Abstract
Colorectal cancer (CRC) is a leading cause of cancer death worldwide. Recent advances in genomic medicine have identified novel gene mutations that contribute to an increased risk of CRC. Here, we describe a diagnosis of colon cancer in a 63-year-old woman and also in her brother. Next-generation sequencing showed that both patients harbored a germline mutation in NF1. The female patient also carried co-mutations in KRAS and NRAS. Furthermore, the NF1 germline mutation was identified in a healthy offspring of the brother. The female patient received three cycles of bevacizumab plus capecitabine/oxaliplatin therapy and achieved stable disease of the primary lesion in the colon and partial response of metastasis in the right abdominal cavity. This study highlights the association of NF1 germline mutations with colon cancer.
Keywords: Colon cancer, NF1 germline mutation, next-generation sequencing, family pedigree, neurofibromin, bevacizumab
Introduction
Neurofibromatosis type 1 (NF1) is a common autosomal dominant disorder occurring in about 1 per 3500 individuals.1 The main manifestations of NF1 are café-au-lait spots, skinfold freckling, neurofibromatosis, optic gliomas, and Lisch nodules of the iris.2 Patients with NF1 have an increased risk for developing malignancies, the most common of which are malignant peripheral nerve sheath tumors and gliomas.3,4
NF1 is caused by germline mutations in the NF1 tumor suppressor gene, located on chromosome 17q11.2.4,5 NF1 is one of the largest genes identified, encompassing a genome size of 282 kb including 57 constitutive exons and at least 3 alternatively spliced exons.6 Neurofibromin, the NF1 gene product, belongs to the family of GTPase-activating proteins and is involved in negative regulation of the Ras-mediated signaling pathway, which controls cell growth and proliferation.7 Hence, a germline mutation in the NF1 gene results in the formation of a truncated or non-functional neurofibromin that can activate or up-regulate the Ras-mediated signaling pathway, leading to the formation of benign or malignant tumors.8 Germline mutations in the NF1 gene have been identified in several tumors including gliomas, malignant peripheral nerve sheath tumors, neurofibromas, pheochromocytomas, and breast cancer.4,9–12 Here, we report two cases of colon cancer in a brother and sister with NF1 germline mutations.
Case Report
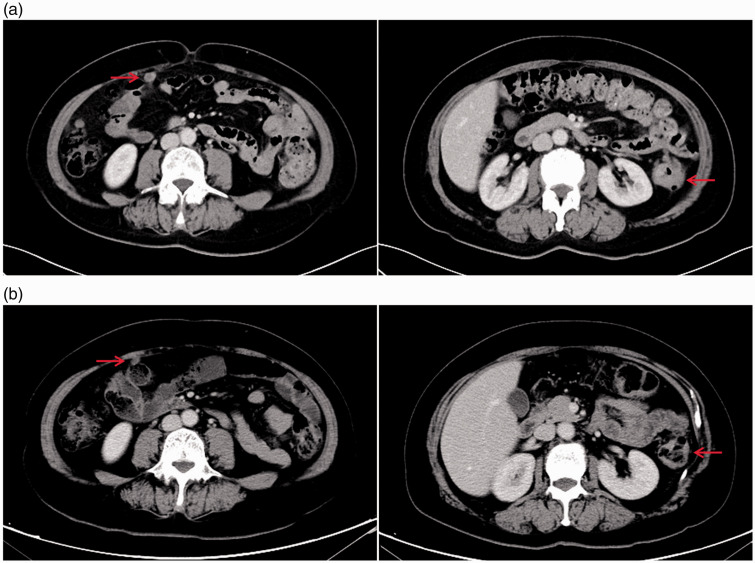
A 63-year-old female patient developed a transverse colon mass and a polyp of the sigmoid colon, which was discovered by colonoscopy during a physical examination on 6 December 2018 (Figure 1). On 10 December 2018, a computed tomography (CT) scan showed multiple nodules in both lungs, a thickened wall of the splenic flexure of the colon, and metastasis in the right abdominal cavity (Figure 2a). Laboratory examination revealed that the levels of CA199 and CA242 were increased to 298.25 IU/mL (normal range: <37.00 IU/mL) and 167.50 U/mL (normal range: 0.00–20.00 U/mL), respectively. Pathological examination revealed a transverse colon adenocarcinoma with sigmoid colon tubulovillous adenomas (Figure 3). On the basis of these imaging and pathology findings, the patient was diagnosed with stage IV adenocarcinoma (cTxNxM1b). Subsequently, the patient received three cycles of treatment with bevacizumab (510 mg, ivgtt, day 1) plus capecitabine (1750 mg, bid, days 1–14)/oxaliplatin (220 mg, ivgtt, day 1) (CapOx) from 26 December 2018 to 11 February 2019. On 3 February 2019, a CT scan showed a thickened wall of the splenic flexure of the colon and metastasis in the right abdominal cavity (Figure 2b), which indicated stable disease (SD) of the primary lesion in the colon and partial response (PR) of metastasis in the right abdominal cavity. The patient’s tumor tissues and blood samples were subjected to next-generation sequencing (NGS) in December 2018. A germline mutation of NF1 L581Ffs*6 (VAF [variant allele frequency]: 24%) and somatic mutations of KRAS A146P (VAF: 10%), NRAS Q61K (VAF: 12%), TP53 R175H (VAF: 13%), and CD274 (PD-L1) T290M (VAF: 11%) were detected.
Figure 1.
Colonoscopy showing a transverse colon mass and polyp of the sigmoid colon.
Figure 2.
Computed tomography (CT) scans pre-chemotherapy (a) and post-chemotherapy (b).
Figure 3.
Hematoxylin eosin (HE) staining. Pathological examination revealed a transverse colon adenocarcinoma with sigmoid colon tubulovillous adenoma.
The patient’s family medical history revealed that her older brother, aged 67 years, had also been diagnosed with sigmoid colon cancer with a tubular adenoma in a cecum polyp 7 years previously. Given the early stage of the tumor at the time of diagnosis, radical resection of the colon cancer had been performed and no recurrence or metastases had been identified to date. The patient's first-degree relatives subsequently consented to genetic testing (Figure 4). The same NF1 L581Ffs*6 germline variation (VAF: 31%) was identified in her older brother. One healthy son of the patient's older brother also carried the same germline mutation, but other healthy relatives did not. This study was approved by the medical ethics committee of Sir Run Run Shaw Hospital. Written informed consent was obtained from all individuals prior to genetic testing.
Figure 4.
Family pedigree of the female patient.
Discussion
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer death worldwide, with over 1.8 million new cases and 881,000 deaths estimated in 2018.13 Among patients with CRC, 75% of cases were sporadic while 25% represented a family history of cancer.14 In addition, approximately 30% of all CRC cases are an inherited form of the disease, including Lynch syndrome, familial adenomatous polyposis, Li–Fraumeni syndrome, MUTYH-associated polyposis, Peutz–Jeghers syndrome, and Juvenile polyposis syndrome,15 resulting from highly penetrant inherited gene mutations. Some germline mutations are associated with an increased risk of CRC, including APC, MSH2, MLH1, and STK11.15
In the present case study, a patient and her brother had a diagnosis of colon cancer. Of note, both patients were diagnosed with intestinal polyps and pathological assessment revealed tubulovillous adenomas. Most tubular adenomas have a low risk of becoming malignant, with only a small proportion progressing to high-grade dysplasia or cancer.16 At present, the most prominent carcinogenic gene associated with familial colorectal adenomatous polyposis is the APC gene.15 Although the classical Mendelian syndrome (Lynch syndrome and familial adenomatous polyposis) is related to high-penetrance gene inheritance,15 many other genes that exhibit moderate penetrance have not been associated with a risk of cancer.17 NF1 mutations are predicted to cause nonsense mutation-mediated mRNA degradation in the cell, which may result in an abnormality or functional loss of the NF1 gene.5 Following a report by Li et al.18 in which 1 out of 22 colorectal tumors had mutated NF1, NF1 has been suggested to play a role in colorectal tumorigenesis. Loss of heterozygosity in the NF1 gene has been identified in primary colorectal tumors.19 Patients carrying NF1 mutations had significantly shorter progression-free survival compared with those having wild-type NF1.20 The NF1 L581Ffs*6 mutation detected in the present case study was a heritable germline variation, and a first-degree relative of the patient had a 50% probability of carrying the same variation. Our patient and her brother carried the same germline mutation of NF1. As expected, one of two of the brother’s sons also carried the germline mutation at the same variant site. Accordingly, it could be speculated that the NF1 germline mutation is a heritable variation that may contribute to the occurrence of familial polyposis and increase the risk of colon cancer.
The large size of the NF1 gene, the existence of pseudogenes, and the diversity of mutation types means that traditional mutation detection in patients with a NF1 mutation is a complicated, time-consuming, and laborious process.21 However, the development of NGS technology has been able to mitigate deficiencies in the single Sanger sequencing method to some extent.22 In addition, possible genotype–phenotype correlations are rarely identified in NF1.23 Although a number of NF1 manifestations have been described,2 none were found in the female patient or in other relatives with NF1 mutations, further supporting the absence of a clear relationship between specific mutations and clinical features. The present case highlights the importance of diagnosis in patients with hereditary cancer syndromes, especially for asymptomatic family members.
NF1 mutations have been found in 45% to 93% of melanomas that promote connective tissue proliferation.24 NF1 deletions in melanoma often co-occur with mutations of the Ras pathway, such as RASA2, PTPN11, and SOS1, indicating that mutations in NF1 may co-associate with additional activating alterations in the RAS/MAP kinase pathway.25 In the present case, a patient with an NF1 germline mutation also carried a co-mutation of KRAS and NRAS, indicating that NF1 loss and RAS were not mutually exclusive in colon cancer. Bevacizumab is a monoclonal antibody against vascular endothelial growth factor (that has a significant clinical benefit in patients with metastatic CRC harboring either mutant or wild-type K-RAS,26 and combination therapy of CapOx plus bevacizumab has shown promising activity and a favorable safety profile in this patient population.27 In our case study, the female patient received bevacizumab plus CapOx therapy and showed SD of the primary lesion in the colon and PR of metastasis in the right abdominal cavity.
In summary, we identified a germline NF1 L581Ffs*6 mutation in three members of a Chinese family, two of whom had been diagnosed with CRC. Given the heredity of NF1 germline mutations, it is necessary to closely follow up with family members of patients who carry such a mutation. Clarification of the pathogenic role of germline mutations may facilitate the planning of appropriate preventive strategies for family members harboring high-risk mutations.
Declaration of conflicting interest
Yihong Zhang and Mei Yang are employees of OrigiMed. The other authors declare that there is no conflict of interest.
Funding
This study was supported by the Zhejiang Natural Sciences Foundation Grant (LY14H160018) and the Ningbo Science and Technology Grant (CN2018027).
ORCID iD
References
- 1.Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol 2009; 61: 1–14; quiz 15-16. DOI: 10.1016/j.jaad.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsipi M, Poulou M, Fylaktou I, et al. Phenotypic expression of a spectrum of Neurofibromatosis Type 1 (NF1) mutations identified through NGS and MLPA. J Neurol Sci 2018; 395: 95–105. DOI: 10.1016/j.jns.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006; 107: 1065–1074. DOI: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 4.D'Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med 2019; 25: 176–187. DOI: 10.1038/s41591-018-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 1990; 249: 181–186. [DOI] [PubMed] [Google Scholar]
- 6.Hinman MN, Sharma A, Luo G, et al. Neurofibromatosis type 1 alternative splicing is a key regulator of Ras signaling in neurons. Mol Cell Biol 2014; 34: 2188–2197. DOI: 10.1128/MCB.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brems H, Beert E, de Ravel T, et al. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol 2009; 10: 508–515. DOI: 10.1016/S1470-2045(09)70033-6. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Lei D, Liang S, et al. Novel phenotypes of NF1 patients from unrelated Chinese families with tibial pseudarthrosis and anemia. Oncotarget 2017; 8: 39695–39702. DOI: 10.18632/oncotarget.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos B, Balmana J, Gardenyes J, et al. Germline mutations in NF1 and BRCA1 in a family with neurofibromatosis type 1 and early-onset breast cancer. Breast Cancer Res Treat 2013; 139: 597–602. DOI: 10.1007/s10549-013-2538-6. [DOI] [PubMed] [Google Scholar]
- 10.Upadhyaya M, Kluwe L, Spurlock G, et al. Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs). Hum Mutat 2008; 29: 74–82. DOI: 10.1002/humu.20601. [DOI] [PubMed] [Google Scholar]
- 11.Pemov A, Li H, Patidar R, et al. The primacy of NF1 loss as the driver of tumorigenesis in neurofibromatosis type 1-associated plexiform neurofibromas. Oncogene 2017; 36: 3168–3177. DOI: 10.1038/onc.2016.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gieldon L, Masjkur JR, Richter S, et al. Next-generation panel sequencing identifies NF1 germline mutations in three patients with pheochromocytoma but no clinical diagnosis of neurofibromatosis type 1. Eur J Endocrinol 2018; 178: K1–K9. DOI: 10.1530/EJE-17-0714. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. DOI: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 14.Rohlin A, Rambech E, Kvist A, et al. Expanding the genotype-phenotype spectrum in hereditary colorectal cancer by gene panel testing. Fam Cancer 2017; 16: 195–203. DOI: 10.1007/s10689-016-9934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology 2010; 138: 2044–2058. DOI: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo AA, Gao J, Rao MS, et al. Composite Epstein-Barr virus-associated B-cell lymphoproliferative disorder and tubular adenoma in a rectal polyp. Int J Surg Pathol 2016; 24: 73–77. DOI: 10.1177/1066896915604736. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Provenzale D, Regenbogen SE, et al. NCCN guidelines insights: Genetic/Familial High-Risk Assessment: Colorectal, version 3.2017. J Natl Compr Canc Netw 2017; 15: 1465–1475. DOI: 10.6004/jnccn.2017.0176. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Bollag G, Clark R, et al. Somatic mutations in the neurofibromatosis 1 gene in human tumors. Cell 1992; 69: 275–281. DOI: 10.1016/0092-8674(92)90408-5. [DOI] [PubMed] [Google Scholar]
- 19.Cawkwell L, Lewis FA, Quirke P. Frequency of allele loss of DCC, p53, RBI, WT1, NF1, NM23 and APC/MCC in colorectal cancer assayed by fluorescent multiplex polymerase chain reaction. Br J Cancer 1994; 70: 813–818. DOI: 10.1038/bjc.1994.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei Z, Shao YW, Lin P, et al. SMAD4 and NF1 mutations as potential biomarkers for poor prognosis to cetuximab-based therapy in Chinese metastatic colorectal cancer patients. BMC Cancer 2018; 18: 479. DOI: 10.1186/s12885-018-4298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao B, Chen S, Chen X, et al. Clinical characteristics and spectrum of NF1 mutations in 12 unrelated Chinese families with neurofibromatosis type 1. BMC Med Genet 2018; 19: 101. DOI: 10.1186/s12881-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Wu H, Shi X, et al. Comparison of next-generation sequencing, quantitative PCR, and Sanger sequencing for mutation profiling of EGFR, KRAS, PIK3CA and BRAF in clinical lung tumors. Clin Lab 2016; 624: 689–696. [DOI] [PubMed] [Google Scholar]
- 23.Ko JM, Sohn YB, Jeong SY, et al. Mutation spectrum of NF1 and clinical characteristics in 78 Korean patients with neurofibromatosis type 1. Pediatr Neurol 2013; 48: 447–453. DOI: 10.1016/j.pediatrneurol.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest 2017; 97: 146–157. DOI: 10.1038/labinvest.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014; 74: 2340–2350. DOI: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 2009; 14: 22–28. DOI: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 27.Schmiegel W, Reinacher-Schick A, Arnold D, et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol 2013; 24: 1580–1587. DOI: 10.1093/annonc/mdt028. [DOI] [PubMed] [Google Scholar]