Abstract
Due to the lack of animal models and difficulty in obtaining specimens, the study of pathogenesis of moyamoya disease (MMD) almost stagnated. In recent years, endothelial progenitor cells (EPCs) have attracted more and more attention in vascular diseases due to their important role in neovascularization. With the aid of paradigms and methods in cardiovascular diseases research, people began to explore the role of EPCs in the processing of MMD. In the past decade, studies have shown that abnormalities in cell amounts and functions of EPCs were closely related to the vascular pathological changes in MMD. However, the lack of consistent criteria, such as isolation, cultivation, and identification standards, is also blocking the way forward. The goal of this review is to provide an overview of the current situation and controversial issues relevant to studies about EPCs in the pathogenesis and etiology of MMD.
Keywords: endothelial progenitor cells, moyamoya disease, neovascularization, pathogenesis
Introduction
Moyamoya disease (MMD) is an idiopathic cerebrovascular disease which was first described by Suzuki and Takaku in 19691. MMD is characterized by progressive stenosis or occlusion at the end of bilateral internal carotid artery (ICA) and/or the beginning of anterior and middle cerebral artery, accompanied by compensatory dilation of the perforating artery, and formation of dense vascular networks (“moyamoya vessels”)2. MMD has been found all over the world, especially in Japan, Korea, and China3. At present, the treatment of MMD is mainly based on revascularization surgery4 which is just a late-stage intervention considering the long development process of this disease. Due to the lack of clear understanding of its etiology and pathogenesis, there is almost no way to carry out any early prevention and intervention for MMD.
Recently, increasing attention has been paid to the important role of endothelium in cerebrovascular biology5–7. In fact, several vascular pathological changes, such as intima hyperplasia, tortuous layering of internal elastic lamina, and abnormal angiogenesis, have already been observed in MMD8. Therefore, the potential role of endothelial progenitor cells (EPCs) in the pathogenesis of MMD has aroused the interest of researchers, especially in maintaining endothelial integrity, function, and postnatal neovascularization. With the help of paradigms provided by studies on EPCs in other vascular diseases (e.g., cardiovascular disease9, cerebral ischemic stroke10), especially the process involving neovascularization, the exploration of EPCs in the mechanism of MMD has made progress in the past decade.
The goal of this review is to provide an overview of the current situation and controversial issues relevant to studies about EPCs in the pathogenesis and etiology of MMD.
What Is Neovascularization?
Neovascularization, the process of new blood vessel growth and development, is an important process under various physiological and pathological conditions such as embryonic development11, wound healing12, ischemia, inflammation, infections13, as well as tumorigenesis14. The molecular, genetic, and cellular mechanisms of vessel growth and their implications are not always the same under different circumstances. Over the years, many related studies have developed the concept of the genesis of new vessels into some similar terms with different connotations.
The term “angiogenesis” describes the initiation of new capillaries from preexisting vessels13, which are stimulated by hypoxia through the activation of numerous growth factors and cytokines15. Arteriogenesis refers to the maturation or regrowth of collateral vessels16, which are usually large enough to be shown on angiography17. Arteriogenesis usually occurs outside the ischemic area in response to the aggregation of blood-derived monocytes in localized arterial stenosis site caused by local shear stress. One of the most important arguments related to arteriogenesis is whether collateral development occurs similar to angiogenesis or it represents the remodeling and dilation of preexisting vascular channels14. Vasculogenesis is an important paradigm for the establishment of embryonic primitive vascular network18, a process of vascular formation in situ by circulating EPCs and vascular progenitor cells19,20. In contrary, “postnatal vasculogenesis” refers to new blood vessel formation in adults21. And the last, neovascularization is the result of several processes, including angiogenesis, arteriogenesis, and vasculogenesis14.
It should be noted that these processes are not completely independent; for example, in the case of a common femoral artery ligation, arteriogenesis will predominate at the site of ligation, whereas angiogenesis will predominate in the ischemic distal bed14. Therefore, it is necessary to study a particular neovascularization event according to the specific pathological conditions in specific diseases.
EPCs in Postnatal Neovascularization
EPCs were first discovered in isolated mononuclear cells (MNCs) from human peripheral blood (PB) by Asahara et al. in 199722. These bone marrow (BM)–derived progenitor cells with high proliferative ability were defined as EPCs, which have the potential to differentiate into endothelial cells (ECs) lines23. EPCs were found to be involved in the physiological process of neovascularization like wound healing and ovarian cycle and subsequent pathological events, such as hypertension24, myocardial infarction25, stroke26, atherosclerosis27, and cancer28.
There have been controversies about the origin of EPCs. At present, it is generally accepted that EPCs originated from mesoderm cells, the same origination as hematopoietic stem cells (HSCs), during embryonic development29. Normally, EPCs retain in a homeostatic BM microenvironment with low oxygen tension and high stromal cell–derived factor-1 (SDF-1) content, which is necessary for maintaining them30. Stimulated by factors such as inflammatory, traumatic, or ischemia-induced hypoxia, EPCs leave the BM and enter the circulation driven by chemokines, matrix metalloproteinase (MMP) 9, vascular endothelial growth factor (VEGF), nitric oxide, and so on, which is called “mobilization”31,32. Regulated by tissue-specific chemokine signaling, EPCs become activated and home to the target tissue. On reaching the site of injury, EPCs begin to adhere to ECs and migrate into vascular and tissue repair sites under the mediation of integrins33. Once EPCs pass through the endodermis, they perform their function by differentiating into ECs and remodeling the vascular extracellular matrix (ECM) components34,35. Although the functional activity of EPCs is mostly under investigation, it is considered that their differentiation involves adhesion to the ECM components controlled by integrins, proliferation and survival induced by growth factors, and maturation and acquisition of the endothelial phenotype36.
EPCs also contribute to the maintenance of the vascular system by producing proangiogenic factors able to enhance the proliferation, survival, and function of mature ECs and other surrounding progenitor cells23. For example, smooth muscle progenitor cells (SMPCs) and smooth muscle cells (SMCs) are key factors in proliferative vascular diseases such as atherosclerosis, intimal hyperplasia, and hypertension37. Studies have found that EPCs were closely related to the source and function of SMPC and SMC38–41. Besides, the abnormalities in the amount and functions of EPCs were also found in chronic ischemic cardiomyopathy42, myocardial infarction25, ischemic stroke10 and infarct models43,44, which prompted the participation of EPCs in vascular occlusive/stenosis process.
As mentioned earlier, EPCs share common precursors (mesoderm cells) with other cell lineages. Therefore, it is feasible to separate EPCs from various sources, such as hematopoietic EPCs (hemogenic endothelium, myeloid cells, mesenchymal stem cells), nonhematopoietic EPCs (umbilical cord blood, PB), and tissue-resident EPCs45. EPCs derived from PB, also known as circulation EPCs (cEPCs), have been studied most because the method to obtain specimens is more convenient and less invasive. A set of methods created by Asahara et al.22 and developed by later researchers were used to isolate and cultivate the cEPCs46. Accordingly, two different types of circulating EPCs (early EPCs and late EPCs) have been identified according to their morphology, appearance time, and cell surface markers47–49. The specific differences between these two kinds of EPCs will be elaborated in the later review.
Abnormal Neovascularization in MMD
According to the definition of MMD, there are at least two impaired vessel growth processes in the course of this disease. The first is the proliferative lesions that cause stenosis/occlusion in major cerebral arteries (e.g., ICA, anterior and middle cerebral artery). Histopathological examination of the end of the carotid artery showed that the luminal stenosis was caused by fibrocellular intimal thickening, tortuosity, and disruption of internal elastic lam50,51. In recent years, the application of neuroimaging techniques such as high-resolution magnetic resonance imaging (MRI) in MMD patients has shown that the artery diameter of the involved segment is narrowed and the symptomatic segment is concentric enhanced52–54, consistent with previous findings of endometrial hyperplasia and medial thinning55,56. More evidences show that MMD is mainly an endometrial hyperplasia disease. The immunohistochemical features of the distal parts of ICAs indicated the proliferation of SMCs or ECs8,51,57. The migration and proliferation of SMCs associated with actin alpha 2 (ACTA2) mutations is considered to be a key mechanism of familial MMD58.
The second refers to the formation of unhealthy perforating arteries, the so-called moyamoya vessels, which are considered a compensation for cerebral ischemia and hypoxia59. Histopathological changes in moyamoya vessels include fibrin deposition in the vessel walls, elastic layer fragments, media weakening, and formation of micro artemia. Immunohistochemical studies have confirmed that many factors related to angiogenesis [VEGF receptors, fibroblast growth factor (FGF) receptor, nestin, and so on] were abnormally expressed in vascular ECs51,60, suggesting an active angiogenetic process. Although moyamoya vessels may supply the lack of perfusions, they are ineffective, fragile neovascularization that gradually disappear over time, leading to adult intracranial hemorrhage61. In conclusion, the excessive formation of collateral vessels that originated from the initial stenosis of the ICA emphasizes that the increase and/or abnormality of neovascularization are involved in the pathophysiological process of the disease62.
Moreover, various proangiogenesis cytokines have also been reported to be associated with MMD, including growth factors (such as VEGF, FGF, platelet-derived growth factor, and hepatocyte growth factor), cytokines related to vascular remodeling and angiogenesis (such as MMP and its inhibitors, hypoxia-inducible factor-1α and cell retinol node Syn-1), and inflammation-related cytokines59.
As described above, MMD is a special disease closely related to the dynamic between arterial proliferation and neovascularization, which may involve the proliferation, migration, differentiation, and maturation of vascular constituent cells and the maintenance of vascular structure. Therefore, the involvement of EPCs in MMD may be more complex than in other cerebrovascular diseases.
Current Studies of EPCs in MMD
Since the first description of EPCs by Asahara et al. in 199721, there have been a lot of studies on EPCs in various diseases, such as hypertension63, cardiovascular disease64, and cerebrovascular diseases26. Especially in cerebral ischemia stroke, EPCs-based cell therapy is now considered an important new therapeutic approach65. EPCs have also attracted attention in the pathogenetic study of MMD. The current studies mainly focused on the aspects given here (Table 1).
Table 1.
Summary of Current Studies About Endothelial Progenitor Cells in Moyamoya Disease.
| Authors | Nation | Year | Subjects | Sample source | Isolation and cultivation methods | Subsets (terminology) of EPCs | Criteria of characterization | Abnormal cell amount (MMD vs. HC) | Abnormal cell function (MMD vs. HC) |
|---|---|---|---|---|---|---|---|---|---|
| Yoshihara et al. | Japan | 2008 | 4 MMD, 26 HC | Peripheral blood | A | cEPCs (circulating CD34+ cells) | CD34+CD45+ | ↑ | |
| Jung et al. | Korea | 2008 | 24 MMD, 48 HC | Peripheral blood | B | Early EPCs (EPC-CFU) and late EPCs (outgrowth cells) | 1. Positive Ac-LDL uptake; 2. Ulex europaeus agglutinin-1, CD31+, vascular endothelium cadherin, CD34+, kinase domain receptor | 1. EPC-CFU: ↓ 2. Outgrowth cells: ↑ |
Early EPCs: proliferation: ↓ Late EPCs: proliferation: ↑, tube formation: ↓ |
| Rafat et al. | Germany | 2009 | 20 MMD, 8 ACVD, 15 HC | Peripheral blood | A | cEPCs | CD34+/CD133+/VEGFR-2+ | ↑ | |
| Kim et al. | Korea | 2010 | 28 MMD, 12 HC | Peripheral blood | B | cEPCs in MMD children | For early EPC: cluster (central core of rounded cells surrounded
by spindle-shaped cell),
CD34+CD133+KDR+
For late EPC: vWF+, cobblestone morphology, positive Ac-LDL uptake |
1. Early EPC and EPC clusters: ↓ 2. Outgrowth cells: ↓ |
Early EPCs: proliferation: ↓ Late EPCs: proliferation: ↓, tube formation: ↓, senescence: ↑ |
| Ni et al. | China | 2011 | 18 MMD, 12 HC | Peripheral blood | C | cEPCs | CD34+, CXCR4 (CD184)+ | ↑ | CD34+CXCR4+ cells: ↑ |
| Lee et al. | Korea | 2015 | 9 MMD, 4 HC | Peripheral blood | B | Late EPCs (ECFCs) | CD34+KDR+CD133+CD31+, CD45+vWF+, positive Ac-LDL uptake | Tube formation: ↓ | |
| Zhang et al. | China | 2016 | 30 MMD with STA-MCA, 27 MMD only conservative treatment | Peripheral blood | A | cEPCs | CD34+CD133+KDR+ | The number of EPCs was decreased significantly after surgery | |
| Phi et al. | Korea | 2017 | 12 MMD, 7 HC | Peripheral blood | B | Late EPCs (ECFCs) | CD34weakKDR+VE-cadherin+CD31+α-SMAweakPDGFR-α and βweak CD45–vWF+ | 1. Tube formation: ↓ 2. MMD ECFCs promote migration of SPCs |
|
| Choi et al. | Korea | 2018 | 5 MMD, 5 HC | Peripheral blood | B | Late EPCs (ECFCs) | CD31+CD34+CD45+CD133+KDR+vWF+ | 1. Tube formation: ↓ 2. Disrupted mitochondrial morphology 3. Mitochondria functional abnormalities |
|
| Bao et al. | China | 2018 | 66 MMD, 81 HC | Peripheral blood | C | cEPCs | CD31+CD45dimCD34brCD133+ | ↑ | |
| Choi et al. | Korea | 2018 | Rat models | ECFCs from control/MMD patients were injected into the CCH rat model | B | Late EPCs (ECFCs) | 1. Less improvement in the restoration of cerebral perfusion and
in behavior 2. Less amount of neovasculogenesis and neurogenesis and more apoptosis |
MMD: moyamoya disease; HC: healthy control; ACVD: atherosclerotic cerebrovascular disease; STA-MCA: superficial temporal; cEPCs: circulating endothelial progenitor cells; EPCs: endothelial progenitor cells; EPC-CFU: endothelial progenitor cells colony-forming unit; ECFCs: endothelial colony-forming cells; PDGFR: platelet derived growth factor recepto; SPC: smooth muscle progenitor cells; PBMNCs: peripheral blood mononuclear cells; Ac-LDL: acetylated low-density lipoprotein; CCH: chronic cerebral hypoperfusion; CFU-EC: colony-forming unit endothelial cells; VEGFR-2: vascular endothelial growth factor receptor-2; KDR: kinase insert domain receptor.
Isolation and culture methods:
A: Density gradient centrifugation to obtain PBMNCs and characterized by flow cytometry; B: density gradient centrifugation to obtain PBMNCs, culture 7days for EPC-CFU, characterized by flow cytometry; 2 months for outgrowth cells, characterized by flow cytometry; C: peripheral whole blood samples characterized by flow cytometry.
EPCs Quantitative Anomaly
After acute cerebral ischemia, cluster differentiation 34 positive (CD34+) cells in the BM of stroke patients were activated6. In addition, transplantation of CD34+ cells66 and BM cells67 has been shown to restore cerebral blood flow in experimental stroke models. In chronic ischemia, CD34+ cell transplantation has also been shown to accelerate the formation of new blood vessels, including collateral vessels, in patients with chronic ischemic heart disease68 and limb ischemia69. In addition, there is a report on the relationship between the hypoplasia of coronary collateral and the decrease of circulating EPCs in patients with myocardial ischemia70. Thus, exploring the difference in the amount of EPCs between MMD patients and normal people may open the window to have a peep at the mechanisms of the complicated angiogenesis in MMD patients.
In 2008, Yoshihara et al.71 found for the first time that the number of CD34+ cells in the PB of MMD patients was significantly higher than that of normal people. Subsequently, researchers used more abundant molecular markers, such as CD133, vascular endothelial growth factor receptor-2 (kinase insert domain receptor) VEGFR-2 (KDR), and CD31, to characterize and count EPCs in PB. Similar results were observed72–74, except in Jung et al.75 and Kim et al.76 In these two studies, the researchers cultured the obtained peripheral blood mononuclear cells (PBMNCs) and subdivided the EPCs into early EPCs/endothelial progenitor cells colony-forming units (EPC-CFU) and late EPCs/outgrowth cells according to the morphological and molecular markers. Jung et al.75 found EPC-CFU numbers were significantly lower in MMD patients than in controls, while outgrowth cells were more in MMD patients. However, Kim et al.76 observed a decrease in both early EPCs and late EPCs. Because of this, the results of the amount of EPCs in the PB of MMD patients are often regarded as “controversial.”
EPCs Functional Abnormality
As mentioned previously, EPCs can be divided into two subpopulations with great differences in morphology and capability. For early EPCs, one of the significant features is the ability to form clusters or colonies in in vitro cultivation. In particular, EPCs show clusters with spindle-shaped cells at the boundary47. Therefore, the formation of clusters and the number of these clusters are considered a definitive measure for evaluating EPCs numbers and differentiation64. As mentioned earlier, Jung et al.75 and Kim et al.76 both found early EPCs and clusters were significantly reduced in MMD patients compared with healthy control.
Late EPCs are closer to mature ECs in phenotype but show surprising tube-forming and proliferative capabilities, which are essential to promote neovascularization and maintain the integrity of vascular structure47. In all relevant studies75–78, the tube-forming ability of EPCs in MMD were decreased, but the results about proliferation function were debatable: Jung et al.75 observed outgrowth cells were more in MMD patients but Kim et al.76 observed outgrowth EPCs in MMD were less. Besides, in 2011, Ni et al.73 found a larger proportion of both CD34+ and C-X-C motif chemokine receptor 4 (CXCR4)-positive cells in the PB pool of EPCs in MMD patients than in healthy controls. CXCR4 is the receptor of SDF-1α, which interacts with SDF-1α for trafficking CD34+ cells or recruiting other vascular wall (progenitor) cells from BM to PB and modulating angiogenesis. Platelet-derived SDF-1α mediates the migration of CD34+ cells to the injured vessel and differentiate into EPCs via binding CXCR479. Therefore, this has been considered as an indirect evidence of the enhanced migration ability of MMD-derived EPCs. However, Kim et al.76 found increased senescent-like phenotype of EPCs from pediatric MMD. Senescent EPCs have been found in impairments in multiple physiological activities, such as migration, differentiation, angiogenic activity, and alterations in growth factor expression80–82.
EPCs are described to contribute to neovascularization not only by differentiating into mature ECs but by paracrine effects, which stimulate angiogenic activity of resting mature ECs, leading to their migration, proliferation, and sprouting. Indeed, in 2017, Phi et al.77 confirmed C-C motif chemokine ligand 5 (CCL5) secreted by MMD endothelial colony-forming cells (ECFCs) significantly augmented the migration activities of SMCs (a main contributor to the hyperplasia of intima in MMD8,57) toward ECFCs.
In addition, retinaldehyde dehydrogenase 2 has been found downregulated in MMD EPCs and was attributed to defective acetyl-histone H3 binding to the promoter region78. The ECFCs from the MMD patients also displayed disrupted mitochondrial morphology like a shorter and more circular shape and functional abnormalities such as decreased oxygen consumption rate, increased intracellular Ca2 + concentration, and increased reactive oxygen species levels83. Except for the above in vitro experiments, the only relevant in vivo experiment found EPCs obtained from MMD brought less improvement in the cerebral perfusion, behavior, and amount of neovasculogenesis and neurogenesis after injection into the chronic cerebral hypoperfusion rat models84.
Related Factors
Few factors related to the quantitative and functional abnormality of EPCs were found. Disease stage75, patient’s age72,74, and serum levels of VEGF72 were found to be inversely correlated to EPCs numbers. In addition, gene enrichment analysis showed the biological processes involving immune response and chemotaxis were significantly enhanced in MMD ECFCs, while biological processes related to cell cycle and deoxyribonucleic acid (DNA) repair were suppressed in MMD ECFCs. In metabolic and signaling pathways, the genes related to the chemokine signaling pathway, ECM–receptor interaction, and cell adhesion molecules were activated in MMD ECFCs, whereas the genes for DNA replication, cell cycle, and mismatch repair were downregulated81. Recently, Nagata et al.85 developed a method to investigated the characteristics of EPCs cultured from patients with MMD under conditions of activated anti-inflammatory and angiogenic monocytes/macrophages and concluded that insufficient production of interleukin 10 from M2 macrophages impairs EPCs differentiation in MMD patients.
Issues About EPCs Study in MMD
EPCs are currently the most studied subtypes of different vascular progenitor cells. Most of these works are related to progenitor cells derived from PB and BM, and many publications show the contribution of EPCs to angiogenesis in tumorigenesis86, wound healing20, and ischemia87, as well as intimal re-endothelialization after vascular wall injury88. However, throughout the 21st century, the study of EPCs has become complicated and hindered by the separation, cultivation, and definition of different angiogenic cell subsets, which are all marked under the banner of EPCs but fail to comply with the necessary standards13,89. The research of EPCs in MMD also faces these problems:
What Are EPCs—The Definition of EPCs
As shown in Table 1, there exist controversies in the quantity, survival, and functionality of the EPCs in different studies. For example, previous literatures all considered the results about the amount of EPCs in the PB of MMD patients were “controversial.” However, the controversial nature of those observations was actually the result of different classification methods and different isolation/cultivation strategies. As shown in Fig. 1, Yoshihara’s study and other three studies71–74 actually just observed the increased amounts of CD34+ or CD34+CD133+KDR+ or CD31+CD45+CD34+CD133+ cells directly obtained from PB of MMD patients, and their results were consistent. However, the investigated targets of the studies of Jung75 and Kim76 were “cultured” early EPCs and late EPCs from PBMNCs. Their results were consistent with early EPCs and opposite on late EPCs. Although all these studies claimed to be conducted in the name of EPCs, the results could not be simply summed up as “controversial” because they lack comparability. In fact, after we distinguished their results in terms of “early EPCs, “late EPCs,” and “EPCs directly from PB (without any further culture)” in Fig. 1, the quantity of EPCs in different studies became almost consistent. Accordingly, these studies only studied one subgroup of EPCs, and could not comprehensively reflect the exact situation of EPCs in the PB of MMD patients. Furthermore, different criteria (Table 1) of EPCs characterization also lead to inconsistent cell composition of “EPCs” in different studies, which may result in the inconsistent conclusions about EPCs cell function. Besides, the related studies are not so much in total, and there are also gaps in the sample size among studies, as well as the characteristics between the samples (such as the onset type, disease stage, age). These may also contribute to the bias in conclusions.
Figure 1.
Different amounts of EPCs in moyamoya disease reported by different studies. EPCs: endothelial progenitor cells; PB: peripheral blood.
Unclear definitions also lead to inconsistent EPCs naming. In the past decade, various names/terms such as “circulating CD34+ cells,” “EPC-CFU,” “outgrowth cells,” “cEPCs,” “early EPCs,” “late EPCs,” colony-forming unit endothelial cells, and “ECFCs” have been adopted by different studies to describe EPCs. There exist inevitable reasons: the research pattern and methods of EPCs in MMD were almost based on researches of EPCs in other diseases such as cardiovascular disease and cerebral ischemic stroke. Even in those pioneering studies, unclear classification standards and lack of unified terms exist. Therefore, these phenomena are inevitable in the pathological study of EPCs in MMD. However, this situation has gradually improved. As shown in Table 1, researchers are gradually using relatively uniform terms and molecular markers to characterize EPCs. In order to solve the inconsistency/confusion of classification and names, at least two aspects must be achieved:
First, unified cell surface markers should be used to characterize EPCs and subgroups of EPCs. Cell surface markers are proteins and carbohydrates attached to cell membranes, providing a clear target for cell recognition. Various types of cell markers have been identified in the EPCs, such as CD34, a hematopoietic stem cell marker present in all types of ECs90,91. The pan-leukocyte marker CD45 is present only on EPCs but not on late EPCs or circulating ECs47,90–92. AC133/CD133 is expressed in HSCs and progenitor cells, early EPCs but not circulating ECs, indicating that prominin (mouse)-like 1 (AC133)/CD133 is an early marker92. On the other hand, there are conflicting reports about the expression of CD133 by late EPCs91,93,94. CD14 is a monocyte lineage marker; various studies have confirmed the presence of CD14 on early EPCs, but not on late EPCs and circulating ECs49,95. VEGFR-2 (mouse flk-1 or human KDR) is an important endothelial marker. VEGFR-2 expression in EPCs was weak, while VEGFR-2 expression was strong in late EPCs and ECs47,49,96. In addition, other markers such as CD36, CD106, and von Willebrand Factor (vWF) are rarely used in literature. Therefore, the true definition of different EPCs needs further study.
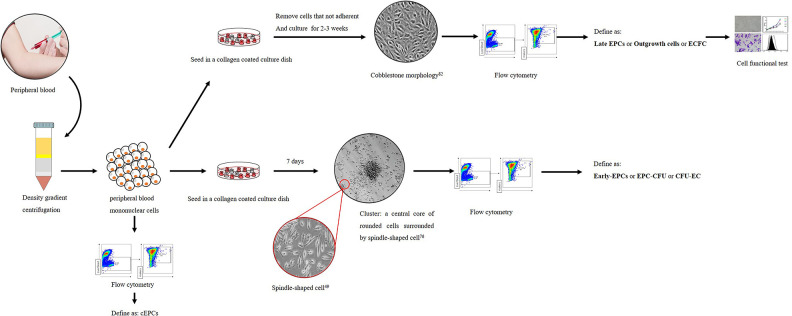
Second, unified isolation and culture methods are needed. We have summarized the isolation and cultivation methods of EPCs from the PB of MMD patients adopted by previous studies in Fig. 2. As show, different isolation and cultivation methods bring out different subsets of EPCs. Founded by Asahara et al. and developed by later researchers, a set of methods were used to isolate and cultivate the cEPCs. In general, after the PBMNCs are obtained from PB via density gradient centrifugation, they are inoculated into a collagen-coated culture dish. After a short period of culture, such as 7 days, clusters surrounded with spindle-like cells at the boundary form. These spindle-like cells are defined as early EPCs. If the PBMNCs are cultured for a longer period, such as 2–3 weeks, a “cobblestone” morphology will appear and these cells are referred to as outgrowth endothelial cells97, or late EPCs47, or ECFCs98. Collectively, these cells are termed as cEPCs. We also recommend techniques for isolating and culturing EPCs summarized by Chopra et al.45
Figure 2.
Methods of isolation, culture, and definition of circulation EPCs in moyamoya disease. EPCs: endothelial progenitor cells; cEPCs: circulation endothelial progenitor cells; EPC-CFU: endothelial progenitor cells colony-forming units; ECFCs: endothelial colony-forming cells; CFU-EC: colony-forming unit endothelial cells.
Why EPCs—Rationality About EPCs in the Pathogenesis of MMD
PB EPCs may contribute to MMD progression; however, other body parts in MMD patients show no obvious vascular atrophy. The microenvironment of brain may play a certain role in MMD. Neovascularization, which encompasses remodeling of existing vessels, angiogenesis, and barrier genesis, is a very complex process that requires coordination of cell-to-cell interaction99. This cellular communication is not limited to signals among vascular cells such as ECs–vascular SMCs or tip cells–stalk cells but indeed includes a network of vascular cells surrounding resident cells of the brain including pericytes, neurons, glia, and oligodendrocytes as well as circulating blood and BM cells100.
Recently, tissue-resident EPCs from large vessels have been considered as prime source for peripheral vascular repair because of their potential for significant cell proliferation, colony formation, drug excretion, and vascular formation101,102. Kawasaki et al. has demonstrated that the lung tissue–resident EPCs, rather than circulating EPCs, play a major role in pulmonary vascular repair of endotoxin-induced injury in the process of pulmonary vascular regeneration in experimental acute respiratory distress syndrome103. In fact, neuronal stem cells (NSCs) from human embryos have also been shown to express several endothelial and hematopoietic markers104. NSCs and peripheral nerve-derived adult pluripotent stem cells can be differentiated into ECs in vitro105–107. Studies using in vivo mouse models have shown that NSCs contribute to neurogenesis and angiogenesis not only in adult neurons but also in nonnerve tissues105. All of the above studies reflect two important findings: first, NSCs and ECs share a common progenitor cell; and second, the local environment is essential to control NSCs to ECs trans-differentiation.
In addition, EPCs may not be the only cells involved in neovascularization of patients with MMD. Aberrant angiogenesis in MMD is an active angiogenetic process that may recruit various cell types such as SMPCs71, SMCs108, circulating ECs109, and/or immune cells110 to cause both stenosis and abnormal collateral formation.
Another important question is the nature of moyamoya vessels. Are they “newly formed” perforator arteries? Or the remodeling and dilatation of preexisting vascular channels? The responsible cells and mechanisms of these two different “neovascularization” processes are different. So far, the “exact identity” of moyamoya vessels has not been determined. The biggest obstacles in the basic research of MMD are difficulty in obtaining specimens and the lack of animal model. Unlike other diseases, MMD has a relatively short history of discovery and research. Further study of its pathological mechanism needs to be based on solid, scientific, and abundant objective observation.
Summary
Accurate and effective progenitor cell research provides a possible prospect for the exploration of the pathogenesis of MMD, but also faces many difficult challenges. More studies are needed to discover accurate mechanisms of EPCs mobilization, migration, (trans)differentiation, and homing to the target areas during the progress of MMD. Despite a large number of unsolved problems, more and more standardized scientific researches are providing some promising results. The aberrant EPCs amounts and impaired EPCs function may be related to the pathogenesis of MMD. However, improved and unified isolation, cultivation, and identification methods are needed to verify the rationality and feasibility of these results. Clinically, the formation of fragile compensatory vascular networks driven by abnormal neovascularization represents the source of cerebral hemorrhage in MMD patients. But at the same time, when using vascularized grafts such as temporal muscle to treat MMD, the expansion of angiogenesis is needed. Thus, a better understanding of the biology of EPCs will provide us with a clearer understanding of MMD and the possibility of early intervention, as well as to apply it to clinical therapy.
Acknowledgments
The authors thank all participants in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (Grant No. 81771280).
Ethical statement: Not applicable
Statement of human rights: Not applicable
ORCID iD: Jianjian Zhang, MD, PhD  https://orcid.org/0000-0001-9882-7978
https://orcid.org/0000-0001-9882-7978
Jincao Chen, MD, PhD  https://orcid.org/0000-0002-9071-9670
https://orcid.org/0000-0002-9071-9670
References
- 1. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20(3):288–299. [DOI] [PubMed] [Google Scholar]
- 2. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226–1237. [DOI] [PubMed] [Google Scholar]
- 3. Goto Y, Yonekawa Y. Worldwide distribution of moyamoya disease. Neurol Med Chir (Tokyo). 1992;32(12):883–886. [DOI] [PubMed] [Google Scholar]
- 4. Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: treatment and outcomes. J Stroke. 2016;18(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pias-Peleteiro J, Perez-Mato M, Lopez-Arias E, Rodriguez-Yanez M, Blanco M, Campos F, Castillo J, Sobrino T. Increased endothelial progenitor cell levels are associated with good outcome in intracerebral hemorrhage. Sci Rep. 2016;6:28724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, Naritomi H. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109(24):2972–2975. [DOI] [PubMed] [Google Scholar]
- 7. Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, Kim M, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke. 2008;39(5):1441–1447. [DOI] [PubMed] [Google Scholar]
- 8. Fukui M, Kono S, Sueishi K, Ikezaki K. Moyamoya disease. Neuropathology. 2000;20(Suppl):S61–S64. [DOI] [PubMed] [Google Scholar]
- 9. Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. [DOI] [PubMed] [Google Scholar]
- 10. Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39(1):69–74. [DOI] [PubMed] [Google Scholar]
- 11. Risau W. Embryonic angiogenesis factors. Pharmacol Ther. 1991;51(3):371–376. [DOI] [PubMed] [Google Scholar]
- 12. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. [DOI] [PubMed] [Google Scholar]
- 14. Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111(12):1556–1566. [DOI] [PubMed] [Google Scholar]
- 15. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. [DOI] [PubMed] [Google Scholar]
- 16. Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10(1):83–97. [DOI] [PubMed] [Google Scholar]
- 17. Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis). J Pathol. 2000;190(3):338–342. [DOI] [PubMed] [Google Scholar]
- 18. Risau W. Differentiation of endothelium. FASEB J. 1995;9(10):926–933. [PubMed] [Google Scholar]
- 19. Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287(3):C572–C579. [DOI] [PubMed] [Google Scholar]
- 20. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. [DOI] [PubMed] [Google Scholar]
- 21. Ribatti D, Vacca A, Nico B, Roncali L, Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100(2):157–163. [DOI] [PubMed] [Google Scholar]
- 22. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. [DOI] [PubMed] [Google Scholar]
- 23. Laurenzana A, Fibbi G, Margheri F, Biagioni A, Luciani C, Del Rosso M, Chilla A. Endothelial progenitor cells in sprouting angiogenesis: proteases pave the way. Curr Mol Med. 2015;15(7):606–620. [DOI] [PubMed] [Google Scholar]
- 24. de Cavanagh EM, Gonzalez SA, Inserra F, Forcada P, Castellaro C, Chiabaut-Svane J, Obregon S, Casarini MJ, Kempny P, Kotliar C. Sympathetic predominance is associated with impaired endothelial progenitor cells and tunneling nanotubes in controlled-hypertensive patients. Am J Physiol Heart Circ Physiol. 2014;307(2): H207–H215. [DOI] [PubMed] [Google Scholar]
- 25. Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. [DOI] [PubMed] [Google Scholar]
- 26. Cesari F, Nencini P, Nesi M, Caporale R, Giusti B, Abbate R, Gori AM, Inzitari D. Bone marrow-derived progenitor cells in the early phase of ischemic stroke: relation with stroke severity and discharge outcome. J Cereb Blood Flow Metab. 2009;29(12):1983–1990. [DOI] [PubMed] [Google Scholar]
- 27. Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217(1):240–248. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Vakil V, Braunstein M, Smith EL, Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U, Norin AJ, et al. Circulating endothelial progenitor cells in multiple myeloma: implications and significance. Blood. 2005;105(8):3286–3294. [DOI] [PubMed] [Google Scholar]
- 29. De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16(2):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balaji S, King A, Crombleholme TM, Keswani SG. The role of endothelial progenitor cells in postnatal vasculogenesis: implications for therapeutic neovascularization and wound healing. Adv Wound Care (New Rochelle). 2013;2(6):283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45(Suppl A):A39–A47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bouvard C, Gafsou B, Dizier B, Galy-Fauroux I, Lokajczyk A, Boisson-Vidal C, Fischer AM, Helley D. Alpha6-integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30(8):1569–1575. [DOI] [PubMed] [Google Scholar]
- 34. Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203(1):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tenreiro MM, Correia ML, Brito MA. Endothelial progenitor cells in multiple myeloma neovascularization: a brick to the wall. Angiogenesis. 2017;20(4):443–462. [DOI] [PubMed] [Google Scholar]
- 37. Lim S, Park S. Erratum: role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2016;49(2):134. [PubMed] [Google Scholar]
- 38. Zhu C, Ying D, Zhou D, Mi J, Zhang W, Chang Q, Li L. Expression of TGF-beta1 in smooth muscle cells regulates endothelial progenitor cells migration and differentiation. J Surg Res. 2005;125(2):151–156. [DOI] [PubMed] [Google Scholar]
- 39. Lao KH, Zeng L, Xu Q. Endothelial and smooth muscle cell transformation in atherosclerosis. Curr Opin Lipidol. 2015;26(5):449–456. [DOI] [PubMed] [Google Scholar]
- 40. Zhu G, Huang L, Song M, Yu Z, Wu X, Zhao X, Jin J, Zhao G, Chen J, Yu S. Over-expression of hepatocyte growth factor in smooth muscle cells regulates endothelial progenitor cells differentiation, migration and proliferation. Int J Cardiol. 2010;138(1):70–80. [DOI] [PubMed] [Google Scholar]
- 41. Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res. 2010;86(3):506–515. [DOI] [PubMed] [Google Scholar]
- 42. Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–1622. [DOI] [PubMed] [Google Scholar]
- 43. Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, Willhauck M, Spitzweg C, Gildehaus FJ, Munzing W, Hannappel E, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117(17):2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang ZT, Hong L, Wang H, Lai HL, Li LF, Yin QL. Application of peripheral-blood-derived endothelial progenitor cell for treating ischemia-reperfusion injury and infarction: a preclinical study in rat models. J Cardiothorac Surg. 2013;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chopra H, Hung MK, Kwong DL, Zhang CF, Pow EHN. Insights into endothelial progenitor cells: origin, classification, potentials, and prospects. Stem Cells Int. 2018;2018:9847015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. [DOI] [PubMed] [Google Scholar]
- 47. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–293. [DOI] [PubMed] [Google Scholar]
- 48. Madonna R, De Caterina R. Circulating endothelial progenitor cells: do they live up to their name? Vascul Pharmacol. 2015;67–69:2–5. [DOI] [PubMed] [Google Scholar]
- 49. Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. [DOI] [PubMed] [Google Scholar]
- 50. Hosoda Y, Ikeda E, Hirose S. Histopathological studies on spontaneous occlusion of the circle of Willis (cerebrovascular moyamoya disease). Clin Neurol Neurosurg. 1997;99(Suppl 2):S203–S208. [DOI] [PubMed] [Google Scholar]
- 51. Chmelova J, Kolar Z, Prochazka V, Curik R, Dvorackova J, Sirucek P, Kraft O, Hrbac T. Moyamoya disease is associated with endothelial activity detected by anti-nestin antibody. Biomed Pap Med Fac Univ Palacky Olomou Czech Repub. 2010;154(2):159–162. [DOI] [PubMed] [Google Scholar]
- 52. Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett. 2015;584:77–82. [DOI] [PubMed] [Google Scholar]
- 53. Kaku Y, Morioka M, Ohmori Y, Kawano T, Kai Y, Fukuoka H, Hirai T, Yamashita Y, Kuratsu J. Outer-diameter narrowing of the internal carotid and middle cerebral arteries in moyamoya disease detected on 3D constructive interference in steady-state MR image: is arterial constrictive remodeling a major pathogenesis? Acta Neurochir (Wien). 2012;154(12):2151–2157. [DOI] [PubMed] [Google Scholar]
- 54. Yu LB, Zhang Q, Shi ZY, Wang MQ, Zhang D. High-resolution magnetic resonance imaging of moyamoya disease. Chin Med J. 2015;128(23):3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oka K, Yamashita M, Sadoshima S, Tanaka K. Cerebral haemorrhage in Moyamoya disease at autopsy. Virchows Archiv A Pathol Anat Histol. 1981;392(3):247–261. [DOI] [PubMed] [Google Scholar]
- 56. Takagi Y, Kikuta K, Nozaki K, Hashimoto N. Histological features of middle cerebral arteries from patients treated for Moyamoya disease. Neurol Med Chir (Tokyo). 2007;47(1):1–4. [DOI] [PubMed] [Google Scholar]
- 57. Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7(11):1056–1066. [DOI] [PubMed] [Google Scholar]
- 58. Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84(5):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fujimura M, Bang OY, Kim JS. Moyamoya disease. Front Neurol Neurosci. 2016;40:204–220. [DOI] [PubMed] [Google Scholar]
- 60. Suzui H, Hoshimaru M, Takahashi JA, Kikuchi H, Fukumoto M, Ohta M, Itoh N, Hatanaka M. Immunohistochemical reactions for fibroblast growth factor receptor in arteries of patients with moyamoya disease. Neurosurgery. 1994;35(1):20–24; discussion 24–25. [DOI] [PubMed] [Google Scholar]
- 61. Funaki T, Takahashi JC, Yoshida K, Takagi Y, Fushimi Y, Kikuchi T, Mineharu Y, Okada T, Morimoto T, Miyamoto S. Periventricular anastomosis in moyamoya disease: detecting fragile collateral vessels with MR angiography. J Neurosurg. 2016;124(6):1766–1772. [DOI] [PubMed] [Google Scholar]
- 62. Bedini G, Blecharz KG, Nava S, Vajkoczy P, Alessandri G, Ranieri M, Acerbi F, Ferroli P, Riva D, Esposito S, Pantaleoni C, et al. Vasculogenic and angiogenic pathways in moyamoya disease. Curr Med Chem. 2016;23(4):315–345. [DOI] [PubMed] [Google Scholar]
- 63. Luo S, Xia W, Chen C, Robinson EA, Tao J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clinical science (London, England: 1979). 2016;130(22):2029–2042. [DOI] [PubMed] [Google Scholar]
- 64. Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49(7):741–752. [DOI] [PubMed] [Google Scholar]
- 65. Bayraktutan U. Endothelial progenitor cells: potential novel therapeutics for ischaemic stroke. Pharmacol Res. 2019;144:181–191. [DOI] [PubMed] [Google Scholar]
- 66. Taguchi A, Matsuyama T, Nakagomi T, Shimizu Y, Fukunaga R, Tatsumi Y, Yoshikawa H, Kikuchi-Taura A, Soma T, Moriwaki H, Nagatsuka K, et al. Circulating CD34-positive cells provide a marker of vascular risk associated with cognitive impairment. J Cereb Blood Flow Metab. 2008;28(3):445–449. [DOI] [PubMed] [Google Scholar]
- 67. Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res. 2004;1009(1–2):26–33. [DOI] [PubMed] [Google Scholar]
- 68. Boyle AJ, Whitbourn R, Schlicht S, Krum H, Kocher A, Nandurkar H, Bergmann S, Daniell M, O’Day J, Skerrett D, Haylock D, et al. Intra-coronary high-dose CD34+ stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006;109(1):21–27. [DOI] [PubMed] [Google Scholar]
- 69. Kudo FA, Nishibe T, Nishibe M, Yasuda K. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int Angiol. 2003;22(4):344–348. [PubMed] [Google Scholar]
- 70. Lambiase PD, Edwards RJ, Anthopoulos P, Rahman S, Meng YG, Bucknall CA, Redwood SR, Pearson JD, Marber MS. Circulating humoral factors and endothelial progenitor cells in patients with differing coronary collateral support. Circulation. 2004;109(24):2986–2992. [DOI] [PubMed] [Google Scholar]
- 71. Yoshihara T, Taguchi A, Matsuyama T, Shimizu Y, Kikuchi-Taura A, Soma T, Stern DM, Yoshikawa H, Kasahara Y, Moriwaki H, Nagatsuka K, et al. Increase in circulating CD34-positive cells in patients with angiographic evidence of moyamoya-like vessels. J Cereb Blood Flow Metab. 2008;28(6):1086–1089. [DOI] [PubMed] [Google Scholar]
- 72. Rafat N, Beck G, Pena-Tapia PG, Schmiedek P, Vajkoczy P. Increased levels of circulating endothelial progenitor cells in patients with Moyamoya disease. Stroke. 2009;40(2):432–438. [DOI] [PubMed] [Google Scholar]
- 73. Ni G, Liu W, Huang X, Zhu S, Yue X, Chen Z, Chen M, Liu X, Xu G. Increased levels of circulating SDF-1alpha and CD34+ CXCR4+ cells in patients with moyamoya disease. European journal of neurology. 2011;18(11):1304–1309. [DOI] [PubMed] [Google Scholar]
- 74. Bao XY, Fan YN, Liu Y, Wang QN, Zhang Y, Zhu B, Liu B, Duan L. Circulating endothelial progenitor cells and endothelial cells in moyamoya disease. Brain Behav. 2018;8(9):e01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jung KH, Chu K, Lee ST, Park HK, Kim DH, Kim JH, Bahn JJ, Song EC, Kim M, Lee SK, Roh JK. Circulating endothelial progenitor cells as a pathogenetic marker of moyamoya disease. J Cereb Blood Flow Metab. 2008;28(11):1795–1803. [DOI] [PubMed] [Google Scholar]
- 76. Kim JH, Jung JH, Phi JH, Kang HS, Kim JE, Chae JH, Kim SJ, Kim YH, Kim YY, Cho BK, Wang KC, et al. Decreased level and defective function of circulating endothelial progenitor cells in children with moyamoya disease. J Neurosci Res. 2010;88(3):510–518. [DOI] [PubMed] [Google Scholar]
- 77. Phi JH, Suzuki N, Moon YJ, Park AK, Wang KC, Lee JY, Choi SA, Chong S, Shirane R, Kim SK. Chemokine Ligand 5 (CCL5) derived from endothelial colony-forming cells (ECFCS) mediates recruitment of smooth muscle progenitor cells (SPCS) toward critical vascular locations in moyamoya disease. PLoS One. 2017;12(1):e0169714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee JY, Moon YJ, Lee HO, Park AK, Choi SA, Wang KC, Han JW, Joung JG, Kang HS, Kim JE, Phi JH, et al. Deregulation of retinaldehyde dehydrogenase 2 leads to defective angiogenic function of endothelial colony-forming cells in pediatric moyamoya disease. Arterioscler Thromb Vasc Biol. 2015;35(7):1670–1677. [DOI] [PubMed] [Google Scholar]
- 79. Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117(2):206–215. [DOI] [PubMed] [Google Scholar]
- 80. Gennaro G, Menard C, Michaud SE, Rivard A. Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation. 2003;107(2):230–233. [DOI] [PubMed] [Google Scholar]
- 81. Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441–1448. [DOI] [PubMed] [Google Scholar]
- 82. Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51(9):1119–1130. [DOI] [PubMed] [Google Scholar]
- 83. Choi JW, Son SM, Mook-Jung I, Moon YJ, Lee JY, Wang KC, Kang HS, Phi JH, Choi SA, Chong S, Byun J, et al. Mitochondrial abnormalities related to the dysfunction of circulating endothelial colony-forming cells in moyamoya disease. J Neurosurg. 2018;129(5):1151–1159. [DOI] [PubMed] [Google Scholar]
- 84. Choi SA, Chong S, Kwak PA, Moon YJ, Jangra A, Phi JH, Lee JY, Park SH, Kim SK. Impaired functional recovery of endothelial colony-forming cells from moyamoya disease in a chronic cerebral hypoperfusion rat model. J Neurosurg Pediatr. 2018;23(2):204–213. [DOI] [PubMed] [Google Scholar]
- 85. Nagata E, Masuda H, Nakayama T, Netsu S, Yuzawa H, Fujii N, Kohara S, Sorimachi T, Osada T, Imazeki R, Matsumae M, et al. Insufficient production of IL-10 from M2 macrophages impairs in vitro endothelial progenitor cell differentiation in patients with Moyamoya disease. Sci Rep. 2019;9(1):16752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21(12):1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. [DOI] [PubMed] [Google Scholar]
- 88. Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287(2): H512–H517. [DOI] [PubMed] [Google Scholar]
- 89. Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116(8):1392–1412. [DOI] [PubMed] [Google Scholar]
- 90. Cheng CC, Chang SJ, Chueh YN, Huang TS, Huang PH, Cheng SM, Tsai TN, Chen JW, Wang HW. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genomics. 2013;14(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–1579. [DOI] [PubMed] [Google Scholar]
- 92. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 93. Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 94. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 95. Cheng SM, Chang SJ, Tsai TN, Wu CH, Lin WS, Lin WY, Cheng CC. Differential expression of distinct surface markers in early endothelial progenitor cells and monocyte-derived macrophages. Gene Expr. 2013;16(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, Kobayashi A, Yamaguchi T, Abe M, Amagasa T, Morita I. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314(3):430–440. [DOI] [PubMed] [Google Scholar]
- 97. Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. [DOI] [PubMed] [Google Scholar]
- 99. Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab. 2014;34(4):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ergul A, Valenzuela JP, Fouda AY, Fagan SC. Cellular connections, microenvironment and brain angiogenesis in diabetes: Lost communication signals in the post-stroke period. Brain Res. 2015;1623:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, Lafont A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26(2):281–286. [DOI] [PubMed] [Google Scholar]
- 102. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–2786. [DOI] [PubMed] [Google Scholar]
- 103. Kawasaki T, Nishiwaki T, Sekine A, Nishimura R, Suda R, Urushibara T, Suzuki T, Takayanagi S, Terada J, Sakao S, Tatsumi K. Vascular repair by tissue-resident endothelial progenitor cells in endotoxin-induced lung injury. Am J Respir Cell Mol Biol. 2015;53(4):500–512. [DOI] [PubMed] [Google Scholar]
- 104. Parati EA, Bez A, Ponti D, de Grazia U, Corsini E, Cova L, Sala S, Colombo A, Alessandri G, Pagano SF. Human neural stem cells express extra-neural markers. Brain Res. 2002;925(2):213–221. [DOI] [PubMed] [Google Scholar]
- 105. Ii M, Nishimura H, Sekiguchi H, Kamei N, Yokoyama A, Horii M, Asahara T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ Res. 2009;105(9):860–868. [DOI] [PubMed] [Google Scholar]
- 106. Oishi K, Kobayashi A, Fujii K, Kanehira D, Ito Y, Uchida MK. Angiogenesis in vitro: vascular tube formation from the differentiation of neural stem cells. J Pharmacol Sci. 2004;96(2):208–218. [DOI] [PubMed] [Google Scholar]
- 107. Yang SY, Strong N, Gong X, Heggeness MH. Differentiation of nerve-derived adult pluripotent stem cells into osteoblastic and endothelial cells. Spine J. 2017;17(2):277–281. [DOI] [PubMed] [Google Scholar]
- 108. Kang HS, Moon YJ, Kim YY, Park WY, Park AK, Wang KC, Kim JE, Phi JH, Lee JY, Kim SK. Smooth-muscle progenitor cells isolated from patients with moyamoya disease: novel experimental cell model. J Neurosurg. 2014;120(2):415–425. [DOI] [PubMed] [Google Scholar]
- 109. Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor Proceedings of the National Academy of Sciences of the United States of America. 2004;101(46):16210–16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Masuda J, Ogata J, Yutani C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke. 1993;24(12):1960–1967. [DOI] [PubMed] [Google Scholar]