Abstract
The aim of this study has been to evaluate the efficacy of the IL-5 receptor blocker benralizumab on chronic rhinosinusitis with nasal polyposis (CRSwNP), associated with severe eosinophilic allergic asthma. Ten patients with severe eosinophilic allergic asthma and CRSwNP were enrolled. Sino-nasal outcome test (SNOT-22), numerical rating scale (NRS), endoscopic nasal polyp score, Lund Mackey CT (computed tomography) score, and blood eosinophil count were measured at baseline and after 24 weeks of treatment with benralizumab. All the above clinical, endoscopic, imaging, and hematological parameters significantly improved after 24 weeks of treatment with benralizumab. In particular, SNOT-22 decreased from 61.10 ± 17.20 to 26.30 ± 19.74 (P < 0.001), NRS decreased from 7.20 ± 1.55 to 3.40 ± 2.22 (P < 0.001), the endoscopic polyp nasal score decreased from 4.20 ± 1.32 to 2.50 ± 1.78 (P < 0.001), the Lund-Mackay CT score decreased from 16.60 ± 5.50 to 6.90 ± 5.99 (P < 0.001), and blood eosinophil count decreased from 807.3 ± 271.1 cells/μL to 0 cells/μL (P < 0.0001). These results strongly suggest that benralizumab exerted a very effective therapeutic action on CRSwNP associated with severe asthma, thus improving nasal symptoms and decreasing polyp size.
Keywords: benralizumab, chronic rhinosinusitis, nasal endoscopy, nasal polyps, severe eosinophilic asthma, SNOT-22, type 2 inflammation
Introduction
Chronic rhinosinusitis (CRS) is a widespread inflammatory disease, localized at level of nose and paranasal sinuses, which affects approximately 12% of people living in the United States, 11% in Europe, 9%–10% in Asia, and 8.5% in Australia.1–4 When associated with nasal polyps (CRSwNP), this upper airway disorder is clinically characterized by nasal obstruction and secretions, smell loss, facial pain, and headache for more than 12 weeks.5,6 Subjective symptoms can be evaluated by a 22 item questionnaire named Sino-Nasal Outcome Test (SNOT-22).7 CRSwNP negatively impacts on health-related quality of life.8 Rhinorrea and nasal blockage also impair sleep quality, thus favoring daytime drowsiness, discomfort, and reduced personal performances with regard to work and school duties. Polyps protruding into nasal cavities can be visualized by rhinoscopy.9 Moreover, inside paranasal sinuses and ostiomeatal complex nasal polyps can cause mucosal abnormalities, which can be detected by computed tomography (CT) scan as partial or complete opacities; these CT changes can be quantified by the widely used Lund-Mackay score.10
CRSwNP is often sustained by a type 2 (T2) immune/inflammatory response, consisting of an eosinophilic infiltration induced by interleukin-5 (IL-5) released from T helper 2 (Th2) lymphocytes and group 2 innate lymphoid cells (ILC2).4,11,12 Such a T2-high inflammation can be associated with elevated levels of immunoglobulins E (IgE), a condition defined as atopy.13,14 In this regard, it is noteworthy that in atopic subjects CRSwNP often represents a relevant comorbidity of severe eosinophilic asthma.15 Indeed, IgE act as additional survival factors for eosinophils,16 even if eosinophilic inflammation does occur independently from atopic status. Asthma severity correlates with the degree of nasal obstruction, hyposmia, and sinus CT scan alterations.17 Furthermore, the presence of nasal polyps has a negative impact on asthma control.18
Standard treatments for CRSwNP include topical corticosteroids and nasal polyp surgery.5 However, many patients report relapsing symptoms and need several surgical polypectomies; therefore, severe CRSwNP is hardly controlled by common therapies.19 Similarly, severe asthma often results to be uncontrolled despite the use of high dosages of inhaled corticosteroids (ICS) and long-acting β2-adrenergic (LABA) bronchodilators.20 Hence, current GINA (Global Initiative for Asthma) guidelines recommend at step 5 the utilization of add-on biological drugs for severe asthma.21 The latter medications appear to be also quite promising for treatment of CRSwNP.22–25 In particular, highly suitable targets for biological therapies addressed against refractory type 2 inflammation underlying severe asthma and CRSwNP include IL-5 and its receptor.26–29 In fact, IL-5 is the key cytokine responsible for maturation, activation, recruitment, and survival of eosinophils.30 For instance, the humanized anti-IL-5 monoclonal antibody mepolizumab was shown to significantly improve SNOT-22 score, Lund-MacKay CT score and lung function in patients with severe eosinophilic asthma and nasal polyps.31 Endoscopic evaluation also documented that a significant reduction of nasal polyp size was induced by either mepolizumab or reslizumab,32,33 another humanized anti-IL-5 monoclonal antibody. Differently from mepolizumab and reslizumab which specifically interact with IL-5, the humanized monoclonal antibody benralizumab binds with its variable Fab fragments to the α subunit of IL-5 receptor (IL-5Rα),28,29 thus preventing IL-5Rα activation by IL-5 itself. Our group recently published a case report referring to an atopic patient complaining of severe eosinophilic asthma and CRSwNP, who experienced an effective therapeutic response to benralizumab in regard not only to asthma control and blood eosinophil count, but also with respect to subjective symptoms and endoscopic evidence of nasal polyposis.34
On the basis of the above considerations we decided to investigate within a real-life context, in a cohort of 10 allergic subjects with severe asthma and CRSwNP, the therapeutic effects of a 24-week treatment with benralizumab on nasal polyps, assessed as changes in symptom score, endoscopic evaluation, and CT scan features.
Patients and methods
Study design
In this observational study we consecutively enrolled all the atopic patients with severe persistent eosinophilic asthma and CRSwNP (five males and five females), referring to the Respiratory Unit and to the Otolaryngology Unit of “Magna Græcia” University Hospital located in Catanzaro, Italy. The diagnosis of CRSwNP was based on clinical criteria, nasal endoscopy and CT, as recommended by the European position paper on rhinosinusitis and nasal polyps.6 All patients met the ERS/ATS criteria that define severe uncontrolled asthma.20 With regard to asthma, the main characteristics of these patients are summarized in Table 1. All enrolled subjects had stopped regular therapy with oral corticosteroids (OCS) at least 2 months before starting treatment with benralizumab, and they had previously undergone surgical removal of nasal polyps, which however occurred more than 6 months before the first benralizumab injection. Benralizumab was prescribed according to the indications of the Italian Drug Agency, and it was administered subcutaneously at the dosage of 30 mg every 4 weeks for the first three doses, and every 8 weeks thereafter.
Table 1.
Baseline characteristics of asthma.
| FEV1 (% predicted) | 49.20 ± 18.93 |
| FEV1/FVC (%) | 54.95 ± 10.08 |
| Total serum IgE (IU/mL) | 494.5 ± 814.3 |
| Blood eosinophils (cells/μL) | 807.3 ± 271.1 |
| ACT score | 14.70 ± 2.111 |
| Daily ICS dosage | >1000 μg of beclomethasone or equivalent |
| LABA use (Yes/No) | 10/0 |
| LAMA use (Yes/No) | 10/0 |
| LTRA use (Yes/No) | 10/0 |
We exclusively used validated questionnaires, scales, and scores. Subjective symptoms and their impact on quality of life were evaluated by SNOT-22 questionnaire, even if this test also measures symptoms which are not cardinal for CRSwNP, such as sneezing, ocular, and otologic symptoms.7 Scores from 0 (non-perceived symptom) to 5 (worst possible perception of that symptom) were assigned for each item. Total score ranges from 0 to 110. A higher score is associated with worse outcomes. Subjective pain was measured by numerical rating scale (NRS).35 This outcome measure attributes an oscillating score ranging from 0 (“no problem”) to 10 (“worst possible”), and it was used in order to get a rapid and synthetic assessment of patient’s perception of the disease. Nasal endoscopies were performed using an Olympus ENF-GP fiber rhinolaryngoscope (Olympus Corp, Tokyo, Japan), and the endoscopic nasal polyp score was measured. This score is based on the size of the polyp and assigns a value from 0 to 3 for each nasal fossa (the score value is directly proportional to the severity of the condition): 0 = no polyps; 1 = small polyps in the middle meatus that do not reach the lower edge of the middle turbinate; 2 = polyps that reach the lower edge of the middle turbinate, but not the lower edge of the lower turbinate; 3 = large polyps that reach and pass the edge of the inferior turbinate.9 Facial massif CT was carried out by an expert radiologist in order to assess the Lund-Mackay CT Score.10 This score evaluates the opacification of the paranasal sinuses (frontal sinus, anterior ethmoidal cells, posterior ethmoidal cells, maxillary sinus, and sphenoid sinus) and the obstruction of the ostiomeatal complex. For each paranasal sinus, a score from 0 (absence of opacification) to 2 (complete opacification) can be chosen. The ostiomeatal complex is assigned a score of either 0 (not obstructed) or 2 (obstructed). Each side is ranked separately. The score ranges from 0 to 24 (a higher score expresses greater opacification). The Lund-Mackay score is usually not utilized as an outcome measure because it is not very sensitive to change. However, despite such a limitation we decided to use this score because it guarantees an objective assessment, which can integrate the diagnostic evaluation provided by rhinoscopy. We also evaluated the adverse events during the first 2 h after drug administration, as well as throughout a once-weekly telephone call, asking to patients if they had experienced headache, pharyngitis, anaphylaxis, and pyrexia, thus soliciting them to refer if any worse variation of their health status had occurred.
This observational investigation met the standards of Good Clinical Practice (GCP) and the principles of the Declaration of Helsinki. All recruited patients gave their written informed consent. Our study was also performed in accordance to what decided by the local Ethical Committee of Calabria Region (Catanzaro, Italy; document n. 66 – 23rd March 2020), which stated that “collection of data regarding observational studies referring to outpatients treated with drugs already approved by regulatory agencies, does not need approval.”
Patients
We included patients over the age of 18 years, who experienced at least two of the known CRSwNP symptoms (nasal obstruction, rhinorrhea, sense of pressure or facial pain, decreased sense of taste or smell) and had a total endoscopic score for nasal polyposis ⩾2, a blood eosinophil count of at least 300 cells/μL at baseline, and a severe persistent asthma characterized by poor symptom control despite high dosages of ICS-LABA combinations and long-acting muscarinic antagonists (LAMA).
Patients were excluded if they met one or more of the following criteria: treatment with either systemic corticosteroid therapy and/or biological drugs, as well as with immunosuppressive agents during the previous 2 months; surgical treatment and functional endoscopic surgery of the paranasal sinuses during the previous 6 months; more than three previous surgical treatments.
Endpoints and assessments
SNOT-22, NRS, endoscopic nasal polyp score, Lund-Mackay CT score, and peripheral blood eosinophils were measured at baseline and 24 weeks after the first injection of benralizumab.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis of the results was performed by Student’s t-test for paired data (SNOT-22, NRS, endoscopic nasal polyp score, Lund-Mackay CT score, peripheral blood eosinophil count). P values <0.05 were considered to be statistically significant. The analysis was performed using Prism Version 8.2.1 (GraphPad Software Inc., San Diego, California).
Results
The mean age of enrolled subjects was 56.40 ± 8.88 years, and the mean BMI was 24.63 ± 4.73 Kg/m2. Three patients were also affected by aspirin-exacerbated respiratory disease (AERD). After 24 weeks of add-on treatment with benralizumab, the SNOT-22 score significantly improved from 61.10 ± 17.20 to 26.30 ± 19.74 (P < 0.001) (Figure 1). When compared to the baseline measurement of 7.20 ± 1.55, NRS significantly decreased to 3.40 ± 2.22 at the 24th week (P < 0.001) (Figure 2). The onset of action of benralizumab was quite rapid, as inferred by both SNOT-22 and NRS scores, which already improved after the second benralizumab injection. These clinical results were associated with a significant improvement of the endoscopic polyp nasal score, which decreased to 2.50 ± 1.78 when compared to the baseline (pre-benralizumab) value of 4.20 ± 1.32 (P < 0.001) (Figure 3). The therapeutic effect of benralizumab was mainly evident at level of polyps present within anterior ethmoidal cells, which represented the most frequent polyp location detected in our patients. Moreover, the Lund-Mackay CT score sharply diminished, with respect to baseline, from 16.60 ± 5.50 to 6.90 ± 5.99 after 24 weeks (P < 0.001) (Figure 4). Such remarkable clinical and imaging improvements were associated with a drastic fall of blood eosinophil count, which dropped from 807.3 ± 271.1 cells/μL to 0 cells/μL after 24 weeks of treatment (P < 0.0001). Cytological sampling documented a sharp reduction of nasal eosinophilic infiltration after treatment with benralizumab (data not shown). With regard to the adverse events, benralizumab was characterized by a very satisfactory profile of safety and tolerability. Indeed, no severe adverse reaction was reported, and only two patients after the first injection experienced mild fever with chills, which rapidly and spontaneously resolved without any need for pharmacologic treatment.
Figure 1.
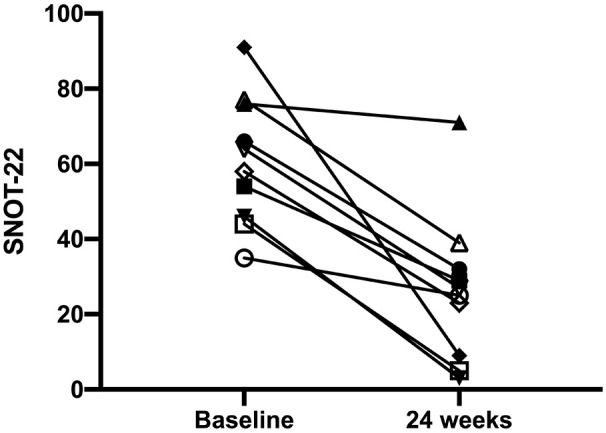
Effects of benralizumab on SNOT-22, evaluated at baseline (before starting anti-IL-5Rα therapy), and 24 weeks after the first drug injection: individual values.
Figure 2.
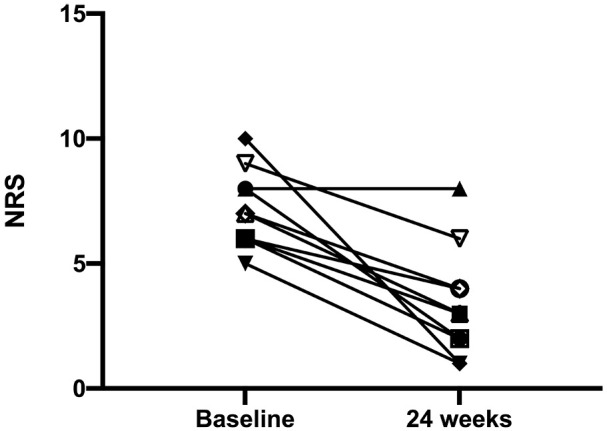
Effects of benralizumab on numerical rating scale (NRS), evaluated at baseline (before starting anti-IL-5Rα therapy), and 24 weeks after the first drug injection: individual values.
Figure 3.
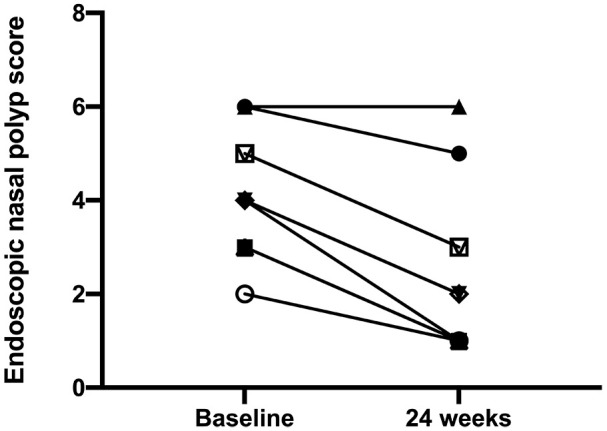
Effects of benralizumab on endoscopic nasal polyp score, evaluated at baseline (before starting anti-IL-5Rα therapy), and 24 weeks after the first drug injection: individual values.
Figure 4.
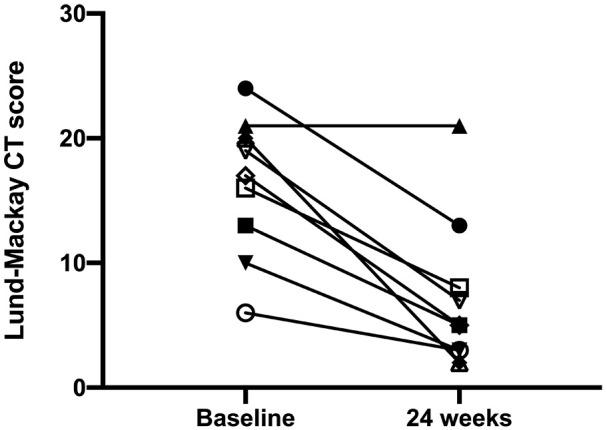
Effects of benralizumab on Lund-Mackay CT score, evaluated at baseline (before starting anti-IL-5Rα therapy), and 24 weeks after the first drug injection: individual values.
Discussion
The results of our real-life observational study show that a 24-week treatment with benralizumab, performed in 10 atopic patients with severe asthma and CRSwNP, induced a marked improvement of subjective nasal symptoms, documented by a significant reduction of both SNOT-22 and NRS scores. These important therapeutic effects of benralizumab were associated with a relevant decrease of nasal polyp size, expressed as a significant reduction of the endoscopic nasal polyp score. Moreover, benralizumab also reduced the opacification of paranasal sinuses, as demonstrated by a significant decrease of Lund-Mackay CT score. Similar therapeutic effects were described in some case reports recently published by us and other authors, showing that benralizumab decreased the size of nasal polyps visualized by rhinoscopy and eliminated the ethmoid sinus shadows detected by CT scan.34,36 Conversely, according to the results of a recent phase 2 randomized controlled trial, no significant difference was detected between treatments with benralizumab and placebo with regard to endoscopic nasal polyp score in patients with eosinophilic chronic rhinosinusitis, though this outcome measure improved in many enrolled subjects.37 However, the duration of this study, lasting only 12 weeks, was too short in order to obtain a convincing evidence about benralizumab inefficacy.
The favorable clinical and instrumental findings arising from our observational investigation were concomitant with a sharp fall of blood eosinophil counts. Eosinophil numbers in peripheral blood and nasal polyp tissue are often concordant, and their concordance is correlated with inadequate control of nasal polyposis.38 In light of the above considerations, the results of our present study are quite interesting because suggest that benralizumab made it possible to achieve the main aims of CRSwNP treatment, including persistent improvements of polyp mass, nasal blockage, rhinorrea, and smell loss. Indeed, amelioration of subjective symptoms is closely related to the attenuation of eosinophilic inflammation, as well as to the reduction of polyp volume and growth. In fact, nasal polyps are often characterized by an intense eosinophilic inflammation associated with high IL-5 expression and elevated IgE levels.39,40 It can thus be argued that in our atopic patients benralizumab was able to suppress IL-5-driven eosinophilia and the consequent development of nasal polyposis. In particular, benralizumab can inhibit IL-5-dependent eosinophilopoiesis which takes place not only in bone marrow but also in situ, for example, within the nasal polyp tissue. Indeed, CD34+ eosinophil progenitor cells obtained from human allergic nasal polyps rapidly differentiated into mature eosinophils when exposed to IL-5.41 Moreover, eosinophil progenitors have been found in both airways and sera of atopic patients.42,43 Inhibitory effects on nasal polyposis can be also exerted by anti-IL-5 monoclonal antibodies such as mepolizumab and reslizumab. SNOT-22 score was reported to be improved by both these drugs.31–33 Mepolizumab also improved nasal polyposis visual analog scale (VAS) score and endoscopic nasal polyp score, as well as significantly decreased the need for surgical treatment of nasal polyps.31,32
Taken together, the findings of our present study indicate that benralizumab had a very positive impact on nasal polyps in atopic patients with severe eosinophilic asthma. Since we have previously shown in these same subjects that benralizumab rapidly and effectively impacted on clinical control of their severe asthma, as well as on lung function and OCS intake,44 it appears that the add-on treatment with this biologic drug can induce an overall improvement in the quality of life of atopic patients with severe asthma and coexistent CRSwNP. On the other hand, in severe asthmatics nasal polyps represent a comorbid condition which can be considered as a very reliable predictor of a positive therapeutic response to benralizumab.45
Conclusion
In conclusion, our preliminary results suggest that benralizumab, similar to mepolizumab and reslizumab,31–33 as well as to the anti-IgE antibody omalizumab and to the dual IL-4/IL-13 receptor blocker dupilumab,46,47 can extend its therapeutic actions to both upper and lower airways, unitedly involved in the pathophysiology of allergic diseases. However, because the main limitation of the present study is the small size of our patient population, further investigations are needed to eventually corroborate and validate such early findings in larger numbers of atopic subjects. Another obvious limitation of our investigation, which is common to all real-life studies, concerns the lack of a randomization design and a placebo control. Therefore, in addition to being characterized by sufficient sample size and duration, hopefully future trials should be also randomized, placebo-controlled, and double-blinded. Such features will possibly allow to definitely confirm in allergic patients the efficacy of benralizumab as add-on biological therapy of CRSwNP associated with severe eosinophilic asthma.
Footnotes
Authorship contribution: All authors contributed to design and carry out the study protocol, as well as to write the text and draw the figures.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was waived by Ethical Committee of Calabria Region (Catanzaro, Italy) because “collection of data regarding retrospective observational studies, which refer to outpatients treated with drugs already approved by regulatory agencies, does not need approval by Ethical Committee”. Document n. 66 – March 23, 2020.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: Not applicable, because this study was not a randomized clinical trial, but rather a real-life, observational investigation.
ORCID iDs: Corrado Pelaia  https://orcid.org/0000-0002-4236-7367
https://orcid.org/0000-0002-4236-7367
Marcello Della Corte  https://orcid.org/0000-0003-3910-8679
https://orcid.org/0000-0003-3910-8679
Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
References
- 1. Schiller JSLJ, Peregoy JA. (2011) Summary health statistics for US adults: National health interview survey, 2011. Vital and Health Statistics 256: 1–218. [PubMed] [Google Scholar]
- 2. Hastan D, Fokkens WJ, Bachert C, et al. (2011) Chronic rhinosinusitis in Europe – an underestimated disease. A GALEN study. Allergy 66: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 3. Shi JB, Fu QL, Zhang H, et al. (2015) Epidemiology of chronic rhinosinusitis: Results from a cross-sectional survey in seven Chinese cities. Allergy 70: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahern S, Cervin A. (2019) Inflammation and endotyping in chronic rhinosinusitis. Medicina 55: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newton JR, Ah-See KW. (2008) A review of nasal polyps. Therapeutics and Clinical Risk Management 4: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fokkens WJ, Lund VJ, Mullol J, et al. (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012; 50: 1–12. [DOI] [PubMed] [Google Scholar]
- 7. Hopkins C, Gillett S, Slack R, et al. (2009) Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology 34: 447–454. [DOI] [PubMed] [Google Scholar]
- 8. Alobid I, Benitez P, Bernal-Sprekelsen M, et al. (2005) Nasal polyposis and its impact on quality of life: Comparison between the effects of medical and surgical treatments. Allergy 60: 452–458. [DOI] [PubMed] [Google Scholar]
- 9. Johansson L, Akerlund A, Holmberg K, et al. (2000) Evaluation of methods for endoscopic staging of nasal polyposis. Acta Oto-Laryngologica 120: 72–76. [DOI] [PubMed] [Google Scholar]
- 10. Lohiya SS, Patel SV, Pawde AM, et al. (2016) Comparative study of diagnostic nasal endoscopy and CT paranasal sinuses in diagnosing chronic rhinosinusitis. Indian Journal of Otolaryngology and Head & Neck Surgery 68: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryu G, Kim DW. (2020) Th2 inflammatory responses in the development of nasal polyps and chronic rhinosinusitis. Current Opinion in Allergy and Clinical Immunology 20: 1–8. [DOI] [PubMed] [Google Scholar]
- 12. Heffler E, Malvezzi L, Boita M, et al. (2018) Immunological mechanisms underlying chronic rhinosinusitis with nasal polyps. Expert Review of Clinical Immunology 14: 731–737. [DOI] [PubMed] [Google Scholar]
- 13. Hentges F, Leonard C, Arumugam K, et al. (2014) Immune responses to inhalant mammalian allergens. Frontiers in Immunology 5: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Froidure A, Mouthuy J, Durham SR, et al. (2016) Asthma phenotypes and IgE responses. European Respiratory Journal 47: 304–319. [DOI] [PubMed] [Google Scholar]
- 15. Heffler E, Blasi F, Latorre M, et al. (2019) The severe asthma network in Italy: Findings and perspectives. The Journal of Allergy and Clinical Immunology: In Practice 7: 1462–1468. [DOI] [PubMed] [Google Scholar]
- 16. Kim IS, Kim MJ, Kim DH, et al. (2013) Different anti-apoptotic effects of normal and asthmatic serum on normal eosinophil apoptosis depending on house dust mite-specific IgE. Molecular Biology Reports 40: 5875–5881. [DOI] [PubMed] [Google Scholar]
- 17. Matsuno O, Ono E, Takenaka R, et al. (2008) Asthma and sinusitis: Association and implication. International Archives of Allergy and Immunology 147: 52–58. [DOI] [PubMed] [Google Scholar]
- 18. Bilodeau L, Boulay ME, Prince P, et al. (2010) Comparative clinical and airway inflammatory features of asthma with or without nasal polyposis. Rhinology 48: 420–425. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Chacon M, Mullol J, Pujols L. (2015) Clinical and biological markers of difficult-to-treat severe chronic rhinosinusitis. Current Allergy and Asthma Reports 15: 19. [DOI] [PubMed] [Google Scholar]
- 20. Chung KF, Wenzel SE, Brozek JL, et al. (2014) International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. European Respiratory Journal 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 21. Global Initiative for Asthma (2020) Available at: http://ginasthma.org/ (accessed 5 June 2020).
- 22. Iqbal IZ, Kao SST, Ooi EH. (2020) The role of biologics in chronic rhinosinusitis: A systematic review. International Forum of Allergy & Rhinology 10: 165–174. [DOI] [PubMed] [Google Scholar]
- 23. Nasta MS, Chatzinakis VA, Georgialas CC. (2020) Updates on current evidence for biologics in chronic rhinosinusitis. Current Opinion in Otolaryngology & Head and Neck Surgery 28: 18–24. [DOI] [PubMed] [Google Scholar]
- 24. Passali D, Bellussi LM, Damiani V, et al. (2020) Chronic rhinosinusitis with nasal polyposis: The role of personalized and integrated medicine. Acta Biomed 91: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naclerio R, Baroody F, Bachert C, et al. (2020) Clinical research needs for the management of chronic rhinosinusitis with nasal polyps in the new era of biologics. A National Institute of Allergy and Infectious Diseases Workshop The Journal of Allergy and Clinical Immunology: In Practice 8: 1532–1549.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pelaia C, Vatrella A, Busceti MT, et al. (2017) Severe eosinophilic asthma: From the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Design, Development and Therapy 11: 3137–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelaia G, Vatrella A, Busceti MT, et al. (2016) Role of biologics in severe eosinophilic asthma - focus on reslizumab. Therapeutics and Clinical Risk Management 12: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pelaia C, Vatrella A, Bruni A, et al. (2018) Benralizumab in the treatment of severe asthma: Design, development and potential place in therapy. Drug Design, Development and Therapy 21: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pelaia C, Calabrese C, Vatrella A, et al. (2018) Benralizumab: From the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. BioMed Research International 2018: 4839230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pelaia C, Paoletti G, Puggioni F, et al. (2019) Interleukin-5 in the pathophysiology of severe asthma. Frontiers in Physiology 10: 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurosawa M, Ogawa K, Sutoh E. (2019) Favorable clinical efficacy of mepolizumab on the upper and lower airways in severe eosinophilic asthma: A 48-week pilot study. European Annals of Allergy and Clinical Immunology 51: 213–221. [DOI] [PubMed] [Google Scholar]
- 32. Bachert C, Sousa AR, Lund VJ, et al. (2017) Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. Journal of Allergy and Clinical Immunology 140: 1024–1031. [DOI] [PubMed] [Google Scholar]
- 33. Gevaert P, Lang-Loidolt D, Lackner A, et al. (2006) Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. Journal of Allergy and Clinical Immunology 118: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 34. Pelaia C, Busceti MT, Vatrella A, et al. (2020) Effects of the first three doses of benralizumab on symptom control, lung function, blood eosinophils, oral corticosteroid intake, and nasal polyps in a patient with severe allergic asthma. SAGE Open Medical Case Reports 8: 2050313X20906963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sedaghat AR, Hoehle LP, Gray ST. (2018) Chronic rhinosinusitis control from the patient and physician perspectives. Laryngoscope Investigative Otolaryngology 3: 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsurumaki H, Matsuyama T, Ezawa K, et al. (2019) Rapid effect of benralizumab for hyperosinophilia in a case of severe asthma with eosinophilic chronic rhinosinusitis. Medicina 55: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagase H, Ueki S, Fujieda S, et al. The roles of IL-5 and IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergology International 69: 178–186. [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Deng J, Yang M, et al. (2019) Concordant systemic and local eosinophilia relates to poorer disease control in patients with nasal polyps. World Allergy Organization Journal 12: 10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yacoub MR, Trimarchi T, Cremona G, et al. (2015) Are atopy and eosinophilic bronchial inflammation associated with relapsing forms of chronic rhinosinusitis with nasal polyps? Clinical and Molecular Allergy 13: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yilmaz I, Bahcecioglu SN, Turk M, et al. (2019) Last station in the eosinophilic asthma with chronic rhinosinusitis and/or nasal polyposis march: Eosinophilic asthma with radiologic findings associated with blood eosinophils. Journal of Asthma 56: 111–117. [DOI] [PubMed] [Google Scholar]
- 41. Cameron L, Christodoulopoulos P, Lavigne F, et al. (2000) Evidence for local eosinophil differentiation within allergic nasal mucosa: Inhibition with soluble IL-5 receptor. The Journal of Immunology 164: 1538–1545. [DOI] [PubMed] [Google Scholar]
- 42. Sehmi R, Howie K, Sutherland DR, et al. (1996) Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. American Journal of Respiratory Cell and Molecular Biology 15: 645–655. [DOI] [PubMed] [Google Scholar]
- 43. Robinson DS, Damia R, Zeibecoglou K, et al. (1999) CD34+/interleukin-5R α messenger RNA+ cells in the bronchial mucosa in asthma: Potential airway eosinophil progenitors. American Journal of Respiratory Cell and Molecular Biology 20: 9–13. [DOI] [PubMed] [Google Scholar]
- 44. Pelaia C, Busceti MT, Vatrella A, et al. (2019) Real-life rapidity of benralizumab effects in patients with severe allergic eosinophilic asthma: Assessment of blood eosinophils, symptom control, lung function and oral corticosteroid intake after the first drug dose. Pulmonary Pharmacology & Therapeutics 58: 101830. [DOI] [PubMed] [Google Scholar]
- 45. Bleecker ER, Wechsler ME, FitzGerald JM, et al. (2018) Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. European Respiratory Journal 52: 1800936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tiotiu A, Oster JP, Roux PR, et al. (2020) Effectiveness of omalizumab in severe allergic asthma and nasal polyposis: A real-life study. Journal of Investigational Allergology & Clinical Immunology 30: 49–57. [DOI] [PubMed] [Google Scholar]
- 47. Matsunaga K, Katoh N, Fujieda S, et al. (2020) Dupilumab: Basic aspects and applications to allergic diseases. Allergology International 69: 187–196. [DOI] [PubMed] [Google Scholar]


