Abstract
Ovarian cancer (OC) is the most aggressive gynecological cancer. Even with the advances in detection and therapeutics, it still remains clinically challenging and there is a pressing need to identify novel therapeutic strategies. In searching for rational molecular targets, we identified metadherin (MTDH), a multifunctional gene associated with several tumor types but previously unrecognized in OC. In this study, we found the MTDH is overexpressed in OC tissues. Through in vitro assays with overexpression cells, we characterized the role of MTDH. We confirmed MTDH stable overexpression significantly increased the expression of TNF-α, IL-6, IL-8, IL-10, and IL-1β. Interestingly, NF-kappa-B (NF-κB) and MTDH were found in a feed-forward loop motif. Thus, our findings support the notion that the MTDH and NF-κB signaling network contributes to OC traits. MTDH represents a new OC-associated gene that can contribute to insights of OC biology and suggests other treatment strategies.
Keywords: metadherin, NF-κB, chronic inflammation, ovarian cancer
Introduction
Ovarian cancer (OC) is the leading cause of gynecological-related deaths and the fifth leading cause of cancer mortality seriously threatening females’ lives and health worldwide1. Extensive efforts have been made to improve treatment; however, the number of cases is increasing worldwide. Thus, there is a pressing need to identify novel therapeutic strategies.
Metadherin (MTDH) is alternatively known as astrocyte elevated gene-12. MTDH is a membrane protein that regulates the homing of tumor cells to the lung endothelium and is a lysine-rich protein associated with tight junctions in prostate epithelial cells3. It is a universally important cancer-associated gene. It has been validated in diverse malignancies, such as breast, lung, esophageal, B-cell lymphoma, brain tumors, gynecologic cancers, and malignant pleural mesothelioma (MPM)4–9. Additionally, MTDH has been identified as the direct target of miR-182-5p contributing to the inhibition of proliferation and metastasis in colorectal cancer10. However, the role of MTDH is still incompletely understood in OC.
The transcription factor NF-κB plays a key role in stress response due to environmental stimuli by regulating genes involved in proliferation, apoptosis, differentiation, inflammation, and immune system regulation. In nonstress conditions, NF-κB is sequestered in the cytoplasm by its repressor IκBα. Because of its importance to many biological processes, NF-κB activation and activity are tightly regulated by a battery of endogenous mechanisms. Aberrant NF-κB activation contributes to the development of various autoimmune, inflammatory, and malignant disorders. Thus, inhibiting NF-κB signaling has potential therapeutic applications in cancer. A previous study reported the activation of EMT in colorectal cancer by MTDH/NF-κB p65 pathway11. However, the role of MTDH/NF-κB p65 pathway is still unclear in OC.
In this study, we explored the expression of MTDH, inflammatory cytokines, and chemokines in OC. In particular, we uncovered a feed-forward regulatory mechanism of MTDH/NF-κB signaling pathway. Our results contribute to insights of OC biology and suggest other treatment strategies in OC.
Materials and Methods
Tissues and Cell Culture
The human ovarian cancer cell line, SKOV-3, was purchased from ATCC (Manassas, VA, USA). Cells were maintained in RPMI 1640 (Gibco Laboratories, Carlsbad, CA, USA) enriched with 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma-Aldrich). Cells were cultured at 37 °C with 5% CO2. SKOV-3 cells were subcultured at 90% confluence.
Study OC tissues were obtained from 18 patients diagnosed in the Department of Gynecology and Obstetrics of China–Japan Friendship Hospital (Beijing, China), between August 2018 and December 2018. All the specimens were reviewed and verified by pathologists and immediately frozen in liquid nitrogen. All subjects gave their informed consent for inclusion before they participated in the study. All experimental protocols were approved by the Ethics Committee of China–Japan Friendship Hospital.
Quantitative Real-Time PCR
We extracted total RNA from 3 × 106 of cells following the mirVanaTM miRNA isolation kit (Ambion, Austin, TX, USA) protocol. Reverse transcription was done using the high-capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA) after removal of residual genomic DNA by DNase I (Invitrogen, Carlsbad, CA, USA).
Specific gene quantitation was determined by TaqMan analysis using QuantStudio 6 Flex PCR system (Thermo Fisher Scientific). β-Actin gene was used as an endogenous housekeeping gene for normalization. Specific gene expression probes including TNF-α, IL-6, IL-8, IL-10, and IL-1β were commercially available from Applied Biosystems (Foster City, CA, USA). All independent PCR-based reactions were performed in triplicate.
The relative expression ratio of each gene was calculated with the comparative CT method (ΔΔCt method)12.
MTDH Overexpression
In order to elucidate the role of MTDH in vitro, we used the lentiviral system (Applied Biological Materials, Montreal, Canada) to stably overexpress MTDH (MTDH-OE) and its negative control vector (Ctrl-OE) in SKOV-3 cells. The overexpression was according to the manufacturer’s instructions.
Western Blotting
For each sample, total protein lysis was separated on 4%–15% precast gels (Bio-Rad, Richmond, CA, USA) and then blotted onto nitrocellulose paper (Bio-Rad). Membranes were blocked with 5% dried nonfat milk powder in TBST for 1 h and incubated with primary antibodies overnight at 4 ºC. After being washed with TBST three times, the membranes were incubated with the goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000; cat. no. STAR124P; Bio-Rad) or goat anti-mouse HRP-conjugated secondary antibody (1:5,000; cat. no. STAR207P; Bio-Rad) for 2 h at room temperature. The band signals of target proteins were visualized using an enhanced chemiluminescence kit (Pierce, Minneapolis, MN, USA). All the experiments were repeated three times. In order to avoid possible problems related to incomplete stripping, all the results are from separate blots.
The primary antibodies are anti-MTDH (1:500, Millipore, Bedford, MA, USA), p-p65 (1:1,000, Cell Signaling Technologies, Beverly, MA, USA), p65 (1:1,000, Cell Signaling Technologies), and β-actin (Sigma-Aldrich).
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was used to verify NF-κB (RELA) interaction with the promoter region of MTDH. SKOV-3 and TOV-112D cell lines were prepared using the Magna ChIP A-Chromatin Immunoprecipitation Kit (Millipore, Billerica, MA, USA). DNA was eluted and purified from complexes, followed by SYBR PCR amplification of three putative binding sites of RELA within the MTDH promoter8. Immune complexes formed with nonspecific IgG were a negative control, while IκBα promoter primers (manufacturer’s kit) were a positive control. Primer pairs flanking these binding sites were used following the method previously described8.
Enzyme-Linked Immunosorbent Assay
Culture medium was centrifuged at 1,000 rpm for 5 min at 4 °C to remove residual cells, immediately after collection. The supernatant was harvested and stored at −20 °C for subsequent analysis. Concentrations of TNF-α , IL-6, IL-8, IL-10, and IL-1β in culture media were assayed using relative enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, MA, USA), according to the manufacturers’ instructions. All samples were assayed in duplicate. Cytokine concentrations are expressed as pg/ml based on relevant standard curves.
Statistics
Means and standard errors (SEs) were calculated from numerical data. Fold changes indicate the difference between experimental and control samples. Bar graphs depict the mean ± SE for a specific experimental run. SPSS Statistics 17 (SPSS Software, Chicago, IL, USA) was used for calculations. Two-tailed, unpaired Student’s t test assessed significance between two conditions. One-way analysis of variance test followed by Tukey’s comparison for multiple groups compared the significance of differences between the means of groups. Two-tailed P-value < 0.05 was considered significant.
Results
MTDH Is Upregulated in Ovarian Cancer Tissues
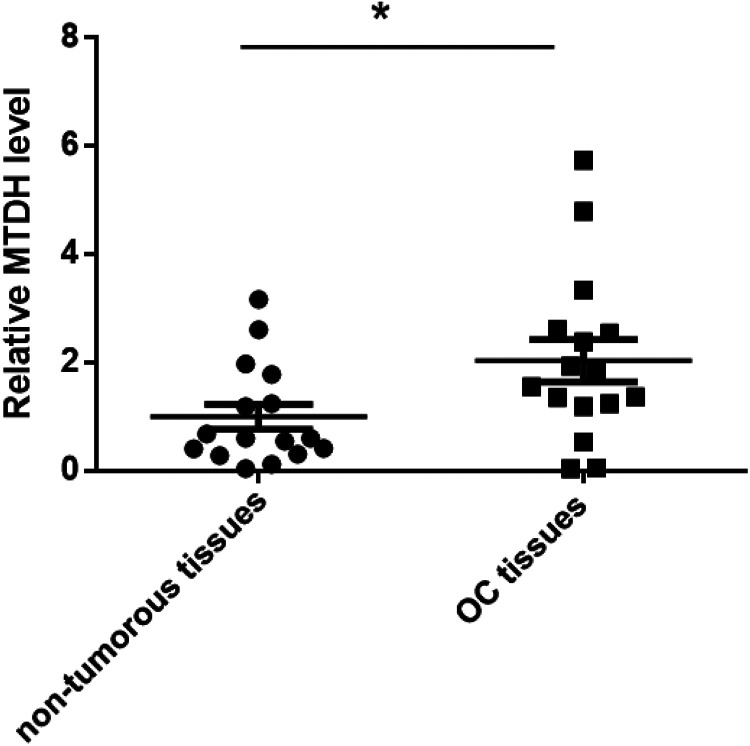
MTDH was reported to be overexpressed in OC13. To decipher the underlying molecular biology and function of MTDH in OC, we first examined the mRNA expression levels of MTDH in the 16 ovarian cancer tissues and the paired nontumorous tissues with Taqman quantitative real-time PCR. Consistently, MTDH showed much higher mRNA level in the OC tissues compared to the paired nontumorous tissues (Fig. 1). These results suggest that MTDH overexpression in OC may be attributed to OC traits.
Figure 1.
MTDH mRNA level is greatly higher in OC tissues. MTDH mRNA levels were detected by qRT-PCR in 16 paired OC tissues and paired nontumorous tissues. *P < 0.05 vs nontumorous tissues. MTDH: metadherin; OC: ovarian cancer; qRT-PCR: quantitative real-time PCR.
MTDH Overexpression Enhances Expression of TNF-α, IL-6, IL-8, IL-10, and IL-1β
Chronic inflammatory conditions contribute to maintaining several signaling pathways that could represent plausible mechanism(s) for MTDH overexpression8. We subsequently investigated the inflammatory cytokine and chemokine expressions in OC. As seen in Figure 2A, TNF-α, IL-6, IL-8, IL-10, and IL-1β mRNA expressions were greatly augmented in OC tissues than the paired nontumorous tissues. In order to explore whether MTDH regulates inflammatory cytokine and chemokine expressions in OC, we stably overexpressed MTDH in SKOV-3 cells (Fig. 2B, C). Subsequently, the MTDH overexpression was investigated to significantly augment TNF-α, IL-6, IL-8, IL-10, and IL-1β mRNA expression after 24 h of TNF-α challenge (Fig. 2D).
Figure 2.
MTDH enhances mRNA and protein expression of TNF-α, IL-6, IL-8, IL-10, and IL-1β. (A) TNF-α, IL-6, IL-8, IL-10, and IL-1β mRNA level is upregulated in OC tissues. mRNA levels were detected by qRT-PCR in 16 paired OC tissues and paired nontumorous tissues. *P < 0.05 vs non-tumorous tissues; (B) MTDH mRNA level is significantly higher after MTDH overexpression. *P < 0.05 vs Ctrl-OE. Results are mean ± SE (n = 3). (C) MTDH protein expression is significantly higher after MTDH overexpression. (D) After cells stably overexpressed MTDH, cells were stimulated or not with TNF-α (10 ng/ml) for 24 h. The mRNA expression of TNF-α, IL-6, IL-8, IL-10, and IL-1β was analyzed by qRT-PCR. The expression of each gene was normalized to the average of nonstimulated cells. *P < 0.05 vs nonstimulated cells; ^P < 0.05 vs Ctrl-OE. Results are mean ± SE (n = 3). (E) The protein levels of TNF-α, IL-6, IL-8, IL-10, and IL-1β in the culture media were assayed with a specific ELISA. *P < 0.05 vs nonstimulated cells; ^P < 0.05 vs Ctrl-OE. Results are mean ± SE (n = 3). Ctrl: control; ELISA: enzyme-linked immunosorbent assay; MTDH: metadherin; OC: ovarian cancer; OE: overexpression; qRT-PCR: quantitative real-time polymerase chain reaction.
Cytokine protein production in culture media of SKOV-3 cells was quantified by ELISA. After 24 h of TNF-α treatment, we observed a significant increase in TNF-α, IL-6, IL-8, IL-10, and IL-1β proteins in the culture media compared with the levels of these cytokines in the Ctrl-OE group (Fig. 2E).
Regulation of MTDH and NF-κB in Ovarian Cancer
TNF-α, IL-6, IL-8, IL-10, and IL-1β are transcriptionally controlled by NF-κB14. It has also been reported that MTDH overexpression contributes to the induction of NF-κB via secondary positive feedback signaling8,15. In this study, to elucidate the underlying molecular network of MTDH and inflammatory condition, we subsequently investigated whether MTDH contributes to the induction of NF-κB. As shown in Figure 3A, MTDH overexpression greatly increased the mRNA level and protein expression of phosphor-p65 compared with the Ctrl-OE group.
Figure 3.
(A) MTDH overexpression leads to the activation of NF-κB (p65) in SKOV-3 cells. qRT-PCR validated the increased mRNA levels of p-p65. Western blotting confirms the increased levels of phosphorylated p65 protein. (B)Chip-qPCR quantification of enrichment of DNA fragments that contain putative NF-κB binding sites. *P < 0.05 vs parent cell line and/or negative control specimen (IgG) (n = 3). Results are mean ± SE (n = 3). ChIP: chromatin immunoprecipitation; Ctrl: control; MTDH: metadherin; OE: overexpression; qRT-PCR: quantitative real-time polymerase chain reaction.
TNF-α stimulation has been reported to induce MTDH transcription in diverse cell types15, suggesting the necessity of additional intracellular mediators interacting with MTDH. However, these precise underlying mechanisms remain incompletely defined in OC. Recently, a footprint of p65 (RELA) subunit has been shown binding to the MTDH promoter8. Using ChIP, we also observed under TNF-α-stimulated conditions an increase in RELA binding at three sites (Fig. 3B). This is consistent with the observation in the MPM8.
Discussion
The normal physiologic role of MTDH still remains elusive16. MTDH acts as a transcriptional cofactor via a nuclear homing domain17. Currently, MTDH has been shown as a critical regulator in a variety of cancers, such as lung cancer, hepatocellular carcinoma, and gastric cancer18–20. The reason is because MTDH can regulate multiple signaling pathways and expression levels of related genes to affect the behavior of tumors, such as NF-κB, PI3K/AKT/MAPK, and Wnt/β-catenin15. Through these signaling pathways, MTDH can also affect the cellular activities including proliferation, angiogenesis, invasion, metastasis, and drug resistance. Despite an extensive literature regarding the multiple functions of MTDH, the precise function of MTDH in OC has not been investigated in detail.
NF-κB is a transcription factor that controls various important genes in many critical physiological responses such as inflammatory responses, proliferation, differentiation, cell adhesion, and apoptosis21. Because of its importance to many biological processes, NF-κB activation and activity are tightly regulated by a battery of endogenous mechanisms. The aberrant NF-κB activation contributes to the development of various autoimmune, inflammatory, and malignant disorders including malignant tumors such as MPM and squamous cell carcinoma8,22. The activation of NF-κB signaling by MTDH has also been reported in hepatocellular carcinoma23. What is more, MTDH was reported as a prognostic apoptosis modulator in mesothelioma induced via NF-kB-mediated signaling8. However, the underlying mechanism and precise function of MTDH/NF-κB signaling still remains unclear.
In this study, we verified that MTDH is upregulated in the OC tissues compared to the paired non-tumorous tissues. Subsequently, we also found TNF-α, IL-6, IL-8, IL-10, and IL-1β mRNA expressions were greatly augmented in OC tissues than the paired non-tumorous tissues. Next, MTDH overexpression greatly increased the mRNA level and protein expression of phosphor-p65 compared with the Ctrl-OE group. Using ChIP analysis, we also validated that NF-κB directly induces MTDH by binding specific promoter regions in OC. We identified a feed-forward loop motif of MTDH and NF-κB in OC. However, it is worthy to verify this model in vivo, add histochemical demonstrations, consider to control the original level of the inflammation factors, and ensure the increased expression of these factors caused by MTDH overexpression.
Taken together, our results suggest MTDH as a rational target to explore for better understanding of OC biology and possibly provide an alternative OC treatment approach.
Acknowledgments
The authors would like to thank the Pathology Division of China–Japan Friendship Hospital in Beijing for the pathologic diagnosis.
Footnotes
Author Contributions: JL conceived the study. CR and JL designed the experiments and wrote the manuscript. CR collected the samples and completed the experimental part of the study. YS, JH, and XW contributed to cell culture and sample collection. RS and YM analyzed the data and revised the manuscript. AM critically discussed the results.
Ethical Approval: Ethical approval was obtained for all experimental procedures from the Ethics Committee of China–Japan Friendship Hospital, Beijing, China.
Statement of Human and Animal Rights: All procedures with human subjects in this study were conducted in accordance with the Human Ethics Committee of China–Japan Friendship Hospital in Beijing, China. This article does not contain any studies with animals.
Statement of Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jing Liang  https://orcid.org/0000-0002-0102-6523
https://orcid.org/0000-0002-0102-6523
References
- 1. Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol. 2016;27(11):2017–2025. [DOI] [PubMed] [Google Scholar]
- 2. Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao W, Volsky DJ, Fisher PB. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21(22):3592–3602. [DOI] [PubMed] [Google Scholar]
- 3. Guo F, Wan L, Zheng A, Stanevich V, Wei Y, Satyshur KA, Shen M, Lee W, Kang Y, Xing Y. Structural insights into the tumor-promoting function of the MTDH-SND1 complex. Cell Rep. 2014;8(6):1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ge X, Lv X, Feng L, Liu X, Gao J, Chen N, Wang X. Metadherin contributes to the pathogenesis of diffuse large B-cell lymphoma. PLoS One. 2012;7(6):e39449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y, Song H, Lin P, Sun X, Yu X, Zhang Y, et al. AEG -1 overexpression: a novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers. Int J Gynecol Cancer. 2011;21(4):602–608. [DOI] [PubMed] [Google Scholar]
- 6. Noch EK, Khalili K. The role of AEG-1/MTDH/LYRIC in the pathogenesis of central nervous system disease. Adv Cancer Res. 2013;120:159–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, Zeng M, Li J, Song L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30(5):894–901. [DOI] [PubMed] [Google Scholar]
- 8. Zhang L, Singh A, Plaisier C, Pruett N, Ripley RT, Schrump DS, Hoang CD. metadherin is a prognostic apoptosis modulator in mesothelioma induced via NF-kappaB-mediated signaling. Transl Oncol. 2019;12(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Li ZY, Hou XX, Wang X, Luo YH, Ying YP, Chen G. Clinical significance and effect of AEG-1 on the proliferation, invasion, and migration of NSCLC: a study based on immunohistochemistry, TCGA, bioinformatics, in vitro and in vivo verification. Oncotarget. 2017;8(10):16531–16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin Y, Zhang ZL, Huang Y, Zhang KN, Xiong B. MiR-182-5p inhibited proliferation and metastasis of colorectal cancer by targeting MTDH. Eur Rev Med Pharmacol Sci. 2019;23(4):1494–1501. [DOI] [PubMed] [Google Scholar]
- 11. El-Ashmawy NE, El-Zamarany EA, Khedr EG, Abo-Saif MA. Activation of EMT in colorectal cancer by MTDH/NF-kappaB p65 pathway. Mol Cell Biochem. 2019;457(1-2):83–91. [DOI] [PubMed] [Google Scholar]
- 12. Fu H, Tan J, Yin Q. Effects of recombinant adeno-associated virus-mediated CD151 gene transfer on the expression of rat vascular endothelial growth factor in ischemic myocardium. Exp Ther Med. 2015;9(1):187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong R, Liu X, Zhang Q, Jiang Z, Li Y, Wei Y, Li Y, Yang Q, Liu J, Wei JJ, Shao C, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget. 2014;5(21):10816–10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emdad L, Das SK, Dasgupta S, Hu B, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res. 2013;120:75–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, Gredler R, Fisher PB, Sarkar D. Astrocyte elevated gene-1 (AEG-1): a multifunctional regulator of normal and abnormal physiology. Pharmacol Ther. 2011;130(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SG, Kang DC, DeSalle R, Sarkar D, Fisher PB. AEG-1/MTDH/LYRIC, the beginning: initial cloning, structure, expression profile, and regulation of expression. Adv Cancer Res. 2013;120:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jian-bo X, Hui W, Yu-long H, Chang-hua Z, Long-juan Z, Shi-rong C, Wen-hua Z. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28(2):455–462. [DOI] [PubMed] [Google Scholar]
- 19. Ke ZF, Mao X, Zeng C, He S, Li S, Wang LT. AEG-1 expression characteristics in human non-small cell lung cancer and its relationship with apoptosis. Med Oncol. 2013;30(1):383. [DOI] [PubMed] [Google Scholar]
- 20. Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119(3):465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. [DOI] [PubMed] [Google Scholar]
- 22. Qin Y, Wang J, Zhu G, Li G, Tan H, Chen C, Pi L, She L, Chen X, Wei M, Li Z, et al. CCL18 promotes the metastasis of squamous cell carcinoma of the head and neck through MTDH-NF-kappaB signalling pathway. J Cell Mol Med. 2019;23(4):2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson CL, Srivastava J, Siddiq A, Gredler R, Emdad L, Rajasekaran D, Akiel M, Shen XN, Guo C, Giashuddin S, Wang XY, et al. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014;74(21):6184–6193. [DOI] [PMC free article] [PubMed] [Google Scholar]