Abstract
Introduction
Musculoskeletal disorders may cause chronic pain, which is associated with deterioration in physical well-being, functions, and quality of life. There are worldwide shortfalls in the care that is provided to the affected patients. Holistic, interdisciplinary care is rare. Monomodal therapeutic approaches dominate when health-care resources are scarce. In this study, we test the patient-relevant outcomes of multimodal treatment for rheumatic diseases that are associated with pain and check for remuneration.
Methods
We performed a retrospective data analysis of an inpatient multimodal treatment. The target parameter was the patient perspective, which we assessed by means of Patient-Reported Outcomes (PRO). We applied the Visual Analogue Scale (mental and physical condition), the Heidelberg Short Early Risk Assessment Questionnaire, the Pain Disability Index, and the pain grading according to Kohlmann/Raspe (N = 375 patients). We also investigated compensation for inpatient treatments with and without multimodal treatments. Moreover, we compared Diagnosis-Related Group remuneration with and without complex treatment.
Results
After implementing a multimodal treatment, improved mental (mood) status was significantly better (Wilcoxon signed-rank test, P < . 001), despite high levels of pain (Kohlmann/Raspe) reported on admission. Apart from the underlying rheumatic disease, 111 patients also reported chronic back pain, which was improved following the treatment (t test, P < . 001). Subjective impairments associated with pain were significantly lower at the end of the hospital stay (Wilcoxon signed-rank test, P < . 001). Compensation for inpatient treatments with multimodal treatments increased noticeably in German hospitals in 2016 to 2019, while remunerations for monomodal treatments show mixed results.
Conclusion
PROs regarding mood, pain, and perceived impairments improved following the multimodal complex treatment. Compensation of hospitals should take into account additional performance requirements of holistic treatments, whereby the promotion and further studies of PROs are recommended.
Keywords: pain, patient satisfaction, holistic care, health policy, multimodal, patient-reported outcome
Background
Chronic pain is a multidimensional problem worldwide. Pain impairs the quality of life of the affected patients who are then looking for treatments to alleviate their symptoms. Hospitals, outpatient care providers, and the pharmaceutical industry seek to develop therapies, while cost bearers aim to save costs and reduce treatment offers. Often, health policy makers create inflexible compensation systems that offer little performance incentive to providers. At the same time, due to pressure from cost bearers, policy makers create barriers to innovative treatment concepts.
In Germany, the care of patients with complex chronic pain disorders has been inadequate for many years, both in outpatient and inpatient settings.1 Surgical interventions also dominate the international health-care services, though multimodal conservative treatments have yielded good results.2 A study on the care situation of pain patients in 15 European countries draws attention to patient dissatisfaction with regular monomodal treatments.3 The number of people with chronic pain is constantly rising and, given the current state of affairs, the condition is likely to affect well over 23 million people in Germany alone.4 Considering this number together with the discussion of effectiveness of multimodal treatments, an evaluation of these is required. This study aims to help close this research gap by analyzing patient-reported outcomes (PROs) of multimodal conservative treatments for rheumatic diagnoses.
Rheumatic diseases are of particular importance due to their increasing prevalence.5 The number of people affected by osteoporosis in the European Union is estimated at 22 million women and 5.5 million men.6 According to the German Society for Rheumatology, approximately 1.5 million people in Germany suffer from inflammatory rheumatic diseases, and 3.5% of the population suffers from fibromyalgia (chronic musculoskeletal pain over the entire body).7 More than 200 rheumatic and musculoskeletal disorders have been classified so far,8 which often occur in a combined fashion or together with other (chronic) illnesses, calling for targeted and comprehensive treatments. What is required is holistic and patient-centered care,9 which is geared toward the individual and his or her complaints.10 In addition to effectively treating the ailments, this can contribute to increasing patient satisfaction.11 Health policy makers should, therefore, aim to develop adequate care concepts that take full account of the needs of pain patients and also financially cover the necessary resources for service providers.
Multimodal treatments, aimed at effective and efficient care for patients with acutely exacerbated chronic pain, are among the range of available options12 and are also reported to increase patient satisfaction.11 Four medical conditions that are treated with multimodal complex treatment are presented below. They were selected because they are associated with pain, impaired physical functions, and worsening quality of life, a combination that covers all aspects requiring complex treatment.
Medical Conditions Associated With Pain
Rheumatoid Arthritis
Rheumatoid arthritis (RA) can be diagnosed based on the presence of a swollen joint, if other potential causes have been ruled out. The type and number of painful and/or swollen joints also play a role. To establish a diagnosis, specific laboratory tests (rheumatoid factor, anti citrullinated protein antibodies [ACPA], and C-reactive protein [CRP]) are also performed, and the duration of symptoms is taken into account.13 RA is often accompanied by depression and anxiety,14,15 especially in cases of persistent pain, impaired physical functions, and inadequate treatment results. Treating depression in patients with RA should, therefore, also be considered in the context of a multimodal treatment.16
Polymyalgia Rheumatica
Polymyalgia rheumatic (PMR) is an inflammatory rheumatic disease that affects primarily the elderly.17 Several diagnostic criteria have been developed over the years18,19 with particular reference to the presence of shoulder pain, neck pain, or pain in the pelvic girdle.20 Joints in hands, knees, shoulders, or hips are often inflamed. General symptoms such as fever, loss of appetite, weakness, and/or weight loss may also be present.21 Laboratory tests often detect elevated CRP values.22 PMR may also cause fatigue, stiffness, fever, and weight loss.23
Ankylosing Spondylitis
Ankylosing spondylitis (AS) is an inflammatory disease of the spine and involves inflammation of tendon insertion sites.24 In advanced stages, the spine becomes rigid, causing loss of mobility. Joints become inflamed. The eyes, kidneys, heart, and lungs are also frequently affected. Many patients diagnosed with AS suffer from severe functional impairments, cardiovascular diseases, diarrhea25, depression, and anxiety, which have a lasting impact on their quality of life.26
The New York diagnostic criteria can be used to establish a diagnosis for investigational studies.27,28 The related clinical criteria are as follows: severe back pain and stiffness, which can be relieved by exercise, for over 3 months. AS can also be associated with limitation of lumbar spine motion in sagittal and frontal planes and decreased chest expansion. Grades 2 to 4 bilateral sacroiliitis or Grades 3 to 4 unilateral sacroiliitis can be used as radiographic criteria. A definite diagnosis of AS is established if all clinical criteria or 1 clinical criterion with 1 radiographic criterion are met.28
Apart from pharmacological treatment,29 physiotherapy and exercise programs30–32 have proven to be of great benefit in the treatment of AS.
Fibromyalgia
Fibromyalgia (FM) can be diagnosed using the classification criteria of the American College of Rheumatology 1990.33 It is defined as chronic pain in multiple body regions and tenderness to pressure of at least 11/18 tender points.
Patients report pain in the axial skeleton, the right and left sides of the body, and above and below the waist, lasting for at least 3 months. FM presents with a large number of accompanying symptoms: headaches, irritable bowel syndrome, cognitive dysfunction, depression, and other impairments are common.34 The origin and etiology of FM are still largely unexplained; however, genetic predispositions are thought to play a role.35 It often takes a long time to diagnose the disease. Many patients do not receive adequate treatment36 and may use multiple medicines (polypharmacy).37 Scientific studies and guidelines focus on the evidence for specific therapeutic procedures, while therapeutic effects of several therapies used simultaneously in a close temporal context have not been adequately examined so far, even though they may bring positive outcomes.38
Multimodal Treatment Concepts
Rheumatic diseases are often associated with a large number of comorbidities,39,40 which are not always treated alongside the main disease.41 These comorbidities may vary widely and include gastrointestinal problems, headaches, cardiovascular diseases, depression, or other diseases of the musculoskeletal system, and so on.42–45 Many multimodal treatment programs described in the literature consist of successive therapeutic interventions.46 However, there are structural requirements for providing a multidisciplinary team comprising various specialists, specialized therapists, and nurses. Moreover, multimodal treatments should consist of therapy procedures that are frequent, performed in a close temporal context, and covering complex disorders.47
Physiotherapeutic measures are important in the treatment of rheumatic diseases. They serve to address the increasing weakness and functional limitations. In multimodal treatment programs, physical therapy should be used in series and in combination. This is to ensure that stimulus-response regulation therapy can influence pain and dysfunction, and that physiological adaptation mechanisms are activated.48 Active movement therapy is significant in this context.49 For example, physiotherapy at close intervals may help address the progression of damage to the musculoskeletal system and functional impairment. Integrating psychotherapeutic measures into multimodal concepts is also recommended.50 Heat therapy, which has been known for millennia, is increasingly being integrated into multimodal treatment concepts for rheumatic diseases. Its integration has been a success.51 In addition, multimodal treatment programs that include guideline-based pharmacotherapy promise good therapeutic results for rheumatic diseases.52
Multimodal Complex Treatments in the German Health-Care System
These treatments were developed in order to offer complex and specialist inpatient treatments, which are provided under the “German Diagnosis-Related Groups System” (G-DRG) to chronically ill patients with acute exacerbation of the disease. As a rule, an interdisciplinary team provides a number of patient-oriented therapeutic procedures in a close temporal context, based on interdisciplinary assessments discussed in team meetings. Within the German health-care system, multimodal inpatient treatments are specified in the German procedure classification (Operationen- und Prozedurenschluessel or Operations- and Procedures- Classifications, translation by the authors [OPS]), an adaptation of the International Classification of Procedures in Medicine (ICPM) of the World Health Organization. The German version of the ICPM is based on a translation of the extended Dutch version ICPM-DE (Dutch extension). Two officially classified multimodal complex treatments, which can be offered in specialized clinics in Germany, are presented below.
Multimodal Nonoperative Complex Treatment of the Movement System
The focus of this complex treatment is an interdisciplinary diagnosis and treatment of complex (multifactorial) diseases of the movement system. A minimum treatment time of 12 days is required, and 5 diagnostic procedures must be used. These can be chosen from the following: neuro-orthopedic structural diagnostics, manual medical functional diagnostics, pain diagnostics and apparatus diagnostics under functional pathological aspects (eg, X-ray, posturography, magnetic resonance imaging, computed tomography [CT], video-based motion analysis, computer-aided motion or force measurement, optimetry), and psychodiagnostics. Three of the following methods must be used as therapy methods: manual medicine, reflex therapy, infiltration therapy/interventional pain therapy, psychotherapy. Furthermore, at least 3 procedures from the therapeutic areas of manual therapy and physiotherapy based on neurophysiology, medical training therapy of physical therapy and relaxation procedures are to be integrated.
In total, a therapy density of at least 30 active and passive individual services from the 2 service groups must be guaranteed, and a therapeutic assessment with interdisciplinary team discussion must take place.
Multimodal Rheumatological Complex Therapy
The team is led by a specialist in internal medicine with a focus on rheumatology, a specialist in orthopedics and trauma surgery with additional training in orthopedic rheumatology, or a specialist in orthopedics with a focus on rheumatology.
Of the therapy areas mentioned below, physiotherapy/physical therapy, occupational therapy, pain therapy, cognitive behavior therapy, and conversation psychotherapy, 3 areas must be applied. The complex treatment is patient-related in different combinations with a therapy density of at least 11 hours per week.
Process-oriented treatment management with standardized findings is to be ensured and disease activity, functional restrictions, and the extent of pain assessed at the beginning and at the end of the inpatient stay. The immediate start of pain therapy, physiotherapy, or physical therapy must be guaranteed
Methods
A retrospective data analysis (N = 375) examines patient-relevant outcomes of multimodal treatment for rheumatic diseases that are associated with pain. To receive this treatment, the main diagnose has to be part of the Major Diagnostic Category (MDC) 8, which covers diseases of the musculoskeletal system and connective tissues.
In order to create a homogenous sample, only patients with a similar degree of chronification (assessed based on patient career, medication and duration of pain) and the following diagnoses were chosen, which all belong to MDC 8: FM, PMR, Morbus Bechterew, RA. The diagnoses of the patients included in the sample were assessed before and at admission, thus confirmed by several specialists, including rheumatologists. Each diagnosis is encoded using the International Statistical Classification of Diseases and Related Health Problems, 10th revision, German modification. This is the official classification for encoding diagnoses in outpatient and inpatient care in Germany.
All patients gave written consent regarding data analysis and could resign from being part of the study at any time. For ethical reasons, no sociodemographic data were asked for. The analysis uses PROs (scales listed below) taken at admission and discharge and is done with SPSS Statistics 25.0 (IBM, 2017). T tests are used for paired samples wherever there is a normal distribution. For nonnormally distributed date, the Wilcoxon test is used to check whether there are significant differences between the 2 measurements. The application for the implementation of the empirical research project was approved by the Research Committee for Scientific Ethical Questions on July 02, 2019, under the reference number 2528. The following scales and scores were used for the project:
Visual Analogue Scale
The Visual Analogue Scale (VAS) is a 1-dimensional measurement tool. It can be used to measure not only pain intensity but also sleep quality and well-being of patients.53–55 It consists of a 100-mm horizontal line on which the patients mark their pain intensity56 with “0” for no pain up to “100” for unbearable pain.
The VAS is in widespread use globally and can be used for patients with rheumatic diseases.57 The instrument fulfills the quality criteria regarding validity, reproducibility, reliability, construct validity, and interpretability58,59 plus is very quick and easy to use.57
Pain Disability Index
The Pain Disability Index (PDI) has been used extensively for many years to assess impairments experienced by patients due to pain. It is suitable for use in various pain-related chronic diseases.60 The instrument is a pain-specific assessment catalogue of 7 items covering the following areas: Family/Home Responsibilities, Recreation, Social Activity, Occupation, Sexual Behavior, Self Care, and Life Support Activities. As a generic instrument, it meets all criteria of validity and reliability.61,62
Pain Grading According to Kohlmann/Raspe
This instrument is based on the patient’s pain assessment via VAS and the Hanover Functional Questionnaire (FFbH), making it a multidimensional instrument. The FFbH is used to identify impairments when performing everyday activities and consists of 18 questions.63 The result is reported on a scale between 0% and 100%. The FFbH is reliable, valid, and sensitive to change. It was shown to be a viable and economical instrument in clinical and epidemiological studies.64 The VAS measuring subjective pain validly and reliably65 has been described above. Combining both values allows calculating the pain grade. Grade 3 stands for high pain intensity and significantly limited physical functions. Grade 1 indicates low pain intensity and adequate physical functions (Figure 1).
Figure 1.
Pain-Grading According to Kohlmann/Raspe.
Heidelberg Short Early Risk Assessment Questionnaire
The validated questionnaire is used for predicting the risk of transition from acute to chronic back pain.66 It consists of 10 core questions with 27 items and covers duration and intensity of pain.
Compensation for Providing Inpatient Care
The German health-care system uses Diagnosis-Related Groups (DRG). These are diagnosis-related flat rates per case paid by health insurance funds for inpatient treatment. DRGs are based on a classification of diagnoses to ensure that similar diseases receive comparable high-standard treatment from a medical point of view, resulting in equal cost. The categorization also takes into account secondary diagnoses, age, and type of discharge from the hospital, as well as other aspects. Every DRG has cost weights, also called relative cost weights, which are used for calculating a price, which ideally is covered by the insurances’ flat rate. Since it is a case-based system, all operating costs for inpatient treatment need to be financed with the revenue that is generated by the DRG(s) treated. This includes all salaries, medicinal products, pharmaceuticals, and the costs of the entire medical and nonmedical infrastructure.11 This scientific investigation analyses the average hospital compensation, with and without complex treatment, for the medical conditions mentioned above, and the developments.
Results
Pain Disability Index
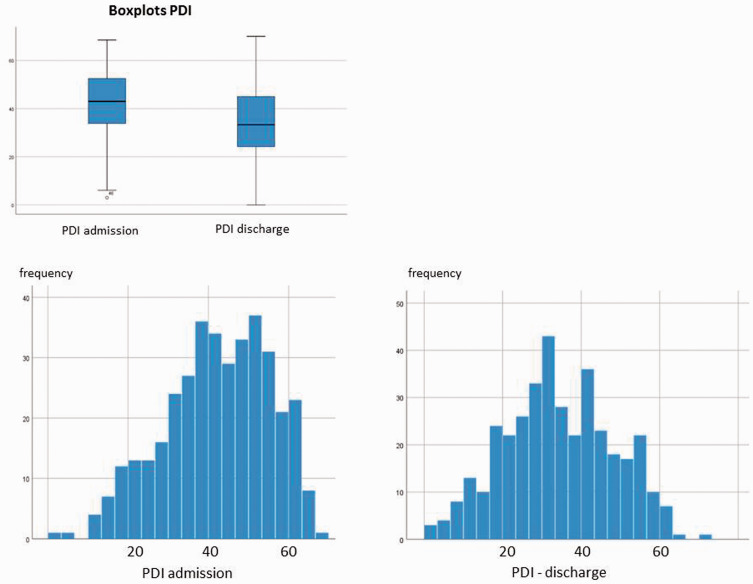
PDI shows a reduction in pain (median on admission =43.00) on discharge (median = 33.30, asymptotic Wilcoxon test: z = −10.854, P < . 001, n = 371) (Table 1). The effect size is r = .56 and corresponds to a strong effect.
Table 1.
PDI Admission and Discharge.
| PDI Admission | PDI Discharge | |
|---|---|---|
| N | ||
| Valid | 371 | 371 |
| Missing | 4 | 4 |
| M | 42.314 | 34.280 |
| Median | 43.000 | 33.300 |
| SD | 13.1646 | 13.9700 |
| Minimum | 3.0 | .0 |
| Maximum | 68.5 | 70.0 |
Abbreviations: SD, standard deviation; PDI, Pain Disability Index.
aPDI discharge < PDI admission.
bPDI discharge > PDI admission.
cPDI discharge = PDI admission.
On admission, there was a deviation from the normal distribution (negative skew). With regard to the severity level, there is, in general, a shift to the left, which indicates improvement in the impairments that are associated with pain (Figure 2).
Figure 2.
PDI. PDI, Pain Disability Index.
VAS Well-Being
The VAS data “well-being” was assessed comprehensively on admission and on discharge. There was a deviation from the normal distribution when VAS assessments were performed (Figure 3). Both show a shift to the left, which suggests a general improvement in the well-being of patients.
Figure 3.
VAS Well-Being. VAS, Visual Analogue Scale.
According to the Wilcox test, the negative ranks clearly outweigh the positive ones. This means that the VAS values went down. The central tendencies of the 2 times of measurement “VAS well-being” differ (asymptotic Wilcoxon test: z = −14 794, P < . 001, n = 375). The effect size is r = .76 and corresponds to a very strong effect (Table 2).
Table 2.
VAS Well-Being Admission and Discharge.
| Ränge | ||||
|---|---|---|---|---|
| N | Middle Rank | Rank Sum | ||
| VAS well-being discharge—VAS well-being admission | Negative ranks | 322a | 198.29 | 63849.00 |
| Positive ranks | 45b | 81.76 | 3679.00 | |
| Bonds | 8c | |||
| Total | 375 | |||
Abbreviation: VAS, Visual Analogue Scale.
aVAS well-being discharge < VAS well-being admission.
bVAS well-being discharge > VAS well-being admission.
cVAS well-being discharge = VAS well-being admission.
Pain-Grading According to Kohlmann/Raspe
The central tendencies of the measurement on admission and upon discharge differ (asymptotic Wilcoxon test: z = −6.600, P < . 001, n = 374) (Table 3). The effect size is r = .34 and corresponds to a low effect.
Table 3.
Pain-Grading According to Kohlmann/Raspe.
| Kohlmann/Raspe Admission | Kohlmann/Raspe Discharge | |
|---|---|---|
| N | ||
| Valid | 374 | 375 |
| Missing | 1 | 0 |
| M | 2.83 | 2.66 |
| Median | 3.00 | 3.00 |
| SD | .418 | .528 |
| Minimum | 1 | 1 |
| Maximum | 3 | 3 |
Abbreviation: SD, standard deviation.
aKohlmann/Raspe discharge < Kohlmann/Raspe admission.
bKohlmann/Raspe discharge > Kohlmann/Raspe admission.
cKohlmann/Raspe discharge = Kohlmann/Raspe admission.
Heidelberg Short Early Risk Assessment Questionnaire
There is a shift to the left, that is, the values decreased, indicating improvement in back pain. The average Heidelberg Short Early Risk Assessment Questionnaire (HKF-R10) was reduced from 83.792 to 69.391 until the patient was discharged and the P value is highly significant (t test, P < .000), equaling a statistically detectable change over time (Table 4; Figure 4).
Table 4.
HKF-R.
| M | N | SD | Standard Error of M | ||
|---|---|---|---|---|---|
| HKF R-10 admission | 83.792 | 111 | 27.0945 | 2.5717 | |
| HKF-R discharge | 69.391 | 111 | 25.2952 | 2.4009 | |
Abbreviations: SD, standard deviation; HKF R, Heidelberg Short Early Risk Assessment Questionnaire.
Figure 4.
HKF-R10 Admission. HKF-R10, Heidelberg Short Early Risk Assessment Questionnaire.
Remuneration Aspects
Analysis of compensation data for approximately 1600 hospitals in Germany from 2016 to 2019 demonstrated, in general, that revenues were lower when conventional unimodal treatment without CT was offered. We have found that revenues were lower especially in relation to the treatment of RA (€3056 in 2016 vs €2594 in 2019). Compensation for the treatment of AS and PMR was somewhat less reduced, but the reduction was still evident. Compensation for FM treatment has improved compared to 2018. When CT was performed, hospital revenues increased noticeably in all years examined (Table 5).
Table 5.
Remuneration Aspects of Fibromyalgia, Polymyalgia Rheumatic, Ankylosing Spondylitis and Rheumatoid Arthritis Versus Complex Treatment.
| Disease | Year | Conventional Treatment | Complex Treatment |
|---|---|---|---|
| Fibromyalgia | 2019 | 3350 | 5364 |
| Fibromyalgia | 2018 | 3081 | 5275 |
| Fibromyalgia | 2017 | 3148 | 5123 |
| Fibromyalgia | 2016 | 3152 | 5010 |
| Polymyalgia rheumatic | 2019 | 2445 | 5364 |
| Polymyalgia rheumatic | 2018 | 2523 | 5275 |
| Polymyalgia rheumatic | 2017 | 2632 | 5123 |
| Polymyalgia rheumatic | 2016 | 2774 | 5010 |
| Ankylosing spondylitis | 2019 | 2445 | 5364 |
| Ankylosing spondylitis | 2018 | 2523 | 5275 |
| Ankylosing spondylitis | 2017 | 2632 | 5123 |
| Ankylosing spondylitis | 2016 | 2774 | 5010 |
| Rheumatoid arthritis | 2019 | 2594 | 5364 |
| Rheumatoid arthritis | 2018 | 2936 | 5275 |
| Rheumatoid arthritis | 2017 | 3063 | 5123 |
| Rheumatoid arthritis | 2016 | 3056 | 5010 |
Outliers in the data do not distort any of the result of the tests performed.
Discussion
The trend toward surgical interventions without a prior attempt to apply conservative treatment has been criticized in recent years.67 The current state of research demonstrates that multimodal treatment programs are effective. However, they are also associated with higher costs for service providers.48,68 This is due to the requirement of providing an interdisciplinary team with specialists from various disciplines but also due to the logistically complex treatment processes, high-intensity therapies, and longer hospital stay. Due to the increase in surgical treatment methods, there is a need for outcomes research to evaluate conservative multimodal treatment concepts,69 which is done in this study.
A high PDI value, as found in this study in patients prior to their admission, suggests a more severe psychological distress, higher pain burden, and greater restrictions when performing everyday activities.60 Subjective patient assessments indicate that the multimodal treatment seems to have had a positive influence on the extent of impairments associated with pain, plus physical and mental well-being. Pain grading according to Kohlmann/Raspe, a multidimensional instrument that takes into account both pain intensity and physical functions of the patient, shows a significant decrease of pain. This result matters because studies demonstrate that also factors other than pain relief, such as improvement in physical functions, are important for the affected patients.70,71
The outcomes of the HKF-R10 indicate that approximately 30% of the patients surveyed also suffer from chronic low back pain. So far, this fact has been given little attention in scientific studies.72 Here, too, the use of CT may have a positive impact.
There are several limitations in this study that future investigations might consider to analyze in more detail. First, we cannot exclude an effect of the setting on the health status, and it is possible that complex cases receive additional attention of the nursing and medical staff, which might improve satisfaction and health conditions. Second, we could not assess sociodemographic data but only controlled for similar degree of chronification and medication.
This results of this study shed light on medical care under everyday conditions using scientific, interdisciplinary methods and examines the outcome changes following routine care73 using PROs. These can be used to assess the therapeutic benefit since they take into account patient-relevant endpoints, which means disease-related changes, and changes due to treatment. Patients in multimodal programs can benefit substantially from the variety of therapies and the frequent procedures that are carried out in quick succession, which can also contribute to high patient satisfaction.74 Studies demonstrate that holistic, multimodal treatment programs may in addition have a positive influence on doctor–patient communication and the patient’s trust in doctors and nurses.75,76
Conclusions
The aim of this study was to test the patient-relevant outcomes of multimodal treatment for rheumatic diseases that are associated with pain and check for remuneration. The results show that PROs regarding mood, pain, and perceived impairments improved following the multimodal complex treatment.
The DRG system established in Germany provides specialized hospitals with the option to offer multimodal treatments in the form of complex treatments. The system takes into account the added value that is created by complex treatments as indicated in the PROs and provides for higher compensation to cover the additional costs. Multimodal complex treatments and the associated financial incentives that are required for quality care of patients with acute exacerbation of chronic conditions thus seem to be a successful model of innovation. Further research including randomized controlled trials testing the clinical effectiveness of such models are warranted.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Tobias Romeyke https://orcid.org/0000-0002-5872-5800
References
- 1.Kröner-Herwig B, Frettlöh J. Behandlung chronischer Schmerzsyndrome: Plädoyer für einen multiprofessionellen Therapieansatz In: Basler HD, ed. Psychologische Schmerztherapie. 6th ed: Berlin, Heidelberg: Springer, 2004, pp. 499–524. [Google Scholar]
- 2.Dysvik E, Kvaløy JT, Stokkeland R, Natvig GKet al. The effectiveness of a multidisciplinary pain management programme managing chronic pain on pain perceptions, health-related quality of life and stages of change—a non-randomized controlled study. Int J Nurs Stud. 2010; 47(7):826–835. [DOI] [PubMed] [Google Scholar]
- 3.Breivik H, Collet B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10(4):287–333. [DOI] [PubMed] [Google Scholar]
- 4.Häuser W, Schmutzer G, Henningsen P, et al. Chronische Schmerzen, Schmerzkrankheit und Zufriedenheit der Betroffenen mit der Schmerzbehandlung in Deutschland. Ergebnisse einer repräsentativen Bevölkerungsstichprobe. . Der Schmerz. 2014; 28(5):483–492. [DOI] [PubMed] [Google Scholar]
- 5.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019; 78(11):1463–1471. [DOI] [PubMed] [Google Scholar]
- 6.Svedbom A, Hernlund E, Ivergård M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013; 8(1-2):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eich W, Bär KJ, Bernateck M, et al. Definition, Klassifikation, klinische Diagnose und Prognose des Fibromyalgiesyndroms. Der Schmerz. 2017; 31(3):231–238. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Heijde D, van’t Hof MA, Van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990; 49(11):916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gusmano MK, Maschke KJ, Solomon MZ. Patient-centered care, yes; patients as consumers, no. Health Aff. 2019; 38(3):368–373. [DOI] [PubMed] [Google Scholar]
- 10.Romeyke T, Nöhammer E, Scheuer HC, et al. Integration of naturopathic medicine into acute inpatient care: an approach for patient-centred medicine under diagnosis-related groups. Complement Ther Clin Pract. 2017; 28:9–17. [DOI] [PubMed] [Google Scholar]
- 11.Romeyke T, Noehammer E, Stummer H. Ensuring quality in interdisciplinary inpatient chronic care. SAGE Open. 2020; 10(2):2158244020914654. [Google Scholar]
- 12.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002; 18:355–365. [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62(9):2569–2581. [DOI] [PubMed] [Google Scholar]
- 14.Covic T, Cumming SR, Pallant JF, et al. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the Hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry. 2012; 12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isik A, Koca SS, Ozturk A, et al. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. 2007; 26:872–878. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Cahalan C, Mensing G, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011; 7(4):216. [DOI] [PubMed] [Google Scholar]
- 17.Michet CJ, Matteson EL. Polymyalgia rheumatica. Br Med J. 2008; 336(7647):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird H, Esselinckx W, Dixon A, et al. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979; 38:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healey LA. Long-term follow-up of polymyalgia rheumatica: evidence for synovitis. Sem Arthritis Rheum. 1984; 13(4):322–328. [DOI] [PubMed] [Google Scholar]
- 20.Buttgereit F, Dejaco C, Matteson EL, Dasgupta B.et al. Polymyalgia rheumatica and giant cell arteritis: a systematic review. J Am Med Assoc. 2016; 315(22):2442–2458. [DOI] [PubMed] [Google Scholar]
- 21.Matteson EL, Dejaco C. Polymyalgia rheumatica. Ann Intern Med. 2017; 166(9):ITC65–ITC80. [DOI] [PubMed] [Google Scholar]
- 22.Nesher G. Polymyalgia rheumatica – diagnosis and classification. J Autoimmun. 2014; 48:76–78. [DOI] [PubMed] [Google Scholar]
- 23.Chuang TY, Hunder GG, Ilstrup DM, et al. Polymyalgia rheumatica: a 10-year epidemiologic and clinical study. Ann Intern Med. 1982; 97(5):672–680. [DOI] [PubMed] [Google Scholar]
- 24.Braun JV, Van Den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011; 70(6):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremander A, Petersson IF, Bergman S, et al. Population‐based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res. 2011; 63(4):550–556. [DOI] [PubMed] [Google Scholar]
- 26.Singh JA, Strand V. Spondyloarthritis is associated with poor function and physical health-related quality of life. J Rheumatol. 2009; 36(5):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler L, Kuipers JG, Schnarr S. Zeidler H. Spondylitis ankylosans: Fortschritte in der medikamentösen Therapie.et al. Dtsch Ärztebl. 2004; 101(21): 1249–54. [Google Scholar]
- 28.Van Der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum. 1984; 27(4):361–368. [DOI] [PubMed] [Google Scholar]
- 29.Baraliakos X, van den Berg, Braun J, et al. van der Heijde D. Update of the literature review on treatment with biologics as a basis for the first update of the ASAS/EULAR management recommendations of ankylosing spondylitis. Rheumatology. 2012; 51(8):1378–1387. [DOI] [PubMed] [Google Scholar]
- 30.Ince G, Sarpel T, Durgun B, Erdogan S.et al. Effects of a multimodal exercise program for people with ankylosing spondylitis. Phys Ther. 2006; 86(7):924–935. [PubMed] [Google Scholar]
- 31.Passalent LA. Physiotherapy for ankylosing spondylitis: evidence and application. Curr Opin Rheumatol. 2011; 23(2):142–147. [DOI] [PubMed] [Google Scholar]
- 32.Niedermann K, Sidelnikov E, Muggli C, et al. Effect of cardiovascular training on fitness and perceived disease activity in people with ankylosing spondylitis. Arthritis Care Res. 2013; 65(11):1844–1852. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990; 33:160–172. [DOI] [PubMed] [Google Scholar]
- 34.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013; 17:356. [DOI] [PubMed] [Google Scholar]
- 35.Clauw DJ. Fibromyalgia: a clinical review. J Am Med Assoc. 2014; 311:1547–1555. [DOI] [PubMed] [Google Scholar]
- 36.Hadker N, Garg S, Chandran AB.Crean SM, McNett M, Silverman SL.et al. Primary care physicians’ perceptions of the challenges and barriers in the timely diagnosis, treatment and management of fibromyalgia. Pain Res Manag. 2011; 16:440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson RL, Kroenke K, Williams DA, et al. Longitudinal observation of treatment patterns and outcomes for patients with fibromyalgia: 12‐month findings from the reflections study. Pain Med. 2013; 14:1400–1415. [DOI] [PubMed] [Google Scholar]
- 38.Romeyke T, Stummer H. Multi-modal pain therapy of fibromyalgia syndrome with integration of systemic whole-body hyperthermia–effects on pain intensity and mental state: a non-randomised controlled study. J Musculoskelet Pain. 2014; 22(4):341–355. [Google Scholar]
- 39.Damjanov N. SP0151. Comorbidity in rheumatic diseases. SP0151. Annas of the Rheumatic Diseases 2014. 73(2):40–1.
- 40.Bilge U, Kaşifoğlu T, Bilge NY, et al. THU0478 Determination of comorbidities in fibromyalgia syndrome 2017.
- 41.Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014; 73:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SC, Landon JE, Solomon DH. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care Res (Hoboken). 2013; 65:1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scrivo R, Gerardi MC, Rutigliano I, et al. Polymyalgia rheumatica and diverticular disease: just two distinct age-related disorders or more? Results from a case-control study. Clin Rheumatol. 2018; v37(9):2573–2577. [DOI] [PubMed] [Google Scholar]
- 44.Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010; 69(6):1165–1168. [DOI] [PubMed] [Google Scholar]
- 45.Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis. 2016; 75(6):965–973. [DOI] [PubMed] [Google Scholar]
- 46.Snijders GF, den Broeder AA, van Riel PLCM, et al. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: between knowing and doing. Scand J Rheumatol. 2011; 40(3):225–231. [DOI] [PubMed] [Google Scholar]
- 47.Romeyke T, Stummer H. Evidence-based complementary and alternative medicine in inpatient care: take a look at Europe . J Evid Based Complement Altern Med. 2015; 20(2):87–93. [DOI] [PubMed] [Google Scholar]
- 48.Crevenna R. Kompendium Physikalische Medizin und Rehabilitation. Springer Berlin, Heidelberg, 2017. [Google Scholar]
- 49.Neill J, Belan I, Ried K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosus: a systematic review. J Adv Nurs. 2006; 56(6):617–635. [DOI] [PubMed] [Google Scholar]
- 50.Lange M, Petermann F. [Influence of depression on fibromyalgia: a systematic review]. Schmerz. 2010; 24:326–333. [DOI] [PubMed] [Google Scholar]
- 51.Romeyke T, Scheuer HC, Stummer H. Fibromyalgia with severe forms of progression in a multidisciplinary therapy setting with emphasis on hyperthermia therapy – a prospective controlled study. Clin Interv Aging. 2015; 10:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marion CE, Balfe LM. Potential advantages of interprofessional care in rheumatoid arthritis. J Manag Care Pharm. 2011; 17(9 Supp B):S25–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDowell I, Newell CA. Guide to Rating Scales and Questionnaires. Oxford: Oxford University Press; 1987. [Google Scholar]
- 54.Jensen MP, Karoly P. Self-report scales and pro-cedures for assessing pain in adults In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. NewYork, NY: Guildford Press; 1992: 135–152. [Google Scholar]
- 55.Steiner M, Streiner DL. Validation of a revised Visual Analogue Scale for premenstrual mood symptoms: results from prospective and retrospective trials. Can J Psychiatry. 2005; 50(6):327–332. [DOI] [PubMed] [Google Scholar]
- 56.Lee K, Keickhefer GM. Measuring human responses using Visual Analogue Scales. West J Nurs Res. 1989; 11:128–132. [DOI] [PubMed] [Google Scholar]
- 57.Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978; 37:378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price DD, McGrath PA, Rafii A, et al. The validation of Visual Analogue Scales as ratio scale measures for chronic and experimental pain. Pain. 1983; 17:45–56. [DOI] [PubMed] [Google Scholar]
- 59.Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007; 60:34–42. [DOI] [PubMed] [Google Scholar]
- 60.Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. 1990; 40(2):171–182. [DOI] [PubMed] [Google Scholar]
- 61.Chibnall JT, Tait RC. The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil. 1994; 75(10):1082–1086. [DOI] [PubMed] [Google Scholar]
- 62.Soer R, Koke AJ, Vroomen PC, et al. Extensive validation of the Pain Disability Index in 3 groups of patients with musculoskeletal pain. Spine (Phila Pa 1976). 2013; 38(9):E562–E568. [DOI] [PubMed] [Google Scholar]
- 63.Kuipers JG, Zeidler H, Köhler L. Medal Rheumatologie Kriterien für die Klassifikation. Diagnose und Aktivität und Prognose rheumatologischer Erkrankungen, Wiskom-Verlag 2006; 2:12–14. [Google Scholar]
- 64.Kohlmann T, Raspe H. Der Funktionsfragebogen Hannover zur Alltagsnahen Diagnostik der Funktionsbeeinträchtigung durch Rückenschmerzen (FFbH-R). Rehabilitation. 1996; 35:I-VIII. [PubMed] [Google Scholar]
- 65.Luria RE. The validity and reliability of the visual analogue mood scale. J Psychiatr Res. 1975; 12:51–57. [DOI] [PubMed] [Google Scholar]
- 66.Neubauer E, Junge A, Pirron P, et al. HKF-R 10 – screening for predicting chronicity in acute low back pain (LBP): a prospective clinical trial. Eur J Pain. 2006; 10(6):559–566. [DOI] [PubMed] [Google Scholar]
- 67.Bäuml M, Kifmann M, Krämer J, et al. Bandscheibenoperationen – Patientenerfahrungen, Indikationsqualität und Notfallkodierung In: Böcken J, Braun B, Meierjürgen R, eds. Gesundheitsmonitor 2016. Bürgerorientierung im Gesundheitswesen. Kooperationsprojekt der Bertelsmann Stiftung und der BARMER GEK. Gutersloh: Verlag Bertelsmann Stiftung; 2016. [Google Scholar]
- 68.Vliet Vlieland TP, Li LC, MacKay C, et al. Does everybody need a team? J Rheumatol. 2006; 33(9):1897–1899. [PubMed] [Google Scholar]
- 69.Flechtenmacher J, Kladny B, Psczolla M. Zehn Forderungen zur Zukunft der konservativen O und U. Orth Unfallchir. 2017; 7:39. [Google Scholar]
- 70.Carbonell-Baeza A, Aparicio VA, Chillón P, Femia P, Delgado-Fernandez M, Ruiz JR.. et al. Effectiveness of multidisciplinary therapy on symptomatology and quality of life in women with fibromyalgia. Clin Exp Rheumatol. 2011; 29(6):S97. [PubMed] [Google Scholar]
- 71.Pergolizzi J, Ahlbeck K, Aldington D, et al. The development of chronic pain: physiological CHANGE necessitates a multidisciplinary approach to treatment. Curr Med Res Opin. 2013; 29(9):1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kothe R. Low back pain in rheumatoid arthritis. Zeitschrift für Rheumatologie. 2017; 76(10):869–875. [DOI] [PubMed] [Google Scholar]
- 73.Pfaff H, Neugebauer EAM, Glaeske G, Schrappe Ml Lehrbuch Versorgungsforschung, 2. Auflage (in print). Stuttgart: Schattauer Verlag; 2017. [Google Scholar]
- 74.Romeyke T, Noehammer E, Scheuer HC, Stummer H. et al. Levels of patient satisfaction on integrative medicine before and after implementation of diagnosis-related groups. Glob Adv Health Med. 2018; 7:2164956118759256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romeyke T, Scheuer HC, Stummer H. Introduction of the German case tariff fee system and its effects on patient satisfaction in inpatient naturopathy. Eur J Integr Med. 2013; 5(2):171–177. [Google Scholar]
- 76.Romeyke T, Noehammer E, Scheuer HC, Stummer H. et al. Patient-centred multidisciplinary inpatient care-have diagnosis-related groups an effect on the doctor-patient relationship and patients’ motivation for behavioural change. Glob J Health Sci. 2016; 8(10):56011. [DOI] [PubMed] [Google Scholar]