Abstract
A diabetic foot ulcer (DFUs) is a state of prolonged chronic inflammation, which can result in amputation. Different from normal skin wounds, various commercially available dressings have not sufficiently improved the healing of DFUs. In this study, a novel self-healing hydrogel was prepared by in situ crosslinking of N-carboxyethyl chitosan (N-chitosan) and adipic acid dihydrazide (ADH) with hyaluronic acid-aldehyde (HA-ALD), to provide a moist and inflammatory relief environment to promote stem cell proliferation or secretion of growth factors, thus accelerating wound healing. The results demonstrated that this injectable and self-healing hydrogel has excellent swelling properties, stability, and mechanical properties. This biocompatible hydrogel stimulated secretion of growth factors from bone marrow mesenchymal stem cells (BM-MSCs) and regulated the inflammatory environment by inhibiting the expression of M1 macrophages and promoting the expression of M2 macrophages, resulting in granulation tissue formation, collagen deposition, nucleated cell proliferation, neovascularization, and enhanced diabetic wound healing. This study showed that N-chitosan/HA-ALD hydrogel could be used as a multifunctional injectable wound dressing to regulate chronic inflammation and provide an optimal environment for BM-MSCs to promote diabetic wound healing.
Keywords: Hydrogel, BM-MSCs, inflammatory micro-environment, diabetic wound
Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia. In 2017, the people suffering from DM reached a population of about 425 million worldwide.1,2 Diabetic foot ulcers (DFUs), which occur in approximately 15% of diabetics patients, are a severe complication of DM.3 Pathophysiological studies of DFUs have indicated that diabetic peripheral vascular disease and neuropathy are the two main factors that contribute to this disease.4–6 This state of prolonged chronic inflammation inhibits wound healing, due to the impaired granulocytosis, chemotaxis, and macrophage function, as well as reduced secretion of growth factors and deregulated neovascularization. In extreme cases, DFUs can even result in amputation.7,8 However, various commercially available dressings have not sufficiently improved the healing of DFUs. Therefore, optimized wound dressing and more effective therapeutic strategies are urgently needed.
Bone marrow mesenchymal stem cells (BM-MSCs) show great promise as an option to treat non-healing wounds due to their multi-lineage differentiation potential. BM-MSCs are proved to have capacities in secreting multiple types of growth factors and differentiating into effector cells, thus promoting vascularization, granulation tissue formation, and re-epithelialization, resulting in improving wound healing.9 Previous studies have identified carriers that were beneficial to adhesion and proliferation of BM-MSCs and were suitable materials for the delivery of BM-MSCs as wound dressings.10,11 However, these carriers cannot work under the chronic inflammatory microenvironment of the diabetic foot due to protease secretion and excessive infiltration of inflammatory cytokines. In the inflammatory microenvironment, growth factors secreted by the effector cells are subject to increased degradation and loss of efficacy. In addition, the chronic inflammatory microenvironment could decrease the bioactivity of BM-MSCs.12,13 A more effective delivery system is critical to inhibition of the chronic inflammatory state, which will enable BM-MSCs to more effectively promote wound healing.
Recently, tissue engineering strategies of combining biomaterials with seed cells, namely combination therapies, using advanced biomaterials to maintain cellular viability, proliferation, and differentiation have received considerable attention.14,15 Hydrogels, which are ideal wound dressing and three-dimensional (3D) cell scaffolds, have many advantages including removal of wound exudate, providing a moist wound environment, preventing secondary infections, and promoting cell adhesion and proliferation. These elements create an optimal environment to encourage high quality wound healing.16,17 However, the beneficial properties are often mitigated by the formation of cracks in the traditional hydrogels. As a result, the integrity of network structures and the mechanical characteristics of bulk gels have limited their practical application. To overcome these disadvantages, functional hydrogels have been designed with self-healing or self-repairing properties.18
The self-repairing materials have the ability to recover the structures and functions after structural damage, which helps to maintain the integrality of the 3D network structures and the mechanical characteristics of the bulk gels. Self-healing hydrogels are capable of sealing the wound site completely to protect against the second injury.19 Furthermore, self-healing hydrogels with shear-thinning properties have the potentials to be used as injectable materials to encapsulate sensitive biological drugs/cells ex vivo, allowing for minimally invasive delivery.20 These novel properties may improve the scope of clinical applications of hydrogels. However, the use of self-healing hydrogels as a carrier to treat DFUs has not been reported.
In this study, an injectable and self-healing polysaccharide-based hydrogel was developed to deliver BM-MSCs to treat DFUs. The hydrogel dressings were comprised primarily of polysaccharides, which enabled excellent control of chemical modification and composition. Chitosan, a cationic polysaccharide, was used as a template because of its biocompatibility, biodegradability, and exhibited hemostatic and antibacterial properties. N-chitosan, a derivative of chitosan, shows greater hemostasis and antimicrobial activity than chitosan.21 Hyaluronic acid (HA), an immunoneutral polysaccharide present in the human body, was also used. Hyaluronic acid was oxidized to form an aldehyde (HA-ALD), which provided a moist environment and allowed absorption of wound exudates while reducing surface dryness and minimizing scar formation.22 We hypothesized that this hydrogel would promote differentiation of BM-MSCs and secretion of factors to inhibit chronic inflammation. The wound healing effects of topical administration of BM-MSCs contained in a hydrogel were evaluated using a diabetic rat model (Scheme 1).
Scheme 1.
A novel injectable hydrogel was prepared by in situ crosslinking of N-chitosan and ADH with HA-ALD, to provide a moist and inflammatory relief environment to promote BM-MSCs proliferation or secretion of growth factors, thus inducing collagen deposition and angiogenesis and accelerating wound healing.
Materials and methods
Materials
Chitosan and streptozotocin (STZ) were purchased from Aladdin, and sodium periodate (NaIO4) was supplied by Sigma-Aldrich. Hyaluronic acid (HA, 100-200k) and adipic acid dihydrazide (ADH) were obtained from Yuanye biology. Cell culture medium, including low glucose Dulbecco's Modified Eagle's Medium (LG-DMEM), fetal bovine serum (FBS), as well as streptomycin-penicillin were supplied by Gibco Life Technologies (USA). Cell Counting Kit 8 (CCK-8) and Calcein-AM/PI kits were obtained from Biobetimes Biotechnology Co., Ltd (Changsha, China). Paraformaldehyde solution (4%) and phosphate buffer saline (PBS) were supplied by Xilong Chemical Co., Ltd. Hematoxylin, eosin stains were purchased from Thermo Fisher Scientific Co., Ltd (Shanghai, China). Antibodies used for immunofluorescence were purchased from Abcam (UK).
Preparation of the hydrogel
The biodegradable multifunctional N-chitosan/HA-ALD hydrogel was prepared according to our previous study.23 Briefly, N-chitosan and HA-ALD were synthesized from chitosan and hyaluronic acid through a series of chemical reactions. Then, the hydrogel was designed by in situ crosslinking of N-chitosan and ADH with HA-ALD. Hydrogels obtained by mixing different proportions of these components had different properties. In this study, 7.5% N-chitosan (W/V) and 7.5% ADH (W/V) were dissolved in deionized water. Then, the same volume of 5% HA-ALD (W/V) was added into the above solution for hydrogel formation through imine and acylhydrazone bonds.
Characterization of hydrogel
To observe the microstructures, the prepared hydrogels were freeze-dried, and the cross-sectional morphology was evaluated using a scanning electron microscope (SEM) (JSM-6700F, JEOL, Japan). To study the swelling properties of hydrogels, samples (2 cm × 2 cm) were dried at 60 °C (Wa), and then soaked in PBS at 37 °C (Ws). The following formula expressed the swelling ratio (SR): SR% = (Ws−Wa) /Wa× 100;
In vitro degradation rate of the hydrogel was studied in PBS with lysozyme (104 units/ml). Hydrogel samples (500 µl) were placed in glass vials. Two milliliters of PBS was gently added to the vials. Pre-weighed (Wi) hydrogel was incubated across a range of times. At pre-determined time points, samples were washed by deionized water and then freeze-dried. Dry weights of the hydrogels were recorded as Wd. Degradation percentage was represented by the following formula: Degradation rate = (Wi−Wd)/Wi × 100%
Isolation and purification of BM-MSCs
BM-MSCs were extracted from healthy 1-week-old Sprague-Dawley (SD) rats. Primary cells were obtained as described in our previous research,24 and cultured in LG-DMEM supplemented with 10% (V/V) FBS and 1% (W/V) penicillin-streptomycin at the atmosphere of 37 °C and 5% CO2. When the cells reached ~80% confluence, the adherent cells were digested with 3 ml 0.25% (W/V) trypsin/EDTA at 37 °C for 3 min and then passaged. Three to four passages of BM-MSCs were used for the following experiments.
In Vitro cell culture experiments
The proliferation rate of BM-MSCs in N-chitosan/HA-ALD hydrogel was determined using CCK-8 assay. BM-MSCs were seeded in the hydrogel-coated 48-well plates at a density of 1 × 105 cells/well. While the control group did not contain hydrogel. After incubation for 1, 4, and 7 days, BM-MSC proliferation was measured using the CCK-8 assay.
To observe the cell viability in the hydrogel, Live/Dead staining was carried out. After 1 and 7 days of culture, 1 mM calcein AM was added into the samples to incubate for 1 h, and then 1 μg/mL PI was added for 5 min at 37°C. Finally, fluorescent photographs were taken by fluorescence microscopy (IX51, Olympus, Japan).
The levels of transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) secreted by BM-MSCs cultured in hydrogel were evaluated using ELISA according to the protocols. BM-MSCs were seeded at a concentration of 1 × 106 cells/mL and cultured for 1 and 3 days. The contents of growth factors secreted by BM-MSCs in the supernatant were measured by the ELISA kits.
Diabetic foot ulcer model preparation and wound closure analysis
All animal experimental protocols were approved by the Animal Care and Use Ethics Committee of The Second Hospital of Jilin University. Male SD rats (200–250 g, 8-weeks-old) were supplied by the Animal Center of Jilin University. Diabetic rat models were prepared by a single intraperitoneal injection of STZ (65 mg/kg). When the blood glucose level exceeded 16.7 mmol/L at 1-week post-STZ injection, it was considered that the DM model was successfully established. Blood glucose levels were recorded throughout the study.
To establish a diabetic foot wound healing model, 45 rats were chosen after 3 weeks of STZ-induced hyperglycemia. After general anesthesia by intraperitoneal pentobarbital sodium (35 mg/kg), a punch biopsy instrument was used to make a 5 mm diameter circular foot skin wound. Rats were randomly divided into the following three groups: control group (untreated, n = 15), hydrogel group (200 μL of hydrogel), and hydrogel + BM-MSCs group (namely, 2×105 BM-MSCs was encapsulated into 100 μL N-chitosan and ADH solution and then mixed with 100 μL HA-ALD solution with stirring).
The wound area was recorded at 0, 3, 6, 9, 12, and 15 days after the procedure using a digital camera. Residual wound areas were expressed using the following equation: Residual wound areas (%) = Sn/So × 100%. S0 represented the initial wound area, and Sn represented the unhealed wound area at different time points.
Growth factor concentrations in wound tissue
To investigate the concentrations of growth factors (e.g., TGF-β, VEGF, and bFGF) in the wound tissues, the wound and surrounding tissues (0.5 cm diameter) were excised 3 and 9 days after the procedure. The samples were homogenized in PBS on the ice at a ratio of 100 mg of tissue per 1 mL of PBS. The samples were centrifuged at 2,500 rpm for 20 min, and then the supernatants were gathered to detect the concentrations of TGF-β, VEGF, and bFGF in the wound tissue by the ELISA kits.
Histological and immunohistochemical analysis
At 6, 12, and 28 days after the operation, the wound tissues were harvested and fixed in 4% paraformaldehyde solution. The samples were embedded in paraffin for routine histological processing, and then about 5 μm sections of the tissues were prepared for histological examination. According to the protocols, sections were stained with H&E, Masson’s trichrome (MT), and antibodies against CD86, CD163, Ki67, and CD31. The maturity of the sub-epithelial dermal matrix of the wound was determined by two trained clinicians based on the immunohistochemical results (Table S1).25 The average optical density values for CD86, CD163, Ki67, and CD31 expression in the tissues were analyzed by Image-Pro Plus software (IPP) according to five randomly selected fields.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD), and statistical analysis was performed using ANOVA with Tukey’s post-hoc analysis (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered to be statistically significant. All experiments were repeated at least three times independently.
Results and discussion
Characterization of the hydrogels
Carboxyethyl functional chitosan (N-chitosan) was synthesized via Michael addition, and HA was chemically modified to contain aldehyde groups (HA-ALD). The self-healing hydrogels were prepared by mixing dilute solutions of N-chitosan, HA-ALD, and ADH (as the cross-linker) in PBS at room temperature. The solution-to-gel phase transformation process is shown in Figure 1(a). This phase change was attributed to acylhydrazone (hydrazine groups of ADH reacted with HA-ALD) and imine bonds formation (amino groups of N-chitosan reacted with HA-ALD).
Figure 1.
(a) Optical images of the gelation progress. The mixture of N-chitosan and ADH was in the solution (sol) state at room temperature, and underwent sol–gel transition approximately 20 s after adding HA-ALD solution. (b) SEM image of the morphology and internal structure of the hydrogel. (c) Swelling properties of the hydrogel. (d) Degradation of the hydrogel.
Patients with DM often suffer from lower limb ulcers. Hydrogel is an ideal wound dressing but limited by the formation of cracks in the traditional hydrogels. As a result, the integrity of network structures and the mechanical characteristics of bulk gels have limited their practical application. To overcome these disadvantages, novel self-healing hydrogels with the ability to recover the structures and functions after structural damage were designed to maintain the integrality of the 3D network structures and the mechanical characteristics of the bulk gels.19 Furthermore, self-healing hydrogels with shear-thinning properties have the potentials to be used as injectable materials to encapsulate sensitive biological drugs/cells ex vivo, allowing for minimally invasive delivery.20 Based on reversible dynamic bonds, acylhydrazone and imine bonds derived from aldehyde groups at HA-ALD reactive with NH2NHCO- groups on ADH and −NH2 groups on N-chitosan. The injectable and self-healing properties of this polysaccharide hydrogel were demonstrated in our previous study.23 Our hydrogel can be injected through a 26-gage needle without clogging quickly. The self-healing property was demonstrated to recover structural and functional damages (Figure S1), which resulted in maintaining the integrality of network structures and the mechanical characteristics of the bulk gel. This hydrogel can tolerate mechanical deformation, such as that caused by limb movements, thus providing a stable connection between the wound area and the repair material. In contrast, accidental external mechanical forces may cause tearing in traditional dressing materials. The self-healing ability of our hydrogel resulted in rapid repair, which allowed for prolonged use.26,27 The gelation time of the hydrogel was approximately 60 s at 25°C. This allowed enough time for stem cells to be encapsulated in the hydrogel.
The micromorphology of the hydrogel was observed by SEM. The hydrogel exhibited a homogeneous interconnected pore structure, and the pore size was about 100–200 nm in diameter (Figure 1(b)). The porous structure of the hydrogel effectively extended the growth space for culturing seed cells and boosted communications among cells. Moreover, when seed cells were implanted in vivo, the interconnected micro-channels allowed for the transport of oxygen, nutrients, and other products to benefit cell migration and proliferation.28 The swelling properties of hydrogels as wound dressings play a critical role in absorbing wound exudates to maintain a clean and moist environment in the wound bed. After exposure to physiological conditions (such as PBS at 37°C), the hydrogel swelled 50.32% in 80 min and 75.77% in 120 min, reaching equilibrium without significant weight loss or structural deformation (Figure 1(c)). The in vitro degradation rate of the hydrogel was evaluated in PBS containing lysozyme (104 units/ml). In the presence of lysozyme, about half of the hydrogel was degraded in the first 7 days, which allowed for granulation tissue to be formed on the surface of skin wounds. The hydrogel was biodegraded entirely in 14 days (Figure 1(d)).
Promoted cell activity in vitro
To investigate the ability of our hydrogel to protect and deliver BM-MSCs, cells were encapsulated into 100 μL N-chitosan and ADH solution and then mixed with 100 μL HA-ALD solution with stirring in the plates. As shown in Figure S2, CCK-8 results clearly showed that the N-chitosan/HA-ALD hydrogel was biocompatible, and BM-MSCs seeded in the hydrogel kept viability for up to 7 days. As shown in Figure 2(a), BM-MSCs can adhere and proliferate well in the hydrogels.
Figure 2.
(a) Representative images of the live/dead assay at 1 and 7 days on the hydrogel (Green: live cells, Red: dead cells). (b–d) The concentrations of the growth factors, TGF-β1, VEGF, and bFGF, secreted by BM-MSCs cultured in hydrogels for 1 and 3 days (Con and Gel represented the control and hydrogel groups, respectively. *p < 0.05, **p < 0.01).
Growth factor concentrations in the wound area have a significant effect on wound healing. In particular, TGF-β1, VEGF, and bFGF promote wound closure and accelerate the formation of new granulation tissue, vascularization, and re-epithelialization.29,30 Our hydrogel could induce growth factors secretion from BM-MSCs. There were no statistical differences in the concentrations of TGF-β1, VEGF, and bFGF secreted by BM-MSCs in the hydrogel group or the control group on the first day. However, on day 3, the concentrations of TGF-β1, VEGF, and bFGF in the hydrogel group were 2.71 ± 0.26 ng/L, 22.17 ± 3.65 ng/L, and 3.63 ± 0.96 ng/L, respectively, which were significantly higher than that in the control group (1.52 ± 0.48 ng/L, 12.33 ± 1.84 ng/L, and 2.23 ± 0.53 ng/L, respectively; Figure 2(b–d)).
The mechanical characteristics of hydrogels give rise to influence on stem cell functions.31 Aggregates of stem cells with enhanced cell-cell contact display greater multipotency and secrete more factors, resulting in favorable cell migration and vitality.32,33 Chitosan-based self-healing hydrogels have been shown to promote cell proliferation better than alginate hydrogels, which may have been due to halo formation in the self-healing hydrogels.34 As a result, our self-healing hydrogel had the ability to restore structure and function after damage, which could preserve the integrality of the network structures and mechanical characteristics of the bulk gels. This improved stability may have contributed to stem cell proliferation and secretion of TGF-β1, VEGF, and b-FGF. Moreover, the components (N-chitosan and HA-ALD) and features of the self-healing hydrogel may have enhanced proliferation of fibroblasts, collagen deposition, angiogenesis, and promoted BM-MSC migration and differentiation, resulting in accelerated wound healing.35,36
Characteristics of the animal model and wound contraction
To evaluate the treatment effect of rat DFUs using BM-MSCs encapsulated in the hydrogel, DM rat models were prepared by intraperitoneal injection of STZ. When the blood glucose levels exceeded 16.7 mmol/L at 1-week post-STZ injection, it was considered that the DM model was successfully prepared. The duration from STZ injection to treatment was 7 weeks. In the first 3 weeks, there was no significant difference in blood glucose levels among the DM rat groups. However, treatment with hydrogel or hydrogel + BM-MSCs resulted in decreased blood glucose levels, particularly in the first 2 weeks after treatment (p < 0.05; Figure S3). No differences in body weight were observed among any of the groups during the experiment (Figure S4).
To determine the rate of wound healing, we recorded general wound closure results. Typical wound closure images for each group at different time points are presented in Figure 3(a). Moreover, residual wound areas were calculated in Figure 3(b), which showed that the remaining regions of wounds covered with hydrogels were significantly smaller than the control group (p < 0.05). This may have been due to the moist wound environment provided by the hydrogel, which could maintain a humid microenvironment and prevent cell death, enhance epidermal migration, promote angiogenesis and collagen deposition.37 In addition, the hydrogel + BM-MSCs group exhibited faster healing at each observation time point compared with other groups. Furthermore, at 15 days after the procedure, the wounds in the hydrogel + BM-MSCs group were almost fully healed. In contrast, the unhealed wound areas were 14.48 ± 3.35% and 41.55 ± 2.67% in the hydrogel and control groups, respectively. These results indicated that BM-MSCs-encapsulated in hydrogel promoted faster wound healing.
Figure 3.
Treatment effects of hydrogel + BMSCs on healing of DFUs. (a) Typical wound closure images of each group at different time points. (b) Time-course of wound changes. (c–e) The concentrations of the growth factors, TGF-β1, VEGF, and bFGF in wound tissue at 3 and 9 days after treatment. (Con, Gel, and G+C represented the control, hydrogel, and hydrogel + BM-MSCs groups, respectively. *p < 0.05, **p < 0.01, ***p < 0.001).
Increased growth factors secretion in vivo
A previous study has shown that TGF-β1 could accelerate re-epithelialization during the healing process by promoting keratinocyte migration.38 VEGF and bFGF are key regulatory growth factors that promote angiogenesis and are essential in wound healing. In addition, bFGF is critical in the prevention of scar formation.39 As shown in Figure 3(c–e), there were no statistical differences in the concentrations of TGF-β1 and bFGF in wound tissue on day 3 among the experimental groups. However, the expression of VEGF on day 3 was 1.45-fold higher in the hydrogel + BM-MSCs than that in the control group (p < 0.01). On day 9, in the control, hydrogel, and hydrogel + BM-MSCs group, the concentrations of TGF-β1 were 25.47 ± 3.37 ng/L, 27.92 ± 4.89 ng/L, and 34.68 ± 8.21 ng/L, respectively; and the concentrations of VEGF were 23.13 ± 1.02 ng/L, 24.47 ± 2.31 ng/L, and 30.31 ± 2.83 ng/L, respectively. Namely, the concentrations of TGF-β1 and VEGF in the hydrogel + BM-MSCs group were significantly higher than those in the hydrogel and control groups. In addition, the expression of bFGF in the hydrogel + BM-MSCs group (3.58 ± 0.64 ng/L) was also superior to the control group (2.45 ± 0.52 ng/L) on day 9 (p < 0.05). Increased secretion of multiple growth factors in the wound tissues treated with hydrogel + BM-MSCs was consistent with our in vitro results. Increased secretion of growth factors has been a critical reason in enhanced wound healing resulting from treatment with hydrogel + BM-MSCs.
Effect of hydrogel on wound healing
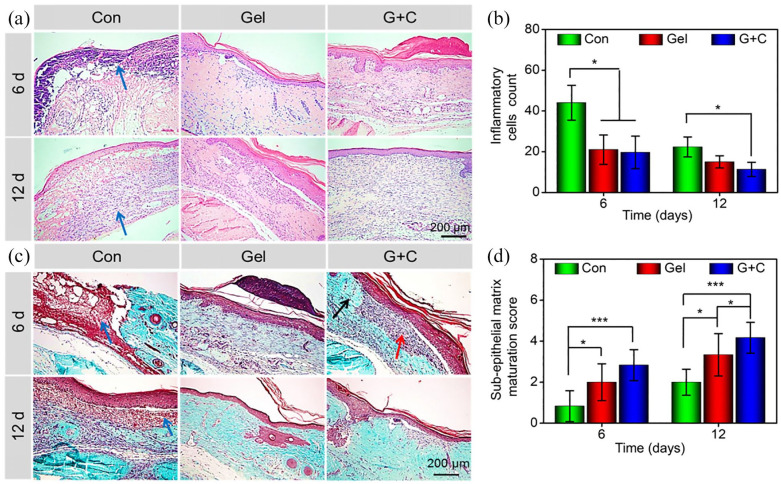
The process of wound healing includes several overlapping stages: for example, hemostasis, inflammation, proliferation, as well as re-epithelialization and remodeling.40,41 The aggregation and activation of inflammatory cells is a critical step in the transition from the inflammatory phase to the repair phase. But the excessive inflammatory reaction will lead to a chronic inflammatory microenvironment and delayed wound healing.42 As displayed in Figure 4(a) and (b), the naked wound surface resulted in necrotic tissue, foreign bodies, and a large number of inflammatory cells at the wound site on day 6. Unlike the control group, a hydrogel with or without BM-MSCs provided a suitable inflammatory environment benefiting for wound healing. These results indicated similar inflammatory cells infiltration in the wound in the hydrogel group and the hydrogel + BM-MSCs group on day 6. At day 12, the wounds covered with hydrogel + BM-MSCs had fewer inflammatory cells infiltrated in the wound area than the control group, which may have been due to the hydrogel-forming a protective film on the wound surface to block external bacteria, and reduced duration of inflammation due to the presence of active BM-MSCs.43,44 As seen in Figure 4(a), H&E staining indicated that hydrogel + BM-MSCs promoted the formation of well-organized granulation tissue on day 6 and 12. In contrast, there was no obvious granulation tissue formation in the control groups on either day 6 or day 12. The level of granulation tissue formation in the hydrogel group was between the hydrogel + BM-MSCs group and control group.
Figure 4.
(a) H&E staining of the wound at 6 and 12 days after treatment (Blue arrows indicated necrotic tissue, foreign bodies, and a large number of inflammatory cells). (b) Inflammatory cell count in the wound area at 6 and 12 days after treatment. (c) Masson trichrome staining to detect new collagen fiber deposition in granulation tissue (Blue arrows indicated necrotic tissue, foreign bodies, and a large number of inflammatory cells; Red arrow indicated fibrous repair (mainly with collagen fibers), and black arrow indicated the original connective tissue of the dermis). At 12 days, the wound tissue in the hydrogel + BM-MSCs group was completely repaired, and was nearly identical in appearance to the surrounding tissue. (d) Sub-epithelial matrix maturation scores of the regeneration tissue at 6 and 12 days after treatment (Con, Gel, and G+C represented the control, hydrogel, and hydrogel + BM-MSCs groups, respectively. *p < 0.05, **p < 0.01, ***p < 0.001).
During the maturation process of wound healing, collagen deposition shows a key role in wound contraction and scar formation, which results from fibroblasts crosslinking with collagen.42 Masson's trichrome staining was used to show collagen deposition in the regenerated granulation tissue. These results demonstrated that the hydrogel + BM-MSCs group formed more collagen fibers than the control group or the hydrogel group at both days 6 and 12 (Figure 4(c)). The maturity of the sub-epithelial dermal matrix of the wound was determined using histological results by two trained clinicians. This evaluation included the scoring of granulation tissue, inflammation, fibroblasts, collagen deposition, and neovascularization (Table S1).25 The sub-epithelial matrix maturation scores of control, hydrogel, and hydrogel + BM-MSCs groups were 0.83 ± 0.75, 2.00 ± 0.89, and 2.83 ± 0.75 after 6 days, respectively; and 2.02 ± 0.63, 3.17 ± 0.75, and 4.17 ± 0.75 after 12 days, respectively. The DFUs treated with a hydrogel containing BM-MSCs exhibited higher maturation scores than the hydrogel or control groups after either 6 or 12 days. In addition, the level of maturation was also greater in the hydrogel group than that in the control group (Figure 4(d)).
Immunohistochemical staining of Ki67 and CD31 was performed to investigate the proliferation of nucleated cells and neovascularization, respectively. Increased expression of Ki67-positive cells was exhibited in the hydrogel + BM-MSCs group, compared with that in the hydrogel and control groups on days 6 and 12. In addition, treatment with hydrogel alone induced significantly higher Ki67 expression than the control group on days 6 and 12 (Figure 5(a, b)). Furthermore, the expression of CD31-positive cells was increased in the hydrogel + BM-MSCs group (Figure 5(c)), which indicated that BM-MSCs stimulated angiogenesis and promoted blood vessel formation in granulation tissue (Figure 5(d)).
Figure 5.
(a) Immunohistochemical staining of Ki67 in the wounds 6 and 12 days after treatment. (b) Quantitative analysis of mean density of Ki67 expression results in the wounds. (c) Immunohistochemical staining of CD31 expression in the wounds 6 and 12 days after treatment. (d) Neovascularization in the wounds (The black arrows represented the neovascularization) (Con, Gel, and G+C represented the control, hydrogel, and hydrogel + BM-MSCs groups, respectively.
*p < 0.05, **p < 0.01).
The hyperglycemia environment of DM delays wound healing by inhibiting cell proliferation and migration, as well as hindering collagen synthesis.45 Therefore, preferable wound contraction and maturation were observed in groups treated with hydrogel, which may have been contributed to the combination of a clean and moist healing environment, and the hypoglycemic effect of chitosan (Figure S3).46,47 In addition, some growth factors, such as TGF-β, VEGF, and bFGF, indicate a critical role in the wound healing process.48 Thus, BM-MSCs may have contributed to effective wound healing in the hydrogel + BM-MSCs group by secreting TGF-β1, bFGF, and VEGF. BM-MSCs can differentiate into effector cells involved in wound healing (e.g., keratinocytes, endothelial cells, and fibroblasts).49 The results of Ki67 and CD31 staining showed that BM-MSCs encapsulated in hydrogel promoted proliferation of nucleated cells, neovascularization, and played an active role in wound healing. The histological and immunohistochemical results indicated that hydrogel with BM-MSCs supported development of granulation tissue, collagen deposition, neovascularization, inhibited excessive inflammation, and accelerated the healing process in DFUs.
Effect of hydrogel on inhibition of chronic inflammation
DFUs are characterized by a chronic inflammatory microenvironment. Macrophages play a critical role in all phases during the healing phases and regulate wound healing progress, which includes two phenotypes: M1 (pro-inflammation) and M2 (pro-healing). The wound microenvironment determines the macrophage functional phenotype.50,51 We evaluated potential mechanisms by which local application of hydrogel and hydrogel + BM-MSCs promoted the healing of DFUs. M1 and M2 macrophages are characterized by expression of CD86 and CD163, respectively.52 As shown in Figure 6(a) and (b), the mean density of CD86-positive cells in the control group was 1.40-fold and 1.46-fold higher than that in the hydrogel and hydrogel + BM-MSCs groups at 6 days, respectively. And the significant difference between the control group and the hydrogel and hydrogel + BM-MSCs groups still existed at 12 days. In contrast, CD86 expression was similar between the hydrogel group and hydrogel + BM-MSCs group (p > 0.05). In addition, the expression of CD163-positive cells in the hydrogel + BM-MSCs group was 1.53-fold and 1.67-fold higher than that in the control group at 6 and 12 days (p < 0.01), respectively, and 1.57-fold higher than that in the hydrogel group at 12 days (p < 0.05) (Figure 6(c) and (d)). These results indicated that the hydrogel regulated the inflammatory microenvironment in diabetic wounds, achieving a better healing effect in response to BM-MSCs.
Figure 6.
Alleviation of chronic inflammation in DFUs. (a, b) Immunofluorescence staining and quantitative statistics of CD86-positive cells at wound sites. (c, d) Immunofluorescence staining and quantitative statistics of CD163-positive cells at wound sites (Con, Gel, and G+C represented the control, hydrogel, and hydrogel + BM-MSCs groups, respectively.
*p < 0.05, **p < 0.01).
Although M1 macrophages show a critical role in host defense, some inflammatory mediators, for instance, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which are released by M1 macrophages, can cause significant tissue damage. M2 macrophages have been shown to enhance wound healing, promote revascularization, and reduce infection, resulting in inhibition of inflammation, and regulation of the immune response.53 Previous studies have shown that BM-MSCs can inhibit M1 macrophage activation, and stimulate M2 macrophage activation, resulting in alleviation of the chronic inflammatory response in the diabetic wound microenvironment.9,50 In the present study, our hydrogel inhibited M1 polarization, and slightly, but not significantly, increased M2 polarization. However, M2 macrophages were increased dramatically in response to the hydrogel that contained BM-MSCs, which agreed with our previous findings that BM-MSCs had an immunomodulatory effect.54,55 In summary, the hydrogel had the ability to modulate chronic inflammation and provided an optimal environment for BM-MSCs to promote wound healing.
Effect of hydrogel on the quality of wound healing
Wound healing of DFUs resulted in scar formation in each group 28 days after treatment. The quality of wound healing was evaluated using H&E staining (Figure 7(a)). The number of regenerative sebaceous glands was significantly increased in the hydrogel + BM-MSCs group (6.67 ± 2.08) than the control group (3.33 ± 1.53) (Figure 7(b)). The hydrogel group showed relatively sparse new sebaceous glands in the center of the wound. However, almost no newborn sebaceous glands were observed in the center of the regenerative skin in the control group. The development of new hair follicles was consistent with that observed for new sebaceous glands. As displayed in Figure 7(c), new hair follicles of the control, hydrogel, and hydrogel + BM-MSCs groups were 3.33 ± 1.52, 4.67 ± 1.52, and 8.00 ± 2.05, respectively. Previous studies have demonstrated that BM-MSCs can differentiate into sebaceous gland cells and hair follicle cells.56 In our study, topically delivered BM-MSCs may have contributed to the formation of the hair follicle and sebaceous gland, which may have significantly improved wound healing quality.
Figure 7.
(a) H&E staining of the distribution of new sebaceous glands (purple arrow) and hair follicles (orange arrow) in the wound after 28 days of treatment. (b) Statistical analysis of new sebaceous glands. (c) Statistical analysis of new hair follicles (Con, Gel, and G+C represented the control, hydrogel, and hydrogel + BM-MSCs groups, respectively. *p < 0.05, n = 3).
Conclusion
In this study, we prepared a novel biocompatible hydrogel with injectable and self-healing properties by in situ crosslinking of N-chitosan and ADH with HA-ALD to provide a moist and inflammatory relief environment to promote stem cell proliferation or secretion of growth factors, thus accelerating DFUs wound healing. This hydrogel promoted the secretion of growth factors from BM-MSCs, including TGF-β1, VEGF, and bFGF. In vivo experiments indicated that this composite hydrogel inhibited chronic inflammation, promoted granulation tissue formation, collagen deposition, and nucleated cell proliferation, and stimulated neovascularization, which resulted in enhanced healing of DFUs. In summary, our N-chitosan/HA-ALD hydrogel regulated chronic inflammation in DFUs and provided an optimal environment for BM-MSCs to enhance wound healing. This formulation represents a potential avenue for the treatment of DFUs.
Supplemental Material
Supplemental material, Supporting_Information for Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers by Haotian Bai, Noh Kyu-Cheol, Zhonghan Wang, Yutao Cui, He Liu, Hou Liu, Yubin Feng, Yue Zhao, Quan Lin and Zuhao Li in Journal of Tissue Engineering
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (Grant Nos. 81772456, 81671804, 51861145311, and 21174048), Scientific Development Program of Jilin Province (Grant Nos. 20200403088SF, 20200802008GH, 20200404202YY, 20190304123YY, 20180201041SF, and 20180623050TC), Program of Jilin Provincial Health Department (Grant Nos. 2019SRCJ001 and 2019SCZT001), Youth Talents Promotion Project of Jilin Province (Grant No. 192004).
ORCID iD: Zuhao Li  https://orcid.org/0000-0003-1909-2999
https://orcid.org/0000-0003-1909-2999
Supplemental material: Supplemental material for this article is available online.
References
- 1. Braffett BH, Gubitosi-Klug RA, Albers JW. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes 2020; 69(5): 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94(3): 311–321. [DOI] [PubMed] [Google Scholar]
- 3. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117(5): 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moura LIF, Dias AMA, Carvalho E, et al. Recent advances on the development of wound dressings for diabetic foot ulcer treatment-A review. Acta Biomater 2013; 9(7): 7093–7114. [DOI] [PubMed] [Google Scholar]
- 5. Zhao Y, Li Z, Song S, et al. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv Funct Mater 2019; 29(31): 1901474. [Google Scholar]
- 6. Liu H, Wang C, Li C, et al. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv 2018; 8(14): 7533–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang ZF, Lu MF, Zhu GH, et al. Acceleration of diabetic-wound healing with PEGylated rhaFGF in healing-impaired streptozocin diabetic rats. Wound Repair Regen 2011; 19(5): 633–644. [DOI] [PubMed] [Google Scholar]
- 8. Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep 2015; 5: 18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dash SN, Dash NR, Guru B, et al. Towards reaching the target: clinical application of mesenchymal stem cells for diabetic foot ulcers. Rejuvenation Res 2014; 17(1): 40–53. [DOI] [PubMed] [Google Scholar]
- 10. Nelson VJ, Dinnunhan MFK, Turner PR, et al. A chitosan/dextran-based hydrogel as a delivery vehicle of human bone-marrow derived mesenchymal stem cells. Biomed Mater 2017; 12(3): 035012. [DOI] [PubMed] [Google Scholar]
- 11. Wise JK, Alford AI, Goldstein SA, et al. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng Pt A 2014; 20(1–2): 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjarnsholt T, Kirketerp-Moller K, Jensen PO, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008; 16(1): 2–10. [DOI] [PubMed] [Google Scholar]
- 13. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366(9498): 1736–1743. [DOI] [PubMed] [Google Scholar]
- 14. Li S, Dong S, Xu W, et al. Antibacterial Hydrogels. Adv Sci (Weinh) 2018; 5(5): 1700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng X, Li J, Zhang X, et al. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Control Release 2019; 302: 19–41. [DOI] [PubMed] [Google Scholar]
- 16. Anisha BS, Biswas R, Chennazhi KP, et al. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int J Biol Macromol 2013; 62: 310–320. [DOI] [PubMed] [Google Scholar]
- 17. Chaires-Rosas CP, Ambriz X, Montesinos JJ, et al. Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton’s jelly cultured in a fibrin hydrogel. J Tissue Eng 2019; 10: 2041731419840622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merino S, Martin C, Kostarelos K, et al. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano 2015; 9(5): 4686–4697. [DOI] [PubMed] [Google Scholar]
- 19. Qu J, Zhao X, Liang Y, et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018; 183: 185–199. [DOI] [PubMed] [Google Scholar]
- 20. Kopecek J, Yang J. Smart self-assembled hybrid hydrogel biomaterials. Angewandte Chemie 2012; 51(30): 7396–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu H, Yu L, Tan S, et al. Novel complex hydrogels based on N-carboxyethyl chitosan and quaternized chitosan and their controlled in vitro protein release property. Carbohydr Res 2010; 345(4): 462–468. [DOI] [PubMed] [Google Scholar]
- 22. Sanad RA, Abdel-Bar HM. Chitosan-hyaluronic acid composite sponge scaffold enriched with Andrographolide-loaded lipid nanoparticles for enhanced wound healing. Carbohydr Polym 2017; 173: 441–450. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y, Wang Z, Jiang Y, et al. Biomimetic composite scaffolds to manipulate stem cells for aiding rheumatoid arthritis management. Adv Funct Mater 2019; 29(30): 1807860. [Google Scholar]
- 24. Bai H, Zhao Y, Wang C, et al. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics 2020; 10(11): 4779–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenhalgh DG, Sprugel KH, Murray MJ, et al. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol 1990; 136(6): 1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 26. Deng ZX, Guo Y, Zhao X, et al. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host-guest interactions. Chem Mater 2018; 30(5): 1729–1742. [Google Scholar]
- 27. Chen Q, Zhu L, Chen H, et al. A novel design strategy for fully physically linked double network hydrogels with tough, fatigue resistant, and self-healing properties. Adv Funct Mater 2015; 25(10): 1598–1607. [Google Scholar]
- 28. Lu Z, Jiang X, Chen M, et al. An oxygen-releasing device to improve the survival of mesenchymal stem cells in tissue engineering. Biofabrication 2019; 11(4): 045012. [DOI] [PubMed] [Google Scholar]
- 29. Ding J, Ma Z, Shankowsky HA, et al. Deep dermal fibroblast profibrotic characteristics are enhanced by bone marrow-derived mesenchymal stem cells. Wound Repair Regen 2013; 21(3): 448–455. [DOI] [PubMed] [Google Scholar]
- 30. Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 2019; 13(9): 10279–10293. [DOI] [PubMed] [Google Scholar]
- 31. Chaudhuri O, Gu L, Klumpers D, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016; 15(3): 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells 2009; 27(3): 724–732. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Q, Nguyen AL, Shi S, et al. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev 2012; 21(6): 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tseng TC, Tao L, Hsieh FY, et al. An injectable, self-healing hydrogel to repair the central nervous system. Adv Mater 2015; 27(23): 3518–3524. [DOI] [PubMed] [Google Scholar]
- 35. Muzzarelli RAA. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohyd Polym 2009; 76(2): 167–182. [Google Scholar]
- 36. Xu HT, Ma L, Shi HF, et al. Chitosan-hyaluronic acid hybrid film as a novel wound dressing: in vitro and in vivo studies. Polym Advan Technol 2007; 18(11): 869–875. [Google Scholar]
- 37. Boateng JS, Matthews KH, Stevens HNE, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008; 97(8): 2892–2923. [DOI] [PubMed] [Google Scholar]
- 38. Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 2013; 70(12): 2059–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hosseinkhani H, Hosseinkhani M, Khademhosseini A, et al. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials 2006; 27(34): 5836–5844. [DOI] [PubMed] [Google Scholar]
- 40. Robert AW, Azevedo Gomes F, Rode MP, et al. The skin regeneration potential of a pro-angiogenic secretome from human skin-derived multipotent stromal cells. J Tissue Eng 2019; 10: 2041731419833391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan U, Bayat A. Microarchitectural analysis of decellularised unscarred and scarred dermis provides insight into the organisation and ultrastructure of the human skin with implications for future dermal substitute scaffold design. J Tissue Eng 2019; 10: 2041731419843710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao L, Niu L, Liang H, et al. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl Mater Inter 2017; 9(43): 37563–37574. [DOI] [PubMed] [Google Scholar]
- 43. Mei SHJ, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Resp Crit Care 2010; 182(8): 1047–1057. [DOI] [PubMed] [Google Scholar]
- 44. Emanuelli T, Burgeiro A, Carvalho E. Effects of insulin on the skin: possible healing benefits for diabetic foot ulcers. Arch Dermatol Res 2016; 308(10): 677–694. [DOI] [PubMed] [Google Scholar]
- 45. Azevedo F, Pessoa A, Moreira G, et al. Effect of topical insulin on second-degree burns in diabetic rats. Biol Res Nurs 2016; 18(2): 181–192. [DOI] [PubMed] [Google Scholar]
- 46. Jo SH, Ha KS, Moon KS, et al. Molecular weight dependent glucose lowering effect of low molecular weight Chitosan Oligosaccharide (GO2KA1) on postprandial blood glucose level in SD rats model. Int J Mol Sci 2013; 14(7): 14214–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu SH, Chang YH, Chiang MT. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. J Agric Food Chem 2010; 58(9): 5795–5800. [DOI] [PubMed] [Google Scholar]
- 48. Yang Y, Xia T, Chen F, et al. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol Pharm 2012; 9(1): 48–58. [DOI] [PubMed] [Google Scholar]
- 49. Ma K, Liao S, He L, et al. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng Pt A 2011; 17(9–10): 1413–1424. [DOI] [PubMed] [Google Scholar]
- 50. Mahdavian Delavary B, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology 2011; 216(7): 753–762. [DOI] [PubMed] [Google Scholar]
- 51. Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019; 8(8): 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ballotta V, Driessen-Mol A, Bouten CVC, et al. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials 2014; 35(18): 4919–4928. [DOI] [PubMed] [Google Scholar]
- 53. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3(1): 23–35. [DOI] [PubMed] [Google Scholar]
- 54. Liu H, Ding JX, Li C, et al. Hydrogel is superior to fibrin gel as matrix of stem cells in alleviating antigen-induced arthritis. Polymers 2016; 8(5): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu H, Ding JX, Wang CY, et al. Intra-articular transplantation of allogeneic BMMSCs rehabilitates cartilage injury of antigen-induced arthritis. Tissue Eng Pt A. 2015; 21(21–22): 2733–2743. [DOI] [PubMed] [Google Scholar]
- 56. Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008; 180(4): 2581–2587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supporting_Information for Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers by Haotian Bai, Noh Kyu-Cheol, Zhonghan Wang, Yutao Cui, He Liu, Hou Liu, Yubin Feng, Yue Zhao, Quan Lin and Zuhao Li in Journal of Tissue Engineering