Abstract
Background
Vibrio cholerae are oxidase-positive bacteria that are classified into various serotypes based on the O surface antigen. V. cholerae serotypes are divided into two main groups: the O1 and O139 group and the non-O1/non-O139 group. O1 and O139 V. cholerae are related to cholera infection, whereas non-O1/non-O139 V. cholerae (NOVC) can cause cholera-like diarrhea. A PubMed search revealed that only 16 cases of necrotizing fasciitis caused by NOVC have been recorded in the scientific literature to date. We report the case of a Japanese woman who developed necrotizing fasciitis caused by NOVC after traveling to Taiwan and returning to Japan.
Case presentation
A 63-year-old woman visited our hospital because she had experienced left knee pain for the past 3 days. She had a history of colon cancer (Stage IV: T3N3 M1a) and had received chemotherapy. She had visited Taiwan 5 days previously, where she had received a massage. She was diagnosed with septic shock owing to necrotizing fasciitis. She underwent fasciotomy and received intensive care. She recovered from the septic shock; however, after 3 weeks, she required an above-knee amputation for necrosis and infection. Her condition improved, and she was discharged after 22 weeks in the hospital.
Conclusions
With the increase in tourism, it is important for clinicians to check patients’ travel history. Clinicians should be alert to the possibility of necrotizing fasciitis in patients with risk factors. Necrotizing fasciitis caused by NOVC is severe and requires early fasciotomy and debridement followed by intensive postoperative care.
Keywords: Necrotizing fasciitis, Vibrio cholerae, Taiwan, Massage, Septic shock, Polymyxin B
Background
Vibrio cholerae are curved gram-negative rod (GNR) bacteria that are oxidase positive. They are classified into various serotypes based on the O surface antigen. V. cholerae serotypes are divided into two main groups: the O1 and O139 group and the non-O1/non-O139 group [1]. O1 and O139 V. cholerae are related to cholera infection, whereas non-O1/non-O139 V. cholerae (NOVC) can cause cholera-like diarrhea. NOVC are found as autochthonous microbes in coastal and marine environments [2]. Outbreaks of cholera-like illness caused by NOVC have been reported in the United States (O141 and O75), former Czechoslovakia (O37), Sudan (O37), Peru (O10, O12), and Mexico (O14) [3–5]. Moreover, NOVC can cause a range of extraintestinal infections, including bacteremia, meningitis, pneumonia, peritonitis, cholangitis, salpingitis, and soft-tissue infection [6]. Seafood, including oysters, fishes, shrimps, clams, mussels, and apple snail, is the most common source of infection (53.9%) [7]. A PubMed search revealed that only 16 cases of necrotizing fasciitis caused by NOVC have been reported in the scientific literature to date. We report the case of a patient who developed necrotizing fasciitis and septic shock caused by NOVC, which necessitated an above-knee amputation of her left leg.
Case presentation
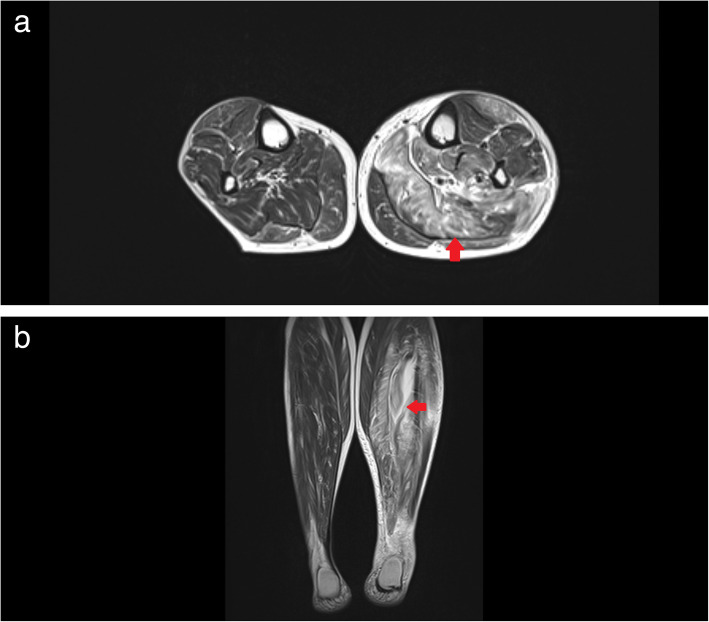
A 63-year-old woman visited Minaminara General Hospital in Nara, Japan, because she had experienced left knee pain for 3 days prior to her visit. She had been diagnosed with colon cancer (Stage IV: T3N3 M1a) 2 years and 5 months previously and had undergone surgery and received chemotherapy. Her most recent dose of chemotherapy was administered 20 days before her initial consultation. She had visited Taiwan 5 days previously, where she had received a massage. After the massage, she developed gradually worsening pain in her lower left leg. On presentation, she was able to walk unaided, and she reported her history of colon cancer and recent travel. As we suspected that the pain in her leg could be due to necrotizing fasciitis, we requested magnetic resonance imaging (MRI) of her left lower leg. The images showed a swollen soleus muscle and posterior tibial muscle, and the T2-weighted image showed hyperintensity of the muscle tissue (Fig. 1). After the MRI, our patient’s condition deteriorated and the following vital signs were observed: blood pressure (BP), 89/50 mmHg; heart rate, 101 beats/min; respiratory rate, 18 breaths/min; and temperature, 36.3 °C. The results of arterial blood gas analysis were as follows: pH, 7.4; PaCO2, 26.7 mmHg; HCO3−, 18.8 mmHg; base excess (BE), − 6.9 mEq/L; and lactate, 3.20 mmol/L. The patient’s laboratory test results were as follows: C-reactive protein (CRP), 42.83 mg/dL; blood urea nitrogen (BUN), 73.3 mg/dL; creatinine, 1.78 mg/dL; procalcitonin, 16.06 ng/mL; N-terminal pro-brain natriuretic peptide (Nt-proBNP), 29,506 pg/mL; and fibrin/fibrinogen degradation products (FDP), 16.7 μg/mL.
Fig. 1.
T2-weighted magnetic resonance images of the patient’s lower legs. a. Coronal image; b. Axial image. These images show that the soleus and posterior tibial muscles on the left lower leg (indicated by red arrows) are swollen and inflamed
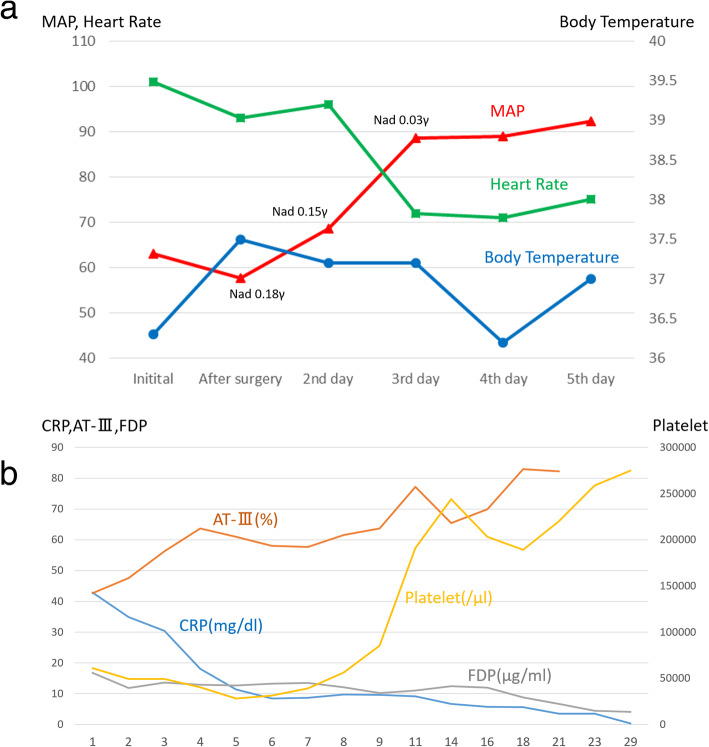
Intravenous infusion of meropenem and noradrenaline was initiated, and the patient underwent emergency surgery. Before the surgery, the compartment pressure of her left leg was measured by simple needle manometry. The pressures were as follows: 63 mmHg, 26 mmHg, 32 mmHg, and 32 mmHg in the anterior, lateral, superficial posterior, and deep posterior compartments, respectively. Some muscle tissues in the anterior and deep posterior compartments were necrotic. For double incision fasciotomy, a relaxation incision was made on her left knee [8], and the affected area was irrigated and debrided (Fig. 2). After the surgery, her blood pressure was low, and therefore, we administered polymyxin B direct hemoperfusion (PMX-DHP), to trap endotoxins, and continuous veno-venous hemodiafiltration (using HEMOFEEL CH-1.3 W, Toray Medical Co., Ltd., Urayasu, Japan). As a slightly curved GNR that was oxidase positive was detected in her blood, we diagnosed her with necrotizing fasciitis and septic shock caused by Vibrio species. We changed the antibiotics from meropenem to ceftriaxone, levofloxacin, and minocycline. We used the PMX-DHP once again and tapered the dose of noradrenalin gradually. We discontinued noradrenalin on Day 4 postoperatively. On Day 6 postoperatively, the organism was identified as NOVC. The susceptibility of antibiotics was confirmed postoperatively on Day 12, and we discontinued levofloxacin (Table 1). Although the patient’s general condition improved, there was a discharge of pus from the postoperative wound. On Day 14 postoperatively, a second debridement was performed. Several muscles in the patient’s left leg, including the anterior tibial muscle, had become necrotic, and the necrosis had spread to her knee. On Day 21 postoperatively, an above-knee amputation was performed. Her vital signs and laboratory data obtained since admission are shown in Fig. 3. Her condition improved and she was discharged 22 weeks after admission.
Fig. 2.
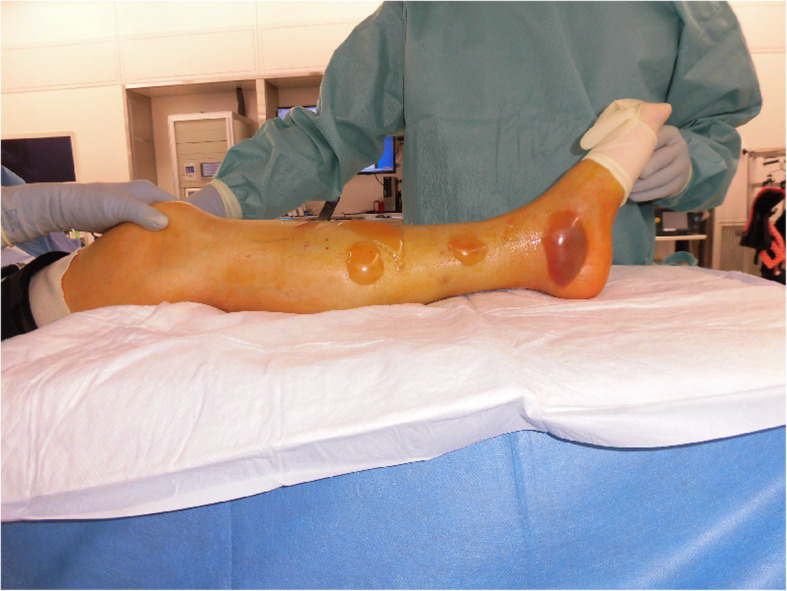
Photographs of lesions in the patient’s leg. The patient’s leg before surgery shows multiple large blisters
Table 1.
Susceptibility of antibiotics
| Antibiotics | Minimal inhibitory concentration |
|---|---|
| Ampicillin | S 2 |
| Piperacillin | S 16 |
| Ceftazidime | S 4 |
| Imipenem/Cilastatin | S ≦1 |
| Amoxicillin/Clavulanate | S 4 |
| Gentamicin | S 2 |
| Minocycline | S ≦1 |
| Chloramphenicol | S ≦2 |
| Sulfamethoxazole-Trimethoprim | S ≦10 |
| Levofloxacin | S ≦0.5 |
| Fosfomycin | R >128 |
Fig. 3.
Change of vital signs and laboratory data during the hospital admission. a. Changes in the patient’s vital signs during days 0–5 of hospitalization. b. Changes in patient’s blood biochemistry during days 1–29 of hospitalization. (AT-III, Antithrombin III; CRP, C-reactive protein; FDP, fibrin/fibrinogen degradation products; MAP, mean arterial pressure; Nad, noradrenaline)
Discussion and conclusion
Sixteen cases of necrotizing fasciitis caused by NOVC have been previously reported (Table 2) [9–17]. The majority of patients were exposed to seawater or had an injury. In rare cases, vigorous massage is one of the risk factors of necrotizing fasciitis [18]. However, the patient in the present case had a risk of NOVC infection because of colon cancer and immunosuppression due to chemotherapy as she received chemotherapy within a month. Thus, in this case, the source of the NOVC remains unknown. As the patient did not report any exposure to sea water or eating seafood, the only potential cause of injury to her left leg was the massage she received. Therefore, we speculate that the massage might have been the source of the NOVC, based on the circumstantial evidence. We administered blood purification therapy using PMX-DHP and veno-venous hemodiafiltration for septic shock. Although no previous studies have reported the use of PMX-DHP for NOVC, a study reported the use of PMX for V. vulnificus [19]. Third-generation cephalosporins, tetracycline, and fluoroquinolone were used for severe Vibrio infections. Tetracycline combined with the fluoroquinolone or a parenteral third-generation cephalosporin followed by oral fluoroquinolones or doxycycline was recommended for invasive NOVC infections [10, 14]. An in vitro study revealed that cefotaxime and minocycline have a synergistic effect in the treatment for V. cholerae infections [20]. As patients with NOVC bacteremia require antibiotic treatment for at least 1 month [14], we administered ceftriaxone and minocycline for 1 month. Necrotizing soft-tissue infections caused by NOVC are more lethal than those caused by V. vulnificus [21].
Table 2.
Clinical characteristics of patients with non-O Vibrio cholerae necrotizing fasciitis
| Year of report (Source) |
Age, Sex | Underlying diseases/risk factors | Treatment | Outcome | Country | O-Antigen | Epidemiologic exposure |
|---|---|---|---|---|---|---|---|
|
1995 (9) |
51 M | Diabetes mellitus | Surgery (amputation, multiple debridement),and antibiotics (ticarcillin/clavulanate, imipenem, gentamicin, clindamycin) | Survived | USA | Exposure of a chronic plantar ulcer to sand in a bathhouse | |
|
1998 (10) |
48 M | Cirrhosis | Surgery, cefotaxime, minocycline, cefotaxime | Survived | Taiwan | ||
|
1998 (10) |
79 F | Cirrhosis, congestive heart failure | Surgery, ceftriaxone | Died | Taiwan | ||
|
2004 (11) |
68 M | Cirrhosis, diabetes mellitus | Surgical debridement, ceftazidime, doxycycline | Died | Taiwan | O56 | |
|
2004 (12) |
70 M | Hepatitis C | Surgery, antibiotics (third-generation cephalosporin, doxycycline) | Died | Taiwan | Handling seafood | |
|
2004 (12) |
63 M | Hepatitis, steroids | Surgery, antibiotics (third-generation cephalosporin, doxycycline) | Survived | Taiwan | Exposure to sea water | |
|
2006 (13) |
52 M | Cirrhosis | Surgery, clindamycin, ceftazidime, tetracycline | Survived | Taiwan | Probable wound infection | |
|
2009 (14) |
57 M | Cirrhosis, hepatitis C, diabetes mellitus | Surgery, antibiotics | Died | Taiwan | Consumption of raw seafood | |
|
2009 (14) |
58 M | Cirrhosis, hepatitis B, hepatitis C, diabetes mellitus | Surgery, antibiotics | Died | Taiwan | Seawater exposure | |
|
2009 (14) |
69 M | Cirrhosis, diabetes mellitus | Surgery, antibiotics | Died | Taiwan | Seawater exposure | |
|
2009 (14) |
70 M | Cirrhosis | Surgery, antibiotics | Survived | Taiwan | Seawater exposure | |
|
2009 (14) |
62 M | COPD | Surgery, antibiotics | Survived | Taiwan | Insect bite wound infection | |
|
2011 (15) |
49 M | HIV, hepatitis C, cirrhosis | Surgical debridement, daptomycin, levofloxacin | Survived | Italy | O137 | Minor abrasion exposed to seawater |
|
2016 (16) |
73 M | Diabetes mellitus | Surgical debridement, piperacillin/tazobactam, fosfomycin | Survived | Austria | Seawater exposure | |
|
2016 (16) |
80 M | Ichthyosis, cellulitis | Surgical debridement, piperacillin/tazobactam, tigecycline, metronidazole | Died | Austria | Seawater exposure | |
|
2016 (17) |
6 3 M | None |
Surgical debridement, penicillin, gentamicin, metronidazole |
Survived | Croatia | O8 | Seawater exposure |
COPD chronic constructive pulmonary disease
To conclude, we treated a woman with necrotizing fasciitis and septic shock caused by NOVC. This case illustrates that early fasciotomy and debridement are necessary for severe necrotizing fasciitis caused by NOVC, and prolonged intensive care may be required after surgery.
Acknowledgements
None.
Abbreviations
- BUN
Blood urea nitrogen
- FDP
Fibrinogen degradation products
- GNR
Gram-negative rod
- MRI
Magnetic resonance imaging
- NOVC
Non-O1/O139 Vibrio cholerae
- Nt-proBNP
N-terminal pro-brain natriuretic peptide
- PMX-DHP
Polymyxin B direct hemoperfusion
Authors’ contributions
KT, TU, TW, KN, and KU treated the patient. KT, TU, HF reviewed the literature and mainly wrote this report. KN, TW, KU reviewed the literature and modified this paper based on specialty (orthopedics, emergency department, infectious disease). All authors have read and approved the manuscript.
Funding
None.
Availability of data and materials
All data are included in this published article.
Ethics approval and consent to participate
This case report was approved by the Ethics Review Committee at Minaminara General Hospital and was conducted in accordance with the Declaration of Helsinki. Consent for participation is not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gardner AD, Venkatraman KV. The antigens of the cholera group of vibrios. J Hyg (Lond) 1935;35:262–282. doi: 10.1017/S0022172400032265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris JG. Non-O group 1 Vibrio cholerae: a look at the epidemiology of an occasional pathogen. Epidemiol Rev. 1990;12:179–191. doi: 10.1093/oxfordjournals.epirev.a036052. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Shimada T, Morris JG, Jr, Sulakvelidze A, Sozhamannan S. Evidence for the emergence of non-O1 and non-O139 Vibrio cholerae strains with pathogenic potential by exchange of O-antigen biosynthesis regions. Infect Immun. 2002;70:2441–2453. doi: 10.1128/IAI.70.5.2441-2453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalsgaard A, Albert MJ, Taylor DN, Shimada T, Meza R, Serichantalergs O, et al. Characterization of Vibrio cholerae non-O1 serogroups obtained from an outbreak of diarrhea in Lima. Peru J Clin Microbiol. 1995;33:2715–2722. doi: 10.1128/JCM.33.10.2715-2722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isaac-Márquez AP, Lezama-Dávila CM, Eslava-Campos C, Navarro-Ocaña A, Cravioto-Quintana A. Serotypes of Vibrio cholerae non-O1 isolated from water supplies for human consumption in Campeche, Mexico and their antibiotic susceptibility pattern. Mem Inst Oswaldo Cruz. 1998;93:17–22. doi: 10.1590/S0074-02761998000100004. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JM, Hollis DG, Gangarosa EJ, Weaver RE. Non-cholera vibrio infections in the United States. Clinical, epidemiologic, and laboratory features. Ann Intern Med. 1978;88:602–606. doi: 10.7326/0003-4819-88-5-602. [DOI] [PubMed] [Google Scholar]
- 7.Deshayes S, Daurel C, Cattoir V, Parienti JJ, Quilici ML, de La Blanchardière A. Non-O1, non-O139 Vibrio cholerae bacteraemia: case report and literature review. Springerplus. 2015;4:575. doi: 10.1186/s40064-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mubarak SJ, Owen CA. Double-incision fasciotomy of the leg for decompression in compartment syndromes. J Bone Joint Surg Am. 1977;59:184–187. doi: 10.2106/00004623-197759020-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wagner PD, Evans SD, Dunlap J, Ballon-Landa G. Necrotizing fasciitis and septic shock caused by Vibrio cholerae acquired in San Diego. California West J Med. 1995;163:375–377. [PMC free article] [PubMed] [Google Scholar]
- 10.Ko W, Chuang Y, Huang G, Hsu SY. Infections due to non-O1 Vibrio cholerae in southern Taiwan: predominance in cirrhotic patients. Clin Infect Dis. 1998;27:774–780. doi: 10.1086/514947. [DOI] [PubMed] [Google Scholar]
- 11.Cheng NC, Tsai JL, Kuo YS, Hsueh PR. Bacteremic necrotizing fasciitis caused by Vibrio cholerae serogroup O56 in a patient with liver cirrhosis. J Formos Med Assoc. 2004;103:935–938. [PubMed] [Google Scholar]
- 12.Tsai YH, Hsu RWW, Huang KC, Chen CH, Cheng CC, Peng KT, et al. Systemic Vibrio infection presenting as necrotizing fasciitis and sepsis. A series of thirteen cases. J Bone Joint Surg Am. 2004;86:2497–2502. doi: 10.2106/00004623-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Chang-Chien CH. Bacteraemic necrotizing fasciitis with compartment syndrome caused by non-O1 Vibrio cholerae. J Plast Reconstr Aesthetic Surg. 2006;59:1381–1384. doi: 10.1016/j.bjps.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Maraki S, Christidou A, Anastasaki M, Scoulica E. Non-O1, non-O139 Vibrio cholerae bacteremic skin and soft tissue infections. Infect Dis (Lond) 2016;48:171–176. doi: 10.3109/23744235.2015.1104720. [DOI] [PubMed] [Google Scholar]
- 15.Ottaviani D, Leoni F, Rocchegiani E, Canonico C, Masini L, Pianetti A, et al. Unusual case of necrotizing fasciitis caused by Vibrio cholerae O137. J Clin Microbiol. 2011;49:757–759. doi: 10.1128/JCM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirk S, Huhulescu S, Allerberger F, Lepuschitz S, Rehak S, Weil S, et al. Necrotizing fasciitis due to Vibrio cholerae non-O1/non-O139 after exposure to Austrian bathing sites. Wien Klin Wochenschr. 2016;128:141–145. doi: 10.1007/s00508-015-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrović K, Rudman F, Ottaviani D, Crnek SŠ, Leoni F, Škrlin J. A rare case of necrotizing fasciitis caused by Vibrio cholerae O8 in an immunocompetent patient. Wien Klin Wochenschr. 2016;128:728–730. doi: 10.1007/s00508-016-1060-3. [DOI] [PubMed] [Google Scholar]
- 18.Jain AKC, Varma AK, Mangalanandan KH, Kumar H, Bal A. Surgical outcome of necrotizing fasciitis in diabetic lower limbs. J Diab Foot Comp. 2010;1:80–84. [Google Scholar]
- 19.Ikeda T, Kanehara S, Ohtani T, Furukawa F. Endotoxin shock due to Vibrio vulnificus infection. Eur J Dermatol. 2006;16:423–427. [PubMed] [Google Scholar]
- 20.Su BA, Tang HJ, Wang YY, Liu YC, Ko WC, Liu CY, et al. In vitro antimicrobial effect of cefazolin and cefotaxime combined with minocycline against Vibrio cholerae non-O1 non-O139. J Microbiol Immunol Infect. 2005;38:425–429. [PubMed] [Google Scholar]
- 21.Tsai YH, Huang TJ, Hsu RWW, Weng YJ, Hsu WH, Huang KC, et al. Necrotizing soft-tissue infections and primary sepsis caused by Vibrio vulnificus and Vibrio cholerae non-O1. J Trauma. 2009;66:899–905. doi: 10.1097/TA.0b013e31816a9ed3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this published article.