Abstract
The development of immune checkpoint inhibitors (ICIs) targeting cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death protein ligand 1 (PD-L1) has revolutionized the treatment strategy in various types of cancers. In addition, recent studies have revealed that tumor microsatellite instability (MSI) status and tumor mutation burden (TMB) contribute significantly to the therapeutic response to anti-PD-1 monoclonal antibody (mAb), which led to an accelerated approval to pembrolizumab for the treatment of MSI-high or mismatch-repair-deficient solid tumors after conventional chemotherapies in 2017 and for the treatment of TMB-high solid tumors in 2020 by the United States Food and Drug Administration (FDA). In the field of gastrointestinal cancers, many clinical trials evaluating the safety and efficacy of various regimens such as ICI monotherapy, the combination of anti-CTLA-4 mAb and anti-PD-1/PD-L1 mAb, and combination of ICI and conventional chemotherapy or tyrosine kinase inhibitor have been reported or are in progress. This review summarizes MSI status and TMB in gastrointestinal, hepatobiliary, and pancreatic cancers, and provides the results of most relevant clinical trials evaluating ICIs. We also discuss the development of biomarkers required for improving the selection of patients with a high probability of benefiting from treatment with ICIs, and potential therapeutic strategies that could help to enhance anticancer responses of ICIs.
Keywords: biliary tract cancer, colorectal cancer, esophageal cancer, gastric cancer, hepatocellular carcinoma, immunotherapy, microsatellite instability, tumor mutation burden
Introduction
The development of immunotherapy with immune checkpoint inhibitors (ICIs) has made a breakthrough in the treatment strategies of various cancer types.1 Immune checkpoints play a key role in maintaining immune homeostasis and preventing autoimmunity by inhibiting the excessive activation of T cells. However, during the carcinogenic process, immune checkpoint mechanisms are often activated to suppress an anti-tumor immune response, which has led to the development of ICIs. Since an initiation of the first clinical trial of anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) monoclonal antibody (mAb) ipilimumab in 2000 and anti-programmed cell death protein 1 (PD-1) mAb nivolumab in 2006, several mAbs targeting CTLA-4, PD-1, or programmed cell death protein ligand 1 (PD-L1) have been approved by the United States Food and Drug Administration (FDA) to treat various types of cancers.2 Furthermore, recent studies have demonstrated that tumor microsatellite instability (MSI) status and tumor mutation burden (TMB) contribute significantly to the therapeutic response to ICI.3 The FDA granted accelerated approval to anti-PD-1 mAb pembrolizumab for the treatment of patients with unresectable or metastatic MSI-high (MSI-H) or mismatch-repair-deficient (dMMR) solid tumors after prior conventional chemotherapies in 2017,4 and for the treatment of patients with unresectable or metastatic TMB-high (TMB-H) solid tumors in 2020.5 In the field of gastrointestinal cancers, many clinical trials evaluating the safety and efficacy of various regimens such as ICI monotherapy, the combination of anti-CTLA-4 mAb and anti-PD-1/PD-L1 mAb, and combination of ICI and conventional chemotherapy or tyrosine kinase inhibitor (TKI) have been reported or are in progress. In the present manuscript, we review the MSI status and TMB in gastrointestinal, hepatobiliary, and pancreatic cancers, report on the results of relevant clinical trials evaluating ICIs, and summarize the prospects of more effective treatment by combining ICI and other therapeutic agents.
Basic immunobiology of CTLA-4 and PD-1/PD-L1
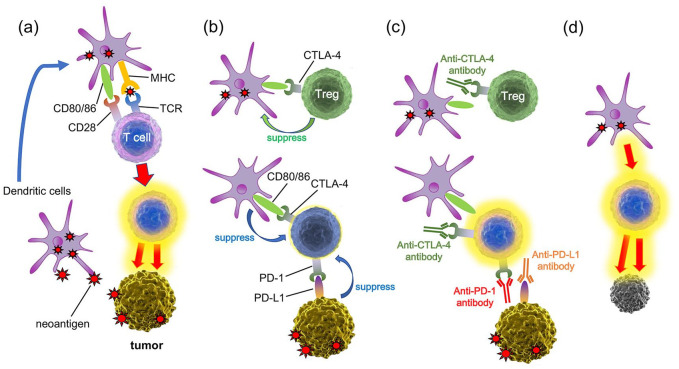
Immune checkpoints play essential roles in preventing T cells overactivation by interacting with antigen presenting cells (APCs) and other cell types. Of the immune checkpoint proteins identified to date, the mechanisms by which CTLA-4 and PD-1/PD-L1 inhibit T cell function are well understood. T cells are activated by the primary signal from recognition of antigens via the T cell receptor (TCR) and the second signal generated by the binding of CD28 to B7 molecules (CD80/CD86) on APCs. CTLA-4 is induced on naïve T cells by antigen activation and is constitutively expressed on regulatory T cells (Tregs).6 CTLA-4 is a homologue of CD28 and binds to CD80/CD86 with approximately 20 times greater affinity, preventing activation of T cells via CD28.7 CTLA-4 on Tregs can also remove CD80/CD86 from APCs, leading to a reduced ability to prime naïve T cells.8 In contrast to CTLA-4, PD-1 is expressed on activated T cells, B cells, and myeloid cells. The engagement of PD-1 by its ligand PD-L1 leads to the transmission of suppressive signals into T cells and the induction of peripheral immune tolerance.9 Similarly for cancer cells, dendritic cells that have recognized neoantigens activate T cells, and the activated T cells attack the tumor (Figure 1a). However, Treg suppresses the function of dendritic cells via CTLA-4 (Figure 1b). CTLA-4 is expressed on activated T cells, and CTLA-4 binds to CD80/86, which suppresses T cell activation. Furthermore, PD-L1 is aberrantly expressed in various tumors, allowing them to escape from host immune surveillance (Figure 1b). Administration of anti-CTLA-4 mAb and anti-PD-1/PD-L1 mAb can cancel these inhibitory mechanisms (Figure 1c), and restore the ability of T cells to attack the tumor (Figure 1d). The intensive study of these immune checkpoint mechanisms led to the approval of ipilimumab in 2011, nivolumab in 2014, and pembrolizumab in 2014 for patients with malignant melanoma.
Figure 1.
The action mechanisms of immune checkpoint inhibitors. (a) Dendritic cells that have recognized neoantigens activate T cells via the TCR and the second signal generated by the binding of CD28 to CD80/CD86, and the activated T cells attack the tumor. (b) Tregs suppresses dendritic cells via CTLA-4, leading to a reduced ability to prime naïve T cells. CTLA-4 on activated T cells binds to CD80/86, which suppresses T cell activation. PD-1 on activated T cells binds to PD-L1 in tumors, leading to the transmission of suppressive signals into T cells. (c) Administration of anti-CTLA-4 mAb and anti-PD-1/PD-L1 mAb can cancel these inhibitory mechanisms, and (d) restore the ability of T cells to attack the tumor.
CTLA-4, cytotoxic T lymphocyte antigen 4; mAb, monoclonal antibody; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; TCR, T cell receptor; Tregs, regulatory T cells.
Microsatellite instability, tumor mutation burden, and response to immunotherapy
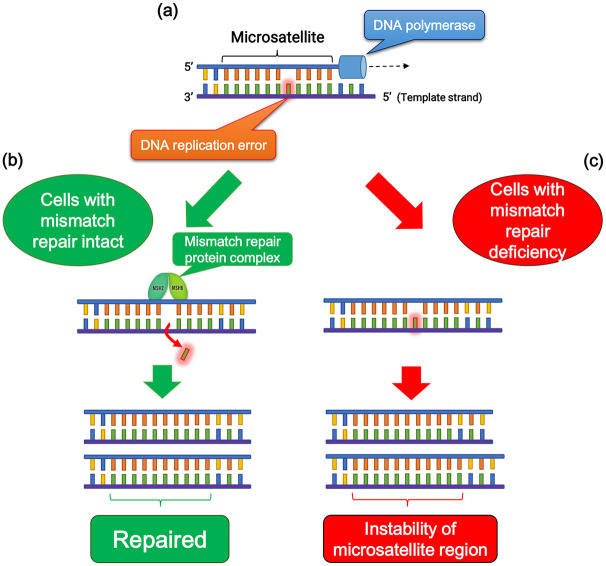
Among human DNA sequences, there are more than 100,000 areas of short tandem repeat sequences termed microsatellites that are particularly susceptible to acquiring errors when the mismatch repair (MMR) pathway is impaired. Cells with an abnormally functioning MMR pathway are unable to correct errors during DNA replication, which causes the creation of an inconsistent number of microsatellite nucleotide repeats, leading to the instability of microsatellite regions (Figure 2).10 MSI reflects the condition of genetic hypermutability that results from impaired DNA MMR and is accompanied by a 100- to 1000-fold increase in the mutation rate.10,11 The presence of MSI is a sign of either sporadic or hereditary dysfunction of the MMR pathway caused by various factors, including mutations in MMR-related genes, inactivation of MMR gene transcription due to hypermethylation of its promoter region.11,12
Figure 2.
Microsatellite stability and instability. (a) DNA replication error is occurred at microsatellite region due to DNA polymerase slippage during replication. (b) When DNA mismatch repair is intact, the replication error is eliminated by mismatch repair pathway, and microsatellite region is repaired accurately. (c) In mismatch repair deficiency, failure of elimination of the replication error leads to the instability of microsatellite lesions.
Llosa and colleagues first reported that colorectal cancers (CRCs) with high infiltration of activated CD8+ cytotoxic T lymphocytes (CTLs) as well as activated Th1 cells characterized by interferon-γ production were MSI-H/dMMR tumors.13 They also demonstrated highly upregulated expression of immune checkpoint proteins in advanced MSI-H/dMMR tumors, which explains why MSI-H/dMMR tumors are not naturally eliminated despite hostile CTL/Th1 microenvironments. Most significantly, their report suggested the utility of MSI status as a predictive marker for the response to PD-1/PD-L1 blockade in cancer patients. Follow-up studies revealed a correlation among MSI status, TMB, and clinical response to treatments with ICIs in various cancers.14–16 High TMB leads to the synthesis of aberrant and potentially immunogenic mutation-associated neoantigens by the cancer cells, which attract CD8+ CTLs and activated Th1 cells to the tumor microenvironment (Figure 3).14 Furthermore, there is a significant correlation between TMB and the response to anti-PD-1/PD-L1 therapy across various types of cancer.15–17 Diaz et al. reported the results of phase II KEYNOTE-158 basket study, in which 77 patients with MSI-H non-CRC across 20 tumor types were enrolled.18 The objective response rate (ORR) was 37.7%, and the 6-month overall survival (OS) and progression-free survival (PFS) rates were 73% and 45%, respectively. Furthermore, Samstein et al. reported an analysis of the clinical and next-generation sequencing (NGS)-based genomic data of 1662 patients with advanced cancer, and demonstrated that high TMB was associated with improved survival in patients receiving treatments with ICIs across a wide variety of cancer types.19
Figure 3.
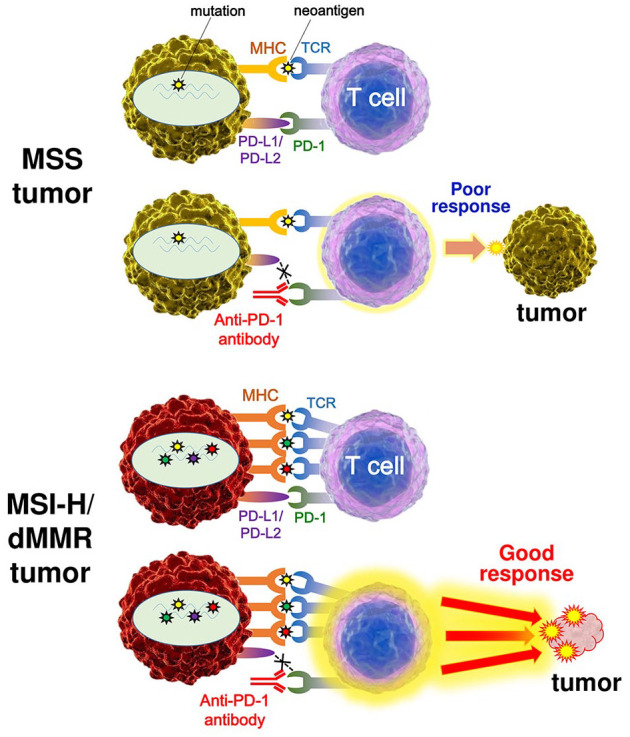
The difference of the response to immune checkpoint therapy between MSS tumors and MSI-H or dMMR tumors. High mutation burden in a MSI-H/dMMR tumor leads to the synthesis of mutation-associated neoantigens presented by MHC class I molecules, which attracts cytotoxic T lymphocytes to the tumor microenvironment via TCR engagement with MHC. Blockade of the PD-1–PD-L1 interaction with an anti-PD-1 antibody results in T cell activation and infiltration into the tumor, leading to objective tumor response.
dMMR, mismatch repair deficiency; MHC, major histocompatibility complex; MSI-H, microsatellite instability-high; MSS, microsatellite-stable; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; TCR, T cell receptor.
As the clinical importance of MSI status and TMB has become broadly recognized, efforts have been made to understand the landscape of MSI status and TMB across various cancer types by NGS-based methods.20–24 Regarding gastrointestinal malignancies, 16.6–19% of colon cancers and 7.5–21.9% of gastric cancers (GCs) were identified as MSI-H, and the frequency of MSI-H/dMMR was lower in patients with advanced-stage cancers.24 In CRC, the frequency of MSI-H has been reported to be 20% in stage I/II, 12% in stage III, and only 4% in stage IV.25 MSI-H CRCs were more frequent in the proximal (right-sided) colon than in the distal (left-sided) colon.26,27 The rates of MSI-H in hepatocellular carcinoma (HCC), biliary tract cancer (BTC), and pancreatic cancer were less than 3% (Table 1). Nakamura et al. recently reported the TMB in tissue samples from 1759 advanced gastrointestinal, hepatobiliary, and pancreatic tumors from a part of the Nationwide Cancer Genome Screening Project in Japan.28 In CRC, TMB-H was observed in 23.6%, including 75.0% of MSI-H and 17.1% of non-MSI-H tumors. In non-CRC, TMB-H was observed in 17.5% of esophageal cancer, 13.3% of GC, 7.4% of HCC, 26.1% of BTC, and 27.9% of pancreatic cancer (Table 1).28 TMB analysis may be useful as an agnostic histologic indicator to identify patients who can benefit from ICIs; however, a universal definition of high TMB may be difficult to establish because the TMB cut-points associated with improved survival varies between cancer types.19
Table 1.
Microsatellite instability status and tumor mutation burden among gastrointestinal, pancreatic, and hepatobiliary cancers.
| Tumor type | dMMR/MSI-H (%) | TMB-high (%) | References |
|---|---|---|---|
| Esophageal cancer | 0–3.3 | 3.5–17.5 | Hause et al., Cortes-Ciriano et al., Salem et al., Bonneville et al., Cancer Genome Atlas Research Network et al.20–23,29 |
| Gastroesophageal junction cancer | 4–8 | 3.1 | Salem et al., Liu et al.22,30 |
| Gastric cancer | 7.5–21.9 | 8.3–13.3 | Hause et al., Cortes-Ciriano et al., Salem et al., Bonneville et al., Nakamura et al., Liu et al., Cancer Genome Atlas Research Network et al.20–23,28,30,31 |
| Small intestinal cancer | 12 | 10.2–30.0 | Salem et al., Nakamura et al.22,28 |
| Gastrointestinal stromal cancer | 0 | 0–6.9 | Salem et al., Nakamura et al.22,28 |
| Right-sided colon cancer | 13.5–27 | 14.6 | Hause et al., Cortes-Ciriano et al., Salem et al., Bonneville et al., Nakamura et al.20–23,28 |
| Left-sided colon cancer | 2.0–2.2 | 3.5 | Salem et al., Liu et al.22,30 |
| Rectal cancer | 2.2–9.2 | 3.0 | Hause et al., Cortes-Ciriano et al., Salem et al., Bonneville et al., Liu et al.20–23,30 |
| Hepatocellular carcinoma | 0–2.9 | 2.2–7.4 | Hause et al., Cortes-Ciriano et al., Salem et al., Bonneville et al., Goumard et al.20–23,32 |
| Biliary tract cancer | 0–3 | 3.7–26.1 | Lee et al., Salem et al., Bonneville et al., Nakamura et al., Ueno et al.10,22,23,28,33 |
| Pancreatic cancer | 0–1.3 | 1.4–27.9 | Cortes-Ciriano et al., Salem et al., Bonneville et al., Liu et al., Hu et al., Lupinacci et al. 21–23,30,34,35 |
| Neuroendocrine tumor/cancer | 0 | 1.3–14.8 | Salem et al., Nakamura et al.22,28 |
dMMR, mismatch repair deficient; MSI-H, microsatellite instability-high; TMB, tumor mutation burden.
From the next section, we discuss the MSI status and critical clinical studies of ICIs for esophageal, gastrointestinal, hepatobiliary, and pancreatic cancers. The results of the most relevant clinical studies are summarized in Table 2. Current ongoing trials related to ICIs identified in ClinicalTrials.gov (https://clinicaltrials.gov/) are summarized in Table 3.
Table 2.
Results of most relevant clinical trials on immune checkpoint inhibitors for gastrointestinal cancers.
| Tumor type | Treatment | Phase | ClinicalTrials.gov identifier | Patient feature | Clinical outcome | Reference |
|---|---|---|---|---|---|---|
| Esophageal cancer | Pembrolizumab versus Paclitaxel, Docetaxel, or Irinotecan | III | KEYNOTE-181 (NCT02564263) |
628 patients with advanced EC that progressed after 1 line of
therapy 222 patients with PD-L1 CPS ⩾10 401 patients with ESCC |
[PD-L1 CPS ⩾10] mOS: 9.3 versus 6.7 months (HR = 0.69 [95% CI: 0.52–0.93]; p = 0.0074) [ESCC] mOS: 8.2 versus 7.1 m (HR = 0.78 [95% CI: 0.63–0.96]; p = 0.0095) |
Shah et al.36 |
| Nivolumab versus
Paclitaxel or Docetaxel |
III | ATTRACTION-3 (NCT02569242) |
Patients with EC who have been refractory or intolerant to one
previous fluoropyrimidine-based and platinum-based
chemotherapy Nivolumab: 210 patients Paclitaxel or Docetaxel: 209 patients |
mOS: 10.9 versus 8.4 month (HR = 0.77 [95% CI: 0.62–0.96; p = 0.019]) |
Kato et al.37 | |
| Gastric and Gastroesophageal junction cancer | Nivolumab versus placebo | III | ATTRACTION-2 (NCT02267343) |
493 patients with advanced GC/GEJc who had been treated with ⩾2 chemotherapy regimens | ORR: 11.2% versus 0% DCR: 40.3% versus 25% mPFS: 1.61 versus 1.45 month (HR = 0.60 [95% CI: 0.49–0.75]; p < 0.0001) mOS: 5.26 versus 4.14 month (HR = 0.63 [95% CI: 0.51–0.78]; p < 0.0001) |
Kang et al.38 |
| Nivolumab + SOX or Nivolumab + CapeOX | II/III | ATTRACTION-4 (NCT02746796) |
<phase II> Patients with previously untreated, unresectable, advanced, or recurrent HER2-negative GC/GEJc Nivolumab + SOX (NS): 21 patients Nivolumab + CapeOX (NC): 18 patients |
ORR: 57.1% (NS) 76.5% (NC) mPFS: 9.7 months (NS) 10.6 months (NC) |
Boku et al.39 | |
| Pembrolizumab | II | KEYNOTE-059 (NCT02335411) |
259 patients with advanced GC/GEJc with ⩾2 prior lines of treatment | ORR: 11.6% (6 CRs) DCR: 28% mDOR: 8.4 months mPFS: 2.0 months mOS: 5.6 months |
Fuchs et al.40 | |
| Pembrolizumab versus Paclitaxel | III | KEYNOTE-061 (NCT02370498) |
592 patients (395 PD-L1 CPS ⩾1) with advanced GC/GEJc that
progressed on first-line chemotherapy with a platinum and
fluoropyrimidine Pembrolizumab arm: 207 GC/89 GEJc (196 PD-L1 CPS ⩾1) Paclitaxel arm: 200 GC/96 GEJc (199 PD-L1 CPS ⩾1) |
Pembrolizumab versus Paclitaxel (PD-L1 CPS
⩾1) ORR: 16% versus 14% mDOR: 18.0 versus 5.2 month mPFS: 1.5 versus 4.1 month (HR = 1.27 [95% CI: 1.03–1.57]) mOS: 9.1 versus 8.3 month (HR = 0.82 [95% CI: 0.66–1.03]) |
Shitara et al.41 | |
| Pembrolizumab or Pembrolizumab + chemotherapy or placebo + chemotherapy | III | KEYNOTE-062 (NCT02494583) |
Randomized study of first-line pembrolizumab or
pembrolizumab + chemotherapy versus
placebo + chemotherapy in 763 patients with PD-L1 CPS ⩾1,
HER2-negative, advanced GC/GEJc (69% GC/30% GEJc) (281 patients [37%] PD-L1 CPS ⩾10) |
Pembrolizumab versus chemotherapy mOS: 10.6 versus 11.1 month (HR = 0.91 [95% CI: 0.69–1.18]) [PD-L1 CPS ⩾10 ]mOS: 17.4 versus 10.8 month (HR = 0.69 [95% CI: 0.49–0.97]) |
Tabernero et al.42 | |
| Colorectal cancer | Pembrolizumab | II | KEYNOTE-164 (NCT02460198) |
63 patients with MSI-H/dMMR mCRC with ⩾1 prior line of therapy | ORR: 58% (2 CRs and 18 PRs) |
Le et al.43 |
| Pembrolizumab | III | KEYNOTE-177 (NCT02563002) | 307 patients with MSI-H/dMMR mCRC were randomly assigned 1:1 to
first-line pembrolizumab or investigator’s choice of mFOLFOX6 or
FOLFIRI ± bevacizumab or cetuximab
(chemotherapy) Pembrolizumab: 153 patients Chemotherapy: 154 patients |
Pembrolizumab versus chemotherapy mPFS: 16.5 versus 8.2 month (HR = 0.60 [95% CI: 0.45–0.80]; p = 0.0002) 12-month PFS: 55.3% versus 37.3% 24-month PFS: 48.3% versus 18.6% ORR: 43.8% versus 33.1% |
Thierry et al. 44 | |
| Nivolumab + low-dose Ipilimumab | II | CheckMate-142 (NCT02060188) |
Preciously treated 119 patients with MSI-H/dMMR mCRC | ORR: 58% DCR: 81% |
Overman et al.45 | |
| Atezolizumab + cobimetinib (MEK1/2 inhibitor) and atezolizumab
monotherapy versus regorafenib |
III | IMblaze370 (NCT02788279) |
Previously treated 363 patients with unresectable mCRC were
randomized to 2:1:1 Atezolizumab + cobimetinib (A+C): 183 patients Atezolizumab monotherapy (A): 90 patients Regorafenib (R): 90 patients |
mOS: 8.9 months (A+C) 7.1 months (A) 8.5 months (R) |
Bendell et al.46 | |
| Hepatocellular carcinoma | Nivolumab | I/II | CheckMate-040 (NCT01658878) |
262 patients with advanced HCC | ORR: 18.2% | Crocenzi et al.47 |
| Nivolumab | III | CheckMate-459 (NCT02576509) |
Nivolumab versus sorafenib as a first-line treatment in 1009 patients with unresectable HCC | HR = 0.85 [95% CI: 0.72–1.02]; p = 0.0752 | Press Release on June 2448 | |
| Pembrolizumab | II | KEYNOTE-224 (NCT02702414) |
104 patients with HCC who had previously treated with sorafenib | ORR: 17% (1 CR and 17 PRs) |
Zhu et al.49 | |
| Pembrolizumab | III | KEYNOTE-240 (NCT02702401) |
Pembrolizumab versus BSC as a second-line
therapy (413 patients) (278 in pembrolizumab and 135 in placebo) |
Pembrolizumab improved OS (HR = 0.78) and PFS
(HR = 0.78) (Not reached the prespecified statistical criteria) ORR: 16.9% versus 2.2% |
Finn et al.50 | |
| Pembrolizumab +Lenvatinib | Ib | KEYNOTE-524 (NCT03006926) |
First-line treatment for advanced unresectable HCC | ORR: 46% (95% CI: 36.0–56.3) mOS: 22.0 months mPFS: 9.3 months (11 CRs and 35 PRs) |
ASCO2020 Virtual Scientific Program51 | |
| Nivolumab + pilimumab | I/II | CheckMate-040 (NCT01658878) |
148 patients with advanced HCC who were previously treated with
sorafenib (arm A) nivolumab/ipilimumab every 3 weeks (arm B) nivolumab/ipilimumab every 2 weeks (arm C) nivolumab/ipilimumab every 6 weeks |
In arm A, the mOS was 23 months and 4 of the 50 patients had a CR | Thomas et al.52 | |
| Atezolizumab + Bevacizumab |
III | IMbrave150 (NCT03434379) |
Randomized study of first-line atezolizumab + bevacizumab
versus sorafenib in 501 patients with
unresectable HCC Atezolizumab + bevacizumab: 336 patients Sorafenib: 165 patients |
Atezolizumab + bevacizumab significantly improved OS (HR = 0.58) and PFS (HR = 0.59) compared with sorafenib | Finn et al.53 | |
| Biliary tract cancers | Pembrolizumab | II | KEYNOTE-158 (NCT02628067) |
104 patients with advanced BTC and prior progression/intolerance on standard therapy | ORR: 6.6% (PD-L1 positive) 2.9% (PD-L1 negative) |
Ueno et al.33 |
| Nivolumab monotherapy or in combination with cisplatin + gemcitabine | I | JapicCTI-153098 | 60 patients with unresectable or recurrent BTC (30 in nivolumab monotherapy and 30 in nivolumab + cisplatin-gemcitabine) |
Nivolumab monotherpy versus
combination mOS: 5.2 versus 15.4 months mPFS: 1.4 versus 4.2 months ORR: 1/30 versus 11/30 |
Ueno et al.54 | |
| Durvalumab with or without tremelimumab | I | NCT01938612 | 107 patients with advanced BTC (42 in durvalumab monotherapy [D] and 65 in durvalumab + tremelimumab [D+T]) |
DCR at 12 weeks: 16.7% (D) 32.2% (D+T) |
Ioka et al.55 | |
| Pancreatic cancer | Ipilimumab | II | - | 27 patients with locally advanced or metastatic pancreas adenocarcinoma | ORR: 0% 1 delayed response |
Royal et al.56 |
| Durvalumab with or without tremelimumab | II | NCT02558894 | 65 patients with advanced pancreatic cancer (33 in durvalumab monotherapy [D] and 32 in durvalumab + tremelimumab [D+T]) |
ORR: 0% (D), 3.1% (D+T) DCR: 6.1% (D), 9.4% (D+T) |
O’Reilly et al. 57 |
BSC, best supportive care; BTC, biliary tract cancer; CapeOX, capecitabine plus oxaliplatin; CI, confidence interval; CPS, combined positive score; CR, complete response; DCR, disease control rate; dMMR, mismatch repair deficient; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; FOLFIRI, leucovorin plus 5-fluorouracil plus irinotecan; GC, gastric cancer; GEJc, gastroesophageal junction cancer; HCC, hepatocellular carcinoma; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; mCRC, metastatic colorectal cancer; mDOR, median duration of response; mFOLFOX6, modified oxaliplatin plus leucovorin plus 5-fluorouracil; mOS, median overall survival; mPFS, median progression-free survival; MSI-H, microsatellite instability-high; ORR, objective response rate; PD-L1, programmed cell death ligand 1; PR, partial response; SOX, S-1 plus oxaliplatin.
Table 3.
Ongoing phase III clinical trials on immune checkpoint inhibitors for gastrointestinal cancers.
| Tumor type | Treatment | ClinicalTrials.gov identifier | Patient feature |
|---|---|---|---|
| Esophageal cancer | Nivolumab versus Docetaxel/Paclitaxel | NCT02569242 | Histologically confirmed unresectable advanced or recurrent
EC Refractory to or intolerant of standard therapy |
| Nivolumab + Ipilimumab or Nivolumab combined with fluorouracil + cisplatin versus fluorouracil + cisplatin | CheckMate 648 (NCT03143153) |
Unresectable advanced, recurrent or metastatic previously untreated ESCC | |
| Pembrolizumab + cisplatin and 5-fluorouracil (5-FU) versus placebo + cisplatin and 5-FU | KEYNOTE-590 (NCT03189719) |
First-line treatment in patients with locally advanced or metastatic esophageal carcinoma | |
| Pembrolizumab versus placebo | KEYNOTE-975 (NCT04210115) |
Patients with esophageal carcinoma who are receiving chemotherapy and radiation therapy | |
| SHR-1210 (camrelizumab) versus
Investigator’s choice standard therapy (Docetaxel or Irinotecan) |
NCT03099382 | Histologically or cytologically confirmed ESCC, locally
advanced, unresectable, recurrent or metastatic
disease Fail to the first-line standard therapy |
|
| SHR-1210 + paclitaxel + cisplatin versus
placebo + paclitaxel + cisplatin |
NCT03691090 | Histologically or cytologically confirmed unresectable local
advanced/recurrent or metastasis ESCC; No previous systemic anti-tumor treatment |
|
| Gastric and Gastroesophageal junction cancer | Nivolumab +S-1 or CapeOX, in comparison with placebo + S-1 or CapeOX | NCT03006705 | Patients with pStage III GC (including GEJc) after D2 or more extensive lymph node dissection (postoperative adjuvant chemotherapy) |
| Nivolumab + Ipilimumab or Nivolumab in combination with oxaliplatin + fluoropyrimidine versus oxaliplatin + fluoropyrimidine | NCT02872116 | Patients with previously untreated advanced or metastatic GC or GEJc | |
| Nivolumab + SOX/CapeOX versus placebo + SOX/CapeOX | ATTRACTION-4 (NCT02746796) |
Patients with unresectable advanced or recurrent GC (including GEJc) that has not been treated with the first-line therapy with systemic antitumor agents | |
| Pembrolizumab versus Paclitaxel | KEYNOTE-063 (NCT03019588) | Asian patients with advanced GC or GEJc who progressed after first-line therapy with platinum and fluoropyrimidine | |
| Pembrolizumab (MK-3475) + Chemotherapy (XP or FP) versus placebo + chemotherapy (XP or FP) | KEYNOTE-585 (NCT03221426) | Neoadjuvant/Adjuvant treatment for patients with GC or GEJc | |
| Pembrolizumab + trastuzumab in combination with standard of care (SOC) chemotherapy versus trastuzumab in combination with SOC chemotherapy | KEYNOTE-811 (NCT03615326) |
Patients with HER2 positive advanced GC or GEJc | |
| Pembrolizumab + chemotherapy (FP or CAPOX regimens) versus placebo + chemotherapy (FP or CAPOX regimens) | KEYNOTE-859 (NCT03675737) | Patients with HER2 negative, previously untreated, unresectable or metastatic GC or GEJc | |
| Colorectal cancer | Nivolumab in combination with standard of care chemotherapy with bevacizumab | CheckMate 9X8 (NCT03414983) | First-line treatment of patients with mCRC |
| Nivolumab alone, Nivolumab in combination with Ipilimumab, or an investigator’s choice chemotherapy | CheckMate 8HW (NCT04008030) | Patients with MSI-H or dMMR mCRC | |
| mFOLFOX6/Bevacizumab combination chemotherapy with or without Atezolizumab or Atezolizumab monotherapy | COMMIT Study (NCT02997228) | First-line treatment of patients with dMMR mCRC | |
| Hepatocellular carcinoma | Durvalumab + Tremelimumab combination therapy and Durvalumab monotherapy versus Sorafenib | HIMALAYA (NCT03298451) | Patients with no prior systemic therapy for unresectable HCC |
| Transarterial chemoembolization (TACE) in combination with durvalumab monotherapy or TACE given with durvalumab + bevacizumab therapy compared with TACE alone | EMERALD-1 (NCT03778957) | Patients with locoregional HCC not amenable to curative therapy | |
| Durvalumab in combination with bevacizumab or durvalumab monotherapy or placebo as adjuvant therapy | EMERALD-2 (NCT03847428) |
Patients with HCC who are at high risk of recurrence after curative hepatic resection or ablation | |
| CS1003 (anti-PD-1 mAb) in combination with lenvatinib and placebo in combination with lenvatinib | NCT04194775 | Patients with no prior systemic treatment and with unresectable HCC | |
| Nivolumab + Ipilimumab versus standard of care (sorafenib or lenvatinib) | CheckMate 9DW (NCT04039607) | Patients with advanced HCC who have not received prior systemic therapy | |
| Adjuvant nivolumab versus placebo | CheckMate 9DX (NCT03383458) | Patients with HCC who are at high risk of recurrence after curative hepatic resection or ablation | |
| Pembrolizumab or placebo given with best supportive care in Asian patients | KEYNOTE-394 (NCT03062358) |
Asian patients with previously systemically treated advanced HCC | |
| Pembrolizumab versus placebo as adjuvant therapy | KEYNOTE-937 (NCT03867084) | Adjuvant therapy in patients with HCC and complete radiological response after surgical resection or local ablation | |
| Lenvatinib in combination with pembrolizumab versus Lenvatinib in combination with placebo | LEAP-002 (NCT03713593) |
First-line therapy of participants with advanced HCC | |
| Cabozantinib in combination with atezolizumab versus the standard of care sorafenib | COSMIC-312 (NCT03755791) |
Patients with advanced HCC who have not received previous systemic anticancer therapy | |
| Adjuvant therapy with atezolizumab + bevacizumab compared with active surveillance | IMbrave050 (NCT04102098) |
Patients with completely resected or ablated HCC who are at high risk for disease recurrence | |
| Biliary tract cancers | Durvalumab in combination with gemcitabine + cisplatin versus placebo in combination with gemcitabine + cisplatin | TOPAZ-1 (NCT03875235) | Patients with first-line advanced BTC |
| KN035 (anti-PD-L1 antibody) compared with standard of care gemcitabine-based chemotherapies | KN035-BTC (NCT03478488) |
Patients with previously untreated locally advanced or metastatic BTC | |
| Pembrolizumab + gemcitabine/cisplatin versus placebo + gemcitabine/cisplatin as first-line therapy | KEYNOTE-966 (NCT04003636) |
Patients with advanced and/or unresectable BTC |
BTC, biliary tract cancer; CapeOX, capecitabine plus oxaliplatin; CAPOX, oxaliplatin combined with capecitabine; dMMR, mismatch repair deficient; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; FP, Cisplatin combined with 5-Fluorouracil; GC, gastric cancer; GEJc, gastroesophageal junction cancer; HCC, hepatocellular carcinoma; HER2, human epidermal growth factor receptor 2; mCRC, metastatic colorectal cancer; mFOLFOX6, modified oxaliplatin plus leucovorin plus 5-fluorouracil; MSI-H, microsatellite instability-high; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; S-1, tegafur-gimeracil-oteracil potassium; SOX, S-1 plus oxaliplatin; XP, capecitabine plus cisplatin.
Esophageal cancer
Although esophageal cancers have a low prevalence of dMMR/MSI-H (0–3.3%), TMB is reported to be relatively high (3.5–17.5%, Table 1). Therefore, the response to ICIs was estimated to be high among gastrointestinal malignancies. The result of the phase III KEYNOTE-181 study evaluated pembrolizumab versus chemotherapy as second-line therapy in 628 patients with advanced/metastatic esophageal squamous cell carcinoma (ESCC) or esophageal adenocarcinoma was presented at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium in 2019.36 Although pembrolizumab did not improve OS or PFS in the overall intent-to-treat population, pembrolizumab was of significant survival benefit in patients with esophageal cancer with PD-L1 combined positive score (CPS) ⩾10 (median OS: 9.3 versus 6.7 months, p = 0.0074). In the ESCC subgroup, median OS was 8.2 months versus 7.1 months (HR = 0.78, p = 0.0095). With pembrolizumab, fewer patients had drug-related adverse events (AEs) of any grade (64% versus 86%) or grade 3–5 (18% versus 41%), compared with chemotherapy. These data support pembrolizumab as a new second-line standard of care for esophageal cancers with PD-L1 CPS ⩾10. The phase III KEYNOTE-590 study, investigating pembrolizumab + chemotherapy as first-line therapy, is ongoing.58
Regarding nivolumab, the result of the global multi-center randomized phase III trial (ATTRACTION-3) evaluating nivolumab versus chemotherapy in patients with unresectable advanced or recurrent ESCC refractory or intolerant to one previous fluoropyrimidine-based and platinum-based drug was recently documented.37 Patients were randomly assigned to either nivolumab or investigator’s choice of chemotherapy (paclitaxel or docetaxel). Nivolumab demonstrated a significant extension in OS compared with chemotherapy (median 10.9 versus 8.4 months; HR = 0.77, p = 0.019). Subgroup analysis revealed that nivolumab provided superior OS regardless of tumor PD-L1 expression. In the nivolumab group, fewer patients had treatment-related AEs of grade 3 or 4 compared with chemotherapy (18% versus 63%). The phase III CheckMate-648 trial evaluating nivolumab + ipilimumab or nivolumab + chemotherapy for advanced esophageal cancer is ongoing.
Gastric or gastroesophageal junction cancer
Among all cancer types, gastrointestinal adenocarcinomas exhibit MSI properties at a comparatively high proportion. Comprehensive molecular analysis of gastrointestinal adenocarcinomas revealed that MSI-H adenocarcinomas are observed primarily in the distal stomach and proximal colon.30 The Cancer Genome Atlas Research Network analyses demonstrated that gastric and gastroesophageal junction adenocarcinomas are divided into four subtypes according to their molecular features: tumors exhibiting chromosomal instability (CIN), MSI-H, Epstein–Barr virus (EBV)-positive, and genomically stable.29,31 Among them, MSI-H tumors account for approximately 22% of GCs, and a small minority of MSI-H GCs are related to a germline mutation in MMR-related genes.30 Pathophysiologically, MSI-H GCs are linked with female sex, older age, intestinal type, and distal location, and almost all sporadic MSI-H GCs exhibit epigenetic silencing of MLH1 in the context of a CpG island methylator phenotype.30,59 MSI-H GCs have a high incidence of somatic mutations, including mutations in genes related to receptor tyrosine kinase-RAS signaling, but generally lack targetable alterations compared with CIN-type GCs having therapeutically targetable amplification in receptor tyrosine kinase. Importantly, MSI-H or EBV-positive GCs have a high interferon-γ gene expression signature levels and highly correlated with PD-L1 positivity.30,60 Therefore, advanced MSI-H GCs with metastases could be suitable targets of anti-PD-1 therapies.
Bang et al. reported the results of the phase III JAVELIN Gastric 300 trial comparing a human PD-L1 antibody avelumab (Bavencio®) versus chemotherapy (paclitaxel or irinotecan) as third-line therapy in patients with advanced GC or gastroesophageal junction cancer (GEJc).61 The trial did not meet its primary endpoint of improving OS (4.6 versus 5.0 months) or the secondary endpoints of PFS (1.4 versus 2.7 months) in the avelumab versus chemotherapy arms, respectively. Recently, the topline results of the phase III JAVELIN Gastric 100 study evaluating avelumab as first-line maintenance therapy following induction chemotherapy in patients with unresectable, locally advanced or metastatic HER-2 negative GC/GEJc versus continuation of chemotherapy or best supportive care were announced.62 While the study showed clinical activity for avelumab, it did not meet the primary endpoints of superior OS compared with the standard of care in the overall intent-to-treat population or the PD-L1-positive population.
Kang et al. reported the efficacy and safety of nivolumab in patients with advanced GC/GEJc who had been previously treated with two or more chemotherapy regimens (ATTRACTION-2).38 In this trial, median OS was 5.26 months in the nivolumab group, compared with 4.14 months in the placebo group (HR = 0.63, p < 0.0001). Recently, the phase II part of ATTRACTION-4 trial, evaluating the safety and efficacy of nivolumab with S-1 + oxaliplatin (SOX) or capecitabine + oxaliplatin (CapeOX) as first-line therapy for unresectable advanced or recurrent human epidermal growth factor receptor 2 (HER2)-negative GC/GEJc was announced.39 Nivolumab combined with SOX/CapeOX was well-tolerated and demonstrated encouraging efficacy (ORR: 57.1% in nivolumab + SOX group and 76.5% in the nivolumab + CapeOX group). ATTRACTION-4 has proceeded to phase III to compare nivolumab + SOX/CapeOX versus placebo + SOX/CapeOX.
Regarding pembrolizumab, Fuchs et al. reported the phase II KEYNOTE-059 trial evaluating the safety and efficacy of pembrolizumab monotherapy in 259 patients with advanced GC/GEJc with ⩾2 prior lines of treatment.40 Pembrolizumab monotherapy demonstrated manageable safety and promising activity (ORR: 11.6%, median response duration: 8.4 months). In the phase III KEYNOTE-061 study, Shitara et al. compared pembrolizumab with paclitaxel in 592 patients with advanced GC/GEJc that progressed on first-line chemotherapy with platinum-based drug and fluoropyrimidine.41 As a result, pembrolizumab did not significantly improve median OS compared with paclitaxel as second-line therapy for advanced GC/GEJc with PD-L1 CPS ⩾1 (9.1 versus 8.3 months). Tabernero et al. recently presented the results of the randomized phase III KEYNOTE-062 study evaluating the first-line pembrolizumab with or without chemotherapy versus chemotherapy alone.42 Initial therapy with pembrolizumab resulted in non-inferior OS compared with standard chemotherapy in patients with PD-L1 CPS ⩾1 (10.6 versus 11.1 months). In patients with PD-L1 CPS ⩾10, median OS was 17.4 months in the pembrolizumab group compared with 10.8 months in the chemotherapy group. This study also evaluated combined treatment with pembrolizumab and standard chemotherapy; however, the combination regimen did not improve survival relative to chemotherapy alone.
Colorectal cancer
CRCs mainly arise from adenoma with inactivated mutation or deletion in the tumor suppressor gene APC (adenoma-carcinoma sequence); however, MSI-H CRCs develop via a different pathway. The Colorectal Cancer Subtyping Consortium classified CRCs into four consensus molecular subtypes (CMSs) with distinguishing biologic features: CMS1 (MSI immune subtype, 14%), CMS2 (canonical subtype, 37%), CMS3 (metabolic subtype, 13%), CMS4 (mesenchymal subtype, 23%), and mixed features (13%).63 Among them, CMS1 tumors are hyper-mutated types and exhibit MSI-H features. Inherited MSI-H CRCs occur due to germline mutations in MMR-related genes such as MLH1 and MSH2, whereas sporadic MSI-H CRCs typically arise from sessile-serrated adenomas/polyps with BRAF V600E mutation and widespread hypermethylation, including MLH1 promoter methylation (serrated pathway). Regardless of the backgrounds (sporadic or hereditary), MSI-H CRCs are frequently diagnosed in the proximal (right-sided) colon and have high growth ability but less metastasis.26,27 Also, these cancers have increased numbers of tumor-infiltrating lymphocytes, mainly comprising Th1 and CTLs, and high expression of PD-L1, along with strong activation of immune evasion pathways,63,64 which results from the presence of numerous neoantigens resulting from the hyper-mutated profile of MSI-H CRCs. Therefore, although recurrent MSI-H CRCs have a worse prognosis, these tumors are good candidates for immune checkpoint therapy.
Le et al. reported the data from cohort B of phase II KEYNOTE-164 study investigating the antitumor activity of pembrolizumab for patients with MSI-H metastatic CRC (mCRC) treated with ⩾1 prior line of therapy.43 Of 63 patients enrolled, the ORR was 32%, the 12-month PFS rate was 41%, and the 12-month OS rate was 76%. The result of phase III KEYNOTE-177 study to evaluate the efficacy and safety of pembrolizumab versus standard-of-care chemotherapy as first-line therapy for MSI-H/dMMR mCRC was recently reported in ASCO 2020.44 Pembrolizumab was clearly superior to chemotherapy for PFS (16.5 versus 8.2 months; p = 0.0002) with fewer treatment-related AEs, suggesting that pembrolizumab is a suitable new standard of care for patients with MSI-H/dMMR mCRC.
With regard to combination therapy, nivolumab + low-dose ipilimumab as first-line therapy in MSI-H/dMMR mCRC (phase II CheckMate-142 trial) demonstrated robust and durable response (ORR = 69% in 45 patients with median follow up of 29.0 months).65 In patients with previously treated MSI-H/dMMR mCRC enrolled in the same trial, the ORR and disease control rates (DCR) of nivolumab + low-dose ipilimumab combination were 58% and 81%, respectively.45 Besides, the result of phase III study comparing atezolizumab (anti-PD-L1 mAb, Tecentriq®) + cobimetinib (MEK1/2 inhibitor) and atezolizumab monotherapy versus standard-of-care regorafenib in chemotherapy-refractory mCRC was reported.46 As a result, atezolizumab + cobimetinib and atezolizumab monotherapy did not demonstrate statistically significant prolonged OS benefit versus regorafenib in intent-to-treat population.46 A randomized phase III study of mFOLFOX6/bevacizumab combination chemotherapy with or without atezolizumab or atezolizumab monotherapy in the first-line treatment of patients with dMMR mCRC is ongoing.66
Hepatocellular carcinoma
After a decade with sorafenib as the only available multi-targeted TKI for HCC, regorafenib as second-line therapy and lenvatinib as another first-line therapeutic agent were finally approved.67 The prognosis of HCC is still poor; however, because of the high potential for intra- and extra-hepatic multiple recurrence and metastasis. Goumard et al. analyzed 122 patients with HCC and found no tumors displaying a typical MSI-H phenotype as defined by PCR-based MSI testing.32 Low levels of MSI, however, were observed in 31.1% (38/122) of HCCs. Furthermore, the rate of MSI was higher in patients with cirrhosis than in those without cirrhosis.32 Some degree of MSI is known to be induced by chronic inflammation, as reported in pancreatitis and ulcerative colitis.68,69 We previously demonstrated that proinflammatory cytokine stimulation-induced transcriptional downregulation of MSH2 via inflammation-mediated microRNA-21 expression in hepatocytes.70 Furthermore, hepatocyte-specific disruption of MSH2 in mice results in the development of liver tumors with the histologic features of HCC. Therefore, although the MSI-H phenotype is rare in HCC, inflammation-mediated dysfunction of the MMR pathway can contribute to an accumulation of mutations during hepatitis-associated tumorigenesis. The CheckMate-040 study revealed that nivolumab induced durable responses in both sorafenib-naïve patients (ORR: 23%, DCR: 63%) and sorafenib-experienced patients (ORR: 16%-19%) with advanced HCC.47 In September 2017, nivolumab was approved by the FDA as a second-line treatment for HCC after sorafenib failure based on a 154-patient subgroup analysis of CheckMate-040.71 However, a randomized phase III study evaluating nivolumab versus sorafenib as a first-line treatment in patients with unresectable HCC (CheckMate-459) revealed that the trial did not achieve statistical significance for its primary endpoint of OS per the pre-specified analysis.48 Pembrolizumab was also granted accelerated approval by the FDA in November 2018, as a second-line treatment after sorafenib failure based on the data from the phase II KEYNOTE-224 trial.49 The results from the phase III KEYNOTE-240 trial, however, demonstrated that although patients treated with pembrolizumab as a second-line treatment achieved a longer OS and PFS versus placebo, the findings were not deemed statistically significant per the prespecified statistical plan.50 Therefore, ICI treatment in combination with TKI or different types of ICI may be promising in the future, rather than the strategy of sequential therapy from TKI to ICI.72 In July 2019, the FDA granted breakthrough therapy designation for pembrolizumab in combination with lenvatinib for the potential first-line treatment of patients with advanced unresectable HCC not amenable to locoregional treatment, based on the results from the phase Ib trial KEYNOTE-524/Study 116.51,73 In March 2020, the FDA granted accelerated approval to the combination of nivolumab and ipilimumab for patients with HCC who have been previously treated with sorafenib based on data from the cohort 4 of CheckMate-040 study.52,74 In addition, the regimen of tremelimumab (anti-CTLA-4 mAb) in combination with durvalumab (anti-PD-L1 mAb) is being evaluated in the ongoing phase III HIMALAYA study in first-line HCC versus sorafenib.75
Recently, the results from the phase III IMbrave 150 study evaluating atezolizumab in combination with bevacizumab (anti-VEGF mAb, Avastin®) for patients with unresectable HCC who have not received prior systemic therapy were reported.53 Atezolizumzb in combination with bevacizumab significantly improved OS and PFS, compared with sorafenib. OS at 12 months was 67.2% with atezolizumab + bevacizumab and 54.6% with sorafenib. Median PFS was 6.8 months and 4.3 months in the respective groups. Grade 3–4 AEs occurred in 56.5% of patients who received atezolizumab + bevacizumab and in 55.1% of patients who received sorafenib. The combination of atezolizumab + bevacizumab was the first regimen that has shown significantly improved OS compared with sorafenib and is considered to replace sorafenib as the novel first-line treatment in the near future. There are various other ongoing trials investigating anti-PD-1/PD-L1 mAb in combination with TKI or anti-CTLA-4 mAb.76
Biliary tract cancer
BTCs are often diagnosed at an advanced stage; therefore, the standard chemotherapy regimen gemcitabine + cisplatin provides limited benefit.77 Therefore, it is essential to investigate the treatment response of ICIs against BTCs and identify a predictive response marker. The rate of MSI-H/dMMR BTCs is reported to be 1–3%.10 Although MSI-H BTCs are rare, anti-PD-1/PD-L1 mAbs exert a specific antitumor activity in a subset of advanced BTCs. Ueno et al. reported the results of phase II, multicohort KEYNOTE-158 study evaluating the antitumor activity and safety of pembrolizumab in 104 patients with advanced BTC and prior progression/intolerance on standard therapy.33 The ORR was 6.6% and 2.9% among those with PD-L1 CPS ⩾1 and CPS <1, respectively. Median PFS was 1.9 months versus 2.1 months and median OS was 7.2 months versus 9.6 months among patients with PD-L1 CPS ⩾1 versus <1, respectively, indicating that pembrolizumab shows durable antitumor activity in a subset of patients with advanced BTC regardless of PD-L1 CPS. Although the OS and PFS of pembrolizumab as a second-line therapy are not entirely satisfactory, it is worth considering because no standard salvage chemotherapy regimen for advanced BTCs in progression after gemcitabine + cisplatin has yet been identified.
The results of the phase I study (JapicCTI-153098) investigating the safety and tolerability of nivolumab monotherapy or in combination with cisplatin + gemcitabine for 60 patients with unresectable or recurrent BTC suggested that nivolimab had a manageable safety profile and signs of clinical activity.54 In the nivolumab monotherapy cohort, mOS and mPFS were 5.2 months and 1.4 months, respectively, and one of 30 patients had an ORR. On the other hand, mOS and mPFS in the combination therapy cohort were 15.4 months and 4.2 months, respectively, and 11 of 30 patients had an ORR. In addition, a recent report of the phase I study of durvalumab with or without tremelimumab suggested that their combination might become a promising regimen for patients with advanced BTC after conventional chemotherapy.55
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is lethal cancer with a poor prognosis despite the recent progress on chemotherapy regimens such as FOLFIRINOX (leucovorin and fluorouracil + irinotecan and oxaliplatin) or gemcitabine + nab-paclitaxel.78 Unfortunately, ICIs including anti-PD-1 or anti-CTLA-4 mAb alone or in combination exhibit little efficacy against PDAC.56,57,79 O’Reilly et al. recently reported the result of phase II randomized clinical trial of 65 patients with metastatic PDAC, evaluating durvalumab with or without tremelimumab. Durvalumab + tremelimumab therapy was tolerated; however, ORR was 3.1% for patients receiving combination therapy and 0% for patients receiving durvalumab monotherapy.57
Poor response of PDAC to ICIs results from highly immunosuppressive tumor microenvironment and stroma, along with low TMB, both of which lead to poor T cell infiltration.80 The MSI-H/dMMR phenotype is indeed very rare in PDAC. Hu et al. reported that the MSI-H/dMMR phenotype was present in only 0.8% (7/833) of patients with PDAC.34 Lupinacci et al. also reported a multicenter study of MSI status in 443 cases with PDAC, including 58 intraductal papillary mucinous neoplasm (IPMN)-associated PDACs.35 In their report, the MSI-H/dMMR phenotype was present in 5 of 385 (1.3%) non-IPMN-associated PDACs and 4 of 58 (6.9%) IPMN-associated PDACs. PDAC has minimal-to-moderate infiltration of CD3, CD4, and CD8 T cells; however, the infiltrates are predominantly present in the stromal area of the tumor and are excluded from the tumoral area of PDACs.81 Furthermore, metastatic PDACs had lower T cell infiltration compared with resectable primary PDACs. It is necessary for improving the responsivity of PDAC to ICIs to elucidate the mechanisms of increasing initial T cell priming, overcoming the immunosuppressive tumor microenvironment, and inhibiting compensatory mechanisms of T cell anergy and exhaustion.79 Although the MSI-H/dMMR phenotype is very rare in PDAC, the ASCO clinical practice guideline recommends routine testing for MSI-H or dMMR, and treatment with pembrolizumab as second-line therapy for patients testing positive for MSI-H or dMMR.82 The National Comprehensive Cancer Network guidelines Version 1.2019 also recommends MSI and/or MMR testing in patients with locally advanced or metastatic PDAC, and treatment with pembrolizumab only for MSI-H or dMMR tumors.83
Prospects of immunotherapy against gastrointestinal malignancies
The clinical experience accumulated to date has provided evidence that anti-PD-1/PD-L1 mAbs are useful in a variety of advanced/metastatic cancer, with improved clinical outcomes compared with conventional chemotherapy. As mentioned, however, in the field of gastrointestinal malignancies, anti-PD-1/PD-L1 mAb monotherapy does not currently exhibit a satisfactory therapeutic efficacy. Three strategies are conceivable to improve the efficacy of ICIs; specifying novel predictive biomarkers, identifying effective combination therapies with other drugs, and overcoming acquired resistance.
Kim et al. reported that patients with MSI-H and EBV-positive metastatic GC had dramatic responses to pembrolizumab.60 ORR was 85.7% in patients with MSI-H tumor and 100% in those with EBV-positive tumor, compared with 6.3% in those with other types of tumor. These results imply the importance of both MSI and EBV testing in the choice of therapy for GC. Also, Shen et al. recently reported that deficiency of AT-rich interaction domain 1A (ARID1A), a subunit of the chromatin remodeling complex SWI/SNF, led to impaired MMR and treatment with ICIs resulted in the prolonged survival of mice bearing ARID1A-deficient tumors.84 Therefore, ARID1A status could also be a potential predictor of response to ICIs.
With regard to identifying the combination regimen of ICIs along with other drugs, many clinical studies are ongoing. As mentioned earlier, clinical studies investigating combination regimens of anti-PD-1/PD-L1 mAb with conventional chemotherapies are ongoing in patients with esophageal cancer,58 GC/GEJc,39 CRC,66 and BTC,54 although the combination of pembrolizumab with standard chemotherapy as a first-line therapy did not improve survival relative to chemotherapy alone in patients with PD-L1 CPS ⩾1, HER2-negative, advanced GC/GEJc.42 Currently, the combined regimens of anti-PD-1/PD-L1 and anti-CTLA-4 mAbs have been evaluated in CRC,45,65 HCC,52,74,75 BTC,54,55 and PDAC.57 The blockade of CTLA-4, which is involved in the regulation of T-cell activation in lymph nodes/tissues and in suppression of dendritic cell activity via Tregs, could act synergistically with blockade of PD-1/PD-L1 that is involved mainly in inhibition of effector T-cell and natural killer (NK) cell activation in peripheral tissues and in induction of Treg differentiation.85,86 As mentioned earlier, clinical trials have shown promising results, especially in the treatment of MSI-H/dMMR mCRC and HCC; however, further studies, particularly in terms of safety, are expected.
Regarding the resistance to immunotherapy, the correlation between increased numbers of intratumoral Treg or tumor-associated macrophages (TAMs) and poor clinical outcomes has been reported in CRC and PDAC.87 Intratumoral Tregs inhibit the activation and proliferation of CD8+ CTLs and effector CD4+ T cells via various means, including release of suppressive molecules such as transforming growth factor-β or interleukin-10, and upregulated expression of immune checkpoints such as PD-1/PD-L1, CTLA-4, lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT).88 The combination therapies of PD-1/PD-L1 or CTLA-4 with mAbs targeting LAG-3, TIM-3, or TIGIT, therefore, could enhance antitumor immune response and overcome acquired resistance, and are currently under clinical investigation.88 The phase Ib REGONIVO study (EPOC1603) evaluating multi-TKI regorafenib + nivolumab in patients with advanced GC or CRC was planned from a basic study in which regorafenib reduces TAMs in tumor models, and was reported in ASCO 2019.89 In the study, 25 patients with GC and 25 patients with CRC were enrolled. The ORR and PFS were 44% and 5.8 months in GC and 36% and 6.3 months in CRC with manageable safety profiles. In addition, Blando et al. reported the presence of a high number of CD68+ macrophages in the tumor stromal area of PDAC.81 Moreover, V-domain immunoglobulin suppressor of T cell activation (VISTA) was predominantly expressed in the macrophages. An activated VISTA pathway decreases T cell responses in the tumor to a higher degree than PD-L1 inhibition, suggesting that PD-1/PD-L1 inhibition might fail in the treatment of PDACs because an untreated VISTA pathway still suppresses the immune response. Combination therapy to increase T cell infiltration, possibly using anti-CTLA-4 mAb + anti-VISTA antibody to target macrophages, may be a prominent treatment strategy for PDAC.
Conclusion
The development of anti-CTLA-4 and anti-PD-1/PD-L1 mAbs has shown remarkable clinical success across several types of malignancies. In the field of gastrointestinal malignancies, however, a small proportion of patients currently benefit from treatment with ICIs. The results of clinical trials are strongly anticipated to lead to the creation of optimal and safe combinations of ICIs and other treatment strategies tailored to the characteristics of each type of cancer. Furthermore, it is necessary to clarify the molecular mechanisms underlying acquired resistance and tumor immune evasion, which could lead to an identification of predictive biomarkers and maximize the therapeutic effect of ICIs.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI Grant Number 19K17458.
ORCID iD: Yuji Eso  https://orcid.org/0000-0003-4426-1491
https://orcid.org/0000-0003-4426-1491
Contributor Information
Yuji Eso, Department of Gastroenterology and Hepatology, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaharacho, Sakyo-ku, Kyoto, 6068507, Japan.
Hiroshi Seno, Department of Gastroenterology and Hepatology, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
References
- 1. Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 2. Tang J, Yu JX, Hubbard-Lucey VM, et al. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov 2018; 17: 854–855. [DOI] [PubMed] [Google Scholar]
- 3. Eso Y, Shimizu T, Takeda H, et al. Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 2020; 55: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type-FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol 2018; 4: 157–158. [DOI] [PubMed] [Google Scholar]
- 5. FDA Drug Approvals and Databases. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. [Google Scholar]
- 6. Nakano S, Eso Y, Okada H, et al. Recent advances in immunotherapy for hepatocellular carcinoma. Cancers (Basel) 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim ES, Kim JE, Patel MA, et al. Immune checkpoint modulators: an emerging antiglioma armamentarium. J Immunol Res 2016; 2016: 4683607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011; 332: 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007; 19: 813–824. [DOI] [PubMed] [Google Scholar]
- 10. Lee V, Murphy A, Le DT, et al. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist 2016; 21: 1200–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seth S, Ager A, Arends MJ, et al. Lynch syndrome - cancer pathways, heterogeneity and immune escape. J Pathol 2018; 246: 129–133. [DOI] [PubMed] [Google Scholar]
- 12. Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer 2015; 15: 181–194. [DOI] [PubMed] [Google Scholar]
- 13. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015; 5: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zappasodi R, Merghoub T, Wolchok JD. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell 2018; 33: 581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019; 30: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wakai T, Prasoon P, Hirose Y, et al. Next-generation sequencing-based clinical sequencing: toward precision medicine in solid tumors. Int J Clin Oncol 2019; 24: 115–122. [DOI] [PubMed] [Google Scholar]
- 17. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diaz L, Marabelle A, Kim TW, et al. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Ann Oncol 2017; 28(suppl 5). [Google Scholar]
- 19. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019; 51: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 2016; 22: 1342–1350. [DOI] [PubMed] [Google Scholar]
- 21. Cortes-Ciriano I, Lee S, Park WY, et al. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017; 8: 15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salem ME, Puccini A, Grothey A, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res 2018; 16: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stadler ZK. Diagnosis and management of DNA mismatch repair-deficient colorectal cancer. Hematol Oncol Clin North Am 2015; 29: 29–41. [DOI] [PubMed] [Google Scholar]
- 26. Fujiyoshi K, Yamamoto G, Takenoya T, et al. Metastatic pattern of stage IV colorectal cancer with high-frequency microsatellite instability as a prognostic factor. Anticancer Res 2017; 37: 239–247. [DOI] [PubMed] [Google Scholar]
- 27. Ogura T, Kakuta M, Yatsuoka T, et al. Clinicopathological characteristics and prognostic impact of colorectal cancers with NRAS mutations. Oncol Rep 2014; 32: 50–56. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura Y, Okamoto W, Shitara K, et al. Large-scale analyses of tumor mutation burdens (TMBs) across various advanced gastrointestinal (GI) malignancies in the nationwide cancer genome screening project, SCRUM-Japan GI-SCREEN. J Clin Oncol 2018; 36: 12094. [Google Scholar]
- 29. Cancer Genome Atlas Research Network; Analysis Working Group; Asan University; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Sethi NS, Hinoue T, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 2018; 33: 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goumard C, Desbois-Mouthon C, Wendum D, et al. Low levels of microsatellite instability at simple repeated sequences commonly occur in human hepatocellular carcinoma. Cancer Genom Proteom 2017; 14: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: Results from the multicohort, phase II KEYNOTE-158 study. Ann Oncol 2018; 29(suppl_8). [Google Scholar]
- 34. Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res 2018; 24: 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lupinacci RM, Goloudina A, Buhard O, et al. Prevalence of microsatellite instability in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology 2018; 154: 1061–1065. [DOI] [PubMed] [Google Scholar]
- 36. Shah MA, Adenis A, Enzinger PC, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase 3 KEYNOTE-181 study. J Clin Oncol 2019; 37: 4010. [Google Scholar]
- 37. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 38. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 39. Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol 2019; 30: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018; 4: e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392: 123–133. [DOI] [PubMed] [Google Scholar]
- 42. Tabernero J, Cutsem EV, Bang Y-J, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol 2019; 37: LBA4007. [Google Scholar]
- 43. Le D, Kavan P, Kim T, et al. Safety and antitumor activity of pembrolizumab in patients with advanced microsatellite instability–high (MSI-H) colorectal cancer: KEYNOTE-164. Ann Oncol 2018; 29(suppl 5). [Google Scholar]
- 44. Thierry A, Kai-Keen S, Won KT, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 study. J Clin Oncol 2020; 38(suppl; abstr LBA4). [Google Scholar]
- 45. Overman MJ, Lonardi S, Wong KYM, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. J Clin Oncol 2019; 37: 635. [Google Scholar]
- 46. Bendell J. atezolizumab combination not superior to regorafenib for refractory mCRC. a late-breaking session at ESMO World Congress on Gastrointestinal Cancer, 20–23 June 2019, Barcelona, Spain. [Google Scholar]
- 47. Crocenzi TS, El-Khoueiry AB, Yau TC, et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol 2017; 35: 4013. [Google Scholar]
- 48. Bristol-Myers Squibb Announces Results from CheckMate -459 Study Evaluating Opdivo (nivolumab) as a First-Line Treatment for Patients with Unresectable Hepatocellular Carcinoma (Press Release on June 24, 2019). [Google Scholar]
- 49. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 50. Finn RS, Ryoo B-Y, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019; 37: 4004. [Google Scholar]
- 51. Phase II trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) for disease progression after PD-1/PD-L1 immune checkpoint inhibitor (ICI) in metastatic clear cell renal cell carcinoma (mccRCC) (Study 111/KEYNOTE-146) (ASCO2020 Virtual Scientific Program Abstract #4519). 2020. [Google Scholar]
- 52. Thomas Y, Yoon-Koo K, Tae-You K, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol 2019; 37(suppl; abstr 412). [Google Scholar]
- 53. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 54. Ueno M, Ikeda M, Morizane C, et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol 2019; 4: 611–621. [DOI] [PubMed] [Google Scholar]
- 55. Ioka T, Ueno M, Oh D-Y, et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). J Clin Oncol 2019; 37: 387. [Google Scholar]
- 56. Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010; 33: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. O’Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol 2019; 5: 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kato K, Shah MA, Enzinger P, et al. KEYNOTE-590: phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol 2019; 15: 1057–1066. [DOI] [PubMed] [Google Scholar]
- 59. Polom K, Marano L, Marrelli D, et al. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018; 105: 159–167. [DOI] [PubMed] [Google Scholar]
- 60. Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018; 24: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 61. Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018; 29: 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. EMD Serono and Pfizer Provide Update on Phase III JAVELIN Gastric 100 Trial (Press Release on November 8, 2019). [Google Scholar]
- 63. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lenz H-J, Lonardi S, Zagonel V, et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) as first-line (1L) therapy in microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): two-year clinical update. J Clin Oncol 2020; 38(15_suppl): 4040. [Google Scholar]
- 66. Lee JJ, Yothers G, Jacobs SA, et al. Colorectal cancer metastatic dmmr immuno-therapy (COMMIT) study (NRG-GI004/SWOG-S1610): a randomized phase III study of mFOLFOX6/bevacizumab combination chemotherapy with or without atezolizumab or atezolizumab monotherapy in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) metastatic colorectal cancer (mCRC). J Clin Oncol 2019; 37. [Google Scholar]
- 67. Eso Y, Marusawa H. Novel approaches for molecular targeted therapy against hepatocellular carcinoma. Hepatol Res 2018; 48: 597–607. [DOI] [PubMed] [Google Scholar]
- 68. Brentnall TA, Chen R, Lee JG, et al. Microsatellite instability and K-ras mutations associated with pancreatic adenocarcinoma and pancreatitis. Cancer Res 1995; 55: 4264–4267. [PubMed] [Google Scholar]
- 69. Brentnall TA, Crispin DA, Bronner MP, et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res 1996; 56: 1237–1240. [PubMed] [Google Scholar]
- 70. Eso Y, Takai A, Matsumoto T, et al. MSH2 dysregulation is triggered by proinflammatory cytokine stimulation and is associated with liver cancer development. Cancer Res 2016; 76: 4383–4393. [DOI] [PubMed] [Google Scholar]
- 71. Finkelmeier F, Waidmann O, Trojan J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther 2018; 18: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 72. Nishida N, Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res 2018; 48: 622–634. [DOI] [PubMed] [Google Scholar]
- 73. Llovet JM, Kudo M, Cheng A-L, et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): phase 3 LEAP-002 study. J Clin Oncol 2019; 37. [Google Scholar]
- 74. FDA Drug Approvals and Databases. FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma. [Google Scholar]
- 75. Kelley RK, Sangro B, Harris WP, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Oncol 2020; 38(suppl; abstr 4508). [Google Scholar]
- 76. Kudo M. Systemic therapy for hepatocellular carcinoma: latest advances. Cancers (Basel) 2018; 10: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009; 101: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 2140–2141. [DOI] [PubMed] [Google Scholar]
- 79. Kabacaoglu D, Ciecielski KJ, Ruess DA, et al. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: current limitations and future options. Front Immunol 2018; 9: 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zheng L, Xue J, Jaffee EM, et al. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013; 144: 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blando J, Sharma A, Higa MG, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A 2019; 116: 1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol 2018; 36: 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tempero MA, Malafa MP, Chiorean EG, et al. Pancreatic adenocarcinoma, Version 1.2019. J Natl Compr Canc Netw 2019; 17: 202–210. [DOI] [PubMed] [Google Scholar]
- 84. Shen J, Ju Z, Zhao W, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 2018; 24: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13: 473–486. [DOI] [PubMed] [Google Scholar]
- 86. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 2019; 38: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: role of tumor-mediated immunosuppression. Semin Cancer Biol. Epub ahead of print 27 July 2019. DOI: 10.1016/j.semcancer.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 89. Shota F, Hiroki H, Naoki T, et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): An open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603). J Clin Oncol 38: 2020; 2053–2061. [DOI] [PubMed] [Google Scholar]