Abstract
Oral mucositis (OM), a common debilitating toxicity associated with chemo- and radiation therapies, is a significant unmet clinical need for head and neck cancer patients. The biological complexities of chemoradiotherapy-induced OM involve interactions among disrupted tissue structures, inflammatory infiltrations, and oral microbiome, whereby several master inflammatory pathways constitute the complicated regulatory networks. Oral mucosal damages triggered by chemoradiotherapy-induced cell apoptosis were further exacerbated by the amplified inflammatory cascades dominantly governed by the innate immune responses. The coexistence of microbiome and innate immune components in oral mucosal barriers indicates that a signaling hub coordinates the interaction between environmental cues and host cells during tissue and immune homeostasis. Dysbiotic shifts in oral microbiota caused by cytotoxic cancer therapies may also contribute to the progression and severity of chemoradiotherapy-induced OM. In this review, we have updated the mechanisms involving innate immunity-governed inflammatory cascades in the pathobiology of chemoradiotherapy-induced OM and the development of new interventional targets for the management of this severe morbidity in head and neck cancer patients.
Keywords: chemotherapy, radiation, inflammatory, microbiota, immunotherapy, tissue homeostasis
Introduction
Oral mucositis (OM) is among the most debilitating side effects of conventional antineoplastic therapies for cancer patients, especially for head and neck cancer patients receiving chemoradiotherapies (Villa and Sonis 2015). OM occurs in about 20% to 40% of cancer patients receiving conventional chemotherapy (Jones et al. 2006; Lalla et al. 2014). In head and neck cancer patients receiving radiotherapy, approximately 80% to 90% of them develop OM, among which about 60% to 70% were recorded as severe OM (grades 3 to 4 by the World Health Organization [WHO] scale) (Elting et al. 2007; Maria et al. 2017). The severe OM often results in reduction and even cessation of cancer treatment, excessive health care cost, and significant negative effects on patient’s quality of life (QOL) due to patients’ extended hospitalization, increased demand for total parenteral nutrition, narcotic analgesia, and antibiotics (Sonis 2004; Bowen and Keefe 2008; Cinausero et al. 2017; Maria et al. 2017). Currently, major roadblocks are the lack of insights into the cellular and molecular mechanisms underlying the pathobiology of chemoradiotherapy-induced OM and few treatment options available for the management of the severe OM (Blakaj et al. 2019; Lalla et al. 2019; Villa and Sonis 2020).
Accumulating evidence has shown that reactive oxygen species (ROS)–mediated inflammatory cascade—in particular, the activated innate immune responses—plays a central role in pathogenesis of chemoradiotherapy-induced OM (Iglesias-Bartolome et al. 2012; Yoshino et al. 2013). Mitochondrial ROS production activates the NLR family pyrin domain containing 3 (NLRP3) inflammasome in response to various bacterial pathogens and tissue damages (Mariathasan and Monack 2007). Blocking mitochondrial ROS/NLRP3 axis protected against radiation-induced OM, suggesting that this pathway plays a role in OM development (Ortiz et al. 2015). Nuclear factor κB (NF-κB) pathway, the downstream of pattern-recognition receptors (PRRs), Toll-like receptors (TLRs), and nucleotide-binding oligomerization domain (NOD)–like receptors, is highly activated by endogenous cell damage–induced molecules and exogenous microbial components (Lotze et al. 2007) and has been shown to play a critical role in pathobiology of OM (Han et al. 2013; Luo et al. 2019). These master inflammatory pathways may synergistically form complex regulatory networks, thus contributing to the complex pathobiology of chemoradiotherapy-induced OM (Maria et al. 2017).
Host–microbe coexistence and interactions play a critical role in maintaining tissue and immune homeostasis at the local mucosal barriers (Moutsopoulos and Konkel 2018). In the healthy oral cavity, the composition and colonization of microbiota are found in complex biofilms, among which the most rich and diverse are tooth-adherent microbial communities (Moutsopoulos and Konkel 2018). In recent years, a growing body of evidence has shown that dysbiotic shifts of oral microflora may contribute to the etiology and severity of chemoradiotherapy-induced OM (Vasconcelos et al. 2016; Hong et al. 2019; Subramaniam and Muthukrishnan 2019; Vesty et al. 2019). Therefore, antimicrobial approaches are emerging as potential modalities for OM management. Herein, we focus on reviewing the progress in studies on the mechanisms underlying the pathobiology of chemoradiotherapy-induced OM and the emerging new therapeutic interventions for this severe morbidity in head and neck cancer patients. To this purpose, we performed a comprehensive search from ClinicalTrials.gov and literatures from PubMed and Web of Science on phase I to III studies on the treatment of chemoradiotherapy-induced OM.
Pathobiology of Chemoradiotherapy-Induced Oral Mucositis
Chemoradiotherapy-induced OM is a biologically complex process involving amplified inflammatory responses, reduced cell proliferation, increased cell senescence/apoptosis, and impaired regenerative potentials in both mucosal and submucosal compartments (Elting et al. 2007; Cinausero et al. 2017; Maria et al. 2017). According to multiple mechanistic models, the complex pathophysiological processes of chemoradiotherapy-induced OM could be divided into 5 overlapped stages, including initiation/primary damage response, message generation, signaling and amplification, ulceration, and healing (Sonis 2004, 2009). The initiation phase occurs immediately after the administration of the cytotoxic agents, which induce primary tissue damage mediated by elevated intracellular ROS levels (Iglesias-Bartolome et al. 2012; Yoshino et al. 2013). The message generation stage involves NF-κB activation, which subsequently upregulates a variety of inflammatory cytokines, such as tumor necrosis factor–α (TNF-α), interleukin (IL)–6, and IL-1β, and stress response genes such as cyclooxygenase-2 (COX-2), inducible NO-synthase, and superoxide dismutase, all of which contribute to the perpetuation of mucosal injuries (Maria et al. 2017). The proinflammatory cytokines, especially TNF-α, further activate NF-κB and then augment the production of proinflammatory cytokines by immune cells such as macrophages, thus leading to a vicious cycle of signal amplification and subsequently the development of epithelial ulceration (Sonis 2004, 2009). The critical role of cytokines and cytokine modulators, stress responders, and cell adhesion molecules, governed by NF-κB signaling, has been implicated in the pathogenesis of chemoradiotherapy-induced OM. In the extremely painful ulcerative stage, oral bacteria colonize the ulcer and stimulate surrounding cells to release cytokines and chemokines for recruitment of inflammatory cells, such as macrophages, mast cells, and neutrophils to produce additional proinflammatory molecules, further contributing to cell apoptosis and tissue damage (Sonis 2009, 2010). The extracellular matrix and submucosal mesenchymal cells interact with innate immune cells to initiate the healing process of chemoradiotherapy-induced OM by activating epithelial cell proliferation and differentiation and oral microbial flora reestablishment (Sonis 2004, 2009, 2010; Vasconcelos et al. 2016; Cinausero et al. 2017). Therefore, such complex interactions between innate immune responses and bacterial microbiome might play a pivotal role in the etiology of chemoradiotherapy-induced OM.
New Pathways Regulating Initiation and Progression of Chemoradiotherapy-Induced Oral Mucositis
Crosstalk between Transforming Growth Factor–β and NF-κB Pathways
Previous studies have identified at least 14 inflammatory and cell apoptotic pathways that are important for pathophysiology of chemoradiotherapy-induced OM (Sonis 2004). NF-κB signaling pathway serves a master hub of inflammatory responses, but it remains largely unknown about the crosstalk among NF-κB and other signaling pathways during the progression of chemoradiotherapy-induced OM. The transforming growth factor–β (TGF-β) family members of cytokines play an important role in regulating a variety of cellular functions and are implicated in tissue homeostasis/remodeling, pathogenesis, and progression of various diseases (Derynck and Budi 2019). Most recently, it has been shown that the TGF-β signaling pathway is highly activated in radiation-induced OM, which leads to inhibition of proliferation and induction of apoptosis in keratinocytes and basal epithelial cells, thus significantly delaying epithelial tissue regeneration and wound healing (Han et al. 2013). Smad7, a signaling antagonist or negative regulator of the TGF-β superfamily, not only blocks TGF-β-mediated cell arrest but also reduces inflammation by suppressing NF-κB activation (He et al. 2002; Hong et al. 2007). In an experimental murine radiation-induced OM model burdened with xenografted head and neck tumors, Smad7 specifically overexpressed in keratinocytes can resist radiation-induced OM through anti-inflammation, promoting epithelial regeneration, and antiapoptosis of keratinocytes (Han et al. 2013; Luo et al. 2019). An N-terminal Tat tag with Smad7 recombinant protein (Tat-Smad7) has been developed, which allows Smad7 to rapidly enter cells (Brooks et al. 2005; Kalvala et al. 2010). Applying Tat-Smad7 complex to oral mucosa shows both preventive and therapeutic effects on radiation-induced OM through activating keratinocyte migration and proliferation, inhibiting cell apoptosis, and blocking TGF-β and NF-κB signaling (Han et al. 2013; Luo et al. 2019). These findings suggest that identification of multiple signaling pathways may provide novel molecular candidates not only for diagnosis but also for the development of novel therapeutic approaches for targeted therapy of chemoradiotherapy-induced OM.
ROS–Mammalian Target of Rapamycin Signaling in Chemoradiotherapy-Induced Oral Mucositis
Increasing evidence suggests that exhaustion of functional somatic stem cells is critical in aging and associated with degenerative phenotypes (Naik et al. 2018). In oral cavity, resident stem cell self-renewal governs mucosal epithelial homeostasis in healthy condition and tissue regeneration during injury (Iglesias-Bartolome et al. 2012; Lei and Chuong 2016). The mammalian target of rapamycin (mTOR) signaling is necessary for cell growth and organ development; however, excessive activation of mTOR cascades can lead to abnormal cell differentiation or senescence, implying that tight control of mTOR signaling level is critical for tissue/organ homeostasis as well as maintenance of cell function by preventing aging (Iglesias-Bartolome and Gutkind 2011; Chen et al. 2015; Chen et al. 2017). Aberrant activation of mTOR signaling pathway plays a pivotal role in oxidative stress or ROS-induced cell senescence and apoptosis (Sharlow et al. 2016; Dermit et al. 2017). A recent study has shown that application of rapamycin, a potent and specific mTOR inhibitor, significantly mitigated the severity of radiation-induced OM in mice through inhibiting radiation-induced cellular senescence, apoptosis, and the loss of proliferative capacity of the oral epithelial stem cell compartment, thus further supporting the notion that mucosal epithelial senescence induced by ROS-mediated aberrant activation of mTOR signaling pathway contributes to a prolonged ulceration and delayed healing process in chemoradiotherapy-induced OM (Iglesias-Bartolome et al. 2012).
On the other hand, mTOR inhibitors have been in clinical trials as oncological drugs to treat patients with metastatic breast cancer, but a variable incidence ranging from 2% to 78% of mTOR inhibitor-associated stomatitis (mIAS), including oral morbidities, has been reported across multiple mTOR inhibitor clinical trials, while grade 3/4 toxicity occurs in up to 9% of patients (Peterson et al. 2016; Chambers et al. 2018). Therefore, it is plausible to take into consideration mTOR inhibitor-associated morbidities in the future development of mTOR inhibitors as interventional drugs for the management of chemoradiotherapy-induced OM.
Wnt/β-catenin Signaling in the Regulation of Cell Cycle in Epithelial Stem Cells
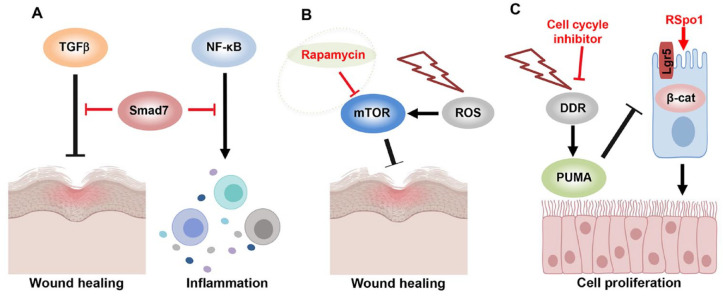
During development of chemoradiotherapy-induced OM, the G1/S check point of cell cycle and DNA damage response (DDR) are highly activated, which causes p53-upregulated modulator of apoptosis-dependent (PUMA-dependent) apoptosis in epithelial stem cells (Wei et al. 2016; Leibowitz et al. 2018). Activation of Wnt/β-catenin by genetic Puma ablation or PUMA inhibition enhances cell proliferation and tissue regeneration following mucosal injury, suggesting that Wnt signaling activators may serve as therapeutic avenue for chemoradiotherapy-induced OM (Fabbrizi et al. 2018; Leibowitz et al. 2018). Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) has been identified as a functional epithelial stem cell marker that is involved in modulating stem cell self-renewal, proliferation, and homeostasis due to its critical role as an effector of R-spondins (Rspo)/Wnt signaling cascades (Barker et al. 2012; Metcalfe et al. 2014; Leung et al. 2018; Raslan and Yoon 2019). It has been reported that the radiosensitivity of Lgr5+ epithelial stem cells is CDK4/6 and DDR dependent, indicating regulation of cell cycle can provide another therapeutic target for chemoradiotherapy-induced OM (Francis et al. 2017; Leibowitz et al. 2018). Zhao et al. (2009) reported that systemic administration of R-Spondin1 (RSpo1) can directly activate canonical Wnt/β-catenin signaling in oral mucosal tissues, thus protecting mice from chemoradiotherapy-induced OM. These findings indicate that multimodes of signaling pathways involved in the initiation and progression of chemoradiotherapy-induced OM can serve as novel therapeutic targets for intervention of this severe antineoplastic therapy-associated morbidity (Fig. 1).
Figure 1.
New pathways regulating inflammation and wound healing in mucositis. (A) Smad7 activation inhibits transforming growth factor–β (TGF-β) signaling for promoting epithelial tissue regeneration and nuclear factor κB (NF-κB) signaling for inflammation resolution. (B) Reactive oxygen species (ROS) induces mammalian target of rapamycin (mTOR) signaling activation in mucositis. Rapamycin, as a specific mTOR inhibitor, promotes wound healing and epithelial cell rejuvenation in mucositis. (C) Chemo- and radiotherapy-induced DNA damage response and cell cycle activation cause apoptosis in Lgr5+ epithelial stem cells. Cell cycle inhibitors or Wnt activator R-Spondin1 (RSpo1) can increase epithelial stem cell survival and tissue regeneration in mucositis. DDR, DNA damage response; PUMA, P53-upregulated modulator of apoptosis; ROS, reactive oxygen species.
Innate and Adaptive Immune Responses in Pathobiology of Chemoradiotherapy-Induced Oral Mucositis
In the healthy mucosa, the coordination of microbiota with innate and adaptive immune responses contributes to the establishment of immune tolerance and epithelial barrier maintenance/tissue homeostasis (Honda and Littman 2016; Thaiss et al. 2016; Brown et al. 2019). On one hand, microbiota are involved in regulating the site-specific phenotypes and functions of both innate and adaptive immune cells, thus contributing to the maintenance of immune tolerance/homeostasis (Honda and Littman 2016; Thaiss et al. 2016). On the other hand, innate and adaptive immune systems coordinate to maintain a balanced microbiota in the mucosa through providing an intact epithelial barrier and immune surveillance on those harmful microbes (Honda and Littman 2016; Thaiss et al. 2016; Brown et al. 2019). In contrast, dysregulation of the 2 interdependent systems can cause miscommunications among microbiota and various types of host cells, thus disturbing the tightly controlled tissue homeostasis and, consequently, the development of different disorders, including chemoradiotherapy-induced OM. In this section, we aim to discuss important immune components that interplay with microbiota to activate downstream signals involved in OM development.
Epithelial Cells
Epithelial cells are the primary barrier between the outside environment and the host, which extensively express innate immune receptors for microbial recognition. As a nonclassical type of innate immune cells, epithelial cells are involved in innate immune responses through secretion of a panel of cytokines (Pott and Hornef 2012). Epithelial cells detect microbial components, pathogen-associated molecular patterns (PAMPs), and endogenous damage-associated molecular patterns (DAMPs), such as alarmin high-mobility group box 1 (HMGB1), through PRRs/TLRs to activate downstream NLRP cascades, leading to inflammasome-dependent release of IL-18 and IL-1β, as well as the subsequent production of antimicrobial peptides (Thaiss et al. 2016; Vasconcelos et al. 2016). During mucositis progression, increased bacterial colonization causes unbalanced PAMPs and DAMPs, which trigger PRR/TLR-mediated activation of downstream NF-κB signaling pathways, leading to augmented inflammatory responses due to the constant release of proinflammatory cytokines (Im et al. 2019). Taken together, restoration of dysregulated epithelial cell functions may serve as a therapeutic approach to mitigate chemoradiotherapy-induced OM due to their critical role in mucosal homeostasis.
Neutrophils
Neutrophils, constituting about 95% of total leukocytes in healthy oral mucosa, act as gatekeepers of oral immunity for microbial surveillance, immunoregulation, and periodontal homeostasis (Moutsopoulos and Konkel 2018). The microbiota influences myelopoiesis from pregnancy to posthematopoiesis, drives neutrophil aging, and then contributes to periodontal immunopathology (Deshmukh et al. 2014; Zhang et al. 2015; Gomez de Aguero et al. 2016). Therefore, a tight control of neutrophils is essential for maintaining a healthy status of oral mucosa because too few or too many neutrophils may create a certain pathophysiological condition that is favorable to the development of chemoradiotherapy-induced OM. Several studies have linked neutropenia with OM development, and neutrophil-based interventions are emerging as a new therapeutic approach for chemoradiotherapy-induced OM (Lee et al. 2016); however, it is still largely unknown whether neutrophils mediate microbial surveillance and perform microbial killing through antimicrobial peptide secretion during chemoradiotherapy-induced OM. Even though certain growth factors, such as granulocyte-macrophage colony stimulating factor (GM-CSF), have been proposed in clinical trials in the treatment of chemoradiotherapy-induced OM through boosting neutrophil functions, it is necessary to further elucidate the pathophysiological roles of neutrophils in chemoradiotherapy-induced OM progression, thus providing new insights into mechanism-directed interventions of this complicated morbidity.
Macrophages
Macrophages are innate immune cells that play key roles in coordinating immune response, inflammation, and tissue remodeling/homeostasis (Martinez et al. 2009). Indeed, tissue-resident macrophages are involved in maintaining homeostasis of mucosal immunity and antimicrobial function, which balances innate immunity and microbiome in oral mucosa (Lavin et al. 2014; De Schepper et al. 2018). During mucositis progression, proinflammatory cytokines produced by classically activated or type 1 macrophages (M1) were found to accumulate in the submucosa of patients following radiation treatment for head and neck cancer (Bonan et al. 2007) and in mice undergoing fractionated radiotherapy (Jaal et al. 2010), suggesting the essential role of macrophages in the initiation and progression of chemoradiotherapy-induced OM. In the late inflammatory phase of mucositis, the lesions are healed and remodeled with the clearance of dead cell debris and pathogens, which is largely attributed to the multiple functions of alternatively activated or type 2 macrophages (M2) (Oronsky et al. 2018). These studies have shed light on the important role of macrophages in the pathobiology of OM, and skewing polarization of macrophages toward a M2 phenotype represents another avenue to mitigate chemoradiotherapy-induced OM.
Innate Lymphoid Cells
Innate lymphoid cells (ILCs), the innate immune cells with similar functions and phenotypes of T lymphocytes, play a crucial role in antagonizing pathogens. In the healthy mucosa, dendritic cells (DCs) and macrophages activate group 1 innate lymphoid cells (ILC1s) against intracellular pathogens through secreting IL-12 and IL-18. In response to extracellular bacteria and fungi, leukocytes can also release IL-23 and IL-1β to induce ILC3 activation. On the other hand, epithelial cells can produce prostaglandin D2 (PGD2), IL-33, and IL-25 to induce ILC2 activation, which is essential for parasite expulsion. In addition, ILC2 and ILC3 can also contribute to tissue maintenance and repair (Geremia and Arancibia-Carcamo 2017). During OM progression, radiotherapy stimulates DCs to release IL-23 and the subsequent activation of ILC3 and IL-22 production, which primes epithelial cells for antimicrobial peptide expression and epithelial tissue repair. ILC3 interplays with epithelial cells while ILC2 suppresses proinflammatory T lymphocytes via anti-inflammatory Th2 cytokines IL-13, IL-4, and IL-5 (Blom et al. 2019; Panda and Colonna 2019).
Adaptive Immune Cells
Naive CD4+ T cells can be activated and differentiate into several subsets of effector cells with distinct biological functions, including T helper type 1 (Th1), Th2, and Th17; regulatory T (Treg); T follicular helper (Tfh); and most recently identified Th9 and Th22 cells (Imam et al. 2018; Loo et al. 2018). Among these T helper cells, Th17 cells can recruit neutrophils to the infectious sites and stimulate various types of cells to protect the host from extracellular bacteria and fungi through the production of a panel of Th17 cytokines such as IL-17A, IL-17F, IL-21, and IL-22. In contrast, Treg cells inhibit both Th1- and Th17-mediated immune responses through the production of anti-inflammatory cytokines such as IL-10 and TGF-β, thus contributing to immune homeostasis (Imam et al. 2018; Loo et al. 2018).
The adaptive immune response, particularly that mediated by CD4+ T cells, also plays a critical role in maintaining mucosal homeostasis through their interaction with microbiota to discriminate harmless and harmful microbes (Honda and Littman 2016; Imam et al. 2018; Brown et al. 2019). Accumulating evidence has shown that microbiota can influence the induction of both Th17 and Tregs in mucosa (Honda and Littman 2016; Brown et al. 2019), while a dysbiotic microbiome triggers Th17 cells to mediate oral mucosal immunopathology in both mice and humans (Dutzan et al. 2018). Even though several lines of evidence have implicated the role of disturbed Th1/Th17/Treg and Th1/Th2 balances in cancer therapy–related intestinal mucositis (Zuo et al. 2015; Mi et al. 2017; Fernandes et al. 2018), less is known about the role of altered adaptive immune responses in chemoradiotherapy-induced OM.
Collectively, these findings have provided evidence that interactions among various types of innate and adaptive immune cells and microbiome integrate a signaling hub that plays a critical role in epithelium homeostasis. In the presence of an altered bacterial flora in chemoradiotherapy-induced OM, innate immune cells dynamically respond to chemo- and radiotherapy and cause amplified inflammatory responses, thus leading to impaired tissue repair/regeneration. Interfering with such interactions between innate immunity and microbiome represents an innovative avenue for OM treatment (Fig. 2).
Figure 2.
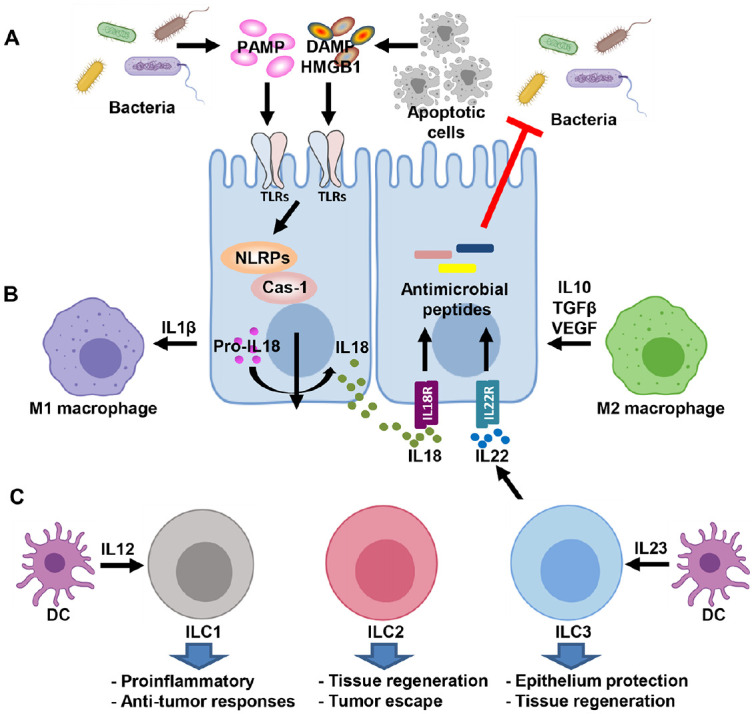
Signaling hub coordinates innate immunity and microbiome in mucositis. (A) Epithelial cells as the primary barrier between the outside environment and the host extensively express innate immune receptors for microbial recognition to maintain tissue/organ homeostasis. (B) Macrophage dynamic polarization plays a key role in the coordination of immune response, inflammation, and tissue remodeling/homeostasis during mucositis progression. (C) Innate lymphoid cells (ILCs) respond to intracellular and extracellular pathogens and contribute to tissue maintenance and repair. DAMPs, damage-associated molecular patterns; DCs, dendritic cells; HMGB1, high-mobility group box 1; IL, interleukin; NLRPs, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing; PAMPs, pathogen-associated molecular patterns; TGFβ, transforming growth factor β; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor.
Emerging Interventional Targets for Chemoradiotherapy-Induced Oral Mucositis
Inflammatory cytokines, specifically IL-1β, IL-6, and TNF-α, derived from both epithelial and immune cells, constitute key factors in the development of chemoradiotherapy-induced OM (Maria et al. 2017). Since NF-κB signaling plays a central role in upregulating proinflammatory cytokines, blocking of NF-κB pathway is an attractive strategy in clinical application for prevention and treatment of chemoradiotherapy-induced OM (Ariyawardana et al. 2019). Herein, we summarized information from ClinicalTrials.gov and related publications regarding clinical trials on anti-inflammatory targets for oral mucositis.
Currently, there are more than 20 clinical trials on the use of anti-inflammatory agents for prevention and treatment of chemoradiotherapy-induced OM in cancer patients, even though most of the trials are still in early phases (Table). Among these agents, benzydamine, Chining decoction, dusquetide, GC4419, and dexamethasone are in phase 3 clinical studies on their efficacy in preventing or treating chemoradiotherapy-induced OM either through mouth rinse or intravenous (IV) injection. Of note, mouth rinse with benzydamine hydrochloride has been recommended by the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) to mitigate moderate radiation-induced OM in head and neck cancer patients without receiving concomitant chemotherapy even though it has not yet been approved for this use by US Food and Drug Administration (FDA) (Lalla et al. 2019). In parallel, around 20 preclinical and phase 2 early clinical studies are also actively ongoing, among which several have received fast-track designation from the FDA. In addition, favorable results of innate immune inhibitors have been reported in several phase 2/3 clinical studies. Dusquetide, an innate defense regulator that can both defend against bacterial infections and dampen the inflammation, has demonstrated beneficial results in increased tissue-healing activities. Golotimod, a synthetic peptide that acts on the TLR pathway for macrocytic phagocytosis and immunomodulation, has been shown to promote bacterial clearance and tissue but to reduce immune responses. Most recently, other inflammatory pathways such as IL6-dependent signaling and the innate immune components of TLR pathways and leukocyte regulation are emerging as new targets for intervening in chemoradiotherapy-induced OM. Collectively, these ongoing clinical trials on the management of chemoradiotherapy-induced OM through targeting specific inflammatory signals, if successful, will contribute to further definition of evidence-based clinical practice guidelines for the effective management of chemoradiotherapy-induced OM in cancer patients.
Table.
Clinical Trials of Anti-inflammatory Agents for the Management of Oral Mucositis.
| Intervention | Phase | Purpose | Application | Results | Identifier |
|---|---|---|---|---|---|
| Benzydamine | III | Treatment | Mouth wash | Inhibiting inflammatory cytokines production | NCT00051441 |
| Chining decoction | III | Prevention | Mouth wash | Inhibiting proinflammatory cytokines IL-6 and TNF-α | NCT02303197 |
| Dusquetide | III | Treatment | IV infusion | Innate defense regulator | NCT03237325 |
| GC4419 | III | Treatment | IV infusion | Superoxide dismutase mimetic | NCT03689712 |
| Dexamethasone | III | Treatment | Mouth wash | T-cell suppression | NCT03839940 |
| Enbrel | II | Treatment | Mouth wash | Anti-TNF activity | NCT00031551 |
| β-Glucan | II | Treatment | Oral | Immunomodulating activities | NCT00289003 |
| Celecoxib | II | Prevention | Oral | Anti-COX-2 | NCT00698204 |
| Amlexanox | II | Treatment | Mouth wash | Inhibiting the synthesis of inflammatory mediators | NCT01083875 |
| Clonidine | II | Treatment | Oral | Inhibiting NF-κB and proinflammatory cytokines | NCT01385748 |
| Golotimod | II | Prevention | SC | Broad effects on the TLR pathway | NCT01247246 |
| IZN-6N4 | II | Prevention | Mouth wash | Anti-inflammation | NCT01400620 |
| Clazakizumab | II | Treatment | IV infusion | A humanized anti-IL-6 antibody | NCT01403064 |
| Lactobacillus CD2 | II | Prevention | Oral | Anti-inflammation | NCT01480011 |
| Quercetin | II | Treatment | Oral | Inhibiting TNF-α expression | NCT01732393 |
| Pentoxifylline | II | Prevention | Oral | Anti-TNF activity | NCT02397486 |
| Brilacidin | II | Prevention | Mouth wash | Host defense protein mimetics | NCT02324335 |
| Melatonin | II | Prevention | Oral | Inhibiting NF-κB and inflammasome pathway | NCT02630004 |
| Mosedipimod | II | Treatment | Oral | Enhancing NK cells and suppressing TLR4 pathway | NCT03200340 |
| Trefoil factor 1 | II | Prevention | Mouth wash | Reducing nitric oxide and inflammatory cytokines | NCT03234465 |
| EC-18 | II | Treatment | Oral | Attenuate the innate immune response | NCT03400340 |
| RRx-001 | II | Treatment | IV infusion | Polarization of tumor associated macrophages | NCT03515538 |
| Chlorine dioxide | II | Treatment | Mouth wash | Bactericide, viricide, and fungicide | NCT03602066 |
| Canakinumab | I | Treatment | SC | Inhibiting IL-1β binding with receptor | NCT02775994 |
| Ectoin | NA | Treatment | Mouth wash | Antioxidant | NCT03932292 |
| CareMin650 | NA | Treatment | Irradiance | Photobiomodulation | NCT03988556 |
The information included in this table was summarized from ClinicalTrials.gov, drug company websites, and published literatures. The purpose of prevention or treatment in the clinical trials was defined by primary study purpose from ClinicalTrials.gov.
COX-2, cyclooxygenase-2; IL, interleukin; IV, intravenous; NA, not available; NF-κB, nuclear factor κB; NK, natural killer; SC, subcutaneous injection; TLR, Toll-like receptor; TNF-α, tumor necrosis factor–α.
In addition to these anti-inflammatory agents undergoing development, several lines of evidence have shown that systemic application of mesenchymal stromal cells (MSCs) could mitigate the severity of chemoradiotherapy-induced OM mucositis in preclinical animal models (Zhang et al. 2012; Schmidt et al. 2014; Chang et al. 2017; Elsaadany et al. 2017). In the past decade, accumulating preclinical and clinical studies have demonstrated the promising efficacy of MSC-based regenerative therapy in treating a wide spectrum of inflammatory and immune-related disorders possibly through multiple modes of actions based on their paracrine secretion of a myriad of trophic growth factors, anti-inflammatory cytokines, and other soluble factors (Zhang et al. 2009; Akiyama et al. 2012; Chen et al. 2015; Chen et al. 2017). Through these biological active factors, MSCs interact with various types of host cells and exert potent immunomodulatory and anti-inflammatory effects on various subtypes of both innate and adaptive immune cells, thus contributing to the establishment of a proregenerative microenvironment that is favorable to tissue regeneration (Fig. 3). Therefore, MSC-based regenerative therapy might be another potential approach for the treatment of chemoradiotherapy-induced OM.
Figure 3.
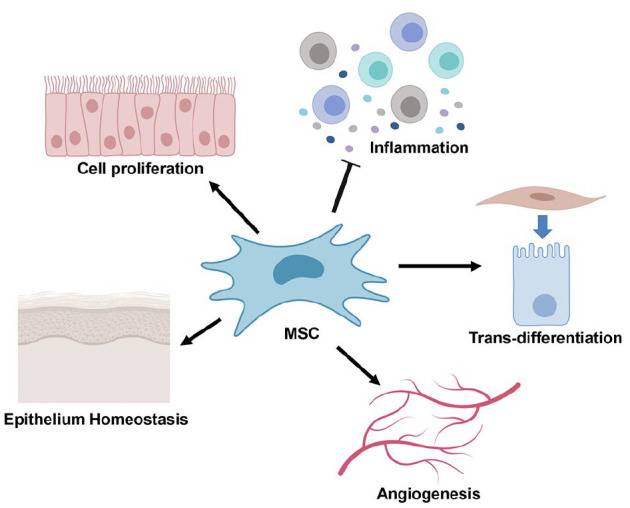
Potential mode of action of mesenchymal stromal cell (MSC)–based regenerative therapy. Exogenous MSCs home to the injury site following in vivo transplantation, where a small proportion of engrafted MSCs transdifferentiate into certain types of cells to replace those damaged ones while some of them interact with various types of host cells and exert multiple biological functions (e.g., proproliferation, proangiogenesis, anti-inflammation, antiapoptosis, and antioxidant) via their secretion of a myriad of trophic factors, thus contributing to the establishment of proregenerative microenvironment that is favorable to tissue regeneration (Akiyama et al. 2012; Zhang et al. 2012; Chang et al. 2017; Elsaadany et al. 2017; Van de Putte et al. 2017).
Conclusion and Perspectives
In the past several decades, substantial studies have explored the potential molecular mechanisms underlying the pathobiology of chemoradiotherapy-induced OM, but there is still lack of effective treatment options for this complex morbidity. Palifermin, a recombinant human keratinocyte growth factor (KGF) (Kepivance), is the only agent approved by the FDA, but its use is currently confined to patients with hematologic malignancies who undergo myelotoxic therapy and transplantation of hematopoietic stem cell (HSCs), whereas the use of palifermin for the treatment of chemoradiotherapy-induced OM in patients with solid tumors remains controversial because of the potential concern of its protumor growth activity and interference with clinical outcomes. To date, around 250 clinical trials on the management of chemoradiotherapy-induced OM have been registered in ClinicalTrials.gov, but clinical outcomes of these clinical studies are still uncertain. With the identification of several novel mechanistic pathways involved in the pathogenesis of chemoradiotherapy-induced OM (Zhao et al. 2009; Han et al. 2013; Luo et al. 2019), it is anticipated that more and more mechanism-based topic and systemic compounds will enter the pipeline for preclinical and clinical development. Importantly, further characterizing the multilayers of interactions between the microbiota and the innate immune system will not only shed light on the elucidation of the complex pathobiology but also lead to the development of new avenues for the treatment of chemoradiotherapy-induced OM.
Author Contributions
C. Chen, Q. Zhang, W. Yu, B. Chang, A.D. Le, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by the Schoenleber funding support (to A.D. Le, Q. Zhang), the National Institute of Dental and Craniofacial Research, National Institutes of Health (NIH/NIDCR) (R00DE025915 and R03DE028026 to C. Chen).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. 2012. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 10(5):544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyawardana A, Cheng KKF, Kandwal A, Tilly V, Al-Azri AR, Galiti D, Chiang K, Vaddi A, Ranna V, Nicolatou-Galitis O, et al. ; Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO). 2019. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 27(10):3985–3995. [DOI] [PubMed] [Google Scholar]
- Barker N, van Oudenaarden A, Clevers H. 2012. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 11(4):452–460. [DOI] [PubMed] [Google Scholar]
- Blakaj A, Bonomi M, Gamez ME, Blakaj DM. 2019. Oral mucositis in head and neck cancer: evidence-based management and review of clinical trial data. Oral Oncol. 95:29–34. [DOI] [PubMed] [Google Scholar]
- Blom B, van Hoeven V, Hazenberg MD. 2019. ILCs in hematologic malignancies: tumor cell killers and tissue healers. Semin Immunol. 41:101279. [DOI] [PubMed] [Google Scholar]
- Bonan PR, Kaminagakura E, Pires FR, Vargas PA, de Almeida OP. 2007. Histomorphometry and immunohistochemical features of grade I (WHO) oral radiomucositis. Oral Dis. 13(2):170–176. [DOI] [PubMed] [Google Scholar]
- Bowen JM, Keefe DM. 2008. New pathways for alimentary mucositis. J Oncol. 2008:907892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vives E. 2005. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 57(4):559–577. [DOI] [PubMed] [Google Scholar]
- Brown EM, Kenny DJ, Xavier RJ. 2019. Gut microbiota regulation of T cells during inflammation and autoimmunity. Ann Rev Immunol. 37:599–624. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Rugo HS, Litton JK, Meiller TF. 2018. Stomatitis associated with mammalian target of rapamycin inhibition: a review of pathogenesis, prevention, treatment, and clinical implications for oral practice in metastatic breast cancer. J Am Dent Assoc. 149(4):291–298. [DOI] [PubMed] [Google Scholar]
- Chang PY, Zhang BY, Cui S, Qu C, Shao LH, Xu TK, Qu YQ, Dong LH, Wang J. 2017. MSC-derived cytokines repair radiation-induced intra-villi microvascular injury. Oncotarget. 8(50):87821–87836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Akiyama K, Wang D, Xu X, Li B, Moshaverinia A, Brombacher F, Sun L, Shi S. 2015. mTor inhibition rescues osteopenia in mice with systemic sclerosis. J Exp Med. 212(1):73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Wang D, Moshaverinia A, Liu D, Kou X, Yu W, Yang R, Sun L, Shi S. 2017. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 27(4):559–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinausero M, Aprile G, Ermacora P, Basile D, Vitale MG, Fanotto V, Parisi G, Calvetti L, Sonis ST. 2017. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front Pharmacol. 8:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper S, Stakenborg N, Matteoli G, Verheijden S, Boeckxstaens GE. 2018. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell Immunol. 330:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermit M, Casado P, Rajeeve V, Wilkes EH, Foxler DE, Campbell H, Critchlow S, Sharp TV, Gribben JG, Unwin R, et al. 2017. Oxidative stress downstream of mTORC1 but not AKT causes a proliferative defect in cancer cells resistant to PI3K inhibition. Oncogene. 36(19):2762–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Budi EH. 2019. Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 12(570). pii: eaav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. 2014. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 20(5):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, et al. 2018. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 10(463). pii: eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaadany B, El Kholy S, El Rouby D, Rashed L, Shouman T. 2017. Effect of transplantation of bone marrow derived mesenchymal stem cells and platelets rich plasma on experimental model of radiation induced oral mucosal injury in albino rats. Int J Dent. 2017:8634540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting LS, Cooksley CD, Chambers MS, Garden AS. 2007. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 68(4): 1110–1120. [DOI] [PubMed] [Google Scholar]
- Fabbrizi MR, Warshowsky KE, Zobel CL, Hallahan DE, Sharma GG. 2018. Molecular and epigenetic regulatory mechanisms of normal stem cell radiosensitivity. Cell Death Discov. 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Wanderley CWS, Silva CMS, Muniz HA, Teixeira MA, Souza NRP, Candido AGF, Falcao RB, Souza M, Almeida PRC, et al. 2018. Role of regulatory T cells in irinotecan-induced intestinal mucositis. Eur J Pharm Sci. 115:158–166. [DOI] [PubMed] [Google Scholar]
- Francis AM, Alexander A, Liu Y, Vijayaraghavan S, Low KH, Yang D, Bui T, Somaiah N, Ravi V, Keyomarsi K, et al. 2017. CDK4/6 inhibitors sensitize Rb-positive sarcoma cells to Wee1 kinase inhibition through reversible cell-cycle arrest. Mol Cancer Ther. 16(9):1751–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Carcamo CV. 2017. Innate lymphoid cells in intestinal inflammation. Front Immunol. 8:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. 2016. The maternal microbiota drives early postnatal innate immune development. Science. 351(6279):1296–1302. [DOI] [PubMed] [Google Scholar]
- Han G, Bian L, Li F, Cotrim A, Wang D, Lu J, Deng Y, Bird G, Sowers A, Mitchell JB, et al. 2013. Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med. 19(4):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li AG, Wang D, Han S, Zheng B, Goumans MJ, Ten Dijke P, Wang XJ. 2002. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. EMBO J. 21(11):2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Littman DR. 2016. The microbiota in adaptive immune homeostasis and disease. Nature. 535(7610):75–84. [DOI] [PubMed] [Google Scholar]
- Hong B-Y, Sobue T, Choquette L, Dupuy AK, Thompson A, Burleson JA, Salner AL, Schauer PK, Joshi P, Fox E, et al. 2019. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lim S, Li AG, Lee C, Lee YS, Lee EK, Park SH, Wang XJ, Kim SJ. 2007. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol. 8(5):504–513. [DOI] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Gutkind JS. 2011. Signaling circuitries controlling stem cell fate: to be or not to be. Curr Opin Cell Biol. 23(6):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. 2012. Mtor inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell. 11(3):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im KI, Nam YS, Kim N, Song Y, Lee ES, Lim JY, Jeon YW, Cho SG. 2019. Regulation of HMGB1 release protects chemoradiotherapy-associated mucositis. Mucosal Immunol. 12(5):1070–1081. [DOI] [PubMed] [Google Scholar]
- Imam T, Park S, Kaplan MH, Olson MR. 2018. Effector T helper cell subsets in inflammatory bowel diseases. Front Immunol. 9:1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaal J, Richter C, Dorr W. 2010. Effect of recombinant human keratinocyte growth factor (Δ23rhukgf, palifermin) on inflammatory and immune changes in mouse tongue during fractionated irradiation. Int J Radiat Biol. 86(10):860–866. [DOI] [PubMed] [Google Scholar]
- Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS. 2006. Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer. 14(6):505–515. [DOI] [PubMed] [Google Scholar]
- Kalvala A, Rainaldi G, Di Primio C, Liverani V, Falaschi A, Galli A. 2010. Enhancement of gene targeting in human cells by intranuclear permeation of the Saccharomyces cerevisiae Rad52 protein. Nucleic Acids Res. 38(14):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, et al. 2014. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 120(10):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalla RV, Brennan MT, Gordon SM, Sonis ST, Rosenthal DI, Keefe DM. 2019. Oral mucositis due to high-dose chemotherapy and/or head and neck radiation therapy. J Natl Cancer Inst Monogr. 2019(53). pii: lgz011. [DOI] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 159(6):1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Yoo N, Kim JH, Sohn KY, Kim HJ, Kim MH, Han MY, Yoon SY, Kim JW. 2016. The therapeutic effect of PLAG against oral mucositis in hamster and mouse model. Front Oncol. 6:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Chuong CM. 2016. Stem cells. Aging, alopecia, and stem cells. Science. 351(6273):559–560. [DOI] [PubMed] [Google Scholar]
- Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L, et al. 2018. Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med. 10(427). pii: eaam7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C, Tan SH, Barker N. 2018. Recent advances in LGR5(+) stem cell research. Trends Cell Biol. 28(5):380–391. [DOI] [PubMed] [Google Scholar]
- Loo TT, Gao Y, Lazarevic V. 2018. Transcriptional regulation of CD4(+) TH cells that mediate tissue inflammation. J Leukoc Biol. 104(6):1069-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. 2007. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 220:60–81. [DOI] [PubMed] [Google Scholar]
- Luo J, Bian L, Blevins MA, Wang D, Liang C, Du D, Wu F, Holwerda B, Zhao R, Raben D, et al. 2019. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin Cancer Res. 25(2):808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria OM, Eliopoulos N, Muanza T. 2017. Radiation-induced oral mucositis. Front Oncol. 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 7(1):31–40. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. 2009. Alternative activation of macrophages: an immunologic functional perspective. Ann Rev Immunol. 27:451–483. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. 2014. LGR5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 14(2):149–159. [DOI] [PubMed] [Google Scholar]
- Mi H, Dong Y, Zhang B, Wang H, Peter CCK, Gao P, Fu H, Gao Y. 2017. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell Physiol Biochem. 42(6):2330–2341. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel JE. 2018. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 39(4):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Cowley CJ, Fuchs E. 2018. Two to tango: dialog between immunity and stem cells in health and disease. Cell. 175(4):908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oronsky B, Goyal S, Kim MM, Cabrales P, Lybeck M, Caroen S, Oronsky N, Burbano E, Carter C, Oronsky A. 2018. A review of clinical radioprotection and chemoprotection for oral mucositis. Transl Oncol. 11(3):771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz F, Acuna-Castroviejo D, Doerrier C, Dayoub JC, Lopez LC, Venegas C, Garcia JA, Lopez A, Volt H, Luna-Sanchez M, et al. 2015. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J Pineal Res. 58(1):34–49. [DOI] [PubMed] [Google Scholar]
- Panda SK, Colonna M. 2019. Innate lymphoid cells in mucosal immunity. Front Immunol. 10:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DE, O’Shaughnessy JA, Rugo HS, Elad S, Schubert MM, Viet CT, Campbell-Baird C, Hronek J, Seery V, Divers J, et al. 2016. Oral mucosal injury caused by mammalian target of rapamycin inhibitors: emerging perspectives on pathobiology and impact on clinical practice. Cancer Med. 5(8):1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J, Hornef M. 2012. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 13(8):684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raslan AA, Yoon JK. 2019. R-spondins: multi-mode wnt signaling regulators in adult stem cells. Int J Biochem Cell Biol. 106:26–34. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Piro-Hussong A, Siegemund A, Gabriel P, Dorr W. 2014. Modification of radiation-induced oral mucositis (mouse) by adult stem cell therapy: single-dose irradiation. Radiat Environ Biophys. 53(4):629–634. [DOI] [PubMed] [Google Scholar]
- Sharlow ER, Leimgruber S, Lira A, McConnell MJ, Norambuena A, Bloom GS, Epperly MW, Greenberger JS, Lazo JS. 2016. A small molecule screen exposes mTOR signaling pathway involvement in radiation-induced apoptosis. ACS Chem Biol. 11(5):1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis ST. 2004. The pathobiology of mucositis. Nat Rev Cancer. 4(4):277–284. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2009. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Ooncol. 45(12):1015–1020. [DOI] [PubMed] [Google Scholar]
- Sonis ST. 2010. New thoughts on the initiation of mucositis. Oral Dis. 16(7):597–600. [DOI] [PubMed] [Google Scholar]
- Subramaniam N, Muthukrishnan A. 2019. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: a prospective analysis in a tertiary care dental hospital. J Investig Clin Dent. 10(4):e12454. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, Elinav E. 2016. The microbiome and innate immunity. Nature. 535(7610):65–74. [DOI] [PubMed] [Google Scholar]
- Van de Putte D, Demarquay C, Van Daele E, Moussa L, Vanhove C, Benderitter M, Ceelen W, Pattyn P, Mathieu N. 2017. Adipose-derived mesenchymal stromal cells improve the healing of colonic anastomoses following high dose of irradiation through anti-inflammatory and angiogenic processes. Cell Transpl. 26(12):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos RM, Sanfilippo N, Paster BJ, Kerr AR, Li Y, Ramalho L, Queiroz EL, Smith B, Sonis ST, Corby PM. 2016. Host-microbiome cross-talk in oral mucositis. J Dent Res. 95(7):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesty A, Gear K, Biswas K, Mackenzie BW, Taylor MW, Douglas RG. 2019. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer [epub ahead of print 25 October 2019]. doi: 10.1007/s00520-019-05084-6. [DOI] [PubMed] [Google Scholar]
- Villa A, Sonis ST. 2015. Mucositis: pathobiology and management. Curr Opin Oncol. 27(3):159–164. [DOI] [PubMed] [Google Scholar]
- Villa A, Sonis ST. 2020. An update on pharmacotherapies in active development for the management of cancer regimen-associated oral mucositis. Expert Opin Pharmacother [epub ahead of print 28 January 2020]. doi:10.1080/ 14656566.2020.1718652. [DOI] [PubMed] [Google Scholar]
- Wei L, Leibowitz BJ, Wang X, Epperly M, Greenberger J, Zhang L, Yu J. 2016. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Investig. 126(11):4076–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino F, Yoshida A, Nakajima A, Wada-Takahashi S, Takahashi SS, Lee MC. 2013. Alteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamsters. PLoS One. 8(12):e82834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, et al. 2015. Neutrophil ageing is regulated by the microbiome. Nature. 525(7570):528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. 2009. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 183(12):7787–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QZ, Nguyen AL, Yu WH, Le AD. 2012. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. J Dent Res. 91(11):1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kim KA, De Vera J, Palencia S, Wagle M, Abo A. 2009. R-spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical wnt/beta-catenin pathway. Proc Natl Acad Sci USA. 106(7):2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Li X, Chang Y, Duan G, Yu L, Zheng R, Xue C, Tang Q. 2015. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. Food Funct. 6(2):415–422. [DOI] [PubMed] [Google Scholar]