Abstract
Chronic pain has a major impact on sufferers and their families. The associated health care costs are substantial. In the context of increasing prevalence, effective treatment options are ever more important. 10 kHz spinal cord stimulation has been shown to effectively provide pain relief, aid in opioid reduction, and improve quality of life in patients with chronic intractable pain. The present review aims to summarize the clinical evidence related to the use of 10 kHz SCS in chronic back and/or leg pain. We searched the PubMed database between 2009 and 2 June 2020 for articles reporting clinical studies that included at least 10 human subjects permanently treated with a 10 kHz SCS system (Senza® system) for chronic back and/or leg pain for a minimum of 3 months. A randomized controlled trial (SENZA-RCT), as well as several prospective and retrospective studies, reported clinical outcomes in subjects with chronic back and leg pain treated with 10 kHz SCS. A high proportion of subjects (60%–80%) reported long-term response to therapy. Pain relief was provided without paresthesia. Other studies showed promising pain relief outcomes in subjects with back pain ineligible for spinal surgery, neuropathic limb pain, and in those with previously failed traditional low-frequency SCS. Most studies reported improved quality of life metrics and/or reduced opioid intake. Level 1 evidence has already been established for the use of 10 kHz SCS in treating chronic back and leg pain, corroborated by real-world, clinical experience. Exploratory studies also show the potential of the therapy in other refractory pain syndromes, although larger studies are desired to validate their findings. Overall, the literature suggests that 10 kHz SCS provides long-term pain relief in a high proportion of patients, along with improved quality of life and reduced opioid consumption.
Keywords: 10 kHz spinal cord stimulation, chronic pain, visual analog scale, quality of life, opioids
Introduction
Chronic pain is a global health problem that causes considerable suffering to individuals and their families. The impact on healthcare resources and public expenditure is major. In two separate surveys carried out in the United States and Europe, around one in five adults reported suffering chronic pain based upon persistence of at least 6 months duration.1,2 Another survey, which did not specify pain duration, estimated prevalence across 10 developed countries as high as 37%.3 Prevalence increases with age,1,4–8 and is rising overall.5,9,10 Chronic pain is well-known to negatively impact people’s well-being,2,3,11–15 social relationships,15–17 daily activities,2,15,16 and work productivity.2,15,16,18 The condition is also an important risk factor for suicidal behavior19 and all-cause mortality.20 The economic burden is estimated to exceed US$500 billion per year in the United States21 and consume around 2%–10% of gross domestic product (GDP) in European countries.22–24
The management of chronic pain is challenging and involves many disciplines. Treatment options include physical therapy, psychological therapies, pharmacology, and surgery.25 Opioid medication is often prescribed as part of a multidisciplinary treatment strategy. Short-term use of opioids seems to be efficacious;26 however, there is little evidence to support its long-term use.27,28 Despite this, long-term prescribing has risen.29
Traditional low-frequency spinal cord stimulation (LF-SCS) has been an alternative treatment option for chronic pain for over 40 years, with failed back surgery syndrome (FBSS) being the most common reason for implantation.30,31 RCTs established strong evidence of efficacy in this indication,32–35 while economic evaluations established its long-term cost-effectiveness.36–38 However, despite the significant evidence base supporting its use in FBSS, the therapy has been underused,38,39 with most FBSS patients undergoing spinal reoperation (>97%).39
During the traditional LF-SCS surgical procedure, one or more thin electrical leads are inserted into the epidural space of the spinal canal, straddling the physiologic midline at the vertebral level that maps to the predominant area of pain. For example, lead tips are typically placed at the midline of T8 T9 for low back stimulation, but stimulation of leg dermatomes (L3–L5) is usually accomplished by placing leads between T9 and T11.40 Leads are then attached to a temporary or permanent stimulation device which delivers electrical pulses to the spinal cord at a fixed frequency in the range of 40–60 Hz with pulse width between 150 and 500 µs.41 Success depends on the pain being masked by stimulation-induced paresthesia.42 It is well-accepted that around half of those treated experience at least 50% pain relief.32,33 However, uncomfortable paresthesia or discomfort related to overstimulation resulting from postural changes is common.34,43,44 Also, some patients adapt to the stimulation after several years, resulting in diminished pain relief.45–50
The last decade has brought several technological advances in traditional LF-SCS. However, success rates have not risen,34 leading to the development of novel, more sophisticated SCS systems, including high-frequency/burst stimulation paradigms,34,51–54 and closed-loop LF-SCS (controlled using evoked compound action potentials (ECAPs)).55
This review is focused on 10 kHz SCS (Senza® system), developed by Nevro Corp. (Redwood City, CA, USA). Over and above operating at a much higher frequency than traditional LF-SCS, 10 kHz SCS delivers lower amplitude (1.0–5.0 mA) and shorter pulse width (30 µs) stimuli, with no invoked sensation of paresthesia.34 During surgery, lead tips are positioned in the anatomical midline at T8 and T9 in a staggered fashion to cover T8–T11 vertebral levels for back and leg pain patients. The patient can remain sedated throughout the entire procedure since paresthesia mapping is not required, making surgery shorter and more straightforward compared with traditional LF-SCS.56
A growing body of evidence has accumulated over the past 5 years related to the use of 10 kHz SCS to treat chronic back and leg pain.57,58 Evidence has also emerged for its use in nonsurgical back pain patients, neuropathic limb pain patients, and in those with previously failed traditional LF-SCS. This chapter aims to summarize the prospective and retrospective clinical studies in these indications, focusing on pain relief outcomes as well as changes in quality of life (QOL) and opioid consumption.
Methods
We searched the PubMed electronic database between 2013 and 2 June 2020, for reports published in English with keywords related to 10 kHz SCS, including spinal cord stimulation, 10 kHz, HF10, high frequency, and kilohertz frequency. Results were limited to articles reporting clinical studies that included at least 10 human subjects permanently treated with a 10 kHz SCS system (Senza system) for chronic back and/or leg pain for a minimum of 3 months. Only articles published in peer-reviewed journals were considered, and studies with questionable methodology59 were not considered.
Results
Chronic back and leg pain
SENZA-RCT study
Kapural et al.34 established Level 1 evidence for the efficacy of 10 kHz SCS in treating chronic back and leg pain in a pivotal, multicenter, RCT published in 2015, with Level 1 evidence defined as a large randomized trial with clear-cut results and low risk of error.60 Subjects had both back pain and leg pain score ⩾5 cm on the visual analog scale (VAS). Most of the cohort had undergone spinal surgery in the past (87%), and just over half of each group reported predominant back pain. After inclusion, they were randomly assigned (1:1) to one of two parallel treatment groups: 10 kHz SCS or traditional LF-SCS (control). The commercially available control device was programmed by the manufacturer’s representatives using any on-label programming parameters, and the sponsor’s personnel programmed the test arm using a fixed waveform of 10 kHz and 30 µs. In total, 93% percent of subjects (90/97) in the 10 kHz SCS group completed a successful trial and were implanted with a permanent system. In the traditional LF-SCS group, this proportion was 88% (81/92). The trial documented outcomes up to 12 months postimplantation. Response to therapy was defined as ⩾50% reduction in pain score.
At the 3-month primary endpoint, 84% of 10 kHz SCS subjects were back pain responders compared with 44% of traditional LF-SCS subjects (p < 0.001 for both noninferiority and superiority). For leg pain response, the corresponding rates were 83% versus 55% (p < 0.001 for both noninferiority and superiority). At 12 months, outcomes were available for 89 and 80 subjects in each group, respectively. Both groups sustained their responder rate out to 12 months. However, the rate remained higher among the 10 kHz SCS subjects (back pain: 79% vs 51%; leg pain: 79% vs 51%; p < 0.001 for both noninferiority and superiority in both pain categories). Moreover, subjects in this group reported a 67% decrease in back pain compared with 44% in the traditional LF-SCS group (back pain: −4.9 vs −3.5 cm, p < 0.001). Similarly, leg pain decreased by 70% versus 49%, respectively (leg pain: −5.0 vs −3.8 cm, p < 0.001).
Analyses of 12-month opioid consumption and patient satisfaction outcome measures also highlighted the advantages of 10 kHz SCS over traditional LF-SCS. Morphine equivalent daily dose (MEDD) decreased more among the former group (−24.8 vs −7.3 mg/day, p = 0.014), and a higher proportion of its subjects were “very satisfied” with their therapy (55% vs 32%, p = 0.002). In total, 83% of 10 kHz SCS subjects were “satisfied” or “very satisfied” with their therapy, and 35% decreased or ceased opioid usage. Furthermore, almost half of the traditional LF-SCS subjects reported uncomfortable stimulation, whereas none of the 10 kHz SCS group reported stimulation-related paresthesia or discomfort.
A later publication of more detailed 12-month secondary outcomes showed that 10 kHz SCS subjects also had a better QOL and functional status compared with their traditional LF-SCS counterparts.61 Measures included the Oswestry Disability Index (ODI), Global Assessment of Functioning (GAF), Short-Form McGill Pain Questionnaire (SF-MPQ-2), Clinician Global Impression of Change (CGIC), Pittsburgh Sleep Quality Index (PSQI), and general survey questions relating to sleeping and driving. On the ODI, 10 kHz SCS subjects improved more (difference in medians (DIM): 6.0 percentage points, p = 0.016). Distribution among the ODI disability subcategories was also more favorable for this group (p = 0.01), with a higher proportion moving into a lower disability category (70% vs 55%). Greater improvements were found in GAF (DIM: 5.0 points, p < 0.01) as well as in the SF-MPQ-2 continuous, intermittent, and neuropathic pain descriptors (DIM: 1.17, p < 0.005; DIM: 1.33, p < 0.005; and DIM: 0.83, p < 0.01, respectively). A higher proportion of 10 kHz SCS subjects were also rated as “better” or “a great deal better” on the CGIC scale (75% vs 56%, p = 0.009), and the number classified in the “good sleeper” category on the global PSQI increased by a larger amount (p = 0.001). More 10 kHz SCS subjects reported sleeping and driving with their device switched on (sleeping: 95% vs 60%, p < 0.001; driving: 94% vs 66%, p < 0.001).
Follow-up was extended for an additional year to evaluate long-term outcomes.62 The 24-month analysis included 85 and 71 subjects in the 10 kHz SCS and traditional LF-SCS groups, respectively. The 10 kHz SCS group sustained its superior response rate for back pain and leg pain compared with the traditional LF-SCS group (back pain: 76% vs 49%, p < 0.001 for both noninferiority and superiority; leg pain: 73% vs 49%, p < 0.001 for noninferiority and p = 0.003 for superiority). Both back pain and leg pain also decreased more in the former group (back pain: −5.0 vs −3.2 cm, p < 0.001 for both noninferiority and superiority; leg pain: −4.7 vs −3.7 cm, p < 0.001 for noninferiority and p = 0.03 for superiority). Other secondary outcomes further reflected the long-term benefits of 10 kHz SCS over traditional LF-SCS. More of the former group reported minimal disability on the ODI (23% vs 10%), being “a great deal better” on the Patient Global Impression of Change scale (PGIC: 34% vs 21%), and being “very satisfied” with their therapy (60% vs 40%). More clinicians also rated subjects in the 10 kHz SCS group as “a great deal better” (CGIC: 41% vs 20%). The distributions among categories for the ODI, PGIC, and CGIC scales also favored 10 kHz SCS (ODI: p = 0.02; PGIC: p = 0.004; CGIC: p = 0.002). A smaller cohort with available data revealed that more traditional LF-SCS subjects used their device programmer daily (35% vs 0%) and carried it around away from home (85% vs 38%).61 It is possible that the higher rate of programmer usage in the traditional LF-SCS group may be mainly related to loss of adequate paresthesia coverage with postural changes, which was reported by 40% of those who experienced paresthesia (95.5%).
Prospective, multicenter, single-arm studies
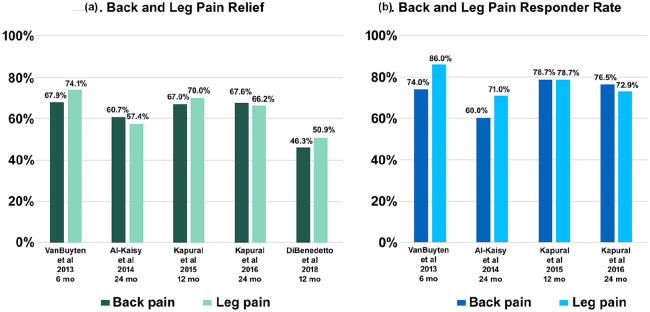
Two prospective, multicenter, single-arm studies evaluated 10 kHz SCS in subjects with a primary diagnosis of chronic back pain (Table 1). In the first study (SENZA-EU), Van Buyten et al.64 presented 6-month data from 83 enrolled subjects. Of these, 82 completed a trial, and 72 (88%) had successful trials followed by permanent implantation. In total, 79% of the implanted group had a diagnosis of FBSS, while the remainder was naïve to spinal surgery. Response was defined as ⩾ 50% reduction in VAS pain score. At 6 months, 74% and 86% of subjects met the criteria for back pain and leg pain response, respectively (Figure 1), and baseline back pain and leg pain decreased by a median of 78% (−5.7 cm, p < 0.001) and 83% (−4.0 cm, p < 0.001), respectively. Almost half of subjects reported > 80% back pain relief. Disability improved (ODI: 55% to 37%, p < 0.001), along with the rate of sleep disturbances per night (3.7–1.3, p < 0.001). In total, 86% of subjects reported opioid use at baseline. By 6 months, 62% of this group had reduced their dosage, and 38% had ceased intake. Overall, 85% of subjects reported satisfaction with their therapy.
Table 1.
Prospective and retrospective studies evaluating 10 kHz SCS in chronic low back and/or leg pain subjects.
| Reference | Study type | Key inclusion | N* | Follow-up period | Outcomes |
|---|---|---|---|---|---|
| Kapural et al.,34 Kapural et al.,62 and Amirdelfan et al.58 | Multicenter RCT | ⩾5 cm VAS back and leg | 90 | 24 months | VAS, responder rate, remitter rate, trial-to-perm ratio, changes in medication use, ODI, GAF, SF-MPQ-2, SF-12, CGIC, PGIC, PSQI, and satisfaction. |
| Stauss et al.63 | Retrospective, multicenter review | Back and leg pain | 1660 | 12 months | VNRS, subject-reported percentage pain relief, responder rate, trial-to-perm ratio, changes in medication use, general function, general QOL and sleep, and satisfaction. |
| Van Buyten et al.64 and Al-Kaisy et al.65 | Prospective, two-center | Primary diagnosis of chronic back pain | 72 | 24 months | VAS, responder rate, trial-to-perm ratio, changes in medication use, ODI, sleep disturbance, and satisfaction. |
| Al-Kaisy et al.66 and Al-Kaisy et al.67 | Prospective, single-center | Predominant chronic back pain, no history of/eligibility for spinal surgery | 20 | 36 months | VAS, responder rate, trial-to-perm ratio, changes in medication use, ODI, SF-36 PCS & MCS, EQ5D TTO, QALY gain, sleep disturbance, and satisfaction. |
| Rapcan et al.68 | Prospective multicenter | FBSS with predominant back pain | 21 | 12 months | VAS, responder rate, trial-to-perm ratio, changes in medication use, performance status, and satisfaction. |
| DiBenedetto et al.69 | Retrospective, single-center, matched cohort study | Chronic back pain with or without leg pain | 32 | 12 months | Changes in medication use, visit volume, interventional procedure volume, NRS, and FPS. |
| Russo et al.70 | Multicenter, retrospective review | Not candidates for LF-SCS or nonresponders | 186 | 6 months | NRS, responder rate, trial-to-perm ratio, and ODI. |
CGIC: Clinician Global Impression of Change; EQ5D: EuroQol 5-Dimensional Questionnaire; FBSS: failed back surgery syndrome; GAF: Global Assessment of Functioning; LF-SCS: low-frequency spinal cord stimulation; MCS: mental component subscale; ODI: Oswestry Disability Index; PCS: physical component subscale; PGIC: Patient Global Impression of Change scale; PSQI: Pittsburgh Sleep Quality Index; QALY: quality-adjusted life year; QOL: quality of life; RCT: randomized controlled trial; SF-MPQ-2: Short-Form McGill Pain Questionnaire; VAS: visual analog scale; VNRS: verbal numerical rating scale; NRS: numerical rating sclae; TTO: time trade-off; SF-12: 12-item short form survey; FPS: functional pain scale.
N*: number of 10 kHz SCS implanted subjects/patients.
Figure .1.
10 kHz SCS benefits for low back and leg pain patients: (a) mean pain relief and (b) responder rate.
Al-Kaisy et al.65 detailed results for 65 subjects at 24 months. The response rate for both back pain and leg pain remained high among this group (60% and 71%, respectively), and the decrease in baseline back pain and leg pain was sustained (back pain: −5.1 cm, p < 0.001; leg pain: −3.1 cm, p < 0.001). Subjects also maintained their improved disability and rate of sleep disturbances (ODI: 55%–40%, p < 0.001; sleep disturbances: 3.7–1.4/night, p < 0.001), and fewer were classified as “crippled” or “severely disabled” (ODI: 90%–49%). In addition, fewer subjects were using opioids (86%–57%, p < 0.001) and consumption went down by 68% (MEDD: 84 to 27 mg/day, p < 0.001). Most subjects were still satisfied with their therapy (>80%). Also noteworthy was the similar level of pain relief experienced by 15 subjects naïve to spinal surgery (back pain: −4.7 cm, p < 0.001; leg pain: −3.1 cm, p < 0.05).
Rapcan et al.68 presented the second study. Twenty-one subjects recruited from four centers completed a successful trial and received a permanent system. All surgeries were carried out by a single implanter. The entire cohort completed 12 months of follow-up. At this time point, 67% of subjects reported therapy response (⩾ 50% reduction in VAS pain score), and baseline pain decreased by 54% (−4.7 cm, p < 0.001). Moreover, 65% of the cohort halved their opioid intake. Performance status (PS) also improved (3.0–1.8 points, p < 0.001).
Retrospective, real-world studies
Two retrospective studies presented results of 10 kHz SCS used to treat chronic low back and leg pain patients in real-world clinical settings (Table 1). Stauss et al.63 examined the database records of a large cohort of 1660 patients with chronic back and leg pain who were trialed and/or permanently implanted with a 10 kHz SCS system in eight centers between April 2014 and January 2018. Of the 1640 patients with available baseline data, 84% had both back and leg pain, back pain was predominant in 27%, leg pain was predominant in 13%, and 16% had other pain. Among the cohort with available trial data (N = 1603), 87% had successful trials. Patients reported combined back and leg pain relief up to 12 months postimplantation and during their last visit (mean 8.9 months).
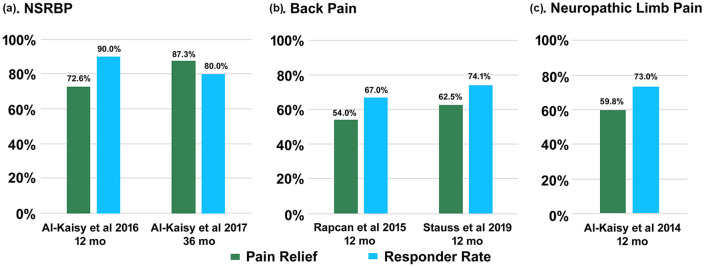
At 3 months, 75% of 844 patients with available data reported ⩾ 50% pain relief. The responder rate was sustained at 12 months (78% of N = 326 with available data) and corroborated by the last visit analysis (74% of N = 1131 with available data; Figure 2). A general survey also administered during the last visit included questions relating to overall changes in medication intake, QOL, and therapy experience. Among the cohort with available data, around a third reported decreased medication intake. In addition, over two-third indicated improved function and sleep, most reported overall better QOL (90%), and the majority were satisfied with their therapy (>80%). Almost all patients noted sleeping and driving with their device switched on (98%). The near-continuous usage of the device reported during sleeping and driving suggests that patients tolerated the therapy very well and found it comfortable. This may be due to the absence of paresthesia.
Figure 2.
Responder rate and mean pain relief in predominant back or leg pain: (a) back pain, (b) nonsurgical back pain, and (c) neuropathic limb pain.
In a smaller, single-center study, DiBenedetto et al.69 compared 32 patients receiving 10 kHz SCS plus conventional medical management (CMM) with 64 case-matched controls receiving only CMM. The study evaluated several outcomes including opioid consumption and health care utilization. Data were collected for 12 months pre- and postimplantation in the 10 kHz SCS + CMM group, and for two consecutive 12-month periods in the CMM group. A 12-month pre/post analysis found opioid reduction only among 10 kHz SCS + CMM patients (MEDD: 92.2–66.0 mg/day, p = 0.001, N = 21) with a between-group difference in favor of this cohort (p = 0.01). Furthermore, opioid dosage decreased in 15 patients in this group (71%) with at least 60% dosage reduction noted in 5 patients (including one who ceased intake). Only one patient in the CMM group reported a dosage reduction of at least 60%. Both groups underwent fewer interventional procedures post versus pre (10 kHz SCS + CMM: 2.5–0.7 procedures, p < 0.001, N = 32; CMM: 2.6–1.7 procedures, p = 0.01, N = 64) with a much larger reduction among 10 kHz SCS + CMM patients (72% vs 35%, p = 0.03). Notably, during the latter 12 months, the 10 kHz SCS cohort underwent less than half the total number of interventional procedures compared with the CMM cohort. Only the CMM patients made fewer office visits (4.9–3.6 visits, p = 0.02, N = 64), but the group difference was not significant. Based on NRS pain scores recorded at baseline and 12 months postimplantation, the 10 kHz SCS + CMM group reported a 46% decrease in low back pain and a 51% decrease in lower extremity pain (low back: −3.1 points, p < 0.001, N = 30; lower extremity: −2.9 points, p = 0.01, N = 16). However, functional pain score was unchanged in both groups.
Chronic back pain ineligible for spinal surgery (“maiden back” or nonsurgical back pain)
A separate, single-center, prospective study by Al-Kaisy et al.66 examined the benefits of 10 kHz SCS in subjects with chronic back pain ineligible for spinal surgery and no history of such intervention. Of 21 enrolled subjects, 20 completed successful trials (95%) and were implanted with a permanent system. All implanted subjects completed 12 months of follow-up. At 6 and 12 months, 75% and 90% of the cohort reported back pain response (⩾ 50% reduction in VAS pain score), respectively, and baseline back pain decreased by 60% (−4.7 cm, p < 0.0001) and 73% (−5.6 cm, p < 0.0001), respectively.
At 12 months, disability score reduced by around half (ODI: −26.0 percentage points, p < 0.0001), and QOL score almost tripled according to the EuroQol 5-Dimensional Questionnaire Time Trade-off (EQ5D TTO) valuation (0.16–0.47 points, p < 0.0001). At the same time point, both the physical component subscale (PCS) and mental component subscale (MCS) of the 36-Item Short-Form Health Survey (SF-36) improved (PCS: p < 0.0005; MCS: p < 0.05). Other metrics revealed 54% fewer sleep disturbances (p < 0.05), 64% opioid reduction (MEDD: 112 to 40 mg/day), cessation of opioids in three subjects, and satisfaction with therapy in at least 70% of subjects (N = 20). Based on the EQ5D TTO valuation, the quality-adjusted life year (QALY) estimated more than the 12-month study period was 0.47.
The follow-up period of the study was extended for another 24 months, and investigators later reported findings from the 36-month assessment.67 Average reduction in back pain intensity was 87%, and the responder rate of the subjects (⩾50% reduction in back pain intensity score) was 80%. Disability score (ODI) further reduced to 19.8 (−33.2 percentage points from baseline; p < 0.0001), and 50% of subjects (N = 10/20) were in the “minimal disability” category. The study reported that subjects continued to reduce and/or eliminate their opioid medication, and that the percentage of subjects not taking opioid medication increased to 88% (N = 15/17). Similarly, further improvements were seen in QOL measures: EQ5D TTO improved to 0.84, SF-36 PCS improved to 48.2, and SF-36 MCS to 56.8 (p < 0.0001).
In a post hoc analysis by Al-Kaisy et al.,71 clinical outcome data were pooled from nonsurgical refractory back pain subjects included in the SENZA-RCT and SENZA-EU studies (N = 12 and N = 14, respectively) and treated with 10 kHz SCS. Outcomes for the combined cohort were analyzed up to 12 months following implantation. The 12-month responder rate for this cohort for both back pain and leg pain was 73%, and baseline back pain and leg pain decreased by 64% (−5.0 cm) and 61% (−4.7%), respectively. Around two-third of the cohort met the criterion for the remission of both back pain and leg pain (VAS ⩽ 3 cm). Disability improved among the subjects over the 12 months (ODI: 52.3%–36.6%), and the proportion of subjects classified on the ODI as crippled or severely disabled dropped markedly from 96% to 42%. Average opioid consumption from baseline to 12 months more than halved among the cohort with available data (85.3 MME–39.8 MME). Overall, more than half of this group decreased or eliminated their opioid intake.
Neuropathic limb pain
Al-Kaisy et al.72 shared their single-center, retrospective experience of using 10 kHz SCS in 15 patients with neuropathic pain in their extremities. Diagnoses included upper or lower neuropathic limb pain, complex regional pain syndrome (CRPS) of the hand or foot, or postsurgical knee pain. Eleven of the 15 enrolled patients (73%) had a successful trial and received a permanent system. All implanted patients completed 6 months of follow-up. At this time point, 73% of the cohort reported ⩾ 50% reduction in pain score (NRS), and the mean reduction in pain was 59% (−4.9 points, p < 0.05). An EQ5D TTO valuation revealed improved QOL: the score doubled at 6 months. In addition, pain-related catastrophic thinking reduced considerably over the same period. Most patients reported satisfaction with their therapy (91%).
Traditional LF-SCS nonresponders
Two studies analyzed subgroups of traditional LF-SCS nonresponders treated with 10 kHz SCS. The first study, by Russo et al.,70 was a multicenter, retrospective review of 256 patients who were not candidates for traditional LF-SCS or were nonresponders. Among the cohort, almost half reported both chronic back and leg pain, and previously failed traditional LF-SCS or peripheral nerve field stimulation (PNFS) was noted in at least 30%. Results were reported for all patients (N = 256), and the subgroup of traditional LF-SCS/PNFS nonresponders (N = 76), up to 6 months implantation. In total, 73% of the former group and 68% of the latter group completed successful trials and received permanent systems. At 6 months, baseline NRS pain score reduced by around 50% in both groups (all patients: −3.8 points, p < 0.001, N = 125; traditional LF-SCS/PNFS nonresponders: −3.5 points, p < 0.001, N = 38). Among the traditional LF-SCS/PNFS nonresponders, 55% reported ⩾50% pain relief, and 8% indicated ⩾80% pain relief. Among all patients, disability improved over the follow-up period (ODI: 41.4%–32.8%, p < 0.001, N = 68). A positive correlation was also confirmed between the improved ODI score and the pain score at 6 months.
Stauss et al.63 also presented a subgroup of traditional LF-SCS nonresponders in their real-world, retrospective review outlined earlier. The subgroup baseline characteristics, trial results, and pain relief outcomes were closely aligned with the primary cohort. At 3 months, 76% of 193 patients with available data were responders. At 12 months, the responder rate was sustained (79% of N = 90 with available data), and confirmed by the last visit evaluation (74% of N = 266 with available data). Answers to the general survey questions administered during the last visit were also well-aligned with the primary cohort: decreased medication intake was reported by 32% of those with available data, improved function by 82%, improved sleep by 70%, better QOL by 88%, satisfaction with therapy by ⩾80%, and driving/sleeping with the device switched on by 98%.
Discussion
Spinal cord stimulation at 10 kHz is associated with improved clinical outcomes in patients with various chronic pain etiologies, while also improving QOL and reducing opioid intake.34,57,62–69,72–83 The primary aim of this review was to summarize the existing clinical evidence for the use of 10 kHz SCS to treat chronic back and/or leg pain.
The pivotal SENZA-RCT, which compared 10 kHz SCS with traditional LF-SCS, underpins the clinical use of the therapy in this population.34,62,73 The study showed long-term statistically superior pain relief among those treated with 10 kHz SCS. Two prospective, single-arm studies found comparable high levels of response to therapy,64,65,68 and two retrospective studies confirmed the efficacy of 10 kHz SCS in real-world, clinical settings.63,69 Overall, the five studies analyzed data from over 1000 subjects. At 12 months, at least 70% of subjects were responders to therapy. Longer term, at 24 months, the responder rate remained high, ranging from 60% to 80%. Most studies reported concomitant improvements in QOL as well as opioid reduction (Table 2). Most subjects (>80%) expressed satisfaction with their therapy.
Table 2.
Studies reporting changes in opioid medication following 10 kHz SCS treatment.
| Reference | N | Baseline dose (mg/day) | Last follow-up dose (mg/day) | % of subjects who reduced/eliminated at last follow-up |
|---|---|---|---|---|
| Kapural et al.34 | 89 | 112.7 | 87.9 | 35.5 |
| Al-Kaisy et al.65 | 65 | 84.0 | 27.0 | 72.0 |
| Al-Kaisy et al.66 | 20 | 112.0 | 40.0 | 88.0 |
| DiBenedetto et al.69 | 21 | 92.2 | 66.0 | 71.4 |
| Stauss et al.63 | 1070 | Not reported | Not reported | 32.1 |
| Rapcan et al.68 | 21 | Not reported | Not reported | 65.0 |
Results from studies that evaluated 10 kHz SCS in other indications are also very encouraging. The prospective study by Al-Kaisy et al.,66 which included subjects with chronic back pain ineligible for spinal surgery, found a markedly high 12-month responder rate. The pooled subanalysis of nonsurgical refractory back pain subjects included in the SENZA-RCT and SENZA-EU studies also found a high rate of response to therapy.71 In another retrospective study by Al-Kaisy et al.,72 almost three-quarters of neuropathic limb pain patients responded to 10 kHz SCS. All three results represent an exciting prospect for patients with limited therapeutic options. In patients with previously failed traditional LF-SCS, 10 kHz SCS may also provide a valuable treatment alternative.63,70 Improved QOL and reduced opioid or medication consumption were common findings among these studies, regardless of indication. However, further investigations are desired to confirm the benefits of 10 kHz SCS in these newer potential treatment populations.
While our review brings together the existing evidence for 10 kHz SCS for the treatment of chronic back and/or leg pain, it has several key limitations. First, this is not a true systematic review and meta-analysis, and as such, it should be interpreted with caution. In addition, apart from the RCT, the prospective studies included in the review were mainly single-arm in design, and some had small sample sizes. Study design of the articles included was not discussed in this review. Furthermore, head-to-head RCTs comparing subjects implanted with the Senza™ 10 kHz SCS system and newer SCS systems such as closed-loop LF-SCS based on ECAPs, have not yet been carried out; therefore, these technologies could not be compared in this review. However, taken together, the included studies may provide insight into the effectiveness of the therapy in different clinical settings and inform future clinical research.
Conclusion
In conclusion, Level 1 evidence exists for the use of 10 kHz SCS to treat chronic back and leg pain. Additional complementary data collected in real-world, clinical settings corroborate its use in this prevalent indication. The evidence base to date also suggests that the therapy improves QOL and reduces opioid consumption. Results in subjects with chronic back pain ineligible for spinal surgery, neuropathic limb pain, and previously failed traditional LF-SCS are promising and warrant further investigation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67: 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10(4): 287–333. [DOI] [PubMed] [Google Scholar]
- 3. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10): 883–891. [DOI] [PubMed] [Google Scholar]
- 4. Pitcher MH, Von Korff M, Bushnell MC, et al. Prevalence and profile of high-impact chronic pain in the United States. J Pain 2019; 20(2): 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fayaz A, Croft P, Langford RM, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016; 6: e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain 2010; 11(11): 1230–1239. [DOI] [PubMed] [Google Scholar]
- 7. Eriksen J, Jensen MK, Sjøgren P, et al. Epidemiology of chronic non-malignant pain in Denmark. Pain 2003; 106(3): 221–228. [DOI] [PubMed] [Google Scholar]
- 8. Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet 1999; 354: 1248–1252. [DOI] [PubMed] [Google Scholar]
- 9. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med 2009; 169: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palmer KT. Back pain in Britain: comparison of two prevalence surveys at an interval of 10 years. BMJ 2000; 320: 1577–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leadley RM, Armstrong N, Reid KJ, et al. Healthy aging in relation to chronic pain and quality of life in Europe. Pain Pract 2014; 14(6): 547–558. [DOI] [PubMed] [Google Scholar]
- 12. Mathias JL, Cant ML, Burke ALJ. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med 2018; 52: 198–210. [DOI] [PubMed] [Google Scholar]
- 13. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med 2003; 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- 14. Rayner L, Hotopf M, Petkova H, et al. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain 2016; 157(7): 1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duenas M, Ojeda B, Salazar A, et al. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016; 9: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011; 27(2): 449–462. [DOI] [PubMed] [Google Scholar]
- 17. West C, Usher K, Foster K, et al. Chronic pain and the family: the experience of the partners of people living with chronic pain. J Clin Nurs 2012; 21(23–24): 3352–3360. [DOI] [PubMed] [Google Scholar]
- 18. Patel AS, Farquharson R, Carroll D, et al. The impact and burden of chronic pain in the workplace: a qualitative systematic review. Pain Pract 2012; 12(7): 578–589. [DOI] [PubMed] [Google Scholar]
- 19. Racine M. Chronic pain and suicide risk: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2018; 87: 269–280. [DOI] [PubMed] [Google Scholar]
- 20. Torrance N, Elliott AM, Lee AJ, et al. Severe chronic pain is associated with increased 10 year mortality. Eur J Pain 2010; 14(4): 380–386. [DOI] [PubMed] [Google Scholar]
- 21. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012; 13: 715–724. [DOI] [PubMed] [Google Scholar]
- 22. Raftery MN, Ryan P, Normand C, et al. The economic cost of chronic noncancer pain in Ireland: results from the PRIME Study, part 2. J Pain 2012; 13(2): 139–145. [DOI] [PubMed] [Google Scholar]
- 23. Gustavsson A, Bjorkman J, Ljungcrantz C, et al. Socio-economic burden of patients with a diagnosis related to chronic pain–register data of 840,000 Swedish patients. Eur J Pain 2012; 16(2): 289–299. [DOI] [PubMed] [Google Scholar]
- 24. Allegri M, Lucioni C, Mazzi S, et al. Social cost of chronic pain in Italy. Glob Reg Health Technol Assess 2015; 2: 33–42. [Google Scholar]
- 25. Institute for Clinical Systems Improvement. Pain; assessment, non-opioid treatment approaches and opioid management (Health Care Guideline). Bloomington, MN: Institute for Clinical Systems Improvement, 2017. [Google Scholar]
- 26. Meske DS, Lawal O, Elder H, et al. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res 2018; 11: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 2015; 162: 276–286. [DOI] [PubMed] [Google Scholar]
- 28. Stannard C. Where now for opioids in chronic pain. Drug Ther Bull 2018; 56(10): 118–122. [DOI] [PubMed] [Google Scholar]
- 29. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009; 18(12): 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gharibo C, Laux G, Forzani BR, et al. State of the field survey: spinal cord stimulator use by academic pain medicine practices. Pain Med 2014; 15(2): 188–195. [DOI] [PubMed] [Google Scholar]
- 31. Moore DM, McCrory C. Spinal cord stimulation. BJA Educ 2016; 16: 258–263. [Google Scholar]
- 32. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005; 56(1): 98–106; discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 33. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007; 132(1–2): 179–188. [DOI] [PubMed] [Google Scholar]
- 34. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology 2015; 123(4): 851–860. [DOI] [PubMed] [Google Scholar]
- 35. Grider JS, Manchikanti L, Carayannopoulos A, et al. Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician 2016; 19(1): E33–E54. [PubMed] [Google Scholar]
- 36. Bala MM, Riemsma RP, Nixon J, et al. Systematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin J Pain 2008; 24(9): 741–756. [DOI] [PubMed] [Google Scholar]
- 37. Taylor RS, Ryan J, O’Donnell R, et al. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain 2010; 26: 463–469. [DOI] [PubMed] [Google Scholar]
- 38. Farber SH, Han JL, Elsamadicy AA, et al. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician 2017; 20(6): E797–E805. [PMC free article] [PubMed] [Google Scholar]
- 39. Lad SP, Babu R, Bagley JH, et al. Utilization of spinal cord stimulation in patients with failed back surgery syndrome. Spine 2014; 39: E719–E727. [DOI] [PubMed] [Google Scholar]
- 40. Kreis P, Fishman S. Electricity and spinal cord stimulation: Spinal cord stimulation—percutaneous implantation techniques. New York: Oxford University Press, 2009. [Google Scholar]
- 41. Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: Hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017; 20(6): 525–533. [DOI] [PubMed] [Google Scholar]
- 42. North RB, Ewend MG, Lawton MT, et al. Spinal cord stimulation for chronic, intractable pain: superiority of “multi-channel” devices. Pain 1991; 44: 119–130. [DOI] [PubMed] [Google Scholar]
- 43. Kuechmann C, Valine T, Wolfe DL. 853 Could automatic position adaptive stimulation be useful in spinal cord stimulation? Eur J Pain 2009; 13: S243. [Google Scholar]
- 44. Levy RM. Anatomic considerations for spinal cord stimulation. Neuromodulation 2014; 17(Suppl. 1): 2–11. [DOI] [PubMed] [Google Scholar]
- 45. Kupers RC, Van den Oever R, Van Houdenhove B, et al. Spinal cord stimulation in Belgium: a nation-wide survey on the incidence, indications and therapeutic efficacy by the health insurer. Pain 1994; 56(2): 211–216. [DOI] [PubMed] [Google Scholar]
- 46. Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine 1996; 21: 1344–1350; discussion 1351. [DOI] [PubMed] [Google Scholar]
- 47. Alo KM, Redko V, Charnov J. Four year follow-up of dual electrode spinal cord stimulation for chronic pain. Neuromodulation 2002; 5(2): 79–88. [DOI] [PubMed] [Google Scholar]
- 48. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 2004; 100(3 Suppl. Spine): 254–267. [DOI] [PubMed] [Google Scholar]
- 49. Sears NC, Machado AG, Nagel SJ, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation 2011; 14(4): 312–318; discussion 318. [DOI] [PubMed] [Google Scholar]
- 50. Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery 2006; 58(3): 481–496; discussion 481–496. [DOI] [PubMed] [Google Scholar]
- 51. Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation 2013; 16(4): 363–369; discussion 369. [DOI] [PubMed] [Google Scholar]
- 52. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) Study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018; 21(1): 56–66. [DOI] [PubMed] [Google Scholar]
- 53. Thomson SJ, Tavakkolizadeh M, Love-Jones S, et al. Effects of rate on analgesia in Kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation 2018; 21(1): 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. North JM, Hong KJ, Cho PY. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia-based stimulation: results of a prospective randomized controlled trial. Neuromodulation 2016; 19(7): 731–737. [DOI] [PubMed] [Google Scholar]
- 55. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol 2020; 19(2): 123–134. [DOI] [PubMed] [Google Scholar]
- 56. Russo M, Van Buyten JP. 10-kHz high-frequency SCS therapy: a clinical summary. Pain Med 2015; 16(5): 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al-Kaisy A, Van Buyten JP, Amirdelfan K, et al. Opioid-sparing effects of 10 kHz spinal cord stimulation: a review of clinical evidence. Ann N Y Acad Sci 2020; 1462(1): 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amirdelfan K, Gliner BE, Kapural L, et al. A proposed definition of remission from chronic pain, based on retrospective evaluation of 24-month outcomes with spinal cord stimulation. Postgrad Med 2019; 131(4): 278–286. [DOI] [PubMed] [Google Scholar]
- 59. North R, Eldabe S. Neuromodulation device comparison studies: coming of age revisited. Pain Med 2018; 19: 2096–2097. [DOI] [PubMed] [Google Scholar]
- 60. Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989; 95: 2S–4S. [PubMed] [Google Scholar]
- 61. Amirdelfan K, Yu C, Doust MW, et al. Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res 2018; 27(8): 2035–2044. [DOI] [PubMed] [Google Scholar]
- 62. Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016; 79: 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stauss T, El Majdoub F, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol 2019; 6: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Van Buyten JP, Al-Kaisy A, Smet I, et al. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation 2013; 16(1): 59–65; discussion 65–66. [DOI] [PubMed] [Google Scholar]
- 65. Al-Kaisy A, Van Buyten JP, Smet I, et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med 2014; 15(3): 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al-Kaisy A, Palmisani S, Smith TE, et al. 10 kHz high-frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof-of-concept study. Neuromodulation 2017; 20(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 67. Al-Kaisy A, Palmisani S, Smith TE, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med 2018; 19: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 68. Rapcan R, Mlaka J, Venglarcik M, et al. High-frequency—spinal cord stimulation. Bratisl Med J 2015; 116: 354–356. [DOI] [PubMed] [Google Scholar]
- 69. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, et al. 10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res 2018; 11: 2929–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Russo M, Verrills P, Mitchell B, et al. High frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6-month Australian clinical experience. Pain Phys 2016; 19(4): 267–280. [PubMed] [Google Scholar]
- 71. Al-Kaisy A, Van Buyten JP, Kapural L, et al. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia 2020; 75(6): 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Al-Kaisy A, Palmisani S, Smith T, et al. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation 2015; 18(1): 18–23; discussion 23. [DOI] [PubMed] [Google Scholar]
- 73. Al-Kaisy A, Van Buyten JP, Carganillo R, et al. 10 kHz SCS therapy for chronic pain, effects on opioid usage: post hoc analysis of data from two prospective studies. Sci Rep 2019; 9: 11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amirdelfan K, Vallejo R, Benyamin R, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of combined neck and arm pain: results from a prospective multicenter study. Neurosurgery 2020; 87(2): 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burgher A, Kosek P, Surrett S, et al. 10 kHz SCS for the treatment of chronic pain of the upper extremities: a post-market observational study. In: Poster presented at 22nd annual meeting of the North American Neuromodulation Society (NANS), Las Vegas, NV, 17–20 January 2019. [Google Scholar]
- 76. Verrills P, Salmon J, Russo M. A multi-center, prospective, clinical trial of high frequency spinal cord stimulation (HF-SCS) at 10 kHz in the treatment of chronic upper limb and neck pain: Australian experience—quality of life outcomes. In: Poster presented at 23nd annual meeting of the North American Neuromodulation Society (NANS), Las Vegas, NV, 23–26 January 2020. [Google Scholar]
- 77. Kapural L, Gupta M, Paicius R, et al. Treatment of chronic abdominal pain with 10-kHz spinal cord stimulation. Clin Transl Gastroenterol 2020; 11(2): e00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sills S. Treatment of painful polyneuropathies of diabetic and other origins with 10 kHz SCS: a case series. Postgrad Med 2020; 132(4): 352–357. [DOI] [PubMed] [Google Scholar]
- 79. Salmon J. High-frequency spinal cord stimulation at 10 kHz for widespread pain: a retrospective survey of outcomes from combined cervical and thoracic electrode placements. Postgrad Med 2019; 131(3): 230–238. [DOI] [PubMed] [Google Scholar]
- 80. Sayed D, Kallewaard JW, Rotte A, et al. Pain relief and improvement in quality of life with 10 kHz SCS therapy: summary of clinical evidence. CNS Neurosci Ther 2020; 26(4): 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sayed D, Salmon J, Khan T, et al. Retrospective analysis of real-world outcomes of 10 kHz SCS in patients with upper limb and neck pain. J Pain Res 2020; 13: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tate J, Stauss T, Li S, et al. A prospective, multi-center, clinical trial of 10 kHz spinal cord stimulation system in the treatment of chronic pelvic pain. Pain Pract. Epub ahead of print 2 July 2020. DOI: 10.1111/papr.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Galan V, Scowcroft J, Chang P, et al. 10 kHz SCS treatment for painful diabetic neuropathy (PDN): results from post hoc analysis of SENZA-PPN study. Pain Manag; Epub ahead of print 11 August 2020. DOI: 10.2217/pmt-2020-0033. [DOI] [PubMed] [Google Scholar]