Abstract
Breast cancer is the most common female cancer diagnosis in the United States (excluding skin cancers), and the second leading cause of female cancer death. This article highlights the role that lifestyle plays in primary breast cancer prevention, breast cancer treatment, and tertiary breast cancer prevention. Current data regarding the benefits of a predominantly plant-based diet in combination with physical activity and maintenance of a healthy body weight will be reviewed. The evidenced-based patient-focused recommendations developed by the World Cancer Research Fund/American Institute for Cancer Research will be discussed in the context of an overall lifestyle strategy. It is our hope that this publication empowers clinicians to provide patients with personalized cancer-protective lifestyle prescriptions.
Keywords: breast cancer, nutrition, exercise, mindfulness, exercise, soy, cancer
‘Approximately 40% of all incident cancer cases in the United States could be prevented through health-related choices . . .’
Approximately 40% of all incident cancer cases in the United States could be prevented through health-related choices such as vaccinations and modifiable lifestyle factors, including body weight, physical activity level, alcohol intake, diet, sun exposure, and tobacco use.1,2
The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) has been at the forefront of synthesizing, interpreting, and evaluating the accumulated evidence on the relationship between cancer risk and diet, nutrition, physical activity, and weight for over three decades.3-5 In 2007, the Continuous Update Project (CUP) was established to develop a rigorous and transparent methodology for comprehensively reviewing, analyzing, and assessing the strength of the evidence regarding each proposed cancer risk factor related to diet, physical activity, and body fatness. In 2018, WCRF/AICR published Diet, Nutrition, Physical Activity and Cancer: A Global Perspective, the WCRF/AICR Third Expert Report.3 The Third Expert Report updated the 10 cancer prevention recommendations, shown in Figure 1, which incorporate statements consistent with a whole-food plant-based diet (WFPB), including consuming a diet rich in whole grains, vegetables, fruit, and beans as well as limiting consumption of red and processed meats, sugar, sugar-sweetened beverages, and fast food and other processed foods.
Figure 1.
American Institute for Cancer Research’s 10 recommendations for cancer prevention.
In this article, we will focus on breast cancer, the most commonly diagnosed female cancer in the United States (excluding skin cancers), and the second leading cause of female cancer death.6 While the cancer prevention recommendations apply to cancer prevention broadly, the factors addressed in these recommendations are directly relevant to the prevention of breast cancer and may have a significant impact on survival after breast cancer diagnosis. Over the past 2 decades, the sheer volume and variability of relevant research on the role of modifiable lifestyle factors on cancer risk and outcomes has often created confusing and contradictory messages. One of the key goals of the CUP is to distinguish evidence from opinion. All literature reviews, data abstraction, and analyses are conducted according to pre-defined cancer site-specific protocols developed as part of the 2007 Second WCRF/AICR Expert Report, Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective.5 The results of the Systematic Literature Review (SLR) are compiled into a SLR report; the SLR report is then externally peer reviewed before being reviewed by an independent panel of experts, the CUP Panel. The CUP panel conducts in-person meetings to discuss each SLR report and judge the strength of the evidence. The causality of observed associations is assessed by the CUP Panel, according to predefined criteria based on modified Bradford Hill criteria.5,7 The criteria for judgement include the number and types of studies, the size and consistency of the effect, the quality of exposure and outcome assessment, the presence of a biological gradient (dose-response), the exclusion of chance bias and confounding, and the presence of biologically plausible candidate mechanisms.5
The conclusions drawn by the CUP Panel form the basis for the WCRF/AICR Cancer Prevention Recommendations. Recommendations are generally only based on evidence of causality regarded as strong, with predefined categories of convincingly or probably causal. If the CUP Panel judges that the evidence fails to meet the criteria for strong evidence, it may be categorized as “limited.” Within the limited category, there are 2 potential designations: suggestive or no conclusion. Limited evidence is generally regarded as insufficient to form the basis for recommendations (Table 1).
Table 1.
Criteria for Continuous Update Project Panel Conclusion.
| Conclusion | Criteria |
|---|---|
| Convincing (strong evidence) | Evidence strong enough to support a judgment of a convincing causal (or protective) relationship, which justifies making recommendations designed to reduce the risk of cancer. The evidence is robust enough to be unlikely to be modified in the foreseeable future as new evidence accumulates. |
| Probable (strong evidence) | Evidence strong enough to support a judgment of a probable causal (or protective) relationship, which generally justifies recommendations designed to reduce the risk of cancer. |
| Limited—suggestive | Evidence that is too limited to permit a probable or convincing causal judgment but is suggestive of a direction of effect. The evidence may be limited in amount or by methodological flaws, but shows a generally consistent direction of effect. This judgement is very rarely sufficient to justify recommendations designed to reduce the risk of cancer; any exceptions to this require special, explicit justification. |
| Limited—no conclusion | Evidence is so limited that no firm conclusion can be made. This judgment represents an entry level and is intended to allow any exposure for which there are sufficient data to warrant Panel consideration, but where insufficient evidence exists to permit a more definitive grading. |
| Substantial effect on risk unlikely (strong evidence) | Evidence is strong enough to support a judgment that a particular food, nutrition, or physical activity exposure is unlikely to have a substantial causal relation to a cancer outcome. The evidence should be robust enough to be unlikely to be modified in the foreseeable future as new evidence accumulates. |
Since the publication of the original WCRF/AICR cancer prevention recommendations in 2007, almost 40 studies have examined the impact of adherence to these recommendations on cancer risk and survival; however, each set of authors derived their own scoring system. This lack of consistency hindered the ability to directly compare results and to summarize the impact of adherence to the recommendations. Therefore, shortly after the publication of the 2018 WCRF/AICR Cancer Prevention Recommendations, a standardized scoring system was proposed to encourage consistency and transparency of scoring.8 Regardless, adherence to a greater number of recommendations is associated with a lower risk of many cancers.
In terms of breast cancer incidence, the VITAL prospective cohort study of over 30 000 postmenopausal women found a 60% reduction in breast cancer incidence in women who met at least 5 of the WCRF/AICR recommendations compared with those who met none.9 Other prospective cohort studies have also reported reductions in breast cancer incidence with adherence to the WCRF/AICR recommendations of 24% (risk ratio [RR] 0.76, 95% CI 0.67-0.87) and 51% (RR 0.49, 95% CI 0.35-0.70).10,11 Furthermore, adherence to the WCRF/AICR recommendations is associated with lower overall mortality in female cancer survivors (hazard ratio [HR]high vs low 0.67, 95% CI 0.49-0.90).12
Primary Breast Cancer Prevention: Nutrition
Plant-based nutrition and dietary recommendations are a large part of the WCRF/AICR recommendations, showing up in some form in 7 of the 10 recommendations.3 Although the data regarding the relationship between specific components of a whole-foods plant-based (WFPB) diet and breast cancer incidence has been mixed (likely due to the heterogeneity of the disease and the dietary components studied), there is a growing body of evidence that breast cancer incidence can be reduced with an overall healthy lifestyle, which includes a high-quality diet consisting of fruits, vegetables, whole grains, and legumes.13,14 There are multiple mechanisms through which plant-based nutrition could decrease both cancer incidence and all-cause mortality. For example, a plant-based diet may help maintain a healthy body mass index (BMI), ensure adequate fiber intake, and optimize phytonutrient and antioxidant intake.15 The mechanisms behind diet and cancer are complex; however, some general principles emerge.
Fiber intake has been shown to be inversely associated with breast cancer incidence. In a meta-analysis of 10 prospective cohort studies with over 700 000 participants, a dose-dependent inverse relationship between fiber intake and breast cancer incidence was observed with a 7% decrease in risk for every 10 g increase in daily fiber intake.16,17 The 2017 WCRF/AICR Breast Cancer CUP report included 11 studies (18591 cases) in a dose-response meta-analysis of fiber intake and postmenopausal breast cancer. The summary RR per 10 g/d increase in fiber intake was 0.95 (95% CI 0.92-0.99), with no evidence of heterogeneity, I2 = 0%, P heterogeneity = .73. There was no evidence of publication bias, P = .69.3
In addition, consumption of fiber-rich foods, which are primarily low-calorie-density foods (vegetables, fruits, whole grains, and legumes,) may help individuals achieve a healthy BMI.18,19 Overweight and obesity is associated with an increased risk of breast cancer, with postmenopausal women with obesity having a 20% to 40% increased risk of developing hormone receptor positive breast cancer when compared with normal weight women.20,21
While adequate macronutrients are crucial for health, plant foods are also a rich source of micronutrients and phytonutrients, many of which play a critical role in cancer prevention due to their anti-inflammatory and/or antioxidant properties.22,23 A thorough description of the different models of tumorigenesis is complex and beyond the scope of this discussion; however, the processes of initiation, promotion, and progression are generally involved.24 Initiation occurs when a cell’s DNA becomes mutated by an initiator. This mutated cell becomes susceptible to promoters which lead to cell proliferation, and subsequent progression to malignancy. Phytochemicals may play a role in cell proliferation, DNA repair, apoptosis, cell differentiation, carcinogen detoxification, epigenetic modulation, activation or inactivation of oncogenes and tumor-suppressor genes, angiogenesis, and mitigation of oxidative stress.25,26 The WCRF/AICR CUP report concluded that consuming non-starchy vegetables, foods high in carotenoids, as well as a diet high in calcium may decrease the risk of breast cancer.3
Although the benefits of a diet rich in vegetables, fruits, whole grains, and legumes are well known, only a minority of adults reach daily targets for fruit and vegetable intake. Sixty percent to 87% percent of adults worldwide report consuming fewer than 5 daily servings of vegetables and fruit.27 There are many resources available on the AICR website (https://www.aicr.org/) and theAmerican College of Lifestyle Medicine (ACLM) website (https://lifestylemedicine.org/), including physician resources and patient education facing materials to help patients adopt health-promoting dietary habits. As lifestyle medicine practitioners, we have a tremendous opportunity to decrease the global disease burden not only by educating our patients about the recommended intake and benefits of plant-based foods but also by providing patients with effective resources and tools.
Soy
Numerous studies involving thousands of women worldwide have shown that dietary intake of soy foods is associated with as much as a 30% decrease in breast cancer incidence.28-30 The Japan Public Health Center Prospective Study on Cancer and Cardiovascular Disease, including more than 20 000 Japanese women, found that those with the highest soy food intake had approximately half the risk of breast cancer when compared to those with the lowest intake.31 Soy intake in childhood and adolescence may be the most protective.32 Women with BRCA gene mutations and gene polymorphisms predisposing to breast cancer may especially benefit from soy.33 Despite the benefits of soy food intake, there is a great deal of confusion among the medical and general population regarding the safety of soy.
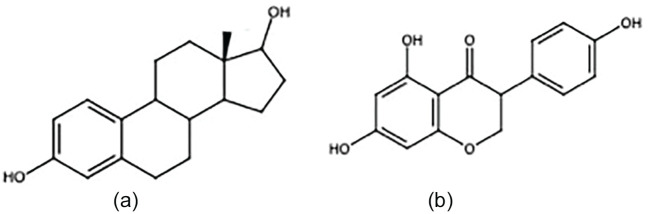
In 2002, the combined estrogen/progesterone hormone replacement therapy (HRT) arm of the Women’s Health Initiative trial was stopped early due to an observed increased risk in breast cancer, coronary artery disease risk, and overall harm.34 In 2004, the estrogen-only arm was also stopped early due to increased stroke risk without cardiovascular benefit.35 At that time, health care providers began to reexamine HRT prescribing habits. Instead of encouraging widespread HRT use, the risks and benefits had to be carefully considered, and the administration of HRT became much more judicious. The increased scrutiny around estrogen led to fear that consumption of soy—a phytoestrogen with a similar molecular structure as estrogen (Figure 2)35—might result in the same deleterious effects.
Figure 2.
The chemical structure of (a) estradiol and (b) genistein.32 Genistein is a soy phytoestrogen isoflavone with a molecular structure similar to estradiol.
The mechanisms behind the protective effects of phytoestrogens are multifaceted. Phytoestrogens may inhibit angiogenesis, proteases, tyrosine kinases, and DNA topoisomerase I and II.36 Phytoestrogens are associated with enhanced immune function, as well as anti-inflammatory and antioxidative effects.37 Heterogeneity in estrogen receptor distribution, binding, and function also plays a role in the differing effects of phytoestrogens versus endogenous/exogenous estrogen. There are 2 types of estrogen receptors (ERs), ER alpha and ER beta. At food consumption doses, phytoestrogens bind primarily to ER beta, producing an antiproliferative, antiestrogen effect. Phytoestrogens may also compete with endogenous estrogen for ER binding.38,39 In addition to phytoestrogens, the soybean is a good source of carbohydrate, protein, essential fatty acids, fiber, and vitamins and minerals (including potassium and iron).40
Primary Breast Cancer Prevention: Physical Activity
Physical activity is associated with a wide variety of benefits. In terms of breast cancer, it not only decreases breast cancer incidence but also decreases breast cancer–related mortality. Numerous studies over the past 20 years have demonstrated that physically active women have a 30% to 40% lower risk of developing breast cancer when compared with sedentary women.41,42 An expert panel at the International Agency for Research on Cancer estimated up to a 40% decrease in breast cancer incidence among the most physically active women, regardless of menopausal status, type, or intensity of activity.43
The benefits of physical activity may be due to metabolic effects, decrease in body fatness, mechanical effects, association with other health promoting lifestyle habits (related to alcohol, smoking, and diet), improved immune system function, hormone modulation, and anti-inflammatory effects.44,45
For cancer prevention, the AICR/WCRF and the American Cancer Society (ACS) recommend getting at least 150 minutes of moderate or 75 minutes of vigorous physical activity per week, along with muscle resistance and flexibility exercises. The ACS adds that any activity over and above usual activities of daily living is beneficial, so while moderate to vigorous exercise is certainly optimal, it is also important to encourage any increase in physical activity during the day such as walking, taking the stairs when possible, and avoiding prolonged periods of sitting.46,47
Lack of physical activity is a major public health problem with only a minority of Americans meeting the recommended exercise guidelines. In a study of over 300 000 Americans, fewer than 25% met published exercise recommendations; increased age and comorbidities were associated with poor compliance.48 Counseling about the importance of physical activity, writing exercise prescriptions, and referral to physical therapy or medically supervised exercise programs when appropriate could have a significant impact on cancer prevention and overall health.
Tertiary Breast Cancer Prevention
While the evidence for the impact of modifiable lifestyle factors on breast cancer risk is strong, the impact on survival after diagnosis is less clear. From 1989 until 2017, the breast cancer death rate has declined by 40%, and by 2019, the American Cancer Society estimated that there are over 3.8 million breast cancer survivors living in the United States.6 Many breast cancer survivors are living for decades beyond their diagnosis, and these individuals have unique medical and psychosocial needs that need to be addressed in the survivorship phase of care. Important issues in breast cancer survivorship care include management of short and long-term side effects from cancer treatment, monitoring for risk of cancer recurrence, screening for new primary breast cancers, and proactive assessment of diet, exercise, and other lifestyle factors.
There is growing evidence that lifestyle factors play an important role in determining rates of recurrence and prognosis among breast cancer survivors. Studies have shown that poor diet, obesity, and inactivity are linked to a higher risk of cancer recurrence and mortality from breast cancer.49-51 In 2018, the American Society of Clinical Oncology (ASCO) launched a survey to assess oncologists’ understanding and practice behaviors related to counseling cancer patients on the role of diet, physical activity, and weight management.52 The study found that the majority of survey respondents agreed that there is strong evidence that overweight/obesity affect cancer outcomes. The majority of survey respondents agreed that the treating physician should make recommendations to cancer survivors regarding nutrition, weight management, and exercise. However, 83% of respondents also felt that additional training was needed to address these issues adequately in cancer patients. Based on results of this ASCO survey, organizations such as the ACLM have an opportunity to contribute in a meaningful way to the care of cancer survivors. By using evidence-based tools to transform health and treat disease, clinicians with expertise in lifestyle medicine have the opportunity to educate and empower cancer survivors to make sustainable lifestyle changes to improve their physical and mental wellbeing after a cancer diagnosis, and also have an impact on disease recurrence and overall mortality from cancer.
The role of diet, weight management, and physical activity in the care of breast cancer survivors will be addressed below. Also note that the previously reviewed guidelines from the WCRF/AICR also apply to breast cancer survivors.3
Tertiary Breast Cancer Prevention: Nutrition
A healthy, plant-based diet is a one of the key aspects of lifestyle medicine. In the breast cancer literature, there have been a number of studies investigating whether the intake of specific dietary nutrients, or specific dietary patterns, have an impact on disease outcome. Although heterogeneity in design and outcome is certainly apparent and further work is needed in this area, encouraging outcomes are emerging. Below we have reviewed data from some of the meta-analyses on this topic, as well as data from three large randomized studies.
In 2016, Schwedhelm and colleagues53 performed a meta-analysis to investigate an association between dietary patterns and specific foods with the overall mortality among cancer survivors. The researchers included 117 studies in their analysis and reported a significant inverse association between adherence to a “high-quality diet” (higher intake of fruits, vegetables, and whole grains, but low in red and processed meat, refined grains, and high-fat foods) and overall mortality among cancer survivors (RR 0.78, 95% CI 0.72-0.85, I2 = 0%). The analysis demonstrated that a Western dietary pattern (characterized by high intake of red and processed meat, refined grains, sweets and desserts, and high-fat dairy products) was associated with an increased risk of mortality in cancer survivors (RR 1.46, 95% CI 1.27-1.68, I2 = 0%). No significant associations between cancer recurrence and dietary patterns were found. When the analysis was limited to breast cancer survivors, there was no evidence that consumption of specific foods or nutrients resulted in an improvement in overall mortality; however, adherence to a “high-quality diet” was associated with a reduced risk of overall mortality (RR 0.76, 95% CI 0.60-0.95; I2 = 4%). In contrast, high adherence to a Western dietary pattern was associated with an increased risk of overall mortality among breast cancer survivors (RR 1.44, 95% CI 1.17-1.77, I2 = 0%). This study also reported that alcohol intake was associated with an increased risk of breast cancer recurrence (RR 1.21, 95% CI 1.06-1.39; I2 = 23%), but not overall mortality.53
In an update of a 2014 meta-analysis, Schwingshackl and colleagues54 investigated the effect of the Mediterranean diet (defined as high consumption of plant-based foods, to include whole grains, vegetables, fruits, nuts, and legumes, with regular intake of fish and seafood, and low intake of red, processed meat, and high-fat dairy products) on risk of overall cancer mortality and recurrence in cancer survivors; they included an additional 27 studies (83 total studies), totaling 2 130 753 subjects. When examining the breast cancer population, the authors reported that the strongest adherence to a Mediterranean diet pattern was inversely associated with breast cancer mortality (RR 0.43, 95% CI 0.21-0.88, n = 1 randomized controlled trial) (RR 0.92, 95% CI 0.87-0.96; I2 = 22%, n = 16 observational studies) and primary breast cancer risk. No association was found between the highest adherence group and breast cancer recurrence.
An analysis of individual components of the Mediterranean diet revealed that the most beneficial effects of this diet were attributable to ingestion of fruits, vegetables, and whole grains,54 contributing to mounting evidence that a plant-based diet is the most beneficial dietary pattern for breast cancer survivors.
The aforementioned WCRF/AICR report concluded that lower dietary fat intake, in particular saturated fat, may be associated with lower breast cancer mortality. In addition, the panel reported that higher intake of foods containing fiber and soy after a breast cancer diagnosis may be associated with an improvement in breast cancer outcome. The WCRF/AICR panel has acknowledged that there is a need for ongoing investigation into the role of nutrition in each stage of breast cancer survivorship, and further research will provide insight into the underlying biological mechanisms3.
In addition to the meta-analyses cited above, there are 3 large randomized trials that provide further insights into the impact of dietary modification on disease outcomes in women with a diagnosis of early stage breast cancer, or in women at risk for breast cancer or other cancers.55-57 These studies include the Women’s Interventional Nutrition Study (WINS), the Women’s Healthy Eating and Living (WHEL) Study, and the Women’s Health Initiative Low Fat Dietary Modification (WHI DM) Trial. The WINS trial was a randomized, prospective, multicenter clinical trial designed to examine the effect of reduced dietary fat intake in women with early stage breast cancer.55 The goal was to reduce dietary fat intake to 15% of total energy intake; at 15 years follow-up, there was no overall survival benefit seen in the reduced dietary fat intake group. However, an exploratory subgroup analysis suggested that patients with hormone receptor negative breast cancers have a significant overall survival benefit (7.5% vs 18.1%, cumulative mortality, RR 0.41, P = .003) with a very-low-fat diet. Relapse-free survival at 15 years was favorably affected by the lower fat diet, which may be related to the participants’ weight loss, and this issue is under further investigation.58
The WHEL study was a multi-institutional, randomized controlled trial with the goal to assess whether a major increase in vegetable, fruit, and fiber intake, and concomitant decrease in dietary fat intake, reduce the risk of recurrent and new primary breast cancer and mortality among women with previously treated early-stage breast cancer. Women who were assigned to the dietary intervention arm of the study did in fact increase intake of fruits and vegetables and decrease calories from fat through the 4-year study. At mean 7.3 years of follow-up, there was no difference in the rate of recurrence or all-cause mortality between the 2 groups. It should be noted that the control group was coached to consume 5 fruits and vegetables a day, which is more than most breast cancer survivors consume; this likely affected the results.56 Longer-term follow-up is needed.
The third randomized trial that provides additional insight into the role of dietary interventions and breast cancer outcome is the WHI DM trial. This trial was a large, randomized study designed to investigate the impact of dietary intervention on the risk of developing breast, colorectal, and other cancers in postmenopausal women. In this study, 48 835 postmenopausal women were randomized to a dietary intervention focused on reduced fat intake (20% of energy) and increased servings of fruits and vegetables, or to a usual diet control; the intervention lasted a median of 8.5 years. The study found that the women in the dietary intervention group did not have a lower risk for breast, colorectal or other cancers.57 However, in a more recent publication with a median follow-up of 16.1 years, there were 3,030 cases of breast cancer diagnosed in the women enrolled in the study. Among the women who developed breast cancer during that time frame, those women who had been previously assigned to the low-fat dietary intervention group had a lower incidence of death from breast cancer.59
The above work provides important background for new studies to clarify the role of dietary interventions in breast cancer care. The ACS and the ASCO recommend that breast cancer survivors be counseled “to achieve a dietary pattern that is high in vegetables, fruits, whole grains, and legumes; low in saturated fats; and limited in alcohol consumption.” The field of lifestyle medicine has demonstrated that adherence to a healthy, plant-based diet is certainly warranted for general health, a recommendation of utmost importance for our breast cancer patients.
Tertiary Breast Cancer Prevention: Obesity
An important focus of recent work surrounds obesity and cancer. In 2013 and 2014, the ASCO made “Obesity and Cancer” one of its core initiatives.60 Data from both randomized controlled trials and observational studies have shown that obesity at the time of a breast cancer diagnosis is linked to an increase in risk for cancer recurrence and mortality.61 Thus, an important component of the ASCO initiative focuses on improving access to weight management programs for cancer survivors. Further research is needed to evaluate whether weight loss interventions in breast cancer survivors will lead to improvements in the risk of disease recurrence and mortality. There are currently a number of studies underway that will provide more information about the benefits of lifestyle interventions in breast cancer survivors. These studies include the German SUCCESS C trial,62 the Italian DIANA 5 trial,63 and the Breast cancer Weight Loss (BWEL) trial.64
Tertiary Breast Cancer Prevention: Exercise
Regular and consistent physical activity is a fundamental pillar of lifestyle medicine. Physical activity also has an important role in breast cancer survivorship. Data from observational studies have demonstrated a link between moderate physical activity and improved outcome from breast cancer. A meta-analysis published by Friedenreich and colleagues65 analyzed 26 studies of breast, colorectal, and prostate cancer patients, and reported a 37% reduction in risk of cancer-specific mortality among the most active versus the least active patients. Schmid and Lietzmann66 published a meta-analysis evaluating the association between physical activity and mortality among breast and colorectal cancer survivors. In their analysis, they identified 16 prospective observational studies of breast cancer survivors and reported a 28% reduction in breast cancer specific mortality (RR 0.72; 95% CI 0.60-0.85), and a 48% reduction in overall mortality (RR 0.52, 95% CI 0.42-0.64) in the most active versus the least active breast cancer survivors. In addition, the breast cancer survivors who increased their physical activity levels after diagnosis also had a lower risk of overall mortality compared with those who had decreased levels of physical activity or were inactive at both time points. The WCRF/AICR panel has also concluded there is evidence that physical activity reduces risk of all-cause mortality and breast cancer specific mortality for survivors.3
In 2018, the American College of Sports Medicine (ACSM) convened a meeting composed of experts in the field of exercise and cancer in order to develop the first set of exercise guidelines for cancer survivors.67,68 The guidelines emphasize that exercise is generally safe for cancer survivors. There are evidence-based recommendations for the role of aerobic, combined aerobic and resistance training, and/or resistance training to address common problems experienced by cancer survivors, including anxiety, depression, fatigue, physical functioning, and health-related quality of life. Thus, this report is an important resource for health care professionals who work with cancer survivors.
Managing Treatment Toxicities
Exercise
In addition to improving long-term outcomes and comorbidities as previously discussed, exercise can lessen toxicities associated with conventional breast cancer treatments and improve quality of life. A Cochrane review including 32 studies and 2626 breast cancer patients actively undergoing adjuvant radiation and/or chemotherapy who were randomized to exercise versus no specified exercise found that physical exercise (whether aerobic and/or resistance exercise interventions) improves cognitive function and physical fitness, reduces fatigue, and improves cancer site-specific quality of life.69 Research by Jones and colleagues70,71 has shown that exercise and physical activity during treatment may even counteract the negative cardiovascular and biological effects of chemotherapy.
While some may feel that discussing exercise with a patient receiving chemotherapy and/or radiation is an unreasonable suggestion or an exercise in futility, in actuality it appears that the opposite is true—our breast cancer patients want to discuss lifestyle modifications, including exercise, as this gives them ownership of a piece of their treatment plan. A study with 205 cancer patients receiving adjuvant chemotherapy and/or radiation found that over 70% of the patients rated information about exercise and fatigue “helpful” or “very helpful,” and those receiving the information were more likely to engage in (and benefit from) exercise alongside their adjuvant therapies.72 The lack of discussion surrounding exercise may also be contributing to the long term trend for low physical activity levels among breast cancer survivors across the 10 years postdiagnosis. In fact, meeting physical activity guidelines prediagnosis was the only factor strongly associated with meeting guidelines at 5 years and 10 years postdiagnosis,73 suggesting that the frequency and/or quality of counseling has room for improvement. Further supporting this notion is the PACT study, which specifically examined adherence rates in exercise programs during breast cancer treatment. In this study, 92 patients with localized breast cancer undergoing chemotherapy were randomly assigned to an 18-week supervised moderate- to high-intensity aerobic and resistance exercise program, which included 2 supervised 1-hour sessions per week in addition to 30 minutes of independent physical activity per day on at least 3 other days. Patients attended 83% of the supervised sessions. Compliance with the duration of the home aerobic exercise was 88%; compliance with the specified intensity of the supervised aerobic exercise was 50% (cycling at ventilatory threshold); and compliance with the muscle strength exercises was 84%.74 If patients desire counseling and compliance rates are high, we have absolutely no reason not to include an exercise prescription in every treatment plan.
Exercise and Cancer-Related Fatigue
Fatigue is the most common symptom experienced by cancer patients during treatment, and likely the symptom for which exercise might be the most beneficial. A recent randomized clinical trial demonstrated the benefits of a simple 12-week home-based walking program in 159 breast cancer patients experiencing fatigue, insomnia, pain, or depression after their first cycle of chemotherapy. The patients were randomized to either the exercise group or the control group, beginning exercise on the first day of their third cycle of chemotherapy. Even though fatigue increased throughout the rest of the course of chemotherapy for both groups, the exercise group experienced significantly less fatigue both during and after completing treatment.75 It also seems that physical fatigue is the aspect of fatigue most relieved by exercise (as opposed to cognitive and affective fatigue).76
As discussed earlier, the recommendation is for 150 minutes per week of moderate-intensity activity (or 75 minutes per week of vigorous activity), along with at least 2 days of strength training.46,47 In breast cancer patients actively receiving adjuvant therapies, 2 separate meta-analyses found that 90 to 120 minutes per week of moderate-intensity exercise significantly improved fatigue, depression, and quality of life when compared with exercise of longer duration or greater intensity, especially when combined with mind-body interventions.77,78 When considering what type of aerobic exercise is best for breast cancer patients, it seems that all types act to decrease fatigue during adjuvant chemotherapy and/or radiation.79
Exercise and Breast Cancer-Related Lymphedema
Patients with upper extremity lymphedema are often discouraged from lifting weights or are hesitant to engage in resistance exercise for fear of exacerbation of symptoms. The Physical Activity and Lymphedema (PAL) randomized clinical trial documented the efficacy of a 12-month, fitness center–based, twice-weekly, progressive resistance exercise program, which included an initial 3 months of direct supervision by a certified fitness professional. A total of 141 breast cancer survivors were randomized to the exercise group or the control group. Those in the exercise group were required to wear a compression sleeve while lifting weights. Lymphedema symptoms decreased by 20% and incident flare-ups (cellulitis or swelling needing therapy) were reduced by 50%, with no effect on arm swelling.80 Building on these findings, the recently published Women in Steady Exercise Research (WISER) Survivor randomized clinical trial examined the effects of weight loss, home-based exercise, and a combination of the 2 programs on clinical lymphedema outcomes among 351 overweight breast cancer survivors with breast cancer–related lymphedema. The women were randomized to a 52-week home-based exercise program of strength/resistance training twice per week along with 180 minutes of walking per week, a weight loss program including 20 weeks of meal replacements and 52 weeks of lifestyle counseling, a combination of the 2 programs, or a control group (facility-based lymphedema care). While the home-based exercise program was found to be safe, they found no significant difference in the 12-month change in percentage of interlimb volume between the groups, concluding that a supervised facility-based resistance exercise program may provide greater lymphedema-specific benefits.81 When comparing these 2 experiences, it is important to note that the WISER trial included only overweight patients, while the PAL trial did not specify that the patients be overweight. The PAL trial also utilized a supervised facility-based program, rather than a home-based program as used in the WISER trial. The lack of significant benefit on limb volume observed in the WISER study is likely due to a combination of these factors. In fact, the weight-lifting program used in the PAL trial is now incorporated into the LIVESTRONG program at YMCAs, a collaboration between the YMCA and the Lance Armstrong Foundation. In our clinic, we focus on both weight loss and supervised strength-training with good clinical outcomes.
Mindfulness-Based Interventions
Stress is an often-forgotten side effect that may be experienced as a result of any aspect of breast cancer diagnosis and treatment. Not only does stress affect quality of life (including psychological, physical, and social aspects), but it is also associated with poorer survival in breast cancer patients.82,83 It is often difficult to identify those patients in need of referral for psychological support to target specific needs, including improved quality of life and mood, and decreased anxiety, depression, and distress. A systematic review of 41 longitudinal studies identified initial sociodemographic, disease-related, and psychosocial factors that later predicted psychological adjustment to living with breast cancer. The predicting factors included income, fatigue, cancer stage, physical functioning, optimism, perceived social support, coping strategies, initial level of psychological functioning, and body image.84 An assessment of these factors should be routine with every encounter, as targeted psychological support can improve both quality and quantity of life.
There are many ways in which women respond and adapt to stress, and as providers it is essential to be aware of effective mindfulness-based interventions to help build resilience. Most of these interventions are easily implemented and safely performed.
Mindfulness-Based Stress Reduction
Mindfulness-based stress reduction (MBSR) is the most scientifically validated stress reduction intervention and was founded by Dr Jon Kabat-Zinn in the 1970s at the University of Massachusetts. MBSR is a structured program which incorporates meditation, yoga, and mindful relaxation techniques, empowering each individual to respond consciously, rather than automatically, to situations and circumstances. MBSR has been shown to improve both psychological (perceived stress, anxiety, depression, fear of recurrence) and physical (pain, fatigue, sleep) aspects of quality of life in breast cancer survivors who have completed all adjuvant therapies.85-87 A systematic review reported that in 15 studies, MBSR was found to have a significant positive effect on multiple aspects of quality of life in women with breast cancer at many stages of treatment.88 A 2019 Cochrane review including 10 randomized clinical trials comparing MBSR versus no intervention in women with breast cancer (most with early stage) found that MBSR may improve quality of life at the end of the intervention but may result in little to no difference over time. They reported that MBSR probably reduces anxiety and depression and improves quality of sleep both at the end of the intervention and up to 6 months later. There was a beneficial effect on fatigue at the end of the intervention but not up to 6 months later.89 This implies what is commonly known—continued practice in necessary for continued, long-term benefit.
Nutritional Interventions
As discussed earlier, a plant-based diet is recommended by the WCRF/AICR. Data for specific foods during chemotherapy and/or radiation is both limited and inconsistent, and we therefore generally recommend the continued use of a whole-food plant-based nutrition plan during adjuvant therapies. Anecdotally, we have observed cases of significantly less than expected radiation-related acute skin toxicity in the majority of patients following a plant-based nutrition plan.
Intermittent fasting during treatment is another area warranting further study. Encouragingly, 24-hour dietary recall data collected from 2413 women with early-stage breast cancer (without diabetes) in the WHEL study revealed that fasting less than 13 hours per night was associated with an increase in the risk of breast cancer recurrence when compared with fasting 13 or more hours per night (HR 1.36, 95% CI 1.05-1.76).90 While this was a single observational study, the result was significant, and implementation is feasible and safe for most women.91
Conclusions
Research to date supports the importance of a healthy diet, physical activity, and healthy weight in the primary prevention, treatment, and tertiary prevention of breast cancer. Further research will provide insights into the benefits of dietary changes, weight loss interventions, physical activity, and other lifestyle changes for our patients after a diagnosis of breast cancer. Such studies will provide oncologists and other clinicians who care for cancer patients with additional tools to promote lifestyle modification with the goal to optimize breast cancer outcomes. Lifestyle medicine is a powerful tool in the fight against breast cancer and other chronic diseases. Although there is much more to be elucidated, the current level of knowledge makes it clear that we must encourage all of our patients to adhere to cancer prevention guidelines such as those of the WCRF/AICR. Following these guidelines not only reduces cancer risk but also reduces the risk of many other noncommunicable chronic diseases, positively affecting individual patient health and also dramatically reducing the global burden of disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Contributor Information
Amber Orman, AdventHealth, Orlando, Florida.
Dianne L. Johnson, MBB Radiology, Jacksonville, Florida.
Amy Comander, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, Massachusetts.
Nigel Brockton, American Institute for Cancer Research, Arlington, Virginia.
References
- 1. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54. [DOI] [PubMed] [Google Scholar]
- 2. McCullough ML, Patel AV, Kushi LH, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1089-1097. [DOI] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund, American Institute for Cancer Research, Continuous Update Project. Diet, nutrition, and physical activity and breast cancer survivors. https://www.wcrf.org/sites/default/files/Breast-cancer-survivors-report.pdf. Published 2018. Accessed March 2, 2020.
- 4. Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523-526. [DOI] [PubMed] [Google Scholar]
- 5. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253-256. [DOI] [PubMed] [Google Scholar]
- 6. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [DOI] [PubMed] [Google Scholar]
- 7. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shams-White MM, Brockton NT, Mitrou P, et al. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: a standardized scoring system. Nutrients. 2019;11:E1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hastert TA, Beresford SA, Patterson RE, Kristal AR, White E. Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1498-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomura SJ, Inoue-Choi M, Lazovich D, Robien K. WCRF/AICR recommendation adherence and breast cancer incidence among postmenopausal women with and without non-modifiable risk factors. Int J Cancer. 2016;138:2602-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris HR, Bergkvist L, Wolk A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and breast cancer risk. Int J Cancer. 2016;138:2657-2664. [DOI] [PubMed] [Google Scholar]
- 12. Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:792-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao Y, Xia J, Li L, et al. Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019;21:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163-170. [DOI] [PubMed] [Google Scholar]
- 16. Chen S, Chen Y, Ma S, et al. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget. 2016;7:80980-80989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong JY, He K, Wang P, Qin LQ. Dietary fiber intake and risk of breast cancer: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2011;94:900-905. [DOI] [PubMed] [Google Scholar]
- 18. Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rolls BJ. Dietary energy density: applying behavioural science to weight management. Nutr Bull. 2017;42:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [DOI] [PubMed] [Google Scholar]
- 22. Beecher GR. Phytonutrients’ role in metabolism: effects on resistance to degenerative processes. Nutr Rev. 1999;57(9 pt 2):S3-S6. [DOI] [PubMed] [Google Scholar]
- 23. Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. J Nutr. 2007;137(3 suppl 1):718S-737S. [DOI] [PubMed] [Google Scholar]
- 24. Vineis P, Schatzkin A, Potter JD. Models of carcinogenesis: an overview. Carcinogenesis. 2010;31:1703-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122-134. [DOI] [PubMed] [Google Scholar]
- 26. Encarnacao JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34:465-478. [DOI] [PubMed] [Google Scholar]
- 27. Murphy MM, Barraj LM, Herman D, Bi X, Cheatham R, Randolph RK. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet. 2012;112:222-229. [DOI] [PubMed] [Google Scholar]
- 28. Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr. 2014;100(suppl 1):423S-430S. [DOI] [PubMed] [Google Scholar]
- 29. Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459-471. [DOI] [PubMed] [Google Scholar]
- 30. Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S; Japan Public Health Center-Based Prospective Study on Cancer Cardiovascular Diseases Group. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906-913. [DOI] [PubMed] [Google Scholar]
- 32. Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89:1920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magee PJ, Rowland I. Soy products in the management of breast cancer. Curr Opin Clin Nutr Metab Care. 2012;15:586-591. [DOI] [PubMed] [Google Scholar]
- 34. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
- 35. Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. [DOI] [PubMed] [Google Scholar]
- 36. Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353-381. [DOI] [PubMed] [Google Scholar]
- 37. Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67:398-415. [DOI] [PubMed] [Google Scholar]
- 38. Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187-210. [DOI] [PubMed] [Google Scholar]
- 40. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8:E754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 suppl):S530-S550. [DOI] [PubMed] [Google Scholar]
- 43. Bianchini F, Kaaks R, Vainio H. Weight control and physical activity in cancer prevention. Obes Rev. 2002;3:5-8. [DOI] [PubMed] [Google Scholar]
- 44. Friedenreich CM, MacLaughlin S, Neilson HK, et al. Study design and methods for the Breast Cancer and Exercise Trial in Alberta (BETA). BMC Cancer. 2014;14:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487-509. [DOI] [PubMed] [Google Scholar]
- 46. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30-67. [DOI] [PubMed] [Google Scholar]
- 47. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bennie JA, De Cocker K, Teychenne MJ, Brown WJ, Biddle SJH. The epidemiology of aerobic physical activity and muscle-strengthening activity guideline adherence among 383,928 US adults. Int J Behav Nutr Phys Act. 2019;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479-2486. [DOI] [PubMed] [Google Scholar]
- 50. Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128-1143. [DOI] [PubMed] [Google Scholar]
- 51. Demark-Wahnefried W, Rock CL. Nutrition-related issues for the breast cancer survivor. Semin Oncol. 2003;30:789-798. [DOI] [PubMed] [Google Scholar]
- 52. Ligibel JA, Jones LW, Brewster AM, et al. Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO Survey of the Oncology Workforce. J Oncol Pract. 2019;15:e520-e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74:737-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4:1933-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767-1776. [DOI] [PubMed] [Google Scholar]
- 56. Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295:629-642. [DOI] [PubMed] [Google Scholar]
- 58. Chlebowski RT, Blackburn GL. Final survival analysis from the randomized Women’s Intervention Nutrition Study (WINS) evaluating dietary intervention as adjuvant breast cancer therapy. Paper presented at: San Antonio Breast Cancer Symposium; December 9-13, 2014; San Antonio, TX. [Google Scholar]
- 59. Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-fat dietary pattern and breast cancer mortality in the Women’s Health Initiative Randomized Controlled Trial. J Clin Oncol. 2017;35:2919-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203-4216. [DOI] [PubMed] [Google Scholar]
- 62. Rack B, Andergassen U, Neugebauer J, et al. The German SUCCESS C Study—the First European Lifestyle Study on Breast Cancer. Breast Care (Basel). 2010;5:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Villarini A, Pasanisi P, Traina A, et al. Lifestyle and breast cancer recurrences: the DIANA-5 trial. Tumori. 2012;98:1-18. [DOI] [PubMed] [Google Scholar]
- 64. Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res. 2016;22:4766-4775. [DOI] [PubMed] [Google Scholar]
- 66. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293-1311. [DOI] [PubMed] [Google Scholar]
- 67. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;(9):CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jones LW, Habel LA, Weltzien E, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jones LW, Fels DR, West M, et al. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila). 2013;6:925-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Windsor PM, Potter J, McAdam K, McCowan C. Evaluation of a fatigue initiative: information on exercise for patients receiving cancer treatment. Clin Oncol (R Coll Radiol). 2009;21:473-482. [DOI] [PubMed] [Google Scholar]
- 73. Mason C, Alfano CM, Smith AW, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Witlox L, Velthuis MJ, Boer JH, et al. Attendance and compliance with an exercise program during localized breast cancer treatment in a randomized controlled trial: the PACT study. PLoS One. 2019;14:e0215517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang HP, Wen FH, Yang TY, et al. The effect of a 12-week home-based walking program on reducing fatigue in women with breast cancer undergoing chemotherapy: a randomized controlled study. Int J Nurs Stud. 2019;99:103376. [DOI] [PubMed] [Google Scholar]
- 76. van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas. 2016;85:104-111. [DOI] [PubMed] [Google Scholar]
- 77. Carayol M, Delpierre C, Bernard P, Ninot G. Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psychooncology. 2015;24:737-747. [DOI] [PubMed] [Google Scholar]
- 78. Carayol M, Bernard P, Boiche J, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. 2013;24:291-300. [DOI] [PubMed] [Google Scholar]
- 79. Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007;16:104-121. [DOI] [PubMed] [Google Scholar]
- 80. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664-673. [DOI] [PubMed] [Google Scholar]
- 81. Schmitz KH, Troxel AB, Dean LT, et al. Effect of home-based exercise and weight loss programs on breast cancer-related lymphedema outcomes among overweight breast cancer survivors: the WISER Survivor Randomized Clinical Trial [published online August 15, 2019]. JAMA Oncol. doi: 10.1001/jamaoncol.2019.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spiegel D. Mind matters in cancer survival. Psychooncology. 2012;21:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brandao T, Schulz MS, Matos PM. Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psychooncology. 2017;26:917-926. [DOI] [PubMed] [Google Scholar]
- 85. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335-1342. [DOI] [PubMed] [Google Scholar]
- 86. Lengacher CA, Johnson-Mallard V, Barta M, et al. Feasibility of a mindfulness-based stress reduction program for early-stage breast cancer survivors. J Holist Nurs. 2011;29:107-117. [DOI] [PubMed] [Google Scholar]
- 87. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18:1261-1272. [DOI] [PubMed] [Google Scholar]
- 88. Zhang X, Liu D, Li Y, et al. Effects of mindfulness-based interventions on quality of life of women with breast cancer: a systematic review. J Comp Eff Res. 2019;8:829-840. [DOI] [PubMed] [Google Scholar]
- 89. Schell LK, Monsef I, Wockel A, Skoetz N. Mindfulness-based stress reduction for women diagnosed with breast cancer. Cochrane Database Syst Rev. 2019;3:CD011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ha K, Song Y. Associations of meal timing and frequency with obesity and metabolic syndrome among Korean adults. Nutrients. 2019;11:E2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marinac CR, Nelson SH, Breen CI, et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]