Abstract
Background and aims:
Alemtuzumab is a humanized monoclonal antibody that depletes CD52-bearing B and T lymphocytes. Clinical trials defined that systemic administration of alemtuzumab reduces disease severity in the relapsing–remitting phase of multiple sclerosis (MS). However, its efficacy in progressive MS patients is limited, which may reflect the inability of alemtuzumab to cross the reconstituted BBB in these patients. Objective: to study whether central nervous system (CNS) delivery of anti-CD52 antibodies reduces disease severity and the neuroinflammatory burden in the experimental autoimmune encephalomyelitis (EAE) model.
Methods:
Anti-CD52 antibodies were administered intrathecally during the acute and chronic phases of EAE. Flow cytometry and immunohistochemistry were utilized to define immunological and pathological parameters.
Results:
We show that subcutaneously administrated anti-CD52 antibodies completely abolish EAE disease severity. CNS delivery of anti-CD52 antibodies during both the acute and chronic phases of EAE moderately reduces disease severity and the neuroinflammatory burden. Our findings further suggest that CNS delivery of anti-CD52 antibodies impacts both the peripheral and CNS immune cell compartments in the EAE model but not in healthy mice.
Conclusion:
Collectively, our findings highlight the therapeutic potential of CNS delivery of alemtuzumab for the treatment of progressive as well as early MS.
Keywords: alemtuzumab, CD52, experimental autoimmune encephalomyelitis, lymphocytes, multiple sclerosis, neuroinflammation
Introduction
Alemtuzumab is a humanized monoclonal antibody directed against CD52, expressed on lymphocytes, which was first approved for the treatment of chronic lymphocytic leukemia. Systemic treatment with alemtuzumab results in rapid and prolonged depletion of T and B lymphocytes. To date, several clinical studies assessed the capacity of alemtuzumab to reduce disease progression in multiple sclerosis (MS). Early studies found that alemtuzumab markedly reduced gadolinium-enhanced magnetic imaging lesions in progressive MS patients, corresponding to a reduced relapse rate.1–4 However, despite its striking impact on newly formed inflammatory lesions, alemtuzumab did not improve clinical disability in these patients, suggesting that alemtuzumab is more beneficial if given early in the course of MS. In agreement, phase II and phase III clinical studies showed that alemtuzumab was more efficient in reducing the annualized relapse rate (ARR), disability score, and lesion burden in naïve as well as prior-treated relapse–remitting MS (RR-MS) patients as compared with subcutaneous interferon (IFN)β-1a.5–7 Importantly, in extension studies, alemtuzumab was found to provide efficacy through 5 years in the absence of continuous treatment.8 In an international cohort study, the effectiveness of alemtuzumab in RR-MS patients was compared with natalizumab, fingolimod, and IFNβ-1a.9 Alemtuzumab was associated with a lower ARR than IFNβ-1a and fingolimod, and a similar ARR to natalizumab. Collectively, these findings indicate that lymphocyte depletion by alemtuzumab efficiently reduces the activity of early MS lesions.
Increasing evidence indicates that the inflammatory reactions in MS are more complex than originally anticipated. Classically, central nervous system (CNS) inflammation was thought to subside in progressive disease stages and neurodegeneration was regarded as continuing in the absence of inflammation. However, post-mortem studies defined that CNS inflammation is still apparent in progressive disease stages, albeit to a lesser extent as in early MS patients, and trapped behind a reconstituted blood–brain barrier.10,11 In progressive MS patients, active tissue injury is mainly associated with pro-inflammatory microglia activation.12,13 Among other potential candidates driving neuroinflammation in progressive disease stages, B and T cells appear to play an essential role. Post-mortem studies defined that both lymphocyte subsets are present in the CNS of progressive MS patients and their presence correlates with the extent of acute axonal injury in these patients.10,11 Moreover, ample evidence indicates the presence of lymphoid follicle-like structures in the meninges of progressive MS patients containing abundant B cells,14–16 suggesting the establishment of a compartmentalized humoral immune response in the progressive phase of MS. In summary, these studies indicate that inflammation is apparent in progressive MS and contributes to ongoing neurodegeneration. While the cells and mechanisms driving inflammation in progressive MS patients remain poorly understood, microglia and B cells are likely culprits, with T cells having a less important impact.17 Therapeutic interventions focused at eliminating the compartmentalized inflammatory reaction observed in the CNS of progressive MS patients may halt ongoing neurodegeneration in these patients.
Monoclonal antibodies such as alemtuzumab are unable to cross an intact blood–brain barrier at an appreciable concentration, which likely explains its inability to improve clinical disability in progressive MS patients.18 For this reason, we sought to determine whether local administration of alemtuzumab in the CNS reduces neuroinflammation and ameliorates disease severity in an animal model of MS; experimental autoimmune encephalomyelitis (EAE). We report that intrathecal treatment with anti-murine CD52 monoclonal antibodies (aCD52 mAb) in both the acute and the chronic phase of EAE slightly reduces disease severity and the presence of inflammatory immune cell infiltrates in the CNS. Intrathecal administration of aCD52 mAb was well tolerated by animals. Our results hold promise for future intervention studies using alemtuzumab to reduce disease progression in progressive MS patients.
Material and methods
Animals
Wild-type C57BL/6J mice were obtained from Envigo. Animals were fed a regular diet and housed in the animal facility of the Biomedical Research Institute of Hasselt University. Mice were maintained on a 12 h light/dark cycle with free access to water and food. All experiments were carried out according to institutional guidelines and were approved by the ethical committee for animal experiments of Hasselt University (approval number 201634).
EAE induction
The induction of EAE was done as described previously.19,20 Following randomization (weight), 11 week old mice were immunized subcutaneously with recombinant myelin oligodendrocyte glycoprotein MOG35–55 emulsified in complete Freund’s adjuvant supplemented with Mycobacterium tuberculosis according to the manufacturer’s guidelines (Hooke Laboratories, Lawrence, USA). Within 2 h and after 24 h, mice were injected intraperitoneally with 50 ng of pertussis toxin (lot number 1008). Immunized mice were weighed and scored daily by following a five-point standardized rating of clinical symptoms: 0, no signs; 1, loss of tail tonus; 2, flaccid tail; 3, hind limb paresis; 4, hind limb paralysis; 5, death. Scoring was performed by an examiner blinded to the experimental protocol.
Anti-CD52 treatment
Healthy (n = 5/group) and EAE (n = 10/group) C57BL/6J mice were treated subcutaneously over 5 consecutive days with anti-murine CD52 (IgG2a, 200 µg/mouse, kindly provided by Genzyme, a Sanofi company) or vehicle (PBS).21 Subcutaneous treatment of EAE animals with anti-CD52 started 10 days post-immunization (dpi). For local administration, healthy and EAE animals were injected intrathecally with 10 µg/mouse of anti-CD52 dissolved in 2 µl of PBS. To this end, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) and fixed in a stereotaxic frame. Using the occipital crest as a reference point, superficial connective tissue and muscles were separated at the midline. Next, a Hamilton 1701 syringe (X046.1) was used to inject vehicle or anti-CD52 in the cisterna magma. Topical ophthalmic ointment was used to prevent ocular dryness/injury. In the EAE experiments, animals were treated once intrathecally at 10 (n = 8/group) or 20 dpi (n = 10/group). Given that the blood/cerebrospinal fluid (CSF) ratio in mice is 40:1 (blood, ±1.2 mL; CSF, ±40 µL), the dilution factor of anti-CD52 mAb in the CSF upon intrathecal administration (CSF, 0.33 mg/mL) is lower than the dilution factor in blood upon subcutaneous administration (blood, 0.17 mg/mL).
Flow cytometry
The spleen was dissociated into single cells by mashing the tissue through a 70-µm cell strainer. Red blood cells from spleens were lysed using 0.83% (w/v) of ammonium chloride. For the detection of immune cell subtypes, the following antibodies were used: Alexa Fluor 647 anti-mouse FOXP3 (Biolegend, 126407), Alexa Fluor 700 anti-mouse CD45 (Biolegend, 103127), Brilliant Violet 510 anti-mouse CD8a (Biolegend, 100751), Brilliant Violet 650 anti-mouse CD19 (Biolegend, 115541), Brilliant Violet 785 anti-mouse Ly-6C (Biolegend, 128041), FITC anti-mouse CD3 (Biolegend, 100203), Pacific Blue anti-mouse CD4 (Biolegend, 100427), PE anti-mouse IL-4 (Biolegend, 504103), PC/Cy7 anti-mouse IFNgamma (Biolegend, 505825), PE/Dazzle 594 anti-mouse IL-17A (Biolegend, 506937), and PerCP/Cy5.5 anti-mouse CD11b (Biolegend, 101227). Viable cells were gated using the Zombie NIR Fixable Viability Kit (Biolegend, 423105). For cell surface staining, cells were incubated with 10% rat serum for 15 min prior to staining with fluorescently labeled antibodies for 15 min on ice. For intracellular staining, cells were fixed, permeabilized, and stained using the Transcription Factor Staining Buffer Set (Thermo Fisher Scientific) according to the manufacturer’s protocol. Flow cytometry was carried out and analyzed on a BD LSRFortessa (BD Biosciences).
Immunostaining
Frozen human brain material from progressive MS patients (n = 4 patients) was obtained from the Netherlands Brain Bank (NBB, Amsterdam, Netherlands). Clinical details of human brain tissue are depicted in Supplemental Material Table S1 online. Animals were transcardially perfused with Ringer’s solution containing heparin, after which spinal cord tissue was isolated and snap-frozen in liquid nitrogen. Cryosections were fixed in acetone for 10 min and blocked with 10% DAKO protein block (Dako, Heverlee, Belgium). For 3,3′ diaminobenzidine (DAB) staining, slides were incubated with rat anti-human CD52 (Bio-Rad, MCA1642). After washing, HRP-conjugated with rabbit-anti-rat HRP (DAKO, P0450) was added. Subsequently, DAB substrate (Dako) was used to stain slides. Sections were counterstained with hematoxylin (Merck, Darmstadt, Germany). For morphological analysis, sections were stained with hematoxylin and eosin using standard protocols. For fluorescence staining, cryosections were incubated with rabbit anti-Iba1 (Wako, NCNP24), rat anti-mouse CD3 (Bio-Rad, MCA500G), rat anti-mouse F4/80 (Bio-Rad, MCA497GA), rat anti-human CD52 (Bio-Rad, MCA1642), mouse anti-human CD3 (Bio-Rad, MCA463), and mouse anti-CD68 (Invitrogen, 14-0688). Cryosections were stained with Alexa Flour secondary antibodies (Invitrogen). Nuclei were visualized using 4,6′-diamidino-2-phenylindole (Invitrogen). Stained sections were visualized using a Nikon Eclipse 80i fluorescence microscope (Nikon, Kingston, UK). For quantification, seven images per animal were used (10× magnification). In all experiments, control staining was performed by omitting the primary antibody. No immunoreactivity was observed in control staining, depicted in Figures 1 and 2, and Supplemental Figure S1 (data not shown). For MS lesions, the normal-appearing white matter (NAWM) was defined based on PLP (normal myelin distribution), HLA-DR (normal microglia branching), and ORO staining (absence of ORO+ cells).
Figure 1.
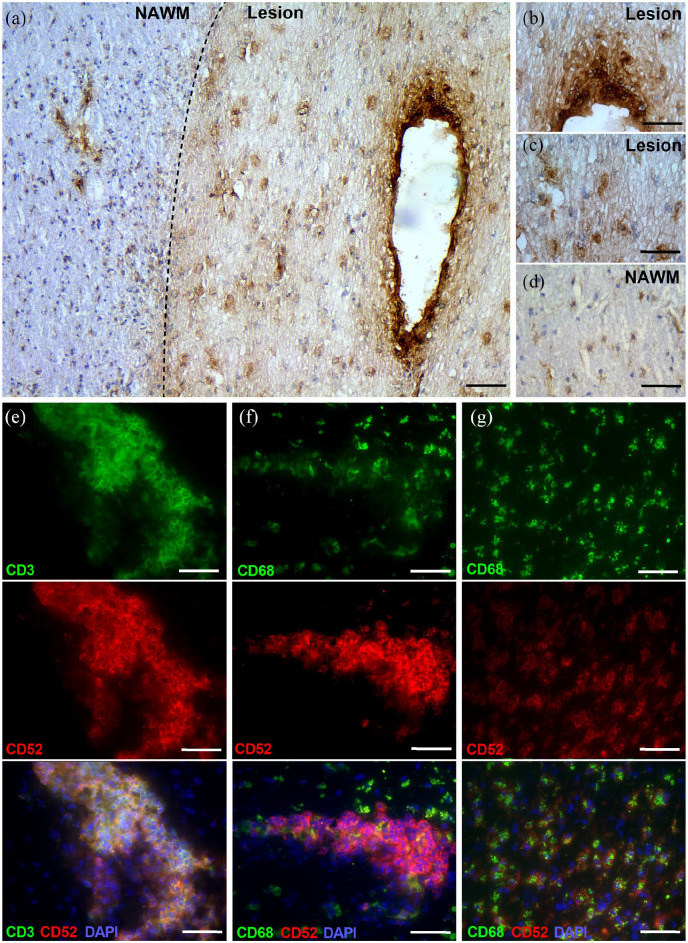
CD52 is expressed by perivascular lymphocytes and parenchymal phagocytes in multiple sclerosis (MS) lesions. (a–d) Representative images of CD52 immunostaining of MS lesions: overview (a), lesion perivascular cuff (b), lesion parenchyma (c), normal-appearing white matter (NAWM, d). (e–g) Representative immunofluorescence images of MS lesions from progressive MS patients stained with CD3/CD52 (e) and CD68/CD52 (f, g). A total of five lesions from four different MS patients were used. Scale bars, 200 µm (a); 100 µm (b–f).
Figure 2.
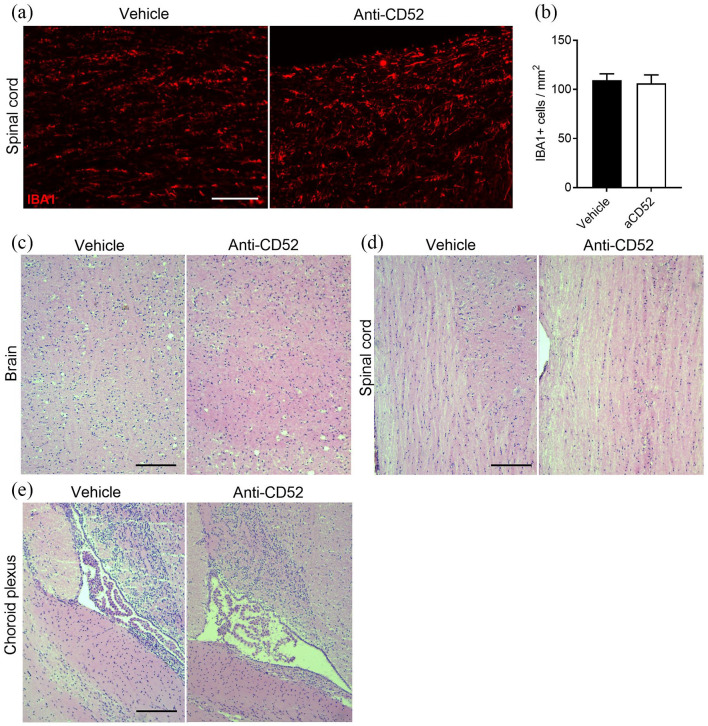
Intrathecally administered anti-CD52 mAb does not impact microglia number and central nervous system morphology. (a, b) Representative immunofluorescent images and quantification of IBA1 staining of spinal cord isolated from healthy mice that received anti-CD52 mAb or vehicle intrathecally. Animals were sacrificed 3 days after surgery. Scale bar, 100 µm. (c–e) Representative images of hematoxylin/eosin staining of the spinal cord (c) and brain (d) parenchyma, and choroid plexus (e) of healthy animals treated with anti-CD52 mAb or vehicle intrathecally. Animals were sacrificed 3 days after surgery (n = 4 animals). Scale bar, 150 µm.
Statistics
Data were analyzed using GraphPad Prism and are reported as mean ± SEM. The D’Agostino and Pearson omnibus normality test was used to test normal distribution. An analysis of variances or two-tailed unpaired Student t-test (with Welch’s correction if necessary) was used for normally distributed data sets. The Kruskal–Wallis or Mann–Whitney analysis was used for data sets which did not pass normality. P values <0.05 (*), <0.01 (**), and <0.001 (***) were taken to indicate statistical significance between groups.
Results
CD52 is expressed by perivascular lymphocytes and parenchymal phagocytes in MS lesions
CD52 is present on the cell surface of the vast majority of lymphoid cells and many other hematopoietic cells. Here, we defined CD52 expression in lesions and adjacent NAWM of progressive MS patients. Our data indicate that CD52 is primarily expressed within MS lesions [Figure 1(a) to (d)]. While CD52 immunoreactivity was highest on perivascular infiltrates [Figure 1(b)], lesional cells faintly expressed CD52 [Figure 1(c)]. In the NAWM, cells resembling microglia, showing long branching processes and a small cellular body, weakly expressed CD52 [Figure 1(d)]. Fluorescent staining showed that perivascular CD3+ T cells and parenchymal CD68+ phagocytes expressed CD52 [Figure 1(e) to (g)], and that CD52 abundance was higher on T cells compared with phagocytes (mean fluorescent intensity, p < 0.001). We did not detect CD20+ B cells within our cohort of MS lesions.
CNS delivery of anti-CD52 mAb does not impact microglia number and CNS morphology
To define the safety of intrathecal injections of anti-CD52 mAb, healthy mice were injected intrathecally with 10 µg/mouse. Three days post-surgery, animals were sacrificed to assess morphological abnormalities. Despite the expression of CD52 on resting microglia [Figure 1(d)], anti-CD52 mAb did not alter the number of IBA1-expressing microglia in healthy mice [Figure 2(a) and (b)]. Similarly, by using a hematoxylin/eosin staining, no morphological changes were observed in the brain and spinal cord parenchyma [Figure 2(c) and (d)] and choroid plexus [Figure 2(e)] in animals treated intrathecally with anti-CD52 mAb. No apparent distress or change in behavior was observed in these animals
CNS delivery of anti-CD52 mAb does not impact peripheral immune cells in healthy mice
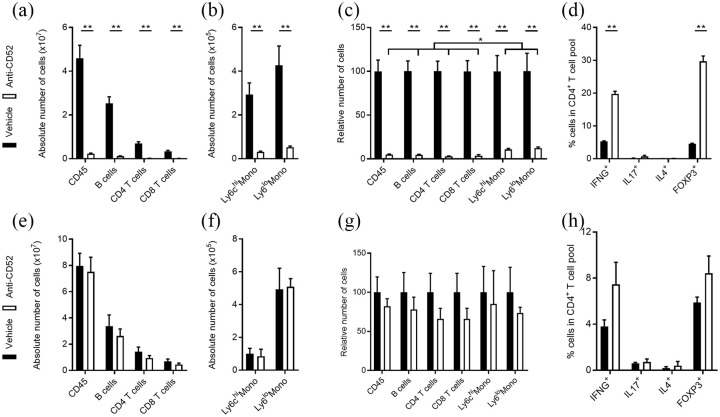
Subcutaneous treatment with anti-CD52 mAb markedly reduces the number of monocytes and lymphocytes in the blood, lymph nodes, and spleen.21–23 Here, we confirm these findings by showing that subcutaneous treatment with anti-CD52 mAb reduces the absolute and relative number of CD45+ leukocytes, CD45+CD3+CD4+ T cells, CD45+CD3+CD8+ T cells, CD45+CD3–CD19+ B cells, and CD11b+Ly6C+ inflammatory and CD11b+Ly6c– patrolling monocytes in the spleen of healthy animals compared with vehicle-treated animals [Figure 3(a) to (c)]. Aside from reducing the number of CD4+ T cells, subcutaneous treatment with anti-CD52 mAb resulted in 6- and 4-fold inductions of CD4+FOXP3+ Tregs and CD4+IFNG+ Th1 cells, respectively [Figure 3(d)]. In contrast to subcutaneous administration and in agreement with the inability of anti-CD52 mAb to cross an intact BBB, intrathecal administration of anti-CD52 mAb did not change the absolute and relative number of CD45+ leukocytes, CD4+ and CD8+ T cells, B cells, and monocytes in the spleen of healthy animals [Figure 3(e) to (g)]. Similarly, no significant changes in the differentiation of splenic CD4+ T cells was observed [Figure 3(h)]. Collectively, these findings indicate that intrathecally administrated anti-CD52 mAb does not impact the peripheral immune cell landscape in healthy animals.
Figure 3.
CNS delivery of anti-CD52 mAb does not impact peripheral immune cells in healthy mice. Flow cytometric analysis of immune cell subsets in the spleen of healthy mice treated subcutaneously (a–d) or intrathecally (e–h) with vehicle (PBS, n = 5) or anti-CD52 mAb (n = 5). Data are depicted as absolute number of immune cell subsets (a, b, e, f), relative number of immune cell subsets (c, g), and percentage of IFNG+, IL17+, IL4+, and FOXP3+ cells in the CD4+ T cell pool (d, h). All replicates were biologically independent.
Data are shown as mean ± SEM.
*p < 0.05.
**p < 0.01.
CNS delivery of anti-CD52 mAb modestly reduces EAE disease severity
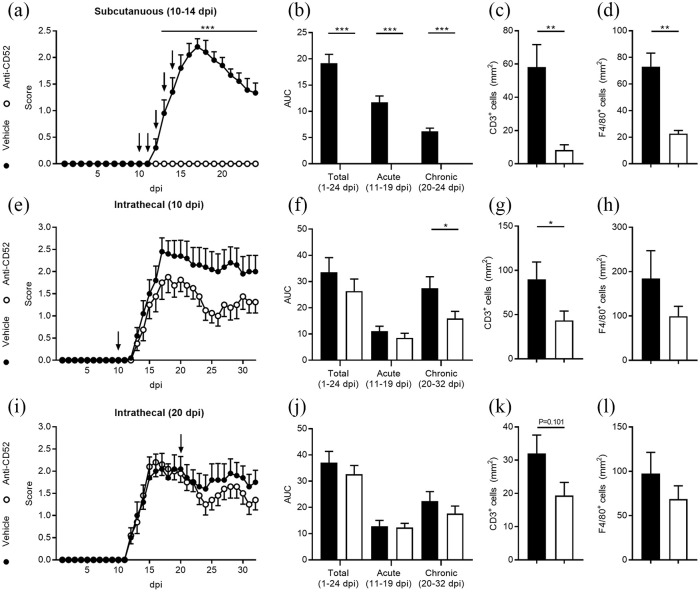
Ample evidence indicates that subcutaneous treatment with anti-CD52 mAb significantly reduces EAE severity.21–23 In agreement with these studies, our data indicate that EAE animals treated subcutaneously with anti-CD52 mAb (10–14 dpi) did not show any signs of neurological dysfunction compared with vehicle-treated animals [Figure 4(a) and (b)]. In concordance with the reduced EAE disease severity, anti-CD52 mAb-treated mice showed a decreased presence of CD3+ T cells and F4/80+ phagocytes in their spinal cord [Figure 4(c) and (d) and Supplemental Figure S1). To define the impact of intrathecally administrated anti-CD52 mAb on EAE disease severity, EAE-affected animals were treated once with anti-CD52 mAb at 10 or 20 dpi. Our data indicate that anti-CD52 mAb reduces EAE disease severity in the chronic disease phase when administered 10 dpi [Figure 4(e) and (f)]. Immunohistochemical analysis further demonstrated a reduced presence of CD3+ T cells in the spinal cord of these animals [Figure 4(g)]. F4/80+ phagocytes showed a non-significant reduction in the spinal cord [Figure 4(h)]. EAE animals treated intrathecally with anti-CD52 mAb at 20 dpi showed a much reduced effect as compared with administration 10 dpi [Figure 4(i) to (l)].
Figure 4.
Central nervous system (CNS) delivery of anti-CD52 mAb modestly reduces experimental autoimmune encephalomyelitis (EAE) disease severity and the number of immune cells in the CNS. EAE animals were treated subcutaneously [10–14 days post-immunization (dpi)] or intrathecally (10 dpi or 20 dpi) with vehicle (n = 10 for all experiments) or anti-CD52 mAb (subcutaneous, n = 10; intrathecal 10 dpi, n = 8; intrathecal 20 dpi, n = 10). Disease severity is depicted by showing the disease course [(a, e, i) arrows represent injections] and cumulative disease score (b, f, j), which was assessed by measuring the area under the curve (AUC). CD3 and F4/80 immunofluorescent staining was used to quantify the number of T cells and phagocytes in the CNS (c, d, g, h, k, l).
Data are represented as mean ± SEM.
*p < 0.05.
**p < 0.01.
***p < 0.001.
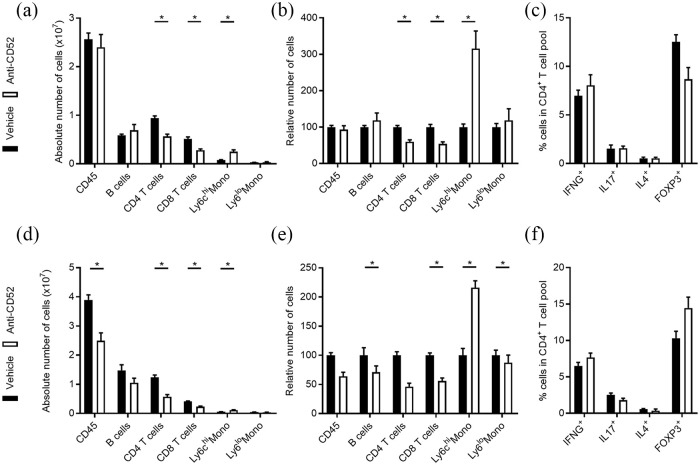
Flow cytometric analysis showed that intrathecal treatment of EAE animals with anti-CD52 mAb at 10 dpi reduces the absolute and relative number of CD4+ and CD8+ T cells [Figure 5(a) and (b)]. However, the reduction in lymphocytes was far less pronounced compared with animals treated subcutaneously with anti-CD52 mAb [Figure 3(a) and (c)]. Interestingly, the number of splenic inflammatory Ly6Chi monocytes was significantly increased upon intrathecal treatment with anti-CD52 mAb at 10 dpi [Figure 5(a) and (b)]. No significant changes in the polarization of CD4+, CD8+, and CD19+ T and B cells were observed [Figure 5(c) and Supplemental Figure S2(a)]. When EAE animals were treated intrathecally with anti-CD52 mAb at 20 dpi, a significant decrease in absolute and relative number of splenic CD4+ and CD8+ T cells and B cells was observed. Again, the reduction in lymphocyte number was far less pronounced compared with subcutaneously treated animals [Figure 3(a) to (d)]. Similar to animals that were intrathecally treated with ani-CD52 mAb at 10 dpi, the number of inflammatory Ly6Chi monocytes was significantly increased in animals treated with anti-CD52 mAb at 20 dpi [Figure 5(d) and (e)]. No significant changes in the polarization of splenic CD4+, CD8+, and CD19+ T and B cells were observed [Figure 5(f) and Supplemental Figure 2(b)].
Figure 5.
Central nervous system delivery of anti-CD52 mAb impacts peripheral immune cells in experimental autoimmune encephalomyelitis (EAE) mice. Flow cytometric analysis of immune cell subsets in the spleen of EAE mice treated intrathecally at 10 days post-immunization (dpi) (a–c) or 20 dpi (d–f) with vehicle or anti-CD52 mAb (n = 4). Data are depicted as absolute number of immune cell subsets (a, d), relative number of immune cell subsets (b, e), and percentage of IFNG+, IL17+, IL4+, and FOXP3+ cells in the CD4+ T cell pool (c, f). All replicates were biologically independent.
Data are represented as mean ± SEM.
*p < 0.05.
Discussion
Systemic administration of alemtuzumab reduces disease severity in RR-MS patients. However, its efficacy in progressive forms of MS is limited, which may reflect the inability of alemtuzumab to cross the reconstituted BBB in progressive MS patients. In this study, we show that a single intrathecal injection of anti-CD52 mAb in either the acute or the chronic phase of EAE modestly reduces disease severity. Reduced disease severity was associated with a decrease in lymphocyte number in the CNS as well as in the spleen. Previous studies demonstrated that five consecutive intraperitoneal or subcutaneous injections of anti-CD52 at disease onset attenuate EAE disease severity.21–25 However, multiple intraperitoneal injections at the disease peak only marginally improved EAE severity,24 and no effect was observed when treatment was initiated in the chronic disease stage.25 These studies emphasize the limited therapeutic efficacy of peripherally administered anti-CD52 antibodies in chronic EAE and are consistent with the inability of alemtuzumab to reduce disease severity in progressive MS patients. Our findings now highlight the therapeutic benefit that intrathecal delivery of alemtuzumab may have in these patients.
We show that intrathecal treatment with anti-CD52 mAb modestly reduces EAE disease severity and the neuroinflammatory burden. Based on our assumption that the anti-CD52 mAb is unable to cross the reconstituted BBB as well as the low ratio of CSF volume to blood volume, we opted for a single low-dose intrathecal injection to eliminate immune cells within the CNS. Retrospectively, intrathecal administration of anti-CD52 mAb is likely more efficient in reducing EAE disease severity when administered multiple times at a higher dose, similar to the subcutaneous treatment regime used in RR-MS patients.1–4 Given that the anti-CD52 mAb was able to enter the circulation in EAE animals once injected in the CSF, this treatment regime will lead to a prolonged and increased concentration of anti-CD52 mAb in the CNS. Moreover, it would more efficiently reduce systemic inflammation once drained to and diluted in the circulation. By using an intrathecal pump system, future studies should define the impact of prolonged intrathecal administration of anti-CD52 during the acute and chronic stage of EAE on disease severity. Such experiments would not only establish whether intrathecal administration at the acute phase is as effective as subcutaneous treatment, but also whether intrathecal anti-CD52 mAb treatment at the chronic stage represents a therapeutic strategy for treating progressive MS patients.
Our data indicate that intrathecally administered anti-CD52 mAb does not impact the number and differentiation of peripheral immune cells in healthy animals. Counterintuitively, a minor decrease in lymphocyte number was observed in EAE animals treated intrathecally with anti-CD52 mAb, indicating that it did not remain within the CNS in these animals. Given that a recent study reported that the BBB shows signs of leakiness in the chronic stages of the EAE model,26 these findings might indicate that the observed peripheral immune cell depletion is a consequence of the experimental model. On the other hand, while the venous route for CSF drainage restricts passage of large monoclonal antibodies to the serum, CNS lymphatics can provide aCD52 mAb access to the periphery,27 in particular in inflammatory settings.28 Interestingly, intrathecally injected rituximab, a monoclonal antibody directed against the B cell marker CD20, was recently found to deplete peripheral B cells in a manner comparable to systemically administered anti-CD20 in the EAE model.29 Similar, in a phase I dose-escalation study of intrathecal rituximab monotherapy in patients with recurrent CNS non-Hodgkin’s lymphoma, rituximab was found to accumulate in the serum.30 Given that progressive MS patients continue to develop inflammatory lesions and display ongoing systemic inflammation, depletion of both peripheral and CNS immune cell subsets upon intrathecal delivery of alemtuzumab might enhance its therapeutic benefit in progressive MS patients. However, in our experimental model, it makes it challenging to distinguish primary CNS effects from secondary peripheral effects upon intrathecal delivery of anti-CD52 mAb.
While CNS delivery of anti-CD52 mAb resulted in a reduced presence of peripheral T and B cells in EAE mice, a marked increase in inflammatory Ly6Chi monocytes was observed. Previous studies defined a disease-promoting role of Ly6Chi monocytes during autoimmune inflammation of the CNS, in particular during the acute inflammatory response.31,32 However, a more recent study showed that Ly6Chi monocytes are protective in an animal model of ischemic stroke by promoting M2 macrophage polarization.33 Similar, selective ablation of Ly6Chi monocytes was demonstrated to impair recovery after liver damage.34 To what extent the protective impact of anti-CD52 mAb in our experiments relies on the increase of these disease-resolving Ly6Chi monocytes remains to be determined. Furthermore, it is unclear why the number of splenic Ly6Chi monocytes but not Ly6Clo monocytes increases upon intrathecal administration of anti-CD52 mAb. A recent study showed that the kinetics underlying the generation and differentiation of monocyte subsets differs in humans.35 Classical monocytes, resembling Ly6Chi monocytes, were the first subset to repopulate the circulation after endotoxin-induced monocytopenia. Hence, our findings might merely reflect the sequence of monocyte repopulation. On the other hand, alemtuzumab has already been reported to selectively expand innate CD56bright NK cells,36 suggesting that leukocyte depletion by anti-CD52 mAb not only ‘reboots’ but also ‘rewires’ the immune system.
Despite the expression of CD52 on resting microglia, our data indicate that CNS delivery of anti-CD52 mAb does not impact microglia number in healthy mice. While these data may implicate the inability of anti-CD52 mAb to penetrate the healthy CNS parenchyma, the observed absence in responsiveness to the cytolytic effects of anti-CD52 mAb may also be associated with the relatively low expression of CD52 on microglia. With respect to the latter, various studies highlighted that the concentration of CD52 antigenic determinants is essential for the cytolytic effects of anti-CD52 mAb.37,38 For instance, in patients with chronic lymphocytic leukemia, alemtuzumab efficiently reduces the number of blood but not skin dendritic cells, which was closely associated with differences in the expression of CD52.37 Moreover, the response to alemtuzumab in patients with different forms of leukemia correlates with the expression level of CD52 on lymphocytes.38 Of note, a recent study defined that systemic anti-CD52 mAb affects microglia morphology in the EAE model without perturbing their function.24 We did not observe visual alterations in microglia morphology in our experiments. This discrepancy may reflect differences in the experimental design and tissue analysis (e.g. divergent treatment regime and analysis of divergent anatomical regions). Regardless of this ambiguity, our findings and those of Ellwardt et al. indicate that intrathecal administration of anti-CD52 mAb does not have adverse effects through the depletion or functional modulation of CNS-resident microglia.
In summary, our findings suggest that the combined peripheral and CNS cell depleting effects of intrathecally administered anti-CD52 mAb renders it suitable for treating chronic as well as acute forms of MS. However, follow-up studies are warranted to certify this claim. Moreover, future studies should define whether single and repeated intrathecal administrations of anti-CD52 mAb lead to severe and fatal adverse events. With respect to the latter, subcutaneous treatment with alemtuzumab can lead to intracranial hemorrhage and secondary autoimmunity, as well as several other severe and fatal conditions.39
Supplementary Material
Acknowledgments
We thank Katrien Wauterickx and Marie-Paule Tulleners for excellent technical assistance.
Footnotes
Author contributions: JFJB, BVW, BVB, and JJAH conceived experiments and designed the study. JFJB, EG, and EW obtained and analyzed data. JFJB and JJAH wrote the manuscript. JFJB, EG, EW, BB, PS, BVW, and JJAH revised the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Sanofi Genzyme, and grants of the Belgian Charcot Foundation, Research Foundation Flanders (FWO), and European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
ORCID iD: Jeroen FJ Bogie  https://orcid.org/0000-0002-0016-1926
https://orcid.org/0000-0002-0016-1926
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jeroen FJ Bogie, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium.
Elien Grajchen, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium.
Elien Wouters, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium.
Bieke Broux, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium.
Piet Stinissen, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium.
Bart Van Wijmeersch, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Diepenbeek, Belgium; Rehabilitation and MS-Centre, Overpelt, Belgium and Hasselt University, Hasselt, Belgium.
Jerome JA Hendriks, Department of Immunology and Infection, Biomedical Research Institute, Hasselt University, Agoralaan Building C, Diepenbeek, 3590, Belgium.
References
- 1. Moreau T, Thorpe J, Miller D, et al. Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet 1994; 344: 298–301. [DOI] [PubMed] [Google Scholar]
- 2. Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999; 354: 1691–1695. [DOI] [PubMed] [Google Scholar]
- 3. Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 1999; 46: 296–304. [DOI] [PubMed] [Google Scholar]
- 4. Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 2006; 253: 98–108. [DOI] [PubMed] [Google Scholar]
- 5. Investigators CT, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801. [DOI] [PubMed] [Google Scholar]
- 6. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 7. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 8. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 10. Lassmann H. Targets of therapy in progressive MS. Mult Scler 2017; 23: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 11. Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009; 132: 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zrzavy T, Hametner S, Wimmer I, et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017; 140: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Loughlin E, Madore C, Lassmann H, et al. Microglial phenotypes and functions in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089–1104. [DOI] [PubMed] [Google Scholar]
- 15. Lovato L, Willis SN, Rodig SJ, et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 2011; 134: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- 17. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 2018; 9: 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avasarala J. It’s time for combination therapies: in multiple sclerosis. Innov Clin Neurosci 2017; 14: 28–30. [PMC free article] [PubMed] [Google Scholar]
- 19. Mailleux J, Vanmierlo T, Bogie JF, et al. Active liver X receptor signaling in phagocytes in multiple sclerosis lesions. Mult Scler 2018; 24: 279–289. [DOI] [PubMed] [Google Scholar]
- 20. Bogie JF, Boelen E, Louagie E, et al. CD169 is a marker for highly pathogenic phagocytes in multiple sclerosis. Mult Scler 2018; 24: 290–300. [DOI] [PubMed] [Google Scholar]
- 21. Turner MJ, Pang PT, Chretien N, et al. Reduction of inflammation and preservation of neurological function by anti-CD52 therapy in murine experimental autoimmune encephalomyelitis. J Neuroimmunol 2015; 285: 4–12. [DOI] [PubMed] [Google Scholar]
- 22. Pant AB, Wang Y, Mielcarz DW, et al. Alteration of CD39+Foxp3+ CD4 T cell and cytokine levels in EAE/MS following anti-CD52 treatment. J Neuroimmunol 2017; 303: 22–30. [DOI] [PubMed] [Google Scholar]
- 23. Barbour M, Wood R, Hridi SU, et al. The therapeutic effect of anti-CD52 treatment in murine experimental autoimmune encephalomyelitis is associated with altered IL-33 and ST2 expression levels. J Neuroimmunol 2018; 318: 87–96. [DOI] [PubMed] [Google Scholar]
- 24. Ellwardt E, Vogelaar CF, Maldet C, et al. Targeting CD52 does not affect murine neuron and microglia function. Eur J Pharmacol 2020; 871: 172923. [DOI] [PubMed] [Google Scholar]
- 25. Simon M, Ipek R, Homola GA, et al. Anti-CD52 antibody treatment depletes B cell aggregates in the central nervous system in a mouse model of multiple sclerosis. J Neuroinflammation 2018; 15: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell L, Koeniger T, Tacke S, et al. Characterization of blood-brain barrier integrity in a B-cell-dependent mouse model of multiple sclerosis. Histochem Cell Biol 2019; 151: 489–499. [DOI] [PubMed] [Google Scholar]
- 27. Ma Q, Ineichen BV, Detmar M, et al. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun 2017; 8: 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cromer WE, Zawieja SD, Tharakan B, et al. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis 2014; 17: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lehmann-Horn K, Kinzel S, Feldmann L, et al. Intrathecal anti-CD20 efficiently depletes meningeal B cells in CNS autoimmunity. Ann Clin Transl Neurol 2014; 1: 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007; 25: 1350–1356. [DOI] [PubMed] [Google Scholar]
- 31. Mildner A, Mack M, Schmidt H, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 2009; 132: 2487–2500. [DOI] [PubMed] [Google Scholar]
- 32. Ajami B, Bennett JL, Krieger C, et al. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14: 1142–1149. [DOI] [PubMed] [Google Scholar]
- 33. Chu HX, Broughton BR, Kim HA, et al. Evidence that Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015; 46: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 34. Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol 2014; 193: 344–353. [DOI] [PubMed] [Google Scholar]
- 35. Patel AA, Zhang Y, Fullerton JN, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 2017; 214: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Auffermann-Gretzinger S, Eger L, Schetelig J, et al. Alemtuzumab depletes dendritic cells more effectively in blood than in skin: a pilot study in patients with chronic lymphocytic leukemia. Transplantation 2007; 83: 1268–1272. [DOI] [PubMed] [Google Scholar]
- 38. Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk Res 1998; 22: 185–191. [DOI] [PubMed] [Google Scholar]
- 39. Holmoy T, Fevang B, Olsen DB, et al. Adverse events with fatal outcome associated with alemtuzumab treatment in multiple sclerosis. BMC Res Notes 2019; 12: 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.