Abstract
NLRP3 inflammasome activation results in severe liver inflammation and injury. Saikosaponin-d (SSd) possesses anti-inflammatory and hepatoprotective effects. This study aimed to determine the protective effects of SSd on carbon tetrachloride (CCl4)-induced acute liver injury in mice, and whether oxidative stress and NLRP3 inflammasome activation participate in the process.
The CCl4 mice model and controls were induced. The mice were treated with SSd at 1, 1.5, or 2.0 mg/kg in a total volume of 100 µl/25 g of body weight. Liver injury was assessed by histopathology. Oxidative stress was determined using mitochondrial superoxide production (MSP), malondialdehyde (MDA) content, and superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities. NLRP3, ASC, and Caspase 1 were determined by real-time PCR and western blot. IL-1β and IL-18 levels were determined by ELISA.
Significantly elevated oxidative stress was induced in the liver by CCl4, as demonstrated by histopathology and increases of MDA and MSP levels and decreases of SOD, GPx, and CAT activities (all P < 0.01). SSd significantly decreased the MDA and MSP levels and increased the activities of SOD, GPx, and CAT (all P < 0.05). The mRNA expression of NLRP3, ASC, and Caspase 1, and the protein expression of Caspase 1-p10, NLRP3, ASC, IL-1β, and IL-18 were significantly increased after CCl4 induction (all P < 0.01). These changes were reversed by SSd (all P < 0.05).
Suppression of the oxidative stress and NLRP3 inflammasome activation were involved in SSd-alleviated acute liver injury in CCl4-induced hepatitis.
Keywords: acute liver injury, carbon tetrachloride, mitochondrial reactive oxygen species, NLRP3 inflammasome, oxidative stress, saikosaponin-d
Introduction
Sustained and progressive hepatocyte impairment will lead to chronic inflammation, fibrosis, or even cirrhosis.1 Carbon tetrachloride (CCl4) is a classical hepatotoxic agent used for the induction of acute and chronic liver injury in animal models, attributed to the reactive oxygen species (ROS).2 The activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome plays an important role in the maturation of effector proinflammatory cytokines.3 NLRP3 inflammasome activation is involved in various liver diseases,4 and NLRP3 overexpression results in severe liver inflammation and fibrosis.3 Therefore, CCl4-induced ROS production will lead to inflammasome activation and liver damage. Modern medicine such as corticosteroids, colchicines, and vaccines have potentially serious adverse side-effects, limiting their clinical application. Therefore, traditional Chinese medicines should be evaluated because of their natural antioxidants that are related to hepatoprotective properties.5 Saikosaponin-d (SSd) is one of the major active pharmacological components of Bupleurum falcatum L. The effects of SSd include anti-inflammation, liver protection, anti-oxidation, anti-fibrosis, and anti-tumor.6–8 Little research has been done on the potential mechanisms of the anti-inflammatory and hepatoprotective effects of SSd, especially on CCl4-induced acute liver injury in mice. Therefore, this study aimed to determine the protective effects of SSd on CCl4-induced acute liver injury in mice, and whether oxidative stress and NLRP3 inflammasome activation participate in the process.
Materials and methods
Materials
Forty-nine male ICR mice (7–9 weeks old, 23–28 g) were obtained from Shanghai SLAC Laboratory Animal Co. (Shanghai, China). CCl4, mito-Tempo (APN17098-1-3), and SSd (purity: 97%) were purchased from Sinopharm (Beijing, China), Abcam (Cambridge, UK), and China’s Drug Supervision and Administration (Shanghai, China), respectively. MitoROS and TRIzol were obtained from Invitrogen (Carlsbad, CA, USA).
Levels of malondialdehyde (MDA) were measured using a kit from Nanjing Jiancheng Bioen-gineering Institute (Nanjing, China). The SOD, GPx, and CAT activities were determined using the kits from Winching (Nanjing, China). The mitochondria fractionation kit, BCA protein assay kit, and RIPA buffer were purchased from Beyotime (Jiangsu, China). The IL-1β and IL-18 ELISA kits were purchased from BD Biosciences (San Diego, USA). The primer sequences for NLRP3, ASC, and Caspase 1 were designed based on their cDNA sequences in GeneBank and synthesized by Shenggong Biotechnology Company (Shanghai, China). The PrimeScript RT Master Mix was from Clontech Laboratories (Mountain View, CA, USA). Primary antibodies against caspase 1 and β-actin were from Santa Cruz Biotechnology (Dallas, TX, USA) and Cell Signaling (Danvers, MA, USA), respectively. The secondary horseradish peroxidase-conjugated IgG was from Proteintech Group (Chicago, IL, USA). The nitrocellulose membranes and the enhanced chemiluminescence western blotting detection system were from Millipore (Billerica, MA, USA). The microscopes were from Olympus (Japan). 7170 automatic biochemistry analyzer was from Hitachi (Tokyo, Japan). The Var10skan Flash fluorescence plate reader and Nanodrop 2000 were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The StepOnePlus real-time PCR system was from Applied Biosystems (Foster City, CA, USA). The scientz-48 homogenizer was from XinZhi Biotechnology (Ningbo, China). The ImagePro Plus software was from Media Cybernetics (Rockville, MD, USA). The GeneTools software used to quantify the protein bands was from SynGene (Cambridge, UK), and the SPSS 21.0 software for statistical analyses was from IBM (Armonk, NY, USA).
Ethical statement
All experiments were performed in accordance with the experimental animal guidelines issued by the Animal Care and Use Committee at the Shanghai University of Traditional Chinese Medicine. This study was approved by the Ethics Committee of the Experimental Animals of Shanghai Municipal Hospital of Traditional Chinese Medicine.
Mouse model of CCl4-induced acute liver injury and SSd treatment
According to a previous study,9 the animals were randomly assigned to 7 groups (n = 7): control, 0.2 mg/kg SSd, CCL4, CCL4+1.0 mg/kg SSd, CCL4+1.5 mg/kg SSd, CCL4+2.0 mg/kg SSd, mito TEMPO . They were housed under controlled temperature and a natural day/night cycle and with free access to food and water.
After 1 week of adaptive feeding, the mice were administered a single intraperitoneal dose of 5% CCl4 at 0.25 ml/kg in a total volume of 100 µl/25 g of body weight, as previously described.10 Control mice were injected intraperitoneally with olive oil. Positive control mice were injected with mito-Tempo at 3 mg/kg.
SSd pretreatment was performed at 24 and 0.5 h before CCl4 injection. SSd was first dissolved in DMSO (20 mg/mL) and then diluted with normal saline. The SSd-treated mice were injected intraperitoneally with SSd solution at 1, 1.5, or 2.0 mg/kg in a total volume of 100 µl/25 g of body weight. The control and CCl4 groups received an identical volume of normal saline. After the intraperitoneal administration of CCl4 for 24 h, the mice were sacrificed by cervical spine dislocation. Liver tissues and blood samples were obtained.
Liver function and histological analysis
The left upper liver tissues were fixed in buffered 10% formalin and then processed for standard (4-µm) paraffin sections, stained with hematoxylin and eosin (H&E), and examined under a microscope to evaluate liver injury. Necrosis areas (%) were calculated using ImagePro Plus.
Serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH) were measured by routine laboratory methods using a biochemistry analyzer.
Malondialdehyde levels
Levels of MDA, an index of membrane lipid peroxidation, were determined,11 according to the manufacturer’s instruction. Liver tissues were homogenized (100 mg/ml) in 10 volumes of 1.15% KCl solution containing 0.85% NaCl and then centrifuged at 1500 ×g for 15 min. The homogenates (200 µl) were added to a reaction mixture consisting of 1.5 ml of 0.8% thiobarbituric acid, 200 µl of 8.1% sodium dodecyl sulfate, 1.5 ml of 20% acetic acid (adjusted to pH 3.5 with NaOH), and 600 µl of distilled H2O. The mixture was heated at 95°C for 40 min. After cooling to room temperature, the samples were cleared by centrifugation (10,000 ×g, 10 min) and the supernatant absorbance was measured at 532 nm, using 1,1,3,3-tetramethoxypropane as an external standard. The level of lipid peroxides was expressed as nmol MDA/mg protein. The protein quantification was completed by the Bradford assay.
SOD, CPx, and CAT activities
Liver tissues were homogenized in cold saline. Homogenates were centrifuged at 1000 ×g for 10 min. The SOD, GPx, and CAT activities in liver tissues were determined according to the kit manufacturer’s instructions, as described previously.11 The mean intra-assay coefficients of variation for SOD, GPx, and CAT were 1.7%, 3.6%, and 1.9%, respectively. The mean inter-assay coefficients of variation for SOD, GPx, and CAT were 3.5%, 6.8%, and 4.9%, respectively.
Measurement of mitochondrial superoxide production
The isolation of mitochondria was performed using a mitochondria fractionation kit, according to the manufacturer’s instructions. Mitochondrial superoxide production was measured by a fluorescence microplate reader following staining with the MitoROS Red mitochondrial superoxide indicator.9 Isolated mitochondria derived from fresh mouse liver were incubated in a final concentration of 5 µM MitoROS dissolved in DMSO at 37°C in the dark for 30 min. Red MitoROS fluorescence was read at 510 nm excitation and 580 nm emission using a Var10skan Flash fluorescence plate reader. The cells were also photographed using a fluorescence microscope. Mitochondrial protein concentrations were assessed using a BCA Protein Assay Kit.
Real-time RT-PCR
Total RNA from liver tissues was extracted by TRIzol. RNA purity was determined using absorbance at 260 and 280 nm (A260/280) using a Thermo Nanodrop 2000. RNA (4 µg) was reverse transcribed to cDNAs using PrimeScript RT Master Mix, according to the manufacturer’s instruction. To determine the mRNA level of each gene, real-time PCR was performed using the SYBR Green Master Mix in a StepOnePlus real-time PCR system for 45 cycles of denaturation at 95°C for 20 s, annealing at 60°C for 20 s, and extension at 72°C for 20 s. Reaction was performed in duplicate for each sample. β-actin was used as an internal control. The expression levels of NLRP3, ASC, and Caspase 1 were calculated using the 2–ΔΔCt method. The primer sequences for NLRP3, ASC, and Caspase 1 were: NLRP3 forward 5′-ACC AGC CAG AGT GGA ATG AC-3′ and reverse 5′-ATG GAG ATG CGG GAG AGA TA-3′; ASC forward 5′-CTT GTC AGG GGA TGA ACT CAA AA-3′ and reverse 5′-GCC ATA CGA CTC CAG ATA GTA GC-3′; Caspase 1 forward 5′-GTA TTC referACG CCC TGT TGG A-3′ and reverse 5′-GCT TCC TCT TTG CCC TCA-3′; and β-actin forward 5′-CTG TAT GCC TCT GGT CGT AC-3′ and reverse 5′-TGA TGT CAC GCA CGA TTT CC-3′.
Western blot
Liver tissues were harvested and homogenized in cold RIPA buffer automatically. The lysates were centrifuged at 12,000 ×g for 15 min at 4°C. Protein concentrations in the supernatants were assessed using a BCA Protein Assay Kit. Proteins (50 µg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% skimmed milk powder (wt/vol) for 2 h at room temperature. Immunoblots were incubated with primary antibodies against caspase 1 (1:500) and β-actin (1:1000) at 4°C overnight. After three washes with TBST, the membranes were incubated with a secondary horseradish peroxidase-conjugated IgG (1:2000) for 1 h at room temperature and washed for 30 min with TBST. Immunoreactive proteins were visualized using the enhanced chemiluminescence western blotting detection system. The signal from the membranes was quantified by a GeneGnome HR scanner using the GeneTools software. The expression levels were normalized to β-actin.
ELISA
Hepatic tissue homogenates were collected and centrifuged at 1000 ×g for 10 min. The levels of IL-1β and IL-18 in liver tissues were determined using ELISA kits according to the manufacturer’s protocol.
Statistical analysis
All statistical analyses were conducted using SPSS 21.0. Data were expressed as means ± standard errors of the mean (SEM). Statistical significance was estimated by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls post hoc test. Two-sided P-values <0.05 were considered statistically significant.
Results
SSd alleviates CCl4-induced acute liver injury
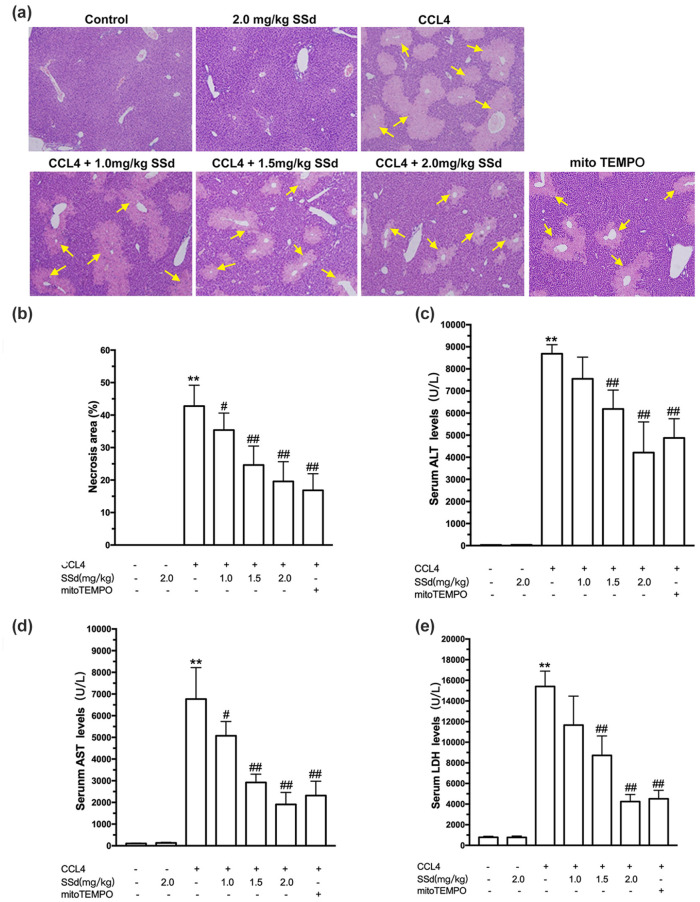
As shown in Figure 1(a), histologically, an increase in damaged hepatocytes at the centrilobular zones and influx of inflammatory cells were observed in the CCl4 group, while the control group showed normal hepatic tissue without massive cell necrosis and loss of hepatocyte architecture around the blood vessels. The CCl4 mice showed an increased necrosis area compared with control mice (P < 0.01). The mice treated with SSd showed alleviated CCl4-induced liver injury and necrosis at concentrations from 1 to 2 mg/kg (all P < 0.05), suggesting a protective effect of SSd on the liver (Figure 1(b)). The positive controls treated with mito-Tempo showed alleviated CCl4-induced damage consistent with the high-dose SSd group.
Figure 1.
Effects of saikosaponin-d (SSd) or mito-Tempo (positive control) on CCl4-induced histopathological damage and hepatic dysfunction. Mice were injected intraperitoneally with the indicated concentrations of SSd or mito-Tempo at 24 and 0.5 h before CCl4 injection. Livers and serums were harvested after intraperitoneal administration of CCl4 for 24 h. (a) Representative micrographs of liver sections stained by hematoxylin and eosin (magnification ×100). The yellow areas indicate the necrotic area (arrows). (b) Necrosis areas (%) were calculated by ImagePro Plus. Serum levels of alanine transaminase (ALT) (c), aspartate transaminase (AST) (d), and lactate dehydrogenase (LDH) (e) were measured by routine laboratory methods. Data are expressed as means ± standard error of mean (SEM) (n = 7/group). **P < 0.01 versus the control group. #P < 0.05, ##P < 0.01 versus the CCl4 group.
Serum levels of ALT (Figure 1(c)), AST (Figure 1(d)), and LDH (Figure 1(e)) were significantly increased in CCl4-induced acute liver injury (all P < 0.01). Liver function was improved by SSd treatment in a dose-dependent manner, as evidenced by the decreases of ALT (1.5 and 2.0 mg/kg, P < 0.01), AST (all doses, P < 0.05), and LDH levels (1.5 and 2.0 mg/kg, P < 0.01). Mito-Tempo had similar effects to those of 2.0 mg/kg SSd.
SSd improves CCl4-induced oxidative stress in the liver
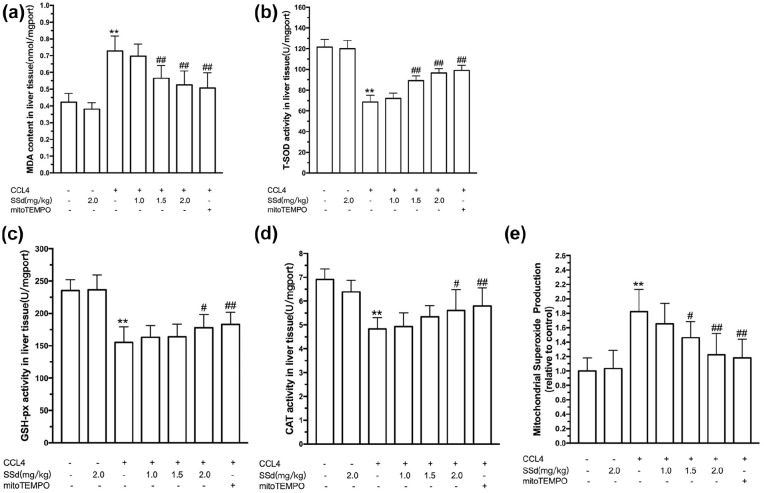
CCl4-induced live injury is often defined as an oxidative hepatocytes damage, which can be assessed using MDA, activities of SOD, GPx, and CAT, and levels of mitochondrial ROS. As shown in Figure 2(a–e), a significantly elevated oxidative stress in the liver was induced by intraperitoneal administration of CCl4, as demonstrated by increases of MDA and MSP levels and decreases of SOD, GPx, and CAT activities (P < 0.01). SSd alleviated CCl4-induced oxidative stress in the liver. SSd significantly decreased the MDA (1.5 and 2.0 mg/kg, P < 0.01) and MSP (1.5 and 2.0 mg/kg, P < 0.05) levels and increased the activities of SOD (1.5 and 2.0 mg/kg, P < 0.01), GPx (2.0 mg/kg, P < 0.05), and CAT (2.0 mg/kg, P < 0.05). The positive control mito-Tempo had effects similar to those of 2.0 mg/kg SSd.
Figure 2.
Effect of saikosaponin-d (SSd) or mito-Tempo (positive control) on CCl4-induced oxidative stress in the liver. Mice were injected intraperitoneally with the indicated concentrations of SSd or mito-Tempo at 24 and 0.5 h before CCl4 injection. Livers and serums were harvested after intraperitoneal administration of CCl4 for 24 h. Liver malondialdehyde (MDA) level (a), superoxide dismutase (SOD) activity (b), glutathione peroxidase (GPx) activity (c), and catalase (CAT) activity (d). Mitochondria were isolated from the liver and used for the determination of mitochondrial superoxide production (MSP) levels (e). Data are expressed as means ± SEM (n = 7/group). **P < 0.01 versus the control group. #P < 0.05, ##P < 0.01 versus the CCl4 group.
SSd alleviated CCl4-induced activation of the NLRP3 inflammasome in the liver
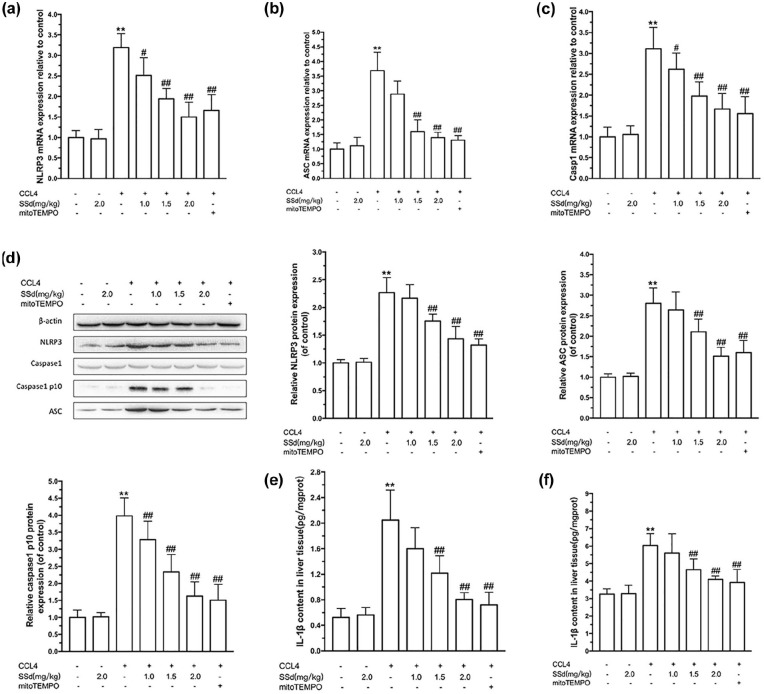
The activation of the NLRP3 inflammasome plays an important role in the pathogenesis of acute liver injury. ASC and Caspase 1 play roles in inflammation and response to oxidative stress. IL-1β and IL-18 are pro-inflammatory cytokines. As shown in Figure 3, the mRNA expression of NLRP3 (Figure 3(a)), ASC (Figure 3(b)), and Caspase 1 (Figure 3(c)), together with the protein expression of Caspase 1-p10 (Figure 3(d)), IL-1β (Figure 3(e)), and IL-18 (Figure 3(f)) were significantly increased after CCl4 induction (all P < 0.01). The protein expression of pro-Caspase 1 remained unchanged.
Figure 3.
Effects of saikosaponin-d (SSd) or mito-Tempo (positive control) on CCl4-induced NLRP3 inflammasome activation in the liver. Mice were injected intraperitoneally with the indicated concentrations of SSd or mito-Tempo at 24 and 0.5 h before CCl4 injection. Livers and serums were harvested after intraperitoneal administration of CCl4 for 24 h. The mRNA expressions of NLRP3 (a), ASC (b), and Caspase 1 (c) were determined by real-time RT-PCR. (d) The protein expressions of NLRP3, ASC, Caspase 1 p10, and pro-Caspase 1 were determined by western bolt. Data are expressed as means ± SEM (n = 7/group). The content of IL1β (e) and IL-18 (f) were examined by ELISA. Data are expressed as means ± SEM (n = 6/group). **P < 0.01 versus the control group. #P < 0.05, ##P < 0.01 versus the CCl4 group.
SSd treatment decreased CCl4-induced NLRP3 (1.0–2.0 mg/kg, P < 0.05) (Figure 3(a)), ASC expression (1.5 and 2.0 mg/kg, P < 0.01) (Figure 3(b)), and Caspase 1 (1.0–2.0 mg/kg, P < 0.05) (Figure 3(c)) in the liver. Protein levels of ASC, Caspase 1-p10, Caspase 1, and NLRP3 showed the same trends as the mRNA levels (Figure 3(d)). SSd also decreased the IL-1β (1.5 and 2.0 mg/kg, P < 0.01) (Figure 3(e)) and IL-18 (1.5 and 2.0 mg/kg, P < 0.01) (Figure 3(f)) levels in the liver tissue. Again, the positive control mito-Tempo had effects similar to those of 2.0 mg/kg SSd, supporting the beneficial effects of SSd on CCl4-induced hepatic injury.
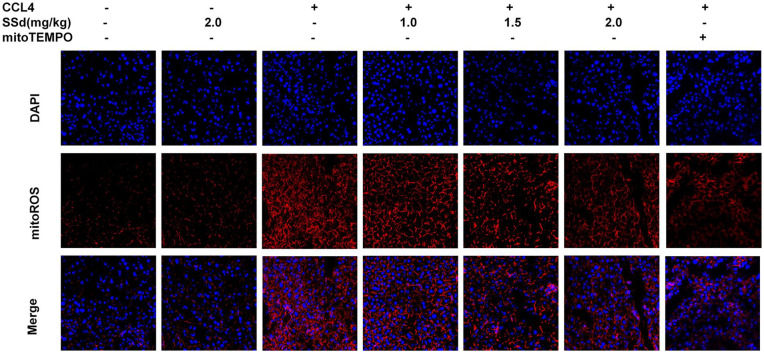
SSd alleviated mitochondrial ROS production
Figure 4 shows that the hepatocytes from mice treated with CCl4 showed higher levels of mitochondrial ROS production. This production was decreased in a dose-dependent manner by SSd, as well as by the mito-Tempo positive control.
Figure 4.
Measurement of mitochondrial superoxide production using fluorescence microscopy after treatment with saikosaponin-d (SSd) or mito-Tempo (positive control) on CCl4-induced liver damage in mice. Mice were injected intraperitoneally with the indicated concentrations of SSd or mito-Tempo at 24 and 0.5 h before CCl4 injection. Livers and serums were harvested after intraperitoneal administration of CCl4 for 24 h. Fluorescence microscopy of hepatocytes stained with 5 µM MitoROS and DAPI. MitoROS specifically stain the production of mitochondrial reactive oxygen species.
Discussion
NLRP3 inflammasome activation results in severe liver inflammation and injury.12 SSd possesses anti-inflammation and hepatoprotective effects.6,7 Nevertheless, the effect of SSd on NLRP3 is poorly known. Therefore, this study aimed to determine the protective effects of SSd on CCl4-induced acute liver injury in mice, and whether oxidative stress and NLRP3 inflammasome activation participate in the process. The results suggest that SSd could alleviate CCl4-induced acute liver injury through suppression of the oxidative stress and NLRP3 inflammasome activation.
SSd has been found to alleviate CCl4-induced hepatocyte injury by inhibiting lipid peroxidation.7 Nevertheless, the downstream molecular mechanism is still mostly unknown. In the present study, the mice treated with SSd showed extensively alleviated CCl4-induced liver injury and necrosis, and improved liver function at concentrations from 1 to 2 mg/kg, suggesting a protective effect of SSd on the liver. Although it was already known that SSd has hepatoprotective effects,6–8 some studies also suggested that it could promote liver damage. Indeed, Chen et al.13 indicated that SSd induced hepatotoxicity via stimulating mitochondrial apoptosis by interrupting the PDGF-βR/p38 pathway in LO2 hepatocytes. Moreover, Zhang et al.14 showed that a 1-week treatment with SSd at 300 mg/kg induced hepatocyte apoptotic death in mice. Nevertheless, it seems that only a large dose of SSd will lead to the induction of hepatotoxicity, while exhibiting a significant hepatoprotective activity at lower concentrations. As previously reported, SSd at 2 mg/kg protects mice from APAP-induced hepatotoxicity mainly through down-regulating NF-κB- and STAT3-mediated inflammatory signaling.6 Meanwhile, the maximum concen-tration of SSd (2 mg/kg) used in the present experiment did not exert any hepatotoxic reaction.
Oxidative stress is regarded as a critical factor in causing a variety of liver diseases.12,15 CCl4, a well-known hepatotoxin, is commonly used in murine models to investigate acute or chronic liver injury.16 This type of liver injury is characterized by typical centrilobular necrosis and remarkable increase of serum transaminases in the presence of increased lipid peroxidation and impairment of antioxidant defense.16 The degradation of cellular membrane is accelerated in the presence of redundant free radicals, and subsequently lead to the breakdown of cell integrity and the release of ALT, AST, and LDH into blood circulation. In the present study, hepatic oxidative stress induced by CCl4 was monitored using MDA, SOD, GPx, and CAT. The increase of MDA, an end product of lipid peroxidation, has been accepted as a key biomarker in liver injury.17 SOD, GPx, and CAT are important antioxidant enzymes that can block the chain of lipid peroxidation.18 The present study showed a significantly elevated oxidative stress in CCl4-induced acute liver injury. Furthermore, a severe increase of damaged hepatocytes at the centrilobular zones and the serum level of ALT, AST, and LDH were observed, as supported by previous studies.19 Importantly, all these detrimental changes were alleviated by SSd in a dose-dependent manner, as supported by previous studies.7,8,14
The activation of NLRP3 inflammasome leads to the maturation of effector proinflammatory cytokines such as pro-IL-1β and pro-IL-18.3 IL-1β and IL-18 play important roles in the progression of fulminant liver failure and are useful indicators of hepatic injury.19 The activation of NLRP3 inflammasome can be observed in various liver diseases, such as NASH, ASH, hepatic I/R and drug-induced liver injury,20–23 and is required for the development of hepatic fibrosis.24 Contradictorily, by using a NLRP3-/- mice model, DeSantis et al.24 showed that the NLRP3-/- mouse had more severe liver injury with higher serum ALT levels, increased activation of IL-18, and reduced activation of IL-1β, suggesting a protective role of NLRP3 inflammasome during alcohol-induced liver injury. The possible explanation for the discrepancy is due to different pathogenic mechanism. Indeed, the NLRP3 inflammasome impacts microbiota in the gut, which may in turn modulate the development of alcohol-induced liver injury. NLRP3-/- mice may have incremental leakage of LPS from the gut, probably attributed to altered microbiota that would impact the degree of liver injury. In the present study, NLRP3 inflammasome activation was a promoting factor in CCl4-induced acute liver injury, which was in accordance with some other models of acute liver injury.12 SSd attenuated the activation of the NLRP3 inflammasome.
It has been reported that ROS are involved in the regulation of NLRP3 inflammasome activation, and scavenging of ROS can block its activation in response to a wide variety of stimuli.12 Recently, it has been demonstrated in a variety of animal and cell models,25–27 that mitochondrial ROS is the key upstream factor in the activation of NLRP3 inflammasome.28 Thus, it could be hypothesized that mtROS-induced NLRP3 inflammasome activation may be playing an important role in CCl4-induced acute liver injury. The anti-oxidative effects of SSd are supported by similar effects being observed with mito-Tempo, a potent anti-oxidative compound that protects hepatocytes against mitochondrial ROS overproduction.29
In conclusion, the hepatoprotective and anti-inflammatory effects of SSd may be attributed to the suppression of the NLRP3 inflammasome activation and ROS production, but the present study was not designed to examine whether the decreased ROS production was a cause or a consequence of suppressed inflammasome. Furthermore, we also did not detect pyroptosis caused by NLRP3 inflammasome activation in CCl4-induced acute liver injury, and whether the hepatoprotective effect of SSd was related to inhibition of hepatocytes pyroptosis was remained to demonstrate. The present results provide new evidence for using SSd and to establish an experimental basis for the development of new drugs for the treatment of acute liver injury. In addition, our previous study reported that SSd was a new kind of phytoestrogen, which could suppress the proliferation and activation of hepatic stellate cells through modulation of estrogen receptor β.30,31 Therefore further studies are still needed to explore the specific action mechanisms of SSd.
Conclusion
Suppression of the oxidative stress and NLRP3 inflammasome activation were involved in SSd-alleviated acute liver injury in CCl4-induced hepatitis.
Footnotes
Animal welfare: The present study followed the experimental animal guidelines issued by the Animal Care and Use Committee at the Shanghai University of Traditional Chinese Medicine.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from the Ethics Committee of the Experimental Animals of Shanghai Municipal Hospital of Traditional Chinese Medicine.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (No.81573775, 81873157), the Natural Science Foundation of Shanghai (No.09ZR1429700), and the Outstanding Academic Leader of Health System of Shanghai (No.XBR2013120). Youth Talent Training Plan of Shanghai Municipal Hospital of Traditional Chinese Medicine (No.2019PY003). The budgetary Foundation of Shanghai University of Traditional Chinese Medicine (No. 18LK074), and Shanghai Sailing Program (No. 19YF1445200).
ORCID iD: Yong Li  https://orcid.org/0000-0001-7971-4871
https://orcid.org/0000-0001-7971-4871
References
- 1. Lee WM. (2012) Acute liver failure. Seminars in Respiratory and Critical Care Medicine 33: 36–45. [DOI] [PubMed] [Google Scholar]
- 2. Hayashi S, Itoh A, Isoda K, et al. (2008) Fucoidan partly prevents CCl4-induced liver fibrosis. European Journal of Pharmacology 580: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wree A, Eguchi A, McGeough MD, et al. (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrasek J, Bala S, Csak T, et al. (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. Journal of Clinical Investigation 122: 3476–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fakurazi S, Sharifudin SA, Arulselvan P. (2012) Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules 17: 8334–8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding Z, Liu S, Wang X, et al. (2014) LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: Implications in atherogenesis. Cardiovascular Research 103: 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan J, Li X, Li P, et al. (2007) Saikosaponin-d attenuates the development of liver fibrosis by preventing hepatocyte injury. Biochemistry and Cell Biology 85: 189–195. [DOI] [PubMed] [Google Scholar]
- 8. Wang BF, Lin S, Bai MH, et al. (2014) Effects of SSd combined with radiation on inhibiting SMMC-7721 hepatoma cell growth. Medical Science Monitor 20: 1340–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang CN, Duan GL, Liu YJ, et al. (2015) Overproduction of nitric oxide by endothelial cells and macrophages contributes to mitochondrial oxidative stress in adrenocortical cells and adrenal insufficiency during endotoxemia. Free Radical Biology and Medicine 83: 31–40. [DOI] [PubMed] [Google Scholar]
- 10. Zhang DG, Zhang C, Wang JX, et al. (2017) Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicology and Applied Pharmacology 314: 39–47. [DOI] [PubMed] [Google Scholar]
- 11. Zhang YQ, Liu YJ, Mao YF, et al. (2015) Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-beta1 signaling. Clinical Nutrition 34: 752–760. [DOI] [PubMed] [Google Scholar]
- 12. Kim HY, Kim SJ, Lee SM. (2015) Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS Journal 282: 259–270. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Zhang F, Kong D, et al. (2013) Saikosaponin D disrupts platelet-derived growth factor-beta receptor/p38 pathway leading to mitochondrial apoptosis in human LO2 hepatocyte cells: A potential mechanism of hepatotoxicity. Chemico-Biological Interactions 206: 76–82. [DOI] [PubMed] [Google Scholar]
- 14. Zhang F, Chen L, Jin H, et al. (2016) Activation of Fas death receptor pathway and Bid in hepatocytes is involved in saikosaponin D induction of hepatotoxicity. Environmental Toxicology and Pharmacology 41: 8–13. [DOI] [PubMed] [Google Scholar]
- 15. Zhang JQ, Shi L, Xu XN, et al. (2014) Therapeutic detoxification of quercetin against carbon tetrachloride-induced acute liver injury in mice and its mechanism. Journal of Zhejiang University SCIENCE B 15:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uehara T, Pogribny IP, Rusyn I. (2014) The DEN and CCl4 -induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Current Protocols in Pharmacology 66: 14.30.1–14.30.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mateos R, Lecumberri E, Ramos S, et al. (2005) Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. Journal of Chromatography B 827:76–82. [DOI] [PubMed] [Google Scholar]
- 18. Zelen I, Djurdjevic P, Popovic S, et al. (2010) Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology 15: 330–336. [PubMed] [Google Scholar]
- 19. Domitrovic R, Jakovac H, Blagojevic G. (2011) Hepatoprotective activity of berberine is mediated by inhibition of TNF-alpha, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology 280: 33–43. [DOI] [PubMed] [Google Scholar]
- 20. Vandanmagsar B, Youm YH, Ravussin A, et al. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine 17: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Csak T, Ganz M, Pespisa J, et al. (2011) Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu P, Duan L, Chen J, et al. (2011) Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Human Gene Therapy 22: 853–864. [DOI] [PubMed] [Google Scholar]
- 23. Williams CD, Antoine DJ, Shaw PJ, et al. (2011) Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicology and Applied Pharmacology 252: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu X, Zhang F, Xiong X, et al. (2015) Tetrameth-ylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway. IUBMB Life 67: 312–321. [DOI] [PubMed] [Google Scholar]
- 25. Guo W, Liu W, Jin B, et al. (2015) Asiatic acid ameliorates dextran sulfate sodium-induced murine experimental colitis via suppressing mitochondria-mediated NLRP3 inflammasome activation. International Immunopharmacology 24: 232–238. [DOI] [PubMed] [Google Scholar]
- 26. Liu D, Xu M, Ding LH, et al. (2014) Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: A novel mechanism of albumin-induced tubulointerstitial inflammation. The International Journal of Biochemistry & Cell Biology 57: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SR, Kim DI, Kim SH, et al. (2014) NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death & Disease 5: e1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou R, Yazdi AS, Menu P, et al. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225. [DOI] [PubMed] [Google Scholar]
- 29. Du K, Farhood A, Jaeschke H. (2017) Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Archives of Toxicology 91: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang P, Ren J, Tang J, et al. (2010) Estrogen-like activities of saikosaponin-d in vitro: A pilot study. European Journal of Pharmacology 626: 159–165. [DOI] [PubMed] [Google Scholar]
- 31. Que RY, Shen YT, Ren JL, et al. (2018) Estrogen receptor-β-dependent effects of saikosaponin-d on the suppression of oxidative stress-induced rat hepatic stellate cell activation. International Journal of Molecular Medicine 41: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]