus drilling in the knee joint, and only 1 study has compared outcomes of these techniques in the ankle joint. Furthermore, only 7 studies were included in this review based on the inclusion and exclusion criteria of our search. These studies were performed in both human and animal models and therefore present methodologic heterogeneity. Further, 4 of the 5 animal studies6–9 were published from the same group. The 2 human studies21,29 involved performing MFx on chondral defects much larger than those typically indicated for this procedure,16 and both of these studies focused on patients who experienced failed marrow stimulation and subsequently underwent TKA. Most importantly, additional basic science and translational research is needed to clarify the outcomes of MFx versus drilling, and drawing clinical conclusions based on currently available studies should be avoided.
Conclusion
Deep drilling of cartilage defects in the knee joint results in improved biological features compared with MFx, including less damage to the subchondral bone and greater access to marrow stroma. Regardless of marrow stimulation technique, the overall quality of cartilage regeneration was poor and did not achieve the characteristics of native hyaline cartilage. Overall, there is a general lack of basic science literature comparing MFx versus drilling for focal chondral defects.
Footnotes
Final revision submitted March 9, 2020; accepted March 31, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.J.S. has received research support from Isto Biologics, consulting fees from DePuy/Medical Device Business Services and Mitek, nonconsulting fees from Flexion Therapeutics, and hospitality payments from Smith & Nephew. E.C.M. has received research support from Arthrex, Breg, Mitek, Ossur, Smith & Nephew, Stryker, and Zimmer Biomet; consulting fees from Zimmer Biomet; and royalties from Elsevier and Zimmer Biomet. M.K.M. has received educational support from Alon Medical Technology, Arthrex, and Quest Medical; nonconsulting fees from Arthrex; and hospitality payments from Zimmer Biomet. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37(suppl 1):148S–155S. [DOI] [PubMed] [Google Scholar]
- 2. Bert JM. Abandoning microfracture of the knee: has the time come? Arthroscopy. 2015;31(3):501–505. [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M, Recker D, Ilgenfritz J, Saris DBF; SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46(6):1343–1351. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85(suppl 2):56–89. [DOI] [PubMed] [Google Scholar]
- 5. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. [DOI] [PubMed] [Google Scholar]
- 6. Chen H, Chevrier A, Hoemann CD, Sun J, Lascau-Coman V, Buschmann MD. Bone marrow stimulation induces greater chondrogenesis in trochlear vs condylar cartilage defects in skeletally mature rabbits. Osteoarthritis Cartilage. 2013;21(7):999–1007. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, Buschmann MD. Characterization of subchondral bone repair for marrow-stimulated chondral defects and its relationship to articular cartilage resurfacing. Am J Sports Med. 2011;39(8):1731–1740. [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Hoemann CD, Sun J, et al. Depth of subchondral bone perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2011;29(8):1178–1184. [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Sun J, Hoemann CD, et al. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;27(11):1432–1438. [DOI] [PubMed] [Google Scholar]
- 10. Choi JI, Lee KB. Comparison of clinical outcomes between arthroscopic subchondral drilling and microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2140–2147. [DOI] [PubMed] [Google Scholar]
- 11. Chubinskaya S, Haudenschild D, Gasser S, Stannard J, Krettek C, Borrelli J., Jr Articular cartilage injury and potential remedies. J Orthop Trauma. 2015;29(suppl 12):S47–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciccotti MC, Kraeutler MJ, Austin LS, et al. The prevalence of articular cartilage changes in the knee joint in patients undergoing arthroscopy for meniscal pathology. Arthroscopy. 2012;28(10):1437–1444. [DOI] [PubMed] [Google Scholar]
- 13. Crawford DC, DeBerardino TM, Williams RJ III. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am. 2012;94(11):979–989. [DOI] [PubMed] [Google Scholar]
- 14. Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242–255. [DOI] [PubMed] [Google Scholar]
- 15. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):1986–1996. [DOI] [PubMed] [Google Scholar]
- 16. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29(9):1579–1588. [DOI] [PubMed] [Google Scholar]
- 17. Gudmann NS, Karsdal MA. Type II collagen In: Karsdal MA, ed. Biochemistry of Collagens, Laminins and Elastin: Academic Press; 2016:13–20. [Google Scholar]
- 18. Gudmann NS, Karsdal MA. Type X collagen In: Karsdal MA, ed. Biochemistry of Collagens, Laminins and Elastin: Academic Press; 2016:73–76. [Google Scholar]
- 19. Henriksen K, Karsdal MA. Type I collagen In: Karsdal MA, ed. Biochemistry of Collagens, Laminins and Elastin: Academic Press; 2016:1–11. [Google Scholar]
- 20. Johnson LL, Spector M. The new microfracture: all things considered. Arthroscopy. 2015;31(6):1028–1031. [DOI] [PubMed] [Google Scholar]
- 21. Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315–2324. [DOI] [PubMed] [Google Scholar]
- 22. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. 2018;46(4):995–999. [DOI] [PubMed] [Google Scholar]
- 23. Kraeutler MJ, Chahla J, Dean CS, et al. Current concepts review update: osteochondral lesions of the talus. Foot Ankle Int. 2017;38(3):331–342. [DOI] [PubMed] [Google Scholar]
- 24. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470(8):2261–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mainil-Varlet P, Aigner T, Brittberg M, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A(suppl 2):45–57. [PubMed] [Google Scholar]
- 26. Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics. 1997;20(6):525–538. [DOI] [PubMed] [Google Scholar]
- 27. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 28. O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness cartilage defects in joint surfaces under the influence of continuous passive motion. J Bone Joint Surg Am. 1988;70(4):595–606. [PubMed] [Google Scholar]
- 29. Sakata K, Furumatsu T, Abe N, Miyazawa S, Sakoma Y, Ozaki T. Histological analysis of failed cartilage repair after marrow stimulation for the treatment of large cartilage defect in medial compartmental osteoarthritis of the knee. Acta Med Okayama. 2013;67(1):65–74. [DOI] [PubMed] [Google Scholar]
- 30. Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–246. [DOI] [PubMed] [Google Scholar]
- 31. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scillia AJ, Aune KT, Andrachuk JS, et al. Return to play after chondroplasty of the knee in National Football League athletes. Am J Sports Med. 2015;43(3):663–668. [DOI] [PubMed] [Google Scholar]
- 34. Seidl AJ, Kraeutler MJ. Management of articular cartilage defects in the glenohumeral joint. J Am Acad Orthop Surg. 2018;26(11):e230–e237. [DOI] [PubMed] [Google Scholar]
- 35. Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. [DOI] [PubMed] [Google Scholar]
- 36. Solheim E, Hegna J, Inderhaug E. Long-term survival after microfracture and mosaicplasty for knee articular cartilage repair: a comparative study between two treatment cohorts. Cartilage. 2020;11(1):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587–1593. [DOI] [PubMed] [Google Scholar]
- 38. Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technique in the management of complete cartilage defects in the knee joint [in German]. Orthopade. 1999;28(1):26–32. [DOI] [PubMed] [Google Scholar]
- 39. Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: a long-term follow-up study. Eur J Radiol. 2012;81(7):1618–1624. [DOI] [PubMed] [Google Scholar]
- 40. Williams RJ, III, Harnly HW. Microfracture: indications, technique, and results. Instr Course Lect. 2007;56:419–428. [PubMed] [Google Scholar]
- 41. Zedde P, Cudoni S, Giachetti G, et al. Subchondral bone remodeling: comparing nanofracture with microfracture. An ovine in vivo study. Joints. 2016;4(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]