Abstract
Mesenchymal stem cell (MSC)-based regenerative therapy is regarded as a promising strategy for the treatment of Parkinson’s disease (PD). However, MSC components may exhibit poor intracranial survivability, particularly in the later stages following cell transplantation, limiting their potential curative effect and also clinical applications. Glial cell line-derived neurotrophic factor (GDNF), which encompasses a variety of transforming growth factor beta super family members, has been reported to enhance motor function and exert neuroprotective effects. However, no previous studies have investigated the effects of GDNF on human primary adipose-derived MSCs (hAMSCs), despite its potential for enhancing stem cell survival and promoting therapeutic efficacy in the treatment of PD. In the present study, we proposed a novel approach for enhancing the proliferative capacity and improving the efficacy of hAMSC treatment. Pre-exposure of engineered hAMSCs to GDNF enhanced the proliferation and differentiation of these stem cells in vitro. In addition, in 6-hydroxydopamine-lesioned mice, a common PD model, intracranial injection of hAMSCs-GDNF was associated with greater performance on behavioral tests, larger graft volumes 5 weeks after transplantation, and higher levels of Nestin, glial fibrillary acidic protein, and Tuj-1 differentiation than those treated with hAMSCs-Vector. Following transplantation of hAMSCs-GDNF into the striatum of lesioned models, we observed significant increases in tyrosine hydroxylase- and NeuN-positive staining. These findings highlight the therapeutic potential of hAMSCs-GDNF for patients with PD, as well as an efficient method for promoting therapeutic efficacy of these delivery vehicles.
Keywords: GDNF, mesenchymal stem cells, Parkinson’s disease, therapeutic efficacy
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and affects millions of people worldwide. It is characterized by typical motor and nonmotor parkinsonian symptoms, affecting approximately 1% of the population over the age of 60. The pathological changes associated with PD include progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc), decreased dopamine (DA) levels in the midbrain striatal system, and abnormal accumulation of alpha-synuclein in dopaminergic neurons1,2. Conventional drug therapy and surgical treatment can only partially improve the clinical symptoms of PD by targeting existing DA neurons. Unfortunately, these methods cannot radically alter the pathological damage associated with this progressive disease.
Recent studies have suggested that human stem cells can aid in the treatment of chronic neurodegenerative disorders. The next generation of cell-based therapies for PD is a potential treatment option for this common neurodegenerative disorder3,4. Although the therapeutic potential of neural stem cells has been investigated in several clinical trials5,6, the clinical translation of neural stem cell therapy is limited by ethical controversies associated with their isolation, undesired immune responses that limit therapeutic efficacy, and their restricted usage to ensure sufficient cellular resources for transplantation trials. Unlike neural stem cells, human mesenchymal stem cells (MSCs) are easily accessible, pose no ethical controversy, and may be highly effective in promoting tissue repair7,8. A recent meta-analysis by Riecke et al. demonstrated that MSC treatment exerts beneficial effects in animal models of PD9. MSCs obtained from healthy donors have also been transplanted into the subventricular zone of human patients with PD10,11. In these studies, patients in the early stages of PD exhibited greater improvement and less pronounced disease progression than patients in the later stages of the disease. Such differences in efficacy may depend on the ability of engrafted cells to survive intracranially12. Munoz et al. reported that 5 days after injection into the substantia nigra in mouse models the MSC-green fluorescent protein (GFP)-luciferase signal decreased by nearly 50%13. Therefore, methods for enhancing stem cell viability are required in order to promote the efficacy of cell replacement therapies for PD.
Glial cell line-derived neurotrophic factor (GDNF) encompasses a variety of transforming growth factor beta super family members, which are involved in the protection and repair of dopaminergic neurons14. Two clinical trials have reported that GDNF may have a positive effect on the recovery of motor impairment when administered into the putamen15,16. Hoban et al. further noted that bone marrow-derived MSC-based GDNF gene delivery exerts neuroprotective effects in inflammation-driven rat models of PD17. Moreover, recent studies have reported that GDNF exerts positive effects on motility and promotes the survival of intracranial neural stem cells in PD18,19. However, no previous studies have investigated the effects on these delivery vehicles, especially on human primary adipose-derived MSC (hAMSC)-GDNF itself, despite its potential for enhancing stem cell survival and promoting therapeutic efficacy in the treatment of PD. In the present study, we found that secreting GDNF not only has positive effect on the viability and neural-like cell differentiation capacity of hAMSCs, but could also promote the therapeutic effectiveness of these delivery vehicles in a 6-hydroxydopamine (6-OHDA)-lesioned mouse model.
Materials and Methods
Cell Expansion
Following approval by the Institutional Review Board of Huazhong University of Science and Technology, early passaged primary hAMSCs (TJH hAMSCs 019) were obtained from patients during neurosurgical procedures, as described in our previous studies20,21. The primary hAMSCs were isolated using the collagenase digestion method (Collagenase-A, ThermoFisher, Carlsbad, CA, USA), following which they were cultured in MSC media (MesenPRO RS basal media with one vial of MesenPRO RS growth supplement [Gibco, Grand Island, NY, USA]; 1% penicillin/streptomycin [Gibco]; and 1% Glutamax [Gibco]). Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Cell Transfection and Pretreatment
To induce the expression of GDNF in hAMSCs, the lentiviral (LV) vector systems were combined with pLVX-hGDNF-ZsGreen-Puro (Viraltherapy Technologies, Wuhan, China) and pLVX-mCMV-ZsGreen-IRES-Puro (Viraltherapy Technologies) hybrid. The LV systems contained internal ribosome entry site (IRES), and the expression of transgenes was driven by CMV promoter. To identify these delivery vehicles in our in vitro and in vivo experiments, we transduced these cells with LV vectors coding for pLVX-Luciferase-Puro-ZsGreen (Genomeditech Biotechnology, Shanghai, China). GDNF expression was assessed via Western blotting. All LV constructs were subsequently packaged as LV vectors in HEK 293 cells (5 × 106) in a 10-cm tissue culture dish. The measurement (TICD50) of viral vector titer was performed according to the recommended protocol. Once collected, hAMSCs (hAMSCs-GDNF-Fluc-GFP, hAMSCs-Vector-Fluc-GFP) were sorted using a Moflo cytometer (Beckman Coulter, Indianapolis, IN, USA). Then, cell lines with stable expression were screened out and stored in liquid nitrogen for subsequent experiments. Cellular proteins were then extracted from the hAMSCs-GDNF-Fluc-GFP (hAMSCs-GDNF-GFP) and hAMSCs-Vector-Fluc-GFP groups (hAMSCs-Vector-GFP) at the passage time of P2, P3, and P4. Western blotting was used to detect GDNF and GFP expression.
MTT Assay
The proliferation capacity of hAMSCs with or without GDNF was determined using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; M2128, Sigma, St Louis, MO, USA). The cells were seeded in 96-well plates (1,000 cells per well) and cultured in MSC complete media with or without GDNF (100 ng/ml; PHC7041, ThermoFisher) for 10 days. The hAMSCs were treated with 5 mg/ml MTT for 3 h at 37 C. Then, 2-propanol was used to dissolve the formazan crystals. Absorbance of each well was read at a wavelength of 570 nm. The cell proliferation was analyzed every 2 days for each group.
Immunofluorescence Staining
Ki67 staining was used to detect the proliferation capacity of hAMSC in response to GDNF. These stem cells were seeded on 24-well plates at 1 × 104 in MSC complete media with or without GDNF (100 ng/ml). After 3 days, the cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 45 min, following which they were pre-incubated in PBS containing 0.3% Triton X-100 with 10% goat serum prior to incubation with Ki67 antibody (1:500; ab15580, Abcam, Cambridge, MA, USA). Alexa Fluor-labeled secondary antibody was added to the sections and incubated for 2 h (1:500; Invitrogen, Carlsbad, CA, USA), and 4′,6-diamidino-2-phenylindole (DAPI) was incubated for 0.5 h (Invitrogen) to visualize markers and cell nuclei. To determine the differentiation capacity of hAMSCs, we performed immunostaining for Nestin (1:500; ab6320, Abcam), glial fibrillary acidic protein (GFAP; 1:500, ab7260, Abcam), and Tuj-1 (1:500; PA5-85874, ThermoFisher), as described above. The number of positive cells was visualized and recorded using an inverted fluorescence microscope (Zeiss, Oberkochen, Germany). All measurements were performed according to the recommended protocol.
Western Blotting
Western blotting was used to detect the expression of GDNF and GFP genes in the modified GDNF and vector groups at P2, P3, and P4 (Fig. 1B). Following pretreatment, hAMSCs were lysed in a radioimmunoprecipitation assay buffer (Sigma) with phenylmethylsulfonyl fluoride at 4 °C and an equal amount of protein (10–30 μg) was loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane. The primary antibodies used were anti-GDNF (1:500, ab18956, Abcam) and anti-β-actin (1:2000, ab8227, Abcam), which were detected by chemiluminescence after incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies. Striatal proteins were harvested from three randomly screened PD mice after 6-OHDA lesioning, following which levels of tyrosine hydroxylase (TH) in the left and right striatum were determined via Western blotting. Primary antibodies were diluted in blocking solution as follows: rabbit anti-mouse TH, 1:500(ab75875, Abcam); rabbit anti-mouse beta-actin, 1:2000(ab8227, Abcam). Moreover, Western blot was also used to detect the expression of GFRa1 in these delivery vehicles. Primary antibodies were diluted in blocking solution as follows: anti-GFRα1, 1:500(ab84106, Abcam) and anti-β-actin, 1:2000(ab8227, Abcam).
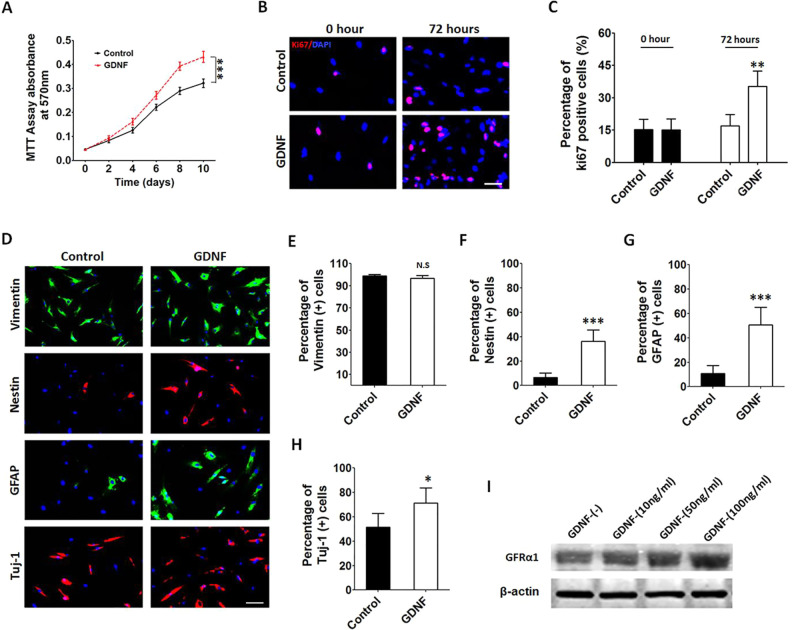
Fig. 1.
Exogenous GDNF promotes the proliferation of hAMSCs and induces hAMSC differentiation in vitro. (A) MTT assays showed that hAMSCs pretreated with GDNF exhibited a greater proliferation capacity when compared with the control group. (B and C) Similar results were confirmed by Ki67 immunofluorescence staining assay, scale bar, 50 μm. (D) Representative immunofluorescence staining pictures of vimentin, Nestin, GFAP, and Tuj-1 in the different groups, scale bar, 50 μm. (E–H) The hAMSCs which were exposed to GDNF displayed a significantly higher percentage of Nestin, GFAP, and Tuj-1 expressions compared to control groups. (I) Western blot assays were used to detect GFRa1 expression in the hAMSCs. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. GDNF: glial cell line-derived neurotrophic factor; GFAP: glial fibrillary acidic protein; hAMSCs: human primary adipose-derived mesenchymal stem cells; MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; SEM: standard error of the mean.
Enzyme-linked Immunosorbent Assay (ELISA)
The concentration of GDNF secreted by hAMSCs was measured using Abcam’s GDNF ELISA kit (ab100525). The samples and standards were placed and incubated overnight at 4 °C. Then, biotinylated GDNF, HRP-streptavidin solution, tetramethylbenzidine substrate reagent, and a stop solution were added sequentially. All measurements were performed according to the recommended protocol. Absorbance of these samples was read at a wavelength of 450 nm.
5-Ethynyl-2′-deoxyuridine (EdU) Assay
The EdU assay was used to evaluate the proliferation capacity of the delivery vehicles loaded with GDNF. hAMSC-GDNF and hAMSC-Vector were seeded on 6-well plates at 5 × 104 for 96 h. These cells were then incubated with isotype control, as well as EdU reagent (Click-iT® EdU Flow Cytometry Assay Kits; Invitrogen) for 4 h and tested via flow cytometry (Beckman Coulter). All measurements were performed according to the recommended protocol. EdU incorporation was detected via flow cytometry. Three replicate experiments were performed for each sample.
In Vivo Studies
Stereotaxic Surgery
Male C57BL/6 J mice (20–25 g, 6 weeks old) were used for the present study. Mice were placed under a 12-h light/dark cycle and raised in a specific pathogen-free environment, with ad libitum access to food and water. All experimental protocols were approved by the Ethical Committee of Huazhong University of Science and Technology (HUST). To investigate the effect of GDNF on the viability and survival of hAMSCs in vivo, 5 × 105 hAMSC-GDNF-Fluc-GFP (hAMSC-GDNF: AP = +0.3 mm, ML = –2.7 mm, DV = –3.5 mm, n = 8) or 5 × 105 hAMSC-Vector-Fluc-GFP (hAMSC-Vector: AP = +0.3 mm, ML = –2.7 mm, DV = –3.5 mm, n =8) were injected into left striatum of the male C57BL/6 J mice. These animals (3/8 in each group) were imaged using an In vivo imaging system (IVIS) small animal imaging system (Perkin Elmer, Waltham, MA, USA) at 0, 2, 4, and 6 weeks following the stem cells injection. The brains of the remaining mouse models were harvested and sectioned to a thickness of 20 µm for analysis 6 weeks post-stem cell transplantation (5/8 in each group). To quantify stem cell differentiation, we calculated the number of cells positive for Nestin (1:500; ab6320, Abcam), GFAP (1:500; ab7260, Abcam), and Tuj-1 (1:500, PA5-85874, ThermoFisher) for each section. The average value of all sections was then calculated for each animal.
To identify the effect of stem cells loaded with GDNF in an animal model of PD, male C57BL/6 J mice were unilaterally lesioned using 6-OHDA, following which they were evaluated using behavioral tests and biochemical analyses. The mouse model of PD was prepared by injecting 6-OHDA solution (2.5 mg/ml) into the left striatum using a 2-μl Hamilton syringe (Reno, NE, USA), as follows: (a) AP = +0.60 mm, ML = –2.0 mm, DV = –3.70 mm; (b) AP = −0.30 mm, ML = –2.30 mm, DV = –2.90 mm, or equal volume of PBS (sham) were injected into the same location within the left striatum. Then, 26 successful unilateral PD mouse models were screened out and randomly divided into 4 groups. Meanwhile, seven sham 6-OHDA lesion mouse models were marked as the sham lesion group. In order to examine the therapeutic effect of hAMSCs, 5 × 105 hAMSCs-Vector-GFP (PD/hAMSCs-Vector, n = 7) and 5 × 105 hAMSCs-GDNF-GFP (PD/hAMSCs-GDNF, n = 7) were injected into the left striatum of PD models (AP = +0.3 mm, ML = −2.2 mm, DV = –3.5 mm) 9 days after 6-OHDA lesioning. Then, we injected the same volume of saline (PD/saline, 2 µl, n = 6) and GDNF (PD/GDNF, 2 µl, 100 µg/ml, n = 6) into the left striatum (AP = +0.3 mm, ML = –2.2 mm, DV = –3.5 mm), as previously described22,23. Afterwards, apomorphine (APO) and rotarod tests were performed. All mice were pretrained for 2 days on the rotarod apparatus in order to ensure stable levels of performance (Fig. 2A). During the test, mice were subjected to a speed of 20 rpm, and the time to fall off the beam was recorded in seconds. The experiment was repeated three times, and the average value was used for analyses. The animals were perfused and sacrificed 5 weeks after 6-OHDA lesioning, following which the brain was sectioned to a thickness of 20 µm (Leica CM 1900, Heidelberg, Germany). These sections were immunostained using primary antibodies including GDNF (1:500; ab18956, Abcam), Nestin (1:500; ab6320, Abcam), GFAP (1:500; ab7260, Abcam), Tuj-1 (1:500, PA5-85874, ThermoFisher), TH (1:500; ab75875, Abcam), and NeuN (1:500; ab128886, Abcam). The brain slices were incubated with primary antibodies for 48 h, following which they were incubated with fluorescence-conjugated secondary antibodies, as described above (Alexa Fluor-labeled secondary antibody, 1:500; Invitrogen).
Fig. 2.
hAMSC-GDNF exhibited a greater survival capacity in vitro and in vivo. (A and B) Immunofluorescence staining and Western blot assays were performed to confirm that GDNF was expressed only in the hAMSC-GDNF group, scale bar, 50 μm. (C) The concentration of GDNF in hAMSC-GDNF was measured using an ELISA kit. (D) The EdU assay was performed to detect the proliferative capacity of hAMSC-vector and hAMSC-GDNF. (E) The hAMSC-GDNF exhibited a significantly higher percentage of proliferation cells compared to hAMSC-vector. (F) Western blot was performed to test GFRα1 in the hAMSC-vector and hAMSC-GDNF. (G) Schematic of hAMSCs with different preconditions (hAMSC-GDNF and hAMSC-vector) that were injected into the left striatum in vivo. (H) Representative pictures showed bioluminescence for the hAMSCs bearing mice on weeks 0, 2, 4, and 6. (I) Bioluminescence signal radiance was significantly decreased in the hAMSC-vector group on weeks 4 and 6 when compared with the hAMSC-GDNF group. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. EdU: 5-Ethynyl-2′-deoxyuridine; ELISA: enzyme-linked immunosorbent assay; GDNF: glial cell line-derived neurotrophic factor; GFP: green fluorescent protein; hAMSCs: human primary adipose-derived mesenchymal stem cells; SEM: standard error of the mean.
Behavioral Testing
To test apomorphine-induced turning behavior (apomorphine-induced rotation, APO), all mice were subcutaneously injected with a solution of 0.05 mg/kg apomorphine hydrochloride (Sigma) dissolved in 1% of ascorbic acid in 0.9% of NaCl, and then placed in metal testing bowls for 45 min. The number of contralateral rotations of the bowl was digitally recorded. Motor coordination and the balance of animals were evaluated using the rotarod tests. All mice were pretrained for 2 days on the rotarod apparatus in order to ensure stable levels of performance (Fig. 2A). During the test, mice were subjected to a speed of 20 rpm, and the time to fall off the beam was recorded in seconds. The experiment was repeated three times, and the average value was used for analyses.
Image Analysis
To quantify the survival of hAMSCs in vivo, the graft volume was calculated using the sum of the hAMSCs’ mass area corrected for section thickness and sample frequency, as follows: graft volume (mm3) = sum of areas (mm2) × 20 µm × 6. For quantification, the boundaries of TH-positive area were drawn and the total TH-positive cells were counted. The number of TH-positive cells was calculated in the nigra for each section and averaged among all sections for a given animal. Data are expressed as the percentage of remaining TH-positive cells in each injected group (PD/saline, PD/GDNF, PD/hAMSC-vector, and PD/hAMSC-GDNF), compared with sham lesion group both in ipsilateral and contralateral side. To detect the therapeutic effect following transplantation of hAMSC-GDNF, the number of TH and NeuN-positive cells per mm2 was calculated within the area of the transplant site for each section and averaged among all sections for a given animal. The brain slices were observed and photographed using an inverted fluorescence microscope. Image J software (National Institutes of Health, Bethesda, MD, USA) was used to analyze data for each animal.
Statistical Analysis
The results are represented as the mean ± standard error of the mean (SEM). All data were analyzed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA). Statistical analyses of the ELISA, EdU, IVIS, immunohistochemistry quantifications, and behavioral tests were performed by Student’s t test for single comparisons, or one-way analysis of variance (ANOVA) with Bonferroni’s test for multiple comparisons, or two-way ANOVA followed by post hoc Fisher’s least significant difference test. The level of statistical significance was set at P < 0.05.
Results
The Exogenous GDNF Promotes the Proliferation and Differentiation of hAMSCs
We performed MTT and Ki67 assays to examine the proliferation capacity of primary hAMSCs delivered with or without GDNF (100 ng/ml; PHC7041, ThermoFisher). The hAMSCs pretreated with GDNF exhibited increased proliferation when compared with the control group (Fig. 3A, MTT assay, GDNF vs Control: P < 0.001; Fig. 3B and C, Ki67 assay, GDNF vs Control: P < 0.01). To examine the effect of GDNF on hAMSC differentiation, the cells were cultured with or without GDNF for 7 days (GDNF and control groups), following which we performed immunofluorescence staining for vimentin, Nestin, GFAP, and Tuj-1. The GDNF group exhibited significant increases in Nestin, GFAP, and Tuj-1 staining when compared with the control group, indicative of greater differentiation (Fig. 3D–H, Nestin, GDNF vs control: P < 0.001; GFAP, GDNF vs control: P < 0.001; Tuj-1, GDNF vs control: P < 0.05). Moreover, the GDNF receptor was detected, and we identified GFRa1 expressed in these delivery vehicles (Fig. 3I). These results support the notion that exogenous GDNF can enhance the proliferation and differentiation of hAMSCs in vitro.
Fig. 3.
hAMSC-GDNF exhibited a better neural-like cell differentiation capacity in vivo. (A) Schematic diagram showing quantification of the differentiation capacity of hAMSCs in the mice brains. (B, E, and H) Representative images showing the number of Nestin-, GDNF-, and Tuj-1-positive cells immunostaining assays. (C and D) When hAMSCs were engineered with GDNF, the expression of Nestin-positive cells was significantly higher than that seen in the hAMSC-vector group in vivo. (F and G) The number of GFAP-positive cells with hAMSC-GDNF was higher than the hAMSC-vector group in vivo. (I and J) When the hAMSCs were loaded with GDNF, the expression of Tuj-1 exhibited a statistically significant increase than hAMSC-vector group. Scale bar, 50 μm. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. GDNF: glial cell line-derived neurotrophic factor; GFAP: glial fibrillary acidic protein; GFP: green fluorescent protein; hAMSCs: human primary adipose-derived mesenchymal stem cells; SEM: standard error of the mean.
hAMSC-GDNF Was Associated with Increased Survivability and Differentiation Capacity In Vitro and In Vivo
To investigate the effect of these delivery vehicles loaded with GDNF, the hAMSCs were engineered to express GDNF for in vitro and in vivo experiments. As shown in Fig. 1A and B, immunofluorescence staining and Western blot assays both indicated that the hAMSCs could secrete GDNF steadily. The concentration of GDNF in hAMSC-GDNF cell media was measured (19.11 + 1.06 ng/ml) using an ELISA Kit (Fig. 1C). The EdU assay indicated that hAMSC-GDNF exhibited a greater proliferation capacity than the hAMSC-vector (Fig. 1D, and E, hAMSC-Vector vs hAMSC-GDNF, P < 0.01). The results of the Western blots indicated that GFRa1 was also expressed in the hAMSC-Vector and hAMSC-GDNF (Fig. 1F). To determine the viability of these cell resources in vivo, 5 × 105 hAMSC-Vector (n = 8) and 5 × 105 hAMSC-GDNF (n = 8) were injected into the left striatum of male C57BL/6 J mice. The hAMSC-bearing mice were then imaged using an IVIS small animal imaging system (3/8 in each group). Our findings indicated that the bioluminescent signal radiance was significantly lower in the hAMSC-Vector group than in the hAMSC-GDNF group at 4 and 6 weeks following transplantation (Fig. 1G–I, hAMSC-Vector vs hAMSC-GDNF: week 4, P < 0.05; week 6, P < 0.01). To evaluate the effects of GDNF on hAMSC differentiation, we counted the number of cells positive for GFP/Nestin, GFP/GFAP, and GFP/Tuj-1 (5/8 in each group; Fig. 4A). As shown in Fig. 4B, E, and H, the hAMSCs loaded with GDNF exhibited a significant increase in differentiation to Nestin-, GFAP-, and Tuj-1-positive cells when compared to the Vector group (Fig. 4C–D, hAMSC-Vector vs hAMSC-GDNF: percentage of Nestin+/GFP+, P < 0.001; Fig. 4F–G, percentage of GFAP+/GFP+, P < 0.001; Fig. 4I–J, percentage of Tuj-1+/GFP+, P < 0.05). These results suggest that loading hAMSCs with GDNF can promote stem cell survival and neural-like cell differentiation capacity in vivo.
Fig. 4.
hAMSC-GDNF alleviates the motor behavior of 6-OHDA-lesioned mice. (A) Schematic of the behavior tests performed in PD mice after 6-OHDA lesioning. (B) Representative images showing the number of TH-positive immunostaining cells in the SNc of 6-OHDA-lesioned mice. (C and D) The number of TH-positive cells in the PD/hAMSC-GDNF group was significantly higher than that in the PD/saline, PD/GDNF, and PD/ hAMSC-vector groups in 6-OHDA-lesioned mice. (E) The average number of rotations per minute was significantly lower in the PD/hAMSC-GDNF group than in the PD/saline, PD/GDNF, and PD/hAMSC-Vector groups. (F) Time to fall displayed a significant difference in the PD/hAMSC-GDNF group compared to other PD groups. The PD/hAMSC-GDNF group exhibited a significantly longer time to fall than the PD/saline, PD/GDNF, and PD/hAMSC-Vector groups. Scale bar, 200 μm. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. 6-OHDA: 6-hydroxydopamine; APO: apomorphine-induced rotation; DAPI: 4′,6-diamidino-2-phenylindole; GDNF: glial cell line-derived neurotrophic factor; hAMSCs: human primary adipose-derived mesenchymal stem cells; PD: Parkinson’s disease; SEM: standard error of the mean; SNc: substantia nigra; TH: tyrosine hydroxylase.
Transplanted hAMSC-GDNF Resulted in a Greater Performance During Behavioral Tests in a 6-OHDA Lesion Mouse Model of PD
We established a mouse model of PD to investigate the therapeutic efficacy of hAMSC-GDNF in vivo. APO-induced rotation and rotarod tests were used in both the PD and sham groups after 6-OHDA lesioning (Supplemental Fig. S1). Then, 26 successful unilateral PD mouse models were screened out and randomly divided into 4 groups (Fig. 2A). To investigate the effect of GDNF secretion on the therapeutic potential of these delivery vehicles, 5 × 105 hAMSC-Vector (PD/hAMSC-vector, n = 7), 5 × 105 hAMSC-GDNF (PD/hAMSC-GDNF, n = 7), and equal volumes of GDNF (PD/GDNF, 2 µl, 0.10 mg/ml, n = 6) or saline (PD/saline, 2 µl, n = 6) were injected into the left striatum of PD model mice. Immunofluorescence staining (Fig. 2B–D) and behavioral tests (Fig. 2E and F) were then performed. TH immunostaining assays revealed that the number of TH-positive cells in the sham lesion group was not significantly different compared to other groups of contralateral side (Fig. 2C). The average number of TH-positive cells in the PD/hAMSC-GDNF group was significantly higher than the number in the PD/saline, PD/GDNF, and PD/hAMSC-vector groups of ipsilateral side (Fig. 2D). Moreover, the TH-positive cells in the PD/ hAMSC-vector group also appeared to be greater than in the PD/saline group (Fig. 2D, PD/hAMSC-GDNF vs PD/saline, P < 0.001; PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.05; PD/hAMSC-vector vs PD/saline, P < 0.05). Similar results were observed when analyzing the APO-induced rotation and rotarod behavioral test results (Fig. 2E and F, PD/hAMSC-GDNF vs PD/saline, P < 0.001 and P < 0.001, respectively; PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.05 and P < 0.05; PD/hAMSC-GDNF vs PD/GDNF, P < 0.01 and P < 0.001; PD/hAMSC-vector vs PD/saline, P < 0.05 and P < 0.05). These results indicate that transplantation of hAMSC has positive effects on improving motor symptoms in the 6-OHDA lesion mouse model of PD. Moreover, secreting GDNF could enhance the positive effects of delivery vehicles.
Secreting GDNF Increased the Survivability and Differentiation Capacity of hAMSC and Enhanced the Therapeutic Potential in a 6-OHDA Mouse Model of PD
We sought to determine whether hAMSCs loaded with GDNF could promote the viability and therapeutic potential of transplanted stem cells in vivo. Immunofluorescence experiments revealed increases in the volume and density of grafts, as well as increases in the number of transplanted cells in the PD/hAMSC-GDNF group, relative to levels observed in the PD/hAMSC-Vector group (Fig. 5A–E, B: graft volume, PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.001; C: number of MSCs per mm2, PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.001; D: TH+ density, contralateral vs ipsilateral: sham lesion, P > 0.05; PD/saline, P < 0.001; PD/GDNF, P < 0.001; PD/hAMSC-Vector, P < 0.01; PD/hAMSC-GDNF, P < 0.05; ipsilateral of PD/hAMSC-GDNF vs ipsilateral of PD/hAMSC-Vector, P < 0.05; ipsilateral of PD/hAMSC-GDNF vs ipsilateral of PD/saline, P < 0.001; E: TH-positive cells: PD/hAMSC-GDNF vs PD/saline, P < 0.001; PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.01; PD/hAMSC-vector vs PD/saline, P < 0.05). In addition, numbers of TH- and NeuN-positive cells were greater in the PD/hAMSC-GDNF group than in the PD/hAMSC-Vector group (Fig. 5F–I, PD/hAMSC-GDNF vs PD/hAMSC-Vector: number of TH-positive cells per mm2, P < 0.001; number of NeuN-positive cells per mm2, P < 0.01). Consequently, the GDNF secreting MSCs, as well as the differentiation capacity of these delivery vehicles in the PD models, were detected. As shown in Fig. 6A, D, G, and J, loading hAMSCs with GDNF can promote stem cell differentiation to Nestin- (Fig. 6E and F, percentage of Nestin+/GFP+, PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.001), GFAP- (Fig. 6H and I, percentage of GFAP+/GFP+, PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.001), and Tuj-1- (Fig. 6K and L, percentage of Tuj-1+/GFP+, PD/hAMSC-GDNF vs PD/hAMSC-Vector, P < 0.01) positive cells, suggesting that these effects of hAMSCs-GDNF may be due to sustained release of GDNF from itself. These results support the notion that GDNF can enhance the survival and therapeutic potential of hAMSCs in a 6-OHDA mouse model of PD.
Fig. 5.
hAMSC-GDNF promotes therapeutic effects in PD models. (A) Representative immunofluorescence staining pictures of TH in the different groups, scale bar, 200 μm. (B–E) Immunostaining assays showed that the volume of grafts, the number, and density of TH-positive cells in the PD/hAMSC-GDNF group were significantly higher than the other groups. (F–I) The number of TH- and NeuN-positive cells in the PD/hAMSC-GDNF group was larger than in the PD/hAMSC-vector group in 6-OHDA lesioned mouse models. Scale bar, 50 μm. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. GDNF: glial cell line-derived neurotrophic factor; GFP: green fluorescent protein; hAMSCs: human primary adipose-derived mesenchymal stem cells; OD: optical density; PD: Parkinson’s disease; SEM: standard error of the mean; TH: tyrosine hydroxylase.
Fig. 6.
GDNF secretion enhanced the neural-like cell differentiation capacity of hAMSC in mouse models of PD. (A, D, G, and J) Representative immunofluorescence staining pictures of GDNF, Nestin, GFAP, and Tuj-1 expression and neural-like cell differentiation in PD/hAMSC-vector and PD/hAMSC-GDNF groups, scale bar, 50 μm. (B and C) Immunofluorescence staining assay indicated that the hAMSCs could secrete GDNF steadily in the PD/hAMSC-GDNF group but not in the PD/hAMSC-vector group. (E & F, H & I, and K & L) The PD/hAMSC-GDNF group showed a higher number of GDNF-, Nestin-, GFAP-, and Tuj-1-positive cells compared to the PD/hAMSC-vector group. Error bars represent SEM. *P < 0.05, **P < 0.01, ***P < 0.001. GDNF: glial cell line-derived neurotrophic factor; GFAP: glial fibrillary acidic protein; hAMSCs: human primary adipose-derived mesenchymal stem cells; SEM: standard error of the mean.
Discussion
Our findings indicate that hAMSCs loaded with GDNF have increased survivability and differentiation capacity relative to those without GDNF. In addition, such treatment was associated with motor function improvements in a PD mouse model. Thus, our findings highlight the need for early-stage clinical trials to investigate the therapeutic potential of hAMSCs-GDNF for PD.
The neuropathological hallmark of PD is the progressive loss of dopaminergic neurons in the SNpc1,2,7. Unfortunately, current therapeutic strategies do not halt disease progression, and many treatments are associated with their own adverse effects and comorbidities. GDNF is a potent survival factor that exerts various effects on neuronal activity24. In addition, GDNF plays a role in the survival and maintenance of mesencephalic DA neurons25,26. A previous in vivo study demonstrated that GDNF promotes the survival and morphological differentiation of dopaminergic neurons27, while several in vitro studies have reported that GDNF protects DA neurons from certain neurotoxins28,29. Gash et al. further observed that GDNF-treated monkeys exhibited improvements in motor symptoms, as well as increased DA levels in the midbrain, larger DA neurons, and increases in TH-positive cells in the ventral tegmental area and substantia nigra on the affected side30. Given the consensus regarding the benefits of GDNF, several clinical trials have investigated its effects on the degree of symptomatic relief in PD. Whone et al. reported that administration of GDNF appeared to be well tolerated and increased DOPA uptake throughout the entire putamen for patients with PD, but it did not show significant clinical improvements against placebo in the long term31,32. The results of these trials are somewhat contradictory, with some studies reporting improvements and others reporting no improvement33–35. Taken together, these findings indicate that the administration of GDNF protein injection directly may be ineffective and that alternative therapies should be considered.
Given the aforementioned evidence, more recent studies have focused on the therapeutic potential of cell grafts36,37. Because of their neuroregenerative potential (e.g., multipotentiality, secretome formation), MSCs are considered a promising cell type for intracranial transplantation. The most commonly investigated MSCs are derived from adipose tissue, the umbilical cord, or bone marrow. Our previous study also found that these delivery vehicles were not tumorigenic in the brain in mice models20,38. In the present study, we used hAMSCs because of their abundance, easy accessibility, and potential for increased tissue repair and neurotrophin secretion. Furthermore, they represent an ethically noncontroversial cell source39,40. Although stem cell transplantation is promising for the treatment of PD, several researchers have expressed concerns regarding the long-term efficacy of such treatment and its application in patients with late-stage PD, among whom results are somewhat unsatisfactory12,13. Thus, there is a need to enhance survivability and promote the therapeutic efficacy of stem cell treatment for PD. GDNF is known to play an important role in neuron survival, growth, migration, and differentiation. Recent studies have also reported that this neurotrophic factor can promote the survival of salivary stem cells and aid in the treatment of radiation-induced xerostomia41,42. Moreover, several researchers demonstrated that Ret/GFRα1 signaling pathway is essential to mediate GDNF’s neuroprotective and neuroregenerative effects43,44. However, few studies have evaluated the effects of GDNF on these delivery vehicles, particularly with regard to cell viability and differentiation.
Several studies have investigated the potential of cell-based GDNF therapy for PD. Chen et al. reported that intrastriatal GDNF-secreting neural progenitor cells can protect DA neurons in 6-OHDA-lesioned rats45. Moreover, overexpression of GDNF increases the viability and proliferation of stem cells. In the present study, hAMSCs treatment improved motor behavior in 6-OHDA-lesioned mice. When hAMSCs were loaded with the GDNF gene, further increases in viability and differentiation were observed in vitro, while improvements in therapeutic efficacy were observed in vivo. As several clinical trials are currently investigating the potential of stem cell therapies for PD46–49, our findings suggest that GDNF should be used to improve stem cell viability and survival in clinical settings. To the best of our knowledge, the present study is the first to demonstrate the positive effect of GDNF on both the survival and neural-like cell differentiation of hAMSCs, and the therapeutic potential of these delivery vehicles in 6-OHDA-lesioned mouse. Loading hAMSCs with GDNF can promote differentiation to Nestin, GFAP, and Tuj-1-positive cells, suggesting that the pluripotent differentiation capacity of hAMSC-GDNF may be due to sustained release of GDNF from itself. Moreover, the 6-OHDA models treated with these modified stem cells (hAMSC-GDNF) exhibited more TH- and NeuN-positive cells in the nigrostriatal pathway than the hAMSC-vector group. Future studies should focus on the clinical potential of this delivery method.
In summary, our in vivo and in vitro experiments reveal that GDNF can be used to efficiently enhance the proliferation and differentiation of hAMSCs in a mouse model of PD. Further studies are required to determine whether hAMSC-GDNF treatment can be applied in the clinical setting.
Supplemental Material
Supplemental Material, S.figure_01 for GDNF Promotes Survival and Therapeutic Efficacy of Human Adipose-Derived Mesenchymal Stem Cells in a Mouse Model of Parkinson’s Disease by Shoujia Sun, Quan Zhang, Man Li, Pan Gao, Kuan Huang, Rajluxmee Beejadhursing, Wei Jiang, Ting Lei, Mingxin Zhu and Kai Shu in Cell Transplantation
Footnotes
Author Contributions: Experimental design and development: MZ, SS, and QZ; experiment execution: MZ, SS, ML, PG, QZ, KH, and WJ; data analysis: MZ, SS QZ, TL, and KS; manuscript preparation: MZ, SS, RB, and KS.
Ethical Approval: This study was approved by the Ethics Committee of Huazhong University of Science and Technology (IRB ID: TJ20161210).
Statement of Human and Animal Rights: The experimental procedure was performed according to the animal care guidelines of the Institutional Animal Care and Use Committees (IRB ID: TJ20161210).
Statement of Informed Consent: Informed consent was obtained from donors according to the Huazhong University of Science and Technology Institutional Review Board.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Natural Science Foundation of China (No.81270865, 81502174), and Innovative Talent Development Fund Project of Wuhan City (2015whcxrczjxm02); the Youth Backbone Project of Wuhan City (2017zqnsk01-11772); Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology (5001540025).
ORCID iD: Mingxin Zhu  https://orcid.org/0000-0002-0985-3857
https://orcid.org/0000-0002-0985-3857
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. [DOI] [PubMed] [Google Scholar]
- 2. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. [DOI] [PubMed] [Google Scholar]
- 3. Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015;11(9):492–503. [DOI] [PubMed] [Google Scholar]
- 4. Barker RA, Gotz M, Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature. 2018;557(7705):329–334. [DOI] [PubMed] [Google Scholar]
- 5. Allen PJ, Feigin A. Gene-based therapies in Parkinson’s disease. Neurotherapeutics 2014;11(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q, Chen W, Tan S, Lin T. Stem cells for modeling and therapy of Parkinson’s disease. Hum Gene Ther. 2017;28(1):85–98. [DOI] [PubMed] [Google Scholar]
- 7. Choi HS, Kim HJ, Oh JH, Park HG, Ra JC, Chang KA, Suh YH. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol Aging. 2015;36(10):2885–2892. [DOI] [PubMed] [Google Scholar]
- 8. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. [DOI] [PubMed] [Google Scholar]
- 9. Riecke J, Johns KM, Cai C, Vahidy FS, Parsha K, Furr-Stimming E, Schiess M, Savitz SI. A meta-analysis of mesenchymal stem cells in animal models of Parkinson’s disease. Stem Cells Dev. 2015;24(18):2082–2090. [DOI] [PubMed] [Google Scholar]
- 10. Mendes PD, Lopes C, Franca DM, Reis JC, Santos ACJ, Darwich RZ. The dilemma of anticoagulating patients with cerebral venous thrombosis who underwent decompressive craniectomy. World Neurosurg. 2018;114:168–171. [DOI] [PubMed] [Google Scholar]
- 11. Venkataramana NK, Pal R, Rao SA, Naik AL, Jan M, Nair R, Sanjeev CC, Kamble RB, Murthy DP, Chaitanya K. Bilateral transplantation of allogenic adult human bone marrow-derived mesenchymal stem cells into the subventricular zone of Parkinson’s disease: a pilot clinical study. Stem Cells Int. 2012;2012:931902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mendes Filho D, Ribeiro PDC, Oliveira LF, de Paula DRM, Capuano V, de Assuncao TSF, da Silva VJD. Therapy with mesenchymal stem cells in Parkinson disease: history and perspectives. Neurologist. 2018;23(4):141–147. [DOI] [PubMed] [Google Scholar]
- 13. Munoz MF, Arguelles S, Guzman-Chozas M, Guillen-Sanz R, Franco JM, Pintor-Toro JA, Cano M, Ayala A. Cell tracking, survival, and differentiation capacity of adipose-derived stem cells after engraftment in rat tissue. J Cell Physiol. 2018;233(10):6317–6328. [DOI] [PubMed] [Google Scholar]
- 14. Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. [DOI] [PubMed] [Google Scholar]
- 15. Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–595. [DOI] [PubMed] [Google Scholar]
- 16. Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102(2):216–222. [DOI] [PubMed] [Google Scholar]
- 17. Hoban DB, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience. 2015;303:402–411. [DOI] [PubMed] [Google Scholar]
- 18. Shi H, Patschan D, Dietz GP, Bahr M, Plotkin M, Goligorsky MS. Glial cell line-derived neurotrophic growth factor increases motility and survival of cultured mesenchymal stem cells and ameliorates acute kidney injury. Am J Physiol Renal Physiol. 2008;294(1):F229–F235. [DOI] [PubMed] [Google Scholar]
- 19. Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, Tayra JT, Shinko A, Wakamori T, Agari T, Date I. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson’s disease. Neurosci Res. 2011;71(1):92–98. [DOI] [PubMed] [Google Scholar]
- 20. Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, Denduluri A, Chaichana KL, Li Q, Zhang H, Levchenko A, et al. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. 2015;6(6):e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu M, Feng Y, Dangelmajer S, Guerrero-Cazares H, Chaichana KL, Smith CL, Levchenko A, Lei T, Quinones-Hinojosa A. Human cerebrospinal fluid regulates proliferation and migration of stem cells through insulin-like growth factor-1. Stem Cells Dev. 2015;24(2):160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aoi M, Date I, Tomita S, Ohmoto T. GDNF induces recovery of the nigrostriatal dopaminergic system in the rat brain following intracerebroventricular or intraparenchymal administration. Acta Neurochir (Wien). 2000;142(7):805–810. [DOI] [PubMed] [Google Scholar]
- 23. Rosenblad C, Martinez-Serrano A, Bjorklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75(4):979–985. [DOI] [PubMed] [Google Scholar]
- 24. Ayanlaja AA, Zhang B, Ji G, Gao Y, Wang J, Kanwore K, Gao D. The reversible effects of glial cell line-derived neurotrophic factor (GDNF) in the human brain. Semin Cancer Biol. 2018;53:212–222. [DOI] [PubMed] [Google Scholar]
- 25. Pascual A, Hidalgo-Figueroa M, Gomez-Diaz R, Lopez-Barneo J. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol. 2011;46(3):R83–R92. [DOI] [PubMed] [Google Scholar]
- 26. Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11(7):755–761. [DOI] [PubMed] [Google Scholar]
- 27. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. [DOI] [PubMed] [Google Scholar]
- 28. Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373(6512):339–341. [DOI] [PubMed] [Google Scholar]
- 29. Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355(4):479–489. [DOI] [PubMed] [Google Scholar]
- 30. Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. [DOI] [PubMed] [Google Scholar]
- 31. Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU, Longpre L, et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain. 2019;142(3):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whone AL, Boca M, Luz M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU, Longpre L, et al. Extended treatment with glial cell line-derived neurotrophic factor in Parkinson’s disease. J Parkinsons Dis. 2019;9(2):301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59(3):459–466. [DOI] [PubMed] [Google Scholar]
- 34. Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11(7):703–704. [DOI] [PubMed] [Google Scholar]
- 35. Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57(2):298–302. [DOI] [PubMed] [Google Scholar]
- 36. Laguna Goya R, Tyers P, Barker RA. The search for a curative cell therapy in Parkinson’s disease. J Neurol Sci. 2008;265(1–2):32–42. [DOI] [PubMed] [Google Scholar]
- 37. Kim HW, Lee HS, Kang JM, Bae SH, Kim C, Lee SH, Schwarz J, Kim GJ, Kim JS, Cha DH, Kim J, et al. Dual effects of human placenta-derived neural cells on neuroprotection and the inhibition of neuroinflammation in a rodent model of Parkinson’s disease. Cell Transplant. 2018;27(5):814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li M, Sun S, Dangelmajer S, Zhang Q, Wang J, Hu F, Dong F, Kahlert UD, Zhu M, Lei T. Exploiting tumor-intrinsic signals to induce mesenchymal stem cell-mediated suicide gene therapy to fight malignant glioma. Stem Cell Res Ther. 2019;10(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwerk A, Altschuler J, Roch M, Gossen M, Winter C, Berg J, Kurtz A, Akyuz L, Steiner B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen Med. 2015;10(4):431–446. [DOI] [PubMed] [Google Scholar]
- 41. Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116(Pt 19):3855–3862. [DOI] [PubMed] [Google Scholar]
- 42. Xiao N, Lin Y, Cao H, Sirjani D, Giaccia AJ, Koong AC, Kong CS, Diehn M, Le QT. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124(8):3364–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drinkut A, Tillack K, Meka DP, Schulz JB, Kugler S, Kramer ER. Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis. 2016;7(9):e2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381(6585):789–793. [DOI] [PubMed] [Google Scholar]
- 45. Chen SS, Yang C, Hao F, Li C, Lu T, Zhao LR, Duan WM. Intrastriatal GDNF gene transfer by inducible lentivirus vectors protects dopaminergic neurons in a rat model of parkinsonism. Exp Neurol. 2014;261:87–96. [DOI] [PubMed] [Google Scholar]
- 46. de Melo-Martin I, Hellmers N, Henchcliffe C. First-in-human cell transplant trials in Parkinson’s disease: the need for an improved informed consent process. Parkinsonism Relat Disord. 2015;21(8):829–832. [DOI] [PubMed] [Google Scholar]
- 47. Parmar M. Towards stem cell based therapies for Parkinson’s disease. Development. 2018;145(1). [DOI] [PubMed] [Google Scholar]
- 48. Barazzetti G, Hurst SA, Mauron A. Adapting preclinical benchmarks for first-in-human trials of human embryonic stem cell-based therapies. Stem Cells Transl Med. 2016;5(8):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lindvall O. Clinical translation of stem cell transplantation in Parkinson’s disease. J Intern Med. 2016;279(1):30–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, S.figure_01 for GDNF Promotes Survival and Therapeutic Efficacy of Human Adipose-Derived Mesenchymal Stem Cells in a Mouse Model of Parkinson’s Disease by Shoujia Sun, Quan Zhang, Man Li, Pan Gao, Kuan Huang, Rajluxmee Beejadhursing, Wei Jiang, Ting Lei, Mingxin Zhu and Kai Shu in Cell Transplantation