Abstract
Objectives
Most Chinese hospitals have customized environmental cleaning policies and systems, with limited data availability based on evidence-based medicine. This study investigated the relationship between multidrug-resistant organism (MDRO) colonization in intensive care unit (ICU) patients and ICU surface bacterial contamination status.
Methods
This cross-sectional study comprised MDRO screening in ICU patients using bacterial cultivation by chromogenic medium; samples were collected before (control group) and after implementation of enhanced cleaning (cleaning group). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was used to identify and analyze microorganisms; the relationships of MDRO colonization with infection and environmental bacteria were analyzed.
Results
In total, 196 patients were enrolled in the study (104 and 92 in control and cleaning groups, respectively); 1042 MDROs were subjected to screening before and after cleaning. After cleaning, the rate of MDRO detection on surfaces of frequently touched objects in ICUs decreased from 31.77% to 13.32%. There were fewer MDRO homologues in the cleaning group than in the control group. Moreover, the cleaning group had a shorter ICU stay and significantly lower mortality rate.
Conclusions
Enhanced environmental cleaning and disinfection could reduce environmental MDRO accumulation and suppress MDRO colonization in ICUs, thereby reducing nosocomial infections and improving adverse patient outcomes.
Keywords: Multi-center, environmental cleaning, disinfection measure, nosocomial infection, intensive care unit, mass spectrometry, multidrug-resistant bacteria, contamination
Introduction
Multidrug-resistant organisms (MDROs) include bacteria that are clinically resistant to ≥3 types of antibacterial drugs;1 these organisms are common causes of nosocomial infections that lead to adverse outcomes. In recent decades, the types and numbers of MDROs have increased rapidly due to the widespread use of antibacterial drugs; MDRO infections have also increased considerably.2,3 MDRO infections are characterized by complexity and refractoriness, which leads to greater mortality and poses a substantial burden on society.
MDRO colonization is an important risk factor that increases the risk of secondary infections, especially for high-risk groups such as patients in intensive care units (ICUs).2 Most ICU patients exhibit poor general and nutritional statuses, low immune function, invasive procedure histories, and long hospital stays; thus, they have reduced stress tolerance and enhanced susceptibility to infection.3 Notably, the risk of nosocomial infections is 5- to 10-fold greater in ICUs than in general hospital wards.4 Most practices to prevent and treat MDRO infections involve environmental objects; the surfaces of these objects are important sources of pathogens in hospitals.5,6 In recent years, the relationship between hospital environmental pollution and nosocomial infections has received increasing attention.7 Failure to maintain a clean environment enhances the risk of MDRO transmission. Notably, the rates of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Acinetobacter baumannii infections have been reduced through systematic improvements to hospital cleaning and disinfection rates.8 Enhanced cleaning practices have been demonstrated to reduce microbial contamination and the rate of S. aureus infection.9
Currently, MDRO environmental contamination in Chinese hospitals is severe. Hospital environmental cleaning is a crucial component of measures for prevention and control of nosocomial infections. Guidelines have been published regarding cleaning and disinfection of environmental surfaces in hospitals;10,11 however, most hospitals in China use customized environmental cleaning policies and systems, rather than protocols established using evidence-based medicine. Nevertheless, there is limited literature regarding the relationship between environmental infection and infectious disease in China.12 Clean hospital environments are safer for patients; thus, a standardized cleaning protocol is urgently needed.
This study assessed the effects of ICU environmental disinfection on the risks of MDRO colonization and infection. Additionally, recommendations for environmental cleaning and disinfection methods were developed and implemented; these methods were evaluated for their abilities to reduce MDRO colonization and infection in ICU patients.
Materials and methods
Ethics approval
This experimental protocol was approved by the Beijing Youan Hospital Research Ethics Committee. Written informed consent to participate was obtained from each included patient, in accordance with the Declaration of Helsinki.
Trial design
Patients were divided into two groups: those surveyed before implementation of enhanced cleaning (control group) and those surveyed after implementation of enhanced cleaning (cleaning group). The surfaces of ICU patients and surrounding frequently touched objects were the research focus in this study. The two patient groups were screened for MDRO colonization at admission to ICU, 48 hours after admission, 7 days after admission, or upon discharge. ICU patients with positive MDRO screening results were selected for peripheral examination of eight frequently touched surfaces: lifting tower or bedside table, bed gear, bed end, patient cuff, suction pipe, monitor panel, bed lifting panel, and urine bag. These surfaces were selected in accordance with the methods used in previous studies.8
Participants
ICU patients in this study had received treatment at one of seven tertiary hospitals (Beijing Youan Hospital, Capital Medical University; Beijing Electric Power Hospital; Dongfang Hospital of Beijing University of Chinese Medicine; Beijing Bo'ai Hospital; Beijing Aerospace General Hospital; Aerospace 731 Hospital; and Beijing Fengtai Hospital) in Beijing, China from 10 October 2014 to 3 February 2017. All patients had been hospitalized in the ICU for more than 7 days and were conscious at the time of sample collection.
Environmental cleaning
All medical staff and cleaning staff in the ICUs of the seven medical centers received training regarding the enhanced cleaning and disinfection methods, as well as the intensity and frequency at which they should be performed. During the baseline period, conventional cleaning methods were performed; one clean cloth was soaked in 500 to 1000 mg/L sodium hypochlorite, then used to wipe patient areas that were frequently touched by health care workers. The cloth was then soaked in sodium hypochlorite disinfectant and reused to clean the next patient area. During the enhanced cleaning period, cleaning and disinfection methods were performed in accordance with the literature.13 Trained nurses were responsible for the daily and terminal cleaning and disinfection of the surfaces of diagnostic and therapeutic equipment and instruments. Trained environmental service workers were responsible for the daily and terminal cleaning and disinfection of the surfaces of other environmental objects. Daily cleaning comprised wiping the surface of environmental objects with quaternary ammonium salt disinfectant wipes (CaviWipes, Metrex, Orange, CA, USA), twice per day; terminal room disinfection comprised wiping with 500 mg/L sodium hypochlorite and the application of ultraviolet irradiation. MDRO monitoring (as described in the “MDRO outcome measurements” section) was carried out to assess the surfaces of frequently touched objects around each patient; when a bed became available, new patients could be accepted only after no MDROs had been detected.
MDRO outcomes measurement
Swabs of patients’ nasal and anal surfaces, as well as swabs of surrounding frequently touched surfaces, were collected for detection of MDROs. Copan transport culture swabs (Copan, Brescia, Italy) were used to collect specimens; color-producing culture medium (HiMedia, Mumbai, India) was used for inoculation and bacterial preservation during transfer to the microbiology laboratory; matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used to perform microbial identification and cluster analysis.
Nasal swabbing was performed as follows: sterile Copan transport culture swabs were stirred in culture medium and inserted into the nostril in a vertical direction. The swab was gently rotated three times, then returned to the HiMedia culture medium and sent to the microbiology laboratory for cultivation. Anal swabbing was performed as follows: sterile Copan transport culture swabs were stirred in culture medium and inserted into the anal canal to a distance of approximately 3 to 4 cm. The swab was gently rotated 360 degrees, then returned to the HiMedia culture medium and sent to the microbiology laboratory for cultivation. Surrounding frequently touched surfaces were swabbed as follows: sterile Copan transport culture swabs were stirred in culture medium and smeared on the surface of the object (five times in a side-to-side manner); they were then returned to the HiMedia culture medium and sent to the microbiology laboratory for cultivation.
Upon arrival at the microbiology laboratory, each cotton swab was used to inoculate ESBL chromogenic medium (CHROMagar, Paris, France), MRSA chromogenic medium (CHROMagar), and VRE chromogenic medium (CHROMagar); the media were cultured in a 37°C incubator for 24 hours for bacterial identification. Bacterial colonies were picked and applied to a MALDI target board; they were then dried, combined with 1 μL of matrix solution, and subjected to MALDI-TOF MS (bioMérieux, Marcy-l'Étoile, France) for identification and typing.
Quality control
Sterile cotton swabs were inserted into the transfer medium, then incubated for 24 hours at 37°C (negative control); swabs of Escherichia coli ATCC 25922 were used as positive control samples.
Data collection
Patient characteristics were recorded, including age, sex, medical conditions, acute physiology and chronic health evaluation (APACHE II score), and length of ICU stay. Additional information collected comprised the frequency, timing, and site of infection.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY, USA). Discrete variables were summarized as frequency (%); continuous variables were summarized as mean and standard deviation or median and interquartile range. Comparisons of continuous variables between groups were performed using Student’s t-test or the Mann–Whitney U test; categorical variables were compared using the chi-squared test. All tests were two-tailed; p < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
In total, 286 patients (131 and 155 in control and cleaning groups, respectively) were screened for inclusion in the study; following exclusion of patients who declined to participate, 196 patients (104 and 92 in control and cleaning groups, respectively) were included. Furthermore, 94 patient bed units were assessed with respect to environmental frequently touched surfaces (48 and 46 in control and cleaning groups, respectively). There were no differences in multiple characteristics between the two groups (Table 1). However, the proportion of high risk factors for infection at ICU admission was higher in cleaning group than in control group; the APACHE II scores were 20.11 ± 7.67 in the control group and 30.40 ± 6.98 in the cleaning group at ICU admission (t = 8.91, p<0.01).
Table 1.
Patient characteristics at intensive care unit admission.
| Variables | Control groupa | Cleaning group | Total | Statisticsb | P |
|---|---|---|---|---|---|
| Age | |||||
| Mean±standard deviation | 67.78±16.06 | 69.1±18.05 | 68.40±16.99 | 0.92 (Rank sum test) | 0.36 |
| Range | 23.00–94.00 | 7.00–94.00 | 7.00–94.00 | ||
| Sex | |||||
| Male | 67 (64.42%) | 68 (73.91%) | 135 (68.88%) | 2.05 | 0.15 |
| Female | 37 (35.58%) | 24 (26.09%) | 61 (31.12%) | ||
| Underlying illness | |||||
| Non-pulmonary infection | 8 (7.69%) | 9 (9.78%) | 17 (8.67%) | 0.27 | 0.60 |
| Cardiovascular disease | 67 (64.42%) | 67 (72.83%) | 134 (68.37%) | 1.59 | 0.21 |
| Nervous system disease | 4 (3.85%) | 4 (4.35%) | 8 (4.08%) | 0.03 | 0.86 |
| Liver and kidney disease | 31 (29.81%) | 5 (5.43%) | 36 (18.37%) | 19.34 | <0.01 |
| Lung infection | 28 (26.92%) | 33 (35.87%) | 61 (31.12%) | 1.82 | 0.18 |
| Others | 49 (47.12%) | 37 (40.22%) | 86 (43.88%) | 0.94 | 0.33 |
| No infection | 36 (34.62%) | 22 (23.91%) | 58 (29.59%) | 2.39 | 0.12 |
| Risk factors for infection when entering the ICU | |||||
| Diabetes | 69 (35.20%) | 34 (32.69%) | 35 (38.04%) | 0.61 | 0.43 |
| Cancer | 19 (9.69%) | 10 (9.62%) | 9 (9.78%) | <0.01 | 0.97 |
| Older than 75 years | 96 (48.98%) | 50 (48.08%) | 46 (50.00%) | 0.07 | 0.79 |
| Immunosuppressant | 6 (3.06%) | 3 (2.88%) | 3 (3.26%) | 0.02 | 0.88 |
| Long bed | 89 (45.41%) | 46 (44.23%) | 43 (46.74%) | 0.12 | 0.73 |
| Cirrhosis | 11 (5.61%) | 9 (8.65%) | 2 (2.17%) | 3.87 | 0.05 |
| Hormone | 11 (5.61%) | 7 (6.73%) | 4 (4.35%) | 0.52 | 0.47 |
| Dialysis | 11 (5.61%) | 9 (8.65%) | 2 (2.17%) | 3.87 | 0.05 |
| Low immune function | 53 (27.04%) | 19 (18.27%) | 34 (36.96%) | 8.64 | |
| Organ transplant | 2 (1.02%) | 2 (2.17%) | 2.28 | 0.13 | |
| Fever | 63 (32.14%) | 21 (20.19%) | 42 (45.65%) | 14.51 | <0.01 |
| Surgery | 26 (13.27%) | 9 (8.65%) | 17 (18.48%) | 4.10 | 0.04 |
| Ventilator | 75 (38.27%) | 29 (27.88%) | 46 (50.00%) | 10.11 | <0.01 |
| Urinary catheter | 108 (55.10%) | 52 (50.00%) | 56 (60.87%) | 2.33 | 0.13 |
| Central venous cannula | 76 (38.78%) | 31 (29.81%) | 45 (48.91%) | 7.51 | 0.01 |
aData shown as n (%), except where indicated.
bComparisons performed using chi-squared test, except where indicated.
ICU, intensive care unit.
MDRO analyses
In total, 1042 MDROs were identified during active screening in 196 patients: 436 were identified in the control group and 606 were identified in the cleaning group. In the control and cleaning groups, 96 and 125 MDROs, respectively (Table 2), were identified during active screening in 94 patients who underwent environmental sampling; the numbers of MDROs did not significantly differ between groups. Notably, 30% to 40% of patients exhibited ≥2 types of MDROs; up to five types of MDROs were detected in a single patient. In both groups, the rate of MDRO colonization decreased from ICU admission until ICU discharge. The rate of MDRO colonization in the control group decreased from 61.83% to 21.37%, while the rate of MDRO colonization in the cleaning group decreased from 71.61% to 31.61%; these rates did not significantly differ between the two groups. As shown in Table 3, 29 patients in the control group exhibited identical bacteria in both clinical specimens and in samples collected nasal or anal cavities during active screening, while 45 patients in the cleaning group exhibited identical bacteria; the proportion of patients with consistent bacteria was higher in the cleaning group than in the control group (p = 0.032).
Table 2.
MDROs swabbed from nasal and anal area.
| Swab location | Control group |
Cleaning group |
P valuea | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Nasal area | |||||
| (+) patients | 30 | 62.50 | 36 | 78.26 | 0.117 |
| Strains | 38 | 63 | |||
| Anal area | |||||
| (+) patients | 41 | 85.42 | 39 | 92.86 | 1.000 |
| Strains | 58 | 62 | |||
| Total strains | 96 | 125 | |||
aP value calculated using chi-squared test.
MDRO, multidrug-resistant organism.
Table 3.
Consistency of detection of pathogenic microorganisms in the intensive care unit and specific bacteria during active screening.
| Time | Totala | Control group | Cleaning group | P valueb |
|---|---|---|---|---|
| Admission to ICU | 19/199c (9.55%) | 6/87 (6.90%) | 13/112 (11.61%) | 0.262 |
| 48 hours in ICU | 15/176 (8.52%) | 8/81 (9.88%) | 7/95 (7.37%) | 0.553 |
| 7 days in ICU | 25/151 (16.56%) | 12/77 (15.58%) | 13/74 (17.57%) | 0.743 |
| Discharge from ICU | 15/122 (12.30%) | 3/56 (5.36%) | 12/66 (18.18%) | 0.032 |
aData shown as n (%).
bP value calculated using chi-squared test.
cNumerator of fraction is number of patients with positive screening results; denominator is real-time number of ICU inpatients.
ICU, intensive care unit.
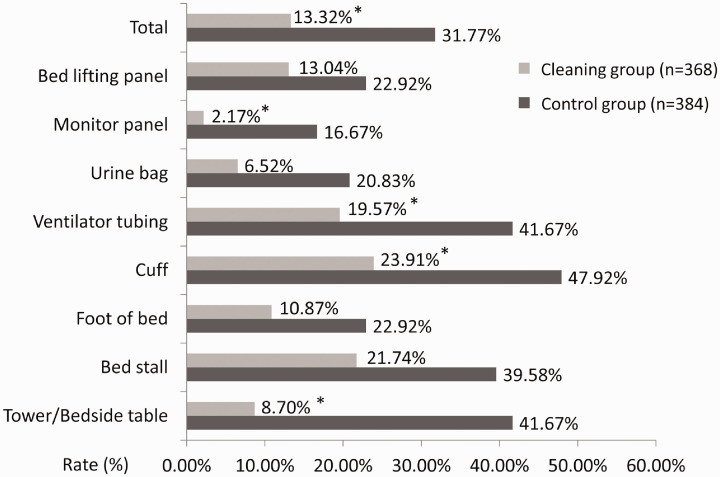
Environmental samples were collected from 384 and 368 frequently touched surfaces of the patients in the control and cleaning groups, respectively; the rate of MDRO detection on the surface of various frequently touched objects decreased from 31.77% to 13.32% (chi-squared value of 36.432, p < 0.001) in the control group. The rate of MDRO detection on the cuff (47.92% vs 23.91%, p = 0.019) significantly differed between the two groups; this rate in the control group was highest among all surfaces sampled in both groups. In the cleaning group, the rates of MDRO detection on the bed lifting panel or bedside table, cuff, ventilator tube, and monitor panel significantly decreased, compared with the control group (p < 0.05, Figure 1).
Figure 1.
Rates of multidrug-resistant organism detection on surfaces of frequently touched objects in intensive care units. Asterisks indicate Cleaning group values that significantly differed from corresponding Control group values.
The types of detected bacteria were compared between groups, including bacteria that were detected repeatedly for each patient. There were more types of bacteria in the control group than in the cleaning group; the number of patients with the same strain in the same hospital was greater in the control group than in the cleaning group. In the control group, 15 patients (31 surfaces) exhibited similar bacteria on the surfaces of multiple frequently touched objects (i.e., those within reach of the bed unit); seven of these patients exhibited similar bacteria at high frequencies in their surroundings: four exhibited Acinetobacter baumannii, one exhibited MRSA, and two exhibited VRE. In the cleaning group, 12 patients (32 surfaces) exhibited similar bacteria on the surfaces of multiple frequently touched objects; four of these patients exhibited similar bacteria at high frequencies in their surroundings. two exhibited MRSA and one exhibited VRE. The number of MDRO homologues was lower in the cleaning group than in the control group, although this difference was not statistically significant.
Patient outcomes
The new infection rate among patients in the cleaning group (14.13%) was lower than the rate among patients in the control group (19.23%). The new infection rate significantly increased from 48 hours to 7 days; this increase tended to be greater in the control group than in the cleaning group, although the difference was not statistically significant (Table 4).
Table 4.
New infection rates of intensive care unit patients.
| Time | Control groupa | Cleaning group | X2 | P |
|---|---|---|---|---|
| 48 hours | 4/104 (3.85%) | 2/92 (2.17%) | 0.460 | 0.498 |
| 48 hours to 7 days | 10/74 (13.51%) | 6/67 (8.96%) | 0.726 | 0.394 |
| >7 days | 6/74 (8.02%) | 5/67 (7.46%) | 0.206 | 0.650 |
| Total | 20/104 (19.23%) | 13/92 (14.13%) | 0.307 | 0.341 |
aData shown as n (%).
The newly infected thousand-day ventilator-associated pneumonia rates of patients in the control and cleaning groups after admission to the ICU were 11.06‰ and 3.6‰, respectively; the thousand-day central line-associated bloodstream infection rates were 2.21‰ and 0.81‰, respectively; and the thousand-day catheter-related urinary tract infection rates were 0.97‰ and 0‰, respectively. These rates significantly differed between the control and cleaning groups (all p < 0.01).
The average number of days in the ICU for all 196 patients was 19.45 ± 18.94 (median of 13 days and maximum of 85 days). The average number of days in the ICU for the control group was 16.34 ± 16.04 (median, 11 days), whereas it was 13.35 ± 12.02 (median, 9 days) for the cleaning group; the interval was shorter in the cleaning group than in the control group (Wilcoxon rank sum test 2.075, p = 0.038). The proportion of ICU patients who died directly or indirectly due to infection was higher in the control group (37/104, 35.57%) than in the cleaning group (22/92, 23.91%; p < 0.05).
Discussion
MDROs are common in ICU patients and the related risk of infection is high;14 the hospital environment is presumed to serve as an important source of MDROs and infections in patients.15 Environmental pollution is closely related to ICU hospital infection outbreaks and the spread of MDROs.5,16 The present study showed that, for patients with MDROs, the presence of MDROs on the surfaces of surrounding frequently touched objects significantly decreased after cleaning. These findings suggest that enhanced environmental cleaning and disinfection measures can reduce the rate of MDRO detection on the surfaces of frequently touched objects around ICU patients, consistent with the results in some prior reports.6,17 Notably, the rates of MDRO detection on the bedside table, cuff, ventilator tubing, and monitor panel significantly decreased after cleaning; although the rates of MDRO detection on bed stalls, bed end, urine bags, and bed lifting panels were reduced, these differences were not statistically significant. The enhanced environmental cleaning measures developed in this study were consistent for both medical and cleaning staff, regardless of the surfaces cleaned. Our study revealed that significant reductions of MDRO detection after cleaning mostly involved surfaces that received contact from medical staff. In contrast, non-significant reductions of MDRO detection after cleaning mostly involved surfaces that received contact from patients, family members, and accompanying staff. A study by Gavalda et al.18 suggested that 13.8 hours after routine cleaning and disinfection, 53.8% of frequently touched objects on patient wards exhibited MDRO colonization. Therefore, environmental cleaning measures should include greater emphasis on hand hygiene among patients and family members; the frequency of cleaning and disinfection should be greater for surfaces that receive contact from patients than for surfaces that receive contact from medical personnel. Furthermore, the frequency of environmental cleaning and disinfection should be further modified in accordance with the frequency at which the surface of each object receives contact from patients; further research is needed to clarify this aspect.
In this study, separate disinfectants containing either chlorine or quaternary ammonium salts were used in a combined approach to reduce MDROs in the environment. A study in Australia regarding frequently touched surfaces in ICUs (e.g., bed units, surrounding items, and furniture)19 revealed that MDROs were detected in 52% of samples, despite use of chlorine-containing disinfectants; another study showed that ultraviolet light, combined with quaternary ammonium disinfectants, was more effective than chlorine-containing disinfectants in terms of reducing patient colonization and risk of infection.20 Thus, improvement of environmental cleaning measures according to target bacteria requires further optimization of daily cleaning and terminal disinfection measures; the effectiveness of these measures requires additional analysis.
Sie et al.21 reported the rates of MDRO colonization in hospitalized patients; notably, patients with MDROs had a higher hospital infection rate than patients without MDROs. In that study, the authors concluded that invasive procedures led to introduction of environmental bacteria into the patients’ bodies. Exogenous infections could also bring autologous bacteria to other parts of the body, thereby causing endogenous infections.22 In patients who undergo invasive procedures and receive antibacterial drug and hormone treatment, the risk of infection with colonized bacteria is significantly increased.23 The present study revealed that the rate of MDRO detection decreased after admission to the ICU; this reduction was more pronounced after cleaning. The numbers of patients with the same bacteria detected in clinical specimens and samples collected from nasal or anal cavities also decreased after cleaning. Notably, MDROs detected in patients in the cleaning group mainly originated from the patients’ own microbiota, rather than from environmental sources. Therefore, enhanced environmental cleaning and disinfection measures may reduce MDRO colonization in ICU patients. However, it remains controversial whether there is a need to perform active screening for MDROs and carry out corresponding reduction measures. Some regions in the United States have implemented legislation that requires active screening for MDROs and the performance of reduction measures; a 2015 expert consensus can serve as a guide for development of environmental cleaning methods.24,25
This study mainly focused on tracking and clustering analysis of the similarities between MDROs in the environment and specific bacteria found through active screening, as well as pathogens that caused infections in patients, using MALDI-TOF MS.26 This approach allows granular typing of bacterial species by analyses of characteristic protein peaks.27 This rapid bacterial analysis enhances traceability efforts and can be used to determine the homology of microorganisms in patients with nosocomial infections. The results of MALDI-TOF MS are reportedly consistent with those of pulsed-field gel electrophoresis (PFGE) and multilocus sequence typing methods with respect to Klebsiella pneumoniae homology analysis.28,29 After cleaning, the presence of VRE strains significantly decreased, presence of K. pneumoniae increased, and fewer types of MRSA were detected. Our study showed that similar types of bacteria were present on patients and their environments, prior to cleaning. After cleaning, similar results were observed. These findings regarding colonization of environmental MDROs were consistent with prior reports.30
Notably, we found that patient characteristics were comparable between groups. However, we found that the APACHE II score was significantly higher in the cleaning group than in the control group, indicating that patients in the cleaning group had more severe conditions. Despite these worse conditions, we found that hospital stay was shorter in the cleaning group than in the control group; the new infection rate was also lower in the cleaning group. Moreover, the cleaning group exhibited a significantly higher survival transfer rate and lower mortality. These results indicate that our enhanced environmental cleaning measures reduced the rate of MDRO detection on the surfaces of frequently touched objects surrounding ICU patients, thus reducing the incidences of multi-drug resistance, ventilator-associated pneumonia, central line-associated bloodstream infection, catheter-related urinary tract infection, nosocomial infection, and resulting mortality.
This study had some limitations. First, because of the urgency and necessity of policy implementation and the prevention and control of MDROs, a randomized controlled trial could not be performed. Thus, we adopted a prospective historical controlled study format, which involved implementation of uniform cleaning and disinfection protocols in seven tertiary hospitals. The control group comprised patients in those hospitals prior to implementation of the enhanced cleaning measures; the cleaning group comprised patients in the same seven hospitals after implementation of the enhanced cleaning measures. However, this historical approach may have introduced some bias. There may have been differences in the climate environment, crowd awareness, diagnosis and treatment approaches, and policy systems. The consistent patient characteristics suggested that the data were reliable. Second, although there is increasing use of MALDI-TOF MS for bacterial homology analysis, which indicates that its results are similar to those of PFGE and multilocus sequence typing, there remain concerns regarding its accuracy. Therefore, the reliability of MALDI-TOF MS for bacterial typing may be inadequate. In future studies, MDROs should be further evaluated using both PFGE and MALDI-TOF MS to enhance the reliability of the findings.
In summary, MDRO-related infections have adverse effects on diagnosis, treatment, and patient prognosis, leading to increased hospital infection rates and increased hospitalization costs. Our findings reveal that implementation of evidence-based environmental cleaning and disinfection protocols can reduce the rate of MDRO-related infections among various patient groups. Hospital environmental hygiene should be viewed in the context of evidence-based medicine; the establishment of standardized environmental cleaning procedures and technical specifications can support hospital environmental infection control efforts.
Acknowledgements
We thank Professor Bin Su at the Beijing Key Laboratory for HIV/AIDS Research for his valuable comments on the draft of the manuscript and for his unreserved support, and also thanks to Beijing MicroFuture Technology Co., Ltd. for its help in Bioinformation data and statistical analysis.
Author contributions
JH, RJ, and XC conceived and designed the experiments. JH, CC, SZ, MC, and HW collected the sample information and contributed to reagents and materials. JH, CC, SZ, and MC performed the experiments. JH, CC, SZ, MC, HW, and XC analyzed the data. JH, RJ, and XC wrote the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Beijing Municipal Administration of Hospitals' Youth Programme (QML20151601 to JH), the NSFC-NIH Biomedical collaborative research program (81761128001 to HW), the National 12th Five-Year Grand Program on Key Infectious Disease Control (2014ZX10001002-001-002 to HW), and the Beijing Municipal of Science and Technology Major Project (D161100000416003 to HW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD
Xinyue Chen https://orcid.org/0000-0002-2180-1750
References
- 1.Landelle C, Marimuthu K, Harbarth S. Infection control measures to decrease the burden of antimicrobial resistance in the critical care setting. Curr Opin Crit Care 2014; 20: 499–506. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet 2017; 389: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JN, Gan TE, Zhu YX, et al. Epidemiology and microbiology of nosocomial bloodstream infections: analysis of 482 cases from a retrospective surveillance study. J Zhejiang Univ Sci B 2015; 16: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y, Chusri S, Sangthong R, et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS One 2019; 14: e0214779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskey CJ. Does improving surface cleaning and disinfection reduce health care-associated infections? Am J Infect Control 2013; 41: S12–S19. [DOI] [PubMed] [Google Scholar]
- 6.Havill NL. Best practices in disinfection of noncritical surfaces in the health care setting: creating a bundle for success. Am J Infect Control 2013; 41: S26–S30. [DOI] [PubMed] [Google Scholar]
- 7.Pogorzelska-Maziarz M, Carter EJ, Manning ML, et al. State health department requirements for reporting of antibiotic-resistant infections by providers, United States, 2013 and 2015. Public Health Rep 2017; 132: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Che J, Lu JX, Li WG, et al. A new high-throughput real-time PCR assay for the screening of multiple antimicrobial resistance genes in broiler fecal samples from China. Biomed Environ Sci 2019; 32: 881–892. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Xu H, Sun J, et al. Shifting trends and age distribution of ESKAPEEc resistance in bloodstream infection, southwest China, 2012-2017. Antimicrob Resist Infect Control 2019; 8: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M, Weinheimer JD, Waites KB, et al. Active surveillance to determine the impact of methicillin-resistant Staphylococcus aureus colonization on patients in intensive care units of a Veterans Affairs Medical Center. Infect Control Hosp Epidemiol 2008; 29: 503–509. [DOI] [PubMed] [Google Scholar]
- 11.Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis 2008; 47: 760–767. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Chen B, Ni X, et al. Computer keyboard and mouse: an intervention study on methicillin-resistant Staphylococcus aureus decontamination in 4 intensive care units. J Crit Care 2017; 37: 266–267. [DOI] [PubMed] [Google Scholar]
- 13.Bloomfield SF, Carling PC, Exner M. A unified framework for developing effective hygiene procedures for hands, environmental surfaces and laundry in healthcare, domestic, food handling and other settings. GMS Hyg Infect Control 2017; 12: Doc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziakas PD, Zacharioudakis IM, Zervou FN, et al. Methicillin-resistant Staphylococcus aureus prevention strategies in the ICU: a clinical decision analysis. Crit Care Med 2015; 43: 382–393. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DJ, Moehring RW, Weber DJ, et al. Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR Disinfection). Lancet Infect Dis 2018; 18: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber DJ, Rutala WA. Understanding and preventing transmission of healthcare-associated pathogens due to the contaminated hospital environment. Infect Control Hosp Epidemiol 2013; 34: 449–452. [DOI] [PubMed] [Google Scholar]
- 17.Carling P. Methods for assessing the adequacy of practice and improving room disinfection. Am J Infect Control 2013; 41: S20–S25. [DOI] [PubMed] [Google Scholar]
- 18.Gavalda L, Pequeno S, Soriano A, et al. Environmental contamination by multidrug-resistant microorganisms after daily cleaning. Am J Infect Control 2015; 43: 776–778. [DOI] [PubMed] [Google Scholar]
- 19.Hu H, Johani K, Gosbell IB, et al. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J Hosp Infect 2015; 91: 35–44. [DOI] [PubMed] [Google Scholar]
- 20.Ghantoji SS, Stibich M, Stachowiak J, et al. Non-inferiority of pulsed xenon UV light versus bleach for reducing environmental Clostridium difficile contamination on high-touch surfaces in Clostridium difficile infection isolation rooms. J Med Microbiol 2015; 64: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sie I, Thorstad M, Andersen BM. Infection control and hand hygiene in nursing homes in Oslo. Tidsskr Nor Laegeforen 2008; 128: 1528–1530. [in Norwegian] [PubMed] [Google Scholar]
- 22.De Macedo CS, Lara FA, Pinheiro RO, et al. New insights into the pathogenesis of leprosy: contribution of subversion of host cell metabolism to bacterial persistence, disease progression, and transmission. F1000Res 2020; 9: F1000 Faculty Rev-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebmann T, Rosenbaum PA. Preventing the transmission of multidrug-resistant Acinetobacter baumannii: an executive summary of the Association for Professionals in Infection Control and Epidemiology's elimination guide. Am J Infect Control 2011; 39: 439–441. [DOI] [PubMed] [Google Scholar]
- 24.Rebmann T, Carrico RM. Preventing Clostridium difficile infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology's elimination guide. Am J Infect Control 2011; 39: 239–242. [DOI] [PubMed] [Google Scholar]
- 25.Rebmann T, Kohut K. Preventing mediastinitis surgical site infections: executive summary of the Association for Professionals in Infection Control and Epidemiology's elimination guide. Am J Infect Control 2011; 39: 529–531. [DOI] [PubMed] [Google Scholar]
- 26.Bader O. MALDI-TOF-MS-based species identification and typing approaches in medical mycology. Proteomics 2013; 13: 788–799. [DOI] [PubMed] [Google Scholar]
- 27.Jadhav S, Bhave M, Palombo EA. Methods used for the detection and subtyping of Listeria monocytogenes. J Microbiol Methods 2012; 88: 327–341. [DOI] [PubMed] [Google Scholar]
- 28.Mencacci A, Monari C, Leli C, et al. Typing of nosocomial outbreaks of Acinetobacter baumannii by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2013; 51: 603–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu MS, Collier S, Liu PY, et al. Sensitivity and specificity of matrix-associated laser desorption/ionization - time of flight mass spectrometry (MALDI-TOF MS) in discrimination at species level for Acinetobacter bacteremia. J Microbiol Methods 2017; 140: 58–60. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Wang X, Wang J, et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: a 4-year quasi-experimental before-and-after study. Antimicrob Resist Infect Control 2019; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]