Abstract
Objective
Microvascular invasion is shown to be an independent risk factor for liver cancer recurrence. Timely treatment may reduce the recurrence rate and prolong total survival time. The aim of this study was to investigate the effectiveness of sorafenib in treating patients with hepatocellular carcinoma (HCC) and microvascular invasion
Methods
A comprehensive literature search was conducted in PubMed, EMBASE, MEDLINE, web of science and Cochrane Library databases for articles published up to December 2019. Two researchers independently reviewed and cross-checked independent reports with sufficient information. A meta-analysis was conducted to assess the impact of sorafenib on mortality in patients with HCC and microvascular involvement.
Results
Four studies were included in the qualitative and quantitative analyses, comprising 955 cancer events and 505 cancer deaths. Meta-analyses showed that sorafenib treatment was associated with an improved survival rate versus no sorafenib treatment in patients with HCC and microvascular invasion (relative risk 1.369, 95% confidence interval 1.193, 1.570).
Conclusions
Sorafenib treatment may improve survival in patients with HCC and microvascular invasion. However, due to the potential for residual confounding, the results should be interpreted with caution.
Keywords: Sorafenib, hepatocellular carcinoma (HCC), microvascular invasion (MVI)
Introduction
Liver cancer is the second leading cause of cancer death worldwide. In particular, hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death, the fifth most common cancer in men and the seventh most common cancer in women.1,2 Partial hepatectomy is the preferred treatment for patients with early HCC,3 but approximately 70% of patients relapse within 5 years following hepatectomy,4 and about 30% of patients with recurrent HCC perform poorly at the time of mid-term diagnosis.5 Hepatocellular carcinoma with microvascular invasion is very common, and microvascular invasion is often associated with early recurrence of tumours and reduced survival. Microvascular invasion refers to the presence of cancer cell nests in the vascular lumen lined by endothelial cells, viewed under the microscope, mainly including branches of the portal vein (including intrathecal vessels).6–8 Microvascular invasion occurs in approximately 15–60% of patients with HCC, and relevant studies have shown that microvascular invasion is an independent risk factor for early recurrence.9–12 Although a few studies have shown that some complementary therapies may help,13,14 there is no commonly accepted adjuvant therapy following hepatectomy.15
Sorafenib is an effective multi-kinase inhibitor that inhibits tumour angiogenesis and proliferation by interfering with the binding of serine/threonine kinases to receptor tyrosine kinases.16 In addition, sorafenib is known to effect both tumour cells and endothelial cells.17 Despite multiple studies, no reliable predictive biomarkers have been identified for the response of patients with HCC to sorafenib, including sorafenib targets such as mitogen activated protein kinase (MAPK)/extracellular-signal regulated kinase (ERK) or vascular endothelial growth factor (VEGF). However, sorafenib is considered to be an effective treatment for advanced liver cancer, and this treatment has been maintained for nearly a decade.18
In view of the global prevalence of liver cancer and the high mortality rate in patients with cancer, it is of great clinical significance to explore sorafenib in the treatment of liver cancer in daily practice. In addition, a modest increase in cancer risk translates into a huge public health and social burden. Thus, the aim of the present study was to investigate the effect of sorafenib on cancer mortality in patients who have HCC with microvascular invasion, via a systematic review of original published reports and meta-analysis of the relevant data.
Materials and methods
Search strategy
In this systematic review and meta-analysis, two researchers (WG and ZT) independently searched the Medline, Embase, PubMed, Cochrane Libraries, and Web of science databases for all relevant articles published between January 2000 and December 2019. Medical subject heading terms used in the search included ‘liver cancer’, ‘hepatocellular carcinoma (HCC)’, ‘hepatic carcinoma’, ‘hepatoma’, ‘sorafenib’, and ‘microvascular invasion’. Titles and abstracts of the retrieved articles were independently screened by the two researchers (WG and ZT) to exclude studies that did not meet the relevant research criteria. The same two researchers then independently reviewed the full text of the remaining articles to determine if they contained the relevant information, and references were examined for any further relevant studies. In cases of incomplete information, attempts were made to contact the corresponding author of the study for more information.
Selection criteria
Selection criteria for inclusion into the present analyses were as follows: (1) study type – cohort or case–control study, or randomized controlled trial; (2) study population – patients with microvascular invasion of HCC; (3) study design – an experimental group who received sorafenib therapy and a group who’s treatment did not include sorafenib; and (4) outcome data – hazard ratio (HR), relative risk (RR), or odds ratio (OR) and corresponding 95% confidence intervals (CIs), or adequate research data for incorporation into the present meta-analysis. There were no language, time, or nationality restrictions. Studies in which each participant acted as their own control (self–controlled studies) were excluded.
Data extraction
For ease of administration, references were merged together using Endnote software, version X9 (Clarivate Analytics, Philadelphia, PA, USA). The two researchers (WG and ZT) independently extracted relevant data from eligible studies, including the study's lead author, publication date, country, research methods, data sources, research time, duration of follow-up, study population characteristics (age and sex), exposure, test dose definition, duration of relevant risk assessment (HR, RR, OR and 95% CI) and relevant confounding factors. To account for any combination therapy, the experimental group was defined as treatment with sorafenib and the control group was defined as treatment without sorafenib.
Quality assessment
The quality of cohort studies was evaluated using the Newcastle–Ottawa scale.19 In this scale, a study is divided into three categories and scored using a star system: subject selection (maximum 4 stars), study group comparability (maximum 2 stars), result/exposure assessment (maximum 3 stars). Studies are categorized into low quality (0–5 stars) or high quality (6–9 stars).
Statistical analyses
Study data were statistically analysed using STATA software, version 15 (StataCorp, College Station, TX, USA). All resultant P values were bilateral, and a P value <0.05 was considered statistically significant. Heterogeneity between studies was assessed using Higgins and Thompson's I2 statistic, where I2 >50% represents high heterogeneity and I2 <50% indicates low heterogeneity.20 Begg’s funnel plot method was used to test for publication bias. DerSimonian and Laird random-effects model or a fixed-effects model was used as the data pooling method.
Results
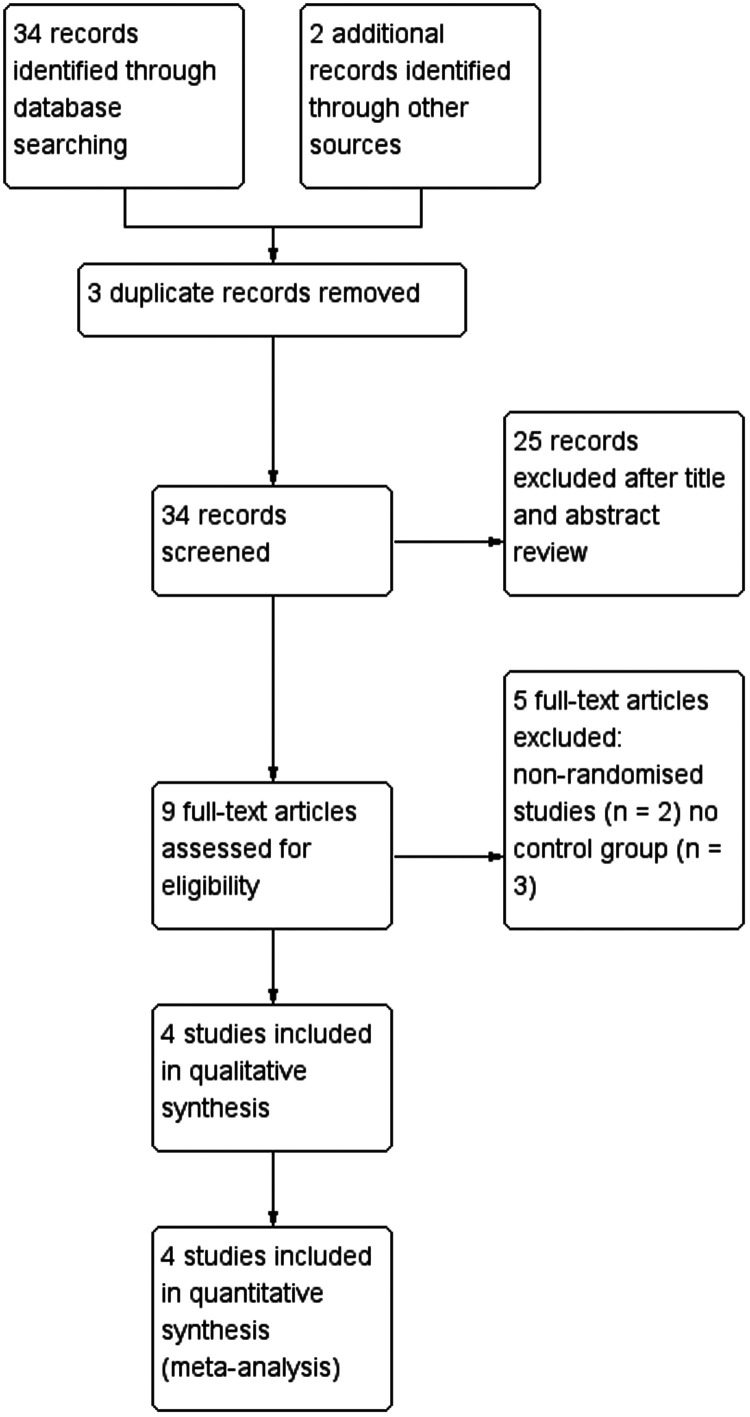
The results of the systematic search process are summarised in Figure 1. Four related studies were reviewed,21–24 with a total of 955 patients with HCC and microvascular invasion, and 505 cancer-related deaths. The characteristics of the included studies are summarized in Table 1. All patients with liver cancer included in the sorafenib treatment group underwent hepatectomy and were treated with sorafenib after surgery.
Figure 1.
Flow chart of article selection.
Table 1.
Characteristics of the four studies included in the meta-analysis.
| Publication | Design | Country | Study population | Total subjects | NOS score | Exposure and control definitions | Median follow-up, months | Adjusting factors |
|---|---|---|---|---|---|---|---|---|
| 21Huang, 2019 | Cohort | China | Patients with HCC and microvascular infiltration | 49 | 6 | Ex: sorafenib following hepatectomy (n = 16); C: no sorafenib following hepatectomy (n = 33) | 22.2 | Study exposure |
| 22Peng, 2019 | Cohort | China | Patients with recurrent HCC and microvascular infiltration following hepatectomy | 127 | 6 | Ex: sorafenib with TACE (n = 55); C: TACE alone (n = 72) | 34.5 | Study exposure and TACE |
| 23Zhang, 2019 | Cohort | China | Patients with HCC and microvascular infiltration | 728 | 7 | Ex: sorafenib following LR (n = 147); C: LR alone (n = 581) | Not reported | Study exposure |
| 24Bi, 2019 | Cohort | China | Patients with HCC and microvascular infiltration | 51 | 6 | Ex: sorafenib following hepatic resection (n = 27); C: hepatic resection alone (n = 24) | Not reported | Study exposure |
Data presented as n prevalence.
HCC, hepatocellular carcinoma; NOS, Newcastle–Ottawa scale; Ex, exposure group; C, controls; TACE, transarterial chemoembolization; LR, liver resection.
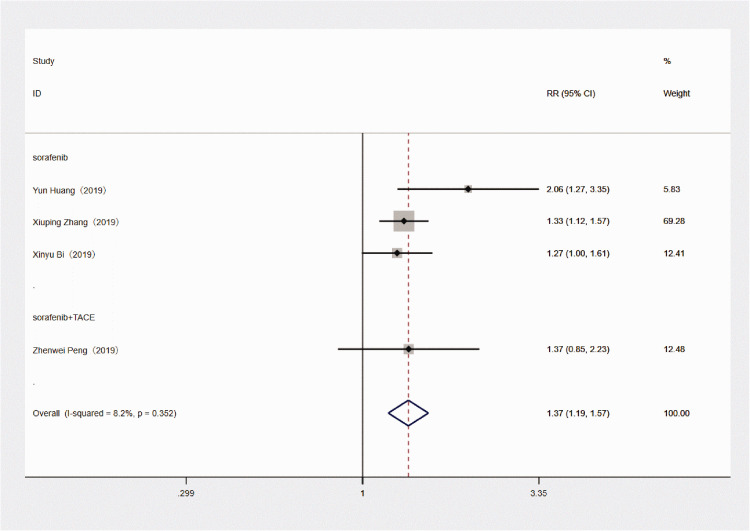
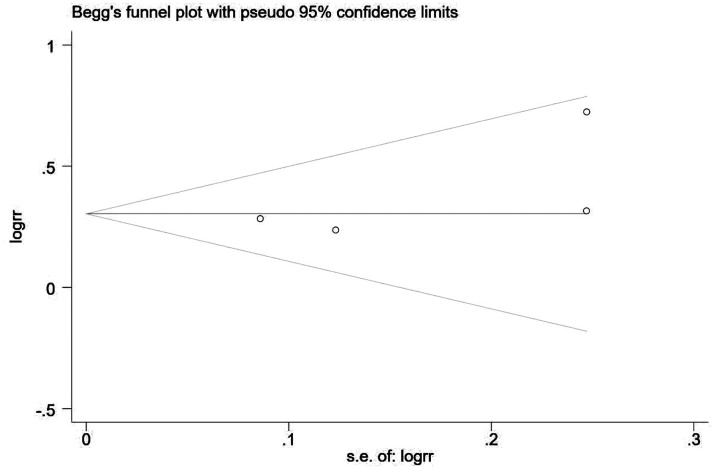
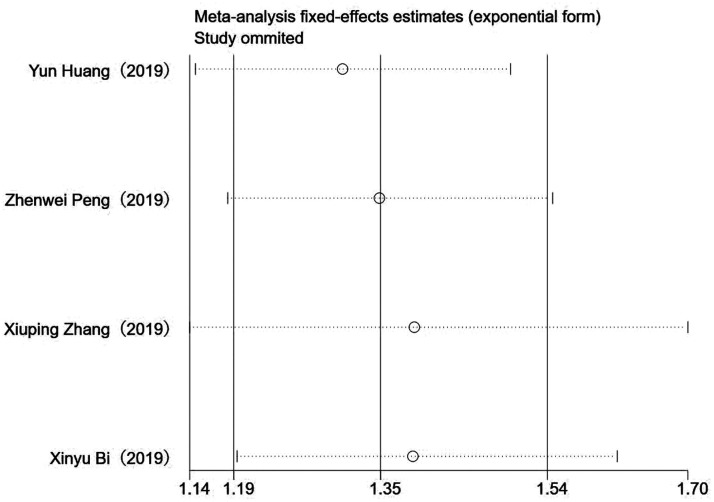
Sorafenib treatment was shown to be associated with significantly improved survival in patients with HCC and microvascular invasion versus patients without sorafenib treatment (RR 1.369, 95% CI 1.193, 1.570; P < 0.001; Figure 2). There was no significant heterogeneity between the four included studies (P = 0.352 >0.01; I2 = 8.2%; Figure 2). A subgroup analysis of the three studies that used sorafenib alone also showed improved survival versus no sorafenib treatment (RR 1.368, 95% CI 1.188, 1.576; P < 0.001), and there was no obvious heterogeneity between the three studies (P = 0.195 >0.01; I2 = 38.3%). Analyses of potential publication bias revealed that there was no publication bias (z = 1.02 >0.05, Pr > z = 0.308; Figure 3). Through the sensitivity analysis, it could be concluded that there is no significant difference among the four studies, with good consistency (Figure 4).
Figure 2.
Forest plot of the relationship between the use of sorafenib and outcome of treatment in patients with hepatocellular carcinoma with microvascular invasion.
Figure 3.
Begg’s funnel plot showing no publication bias in the four included studies.
Figure 4.
Sensitivity analysis of the four included studies.
Discussion
Hepatocellular carcinoma is one of the most common cancers in the world.25 With improvements in technology, the treatment of liver cancer has made great progress. Surgical resection remains the first-line treatment for early and intermediate HCC, however, the recurrence rate following tumour resection is high, and the long-term survival rate of patients is low.26,27 Microvascular invasion is a manifestation of tumour invasion of endothelial cells, and microvascular invasion often leads to poor prognosis.9,10 Hepatectomy is a safe and effective method to treat liver cancer, however, microvascular invasion greatly increases the risk of cancer recurrence following hepatectomy. Several studies have demonstrated that radiofrequency ablation and transarterial chemoembolization are effective treatments for recurrence after hepatectomy and for patients with hepatocellular carcinoma with venular invasion.28–30 A study involving an orthotopic mouse model found that sorafenib prevented the recurrence and metastasis of liver tumours.31 Another study revealed a positive effect with sorafenib adjuvant chemotherapy after surgery in patients with liver cancer with microvascular invasion.32 Therefore, theoretically, the anti-angiogenesis, apoptosis and anti-proliferation effects of sorafenib make it an ideal drug choice following hepatectomy, but there are few relevant published studies to support the theory.
Although the mechanism behind the action of sorafenib is unclear, related studies suggest that sorafenib may be an enzyme inhibitor, whose effect is to inhibit vascular endothelial hyperplasia and thus inhibit microvascular invasion. For example, VEGF is a major factor in the mechanism of tumour angiogenesis and sorafenib has been shown to block serine/threonine kinases (c-RAF and b-RAF) and receptor tyrosine kinases (VEGF receptors 2 and 3, platelet-derived growth factor receptor levels, and FMS tyrosine kinase 3).33 Sorafenib was also shown to significantly inhibit the growth and migration of cancer cells in a mouse model.31 Wang et al.34 demonstrated that sorafenib, as an adjuvant therapy for liver cancer, can prevent early recurrence after hepatectomy. Studies have also found that use of sorafenib can significantly reduce the recurrence rate and improve the survival rate in patients with liver cancer.34–37 However, the study results vary, and it has been shown that although sorafenib can reduce the mortality of patients after radical hepatectomy, and reduce the operation time, it may not reduce the risk of tumour recurrence.38
At present, there are few relevant studies on the efficacy of sorafenib in patients with liver cancer with venular metastasis, and the sample sizes in existing studies are relatively small. Thus, a meta-analysis based on relevant studies was conducted in the present study, in which the efficacy of sorafenib in treating patients with HCC with microvascular invasion was systematically reviewed in four studies. Relevant literature searches were conducted in multiple databases in the hope of retrieving articles as comprehensively as possible, and rigorous scientific methods were used to extract valid data in relevant articles. The present meta-analysis analysis of four studies, involving 955 patients with hepatocellular carcinoma with microvascular invasion, found that the use of sorafenib significantly improved the survival rate of patients with hepatocellular carcinoma with venular invasion compared with patients not treated with sorafenib (RR 1.369; 95% CI 1.193, 1.570; P <0.001), and there was no statistically significant heterogeneity between the studies (I2 = 8.2%). Since there was no significant heterogeneity between studies, any sources of heterogeneity were not further explored. In addition, further subgroup analysis did not show significant heterogeneity, and the positive therapeutic effect of sorafenib was supported.
The results of the present systematic analysis may be limited by the following factors. For example, the adjustments included in the study may be incomplete and inconsistent. Confounders, such as the patient's own status (tumour stage and related complications) and the lack of detail on sorafenib use (dose and duration) in most studies will affect the overall analysis. In addition, treatment information in the studies included in the present analysis was obtained through prescription information in the patient's medical records, and any differences between the prescribed dose and the actual dose may affect the outcome. Thus, given the potential for residual confounding, the results should be interpreted with caution. Further prospective studies are required to confirm the present results and to elucidate the prognostic benefits of sorafenib.
In conclusion, the use of sorafenib was associated with reduced mortality in patients with HCC with venular invasion. However, due to the limitation of relevant research design, and considering the presence of confounding factors, the conclusions should be interpreted with caution.
Acknowledgements
Many thanks to Ms. Han Yanhuan for her support and help in Mr. Gu Wang's writing. Ms. Han was Mr. Gu Wang's favourite person and she will be greatly missed. There is a pre-printed version of the present study, DOI: 10.21203/rs.3.rs-23398/v1.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Wang Gu https://orcid.org/0000-0002-8869-2691 Zhong Tong https://orcid.org/0000-0002-6494-7119
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362: 1907–1917. [DOI] [PubMed] [Google Scholar]
- 4.Onoe T, Yamaguchi M, Irei T, et al. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg Endosc Epub ahead of print 18 December 2019. DOI: 10.1007/s00464-019-07246-3. [DOI] [PubMed]
- 5.Ou DP, Yang LY, Huang GW, et al. Clinical analysis of the risk factors for recurrence of HCC and its relationship with HBV. World J Gastroenterol 2005; 11: 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung TK, Lai CL, Wong BCY, et al. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther 2006; 24: 573–583. [DOI] [PubMed] [Google Scholar]
- 7.Cortese S, Morales J, Martín L, et al. Hepatic resection with thrombectomy in the treatment of hepatocellular carcinoma associated with macrovascular invasion. Cir Esp 2020; 98: 9–17. [DOI] [PubMed] [Google Scholar]
- 8.Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol 2017; 143: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009; 10: 35–43. [DOI] [PubMed] [Google Scholar]
- 10.Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014; 14: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011; 254: 108–113. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013; 20: 325–339. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Wang W, Yao X, et al. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget 2017; 8: 79971–79981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JJ, Wang K, Zhang CZ, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 2016; 23: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 15.Kudo M, Kitano M, Sakurai T, et al. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis 2015; 33: 765–770. [DOI] [PubMed] [Google Scholar]
- 16.Sanoff HK, Chang Y, Lund JL, et al. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist 2016; 21: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008; 7: 3129–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Zhang Z, Zhou Y, et al. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? Onco Targets Ther 2019; 12: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Z, Chen S, Xiao H, et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology 2019; 292: 237–247. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XP, Chai ZT, Gao YZ, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB (Oxford) 2019; 21: 1687–1696. [DOI] [PubMed] [Google Scholar]
- 24.Bi X, Gao J, Cai J. Sorafenib versus transarterial chemoembolization as adjuvant therapies for patients with hepatocellular carcinoma and microvascular invasion. J Clin Oncol 2019; 37: 244–244. [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M. and American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011, 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002; 35: 519–524. [DOI] [PubMed] [Google Scholar]
- 28.Feng X, Xu R, Du X, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC stage 0–B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol 2014; 109: 1891–1899. [DOI] [PubMed] [Google Scholar]
- 29.Yao Q, Zhang H, Xiong B, et al. Combination of sorafenib and TACE inhibits portal vein invasion for intermediate stage HCC: a single center retrospective controlled study. Oncotarget 2017; 8: 79012–79022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TY, Lin CC, Chen CY, et al. Combination of transcatheter arterial chemoembolization and interrupted dosing sorafenib improves patient survival in early-intermediate stage hepatocellular carcinoma: a post hoc analysis of the START trial. Medicine (Baltimore) 2017; 96: e7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng YX, Wang T, Deng YZ, et al. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology 2011; 53: 483–492. [DOI] [PubMed] [Google Scholar]
- 32.Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009; 137: 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai WT, Cheng AL, Shiau CW, et al. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol 2011; 55: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 34.Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res 2014; 44: 523–531. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Hou Y, Cai XB, et al. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 2016; 22: 4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang L, Wen T, Xu M, et al. Sorafenib combined with hepatectomy in patients with intermediate-stage and advanced hepatocellular carcinoma. Arch Med Sci 2017; 6: 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia F, Wu LL, Lau WY, et al. Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J Gastroenterol 2016; 22: 5384–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends 2014; 8: 333–338. [DOI] [PubMed] [Google Scholar]